Abstract
Proper immune responses are needed for controlling pathogen infection at mucosal surfaces. IL-22-producing CD4+ T cells play an important role in controlling bacterial infection in the gut; however, transcriptional regulation of these cells remains elusive. Here, we show that mice with targeted deletion of the fourth DNA-binding zinc finger of the transcription factor Ikaros had increased IL-22- but not IL-17-producing CD4+ T cells in the gut. Adoptive transfer of CD4+ T cells from these Ikaros mutant mice conferred enhanced mucosal immunity against Citrobacter rodentium infection. Despite an intact in vivo thymic-derived Treg compartment in these Ikaros mutant mice, TGF-β, a cytokine well known for induction of Tregs, failed to induce Foxp3 expression in Ikaros mutant CD4+ T cells in vitro but instead promoted IL-22. Aberrant upregulation of IL-21 in CD4+ T cells expressing mutant Ikaros was at least in part responsible for the enhanced IL-22 expression in a Stat3-dependent manner. Genetic analysis using compound mutations further demonstrated that the aryl hydrocarbon receptor (Ahr), but not RORγt, was required for aberrant IL-22 expression by Ikaros mutant CD4+ T cells, whereas forced expression of Foxp3 was sufficient to inhibit this aberrant cytokine production. Together, our data has uncovered new functions for Ikaros in maintaining mucosal immune homeostasis by restricting IL-22 production by CD4+ T cells.
INTRODUCTION
Mucosal immunity requires the concerted action of innate and adaptive immune systems, among which interleukin (IL)-22-mediated CD4+ T helper cell responses (e.g., Th17 and/or Th22 cells) are particularly important for the host to control bacterial infections in the gut, while Tregs are important to limit inflammation and maintain homeostasis. Citrobacter rodentium is a murine pathogen that models human enterohemorrhagic and enteropathogenic Escherichia coli infections, which are responsible for the deaths of several hundred thousand children each year(1). Clearance of C. rodentium requires both the innate and adaptive immune responses(2, 3). While RORγt+ group 3 innate lymphoid cells (ILC3s) are essential for protection against infection(4–7), CD4+ T cell production of IL-22 is important for the host to control C. rodentium infection(8, 9). Indeed, transferring either IL-22-producing innate lymphoid cells (e.g., ILC3s)(4) or CD4+ T cells (e.g., Th22)(8) protects mice from C. rodentium infection, thereby highlighting the crucial role of IL-22 in mucosal immunity. Various proinflammatory cytokines (e.g., IL-6, IL-21, and IL-23) promote IL-22-producing CD4+ T cell responses(10–15). In contrast, TGF-β has been shown to inhibit IL-22 production by CD4+ T cells(16–18).
The differentiation and function of CD4+ T cells is influenced by multiple transcription factors induced and/or activated by signals stemming from the local cytokine microenvironment. The activation of the nuclear receptor RAR-related orphan receptor gamma t (RORγt) in response to transforming growth factor (TGF)-β in addition to Stat3-activating cytokines (e.g., IL-6, IL-21, and IL-23) is crucial for expression of the genes currently defining the Th17 cell program (e.g., IL-17 and/or IL-22)(10–15). Though also induced by TGF-β, the transcription factor forkhead box protein 3 (Foxp3), a lineage marker for regulatory T cells (Tregs), is able to suppress Th17 cell differentiation through antagonism of RORγt transcriptional activity in part via physical interaction between the proteins(19–21). Among the transcription factors implicated thus far in Th17 cell differentiation, the ligand-dependent aryl hydrocarbon receptor (Ahr), best known to mediate the effects of environmental toxins (e.g., dioxin), is essential for IL-22 expression and thought to enhance the expression of IL-17 by CD4+ T cells in vitro(22–24). The activation of transcription factor Ahr, together with RORγt, induces IL-22 transcription(6), whereas c-Maf has been shown to repress IL-22 expression by CD4+ T cells(16).
Ikaros is a highly conserved zinc finger protein with four amino (N)-terminal DNA binding zinc fingers and two carboxyl (C)-terminal zinc fingers that mediate dimerization(25, 26). Ikaros is required for lymphocyte development, as its deletion completely abrogates fetal T- and B-lymphocytes as well as adult B cells(27). Although Ikaros null mice display post-natal T cells, their development is perturbed and results in clonal expansion of abnormal T cells(27). Depending on the context, Ikaros has been shown to function either as a transcriptional activator or repressor (i.e., Ikaros promotes expression of Il10 or represses Il2, Tbx21, and Ifng)(28–32). While N-terminal zinc fingers 2 and 3 have been shown to be required for DNA-binding and function(33), recent studies have suggested that Ikaros zinc fingers 1 and 4 play important roles in regulating target gene specificity(34). Specifically, it was shown that the two flanking DNA-binding zinc fingers regulate distinct sets of genes by modulating binding to different genomic sites(34). Of note, despite thousands of Ikaros-binding sites identified by ChIP-seq, only a small number of genes were found to be either upregulated or downregulated in the absence of full-length wildtype Ikaros (i.e., Ikaros lacking zinc finger 1 or 4) by RNA-seq, suggesting that Ikaros may directly regulate only a limited number of target genes involved in different hematopoietic lineages, developmental stages and biological functions(34). Recent studies have implicated Ikaros in the regulation of different stages of T cell development and in several T cell subsets(28, 30, 35). These studies, taken together, indicate the complex roles of Ikaros in multiple stages of T cell differentiation and function that are still incompletely understood. Specifically, the function of Ikaros in regulating IL-22-mediated T cell responses in gut immunity is unknown. Here, we uncover a critical function of Ikaros in mucosal homeostasis and immunity by restricting IL-22 producing CD4+ T cells and regulating different Treg compartments. We identify a unique role for Ikaros in regulating IL-22 single-producing cells, versus IL-17 and IL-22 double-producing Th17 cells.
To determine the role of Ikaros in CD4+ T cell responses both in vitro and in vivo under the steady state and during infection, we utilized two Ikaros mutant mouse strains with germline targeted deletion of Ikzf1 exons encoding zinc finger 1 (Ikzf1ΔF1/ΔF1) or 4 (Ikzf1ΔF4/ΔF4)(34). Of note, unlike Ikaros null mice (Ikzf1−/−) with developmental perturbation of various immune compartments, Ikzf1ΔF1/ΔF1 and Ikzf1ΔF4/ΔF4 mice have fewer and distinct global immune defects(34), thus making them an appropriate model system to dissect the function of Ikaros in CD4+ T cells. By using a series of genetic and pharmacological experiments, our data reveal new functions of Ikaros in the regulation of cytokine production and transcription factor expression and/or activity in CD4+ T cells, and thus suggest a new role for Ikaros in limiting CD4+ T cell immune responses in vivo during mucosal intestinal infection that is controlled by IL-22.
MATERIALS AND METHODS
Mice
All mice used in this study were maintained in Specific Pathogen Free (SPF) facilities at Northwestern University. The mice were littermate controlled and were 6–10 weeks old unless otherwise indicated in the text. Ikzf1ΔF4/ΔF4, Ikzf1ΔF1/ΔF1, Ikzf1+/−, Rorcgfp/gfp, Ahr−/−, Stat3f/f mice were described previously(27, 34, 36–38) and were all fully backcrossed to C57BL/6 background. Cd4-cre and Rag1−/− mice were purchased from Taconic Farms or Jackson laboratory. All studies with mice were approved by the Animal Care and Use Committee of Northwestern University.
Isolation of Intestinal Lamina Propria Lymphocytes and in vitro T Cell Differentiation
The isolation of intestinal lamina proprial cells and flow cytometry were done as previously described(6). Splenocytes were made into single cell suspension and CD4+ T cells were purified with CD4+ T cell isolation kit (Miltenyi) with 90–95% purity. 24-well-plates were coated with anti-hamster antibody (MP Biomedical). CD4+ T cells were cultured in IMDM medium (Sigma Aldrich) supplied with soluble hamster-anti-mouse CD3 (0.25 µg/ml unless otherwise indicated in the text), hamster-anti-mouse CD28 (1 µg/ml), Gentamicin (50 µg/ml), anti-mouse IL-4 (11B11, 2 µg/ml, BioXCell), and anti-mouse IFN-γ XMG1.2, 2 µg/ml, BioXCell). For iTreg cell differentiation, 5 ng/ml TGF-β was added to the culture. For Th17 cell differentiation, TGF-β was added at 5 ng/ml and IL-6 was added at 20 ng/ml. In some experiments, FICZ was added at a concentration of 200 nM. For blocking RORγt or Stat3 activity, cells were cultured with 10 µM Digoxin (Sigma) or 15 µM STA21 (Santa Cruz), respectively, with controls of DMSO. For IL-21 neutralization, the cells with cultured with 12.5 µg/ml anti-IL21 (R&D Systems) or control IgG.
Flow Cytometry, Antibodies and ELISA
CD16/32 antibody was used to block the non-specific binding to Fc receptors before surface stainings on all gut LPL and some splenocyte experiments. Antibodies were either purchased from eBioscience, BD Pharmingen, or R&D Systems. For nuclear staining, cells were fixed and permeabilized using a Mouse Regulatory T Cell Staining Kit (eBioscience). For cytokine production, cells were stimulated ex vivo by 50 ng/ml PMA and 500 ng/ml ionomycin for 4 hours. Brefeldin A (2 µg/ml) was added 2 hours before cells were harvested for analysis. Dead cells in the gut were excluded from the analysis by using Live and Dead violet viability kit (Invitrogen). Live lymphoctyes isolated from the spleen or thymus were gated on using FSC/SSC. Flow cytometry data were collected using the FACSCantoII or FACSCalibur (BD Biosciences), and analyzed by FlowJo software (Tree Star Inc.). The amount of IL-21 in the culture supernatant was measured by ELISA kit from R&D Systems. The assays were performed in duplicate according to the manufacturer's instruction.
Realtime RT-PCR
RNA from sorted cell populations was isolated with Trizol reagent (Invitrogen). cDNA was synthesized using GoScript™ Reverse Transcription kit (Promega). Real-time RT-PCR was performed using SYBR Green (Biorad) and different primer sets (Table S1).
Reactions were run using the MyiQ™2 Two-Color Real-Time PCR Detection System (Biorad). The results were analyzed by the comparative CT method and displayed as relative gene expression values normalized to β-actin.
Chromatin Immunoprecipitation (ChIP) Assay
Approximately 5×106 cells CD4+ T cells under the indicated polarizing conditions were fixed in 1% formaldehyde in IMDM medium (Sigma Aldrich) for 10 minutes at room temperature. The reaction was stopped by adding glycine solution (final concentration, 0.125 M). Fixed cells were then washed 3 times with cold PBS, and resuspended in lysis buffer (1% SDS, 10mM EDTA, 50mM Tris-HCL pH 8.1) with protease inhibitor mixture (Sigma). Chromatin was sheared by water Bioruptor using 4 cycles of 15 minutes each at 4°C, and the insoluble fraction was removed by centrifugation. Then, chromatin immunoprecipitation was performed by Millipore ChIP kit (cat# 17–295) using Millipore H3K4Me3 or control IgG antibody (cat# 17–614) according to protocol. After proteinase K digestion, DNA was extracted using Phenol-Chloroform (Invitrogen), and precipitated for quantitative real-time PCR analyses using specific primers (Table S1).
Plasmids, Retrovirus production and Transduction of Primary CD4+ T Cells
MIG (MSCV-IRES-GFP) plasmid expressing Foxp3 has been described previously(19). Plasmids were transfected into Phoenix cells for viral preparation, and supernatant containing retroviruses was collected at 48 and 72 hours and used for primary CD4+ T cell transduction. Briefly, CD4+ T cells were stimulated with anti-CD3 and anti-CD28 in the presence of Gentamicin and anti-IFN-γ and anti-IL-4 for 24 hours. Cells were then transduced on two consecutive days by spin infection at 2500 rpm for 90 minutes at 30°C supplied with supernatant containing retroviruses together with 8 µg/ml polybrene. After the second transduction, the retroviral supernatant was then replaced with T cell culture medium with cytokines required for CD4+ T cell differentiation before analysis.
T Cell Reconstitution and C. rodentium Infection
CD4+ T cells from pooled spleens of littermate WT or Ikzf1ΔF4/ΔF4 mice were purified with CD4+ T cell isolation kit (Miltenyi), and transferred by retro-orbital i.v. injection (2 × 106 cells per mouse) into littermate Rag1−/− recipients. Reconstitution efficiency was evaluated 2 weeks post-injection, and mice were subsequently inoculated by gavage with 1010 CFU C. rodentium in 200 µl phosphate-buffered saline (PBS). C. rodentium strain DBS100 (ATCC 51459; American Type Culture Collection) was prepared by culturing in LB broth overnight and bacterial concentration was determined by measuring the optical density at 600 nm (OD600). Body weight was measured daily. Fecal pellets were collected, weighed and then homogenized in sterile PBS. Serially diluted homogenates were plated on MacConkey agar plates. C. rodentium colonies were identified based on morphology after 18–24 hours of incubation at 37°C. Tissues from middle colon were dissected and fixed with 10% formalin. Tissues were then embedded in paraffin, sectioned at 4 µm, and stained with hematoxylin and eosin. Sections were then blindly analyzed by a trained gastrointestinal pathologist for lamina propria inflammation, goblet cell loss, abnormal crypts, crypt abscesses, mucosal erosion or ulceration, submucosal spread to transmural inflammation, and neutrophil counts. Disease score was assessed as previously described(39–41).
Statistical Methods
Unless otherwise noted, statistical analysis was performed with the unpaired two-tailed Student’s t test on individual biological samples with GraphPad Prism. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
RESULTS
Ikzf1ΔF4/ΔF4 CD4+ T Cells in Vivo Have Increased IL-22 Expression and Differentially Regulated In Vivo Treg Compartments
Members of the Ikaros family of transcription factors have been implicated in Treg and Th17 cell differentiation in vitro(35, 42–44). We hypothesized that Ikaros may play an important role in vivo, especially in mucosal tissues where Th17 cells and Tregs are abundantly present. To understand the role of Ikaros in vivo, we have used recently developed Ikaros mutant mice with genetic deletion of exons encoding either DNA-binding zinc finger 1 (Ikzf1ΔF1/ΔF1) or DNA-binding zinc finger 4 of Ikaros (Ikzf1ΔF4/ΔF4)(34). Two benefits of using these mice rather than Ikaros null mice are the ability to study the phenotypes of homozygous mutant Ikzf1ΔF1/ΔF1 and Ikzf1ΔF4/ΔF4 mice in a C57BL/6 background and the knowledge that these finger mutants disrupt expression of only a subset of Ikaros target genes(34), thus potentially avoiding some confounding global defects of Ikaros null mice.
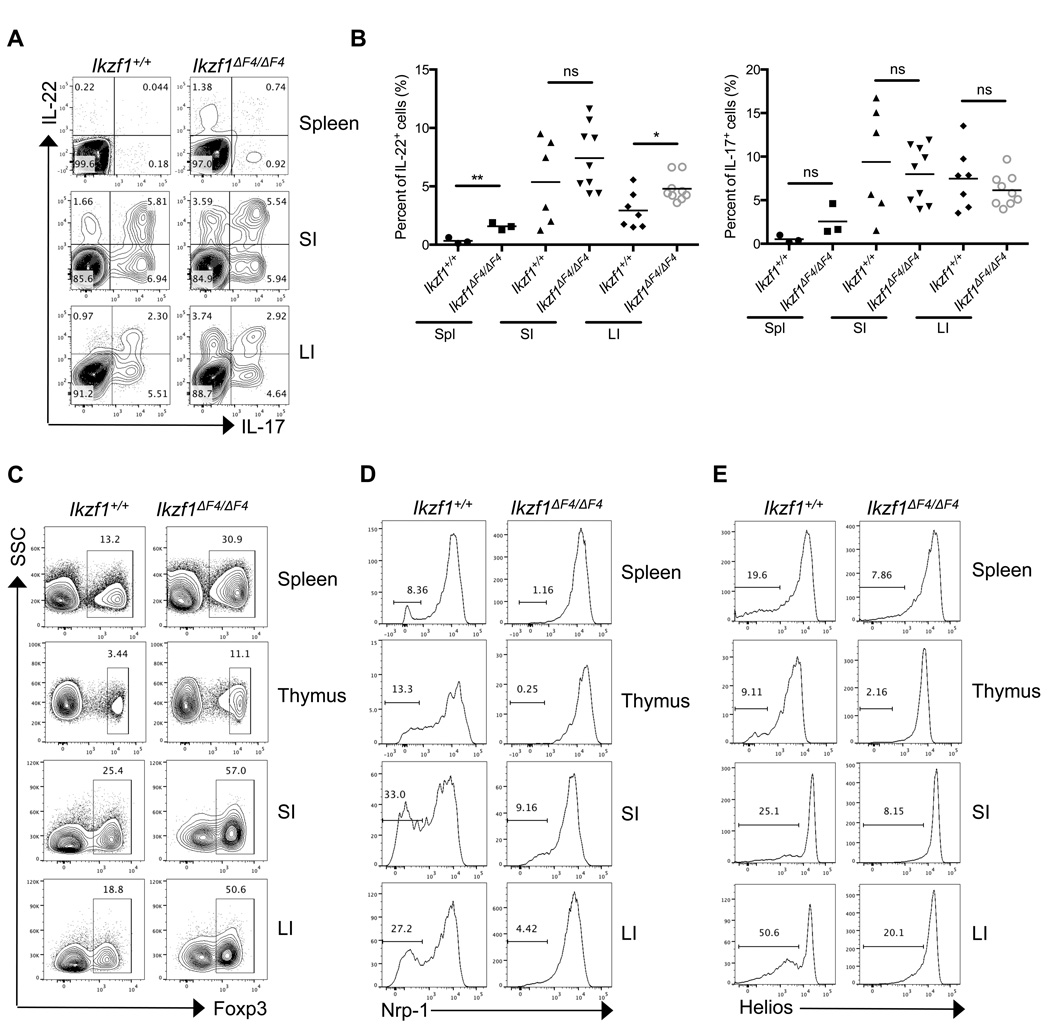
We first examined the T cell composition in the spleen of Ikaros zinc finger 1 or 4 mutant mice. Although the percentages of T cells (i.e., CD3+ cells) and CD4+ T cells were increased due to downregulation of B cells (i.e., CD19+ cells) (Supplemental Fig. 1A, 1B)(34), we found that the total number of T-lymphocytes (e.g., CD3+ cells) as well as the number of CD4+ T cells in the Ikaros mutant mice were reduced (Supplemental Fig. 1C, 1D), consistent with a decrease in total splenocyotes in the Ikaros isoform mutants (Supplemental Fig. 1E). To further investigate the T cell function in vivo, we examined CD4+ T cell cytokine expression under steady-state conditions. In agreement with the abundant presence of naturally occurring splice isoform of Ikaros lacking DNA-binding zinc finger 1 (e.g., Ik-2) in T cells(26, 45, 46), CD4+ T cells isolated from the spleen and intestines of Ikzf1ΔF1/ΔF1 mice had similar production of IL-17 and IL-22 compared to those from wildtype littermate mice (Supplemental Fig. 1F). However, CD4+ T cells from the spleen and intestines of Ikzf1ΔF4/ΔF4 mice aberrantly expressed higher levels of IL-22, but not IL-17 compared to those from wildtype littermate mice (Fig. 1A, 1B). Given the relatively normal function of CD4+ T cells in Ikzf1ΔF1/ΔF1 mice, we thus focused our remaining studies by using Ikzf1ΔF4/ΔF4 mice to elucidate the role of Ikaros in CD4+ T cells. Considering the elevated cytokine production by CD4+ T cells under steady state conditions in Ikzf1ΔF4/ΔF4 mice, we next examined the regulatory T cell (Treg) compartment. Total Foxp3+ Tregs isolated from the spleen, thymus, and intestines of Ikzf1ΔF4/ΔF4 mice were increased in the absence of wildtype full-length Ikaros in Ikzf1ΔF4/ΔF4 mice (Fig. 1C, Supplemental Fig 1G), suggesting that the cytokine upregulation (i.e., IL-22) observed in Ikzf1ΔF4/ΔF4 mice was not due to a general loss of Tregs in vivo. Indeed, no overt spontaneous autoimmunity was observed in Ikzf1ΔF4/ΔF4 mice (data not shown.)
Figure 1. Absence of full-length wildtype Ikaros results in aberrant IL-22 expression by CD4+ T cells and altered Treg compartments in vivo.
CD4+ T cells were isolated from the spleen, thymus, small intestinal lamina propria (SI), and large intestinal lamina propria (LI) of steady-state littermate mice of the indicated genotypes. Cytokine (A,B) or Foxp3 (C–E) expression was measured by intracellular staining and flow cytometry. Neuropilin-1 (D) or Helios (E) was measured on cells that were gated on CD4+TCRβ+Foxp3+. Data (A,C,D,E) are representative of at least three independent experiments. Data (B) are compiled from independent experiments (each dot represents one mouse) and mean ± SEM are shown of cytokine expressing cells gated on total CD4+TCRβ+ cells from the indicated organs.
To better assess the Foxp3+ Tregs in vivo, we co-stained Foxp3+ CD4+ T cells with intracellular marker Helios or surface marker neuropilin-1 (Nrp-1), both of which have been identified to be expressed at high levels on thymic-derived Tregs (tTregs) but not on Tregs induced by TGF-β (iTregs) or peripherally-derived Tregs (pTregs)(43, 47–49). While total Foxp3+ T cells were markedly enhanced under in vivo steady state conditions, the Foxp3+Helios− and Foxp3+Nrp-1− populations that indicate the peripherally-derived Tregs were reduced in the Ikzf1ΔF4/ΔF4 mice (Fig. 1D, 1E). These data indicate that Ikaros may differentially regulate tTregs and pTregs in vivo.
Ikzf1ΔF4/ΔF4 CD4+ T Cells are Functional In Vivo and Display Increased IL-22 Expression and Enhanced Protection Against C. rodentium Infection
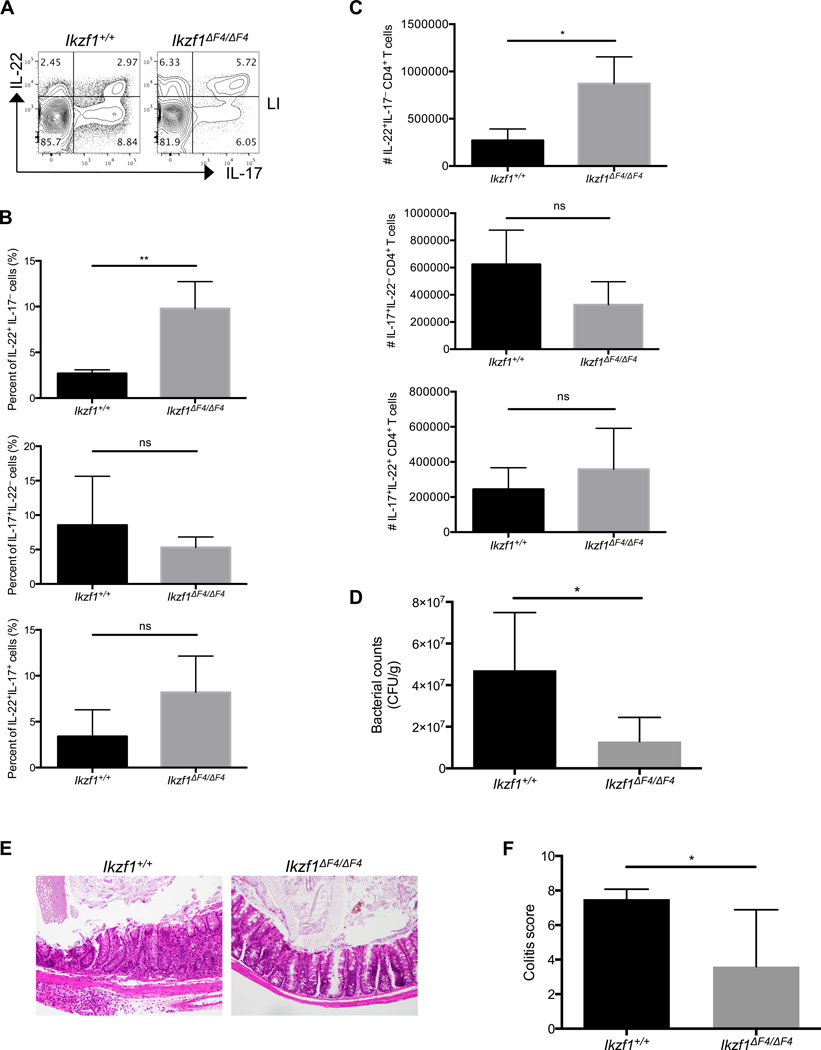
Although less severe than the Ikaros null mice, the Ikzf1ΔF4/ΔF4 mice also have various deficiencies in the immune system (e.g., early development of thymic lymphoma, lack of lymph nodes and Peyer’s patches and fetal lymphoid tissue inducer cells (LTis)(34) that might complicate more detailed in vivo studies in these mice. To further examine the role of Ikaros in T cell-mediated mucosal immunity, we therefore chose a T cell adoptive transfer model to determine the in vivo function of the CD4+ T cells lacking full-length wildtype Ikaros upon bacterial infection. Based on our observations of enhanced IL-22 production by Ikaros mutant T cells, we chose Citrobacter rodentium, which is a murine bacteria that requires IL-22 for its clearance(4, 8, 50). Specifically, we examined the T cell responses directly in the gut of Rag1−/− mice that were initially reconstituted with wildtype or Ikzf1ΔF4/ΔF4 mutant total CD4+ T cells and then subsequently subjected to C. rodentium infection. While there was no significant difference in the weight changes among the reconstituted Rag1−/− mice (data not shown), the mice that received Ikaros mutant CD4+ T cells showed increased percentage and number of IL-22-producing CD4+ T cells in the gut compared to the mice reconstituted with wildtype CD4+ T cells (Fig. 2A–C). Furthermore, Rag1−/− mice reconstituted with Ikzf1ΔF4/ΔF4 CD4+ T cells displayed enhanced clearance of the bacteria and alleviated intestinal histopathology after infection compared to those reconstituted with wildtype CD4+ T cells (Fig. 2D–F). These data indicate that the enhanced generation (e.g., expansion and/or differentiation) of IL-22-producing CD4+ T cells in the absence of functional Ikaros confers protection against mucosal pathogen infection.
Figure 2. Aberrant IL-22 expression by Ikzf1ΔF4/ΔF4 T cells in vivo protects against C. rodentium infection.
Littermate Rag1−/− mice were reconstituted with CD4+ T cells isolated from littermate mouse spleens of the indicated genotypes, and then were infected with C. rodentium for 9 days. CD4+ T cells (CD4+CD3+TCRβ+) were isolated from gut lamina propria at day 9, counted, stimulated by PMA/ionomycin/Brefeldin A for 4 hours, and cytokine expression was measured by intracellular staining and flow cytometry (A,B,C). Data (A) are representative FACS plots of two independent experiments. Fecal bacterial counts (D) were measured at day 7 and normalized per gram of feces. Mid-colon sections were stained for H&E, and then scored for disease severity (E,F). Panels (B,C,D,F) show pooled data of two independent experiments (Ikzf1+/+ n=4; Ikzf1ΔF4/ΔF4 n=6). Mean ± SEM are shown.
Ikaros Regulates the Expression of Foxp3, IL-17, and IL-22 in CD4+ T Cells
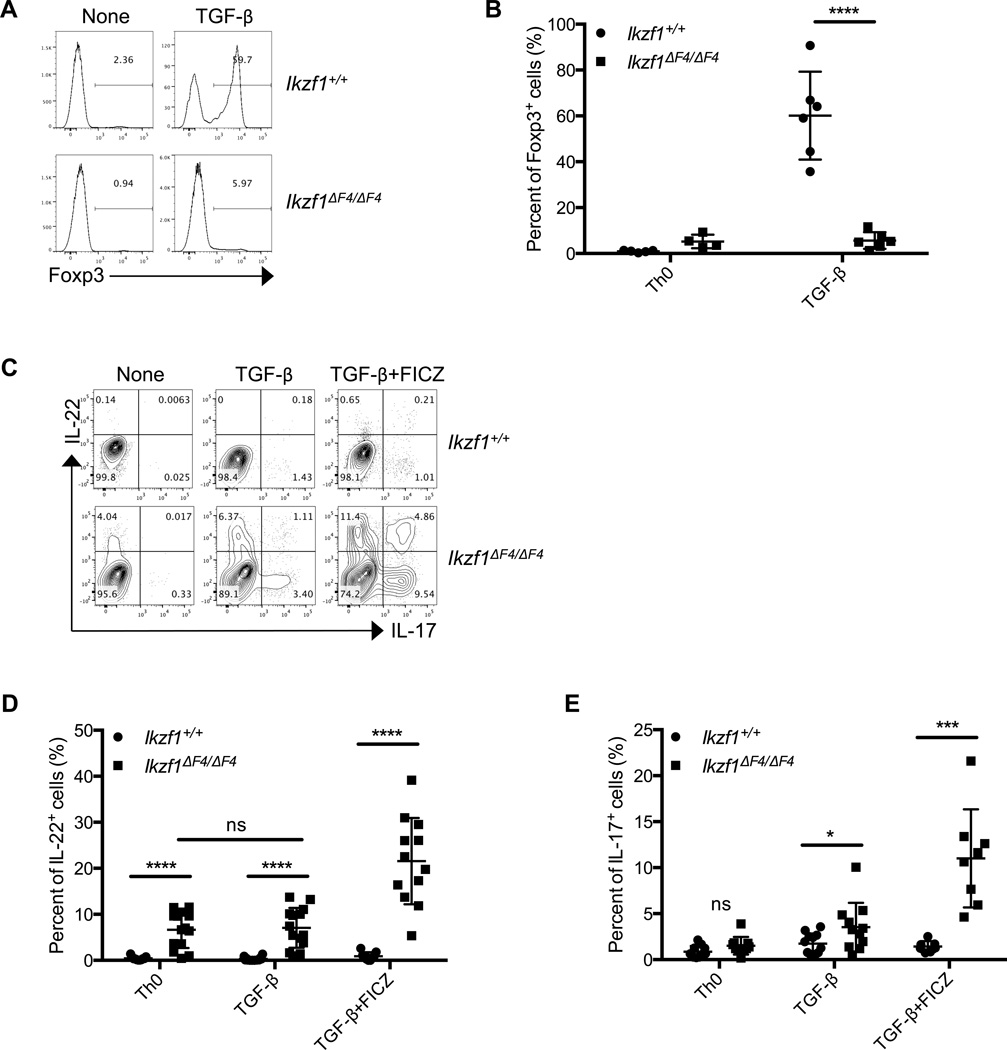
To further understand the cell-intrinsic mechanistic role of Ikaros in CD4+ T cell differentiation and/or cytokine regulation, we turned to an in vitro culture system. Considering our data demonstrating a marked decrease in Foxp3+Nrp-1− or Foxp3+Helios− cells that have been suggested to be pTregs and iTregs(43, 47, 48)(Fig. 1D, 1E), we first examined the iTreg cells under in vitro polarizing conditions. As expected, upon stimulation with TGF-β in vitro, both wildtype bulk CD4+ and naïve CD4+ T cells (i.e., CD4+CD25−CD62LhiCD44lo cells) were readily differentiated into inducible Tregs (iTregs) as measured by upregulation of Foxp3 (Fig. 3A, 3B, Supplemental Fig. 2A, 2B). Consistent with the in vivo defects in pTregs (Fig. 1D, 1E), Ikzf1ΔF4/ΔF4 CD4+ T cells had marked decreases in Foxp3 protein and mRNA expression compared to wildtype cells under iTreg conditions in vitro (i.e., TGF-β) (Fig. 3A, 3B, Supplemental Fig. 2A, 2B). Together, these data indicate that Ikaros is required for Foxp3 expression induced by TGF-β.
Figure 3. Perturbed expression of Foxp3, IL-22 and IL-17 in Ikzf1ΔF4/ΔF4 CD4+ T cells in vitro.
Bulk CD4+ T cells were purified from littermate mice of the indicated genotypes and activated by anti-CD3/CD28 with or without TGF-β and/or Ahr ligand FICZ for 96 hours in culture. Foxp3 (A,B) and IL-22 and IL-17 (C–E) protein expression were measured after 96 hours by intracellular staining and flow cytometry. Data are representative of at least four independent experiments. Data (B,D,E) are compiled from independent experiments (each dot is one mouse) and mean ± SEM are shown.
Given the potential for a reciprocal balance between Treg and Th17 cell differentiation(15, 19), we next examined the Th17 cell-related cytokine expression (i.e., IL-17 and IL-22) in Ikzf1ΔF4/ΔF4 CD4+ T cells. Strikingly, total CD4+ T cells isolated from Ikzf1ΔF4/ΔF4 mice that contain both naïve and memory T cells expressed high levels of IL-22 compared to wildtype CD4+ T cells without exogenous cytokine stimulation in the Th0 condition (i.e., only anti-CD3 and -CD28 stimulation) (Fig. 3C, 3D). In contrast, naïve CD4+ T cells (CD4+CD25−CD62LhiCD44lo) purified from Ikzf1ΔF4/ΔF4 mice did not appear to have upreregulated expression of IL-22 in the Th0 condition (Supplemental Fig. 2C). Furthermore, naïve CD4+ T cell (i.e., CD4+CD62LhiCD44lo) percentages in Ikzf1ΔF4/ΔF4 splenocytes were decreased and memory CD4+ T cell (i.e., CD4+CD62LloCD44hi) percentages were increased compared with wildtype splenocytes (Supplemental Fig. 2D, 2E). These data suggest a potential difference of Ikaros in the regulation of IL-22 in naïve vs. memory CD4+ T cells in the Th0 condition. Of note, lower cell viability of naïve CD4+ T cells was observed in vitro in the Th0 conditions (data not shown). Thus, we cannot rule out the possibility that the lack of IL-22 expression by naïve Ikzf1ΔF4/ΔF4 CD4+ T cells in the Th0 condition might be due to reduced general cell fitness. Nevertheless, even in the presence of TGF-β, a cytokine that has been shown to inhibit IL-22 expression(16–18), both bulk and naïve Ikzf1ΔF4/ΔF4 CD4+ T cells aberrantly expressed IL-22 (Fig. 3C–E, Supplemental Fig. 2C). The expression of the key Th17 cell transcription factor RORγt was also upregulated in Ikzf1ΔF4/ΔF4 CD4+ T cells under Th0 and iTreg cell-polarizing conditions (Supplemental Fig. 2F). Together, these data suggest that Ikaros in a zinc finger 4-dependent manner is required to inhibit aberrant expression of Th17 cell-associated cytokine (especially IL-22) expression by CD4+ T cells in vitro.
Ahr activation by ligand 6-formylindolo[3,2-b]carbazole (FICZ) has been shown to enhance IL-17 and IL-22 production by CD4+ T cells(22–24). Thus, we tested the effect of Ahr ligand on cytokine production by Ikzf1ΔF4/ΔF4 CD4+ T cells. Addition of FICZ to the cultures that contained TGF-β further enhanced the upregulation of IL-22 and IL-17 in Ikzf1ΔF4/ΔF4 CD4+ T cells (Fig. 3C–E). It is worth mentioning that under Th17 cell-polarizing conditions that allow optimal expression of both IL-17 and IL-22 (e.g., IL-6, TGF-β and FICZ)(14), Ikzf1ΔF4/ΔF4 CD4+ T cells had no statistical differences in IL-17 production compared to wildtype CD4+ T cells (Supplemental Fig. 2G, 2H,), consistent with our in vivo data during C. rodentium infection (Fig. 2B, 2C, middle panels). However, IL-22 production by Ikzf1ΔF4/ΔF4 CD4+ T cells was still markedly enhanced under Th17 cell-polarizing conditions, particularly by the IL-22 single-producing cells (Supplemental Fig. 2C, 2G, 2H). These data underscore the important negative and selective regulatory role of Ikaros in the regulation of IL-22 in CD4+ T cells and point to a differential regulation of IL-17 and IL-22.
To determine whether this aberrant IL-22 upregulation was a loss-of-function or gain-of-function phenotype due to the expression of the mutant form of Ikaros lacking zinc finger 4, we conducted the same in vitro experiments on CD4+ T cells isolated from Ikzf1+/− mice, which had been fully backcrossed on a C57BL/6 background. Due to embryonic lethality with Ikaros null mice on C57BL/6 background(34), we used CD4+ T cells from the heterozygotes to examine Th17 cell-associated cytokine expression levels. Consistent with the data in Ikaros zinc finger 4 mutant cells, the expression of IL-22 was also elevated in Ikzf1+/− CD4+ T cells compared to wildtype littermate controls under Th0 or iTreg polarizing conditions, and further enhanced upon addition of FICZ (Supplemental Fig. 2I), suggesting a loss-of-function phenotype of Ikaros in Ikzf1ΔF4/ΔF4 mice.
Ahr is Essential for Aberrant IL-22 Expression in Ikzf1ΔF4/ΔF4 CD4+ T cells
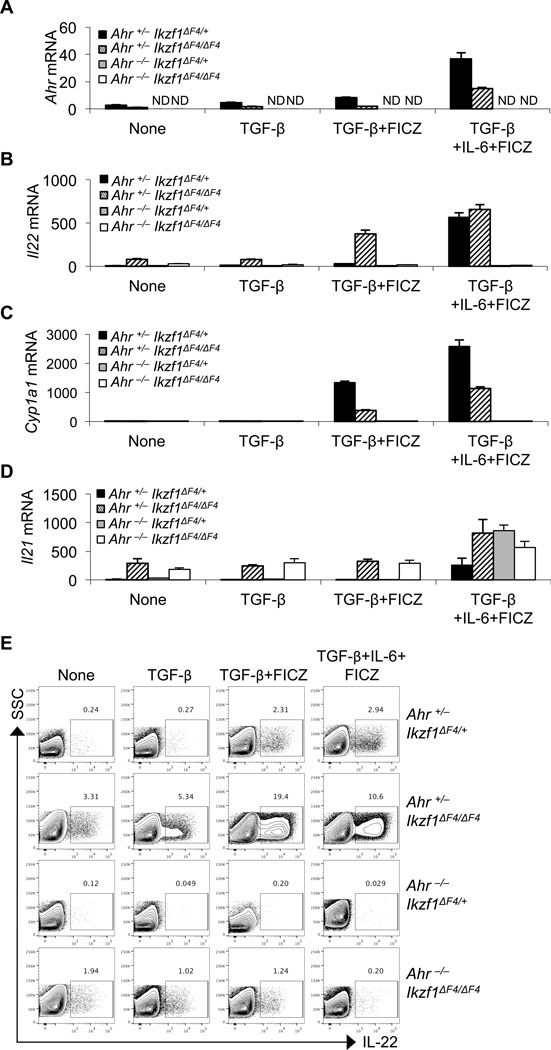
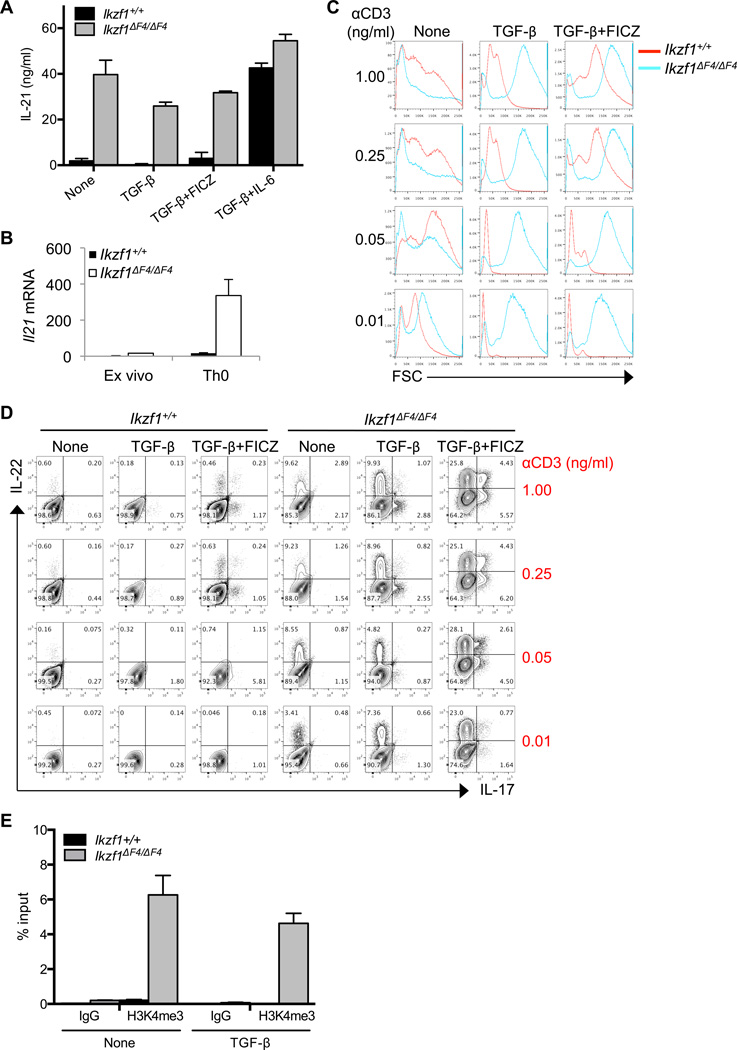
Upregulation of IL-22 in Ikaros mutant CD4+ T cells prompted us to examine the expression and activation of Ahr, a key regulator that cooperates with RORγt to enhance the Il22 gene transcription(6, 51). Unexpectedly, the Ahr expression induced by TGF-β, TGF-β plus FICZ, or TGF-β and IL-6 plus FICZ was greatly reduced in Ikzf1ΔF4/ΔF4 CD4+ T cells compared to control cells despite the upregulation of Il22 (Fig. 4A, 4B). These data suggest that Ikaros may function as a positive regulator for Ahr transcription, consistent with the direct binding of Ikaros at the Ahr promoter(35). Consistent with the reduced expression of Ahr, Cyp1a1, a known target gene of Ahr, was downregulated in Ikzf1ΔF4/ΔF4 CD4+ T cells (Fig. 4C). Notably, the aberrant Il21 expression in Ikzf1ΔF4/ΔF4 CD4+ T cells is independent of Ahr (Fig. 4D).
Figure 4. Regulation of Ahr expression and its role for aberrant IL-22 expression in Ikzf1ΔF4/ΔF4 CD4+ T cells.
CD4+ T cells were purified from littermate mice of the indicated genotypes and activated by anti-CD3/CD28 with or without TGF-β, IL-6, and/or Ahr ligand FICZ. mRNA expression (A–D) was measured by realtime RT-PCR after 48 hours in culture, and protein expression (E) was measured after 96 hours in culture by intracellular staining and flow cytometry. Mean ± SD (A–D) of experimental triplicates are shown. Data are representative of at least two independent experiments.
Considering the paradoxical upregulation of IL-22 with decreased expression of Ahr in Ikzf1ΔF4/ΔF4 CD4+ T cells, we questioned whether Ahr was at all required for the observed IL-22 expression in these cells. To this end, we generated Ahr−/−Ikzf1ΔF4/ΔF4 mice to genetically ablate Ahr expression in Ikaros mutant mice. When these compound-mutant CD4+ T cells were cultured with TGF-β, TGF-β plus FICZ, or TGF-β and IL-6 plus FICZ, the aberrant production of IL-22 was markedly reduced (compare the IL-22 production by CD4+ T cells from Ahr+/−Ikzf1ΔF4/ΔF4 mice and from Ahr−/−Ikzf1ΔF4/ΔF4 mice) (Fig. 4B, 4E, Supplemental Fig. 3). Together, these data demonstrate that Ahr is essential for IL-22 upregulation also in Ikaros mutant CD4+ T cells as reported in wildtype cells. The expression level of Ahr correlated with Cyp1a1 but surprisingly not the IL-22 expression in Ikaros mutant CD4+ T cells, suggesting that Ikaros may not only regulate Ahr expression but also its activity in a target gene-specific manner in CD4+ T cells.
Ikaros Limits IL-21 Expression in CD4+ T Cells
Both IL-6 and IL-21 have been shown to promote Th17 cell differentiation and to inhibit iTreg cell differentiation in vitro(52). Thus, we investigated whether these cytokines are responsible for the aberrant upregulation of IL-17 and/or IL-22 in Ikzf1ΔF4/ΔF4 CD4+ T cells. We did not detect IL-6 secreted by Ikzf1ΔF4/ΔF4 CD4+ T cells (data not shown), consistent with the production of IL-6 by antigen presenting cells but not by T cells(14). In wildtype CD4+ T cells, IL-21 can be induced under Th17 cell-polarizing condition (i.e., IL-6 plus TGF-β). In contrast, IL-21 was markedly increased in Ikzf1ΔF4/ΔF4 CD4+ T cells even in the absence of any exogenous cytokines (Fig. 5A, 5B). This is consistent with the crucial role of IL-21 in promoting IL-17 and IL-22 expression by CD4+ T cells in an autocrine manner(11, 12, 19). Interestingly, CD4+ T cells directly isolated ex vivo from the spleen of Ikzf1ΔF4/ΔF4 mice expressed slightly higher levels of IL-21 than wildtype cells (Fig. 5B); however, stimulation with anti-CD3 and anti-CD28 in vitro (i.e., Th0 condition) substantially increased the production of IL-21 by Ikzf1ΔF4/ΔF4 CD4+ T cells (Fig. 5B), suggesting that aberrant upregulation of IL-21 in Ikzf1ΔF4/ΔF4 CD4+ T cells is dependent on the T cell receptor (TCR) signaling pathway.
Figure 5. Aberrant cytokine expression by Ikzf1ΔF4/ΔF4 requires TCR activation.
CD4+ T cells were purified from Ikzf1ΔF4/ΔF4 or wildtype littermate mice, activated by anti-CD3/CD28, and cultured with the indicated cytokines. IL-21 protein expression (A) was measured by ELISA after 96 hours. Mean ± SD (A) of experimental triplicates are shown. CD4+ T cells were purified from littermate mice of the indicated genotypes and mRNA measured either directly ex vivo or after 12 hours activated by anti-CD3/CD28 in vitro culture (B) and mean ± SD of experimental triplicates are shown. CD4+ T cells were purified from littermate mice of the indicated genotypes and activated by anti-CD28 (at constant concentration of 1 µg/ml) and varying concentrations of anti-CD3 with or without TGF-β and with or without Ahr ligand FICZ. Forward scatter (FSC) (C) and cytokine expression (D) were measured after 96 hours by flow cytometry and/or intracellular staining. CD4+ T cells were purified from Ikzf1ΔF4/ΔF4 or wildtype littermate mice, activated by anti-CD3/CD28, cultured with or without TGF-β for 24 hours, and H3K4me3 chromatin marks (E) were measured by ChIP. Mean ± SD (E) of experimental triplicates are shown. Data are representative of three independent experiments.
To determine the Ikaros zinc finger 4 mutant CD4+ T cells’ response to TCR activation, we performed TCR titrations on in vitro cultured cells and measured cytokine expression. We used the forward scatter (FSC) as surrogate readout for T cell activation. Consistent with the literature on Ikaros null CD4+ T cells(53), we observed that Ikzf1ΔF4/ΔF4 CD4+ T cells had a decreased threshold for TCR activation (Fig. 5C) and produced elevated levels of cytokines (i.e., IL-17 and IL-22) at low concentrations of anti-CD3 (Fig. 5D). We also observed increased histone H3 K4 trimethylation marks (H3K4me3) at the promoter of the Il21 locus in Ikzf1ΔF4/ΔF4 CD4+ T cells in the Th0 (i.e., none) and iTreg (i.e., TGF-β)-polarizing conditions, consistent with an opened chromatin and enhanced expression of Il21 in the absence of Ikaros (Fig. 5E, 5A, 5B). Together, these data indicate that Ikaros negatively regulates IL-21 expression in CD4+ T cells.
Unrestricted IL-21 Contributes to Aberrant Expression of IL-22 and Downregulation of TGF-β-induced Foxp3 in Ikaros Mutant CD4+ T Cells
To test if the aberrant IL-21 expression in Ikzf1ΔF4/ΔF4 CD4+ T cells was involved in and/or required for the observed aberrant IL-22 expression, we blocked IL-21 with neutralizing antibodies. Blocking IL-21 in the cultures greatly reduced the polarization of Ikzf1ΔF4/ΔF4 CD4+ T cells toward a Th17 cell-like phenotype by suppressing the expression of both IL-17 and IL-22 (Fig. 6A). In contrast, neutralizing antibodies against IL-6 had no effect (data not shown). Neutralizing IL-21 completely relieved the inhibitory effect of exogenously added IL-21 on TGF-β-induced Foxp3 in wildtype cells, and it also partially restored the Foxp3 expression in Ikzf1ΔF4/ΔF4 CD4+ T cells (Fig. 6B), suggesting that one potential mechanism underlying Foxp3 downregulation in Ikzf1ΔF4/ΔF4 CD4+ T cells is in part due to aberrant expression of IL-21. IL-21 has been shown to promote Th17 cell differentiation by activation of the Stat3 pathway(10, 54). Indeed, blocking Stat3 in CD4+ T cells by the pharmacological agent STA-21 (55) (Fig. 6C) or by genetic deletion (Fig. 6D) eliminated the aberrant IL-17 and IL-22 expression in Ikzf1ΔF4/ΔF4 CD4+ T cells, but only partially restored TGF-β-induced Foxp3 expression (data not shown). Together, these data suggest that Ikaros, through zinc finger 4, regulates Th17 cell-related cytokine production and TGF-β-induced Foxp3 expression via inhibition of IL-21 and Stat3-dependent pathways.
Figure 6. Aberrant expression of IL-21 in Ikzf1ΔF4/ΔF4 CD4+ T cells promotes Th17 cell-like phenotype via Stat3 pathway.
In vitro neutralization assays for IL-21 (A,B) and Stat3 inhibition (C) or genetic deletion (D) were conducted on CD4+ T cells purified from littermate mice of the indicated genotypes, activated by anti-CD3/CD28, and cultured with or without TGF-β, Ahr ligand FICZ, and neutralizing antibodies (A,B) or Stat3 inhibitor STA-21 (C) for 96 hours. IL-17 and IL-22 expression (A,C,D) or Foxp3 (B) expression was measured by intracellular staining and flow cytometry. Data are representative of at least two independent experiments.
RORγt is Required for Aberrant IL-17 but Not IL-22 Production in Ikzf1ΔF4/ΔF4 CD4+ T Cells
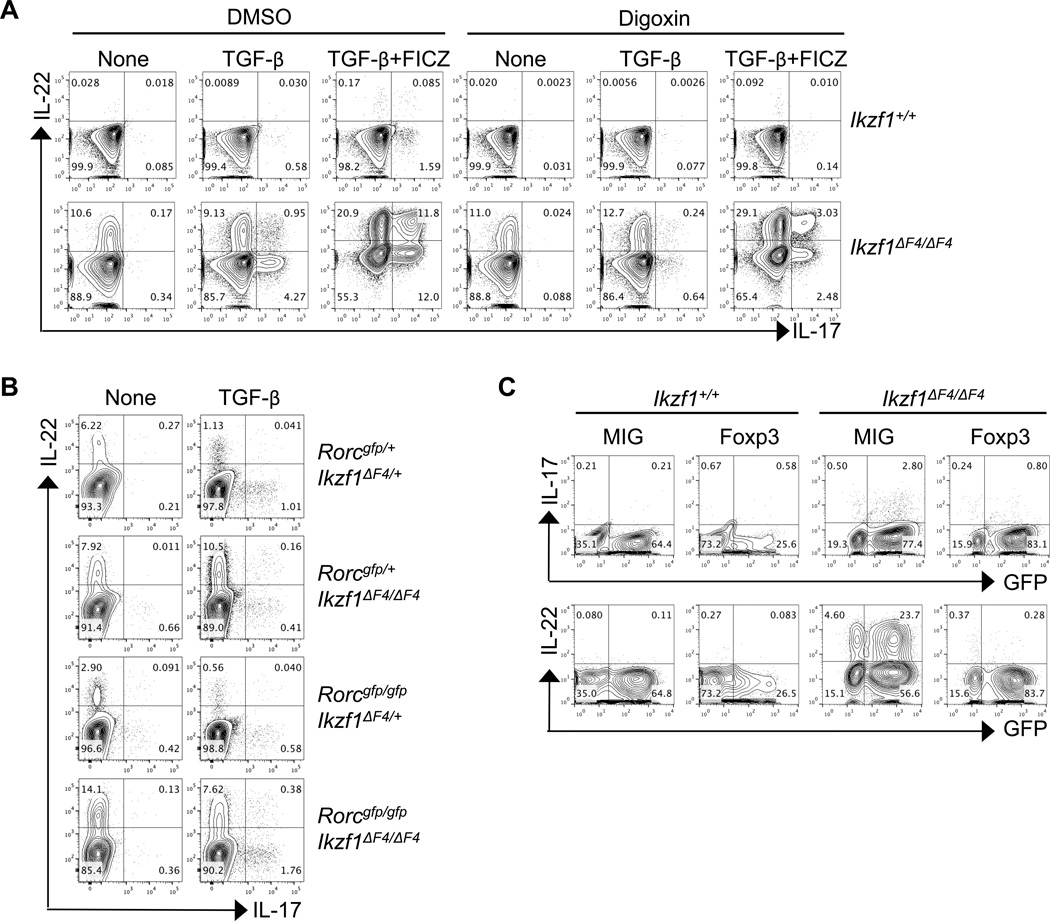
RORγt is a key transcription factor that promotes transcription of both IL-17 and IL-22 in wildtype CD4+ T cells(13). To investigate the potential role of RORγt in aberrant production of Th17 cell-associated cytokines by Ikzf1ΔF4/ΔF4 CD4+ T cells, we cultured CD4+ T cells with TGF-β or TGF-β plus FICZ with or without digoxin, a pharmacological agent that antagonizes RORγt activity and is known to suppress Th17 cell differentiation(56, 57). Intriguingly, inhibition of RORγt activity greatly diminished the aberrant IL-17 but had minimal effect on IL-22 expression in Ikzf1ΔF4/ΔF4 CD4+ T cells, and especially IL-22+IL-17− cells were not reduced by digoxin-mediated RORγt activity blockade (Fig. 7A).
Figure 7. RORγt differentially regulates and Foxp3 suppresses aberrant Th17-associated cytokines in Ikzf1ΔF4/ΔF4 CD4+ T cells.
CD4+ T cells were purified from littermate mice of the indicated genotypes and activated by anti-CD3/CD28 with or without TGF-β (A, B) and with or without Ahr ligand FICZ (A) together with or without RORγt inhibitor Digoxin or DMSO as a control (A). Cytokine expression (A,B) was measured after 96 hours by intracellular staining and flow cytometry. CD4+ T cells were purified from littermate mice of the indicated genotypes, activated by anti-CD3/CD28, transduced with empty vector MIG plasmid or Foxp3-expressing retroviral plasmid, and skewed with TGF-β for 96 hours (C). IL-17, IL-22, and GFP expression (C) was measured by intracellular staining and flow cytometry. Data are representative of at least three independent experiments.
To test whether the residual activity of RORγt in the presence of digoxin was sufficient to support the observed IL-22 expression in Ikaros mutant CD4+ T cells, we genetically ablated RORγt expression in Ikaros mutant mice by generating Rorcgfp/gfpIkzf1ΔF4/ΔF4 mice. Consistent with the data using the RORγt pharmacological inhibitor (Fig. 7A), when CD4+ T cells were cultured with or without TGF-β, the production of IL-22 in Ikaros mutant mice was still aberrantly upregulated when RORγt was genetically deleted (compare the IL-22 production by CD4+ T cells from Rorcgfp/+Ikzf1ΔF4/ΔF4 mice and from Rorcgfp/gfpIkzf1ΔF4/ΔF4 mice) (Fig. 7B). Of note, IL-17 but not IL-22 production was markedly reduced in the Ikaros mutant CD4+ T cells on RORγt heterozygous background compared to wildtype background (i.e., Rorcgfp/+Ikzf1ΔF4/ΔF4 versus Ikzf1ΔF4/ΔF4) (Fig. 7B, Fig. 3C), consistent with the dependency of IL-17 on RORγt(13). Together, these data indicate a selective requirement for RORγt in upregulation of IL-17 but not IL-22 in Ikaros mutant CD4+ T cells and underscore the differential regulation of the IL-22 single-producing cells versus IL-17 and IL-22 double-producing cells (e.g., Th17 cells).
Foxp3 Inhibits Aberrant IL-22 Expression in Ikzf1ΔF4/ΔF4 CD4+ T cells
Foxp3 has been shown to inhibit Th17-associated cytokine expression by antagonizing RORγt activity(19). Given that Foxp3 expression is decreased in TGF-β-skewed Ikzf1ΔF4/ΔF4 CD4+ T cells, we next forced expression of Foxp3 in Ikzf1ΔF4/ΔF4 CD4+ T cells by retroviral transduction. Indeed, ectopic expression of Foxp3 in Ikzf1ΔF4/ΔF4 CD4+ T cells suppressed the aberrant expression of Th17 cell-associated cytokines (especially IL-22 cytokine production) (Fig. 7C). Notably, downregulation of IL-22 was also observed in the non-transduced cells (e.g., GFP−cells) when Foxp3 was expressed in Ikzf1ΔF4/ΔF4 CD4+ T cells, suggesting a by-stander effect on inhibition of IL-22 expression. Together, these data indicate that lack of Foxp3 induction is at least in part responsible for aberrant upregulation of proinflammatory cytokine production (i.e., IL-17 and IL-22) by Ikzf1ΔF4/ΔF4 CD4+ T cells.
DISCUSSION
The role of Ikaros and its family members in immune cell development has been increasingly recognized over the past decade. In CD4+ T cells, Ikaros has been identified as a key regulator for Th1, Th2, and Th17 cell subsets(29, 35, 58, 59), and family members Helios, Eos and Aiolos have been shown to play important roles in the Treg cell compartments(35, 42–44, 60). Our studies uncover a new role for Ikaros in the differential regulation of various Treg compartments (e.g., tTregs, pTregs and iTregs). We further demonstrate a unique role of Ikaros in inhibiting IL-22-producing CD4+ T cells (especially IL-22+IL-17− CD4+ T cells) in vitro and in vivo in mucosal immunity both under the steady state and during bacterial infection.
In contrast to our study, a recent report showed that Ikaros positively regulates Th17 cell differentiation (e.g., IL-17 and IL-22 production)(35). The precise reasons underlying this discrepancy are unknown and most likely due to differences in the in vitro culturing conditions, mouse genetic backgrounds, and/or organ(s) assessed in vivo. For example, using Ikaros null mice on a mixed genetic background will most likely confound the data, consistent with a reduced Ahr affinity for ligand in certain mouse strains (i.e., DBA/2J and 129/SvJ)(61). In addition, Th17 cells (e.g., CD4+ T cells producing IL-17) in Ikaros null mice were only examined in the thymus(35), but not in other organs such as the gut where most in vivo Th17 cells are located(13). Finally, differential regulation of IL-17 and IL-22 production on a per cell basis by Ikaros was not determined in the previous study(35). Nevertheless, these studies ((35), and our findings) underscore the complex role of Ikaros in regulating Th17 cell-associated cytokine expression.
Previous data have shown that of the four N-terminal DNA-binding zinc fingers, fingers 2 and 3 are essential for the protein-DNA binding process and fingers 1 and 4 may modulate the binding of Ikaros to DNA(33, 34, 62). Our data showed that the absence of the fourth DNA-binding zinc finger of Ikaros (i.e., Ikzf1ΔF4/ΔF4) led to impaired induction of Foxp3 (i.e., iTreg cells) and aberrant upregulation of IL-17 and IL-22 expression upon TCR activation and TGF-β stimulation in T cells. Our data further showed that in bona fide Th17 cell-polarizing conditions (i.e., IL-6, TGF-β and/or FICZ), Ikzf1ΔF4/ΔF4 CD4+ T cells still produced more IL-22 but not IL-17. The precise mechanism that controls IL-17 expression by Ikzf1ΔF4/ΔF4 CD4+ T cells in different differentiation conditions (i.e., Th17 condition vs. Th0 and iTreg conditions) remains to be determined.
We favor a model that the absence of wildtype Ikaros resulted in unrestricted or de-repressed IL-21 expression that under Th0 condition (i.e., anti-CD3/CD28) promoted production of IL-22 by CD4+ T cells and together with the exogenous addition of TGF-β skewed the cells into a Th17 cell-like state (i.e., production of IL-17 and IL-22). Consistent with this notion, neutralizing IL-21 or blocking the Stat3 pathway that is activated by IL-21 efficiently inhibited the aberrant cytokine production (i.e., IL-22 and IL-17) in Ikzf1ΔF4/ΔF4 cells. Furthermore, severely impaired induction of Foxp3 at least in part accounted for the aberrant upregulation of IL-17 and IL-22 cytokine production by Ikzf1ΔF4/ΔF4 CD4+ T cells upon TGF-β treatment. Indeed, forced expression of Foxp3 can completely block aberrant expression of IL-17 and IL-22, consistent with a negative role of Foxp3 in regulation of RORγt and/or Ahr activity(19). C-Maf has been suggested to mediate the TGF-β-dependent suppression of IL-22 production by CD4+ T cells(16); however, we found no reduction in the expression of this transcription factor in Ikzf1ΔF4/ΔF4 CD4+ T cells compared to wildtype cells (data not shown).
The precise mechanisms underlying the differential regulation of distinct Treg compartments remain to be determined. Intriguingly, although the pTregs and iTregs were decreased, the tTregs were enhanced in the Ikzf1ΔF4/ΔF4 mice. These findings are consistent with the requirement for stronger TCR activation during tTreg development(63), and with the reduced TCR activation threshold observed in the Ikzf1ΔF4/ΔF4 mice, consistent with previously reported findings in Ikaros null CD4+ T cells(53). Blocking IL-21 in the Ikzf1ΔF4/ΔF4 CD4+ T cells only partially restored the Foxp3 expression induced by TGF-β (i.e., iTregs), suggesting the contribution of additional mechanism(s) to the defects of iTreg differentiation in the absence of wildtype Ikaros. In contrast to tTregs, iTreg cell differentiation has been shown to require suboptimal TCR signaling(64). Thus, besides aberrant expression of IL-21, the enhanced TCR strength may further contribute to the iTreg defects observed in Ikzf1ΔF4/ΔF4 mice.
Our previous findings have shown that optimal expression of IL-22 in wildtype CD4+ T cells requires the presence of both transcription factors RORγt and Ahr that work in a cooperative manner(6). Intriguingly, our data showed that the aberrant production of IL-17 but not IL-22 by Ikzf1ΔF4/ΔF4 CD4+ T cells was dependent on RORγt. Although the precise mechanisms underlying the bypass of a requirement for RORγt in the regulation of Il22 transcription in Ikzf1ΔF4/ΔF4 CD4+ T cells remain to be further determined, all together our data support a model of differential regulation of Th17 cell-associated cytokine transcription by Ikaros.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the entire L.Z. laboratory for their help and suggestions. We thank the Mouse Histology and Phenotyping Laboratory (Northwestern University) for their services and assistance. We thank Drs. S. Swaminathan and C. Goolsby at Flow Cytometry Facility (Northwestern University) for cell sorting support. We also thank Dr. L. Molinero for advice on the ChIP analysis, Dr. C. Mullighan and Dr. O. Cen for mice.
Footnotes
The work was supported by the National Institutes of Health (AI089954 and AI091962 to LZ), by a Cancer Research Institute Investigator Award (LZ), and by a Skin Disease Research Center (Northwestern University) Pilot and Feasibility Award (LZ). Liang Zhou is a Pew Scholar in Biomedical Sciences, supported by the Pew Charitable Trusts, and an Investigator in the Pathogenesis of Infectious Disease, supported by Burroughs Wellcome Fund. Jennifer Heller is supported by NIH training grant (T32 NIH T32 GM08061). The authors have no financial conflict of interest.
REFERENCES
- 1.Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PloS one. 2013;8:e72788. doi: 10.1371/journal.pone.0072788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bry L, Brenner MB. Critical role of T cell-dependent serum antibody, but not the gut-associated lymphoid tissue, for surviving acute mucosal infection with Citrobacter rodentium, an attaching and effacing pathogen. Journal of immunology. 2004;172:433–441. doi: 10.4049/jimmunol.172.1.433. [DOI] [PubMed] [Google Scholar]
- 3.Maaser C, Housley MP, Iimura M, Smith JR, Vallance BA, Finlay BB, Schreiber JR, Varki NM, Kagnoff MF, Eckmann L. Clearance of Citrobacter rodentium requires B cells but not secretory immunoglobulin A (IgA) or IgM antibodies. Infection and immunity. 2004;72:3315–3324. doi: 10.1128/IAI.72.6.3315-3324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nature immunology. 2011;12:383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 5.Tumanov AV, Koroleva EP, Guo X, Wang Y, Kruglov A, Nedospasov S, Fu YX. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell host & microbe. 2011;10:44–53. doi: 10.1016/j.chom.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36:92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo X, Qiu J, Tu T, Yang X, Deng L, Anders RA, Zhou L, Fu YX. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity. 2014;40:25–39. doi: 10.1016/j.immuni.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basu R, O'Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, Weaver CT. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity. 2012;37:1061–1075. doi: 10.1016/j.immuni.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annual review of immunology. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nature immunology. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 11.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 13.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 14.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 16.Rutz S, Noubade R, Eidenschenk C, Ota N, Zeng W, Zheng Y, Hackney J, Ding J, Singh H, Ouyang W. Transcription factor c-Maf mediates the TGF-beta-dependent suppression of IL-22 production in T(H)17 cells. Nature immunology. 2011;12:1238–1245. doi: 10.1038/ni.2134. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, Ouyang W. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 18.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nature immunology. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 19.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang F, Meng G, Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nature immunology. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 23.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 25.Georgopoulos K, Moore DD, Derfler B. Ikaros, an early lymphoid-specific transcription factor and a putative mediator for T cell commitment. Science. 1992;258:808–812. doi: 10.1126/science.1439790. [DOI] [PubMed] [Google Scholar]
- 26.Hahm K, Ernst P, Lo K, Kim GS, Turck C, Smale ST. The lymphoid transcription factor LyF-1 is encoded by specific, alternatively spliced mRNAs derived from the Ikaros gene. Molecular and cellular biology. 1994;14:7111–7123. doi: 10.1128/mcb.14.11.7111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JH, Nichogiannopoulou A, Wu L, Sun L, Sharpe AH, Bigby M, Georgopoulos K. Selective defects in the development of the fetal and adult lymphoid system in mice with an Ikaros null mutation. Immunity. 1996;5:537–549. doi: 10.1016/s1074-7613(00)80269-1. [DOI] [PubMed] [Google Scholar]
- 28.Umetsu SE, Winandy S. Ikaros is a regulator of Il10 expression in CD4+ T cells. Journal of immunology. 2009;183:5518–5525. doi: 10.4049/jimmunol.0901284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quirion MR, Gregory GD, Umetsu SE, Winandy S, Brown MA. Cutting edge: Ikaros is a regulator of Th2 cell differentiation. Journal of immunology. 2009;182:741–745. doi: 10.4049/jimmunol.182.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas RM, Chen C, Chunder N, Ma L, Taylor J, Pearce EJ, Wells AD. Ikaros silences T-bet expression and interferon-gamma production during T helper 2 differentiation. The Journal of biological chemistry. 2010;285:2545–2553. doi: 10.1074/jbc.M109.038794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas RM, Chunder N, Chen C, Umetsu SE, Winandy S, Wells AD. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. Journal of immunology. 2007;179:7305–7315. doi: 10.4049/jimmunol.179.11.7305. [DOI] [PubMed] [Google Scholar]
- 32.Bandyopadhyay S, Dure M, Paroder M, Soto-Nieves N, Puga I, Macian F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2007;109:2878–2886. doi: 10.1182/blood-2006-07-037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cobb BS, Morales-Alcelay S, Kleiger G, Brown KE, Fisher AG, Smale ST. Targeting of Ikaros to pericentromeric heterochromatin by direct DNA binding. Genes & development. 2000;14:2146–2160. doi: 10.1101/gad.816400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schjerven H, McLaughlin J, Arenzana TL, Frietze S, Cheng D, Wadsworth SE, Lawson GW, Bensinger SJ, Farnham PJ, Witte ON, Smale ST. Selective regulation of lymphopoiesis and leukemogenesis by individual zinc fingers of Ikaros. Nature immunology. 2013;14:1073–1083. doi: 10.1038/ni.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong LY, Hatfield JK, Brown MA. Ikaros sets the potential for Th17 lineage gene expression through effects on chromatin state in early T cell development. The Journal of biological chemistry. 2013;288:35170–35179. doi: 10.1074/jbc.M113.481440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 37.Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science. 2004;305:248–251. doi: 10.1126/science.1096472. [DOI] [PubMed] [Google Scholar]
- 38.Raz R, Lee CK, Cannizzaro LA, d'Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2846–2851. doi: 10.1073/pnas.96.6.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostanin DV, Pavlick KP, Bharwani S, D'Souza D, Furr KL, Brown CM, Grisham MB. T cell-induced inflammation of the small and large intestine in immunodeficient mice. American journal of physiology. Gastrointestinal and liver physiology. 2006;290:G109–G119. doi: 10.1152/ajpgi.00214.2005. [DOI] [PubMed] [Google Scholar]
- 40.Pavlick KP, Ostanin DV, Furr KL, Laroux FS, Brown CM, Gray L, Kevil CG, Grisham MB. Role of T-cell-associated lymphocyte function-associated antigen-1 in the pathogenesis of experimental colitis. International immunology. 2006;18:389–398. doi: 10.1093/intimm/dxh378. [DOI] [PubMed] [Google Scholar]
- 41.Qiu J, Guo X, Chen ZM, He L, Sonnenberg GF, Artis D, Fu YX, Zhou L. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39:386–399. doi: 10.1016/j.immuni.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, Jinasena D, Sharma SM, McCadden EM, Getnet D, Drake CG, Liu JO, Ostrowski MC, Pardoll DM. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009;325:1142–1146. doi: 10.1126/science.1176077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. Journal of immunology. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, Rangachari M, Zhu C, Xiao S, Seavitt J, Georgopoulos K, Kuchroo VK. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nature immunology. 2012;13:770–777. doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molnar A, Wu P, Largespada DA, Vortkamp A, Scherer S, Copeland NG, Jenkins NA, Bruns G, Georgopoulos K. The Ikaros gene encodes a family of lymphocyte-restricted zinc finger DNA binding proteins, highly conserved in human and mouse. Journal of immunology. 1996;156:585–592. [PubMed] [Google Scholar]
- 46.Klug CA, Morrison SJ, Masek M, Hahm K, Smale ST, Weissman IL. Hematopoietic stem cells and lymphoid progenitors express different Ikaros isoforms, and Ikaros is localized to heterochromatin in immature lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:657–662. doi: 10.1073/pnas.95.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ, Ruminski P, Weiss D, Von Schack D, Bluestone JA. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. The Journal of experimental medicine. 2012;209:1713–1722. S1711–S1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG, Yang Y, Floess S, Huehn J, Oh S, Li MO, Niec RE, Rudensky AY, Dustin ML, Littman DR, Lafaille JJ. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. The Journal of experimental medicine. 2012;209:1723–1742. S1721. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DA, Ziegler SF. Regulatory T cells: recommendations to simplify the nomenclature. Nature immunology. 2013;14:307–308. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 50.Ota N, Wong K, Valdez PA, Zheng Y, Crellin NK, Diehl L, Ouyang W. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nature immunology. 2011;12:941–948. doi: 10.1038/ni.2089. [DOI] [PubMed] [Google Scholar]
- 51.Qiu J, Zhou L. Aryl hydrocarbon receptor promotes RORgammat(+) group 3 ILCs and controls intestinal immunity and inflammation. Seminars in immunopathology. 2013;35:657–670. doi: 10.1007/s00281-013-0393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Winandy S, Wu L, Wang JH, Georgopoulos K. Pre-T cell receptor (TCR) and TCR-controlled checkpoints in T cell differentiation are set by Ikaros. The Journal of experimental medicine. 1999;190:1039–1048. doi: 10.1084/jem.190.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. The Journal of biological chemistry. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chalmin F, Mignot G, Bruchard M, Chevriaux A, Vegran F, Hichami A, Ladoire S, Derangere V, Vincent J, Masson D, Robson SC, Eberl G, Pallandre JR, Borg C, Ryffel B, Apetoh L, Rebe C, Ghiringhelli F. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 56.Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, Chow J, Manel N, Ciofani M, Kim SV, Cuesta A, Santori FR, Lafaille JJ, Xu HE, Gin DY, Rastinejad F, Littman DR. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature. 2011;472:486–490. doi: 10.1038/nature09978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fujita-Sato S, Ito S, Isobe T, Ohyama T, Wakabayashi K, Morishita K, Ando O, Isono F. Structural basis of digoxin that antagonizes RORgamma t receptor activity and suppresses Th17 cell differentiation and interleukin (IL)-17 production. J Biol Chem. 2011;286:31409–31417. doi: 10.1074/jbc.M111.254003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Movassagh M, Laderach D, Galy A. Proteins of the Ikaros family control dendritic cell maturation required to induce optimal Th1 T cell differentiation. International immunology. 2004;16:867–875. doi: 10.1093/intimm/dxh090. [DOI] [PubMed] [Google Scholar]
- 59.Gregory GD, Raju SS, Winandy S, Brown MA. Mast cell IL-4 expression is regulated by Ikaros and influences encephalitogenic Th1 responses in EAE. The Journal of clinical investigation. 2006;116:1327–1336. doi: 10.1172/JCI27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raffin C, Pignon P, Celse C, Debien E, Valmori D, Ayyoub M. Human memory Helios- FOXP3+ regulatory T cells (Tregs) encompass induced Tregs that express Aiolos and respond to IL-1beta by downregulating their suppressor functions. Journal of immunology. 2013;191:4619–4627. doi: 10.4049/jimmunol.1301378. [DOI] [PubMed] [Google Scholar]
- 61.Thomas RS, Penn SG, Holden K, Bradfield CA, Rank DR. Sequence variation and phylogenetic history of the mouse Ahr gene. Pharmacogenetics. 2002;12:151–163. doi: 10.1097/00008571-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Kim J, Sif S, Jones B, Jackson A, Koipally J, Heller E, Winandy S, Viel A, Sawyer A, Ikeda T, Kingston R, Georgopoulos K. Ikaros DNA-binding proteins direct formation of chromatin remodeling complexes in lymphocytes. Immunity. 1999;10:345–355. doi: 10.1016/s1074-7613(00)80034-5. [DOI] [PubMed] [Google Scholar]
- 63.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annual review of immunology. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oliveira VG, Caridade M, Paiva RS, Demengeot J, Graca L. Sub-optimal CD4+ T-cell activation triggers autonomous TGF-beta-dependent conversion to Foxp3+ regulatory T cells. European journal of immunology. 2011;41:1249–1255. doi: 10.1002/eji.201040896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.