Abstract
Objective:
This study examined the association of sleep before and during a chemotherapy (CT) cycle for breast cancer with symptoms and mood during a CT cycle.
Methods:
Twenty women undergoing CT for breast cancer completed the Pittsburgh Sleep Quality Inventory (PSQI) 1 hour prior to a CT infusion. For 3 weeks following infusion, participants estimated sleep efficiency (SE), minutes to sleep (sleep latency, SL), number of nocturnal awakenings (sleep fragmentation, SF) and sleep quality (SQ) each morning and rated symptoms (nausea, fatigue, numbness, difficulty thinking) and mood three times daily (morning, afternoon, and evening) via ecological momentary assessments (EMAs) using automated handheld computers.
Results:
The results showed that disturbed sleep (PSQI score ≥5) prior to CT infusion was associated with greater fatigue, and more negative and anxious mood throughout the 3 week CT cycle, and good pre-CT infusion sleep (PSQI score <5) buffered anxious mood in the first days following infusion. Time lagged analyses controlling for mood/symptom ratings reported the previous evening revealed that longer SL and greater SF were associated with greater daytime fatigue; poorer SQ and greater SF were antecedents of worse morning negative mood, and greater SF was associated with feeling more passive and drowsy. No evening symptom or mood ratings were related to subsequent sleep quality.
Conclusions:
These findings suggest that disturbed sleep before and after a CT infusion exacerbates fatigue, and negative, anxious, and drowsy mood during a CT cycle. Reducing sleep disturbance may be an important way to improve quality of life during chemotherapy.
Keywords: chemotherapy, breast cancer, fatigue, sleep, ecological momentary assessment, QOL
Introduction
Chemotherapy (CT) is a commonly used treatment for breast cancer, and is associated with a number of negative side effects including fatigue, nausea, neuropathy, cognitive impairment, disturbed sleep, and depressed mood.[1] Understanding the patient experience and the interplay between these symptoms is essential to developing targeted interventions to improve quality of life (QOL) during CT.
Disturbed sleep is one of the most common cancer-related symptoms, with an estimated 30-50% of newly diagnosed or recently treated cancer patients experiencing symptoms of insomnia.[2, 3] Prolonged sleep disturbance is associated with greater fatigue, distress, and depression and decreased physical activity and health-related QOL.[4-7] To date, the temporal nature of the relationship between sleep and common CT-related symptoms remains unclear.[8] Both directions of effect are intuitively plausible: increased cancer-related symptoms may result in disturbed sleep, while disturbed sleep also may worsen cancer-related symptoms.
One recent study identified a cascading effect in which increased sleep disturbance led to greater fatigue, which led to greater depressed mood in a sample of women receiving CT for gynecologic cancer.[9] This study noted that fatigue did not predict subsequent sleep disturbance. Additionally, a time-lagged study of individuals with chronic pain and insomnia found that better sleep quality was predictive of morning pain relief, while presleep pain did not predict subsequent sleep quality.[10] Though these studies suggest that sleep may drive subsequent symptoms, they did not examine the relationship of sleep with other common CT-related symptoms (e.g., nausea, cognitive impairment, neuropathic numbness, or depressed mood) and did not consider patient experience during a complete CT cycle. Thus, a time-lagged examination of sleep and the most common cancer-related symptoms may reveal areas to intervene to improve QOL.
The Present Study
The present study used self-report measures and ecological momentary assessment (EMA) to assess the temporal association between sleep and cancer-related symptoms during a CT cycle. EMA allows patients to record their symptoms in “real time” in their natural environment, providing a potentially clearer picture of patient experience compared to traditional retrospective self-report measures.[11, 12] The only known published study to use EMA during active cancer treatment did not assess many of the most common CT-related symptoms including nausea, cognitive impairment, and neuropathic numbness.[9] Thus, the present study examined the interplay between sleep and cancer-related symptoms during a cycle of CT.
The present study had three hypotheses. First, we hypothesized that sleep prior to a CT infusion (pre-CT) would moderate cancer-related symptoms and mood during the subsequent three-weeks; such that good pre-CT sleep (Pittsburgh Sleep Quality Index (PSQI) < 5) would buffer cancer-related symptoms and negative mood, particularly in the first few days after CT when symptoms tend to be most severe.[13] Second, we hypothesized that sleep disturbances during the three-weeks after CT infusion would be positively associated with subsequent cancer-related symptoms and worse mood, independent of symptoms and mood reported prior to sleep. Third, we hypothesized that cancer-related symptoms and mood reported just prior to sleep would not be significantly associated with subsequent sleep quality, independent of patient’s sleep quality the previous night.
Method
Participants
Participants were women with stage I, II or III breast cancer undergoing either neoadjuvant or adjuvant CT at MD Anderson in 2002, who had received at least one cycle of CT with at least two left, had no evidence of distant metastases, were able to read and speak English, and lived within 90 miles of the hospital. Patients were excluded if they had any surgical procedures not related to their breast cancer in the preceding year or had any medical conditions likely to affect outcome measures such as an autoimmune disease, endocrine abnormalities, current or past diagnosis of drug and/or alcohol dependence, current diagnosis of thought disorder, or were undergoing psychiatric or psychological counseling.
Procedure
Twenty-one women were recruited prior to a CT visit. Informed consent was obtained, baseline questionnaires (demographic information, the Pittsburgh Sleep Quality Index (PSQI), and other measures (to be reported in subsequent manuscript) were administered, and participants were given instructions on how to complete EMAs using the personal palm computer (PPC). Patients were asked to carry the device with them at all times until their next CT visit (approximately 3 weeks later).
During the EMA data collection period, the PPC randomly prompted patients with a beep four times a day within four set time blocks (wake-time, morning, afternoon, evening). The PPC was programmed to allow at least 2 hours between each assessment (Figure 1). The prompting signal lasted approximately 60 seconds. If there was no response, it sounded again 5 minutes later. The assessment was considered missed if there was no response to the second prompt. When the PPC was activated for the wake-time assessment, patients were asked questions about their sleep the previous evening. For the remaining three daily assessments, patients rated their mood and symptom severity. Each EMA took 2-4 minutes to complete. Participants were compensated based on the percentage of EMAs that they completed during assessment period.
Figure 1.
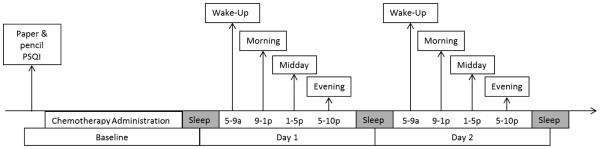
Study design and EMA timing.
This study was approved by the Institutional Review Board of MD Anderson Cancer Center.
Measures
Pre-CT Sleep Assessment
Sleep disturbances were assessed using the Pittsburgh Sleep Quality Index (PSQI).[14] The PSQI assesses quality of sleep and sleep disturbances over a 1-month period. A total score is derived with a score of 5 or greater associated with being a “poor” sleeper.[14] In this study sample, the internal reliability was acceptable (Cronbach’s alpha=0.73).
Post-CT EMAs
EMAs were designed specifically for this study using the Casio E-100 running Windows CE PPC and a custom-programmed software system. Participants completed assessments on the PPC using a stylus pen. All EMAs were date and time stamped.
Wake-time assessments were programmed to prompt patients within 30 minutes of their average wake up time. The wake-time assessment asked patients to estimate: 1) minutes they spent in bed (scored as “total sleep time;” TST), 2) minutes it took them to fall asleep (scored as “sleep latency”; SL); 3) the number of nocturnal awakenings (scored as “sleep fragmentation”; SF); 4) the duration of nocturnal awakenings (scored as “sleep fragmentation duration”; SFD), and 5) their overall quality of sleep (SQ) the previous night rated from 1 (very bad) to 10 (very good). Sleep efficiency (SE) was calculated by subtracting SL and SFD from TST, and dividing this by TST. To reduce the number of analyses, the present study focused on SE, SL, SF, and SQ.
Three daily assessments (delivered morning, afternoon, and evening) asked patients to rate their current mood using the Circumplex Model of Emotion Measure (CMEM). The CMEM is a theory-driven measure based on the conceptualization of mood as a combination of a two “core” dimensions: arousal and valence, which has been supported by behavioral, cognitive, and neurophysiological research.[15, 16] This measure was chosen because in other patient populations CMEM mood states were associated with physical symptoms.[17] The CMEM is comprised of 48 mood adjectives that are divided into eight categories (active, inactive, pleasant, unpleasant, calm, anxious, peppy, and drowsy).[18] To minimize the length of each EMA, the PPC software presented two adjectives from each of the eight categories at each assessment. The PPC selected the adjectives based on an algorithm that ensured each adjective was presented only once per day and the adjectives were presented in as many different pair combinations as possible. Participants rated the degree to which the adjective described the way they were feeling at the moment from 1 (not at all) to 5 (extremely). Four bipolar mood-vector scores were derived (i.e., active/inactive, pleasant/unpleasant, calm/anxious, peppy/drowsy), by subtracting ratings of negative adjectives from ratings of the opposing positive adjective. Thus, the mood-vector scores range from −5 to +5, with positive scores indicating more active, pleasant, calm, and peppy mood.
Patients then rated the current severity of four symptoms (nausea, fatigue, difficulty concentrating, and numbness) using a 1 (not present) to 10 (as bad as you can imagine) scale. Symptoms were derived from focus groups conducted prior to the start of the present study, which included a total of 50 stage II and III breast cancer patients who recently completed chemotherapy, and were asked to describe the most severe cancer-related symptoms they experienced during chemotherapy. A number of studies also indicate that these are the most common symptoms women with breast cancer experience during CT.[19]
Analyses
Taking into account the dependent nature of nested longitudinal EMA data (i.e., assessments nested within days and days nested within participants) the PROC MIXED procedure in SAS was used to conduct linear multilevel modeling (LMM) analyses.[20] LMM efficiently handles unbalanced designs and missing data without excluding participants or imputing values.[21] LMMs were used to: 1) estimate the effects of pre-CT sleep quality (i.e., PSQI scores) and day (i.e., assessment day 1 through day 21) on EMA symptom ratings and mood-vector scores recorded across the 21-day post-CT assessment period; 2) estimate the effects of sleep quality during the EMA monitoring period (i.e., the daily wake-time EMA SE, SL, SF, and SQ) on morning, midday, and evening EMA symptom ratings and mood-vector scores controlling for the symptom and mood ratings reported at the previous days’ evening assessment; and 3) estimate the effects of the EMA symptom ratings and mood-vector scores at the evening assessment on sleep indices (SE, SL, SF, and SQ) reported the following morning (which assessed the previous nights’ sleep), controlling for the sleep indices reported at the previous days’ wake-time assessment. The covariates in models 2 and 3 were included in an effort to establish direction of effect. Specifically, in model 2, the effect of sleep on symptoms and mood throughout the day could be examined independent of the effect of pre-sleep mood and symptoms. Similarly, in model 3, the effect of evening symptoms and mood on sleep could be examined independent of the effect of the previous night’s sleep.
For the first hypothesis, the Bonferroni method was used to correct for testing 8 models (four mood and four symptom variables regressed on pre-CT PSQI), taking into consideration the average correlation of the outcome variables (mean r = 0.17), and alpha was adjusted to 0.009.[22] For the second hypothesis, the Bonferroni method was used to correct for testing 96 mixed models (four mood and four symptom variables at three daily time points regressed onto four sleep indices), with the average correlation of the outcome variables taken into consideration (mean r = 0.17), and alpha was adjusted to 0.001. For the third hypothesis, the Bonferroni method was used to correct for testing 32 mixed models (four sleep indices regressed onto four mood and four symptom variables at the evening assessment time point), with the average correlation of the outcome variables taken into consideration (mean r = 0.18), and alpha was adjusted to 0.003. Using G*Power, it was determined that a sample of size 20 patients who completed 40 repeated measurements would enable us to detect a small effect size (f 2 > 0.02) with 95% confidence with 80% power.[23]
Results
Participant Characteristics
Of the 21 women who enrolled in the current study, one did not complete any wake-time EMAs and was excluded from the analyses. Participant characteristics are shown in Table 1. The EMA monitoring period was during the first cycle of a treatment schedule for 20% of women, second cycle for 40% of women, third cycle for 15% of women, and the fourth cycle for 25% of women. Pre-CT assessment revealed that the majority of participants experienced disturbed sleep over the previous month (PSQI; mean = 6.57 (SD = 4.11); 55% reporting scores ≥ 5). CT regimen and number of prior CT cycles were not related to baseline PSQI scores.
Table 1.
Patient Characteristics
| Characteristic | N=20 | |
|---|---|---|
|
| ||
| Mean Age M (SD) | 54.70(10.29) | |
|
| ||
| Ethnicity No. (%) | ||
| White | 13 | 65 |
| African American | 3 | 15 |
| Asian/Pacific Islander | 2 | 10 |
| Unknown | 2 | 10 |
|
| ||
| Marital Status No. (%) | ||
| Married/Cohabitating | 13 | 35 |
| Divorced/Widowed | 4 | 20 |
| Never Married | 2 | 10 |
| Unknown | 1 | 5 |
|
| ||
| Education No. (%) | ||
| High School | 2 | 10 |
| Some College | 3 | 15 |
| College Graduate | 9 | 45 |
| Graduate Degree | 6 | 30 |
|
| ||
| Type of Chemotherapy No. (%) |
||
| FAC | 16 | 80 |
| Paclitaxel | 1 | 5 |
| Taxotere | 3 | 15 |
|
| ||
| Previous Cycles of Chemotherapy M (SD) |
3.70 (2.72) | |
|
| ||
| Stage of Disease No. (%) | ||
| I | 1 | 5 |
| II | 15 | 75 |
| III | 4 | 20 |
|
| ||
| Previous Radiation | 16 | 80 |
| Treatment No. (%) | ||
|
| ||
| Previous Hormone Therapy No. (%) |
13 | 65 |
|
| ||
| Menopausal Status No. (%) | 16 | 80 |
Abbreviations: Fluorouracil, Adriamycin, Cyclophosphamide = FAC
Assessment Completion
All participants completed the PSQI at the orientation visit pre-CT infusion. Participants completed 923 assessments during the EMA monitoring period. Participants had their PPC for an average of 20.15 days (SD = 1.84), were prompted to complete an average of 80.6 assessments, and completed an average of 46.15 assessments, demonstrating an average compliance rate of 57%. An average of 12.55 (SD =5.09) wake-up, 12.15 (SD = 4.32) morning, 10.75 (SD = 4.66) midday, and 10.70 (SD = 4.92) evening assessments per person were completed during the assessment period. Compliance rate was not associated with EMA sleep, symptom, or mood ratings with the exception of SQ. Higher wake-up assessment compliance rate was associated with higher average SQ ratings (r = 0.46, p = 0.04).
Descriptive Statistics: Symptoms, mood, and sleep during CT cycle
During the EMA period, women reported relatively low symptom severity, with fatigue receiving the highest severity rating (3.77 (SD = 2.52)), and tended to report experiencing a positive (2.90 (SD = 2.41)) and calm mood (2.88 (SD = 2.51)). Mixed models regressing days of EMA monitoring onto each symptom and mood variable indicated that morning, midday, and evening nausea decreased significantly over the 21-day EMA monitoring period following CT infusion (p’s < 0.0001). Additionally, morning and afternoon ratings of active mood increased over the 21-day EMA monitoring period (p’s < 0.01). Conversely, afternoon and evening symptoms of anxious mood increased slightly over the 21-day EMA monitoring period (p’s < 0.04), as did evening ratings of difficulty thinking (p = 0.01). All other symptom and mood variables remained stable over time (p’s > 0.05).
During the EMA period, women averaged 85.9% (SD = 16.99) SE, and reported a mean of 20.73 (SD = 20.46) minutes to fall asleep (SL), with women reporting greater than 30 minutes to fall asleep in 18% of wake-time assessments. Women also reported an average of 2.05 (SD = 1.56) nocturnal awakenings (SF), and rated sleep quality (SQ) as 6.32 (SD = 2.00) on a 10 point scale. SE increased (p = .03), and SL decreased (i.e., women fell asleep faster; p = 0.03) over time, while SF and SQ remained stable during the EMA period (p’s > 0.09).
Hypothesis 1: The association of pre-CT sleep with symptoms/mood during CT cycle
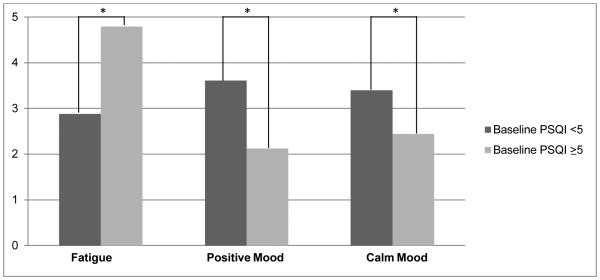
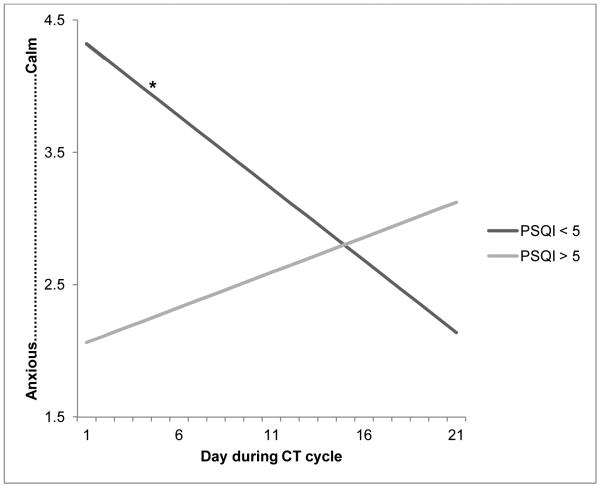
Using the adjusted alpha of 0.009, no significant associations between pre-CT sleep and symptoms/mood during CT cycle were found. However, several associations reached p < 0.05. Women who reported disturbed pre-CT sleep (PSQI ≥ 5) tended to report greater overall fatigue (p = 0.04), less positive/more negative (p = 0.02) and less calm/more anxious mood (p = 0.02) compared to women who indicated fewer pre-CT sleep problems (PSQI < 5; Figure 2). Additionally, there was an interaction between pre-CT sleep disturbances and time in predicting calm/anxious mood-vector scores (p = 0.03). To better understand the interaction, we conducted simple slope analyses using the method outlined by Preacher and colleagues. [24] Analyses revealed that for women who reported good pre-CT sleep, calm/anxious mood ratings became less calm/more anxious over time (r = −0.11, p = 0.05), while women with worse pre-CT sleep tended to report relatively consistent calm/anxious ratings over time (r = 0.05, p = 0.31; Figure 3). There was no association between pre-CT PSQI scores and nausea, difficulty thinking, and numbness across the 3-week CT cycle.
Figure 2.
Association of baseline PSQI with average fatigue and positive/negative and calm/anxious mood ratings during EMA monitoring period.
*p < 0.05
Figure 3.
Simple slopes analysis of baseline PSQI and time with calm/anxious mood-vector rating during EMA monitoring period
Note: For women with good pre-CT sleep (PSQI < 5), calm/anxious mood ratings became less calm/more anxious over the 21-day CT cycle, while calm/anxious ratings did not significantly change for women with poor pre-CT sleep (PSQI ≥ 5).
*p = 0.05
Hypothesis 2: The association of sleep with subsequent symptoms/mood during CT cycle
Using the adjusted alpha of 0.001, greater SL and SF reported at the wake-time assessment were associated with more fatigue at the morning assessment, independent of fatigue reported at the previous days’ evening assessment (p’s < 0.001; Table 2). Additionally, greater SF and poorer SQ reported at the wake-time assessment were associated with greater negative mood at the morning assessment, independent of negative mood ratings reported at the previous days’ evening assessment (p’s < 0.001). Finally, poorer SQ reported at the wake-time assessment was associated with feeling more passive and drowsy at the morning assessment, independent of passive and drowsy mood ratings reported at the previous days’ evening assessment (p’s < 0.001). SE was not associated with any subsequent symptoms.
Table 2.
Sleep associated with subsequent symptom and mood
|
Sleep
Efficiency β |
Sleep
Latency β |
Sleep
Fragmentation β |
Sleep
Quality β |
|
|---|---|---|---|---|
| Nausea | ||||
| Morning | −0.003 | 0.009** | 0.078 | −0.066 |
| Midday | 0.002 | −0.003 | 0.073 | −0.070 |
| Evening | −0.0003 | 0.002 | 0.106* | 0.013 |
| Fatigue | ||||
| Morning | −0.019** | 0.021*** | 0.228*** | −0.160** |
| Midday | −0.002 | 0.005 | 0.184** | −0.088 |
| Evening | −0.001 | 0.005 | 0.144 | −0.030 |
| Numbness | ||||
| Morning | −0.0003 | −0.001 | 0.004 | 0.013 |
| Midday | 0.001 | 0.001 | −0.038 | 0.027 |
| Evening | 0.002 | −0.001 | −0.081* | 0.040 |
| Difficulty Thinking | ||||
| Morning | −0.011** | 0.008** | 0.094* | −0.064 |
| Midday | −0.005 | 0.007* | 0.114* | −0.012 |
| Evening | 0.0003 | 0.003 | −0.014 | −0.005 |
| Positive/Negative | ||||
| Morning | 0.017 | −0.017* | −0.352*** | 0.327*** |
| Midday | 0.007 | 0.007 | −0.275* | 0.109 |
| Evening | 0.001 | −0.010 | −0.313* | 0.198 |
| Active/Passive | ||||
| Morning | 0.020 | −0.016 | −0.201 | 0.366*** |
| Midday | 0.020 | −0.003 | −0.226* | 0.017 |
| Evening | −0.012 | 0.009 | −0.113 | 0.002 |
| Calm/Anxious | ||||
| Morning | 0.005 | −0.006 | −0.078 | 0.254* |
| Midday | −0.018 | 0.016 | 0.017 | 0.232* |
| Evening | −0.005 | −0.009 | −0.193 | 0.200 |
| Peppy/Drowsy | ||||
| Morning | 0.021 | −0.020* | −0.387** | 0.395*** |
| Midday | 0.019 | −0.008 | −0.227 | 0.149 |
| Evening | 0.003 | −0.003 | −0.225 | 0.133 |
Standardized β weights are from linear multilevel modeling analyses in which each symptom mood-vector rating at each of the three daily time points were regressed onto each sleep index, controlling for the respective symptom and mood-vector rating reported at the previous days' evening assessment.
p<0.05
p<0.01
p<0.001
Hypothesis 3: The association of evening symptoms/mood with subsequent sleep during CT cycle
Greater evening numbness was marginally associated with longer SL (β = 4.198, p = 0.025), and greater evening fatigue was marginally associated with poorer SE (β = −1.23, p = 0.053) reported at wake-time assessment the next morning, though these associations did not meet the adjusted alpha of 0.003. All other evening mood and symptom predictors were not associated with sleep (p’s > 0.05).
Discussion
The present study is the only known study to employ EMA to examine patient’s perceptions of their experience during a complete CT cycle. Using an adjusted alpha of 0.009 for our first hypothesis, no significant associations between pre-CT sleep and symptoms/mood assessed during the three-week CT cycle were found. However, results suggested partial support for this hypothesis may have been found with greater power. Specifically, there was a trend (p < 0.05) for disturbed pre-CT sleep to be associated with greater fatigue and negative mood during the CT cycle.
Interestingly, calm/anxious mood ratings tended to become less calm for women with good pre-CT sleep, while calm/anxious mood ratings remained stable for those with disturbed pre-CT sleep. This finding may be best explained by regression to the mean, as women with good pre-CT sleep reported very calm mood (M = 4.32 on a −5 to +5 scale) immediately after CT infusion, which decreased by an average of 2.18 points by the end of the CT cycle. Conversely, women with poor pre-CT sleep reported a relatively less calm mood immediately after CT infusion (M = 2.06 on a −5 to +5 scale), which increased by an average of 1.06 points by the end of the CT cycle. The biggest difference in mood ratings between those with good and poor pre-CT sleep is during the first few days after CT infusion, and is in favor of those with good pre-CT sleep (Figure 3). Thus, good sleep prior to CT infusion may serve as a buffer against anxious mood during the days immediately following CT infusion.
In keeping with previous research, women tended to report sleep disturbances pre-CT infusion, with 55% of the sample scoring above the clinical cut off (≥ 5) on the PSQI.[2, 4] Thus, many women may be at increased risk for greater fatigue and mood disturbance during CT due to problematic sleeping patterns prior to treatment administration. Indeed, several longitudinal studies indicate that health-related QOL and fatigue are associated with sleep disturbances before, during, and after treatment for breast cancer.[3-6]
Results partially supported our second hypothesis that disturbed sleep during the three-week CT cycle would be positively associated with cancer-related symptoms and worse mood. Using an adjusted alpha of 0.001, greater SL and SF were associated with greater morning fatigue; poorer SQ and greater SF were associated with more negative morning mood; and poorer SQ was associated with greater passive and drowsy morning mood. A number of additional associations were found at the p < 0.01 levels, including a positive association between SL and both morning nausea and difficulty thinking, and a negative association between SE and morning fatigue and difficulty thinking (Table 2). Importantly, these analyses controlled for symptom and mood ratings provided at the evening assessment, suggesting that the relationship between sleep and daytime cancer-related symptoms and mood exists independent of the patient’s symptom and mood the previous evening.
Our third hypothesis, that cancer-related symptoms and mood reported prior to sleep would not be related to sleep quality, was supported. Using an adjusted alpha of 0.003, no symptom or mood ratings provided at the evening assessment were significantly associated with sleep outcomes the next morning, though greater evening numbness was marginally associated with longer SL (p = 0.03), and greater evening fatigue was marginally associated with poorer SE (p = 0.05). Thus, interventions aimed at reducing sleep disturbance may be the most effective method by which to improve QOL during CT.
There are a number of factors that that may explain how sleep disruption leads to worse symptom severity and mood. Evidence suggests that disturbed sleep/wake cycles are associated with disrupted cortisol rhythms and increased inflammation, which in turn have been associated with fatigue, pain, and depression.[25, 26] However, the associations may be more complex and non-linear. Recent studies examining clusters of cancer-related symptoms (e.g., fatigue, nausea, depressed mood, cognitive difficulties, and disturbed sleep) and biological outcomes find that increased inflammation likely represents a shared mechanism underlying the effects of cancer, chemotherapy, and the manifested cluster of symptoms.[19, 27, 28] Lyon and colleagues note that future research is needed to better understand the mechanisms driving the interplay among cancer progression, treatment, and symptoms, and propose epigenetic alternations at the deoxyribonucleic acid (DNA) level may be a key mechanism underlying the associations.[29]
As this study was among the first to use EMA during active CT, these findings can also provide an important depiction of the patient experience during treatment. First, in keeping with previous research, patients rated fatigue as the most severe symptom. Additionally, similar to previous research suggesting symptoms abate during the CT cycle, patients in the present study reported less nausea, better sleep outcomes, and indicated feeling more active as time progressed.[30, 31] Conversely, anxiety and cognitive difficulty appeared to worsen over the CT cycle, consistent with previous research suggesting cancer-related anxiety and CT-related cognitive impairment can be long lasting with delayed onset.[32, 33] Interestingly, women in the present study reported falling asleep relatively quickly with few instances of awakening in the night, which resulted in relatively efficient sleep. Only 18% of wake-time assessments indicated clinically problematic SL (>30 minutes), and women reported far fewer instances of SF (2.05 (SD = 1.56)) compared to the 22 nocturnal awakenings evidenced by actigraphy in women undergoing CT in a study by Berger and colleagues.[34] Further, one study found that sleep diaries indicated only 20% of the nocturnal awakenings suggested by actigraphy, making it possible that women in the present study were unaware of nocturnal awakenings.[35] The SE reported in the present study (M = 85.9%, SD = 16.99) is comparable to that of other studies examining self-reported SE during active CT (82%-90%).[30] However, despite their reported normal SE, brief SL, and little SF, patients tended to report less than satisfying SQ (6.32 (SD = 2.00) on a 10 point scale), which is consistent with previous research using daily self-report methods.[5, 30]
There are several limitations to recognize in this study. First, the present study does not include an objective measure of sleep, such as actigraphy. Though several studies suggest that sleep indices obtained via self-report (e.g., daily diary) are very similar to those obtained via actigraphy,[35-37] it is possible that patients’ inadvertently under- or over-reported the number of minutes to sleep and nocturnal awakenings.[30] Indeed, women who tended to report higher SQ also tended to be more compliant with EMA sleep assessments, raising the possibility that subjective reports of SQ may be positively skewed. Additionally, due to the subjective nature of the sleep data, individuals’ rating of their sleep may be based on their current mood or symptoms (e.g., “I must have slept poorly because I feel very fatigued this morning”), making misattributions possible. Second, the relatively low compliance rate (57%) in the present study suggests that using EMA throughout a CT cycle (i.e., 21 days) may not be feasible. Most studies using EMA in patient populations report compliance rates of >75% and observation periods of 3-7 days.[11, 38] It is possible that a shorter data collection time, such as staggering 6 days of data collection across the beginning, middle, and end of the CT cycle (2 days each) may increase patient compliance and therefore provide richer data. Third, the sample size, though comparable to other studies utilizing EMA, was small.[11, 38, 39] Fourth, the majority of participants were white, non-Hispanic, married, and highly educated. Thus, future research is needed to test the generalizability of these findings to more diverse populations.
In spite of these limitations, the present study represents the first attempt to characterize the patient experience of a range of CT-symptoms in real time throughout a cycle of CT and to examine the interplay between common symptoms during active cancer treatment. These findings suggest that disturbed sleep before and during a three-week CT cycle exacerbates treatment-related symptoms including fatigue, and negative, anxious, and drowsy mood, and may also relate to increased nausea and cognitive problems. Designing and implementing interventions to reduce sleep disturbance, particularly in patients with sleep difficulties prior to treatment, may be an essential step toward reducing treatment-related symptoms and improving patient health, wellbeing, and QOL during chemotherapy.
Acknowledgments
This project was supported by a research grant from the National Institutes of Health/National Institute of Mental Health (5R21MH062031-02), and a cancer prevention fellowship for Chelsea G. Ratcliff from the National Cancer Institute (R25T CA57730, Shine Chang, PhD, Principal Investigator).
Footnotes
The authors have no conflict of interest to disclose.
References
- 1.Byar KL, et al. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. in Oncology Nursing Forum. Onc Nurs Society. 2006 doi: 10.1188/06.ONF.E18-E26. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Supportive Care in Cancer. 2006;14(3):201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu L, et al. The longitudinal relationship between fatigue and sleep in breast cancer patients undergoing chemotherapy. Sleep. 2012;35(2):237–45. doi: 10.5665/sleep.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, et al. Decreased health-related quality of life in women with breast cancer is associated with poor sleep. Behav Sleep Med. 2013;11(3):189–206. doi: 10.1080/15402002.2012.660589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanford SD, et al. Longitudinal prospective assessment of sleep quality: before, during, and after adjuvant chemotherapy for breast cancer. Support Care Cancer. 2013;21(4):959–67. doi: 10.1007/s00520-012-1612-7. [DOI] [PubMed] [Google Scholar]
- 6.Phillips KM, et al. Characteristics and correlates of sleep disturbances in cancer patients. Support Care Cancer. 2012;20(2):357–65. doi: 10.1007/s00520-011-1106-z. [DOI] [PubMed] [Google Scholar]
- 7.Berger AM, et al. Circadian rhythms, symptoms, physical functioning, and body mass index in breast cancer survivors. J Cancer Surviv. 2012;6(3):305–14. doi: 10.1007/s11764-012-0218-x. [DOI] [PubMed] [Google Scholar]
- 8.Roscoe JA, et al. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12(Suppl 1):35–42. doi: 10.1634/theoncologist.12-S1-35. [DOI] [PubMed] [Google Scholar]
- 9.Jim HS, et al. Lagged relationships among sleep disturbance, fatigue, and depressed mood during chemotherapy. Health Psychol. 2013;32(7):768–74. doi: 10.1037/a0031322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang NK, et al. Deciphering the temporal link between pain and sleep in a heterogeneous chronic pain patient sample: a multilevel daily process study. Sleep. 2012;35(5):675–87A. doi: 10.5665/sleep.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacker ED, Ferrans CE. Ecological momentary assessment of fatigue in patients receiving intensive cancer therapy. Journal of Pain and Symptom Management. 2007;33(3):267–275. doi: 10.1016/j.jpainsymman.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz JE, Stone AA. Strategies for analyzing ecological momentary assessment data. Health Psychology. 1998;17(1):6. doi: 10.1037//0278-6133.17.1.6. [DOI] [PubMed] [Google Scholar]
- 13.Berger AM, Higginbotham P. Correlates of fatigue during and following adjuvant breast cancer chemotherapy: a pilot study. Oncol Nurs Forum. 2000;27(9):1443–8. [PubMed] [Google Scholar]
- 14.Buysse DJ, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 15.Posner J, Russell JA, Peterson BS. The circumplex model of affect: an integrative approach to affective neuroscience, cognitive development, and psychopathology. Dev Psychopathol. 2005;17(3):715–34. doi: 10.1017/S0954579405050340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posner J, et al. The neurophysiological bases of emotion: An fMRI study of the affective circumplex using emotion-denoting words. Hum Brain Mapp. 2009;30(3):883–95. doi: 10.1002/hbm.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Affleck G, et al. Mood states associated with transitory changes in asthma symptoms and peak expiratory flow. Psychosomatic Medicine. 2000;62(1):61–68. doi: 10.1097/00006842-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Larsen RJ, Diener E. Promises and problems with the circumplex model of emotion. 1992 [Google Scholar]
- 19.Wood LJ, Weymann K. Inflammation and neural signaling: etiologic mechanisms of the cancer treatment-related symptom cluster. Curr Opin Support Palliat Care. 2013;7(1):54–9. doi: 10.1097/SPC.0b013e32835dabe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Littell RC, et al. SAS for Mixed Models. 2nd SAS Institute Inc; Cary, NC: 2006. [Google Scholar]
- 21.Gibbons RD, et al. Random regression models: a comprehensive approach to the analysis of longitudinal psychiatric data. Psychopharmacology Bulletin. 1988;24:438–443. [PubMed] [Google Scholar]
- 22.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Statistics in Medicine. 1997;16(22):2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 23.Faul F, et al. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 24.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31(4):437–448. [Google Scholar]
- 25.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley KW, et al. Cytokine-induced sickness behavior. Brain, behavior, and immunity. 2003;17(1, Supplement):112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 27.Palesh O, et al. Sleep disruption in breast cancer patients and survivors. J Natl Compr Canc Netw. 2013;11(12):1523–30. doi: 10.6004/jnccn.2013.0179. [DOI] [PubMed] [Google Scholar]
- 28.Mantovani A, et al. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 29.Lyon D, et al. Potential Epigenetic Mechanism(s) Associated With the Persistence of Psychoneurological Symptoms in Women Receiving Chemotherapy for Breast Cancer: A Hypothesis. Biol Res Nurs. 2013 doi: 10.1177/1099800413483545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enderlin CA, et al. Sleep across chemotherapy treatment: a growing concern for women older than 50 with breast cancer. Oncol Nurs Forum. 2010;37(4):461–A3. doi: 10.1188/10.ONF.461-468. [DOI] [PubMed] [Google Scholar]
- 31.Berger AM, et al. Adherence, sleep, and fatigue outcomes after adjuvant breast cancer chemotherapy: results of a feasibility intervention study. Oncol Nurs Forum. 2003;30(3):513–22. doi: 10.1188/03.ONF.513-522. [DOI] [PubMed] [Google Scholar]
- 32.Dumas JA, et al. Chemotherapy altered brain functional connectivity in women with breast cancer: a pilot study. Brain Imaging Behav. 2013 doi: 10.1007/s11682-013-9244-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reece JC, et al. Course of depression, mental health service utilization and treatment preferences in women receiving chemotherapy for breast cancer. Gen Hosp Psychiatry. 2013;35(4):376–81. doi: 10.1016/j.genhosppsych.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 1998;25(1):51–62. [PubMed] [Google Scholar]
- 35.Berger AM, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psycho-Oncology. 2009;18(6):634–646. doi: 10.1002/pon.1438. [DOI] [PubMed] [Google Scholar]
- 36.Carney CE, Lajos LE, Waters WF. Wrist actigraph versus self-report in normal sleepers: Sleep schedule adherence and self-report validity. Behavioral Sleep Medicine. 2004;2(3):134–143. doi: 10.1207/s15402010bsm0203_2. [DOI] [PubMed] [Google Scholar]
- 37.Grutsch JF, et al. Validation of actigraphy to assess circadian organization and sleep quality in patients with advanced lung cancer. J Circadian Rhythms. 2011;9:4. doi: 10.1186/1740-3391-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Curran SL, Beacham AO, Andrykowski MA. Ecological Momentary Assessment of Fatigue Following Breast Cancer Treatment. Journal of Behavioral Medicine. 2004;27(5):425–444. doi: 10.1023/b:jobm.0000047608.03692.0c. [DOI] [PubMed] [Google Scholar]
- 39.Rumble ME, et al. Contribution of cancer symptoms, dysfunctional sleep related thoughts, and sleep inhibitory behaviors to the insomnia process in breast cancer survivors: a daily process analysis. Sleep. 2010;33(11):1501–9. doi: 10.1093/sleep/33.11.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]