Abstract
Respiratory virus infections are often pathogenic, driving severe inflammatory responses. Most research has focused on localized effects of virus infection and inflammation. However, infection can induce broad-reaching, systemic changes that are only beginning to be characterized. In the current study, we assessed the impact of acute pneumovirus infection in C57Bl/6 mice on bone marrow hematopoiesis. We hypothesized that inflammatory cytokine production in the lung upregulates myeloid cell production in response to infection. We demonstrate a dramatic increase in the percentages of circulating myeloid cells, which is associated with pronounced elevations in inflammatory cytokines in serum (IFNγ, IL-6, CCL2), bone (TNFα and lung tissue (TNFα, IFNγ, IL-6, CCL2, CCL3, G-CSF, osteopontin). Elevated hematopoietic stem/progenitor cell (HSPC) percentages (Lineage−Sca-I+c-kit+) were also detected in the bone marrow. This increase was accompanied by an elevation in the proportions of committed myeloid progenitors, as determined by colony forming unit assay. However, no functional changes in hematopoeitic stem cells occurred, as assessed by competitive bone marrow reconstitution. Systemic administration of neutralizing antibodies to either TNFα or IFNγ blocked expansion of myeloid progenitors in the bone marrow and also limited virus clearance from the lung. These findings suggest that acute inflammatory cytokines drive production and differentiation of myeloid cells in the bone marrow by inducing differentiation of committed myeloid progenitors. Our findings provide insight into the mechanisms via which innate immune responses regulate myeloid cell progenitor numbers in response to acute respiratory virus infection.
Introduction
Respiratory viruses induce a variety of symptoms and pathologies, with important impacts on health. Most research has focused on characterizing the inflammatory response and disease processes at the site of infection in the airways and lung tissue, but emerging evidence suggests that this inflammatory response does not remain compartmentalized to the lung [1–3]. Rather, localized viral infection can have systemic effects, including elevated circulating cytokines levels and alterations in bone marrow hematopoiesis [1–3]. The systemic response to respiratory viral infections and the impact on disease outcomes remains poorly understood. In our investigation, we gain new insights into the impact of viral lung infection in vivo on systemic immune responses by assessing changes in cytokine levels and alterations in bone marrow hematopoiesis.
Hematopoiesis proceeds through a tightly regulated hierarchy of cell stages, whereby hematopoietic stem cells (HSCs) differentiate through committed multipotent progenitor (MPP) and lineage-specific progenitor stages, before differentiating into mature hematopoietic lineages. During differentiation, hematopoietic stem/progenitor cells (HSPCs) progressively lose multi-lineage potential as they undergo commitment to specific lineages. The regulation of HSPC populations by inflammatory signals and infection has been extensively reviewed [3–5]. Recent findings suggest that, rather than acting as quiescent bystanders, HSPC populations are modulated by inflammatory cytokine stimulation (including IFNγ [6–12] and TNFα [13–16], which feature prominently in respiratory virus infection [17–19]). Inflammatory cytokine stimulation and/or direct interaction of HSPCs with pathogens [3–5] may modulate bone marrow homeostasis [20,21]. Thus, HSPCs respond rapidly and appropriately to distinct inflammatory signals. While a growing body of literature suggests a role for inflammatory cytokines in modulating hematopoiesis, the majority of these studies have been conducted through direct administration of individual cytokines. Relatively few studies have assessed changes during active infection, particularly using assays that quantify HSC and downstream progenitor function. As such, the mechanisms underlying HSPC regulation remain unclear, but have important implications for disease management, particularly as new therapies are being developed targeting inflammatory mediators in disease settings [22].
In the current study, we use pneumonia virus of mice (PVM) in an acute model of respiratory infection [23]. PVM (Family Paramyxoviridae, Genus Pneumovirus) is a natural mouse pathogen related to human respiratory syncytial virus (RSV). PVM infection reproduces many of the clinical and pathological features of severe RSV infection seen in human infants, inducing impaired respiratory function and pro-inflammatory chemokine production [24] and myeloid cell recruitment to the lung [25,26]. In humans, early life exposure to RSV has long-term effects, being associated with increased asthma susceptibility later in life [27,28]. Similarly, PVM infection in early-life can induce an asthmatic phenotype in mice [29], or drive spontaneous asthma-like pathology in the context of TLR7-deficiency [30]. The local response to PVM has been extensively studied and specifically highlights a role for CCL3 in myeloid cell recruitment, viral clearance and clinical outcome [25]. In contrast, there are comparatively few investigations that focus on systemic or hematopoietic responses to acute PVM infection.
In the current study, we demonstrate that acute pneumovirus infection results in a profound increase in myeloid cells in the lung, circulation, spleen and bone marrow, defined by cell surface expression of CD11b, Ly6G and Gr-1 lineage markers. This increase occurs despite restriction of PVM replication to the lung tissue, occurs before the onset of severe symptoms and is associated with increases in both local and systemic inflammatory cytokine levels. We also observe a dramatic increase in Sca-I expression across multiple hematopoietic lineages and increased bone marrow HSPC proportions (assessed using flow cytometry). Functional assays demonstrate this occurs through increased committed myeloid progenitor numbers, with no changes in HSC numbers. Furthermore, systemic administration of neutralizing anti-TNFα or anti-IFNγ antibodies limited myeloid progenitor expansion in the bone marrow, and interfered with viral clearance in the lung demonstrating a central role for viral-induced inflammatory responses in promoting myeloid cell development.
Materials & Methods
Animals, PVM infection and clinical assessment
C57Bl/6 male mice (8–12 weeks of age) were received from University of Newcastle Animal Services Unit and experiments were performed in the Hunter Medical Research Institute (HMRI) animal facility, under specific pathogen-free conditions, following review and approval from the local animal care and ethics committee. Mice were infected by intranasal instillation of 100 pfu PVM strain J3666 in DMEM + 10% FCS, as previously described [31]. This inoculum induces lethal disease, which would require euthanization by approximately day 8–10. Control animals were sham administered DMEM + 10% FCS. Following infection, mice were monitored daily for weight loss and clinical symptoms scored as follows: 1 = no signs of illness, 2 = consistently ruffled fur, 3 = piloerection, deeper breathing and decreased alertness. Animals were euthanized on days 3, 6 or 8, as indicated in the text.
For competitive bone marrow reconstitution experiments, CD45.1 recipient animals were purchased from the Animal Resources Centre (Perth, Australia) and housed at Australian BioResources (Moss Vale, Australia). C57Bl/6 (CD45.2) and CD45.1 X CD57Bl/6 (CD45.1/2) donor mice were infected by intranasal instillation of 100 pfu PVM strain J3666. Animals were then euthanized on day 8, bone marrow cells collected and red blood cell lysis performed using ammonium chloride solution. Cells from PVM-infected donors were mixed 1:1 with sham-infected donors of reciprocal genotypes (ie. PVM-infected CD45.2 with sham-infected CD45.1/2 and vice versa) and injected into lethally-irradiated CD45.1 recipient animals (1100 rad; RS2000 X-ray Irradiator, RadSource; Suwanee, GA). Donor cell mixtures and blood samples then were assessed by flow cytometry, at the timepoints indicated.
To assess the role of TNFα or IFNγ, PVM-infected mice were injected intraperitoneally on days 3 and 6, with 200µg (in 200uL PBS) of either anti-TNFα (clone XT3.11; BioXCell; West Lebanon, NH), anti-IFNγ (clone R4–6A2; BioXCell) or isotype rat IgG1 controls (clone HRPN; BioXCell), respectively and euthanized on day 8 post-infection.
Flow cytometry processing, antibody staining and cell enrichment
Lung tissue, blood, spleen and bone marrow samples were collected and processed to single cell suspensions prior to staining. Lung tissue was digested in HEPES buffer containing collagenase D (Sigma-Aldrich; St Louis, MO) and DNAse for 1 hour, then forced through a 70µm strainer. Cardiac puncture blood was collected in EDTA-coated microvette collection tubes (Sarstedt; Numbrecht, Germany). Spleen samples were forced through 70µm strainers. Bone marrow cells were isolated by flushing femurs with PBS/2% FCS.
Following isolation, red blood cell lysis was performed using ammonium chloride solution. All cells were treated with anti-FcγRIII/II (Fc block) in PBS/2% FCS for 20 minutes prior to staining with combinations of the following fluorochrome conjugated antibodies as indicated in the text (all antibodies from BD Biosciences unless otherwise indicated; San Jose, CA): APC-conjugated lineage cocktail (containing CD3e (145-2C11); CD11b (M1/70); B220 (RA3–6B2); Ly-76 (TER-119); Gr-1 (RB6–8C5)), PE/Cy7-conjugated Sca-1 (D7), PerCP-Cy5.5-conjugated c-kit (2B8), PE-conjugated CD150 (TC15-12F12.2; BioLegend; San Diego, CA), FITC-conjugated CD48 (HM48-1; BioLegend), FITC-conjugated Ly6G (1A8), PE-conjugated F4/80 (BM8; BioLegend), PerCP-Cy5.5-conjugated CD11b (M1/70), APC-conjugated Gr-1 (Ly6C/G; RB6–8C5), FITC-conjugated CD45 (30-F11), PE-conjugated CD3e (145-2C11), FITC-conjugated B220 (RA3–6B2), PerCP-conjugated CD8a (56-6.7) and APC-conjugated CD4 (RM4–5). Competitive reconstitution samples were also stained using: FITC-conjugated CD45.1 (A20) and PE-conjugated CD45.2 (104). For flow cytometry assessment, samples were fixed overnight in PBS/2% FCS + 0.1% PFA and collected on a BD FACSCanto II flow cytometer, then analysed using FACSDiva software (BD BioSciences).
For cell enrichment experiments, lung tissues were digested, treated with anti-FcγRIII/II (Fc block) and stained with FITC-conjugated Ly6G (1A8) and APC-conjugated Gr-1 (Ly6C/G; RB6–8C5) antibodies, as described. Cells were then sequentially magnetically enriched using first anti-FITC (Ly6G+) microbeads, followed by anti-APC microbeads (Gr-1+Ly6G−), according to manufacturers specifications (Miltenyi). Enrichment was confirmed by flow cytometry on a BD FACSCanto II flow cytometer. Cells were prepared by cytospin and stained by modified Wright Geimsa stain, visualised on an Olympus BX51 microscope and imaged on a DP73 digital camera (Olympus) or resuspended in Trizol Reagent (Invitrogen) for RNA isolations.
Cytokine assessment
Lung tissue was disrupted in radioimmunoprecipitation assay buffer (RIPA; Sigma-Aldrich) with protease/phosphatase inhibitor cocktail (Cell Signaling Technology; Danvers, MA), on a Tissuelyser LT tissue disruptor (Qiagen; Valencia, CA) at 50 Hz for 5 minutes and stored at −80C. Serum was collected by cardiac puncture and centrifugation, after allowing blood to fully clot on ice. Cytokine levels for IL-6, IL-10, CCL2 (MCP-1), IFNγ, TNFα and IL-12p70 were assessed using the mouse inflammation cytometric bead array kit (BD BioSciences), according to manufacturers specifications on a BD FACS Canto II flow cytometer and analysed using FCAP Array software (BD BioSciences)
RNA isolation, reverse-transcription (RT)-PCR and quantitative PCR (qPCR) assessment
Lung tissue was placed in RNALater (Invitrogen; Carlsbad, CA) and stored at −80C. Unflushed femurs were disrupted in Trizol Reagent (Invitrogen), on a Tissuelyser LT tissue disruptor (Qiagen) at 50 Hz for 5 minutes and stored at −80C. Total RNA was isolated by phenol-chloroform separation and isopropanol precipitation and quantified on a Nanodrop 1000 spectrophotometer (Nanodrop; Wilmington, DE). cDNA was prepared by RT-PCR using random hexamer primers (Invitrogen) and MMLV reverse transcriptase (Invitrogen), on a T100 thermal cycler (Bio-Rad; Hercules, CA). Relative qRT-PCR quantification was performed on a Via7 real-time PCR machine (Life Technologies; Carlsbad, CA), using SYBR reagents. Measured cDNA levels were normalized to the housekeeping gene HPRT. Primer sets (Table I) were designed across exon boundaries to specifically amplify mRNA products.
Table I.
Primer sequences used for qPCR analysis
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| PVM SH | gcc tgc atc aac aca gtg tgt | gcc tga tgt ggc agt gct t |
| TNFα | acc acg ctc ttc tgt cta ctg aac t | gcg ttg gcg cgc tgg ctc agc cac t |
| IFNγ | tct tga aag aca atc agg cca tca | gaa tca gca gcg act cct ttt cc |
| CCL2 | cca act ctc act gaa gcc agc tct | tca gca cag acc tct ctc ttg agc |
| IL-6 | aga aaa caa tct gaa act tcc aga gat | gaa gac cag agg aaa ttt tca ata gg |
| CCL3 | cct ctg tca cct gct caa ca | gat gaa ttg gcg tgg aat c |
| G-CSF | gtg ctg ctg gag cag ttg t | tcg gga tcc cca gag agt |
| Sca-I | gtc tgt gtt act cag gag gca gca | tgc tac att gca gag gtc ttc ctg |
| Osteopontin | act ttc act cca atc gtc cct aca | ggc atc agg ata ctg ttc atc aga |
| GM-CSF | tac ttt tcc tgg gca ttg tgg tct | ccc gta gac cct gct cga ata tct |
| IL-3 | gaa gct ccc aga acc tga act caa | gca gat gta ggc agg caa cag tta |
| Arginase | gct cca agc caa agt cct tag aga t | agg agc tgt cat tag gga cat caa c |
| iNOS (NOS2) | agc gag gag cag gtg gaa gac tat | cca tag gaa aag act gca ccg aag |
| IL-10 | cat ttg aat tcc ctg ggt gag aag | gcc ttg tag aca cct tgg tct tgg |
| HPRT | agg cca gac ttt gtt gga ttt gaa | caa ctt gcg ctc atc tta ggc ttt |
Note: All primer sequences are indicated in 5’ – 3’ orientation.
Colony-forming unit (CFU) assays
Bone marrows were initially processed to single cell suspensions as described above. Cells were then plated in duplicate at 2×104 cells/plate, in Methocult GF3534 (Stem Cell Technologies; Vancouver, Canada), containing FBS, BSA, rh-Insulin, human transferrin, 2-mercaptoethanol, rm-SCF and rm-IL6. Plates were cultured for 7-days and myeloid colonies (CFU-G/M/GM) were counted by light microscopy, according to manufacturer’s instructions.
Statistical analysis
P values were calculated using unpaired two-way Student’s t test.
Results
Acute PVM respiratory infection induces systemic increases in myeloid cells
Inoculation of C57Bl/6 mice with PVM (100 pfu) resulted in rapid and significant weight loss (detected at day 6 post-inoculation) and the onset of clinical symptoms immediately prior to sacrifice on day 8 (Figure 1A and 1B). Virus was detected in lung tissue as early as day 3 post-inoculation, with a peak viral load at day 6 coinciding with the onset of weight loss at this inoculum (Figure 1C). Importantly, PVM virus was not detected in spleen or bone marrow at any timepoint by qPCR (data not shown). Leukocyte populations in lung, blood, spleen and bone marrow were evaluated at days 6 and 8 post-inoculation and compared to sham-inoculated controls.
Figure 1. Acute PVM Infection Induces Increased Systemic Myeloid Cell Percentages.
C57Bl/6 mice were infected via intranasal instillation of 100 pfu of pneumovirus of mice (PVM). Animals were monitored daily for A) weight loss and B) clinical scores. At days indicated post-infection, C) lung PVM viral load was assessed by qPCR, normalized to standards and expressed as copies PVM/copies HPRT. Cell populations were quantified at days 6 and 8 post-inoculation by flow cytometry in D/H) lung, E/I) blood, F/J) spleen and G/K) bone marrow, presented as percentage of CD45+ cells for lung, blood and spleen and percentage of total cells for bone marrow. (Data is represented as mean +/− SEM, representative of >3 replicate experiments, n=4–6 animals/group. * represents p<0.05, ** p<0.01, *** p<0.001 compared to sham).
Increased myeloid cell percentages were detected in lung tissue by flow cytometry using the markers Gr-1 and CD11b, compared to sham–infected controls (Figure 1D/H). Specifically, the total percentage of Gr-1+ cells was increased in PVM-infected mice at day 6, with a further increase by day 8 (Figure 1D/H). All Gr-1+ cells co-expressed CD11b in both sham and PVM-infected mice. Further subdividing the Gr-1+ population, two subpopulations (Gr−1+Ly6G+ (SSChi) and Gr-1+Ly6G− (SSClo)) were evident, both of which were increased after PVM infection. A slight increase was also observed in the percentage of Gr-1−CD11b+ cells within the lung, after PVM infection. These staining profiles are characteristic of neutrophils (Gr-1+Ly6G+), infiltrating monocytes/macrophages (Gr-1+Ly6G−) and interstitial macrophages (Gr-1−CD11b+) respectively, based on previous studies [32]. This increased cell proportion (displayed as % of total CD45+ cells) coincided with a consistent increase in total cell numbers isolated from PVM-infected lungs following processing, compared to sham controls, indicating a marked increase in neutrophil and monocyte infiltration into the lung.
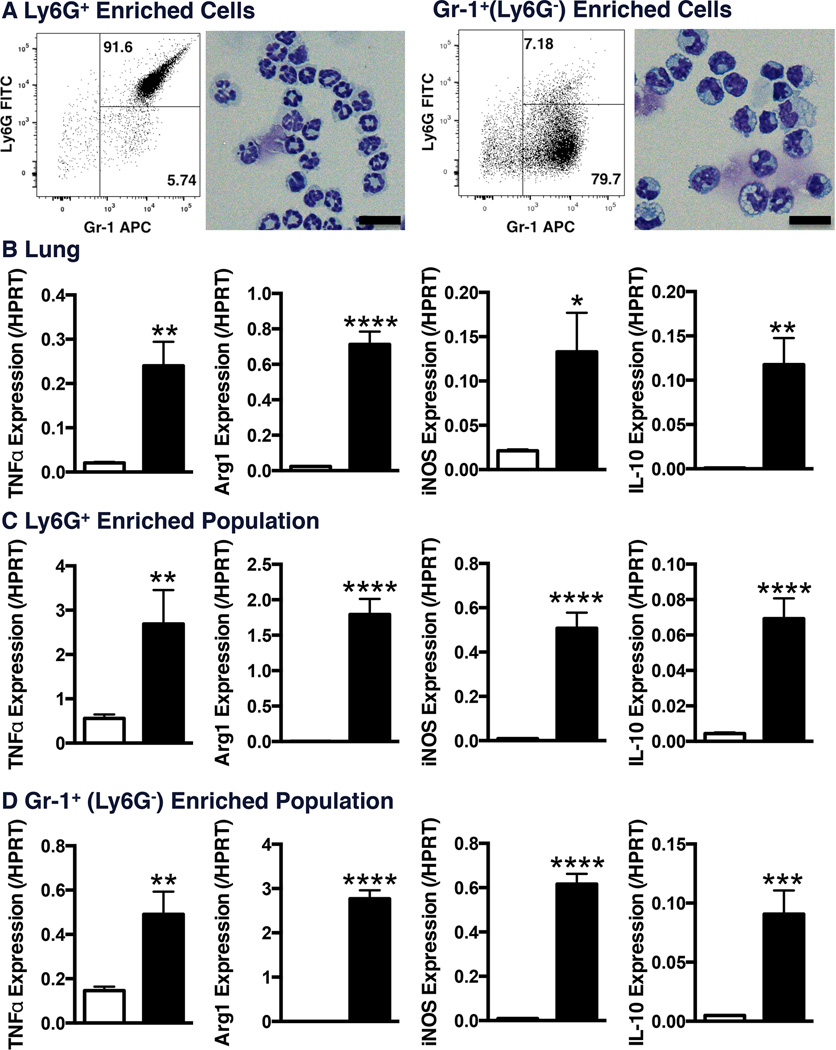
Magnetic enrichment of the Gr-1+Ly6G+and Gr-1+Ly6G− populations from lung tissue for histological assessment at day 8 post-inoculation confirmed granulocytic and monocytic morphology, respectively (Figure 2A). These surface marker profiles and morphology overlap with recently described myeloid-derived suppressor cells (MDSC) populations. Characterisation of mRNA expression in total lung samples and isolated cell populations revealed increased TNFα expression following PVM infection, as well as increased levels of Arg1, iNOS and IL-10 expression (Figure 2B/C/D), which are expressed by MDSCs [33–35].
Figure 2. Characterization of Induced Lung Myeloid Cell Populations.
C57Bl/6 mice were infected via intranasal instillation of 100 pfu of pneumovirus of mice (PVM). At day-8 post-inoculation, digested lung samples were stained and magnetically enriched to isolate Ly6G+ and Gr-1+(Ly6G) populations. A) Cytospin samples were stained by modified Wright Geimsa stain (Scale bar corresponds to 20µm). qPCR quantification of indicated genes were performed on RNA samples from B) total lung tissue, C) Ly6G+ or D) Gr-1+(Ly6G) enriched cells. (Data is represented as mean +/− SEM, representative of 2 replicate experiments, n=3 samples pooled from 2 mice each/group. * represents p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001 compared to sham).
To assess systemic effects on myeloid cell levels, we evaluated peripheral blood, spleen and bone marrow. In blood and spleen, Gr-1+Ly6G+ myeloid cells (primarily neutrophils [36]) were dramatically increased at the day 8 endpoint over sham-inoculated controls (Figure 1I/J). In contrast, both Gr1+Ly6G− (monocyte/macrophage) and lymphoid cell percentages were unaffected, with only a slight increase in CD3+CD8+ lymphocytes after PVM infection (Figure 1J and data not shown). Similar changes were also seen in the blood at day 6 post-inoculation, although again to a lesser extent (Figure 1E), while myeloid cells percentages in the spleen were decreased at day 6 post-inoculation (Figure 1F). In bone marrow, Gr-1+Ly6G+ (neutrophil) percentages were unaffected by infection, while Gr1+Ly6G− (monocyte/macrophage) percentages were increased (Figure 1G/K). Total bone marrow cell counts were unaffected by PVM infection.
PVM infection in the lung results in both localized and systemic increases in inflammatory mediators
To identify mechanisms driving increased myeloid cell percentages during acute infection, we assessed both local and systemic inflammatory mediator levels. In the lung, PVM infection induced expression of cytokines TNFα, IL-6, IFNγ and CCL2 (Figure 3A). IL-6, IFNγ and CCL2 were also detected in serum following infection (Figure 3B), while serum TNFα levels were variable, around the limit of detection in our assay (data not shown). Quantitative PCR of lung tissue confirmed increased levels of these cytokines, and likewise documented expression of CCL3, G-CSF and osteopontin, with kinetics corresponding to the timing of peak viral load (Figure 3A and data not shown). In total bone samples, TNFα transcript levels progressively increased 8 days post-inoculation (Figure 3C). In separate experiments, the increase in TNFα expression was observed in isolated bone marrow samples, as well as samples isolated from flushed bone (data not shown). Assessment of GM-CSF and IL-3 transcript levels by qPCR in lung, spleen and bone failed to identify any changes in expression following infection (data not shown).
Figure 3. PVM Infection Induces Local and Systemic Inflammatory Cytokines.
Inflammatory cytokine expression determined at day 8 post-infection by cytokine bead array in A) lung homogenates and B) serum samples. mRNA expression assessed by qPCR and normalized to HPRT, in A) lung, and C) bone. (Data is represented as mean +/− SEM, representative of 2 replicate experiments, n=4–8 animals/group. * represents p<0.05, ** p<0.01, *** p<0.001 compared to sham).
PVM infection results in increased HSPC numbers, specifically committed myeloid progenitors, in the bone marrow
We observed an increase in the percentages of HSPCs, based on LSK (Lineage−Sca-I+ckit+) staining of cells from bone marrow at day 8 of infection (Figure 4A and 4C); increased LSK percentages were not present at day 3 post-infection and minor increases were observed at day 6 (Figure 4B). The LSK cell gate is enriched for HSCs, and is commonly used to quantify stem cell numbers, but also includes more committed MPPs [37]. Further assessment using the signalling lymphocyte activation molecule (SLAM) markers (CD48 and CD150) demonstrate that the observed increase in the LSK population results from a minor increase in CD48−CD150+ cells (a population further enriched for HSCs) and a major increase in the percentages of CD48+CD150− cells, which are enriched for MPP populations (Figure 4B/C) [38]. Further, in the spleen the percentages of LSK cells, specifically within the CD48+CD150− MPP gate, was also increased (Figure 4D). Interestingly, proportions of LSK cells (specifically the MPP subset) were also increased in a low dose infection model (12 pfu) at day 8 (Supplementary Figure 1). These findings indicate that MPP proportions are increased following viral infection, but do not require severe disease.
Figure 4. Increased HSPC Populations After PVM Infection.
Hematopoietic stem/progenitor cell (HSPC) percentages were assessed at days 6 and 8 post-inoculation by flow cytometry using LSK (LinSca-I+c-kit+) and SLAM (CD48 CD150) marker staining in A/B/C) bone marrow and D) spleen. A) Representative graphs from bone marrow display c-kit and Sca-I expression have been gated to display only Lineage events. (Data is represented as mean +/− SEM, representative of >3 replicate experiments, n=3–5 animals/group. * represents p<0.05, ** p<0.01, *** p<0.001 compared to sham).
To characterize this increase in HSPCs, we assessed the number of committed myeloid progenitors using a colony-forming unit (CFU) assay. Following PVM infection, the total number of bone marrow myeloid CFU were increased slightly (but significantly) at day 6 and increased approximately 2-fold at day 8, compared to sham-infected controls (Figure 5A). This increase occurred for bi-potent granulocyte/macrophage (CFU-GM) progenitors, as well as committed monocyte (CFU-M) progenitor subsets at day 6, and also for the granulocyte (CFU-G) progenitors at day 8 (Figure 5A). Interestingly, although increased HSPCs were also observed in the spleen by flow cytometry, no difference was observed in myeloid CFU numbers in either spleen or lung tissue (data not shown), suggesting that acute PVM infection fails to induce significant extramedullary hematopoiesis.
Figure 5. Increased Myeloid Multipotent Progenitors, but Not HSCs, After PVM Infection.
Committed myeloid progenitors were assessed at days 6 and 8 post-inoculation by A) colony-forming unit (CFU) assay on bone marrow cells. Hematopoietic stem cells (HSCs) were assessed by competitive bone marrow reconstitution using sham- and PVM-infected bone marrow (1:1) transplanted into lethally irradiated recipients. Relative donor contribution was assessed in B) bone marrow at transfer (day 0) and final endpoint (week 7 post-transplant) and C) blood samples assessed over 6-weeks post-transplant. (Data is represented as mean +/− SEM, representative of 3 replicate experiments, n=3–5 animals/group (CFU assay) and one experiment, n=4–5 animals/group (competitive reconstitution). * represents p<0.05, ** p<0.01).
To assess changes in HSC number following PVM infection, competitive reconstitution experiments were performed using the CD45.1/2 system. Bone marrow cells from sham-infected and PVM-infected donors were mixed 1:1 and injected into lethally-irradiated recipient animals. In donor cell mixtures, ratios of total cell numbers from each donor were confirmed at ∼50%, while consistent with our previous observations ∼75–80% of the LSK population (in the 1:1 mixture) were from PVM-infected donors (Figure 5B). Donor contributions to hematopoiesis were monitored in peripheral blood for 6 weeks post-reconstitution and total donor reconstitution levels of >95% were achieved. Assessment of relative donor contributions revealed no difference in the relative contributions of sham versus PVM-infected donors to reconstitution (Figure 5C; right panel). Further, at the 7-week endpoint, bone marrow exhibited a ∼50% contribution from PVM-infected and sham-infected donor cells to both total bone marrow and LSK subsets (Figure 5B). These findings indicate that the increase in phenotypic LSK cells following acute PVM infection results from an increase in the number of committed myeloid progenitors, while functional HSC numbers remain unaltered.
Increased Sca-I expression on hematopoietic lineages following PVM infection
Of interest, we also observed increased Sca-I expression in the bone marrow, lung and spleen by flow cytometry and qPCR assessment following PVM infection. In total bone and lung samples, Sca-I mRNA levels increased progressively throughout the time-course of PVM infection (Figure 6A and 6B). Further, Sca-I expression was increased across all hematopoietic (CD45+) cell subsets assessed by Sca-I staining mean fluorescence intensity (MFI) in bone marrow (Figure 6A). In the lung, Sca-I surface staining occurred on CD3+ T cell populations, as previously described [39], as well as mature myeloid populations, presented as the percentage of Sca-I+ (/CD45+) cells and Sca-I mean fluorescence intensity (MFI) (Figure 6B). In the spleen, increased Sca-I mRNA levels were also seen at day 8 post-infection, as well as increased Sca-I expression assessed by staining MFI (Figure 6C). These findings demonstrate that Sca-I expression is increased across a range of hematopoietic lineages.
Figure 6. Increased Sca-I Expression After PVM Infection.
Sca-I expression was assessed at day 8 post-infection by qPCR and flow cytometry in A) bone, B) lung and C) spleen. (Data is represented as mean +/− SEM, representative of >3 replicate experiments, n=3–5 animals/group. * represents p<0.05, ** p<0.01, *** p<0.001 compared to sham).
Systemic administration of TNFα-neutralizing antibodies impairs viral clearance and reduces myeloid progenitor induction
To determine a mechanism underlying the viral-induced increases in myeloid cell production, we systemically (i.p.) administered a neutralizing antibody against TNFα (or a rat IgG1 isotype control) on days 3 and 6 post-inoculation into PVM-infected mice. The intervention timepoints were chosen based on our kinetic analysis, as TNFα expression increased in lung tissue and bone between days 3 and 6 post-infection (Figure 3C). Antibody administration had no effect on weight loss or clinical symptoms, compared to isotype control (data not shown).
At the day 8 endpoint, increased virus recovery occurred from the lungs of mice receiving anti-TNFα (Figure 7A), although no differences were observed in myeloid cell infiltration into the lung (data not shown). Assessment of lung and serum cytokine levels demonstrate that anti-TNFα administration partially reduced levels of TNFα in lung homogenates, but had no effect on the induction of other inflammatory mediators within the lung or serum (Figure 7B).
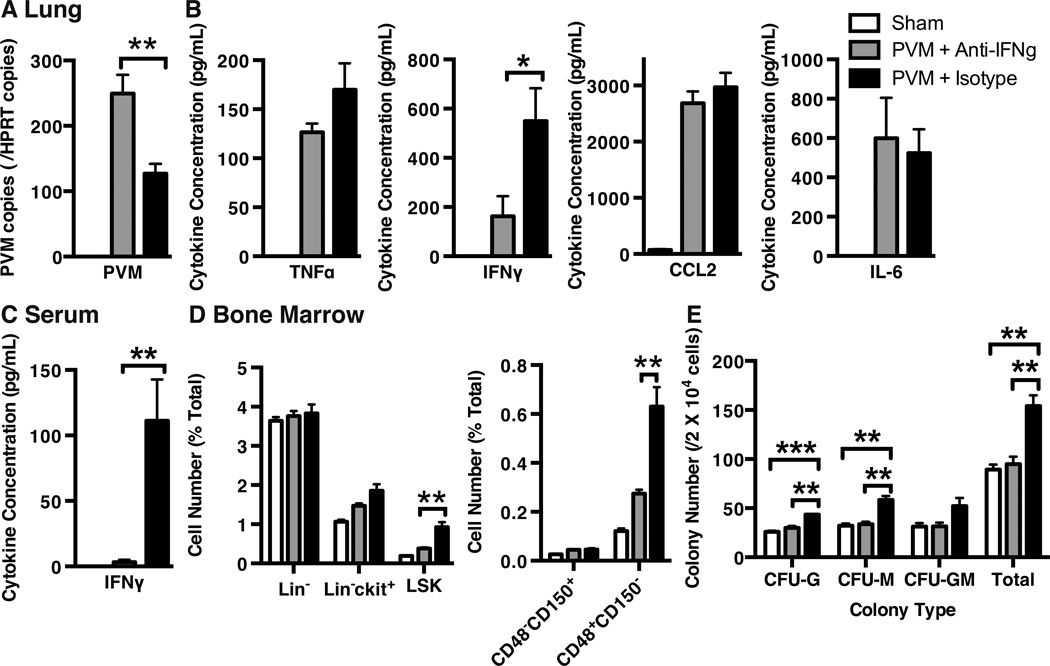
Figure 7. Anti-TNFα Administration Results in Increased Viral Load and Decreased Induction of LSK and CFU Populations.
C57Bl/6 mice were infected via intranasal instillation of 100 pfu of pneumovirus of mice (PVM) and administered anti-TNFα antibody (or isotype controls) i.p. on days 3 and 6 post-infection. At the day 8 endpoint, A) lung PVM viral load was assessed by qPCR and B) tissue cytokines were assessed by cytokine bead array. C) Hematopoietic progenitor percentages were assessed by flow cytometry and D) colony-forming assay. (Data is represented as mean +/− SEM, representative of 3 replicate experiments, n=3–7 animals/group. * represents p<0.05, ** p<0.01).
Despite increased virus recovery, mice receiving anti-TNFα antibody responded with decreased induction of LSK cells in the bone marrow, particularly within the CD48+CD150− MPP cell subset (Figure 7C). Further, anti-TNFα administration resulted in striking decreases in the numbers of myeloid CFU in the bone marrow, with numbers at the baseline levels observed in sham-infected animals (Figure 7D). Taken together, these findings demonstrate a role for TNFα in virus-induced bone marrow HSPC expansion, myeloid cell production and virus clearance in the lung.
Systemic administration of IFNγ-neutralizing antibodies impairs virus clearance and reduces myeloid progenitor induction
In a separate set of experiments, we also assessed the role of IFNγ in the observed changes following acute PVM infection. IFNγ-neutralizing antibody (or a rat IgG1 isotype control) was also administered systemically on days 3 and 6 post-infection, based on kinetics of IFNγ production following infection (Figure 3C).
Similar to our findings with anti-TNFα administration, anti-IFNγ resulted in increased PVM viral recovery from the lungs at day 8 (Figure 8A), but had no effect on myeloid cell infiltration into the lung (data not shown). Assessment of cytokine levels in the lung and serum revealed a striking decrease in IFNγ protein levels following antibody treatment (Figure 8B and 8C). In the bone marrow, anti-IFNγ resulted in dramatic decreases in LSK cell induction and MPP cell numbers (among the LSK population) (Figure 8D). Functional assessment of CFUs in the bone marrow revealed an ablation of myeloid progenitor induction following anti-IFNγ administration (Figure 8E), similar to our findings with anti-TNFα. These findings suggest that IFNγ plays a role analogous to that played by TNFα following lung PVM infection, driving increases in myeloid progenitors, as well as virus clearance in the lung.
Figure 8. Anti-IFNγ Administration Results in Increased Viral Load, Decreased Induction of LSK and CFU Populations and Decreased Sca-I.
C57Bl/6 mice were infected via intranasal instillation of 100 pfu of pneumovirus of mice (PVM) and administered anti-IFNγ antibody (or isotype controls) i.p. on days 3 and 6 post-infection. At the day 8 endpoint, A) lung PVM viral load was assessed by qPCR and B) tissue cytokines were assessed by cytokine bead array. C) Serum cytokine levels were assessed by cytokine bead array. D) Hematopoietic progenitor percentages were assessed by flow cytometry and E) colony-forming assay. (Data is represented as mean +/− SEM, representative of 3 replicate experiments, n=3–7 animals/group. * represents p<0.05, ** p<0.01, *** p<0.001).
Discussion
Previous research has primarily focussed on localized myeloid cell infiltration, inflammatory cytokine release and tissue damage following PVM infection [24–26,39,29]. However, numerous clinical studies have documented increased systemic inflammatory cytokine levels in virally-infected patients, including increased serum IL-2, IL-4 and IFNγ following RSV infection [1] and increased serum CCL2 levels in RSV-infected asthmatics [40] (while serum TNFα levels remain low [41]). Further, in severe influenza infection, IL-6, IFNγ and IL-10 levels are raised [2,42]. Similar to these clinical findings, we observed increased serum levels of IFNγ, CCL2 and IL-6, as well as increased TNFα transcript in the bone, following PVM infection. Of these factors, we demonstrate an important role for TNFα and IFNγ, in modulating hematopoiesis in response to infection.
In parallel with elevated systemic cytokines, we observed increased systemic myeloid cell percentages following PVM infection. Specifically, neutrophil proportions (Gr1+Ly6G+) were dramatically increased in the blood and spleen, while inflammatory monocytes/macrophages (Gr1+Ly6G−) were increased in the bone marrow. We speculate that the mild changes in the bone marrow may reflect the rapid egress of newly generated cells from the marrow niche into the circulation. Interestingly, increased systemic myeloid cell numbers (reported as CD11b+Gr1+) in the lung, blood and bone marrow was also recently reported in severe H5N1/H1N1 influenza infections in mice [43].
While the staining profiles of these cell populations are consistent with traditional neutrophil and monocyte/macrophage populations, they also overlap with recently described MDSC populations. MDSCs suppress T cell responses and have been best characterized in cancer models (reviewed in [44]). They have also been described in infectious disease models, including lung fungal infection [45] and viral infections [46,47]. Importantly, MDSCs are nearly indistinguishable from pro-inflammatory myeloid cell populations by histology and surface marker staining and controversy remains over whether they exist as distinct cells lineages or are alternative activation states of these cell populations [48]. We demonstrate increased TNFα expression following PVM infection, consistent with the presence of proinflammatory myeloid cells (Figure 2C/D). Interestingly, we also detected increased levels of Arg1, iNOS and IL-10 expression, which are expressed by MDSC populations [33–35]. However, IL-10 protein levels were not detected in lung homogenate samples assessed by cytometric bead array. These findings demonstrate that this induced myeloid population may include a mixed population including MDSCs and inflammatory myeloid cells.
We also observed increased HSPCs in the bone marrow, following PVM infection, with increased committed progenitors (MPPs) assessed by flow cytometry (LSK CD48+CD150−) and colony-forming assays. Importantly, these findings occur in the absence of detectable systemic PVM virus in the spleen and bone (by qPCR), suggesting changes are not driven by direct interactions between pathogen and HSPCs (e.g. TLR stimulation of HSPCs), as has been proposed in other models [3,4]. Rather, we suggest an indirect feedback mechanism, whereby anti-viral responses initiated in the lung signal via pro-inflammatory cytokines to the bone marrow, promoting myeloid cell production. This also fits with data from a low-dose PVM recovery model (Supplementary Figure 1), where increases in MPP levels coincided with the kinetics of increased lung inflammatory cytokine expression.
Importantly, our initial observations of increased LSK cell percentages (as well as a minor increase in LSK CD48−CD150+) suggested the possibility of a PVM-induced increase in bone marrow HSC numbers. However, previous studies have demonstrated that inflammatory cytokines (including both TNFα and IFNγ) increase Sca-I expression on multiple hematopoietic cells [49], making conclusions based on phenotypic analysis alone problematic. Furthermore, recent evidence suggests that Sca-I induction on myeloid precursors plays an important functional role in driving the granulopoietic response to bacterial infection [50,51]. Increased Sca-I expression results in an apparent increase in phenotypic HSCs (if HSCs are defined based on LSK surface staining alone), emphasizing the importance of performing functional assays to quantify HSCs. Indeed, we observed striking increases in Sca-I expression across multiple hematopoietic lineages in the lung, spleen and bone marrow. While we observed increased numbers of committed myeloid progenitors (CFU-GM, CFU-G, CFU-M), no differences in bone marrow reconstitution capacity were detected. These results indicate that HSC numbers are not affected by PVM infection. Rather, hematopoiesis is altered through an increase in committed MPPs and myeloid progenitors.
As altered cytokine levels are frequently observed in viral-infected patients (as mentioned above) and inflammatory cytokines have been proposed as a mechanism regulating HSPC proliferation and differentiation, we assessed levels in our model. Previous research on PVM pathogenesis identified a role for CCL3 (MIP-1α) in myeloid cell recruitment to the lungs [25]. Interestingly, CCL3-induced recruitment of neutrophils was coordinated by IFNγ [25]. The macrophage chemokine CCL2 (MCP-1) is also induced by PVM infection [24,26]. While these chemokines drive local recruitment of mature myeloid cells, it remains unclear what drives increased systemic myeloid cell numbers. GM-CSF, G-CSF and IL-3 have well-established roles regulating myeloid progenitor cell differentiation [52–54], while osteopontin can induce myeloid cell migration [55,56]. IL-6 acts as a potent pro-inflammatory cytokine and recent evidence suggests its production in the bone marrow environment may induce emergency myelopoiesis [57]. Based on these previous reports, we assessed the levels of these cytokines in our infection model. We observed increased TNFα, IFNγ, CCL2, CCL3, IL-6, G-CSF and osteopontin levels in lung tissue, as well as increased circulating levels of IFNγ, CCL2 and IL-6 and increased TNFα mRNA in bone. Based on the proposed roles for TNFα and IFNγ in regulating and inducing HSPC differentiation, we targeted these molecules using blocking antibodies.
TNFα induces HSC proliferation, while limiting long-term engraftment potential [13,14] and plays a role in the maintenance of baseline homeostasis in the absence of infection [15,16]. In antibody-blocking experiments, anti-TNFα antibody administration decreased both MPP induction (LSK CD48+ CD150−) and myeloid CFU induction. This decrease in myeloid HSPC induction occurred despite increased PVM viral load within the lung, highlighting a role for TNFα in infection-driven myeloid cell production and a disconnect between viral levels and HSPC induction. Our results differ slightly from other observations on the effects of anti-TNFα interventions in mouse models of RSV infection. In one study, mice were primed with recombinant vaccinia virus and subsequently infected with RSV (or influenza), causing rapid weight loss over the first 4-days post-infection [58]. Systemic blockade of TNFα resulted in decreased weight loss, reduced lung infiltration and pathology, decreased IFNγ production from CD4+ T cells, but had no effect on viral load [58], quite different from our observations. Another study assessed RSV infection in unprimed mice, which demonstrated minimal weight loss and pathology [59]. In this model, TNFα depletion resulted in very minor decreases in peak weight loss and slightly reduced cell recruitment to the lung, while also limiting the number of RSV-specific T cells, resulting in increased viral load [59]. Further, previous work demonstrated that anti-TNFα treatment in RSV-infected mice resulted in increased weight loss and delayed recovery [17]. Importantly, none of these studies assessed the impact of anti-TNFα on bone marrow hematopoiesis or myeloid progenitor numbers. Taken together, these findings suggest that the effect of anti-TNFα may differ depending on the timing of treatment or infection and the severity of disease.
IFNγ also has an impact on hematopoietic stem cell (HSC) proliferation and survival, with varying effects reported, including apoptosis induction [10,11], impaired proliferation [9] and promotion of proliferation and differentiation [6–8,12]. As such, it remains unclear what effect IFNγ has on HSPC function and whether effects vary based on disease-specific conditions. Further, IFNγ exposure induces Sca-I expression on isolated Lineage−c-kit+ cells [60]. Antibody-mediated blocking of IFNγ decreased LSK cell induction (which may in part be explained by decreased IFNγ-mediated Sca-I induction) and blocked induction of myeloid CFUs, demonstrating a role for IFNγ in driving increased myeloid progenitor numbers. As with our observations using anti-TNFα, anti-IFNγ also resulted in increased PVM viral load in the lung, demonstrating a requirement for IFNγ in the appropriate clearance of lung viral infection. Taken together, these experiments suggest that TNFα and IFNγ may function through a similar pathway to induce increased myeloid cell progenitors in the bone marrow following viral infection.
While both anti-TNFα and anti-IFNγ treatment resulted in reduced MPP proportions and increased viral recovery, our study cannot make conclusions over whether a direct link exists between these observations. TNFα and IFNγ are each important mediators of antiviral defense and the observed increase in viral levels may reflect localized effects within the lung. Further, while the interventions impacted on myeloid cell production in the marrow, no change was observed in myeloid cell recruitment to the lung. This recruitment is likely driven by chemokine production in the lung, which was unaltered by either anti-TNFα or anti-IFNγ treatment. Thus, while our observations clearly demonstrate a role for both TNFα and IFNγ in the upregulation of myeloid cell production following lung infection, the direct impact of this increase on antiviral immunity remains unclear.
Intriguingly, recent evidence in long-term autoimmune colitis models [52] revealed very similar alterations to those observed in our model. Induction of colitis resulted in increased HSC populations in the spleen and bone marrow (assessed by flow cytometry), through an IFNγ-dependent mechanism [52]. Of note, increased HSC numbers was not confirmed functionally. Induced HSC populations were skewed towards the myeloid lineage, and GM-CSF induced extramedullary hematopoiesis within the spleen and colon [52] (which was not observed in PVM infection). The similarity between this report and our current findings highlight the key role for inflammatory cytokines in regulating hematopoiesis and myeloid cell production, and demonstrate a conserved mechanism underlying myeloid cell production across a range of inflammatory conditions. Further, differences between disease models, may highlight tailored responses, which are modulated by the duration, cause and dynamics of disease.
In summary, our study provides evidence that localized pneumovirus infection drives systemic inflammatory responses, promoting increased myeloid cell production and differentiation in the bone marrow, and increased systemic myeloid cell percentages. Increased myeloid cell production occurs in the absence of viremia and is associated with responses to the cytokines TNFα and IFNγ. This paradigm may provide a more general mechanism whereby the innate immune system recognizes localized infection and up-regulates myeloid cell differentiation, so as to respond appropriately and augment pathogen clearance.
Supplementary Material
Acknowledgements
The authors would like to thank the Analytical Biomolecular Research Facility (ABRF) at the University of Newcastle for flow cytometry support.
Financial Support:
This work was supported by project grants from the National Health and Medical Research Council (NHMRC) of Australia, the NIAID Division of Intramural Research and a start-up grant from the University of Newcastle. SM is supported by fellowships from the Canadian Institutes of Health Research (CIHR) and the University of Newcastle.
Footnotes
Authorship Statements
S.M. designed research, performed research, collected data, analysed and interpreted data, performed statistical analysis and wrote the manuscript. N.G.H. performed research, collected and analysed data and wrote the manuscript. H.L.T. performed research, collected data, analysed and interpreted data and wrote the manuscript. J.S. performed research, collected data, analysed data and wrote the manuscript. M.P. interpreted data and wrote the manuscript. B.D. performed research, collected data, analysed data and wrote the manuscript. H.F.R. contributed vital reagents, interpreted data and wrote the manuscript. P.S.F. designed research, interpreted data and wrote the manuscript.
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Chen ZM, Mao JH, Du LZ, Tang YM. Association of cytokine responses with disease severity in infants with respiratory syncytial virus infection. Acta Paediatr. 2002;91(9):914–922. doi: 10.1080/080352502760272588. [DOI] [PubMed] [Google Scholar]
- 2.Paquette SG, Banner D, Zhao Z, Fang Y, Huang SS, Leomicronn AJ, Ng DC, Almansa R, Martin-Loeches I, Ramirez P, Socias L, Loza A, Blanco J, Sansonetti P, Rello J, Andaluz D, Shum B, Rubino S, Lejarazu ROde, Tran D, Delogu G, Fadda G, Krajden S, Rubin BB, Bermejo-Martin JF, Kelvin AA, Kelvin DJ. Interleukin-6 is a potential biomarker for severe pandemic H1N1 influenza A infection. PLoS One. 2012;7(6):e38214. doi: 10.1371/journal.pone.0038214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King KY, Goodell MA. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat Rev Immunol. 2011;11(10):685–692. doi: 10.1038/nri3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends in immunology. 2011;32(2):57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119(13):2991–3002. doi: 10.1182/blood-2011-12-380113. [DOI] [PubMed] [Google Scholar]
- 6.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465(7299):793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brugger W, Mocklin W, Heimfeld S, Berenson RJ, Mertelsmann R, Kanz L. Ex vivo expansion of enriched peripheral blood CD34+ progenitor cells by stem cell factor, interleukin-1 beta (IL-1 beta), IL-6, IL-3, interferon-gamma, and erythropoietin. Blood. 1993;81(10):2579–2584. [PubMed] [Google Scholar]
- 8.Caux C, Moreau I, Saeland S, Banchereau J. Interferon-gamma enhances factor-dependent myeloid proliferation of human CD34+ hematopoietic progenitor cells. Blood. 1992;79(10):2628–2635. [PubMed] [Google Scholar]
- 9.de Bruin AM, Demirel O, Hooibrink B, Brandts CH, Nolte MA. Interferon-gamma impairs proliferation of hematopoietic stem cells in mice. Blood. 2013;121(18):3578–3585. doi: 10.1182/blood-2012-05-432906. [DOI] [PubMed] [Google Scholar]
- 10.Selleri C, Sato T, Anderson S, Young NS, Maciejewski JP. Interferon-gamma and tumor necrosis factor-alpha suppress both early and late stages of hematopoiesis and induce programmed cell death. Journal of cellular physiology. 1995;165(3):538–546. doi: 10.1002/jcp.1041650312. [DOI] [PubMed] [Google Scholar]
- 11.Zeng W, Miyazato A, Chen G, Kajigaya S, Young NS, Maciejewski JP. Interferon-gamma-induced gene expression in CD34 cells: identification of pathologic cytokine-specific signature profiles. Blood. 2006;107(1):167–175. doi: 10.1182/blood-2005-05-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao X, Ren G, Liang L, Ai PZ, Zheng B, Tischfield JA, Shi Y, Shao C. Brief report: interferon-gamma induces expansion of Lin(−)Sca-1(+)C-Kit(+) Cells. Stem Cells. 2010;28(1):122–126. doi: 10.1002/stem.252. [DOI] [PubMed] [Google Scholar]
- 13.Quinton LJ, Nelson S, Boe DM, Zhang P, Zhong Q, Kolls JK, Bagby GJ. The granulocyte colony-stimulating factor response after intrapulmonary and systemic bacterial challenges. The Journal of infectious diseases. 2002;185(10):1476–1482. doi: 10.1086/340504. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Liu Y, Liu Y, Zheng P. Mammalian target of rapamycin activation underlies HSC defects in autoimmune disease and inflammation in mice. J Clin Invest. 2010;120(11):4091–4101. doi: 10.1172/JCI43873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rebel VI, Hartnett S, Hill GR, Lazo-Kallanian SB, Ferrara JL, Sieff CA. Essential role for the p55 tumor necrosis factor receptor in regulating hematopoiesis at a stem cell level. J Exp Med. 1999;190(10):1493–1504. doi: 10.1084/jem.190.10.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rezzoug F, Huang Y, Tanner MK, Wysoczynski M, Schanie CL, Chilton PM, Ratajczak MZ, Fugier-Vivier IJ, Ildstad ST. TNF-alpha is critical to facilitate hemopoietic stem cell engraftment and function. J Immunol. 2008;180(1):49–57. doi: 10.4049/jimmunol.180.1.49. [DOI] [PubMed] [Google Scholar]
- 17.Neuzil KM, Tang YW, Graham BS. Protective Role of TNF-alpha in respiratory syncytial virus infection in vitro and in vivo. The American journal of the medical sciences. 1996;311(5):201–204. doi: 10.1097/00000441-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 18.van Schaik SM, Obot N, Enhorning G, Hintz K, Gross K, Hancock GE, Stack AM, Welliver RC. Role of interferon gamma in the pathogenesis of primary respiratory syncytial virus infection in BALB/c mice. Journal of medical virology. 2000;62(2):257–266. doi: 10.1002/1096-9071(200010)62:2<257::aid-jmv19>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.van Benten IJ, Drunen CMvan, Koevoet JL, Koopman LP, Hop WC, Osterhaus AD, Neijens HJ, Fokkens WJ. Reduced nasal IL-10 and enhanced TNFalpha responses during rhinovirus and RSV-induced upper respiratory tract infection in atopic and non-atopic infants. Journal of medical virology. 2005;75(2):348–357. doi: 10.1002/jmv.20277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Souza PP, Lerner UH. The role of cytokines in inflammatory bone loss. Immunological investigations. 2013;42(7):555–622. doi: 10.3109/08820139.2013.822766. [DOI] [PubMed] [Google Scholar]
- 21.Braun T, Schett G. Pathways for bone loss in inflammatory disease. Current osteoporosis reports. 2012;10(2):101–108. doi: 10.1007/s11914-012-0104-5. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg HF, Domachowske JB. Inflammatory responses to respiratory syncytial virus (RSV) infection and the development of immunomodulatory pharmacotherapeutics. Current medicinal chemistry. 2012;19(10):1424–1431. doi: 10.2174/092986712799828346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyer KD, Garcia-Crespo KE, Glineur S, Domachowske JB, Rosenberg HF. The Pneumonia Virus of Mice (PVM) model of acute respiratory infection. Viruses. 2012;4(12):3494–3510. doi: 10.3390/v4123494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonville CA, Bennett NJ, Koehnlein M, Haines DM, Ellis JA, DelVecchio AM, Rosenberg HF, Domachowske JB. Respiratory dysfunction and proinflammatory chemokines in the pneumonia virus of mice (PVM) model of viral bronchiolitis. Virology. 2006;349(1):87–95. doi: 10.1016/j.virol.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Bonville CA, Percopo CM, Dyer KD, Gao J, Prussin C, Foster B, Rosenberg HF, Domachowske JB. Interferon-gamma coordinates CCL3-mediated neutrophil recruitment in vivo. BMC immunology. 2009;10:14. doi: 10.1186/1471-2172-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watkiss ER, Shrivastava P, Arsic N, Gomis S, van Drunen Littel-van den Hurk S. Innate and adaptive immune response to pneumonia virus of mice in a resistant and a susceptible mouse strain. Viruses. 2013;5(1):295–320. doi: 10.3390/v5010295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. American journal of respiratory and critical care medicine. 2000;161(5):1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 28.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354(9178):541–545. doi: 10.1016/S0140-6736(98)10321-5. [DOI] [PubMed] [Google Scholar]
- 29.Siegle JS, Hansbro N, Herbert C, Rosenberg HF, Domachowske JB, Asquith KL, Foster PS, Kumar RK. Early-life viral infection and allergen exposure interact to induce an asthmatic phenotype in mice. Respir Res. 2010;11:14. doi: 10.1186/1465-9921-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaiko GE, Loh Z, Spann K, Lynch JP, Lalwani A, Zheng Z, Davidson S, Uematsu S, Akira S, Hayball J, Diener KR, Baines KJ, Simpson JL, Foster PS, Phipps S. Toll-like receptor 7 gene deficiency and early-life Pneumovirus infection interact to predispose toward the development of asthma-like pathology in mice. The Journal of allergy and clinical immunology. 2013;131(5):1331–1339. doi: 10.1016/j.jaci.2013.02.041. e1310. [DOI] [PubMed] [Google Scholar]
- 31.Garvey TL, Dyer KD, Ellis JA, Bonville CA, Foster B, Prussin C, Easton AJ, Domachowske JB, Rosenberg HF. Inflammatory responses to pneumovirus infection in IFN-alpha beta R gene-deleted mice. J Immunol. 2005;175(7):4735–4744. doi: 10.4049/jimmunol.175.7.4735. [DOI] [PubMed] [Google Scholar]
- 32.Zaynagetdinov R, Sherrill TP, Kendall PL, Segal BH, Weller KP, Tighe RM, Blackwell TS. Identification of myeloid cell subsets in murine lungs using flow cytometry. American journal of respiratory cell and molecular biology. 2013;49(2):180–189. doi: 10.1165/rcmb.2012-0366MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youn JI, Collazo M, Shalova IN, Biswas SK, Gabrilovich DI. Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Journal of leukocyte biology. 2012;91(1):167–181. doi: 10.1189/jlb.0311177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in immunology. 2002;23(11):549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 35.Goulart MR, Pluhar GE, Ohlfest JR. Identification of myeloid derived suppressor cells in dogs with naturally occurring cancer. PloS one. 2012;7(3):e33274. doi: 10.1371/journal.pone.0033274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G–based strategy to analyze the mouse splenic myeloid compartment. Cytometry Part A : the journal of the International Society for Analytical Cytology. 2012;81(4):343–350. doi: 10.1002/cyto.a.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kondo M, Wagers AJ, Manz MG, Prohaska SS, Scherer DC, Beilhack GF, Shizuru JA, Weissman IL. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 38.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 39.Davidson S, Kaiko G, Loh Z, Lalwani A, Zhang V, Spann K, Foo SY, Hansbro N, Uematsu S, Akira S, Matthaei KI, Rosenberg HF, Foster PS, Phipps S. Plasmacytoid dendritic cells promote host defense against acute pneumovirus infection via the TLR7-MyD88-dependent signaling pathway. J Immunol. 2011;186(10):5938–5948. doi: 10.4049/jimmunol.1002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gotera J, Giuffrida M, Mavarez A, Pons H, Bermudez J, Maldonado M, Espina LM, Mosquera J, Valero N. Respiratory syncytial virus infection increases regulated on activation normal T cell expressed and secreted and monocyte chemotactic protein 1 levels in serum of patients with asthma and in human monocyte cultures. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2012;108(5):316–320. doi: 10.1016/j.anai.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 41.Wang CM, Tang RB, Chung RL, Hwang BT. Tumor necrosis factor-alpha and interleukin-6 profiles in children with pneumonia. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 1999;32(4):233–238. [PubMed] [Google Scholar]
- 42.Morichi S, Kawashima H, Ioi H, Yamanaka G, Kashiwagi Y, Hoshika A. High production of interleukin-10 and interferon-gamma in influenza-associated MERS in the early phase. Pediatrics international : official journal of the Japan Pediatric Society. 2012;54(4):536–538. doi: 10.1111/j.1442-200X.2011.03483.x. [DOI] [PubMed] [Google Scholar]
- 43.Long JP, Kotur MS, Stark GV, Warren RL, Kasoji M, Craft JL, Albrecht RA, Garcia-Sastre A, Katze MG, Waters KM, Vasconcelos D, Sabourin PJ, Bresler HS, Sabourin CL. Accumulation of CD11b(+)Gr-1(+) cells in the lung, blood and bone marrow of mice infected with highly pathogenic H5N1 and H1N1 influenza viruses. Archives of virology. 2013;158(6):1305–1322. doi: 10.1007/s00705-012-1593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pillay J, Tak T, Kamp VM, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cellular and molecular life sciences : CMLS. 2013;70(20):3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang C, Lei GS, Shao S, Jung HW, Durant PJ, Lee CH. Accumulation of myeloid-derived suppressor cells in the lungs during Pneumocystis pneumonia. Infection and immunity. 2012;80(10):3634–3641. doi: 10.1128/IAI.00668-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fortin C, Huang X, Yang Y. NK cell response to vaccinia virus is regulated by myeloid-derived suppressor cells. J Immunol. 2012;189(4):1843–1849. doi: 10.4049/jimmunol.1200584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Santo C, Salio M, Masri SH, Lee LY, Dong T, Speak AO, Porubsky S, Booth S, Veerapen N, Besra GS, Grone HJ, Platt FM, Zambon M, Cerundolo V. Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. The Journal of clinical investigation. 2008;118(12):4036–4048. doi: 10.1172/JCI36264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer research. 2009;69(4):1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malek TR, Danis KM, Codias EK. Tumor necrosis factor synergistically acts with IFN-gamma to regulate Ly-6A/E expression in T lymphocytes, thymocytes and bone marrow cells. J Immunol. 1989;142(6):1929–1936. [PubMed] [Google Scholar]
- 50.Shi X, Siggins RW, Stanford WL, Melvan JN, Basson MD, Zhang P. Toll-like receptor 4/stem cell antigen 1 signaling promotes hematopoietic precursor cell commitment to granulocyte development during the granulopoietic response to Escherichia coli bacteremia. Infection and immunity. 2013;81(6):2197–2205. doi: 10.1128/IAI.01280-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melvan JN, Siggins RW, Stanford WL, Porretta C, Nelson S, Bagby GJ, Zhang P. Alcohol impairs the myeloid proliferative response to bacteremia in mice by inhibiting the stem cell antigen-1/ERK pathway. J Immunol. 2012;188(4):1961–1969. doi: 10.4049/jimmunol.1102395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Griseri T, McKenzie BS, Schiering C, Powrie F. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity. 2012;37(6):1116–1129. doi: 10.1016/j.immuni.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas J, Liu F, Link DC. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Current opinion in hematology. 2002;9(3):183–189. doi: 10.1097/00062752-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Kindler V, Thorens B, Kossodo Sde, Allet B, Eliason JF, Thatcher D, Farber N, Vassalli P. Stimulation of hematopoiesis in vivo by recombinant bacterial murine interleukin 3. Proc Natl Acad Sci U S A. 1986;83(4):1001–1005. doi: 10.1073/pnas.83.4.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koh A, Silva APda, Bansal AK, Bansal M, Sun C, Lee H, Glogauer M, Sodek J, Zohar R. Role of osteopontin in neutrophil function. Immunology. 2007;122(4):466–475. doi: 10.1111/j.1365-2567.2007.02682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Burdo TH, Wood MR, Fox HS. Osteopontin prevents monocyte recirculation and apoptosis. Journal of leukocyte biology. 2007;81(6):1504–1511. doi: 10.1189/jlb.1106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schurch CM, Riether C, Ochsenbein AF. Cytotoxic CD8+ T cells stimulate hematopoietic progenitors by promoting cytokine release from bone marrow mesenchymal stromal cells. Cell stem cell. 2014;14(4):460–472. doi: 10.1016/j.stem.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 58.Hussell T, Pennycook A, Openshaw PJ. Inhibition of tumor necrosis factor reduces the severity of virus-specific lung immunopathology. European journal of immunology. 2001;31(9):2566–2573. doi: 10.1002/1521-4141(200109)31:9<2566::aid-immu2566>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 59.Tregoning JS, Pribul PK, Pennycook AM, Hussell T, Wang B, Lukacs N, Schwarze J, Culley FJ, Openshaw PJ. The chemokine MIP1alpha/CCL3 determines pathology in primary RSV infection by regulating the balance of T cell populations in the murine lung. PloS one. 2010;5(2):e9381. doi: 10.1371/journal.pone.0009381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang P, Nelson S, Bagby GJ, Siggins R, 2nd, Shellito JE, Welsh DA. The lineage-c-Kit+Sca-1+ cell response to Escherichia coli bacteremia in Balb/c mice. Stem Cells. 2008;26(7):1778–1786. doi: 10.1634/stemcells.2007-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.