Abstract
The Lecanoromycetes is the largest class of lichenized Fungi, and one of the most species-rich classes in the kingdom. Here we provide a multigene phylogenetic synthesis (using three ribosomal RNA-coding and two protein-coding genes) of the Lecanoromycetes based on 642 newly generated and 3329 publicly available sequences representing 1139 taxa, 317 genera, 66 families, 17 orders and five subclasses (four currently recognized: Acarosporomycetidae, Lecanoromycetidae, Ostropomycetidae, Umbilicariomycetidae; and one provisionarily recognized, ‘Candelariomycetidae’). Maximum likelihood phylogenetic analyses on four multigene datasets assembled using a cumulative supermatrix approach with a progressively higher number of species and missing data (5-gene, 5+4-gene, 5+4+3-gene and 5+4+3+2-gene datasets) show that the current classification includes non-monophyletic taxa at various ranks, which need to be recircumscribed and require revisionary treatments based on denser taxon sampling and more loci. Two newly circumscribed orders (Arctomiales and Hymeneliales in the Ostropomycetidae) and three families (Ramboldiaceae and Psilolechiaceae in the Lecanorales, and Strangosporaceae in the Lecanoromycetes inc. sed.) are introduced. The potential resurrection of the families Eigleraceae and Lopadiaceae is considered here to alleviate phylogenetic and classification disparities. An overview of the photobionts associated with the main fungal lineages in the Lecanoromycetes based on available published records is provided. A revised schematic classification at the family level in the phylogenetic context of widely accepted and newly revealed relationships across Lecanoromycetes is included. The cumulative addition of taxa with an increasing amount of missing data (i.e., a cumulative supermatrix approach, starting with taxa for which sequences were available for all five targeted genes and ending with the addition of taxa for which only two genes have been sequenced) revealed relatively stable relationships for many families and orders. However, the increasing number of taxa without the addition of more loci also resulted in an expected substantial loss of phylogenetic resolving power and support (especially for deep phylogenetic relationships), potentially including the misplacements of several taxa. Future phylogenetic analyses should include additional single copy protein-coding markers in order to improve the tree of the Lecanoromycetes. As part of this study, a new module (“Hypha”) of the freely available Mesquite software was developed to compare and display the internodal support values derived from this cumulative supermatrix approach.
Keywords: Classification; Cumulative supermatrix, Lecanoromycetes; Lichenized fungi; Maximum likelihood; Multi-gene phylogeny; Systematics
1. Introduction
The Lecanoromycetes (formally introduced by Eriksson and Winka, 1997) is the third largest known class of fungi (after Agaricomycetes and Dothideomycetes), with more than 14,000 recognized species, of which the majority (over 95%) are lichenized (Kirk et al., 2008). Besides lichenicolous species, only a few truly non-lichenized lineages evolved in the Lecanoromycetes, namely Odontotremataceae and Stictidaceae in the Ostropomycetidae (Baloch et al., 2010, 2012; Lutzoni et al., 2001, 2004; Schoch et al., 2009; Wedin et al., 2006). Members of the Lecanoromycetes typically form bi-membered symbiotic associations with coccoid and filamentous green algae from the Trebouxiophyceae and Ulvophyceae, respectively (e.g., Asterochloris, Coccomyxa, Dictyochloropsis s.l., Phycopeltis, Trebouxia, and Trentepohlia) or cyanobacteria from the orders Chroococcales, Nostocales, and Stigonematales (e.g., Nostoc, Rhizonema, Scytonema, and Stigonema). A small fraction of species form tri-membered symbioses involving two photobionts, usually a green alga and a cyanobacterium, in which the cyanobacterium (secondary photobiont) is located in internal or external compartments of the thallus called cephalodia (overview and relevant literature is included in Gueidan et al., 2014 and Voytsekhovich et al., 2011b).
The Lecanoromycetes are predominantly characterized by ascohymenial ascoma ontogeny resulting in apothecial fruiting bodies of various structures and shapes, amyloid asci with an apical thickening and two-layered wall, and a hamathecium formed by unbranched to branched and anastomosing interascal hyphae (Gueidan et al., 2014). Most members of the class produce a wide variety of secondary substances such as depsidones, terpenoids and xanthones (e.g., Culberson and Culberson, 1994; Elix and Stocker-Wörgötter, 2008; Huneck and Yoshimura, 1996), which are of great biological and systematic significance. Members of the Lecanoromycetes are distributed worldwide, primarily in terrestrial habitats (rarely aquatic or semiaquatic), where they grow on various substrates (tree bark, wood, leaves, rocks, soil, mosses, and other lichens). An overview on the biology of the Lecanoromycetes is provided in Gueidan et al. (2014) and the literature therein.
Molecular phylogenetic studies have substantially challenged the traditional classification of Ascomycota that were based on, e.g., ascoma morphology and development, ascus type and dehiscence, and ascospores, by revealing the non-monophyly of taxa at various taxonomic levels and providing an alternative framework for taxonomic revisions and recircumscriptions (Gueidan et al., 2014). Currently five subclasses and 17 orders are recognized in the Lecanoromycetes. Acarosporomycetidae, Lecanoromycetidae, Ostropomycetidae, and Umbilicariomycetidae have been formally described (Bendiksby and Timdal, 2013; Reeb et al. 2004; Hibbett et al. 2007), whereas ‘Candelariomycetidae’ is currently a provisionary name awaiting confirmation from a large-scale study with exhaustive taxon and locus sampling (Bendiksby and Timdal, 2013; Gaya et al., 2012; Gueidan et al., 2014; Hodkinson and Lendemer, 2011; Lumbsch and Huhndorf, 2010; Miadlikowska et al., 2006; Printzen, 2010; Schmull et al., 2011).
Until now the majority of phylogenetic studies on the Lecanoromycetes relied on various combinations of two or three ribosomal RNA genes, i.e., nucLSU, nucSSU and mitSSU. Recently, protein-coding markers (usually RPB1 and RPB2, but also beta-tubulin, GAPDH, and MCM7) have been more frequently used for phylogenetic inference within Lecanoromycetes (e.g., Baloch et al., 2010; Crespo et al., 2010; Hofstetter et al., 2007; Lumbsch et al., 2007b; Miadlikowska et al., 2006; Myllys et al., 2005; Otálora and Wedin, 2013; Otálora et al. 2013a; Parnmen et al., 2012; Reeb et al., 2004; Rivas Plata et al., 2012a, 2012b; Schmitt et al., 2010). Only five studies (Hofstetter et al., 2007; Lumbsch et al., 2004; Miadlikowska et al., 2006; Reeb et al., 2004; Wedin et al., 2005) were designed specifically to reconstruct phylogenetic relationships within the Lecanoromycetes at the family and higher ranks as a framework for the evaluation of existing classifications. Two additional studies utilized a broad taxon sampling across the Lecanoromycetes to address the phylogenetic affiliation of the genera, Lecidea s.l. (Schmull et al., 2011) and Hypocenomyce s.l. (Bendiksby and Timdal, 2013). Lumbsch et al. (2004) presented a Bayesian phylogeny based on a combined nucLSU and mitSSU dataset for 86 individuals representing 86 species and 59 genera from the Lecanoromycetes, and provided an overview of coexisting classifications of the Lecanoromycetes at the order level. Reeb et al. (2004) conducted a phylogenetic study addressing the circumscription and placement of the Acarosporaceae based in part on Bayesian and ML analyses of combined nucSSU, nucLSU and RPB2 sequences obtained from 82 species. With the study by Lutzoni et al. (2004), this was the first use of RPB2 sequences to resolve relationships within the Lecanoromycetes. The study by Wedin et al. (2005) as stated by the authors, focused on putatively basal and erroneously classified groups based on morphological and preliminary phylogenetic analyses, using a similar number of taxa as the previous studies (83 individuals representing 80 species and 61 genera in the Lecanoromycetes).
The first phylogenetic study on lichen-forming fungi based on four loci (nucLSU, nucSSU, mitSSU, and RPB2) was by Lutzoni et al. (2004). With the addition of RPB1 to these four loci, Miadlikowska et al. (2006) were the first to use a five-locus supermatrix to resolve phylogenetic relationships within Lecanoromycetes, while James et al. (2006) used a six-gene phylogeny to unveil the relationships of lichen-forming fungi across the entire kingdom. Hofstetter et al. (2007) provided a foundation for future multilocus phylogenetic studies for Fungi by evaluating the resolving power and statistical support delivered by protein-coding genes (RPB1 and RPB2) compared to the three commonly used ribosomal RNA-coding genes (nucLSU, nucSSU and mitSSU). Maximum likelihood analyses performed by Hofstetter et al. (2007) on each locus separately and in various combinations indicated that among available markers, the optimal loci (single or combined) to use for molecular systematics of the Lecanoromycetes were the protein-coding genes (RPB1 and RPB2). A gene-by-gene assessment of phylogenetic informativeness (sensu Townsend 2007) within a broader, Ascomycota-wide, study by Schoch et al (2009) yielded higher levels of informativeness for these two protein19 coding genes (and TEF1) as compared with the ribosomal genes.
The first implementation of a cumulative supermatrix approach was by Miadlikowska et al. (2006). Three five-locus datasets for the Lecanoromycetes were assembled with a progressively higher number of taxa (from 111 to 274 representing 10 orders, and 43 of the 64 families recognized by Eriksson at the time [2006]) and an increasing amount of missing data (with the inclusion of taxa with only four and three of the five targeted genes). That study showed that several non-monophyletic taxa at different ranks needed to be recircumscribed, and confirmed that ascus morphology cannot be applied consistently to shape the classification across the Lecanoromycetes. The authors concluded that the cumulative addition of taxa with increasing amount of missing data resulted in the expected decay of phylogenetic support values, but also improved statistical support for many internodes (see also Crespo et al., 2010 and Gaya et al., 2012). However, the addition of 43 taxa by Schmull et al. (2011) with mainly two non31 protein-coding loci to the Miadlikowska et al. (2006) supermatrix, caused a rearrangement of some phylogenetic relationships and a substantial decrease in their stability even if 5.8S sequences were added to the five-locus supermatrix. A more detailed overview on the progress in reconstructing phylogenetic relationships within the Lecanoromycetes and their implications for classification can be found in Gueidan et al. (2014), Lumbsch et al. (2004), Lutzoni et al. (2004), Miadlikowska et al. (2006), Printzen (2010), Reeb et al. (2004), and Wedin et al. (2005).
Despite a high number of phylogenetic studies focusing on various groups in the Lecanoromycetes, a comprehensive large-scale phylogeny with a dense taxon sampling, especially at the family and generic levels, coupled with the use of a larger number of protein coding genes is still needed. Numerous important relationships spanning all ranks, including the class Lecanoromycetes itself, are unsettled. A robust phylogeny is essential for a major and stable improvement of the classification of this class.
The main objectives of this study were to: 1) generate the most comprehensive phylogeny for the Lecanoromycetes to date by utilizing all sequence data available in GenBank (taxa represented by at least two of the five targeted genes: nucLSU, nucSSU, mitSSU, RRPB1 and RPB2) and by complementing the data with new sequences to increase taxon sampling and to reduce the amount of missing data; 2) evaluate the existing classification (Lumbsch and Huhndorf, 2010) and recently proposed changes at the family and higher ranks; 3) resolve phylogenetic placement of taxa with uncertain or unknown affinities and identify taxa that require further systematic revision; and 4) revisit the distribution of photobionts across Lecanoromycetes and their utility in lichen systematics and evolutionary studies.
Using a cumulative supermatrix approach (Miadlikowska et al., 2006; Gaya et al., 2012) we assembled four multigene datasets with a progressively higher number of taxa and missing data (5-gene, 5+4-gene, 5+4+3-gene and 5+4+3+2-gene datasets). Internodal support estimated with maximum likelihood bootstrap analyses for each dataset was displayed graphically on the optimal and most comprehensive tree using a newly developed module called “Hypha” (Oliver et al., 2013), implemented in Mesquite (Maddison and Maddison, 2011). Phylogenetic confidence revealed by each taxon subset of the complete supermatrix was compared and discussed in the context of missing data and stability of phylogenetic relationships. Phylogenetic relationships from the present, and recently published, studies and their potential implications for classification of the Lecanoromycetes were examined and depicted schematically at the family and higher ranks. Available records on photobiont-mycobiont associations across the Lecanoromycetes were summarized.
2. Materials and Methods
2.1 Gene and taxon sampling
Sequence data were generated and gathered for five genes (three ribosomal RNA-coding and two protein-coding): ca. 0.6 kb of the mitochondrial small subunit (mitSSU); ca. 1.6 kb of the large subunit (nucSSU); ca. 1.4 kb of the large subunit (nucLSU); ca. 0.7–1.2 kb of the largest subunit of the RNA polymerase II gene (RPB1) region A-F and ca. 1.5 kb of RPB1 region F-G (two amplicons, considered as a single gene in all phylogenetic analyses); ca. 0.8–1.0 kb of the second largest subunit of RNA polymerase II gene (RPB2) region 5–7 and ca. 1.0 kb of RPB2 region 7–11 (two amplicons, considered as a single gene in all phylogenetic analyses). Using a predefined list of 649 genera currently included in the class Lecanoromycetes according to Myconet (Lumbsch and Huhndorf, 2010), the WASABI database (http://ocid.nacse.org/research/aftol; Kauff et al., 2007) of the Assembling the Fungal Tree of Life project (AFToL; www.aftol.org) and GenBank were searched for available sequence data, including unpublished sequences generated at Duke University (Lutzoni lab) as part of the AFTOL project. These sequences were complemented with sequences newly generated in the Finnish Museum of Natural History, University of Helsinki (S. Stenroos' team), for the purpose of this study. All database searches, sequence collection, filtering, and assembly were done using automated scripts written in Biopython (Cock et al., 2009).
From these sequences we assembled Operational Taxonomic Units (OTUs) by combining the available sequence data for each locus, giving preference to longer sequences, as well as sequences for different loci obtained from the same voucher specimen, and retaining in most cases a maximum of two OTUs per species with at least two of the five targeted genes. Manual corrections were applied in order to remove low-quality sequence data or mislabelled sequences.
A total of 1307 OTUs representing 1139 infrageneric taxa (including 47 unidentified specimens or doubtful identifications) and 317 genera from 66 of the 75 currently recognized families, as well as all seventeen currently recognized orders and all five subclasses (Acarosporomycetidae, ‘Candelariomycetidae’, Lecanoromycetidae, Ostropomycetidae and Umbilicariomycetidae) were compiled. Members of nine families were not included in this study due to a lack of DNA sequences (Biatorellaceae, Calycidaceae, and Pachyascaceae from the Lecanorales) or because they were represented by only one of the five targeted genes (Anamylopsoraceae, Miltideaceae, Myeloconidaceae, Phaneromycetaceae, Protothelenellaceae, and Thelenellaceae from the Ostropomycetidae). In the resulting supermatrix (which includes all specimens with at least 2 genes sequenced), the Acarosporomycetidae are represented by seven of eight genera and the ‘Candelariomycetidae’ by four of five genera currently recognized in these two subclasses. We sampled 211 genera out of approximately 393 classified within the Lecanoromycetidae as follow: Caliciales (19), Lecanorales (123; including two of six genera incertae sedis), Lecideales (6), Leprocaulales (1), Peltigerales (42), Rhizocarpales (2), and Teloschistales (16), as well as two families of uncertain placement in the Lecanoromycetidae, Sporastatiaceae (one of two genera) and the monogeneric Ropalosporaceae. From the Ostropomycetidae, we included 83 of approximately 180 genera from the Baeomycetales (3), Ostropales (44), Pertusariales (16), Sarrameanales (1), and Trapeliales (10), as well as four families of uncertain placement, Arctomiaceae (all three genera), Hymeneliaceae (four of five genera) and the monogeneric Arthrorhaphidaceae and Schaereriaceae. The Umbilicariomycetidae are represented by nine of 14 genera classified in this subclass. In addition, three of the 28 genera of uncertain placement within Lecanoromycetes (Lumbsch and Huhndorf, 2010) were included. The outgroup consisted of ten taxa representing nine genera and three classes (Lichinomycetes, Geoglossomycetes and Leotiomycetes) outside Lecanoromycetes (Miadlikowska et al., 2006) for a grand total of 1317 OTUs. Our taxon/gene supermatrix for this project included 85 cells, of which 29 included sequences retrieved from GenBank, 642 (16% of all sequences used for this study) included new sequences generated as part of this study, and 2614 were empty, i.e., missing data. Information about sequence data used in this study including sequences newly deposited in GenBank is provided in Supplemental Tables S1 and S2.
2.2 Molecular data acquisition
Herbarium material preferably not older than five years was selected for the molecular study. For DNA extractions, small fragments of the specimens were removed and placed in 1.5 ml Eppendorf tubes. For the extractions DNeasy Blood and Tissue Kit (Qiagen) were used. The manufacturer’s protocol was followed, except for the grinding of the thallus fragments, which was done in a small amount of lysis buffer instead of liquid nitrogen. Amplification of the target loci used the illustra PuReTaq Ready-To-Go™ PCR Beads (GE Healthcare) protocol. Twenty-five µl PCR samples were prepared by adding 19 µl of sterile water, 1 µl of each primer at 10 µM concentration, and 4 µl template DNA to the tubes containing the beads. The primers used and the PCR settings are summarized in Supplemental Table S3. The reactions were run on PTC-100 and PTC-200 Thermocyclers (MJ Research). PCR products were purified using the GFX PCR DNA and Gel Band Purification Kit (GE Healthcare) or Montage SEQ96 Sequencing Reaction Cleanup Kit (Millipore) and sequenced on a MegaBACE 1000 sequencer (Amersham). Sequencing used BigDye® Terminator v1.1 Cycle Sequencing Kit and the same primers as for amplification with additionnal internal sequencing primers when needed (Supplemental Table S3). Alternatively, the sequencing service provided by Macrogen Inc. (http://www.macrogen.com) was used. Sources for laboratory protocols and primers used for generating the new sequences as part of the AFToL1 project can be found in Lutzoni et al. (2004) and Hofstetter et al. (2007).
2.3 Sequence alignments and topological incongruence assessment
All newly generated sequences were subjected to BLAST searches for a preliminary verification of their identity. They were then assembled and edited using the software package Sequencher™ 4.1 (Gene Codes Corporation, Ann Arbor, MI, USA). The single gene alignments were initially generated using the WASABI aligning tool (Kauff et al., 2007) or MUSCLE 3.8.31 (Edgar, 2004) as implemented in Seaview 4 (Galtier et al., 1996; Gouy et al., 2010). Alignments including up to 1500 OTUs were manually optimized with MacClade 4.08 (Maddison and Maddison, 2003), whereas alignments with higher number of OTUs were optimized with Mesquite 2.75 (Maddison and Maddison, 2011). The “Nucleotide with AA color” option was used for guiding the RPB1 and RPB2 alignments. The nuclear ribosomal loci were aligned according to secondary structure as described in Miadlikowska et al. (2006). Ambiguously aligned regions (sensu Lutzoni et al., 2000) and introns were delimited manually and excluded from subsequent analyses.
The resulting single-locus datasets (mitSSU, 1494 sequences; nucLSU, 1347 sequences; nucSSU, 689 sequences; RPB1 A-F, 551 sequences; RPB1 F-G, 106 sequences; RPB2 5-7, 392 sequences; RPB2 7-11, 358 sequences) were examined for topological incongruence among loci using the program compat3 (available at www.lutzonilab.net/downloads). For each individual locus, 500 bootstrap replicates (Felsenstein, 1985) were generated with RA x ML 7.2.8 (Stamatakis, 2006; Stamatakis et al., 2008) and all pairwise comparisons between loci were calculated with compat3. A conflict between two loci was assumed when a clade was supported as monophyletic with a bootstrap frequency ≥75% in one tree, but supported as non7 monophyletic in another (Mason-Gamer and Kellogg, 1996). After removing conflicting sequences, the assessment of congruence was repeated, and a total of three rounds of conflict evaluation were carried out to achieve an acceptable level of topological incongruence (i.e., below 75% of bootstrap support) among loci.
Some of the sequences that were removed after evaluation in congruence tests, belonged to misidentified taxa (for which we did not have access to specimens) or were obvious contaminants. A total of 52 GenBank sequences (Supplemental Table S4) and 42 unpublished sequences were excluded from the alignments, even if only some of them were revealed by BLAST searches as obvious contaminants (i.e., classified outside of Lecanoromycetes). Despite careful examinations and congruence analyses, our final datasets contained the following errors, due to the large number of infrageneric taxa (1139) and sequences included (3971), which were introduced throughout various steps during the data preparation: duplicated sequences (six cases), duplicated OTUs (four cases), and erroneous sequences (very likely representing lichen20 associated fungi [endolichenic fungi; Arnold et al., 2009]) from GenBank (seven sequences) and unpublished (ten sequences) (Supplemental Table S1; Fig. 1). Erronous sequences were flagged in GenBank as such and the newly generated unpublished sequences were submitted to GenBank as lichen-associated, unidentified fungi.
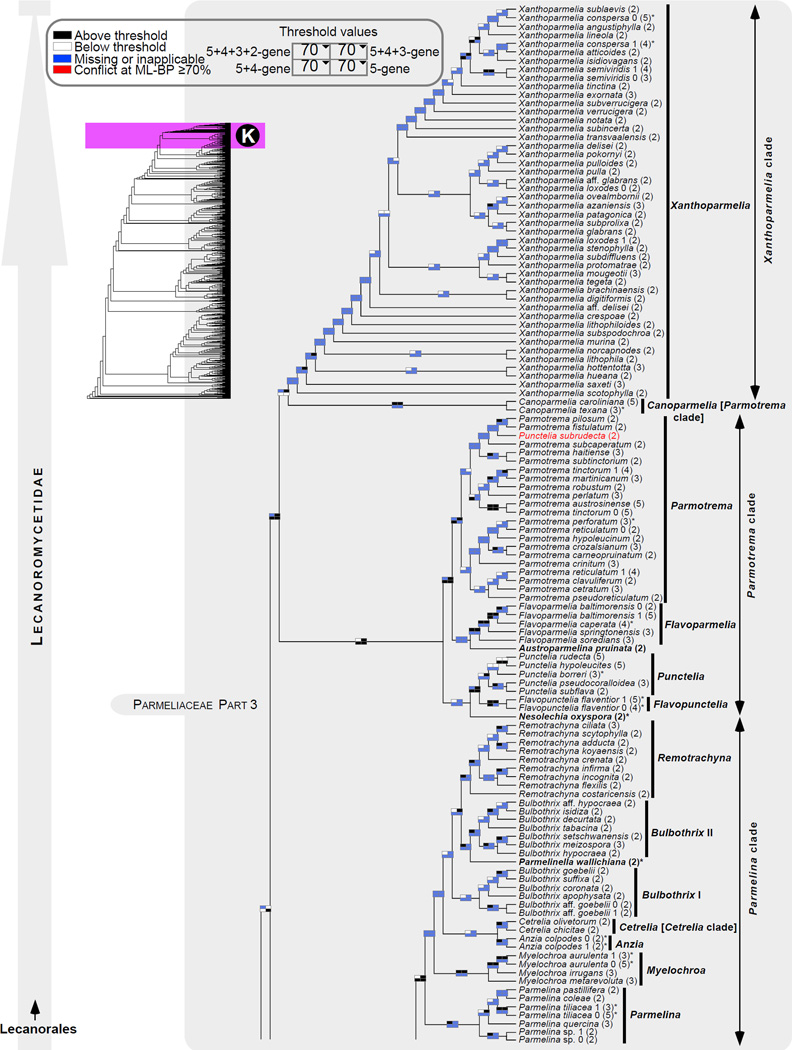
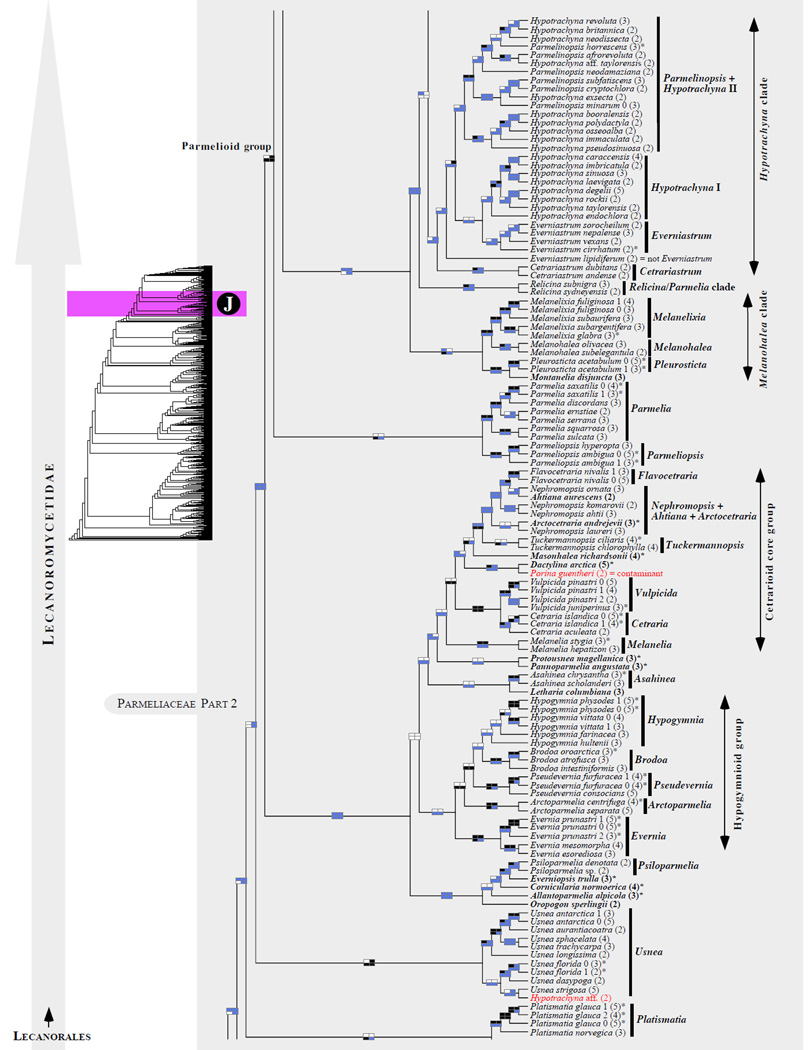
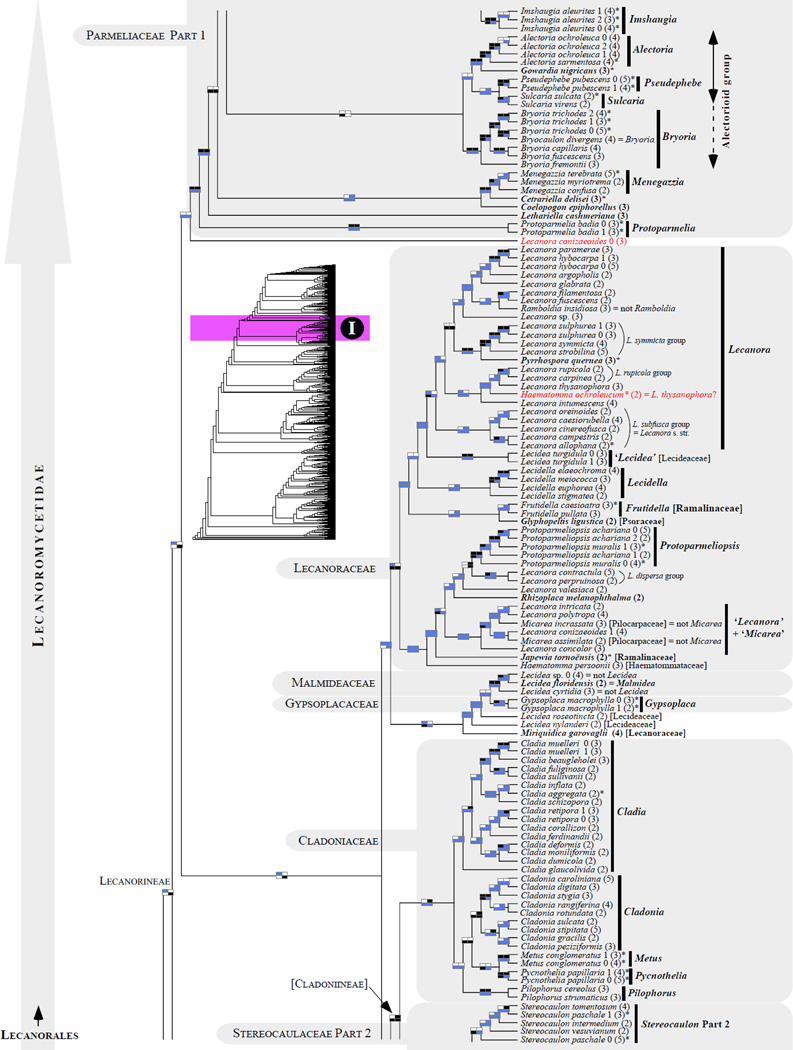
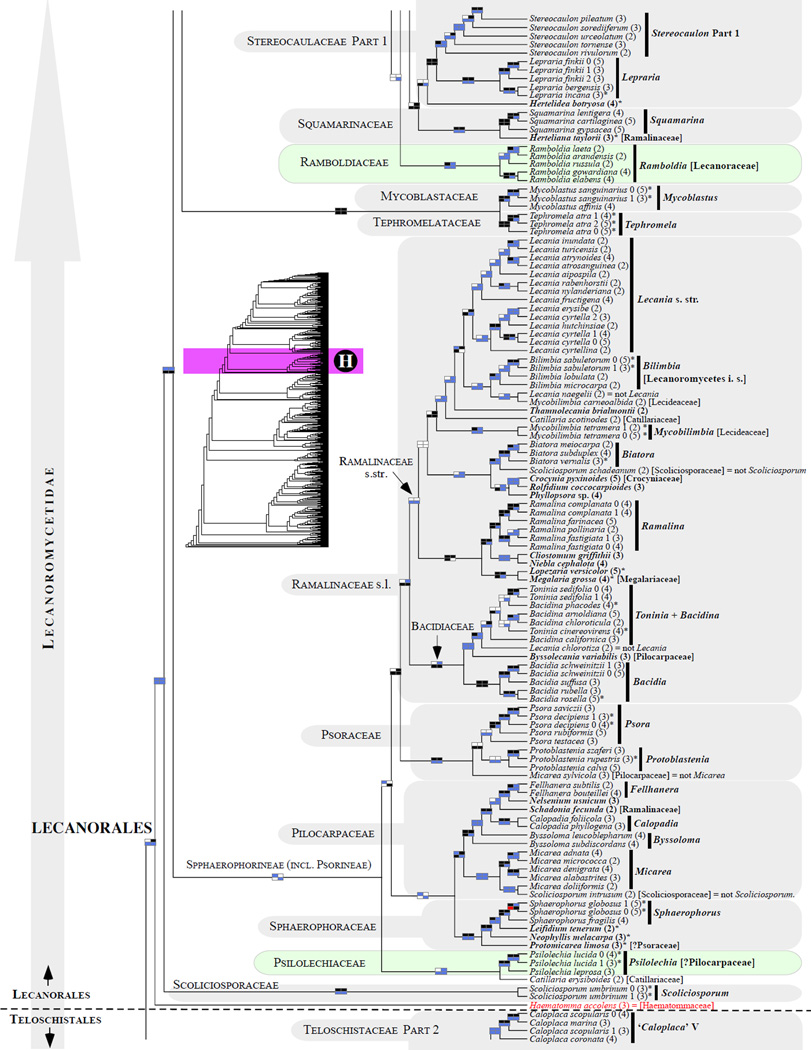
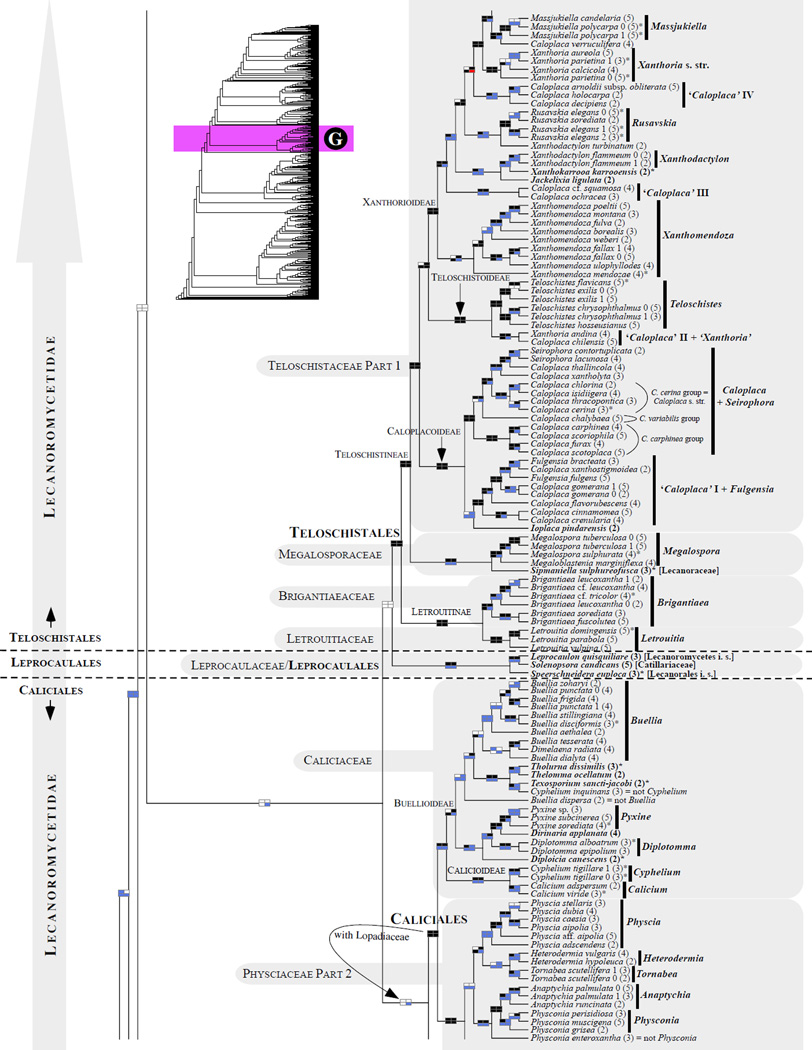
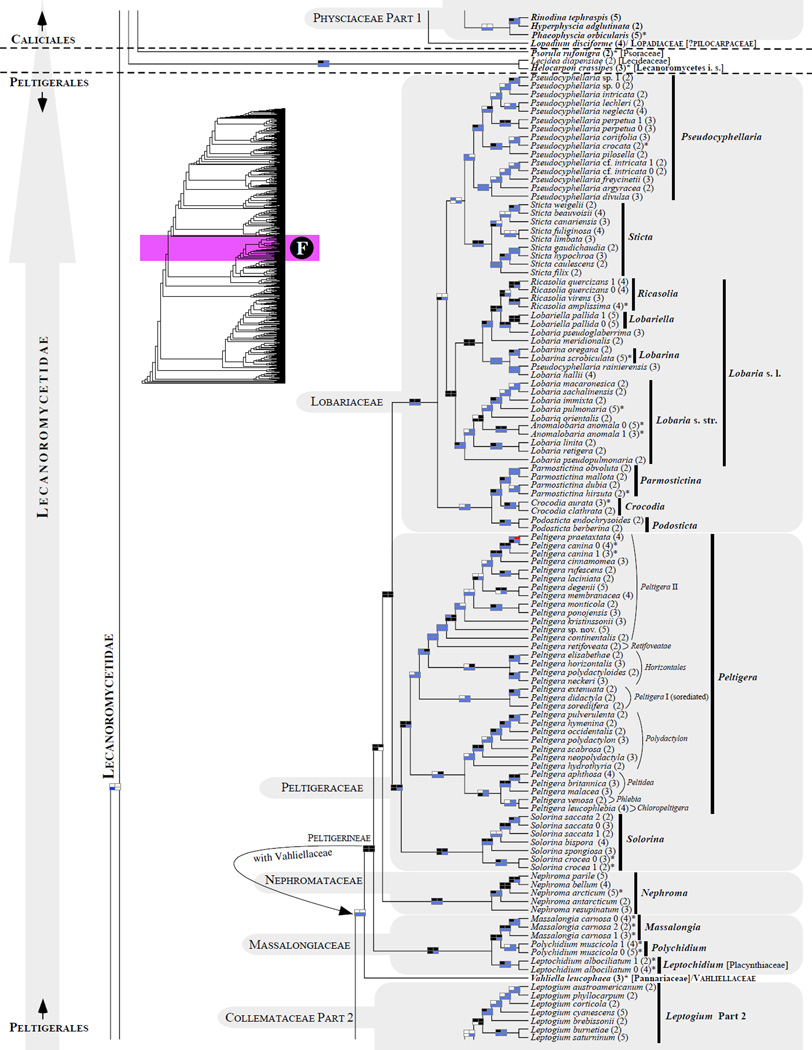
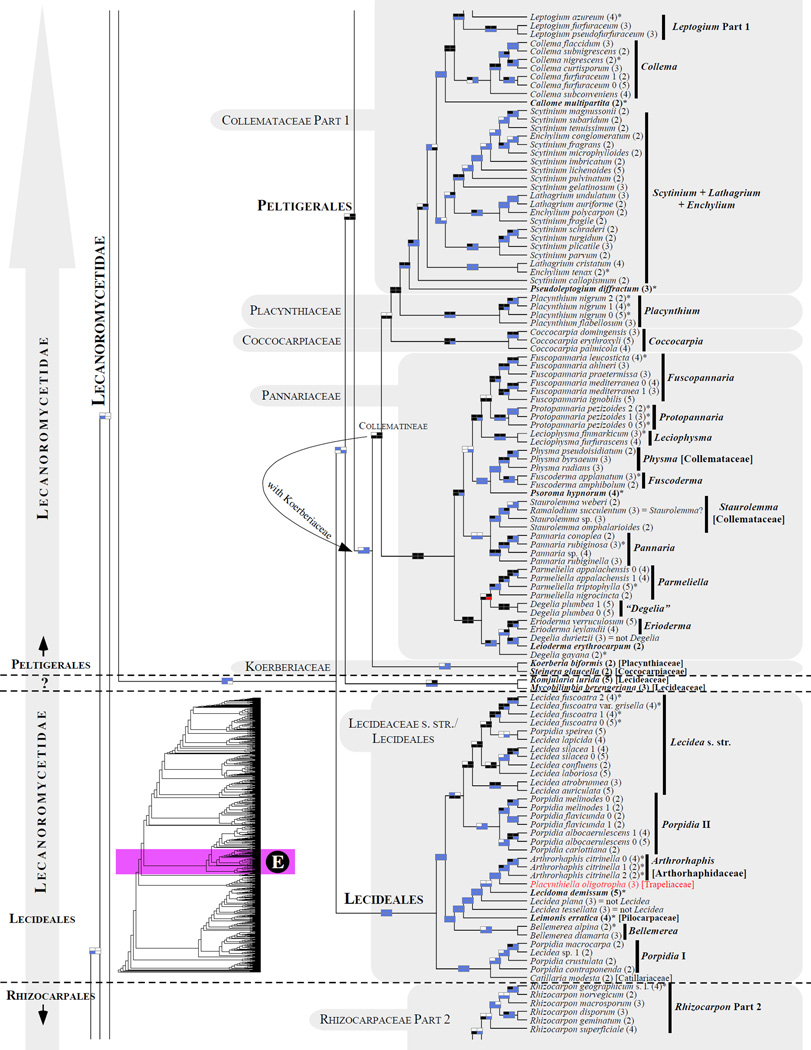
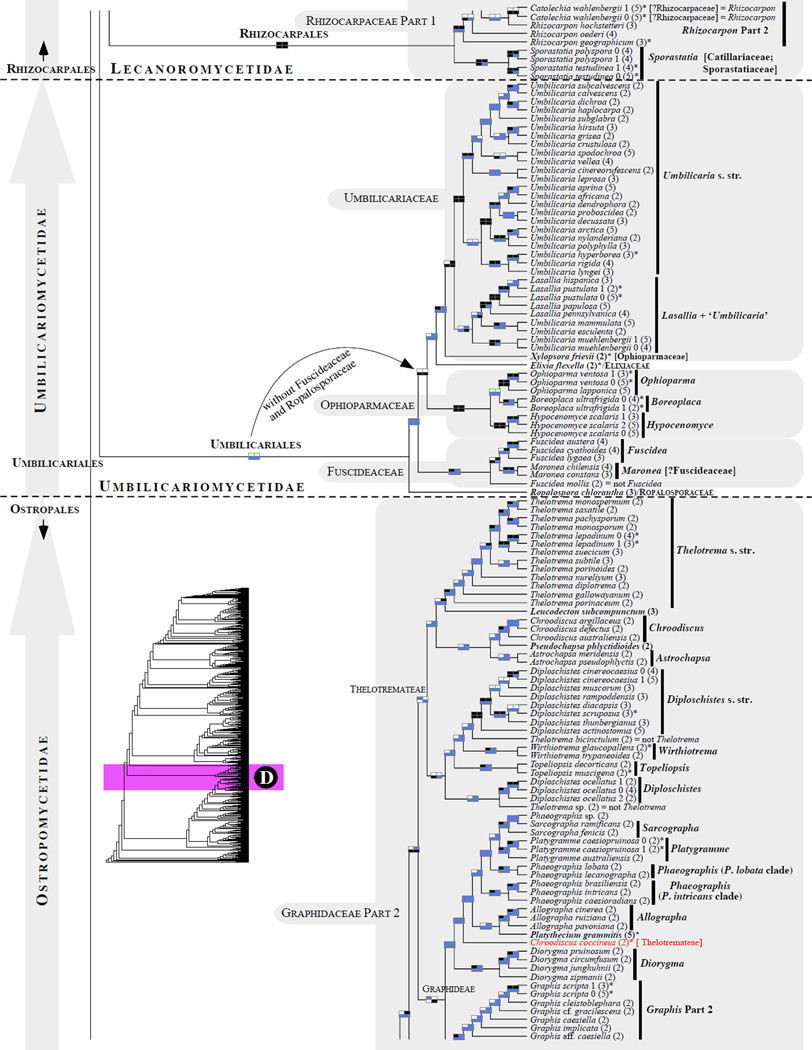
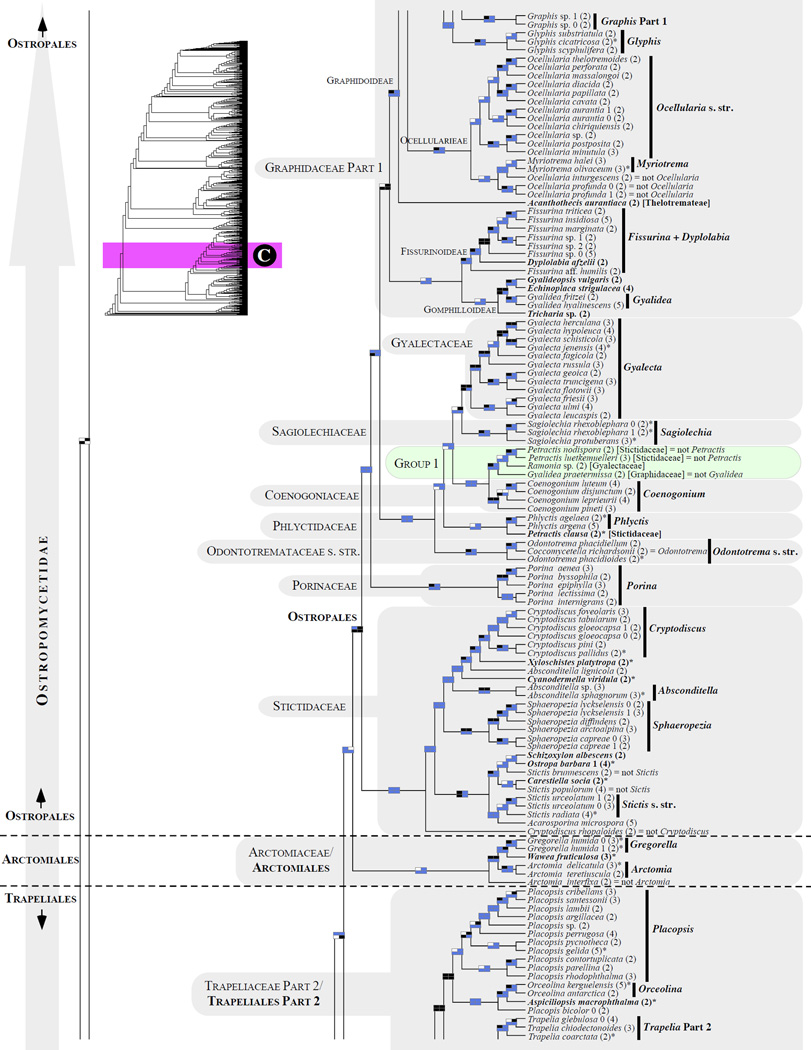
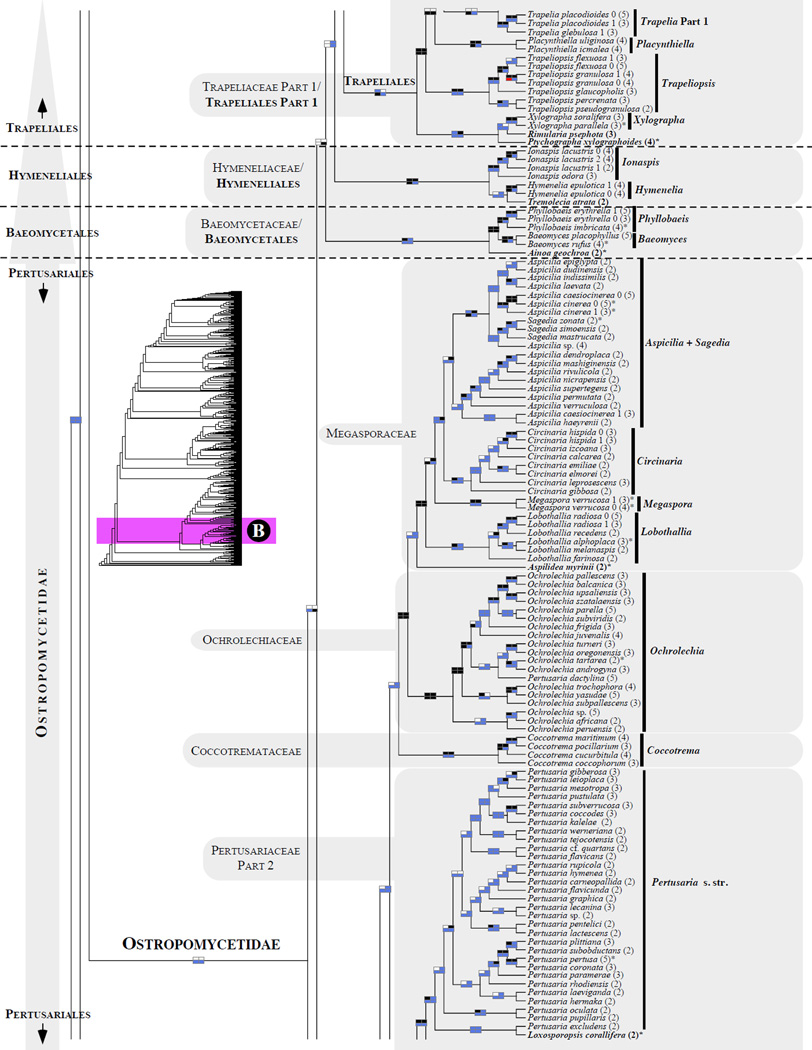
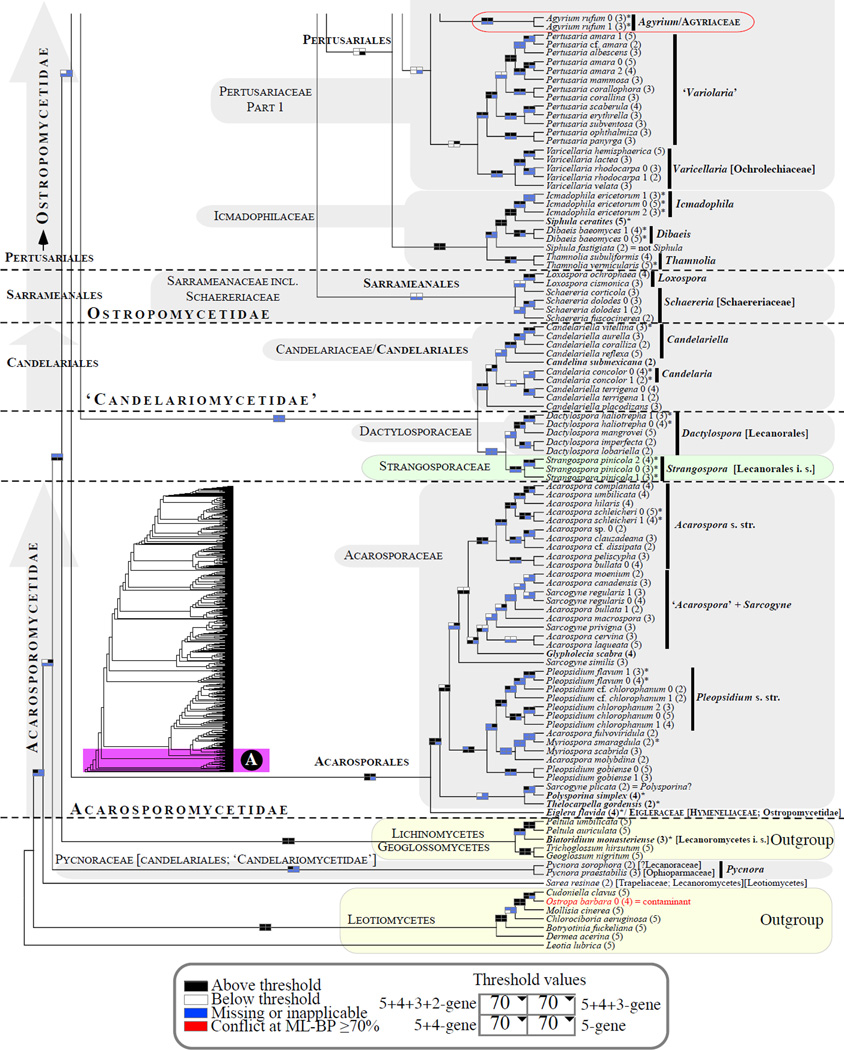
Figure 1.
Phylogenetic relationships among 1307 putative members of the Lecanoromycetes based on maximum likelihood analyses of the combined mitSSU, nucLSU, nucSSU, RPB1 and RPB2 sequences (5+4+3+2-locus dataset) and 10 species used as outgroup (Geoglossomycetes, Lichinomycetes and Leotiomycetes). Numbers in parentheses after taxon names indicate the numbers of genes in our supermatrix for each OTU. Numbers (0, 1, 2) after taxon names indicate presence of multiple specimens from the same taxon. Stars indicate type species. The four-box grids associated with each internode indicate maximum likelihood bootstrap support based on different datasets. Black boxes indicate bootstrap support ≥70%; white boxes indicate bootstrap support <70%; blue boxes indicate cases where internodal support is not applicable due to at least one of the (usually two) immediately downstream branches being absent (due to missing taxa) or the node was not recovered by the bootstrap analysis (not present in the majority-rule consensus tree with all compatible groupings included; 1% treshold); and red boxes indicate conflicting relationships at bootstrap support ≥70%. If in disagreement, the current classification is provided in square brackets. Taxon names in bold indicate that a genus is represented by only one OTU. Grey shadings delimit existing families.Orange shadings highlight new orders. Green shadings delimit new, or potentially, new families. Yellow shadings indicate outgroup taxa. Agyriaceae is delimited by a red line because it is a family embedded within another family (Pertusariaceae). Taxa in red represent obvious phylogenetic misplacements resulting, most likely, from erroneous sequences (see also Supplemental Table S1), misidentified specimens, or stochastic error. Porina guentheri (2) and Ostropa barbara 0 (4) are represented by erronous sequences; Placynthiella oligotropha 0 (3) is represented by an erroneous RPB1 sequence; Chroodiscus coccineus is represented by an erronous nucLSU sequence; Haematomma accolens 0 (3) is represented by an erroneous nucSSU sequence; and H. ochroleucum 0 (2) might represent sterile Lecanora thysanophora. Punctelia subrudecta 0 (2), Hypotrachyna aff. 1 (2), and Lecanora conizaeoides 0 (3), are represented by correct sequences, according to the BLAST searches, and therefore the reason for their unexpected placements is unknown.
2.4 Datasets and phylogenetic analyses
The conflict-free, single-locus alignments were concatenated for the subsequent phylogenetic analyses using a cumulative supermatrix approach as introduced in Miadlikowska et al. (2006) to allow broad and inclusive taxon sampling. A dataset of 183 taxa with all five genes was initially assembled; this 5-gene dataset was expanded with taxa for which at least four of the five-targeted genes were available, resulting in a 5+4-gene dataset of 388 taxa. Subsequently, taxa with at least three genes were added to form a 5+4+3-gene dataset of 764 taxa and, finally, taxa with at least two genes were added to form a 5+4+3+2-gene dataset of 1317 OTUs, for a total of four different datasets (with an increasing number of missing sequences) that were analyzed separately (Table 1). The entire supermatrix (5 + 4 + 3 + 2-genedataset) was deposited in TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S15652).
TABLE I.
Summary of datasets including number of OTUs, number of analyzed sites (before slash), total number of sites (after slash), and the percentage of analyzed sites (in parentheses) for each gene separately, and combined, in comparison with datasets used in Miadlikowska et al. (2006). The 5-gene dataset includes taxa for which complete or partial data from all five genes (nucSSU, nucLSU, mitSSU, RPB1 and RPB2) were available. The 5+4-gene, 5+4+3-gene, and 5+4+3+2-gene datasets include taxa for which at least four, three, or two genes were included, respectively.
| Datasets | Newly added OTUs or sequences to GenBankc |
Missing sequencesc |
5-gene dataset/ 2006 |
5-gene dataset/ this study |
5+4-gene dataset/ 2006 |
5+4-gene dataset/ this study |
5+4+3-gene dataset/ 2006 |
5+4+3-gene dataset/ this study |
5+4+3+2- gene dataset/ this study |
|---|---|---|---|---|---|---|---|---|---|
| No. of OTUs/percent of missing data | NA | NA | 111/0% | 183 (+72)/0% | 188/26% | 388 (+200)/10% | 274/37% | 764 (+490)/25% | 1317 (+553)/54% |
| nucSSU | 143 OTUs | 701 | 1125/7445 (15%) | 1494/15913 (9%) | 1085/7228 (15%) | 1494/15913 (9%) | 1071/7215 (15%) | 1494/15913 (9%) | 1494/15913 (9%) |
| nucLSU | 162 OTUs | 127 | 1141/5162 (22%) | 1061/9363 (11%) | 1121/5151 (22%) | 1061/9363 (11%) | 1122/5096 (22%) | 1061/9363 (11%) | 1061/9363 (11%) |
| mitSSU | 174 OTUs | 127 | 471/2635 (17%) | 429/3642 (12%) | 437/2691 (16%) | 429/3642 (12%) | 445/2862 (15%) | 429/3642 (12%) | 429/3642 (12%) |
| RPB1 | 104 (114a) OTUs | 780 (2022a) | 2688/3159 (85%) | 2676/3172 (84%) | 2676/3243 (82%) | 2676/3172 (84%) | 2673/3229 (83%) | 2676/3172 (84%) | 2676/3172 (84%) |
| RPB2 | 59 (80b) OTUs | 879 (1924b) | 1932/2291 (84%) | 1773/2307 (77%) | 1851/2349 (79%) | 1773/2307 (77%) | 1803/2409 (75%) | 1773/2307 (77%) | 1773/2307 (77%) |
| Combined | 642 seq. | 2614 | 7357/20692 (35%) | 7433/34397 (22%) | 7170/20662 (35%) | 7433/34397 (22%) | 7114/20811 (34%) | 7433/34397 (22%) | 7433/34397 (22%) |
RPB1 (A–F) and RPB1 (F–G) separately
RPB2 (5–7) and RPB2 (7–11) separately
In the most inclusive (5+4+3+2-gene) dataset assembled for this study.
Maximum likelihood analyses were carried out on each of the four datasets, with nine partitions assigned (nucSSU, nucLSU, mitSSU, RPB1 1st/2nd/3rd and RPB2 1st/2nd/3rd) using RAxML 7.2.8. The fast bootstrap combined with the tree search algorithm (option -f a of RAxML) was used, implementing a GTRGAMMAI model as provided by the program, and calculating 1000 bootstrap replicates.
Internodal support resulting from bootstrap analyses of the four datasets was depicted on the 5+4+3+2-gene phylogeny using the box scheme (Fig. 1) as implemented in the newly developed “Hypha” module (Oliver et al., 2013) part of Mesquite (Maddison and Maddison, 2011).
3. Results and Discussion
3.1 Datasets, phylogenetic inference and confidence
The number of taxa in each multigene dataset was much higher than in our previous study (Miadlikowska et al., 2006), ranging from 72 newly added OTUs for the 5-gene dataset, to 490 newly added OTUs for the 5+4+3-gene dataset (Table 1). Extra 553 OTUs were added by including OTUs for which sequences were available for only two of the five genes. In our previous phylogenetic study of the Lecanoromycetes (Miadlikowska et al., 2006) we did not include taxa with fewer than three of the five targeted genes. This strategy elevated our number of OTUs from 274 in 2006 to 1317 in this study. Of the 3971 sequences incorporated in the most inclusive (5+4+3+2-gene) dataset, 642 were newly generated. This allowed us to reduce the proportion of missing sequences, in each dataset that included OTUs with fewer than five genes, by more than 10%, compared to Miadlikowska et al. (2006) (Table 1). The highest number of new sequences was obtained for the mitochondrial ribosomal SSU (174) and nuclear ribosomal LSU and SSU with162 and 142, respectively. RPB2 and RPB1 genes had the lowest sequencing success (59 and 104 new sequences, respectively) and, consequently, these genes had the highest number of missing sequences (more than half of the OTUs; Table 1). Contrary to Miadlikowska et al. (2006), the ambiguously aligned regions were delimited only in the most OTU inclusive (5+4+3+2–gene) dataset, and were not re-adjusted in the less OTU inclusive datasets due to the large size of the datasets, which were difficult to handle manually. Similar to other studies (Hofstetter et al., 2007; Miadlikowska et al., 2006), RPB1 and RPB2 provided the greatest proportion of unambiguously aligned sites (> 75% of the sites), whereas only a small fraction of the RNA coding sites (9–12%) could be aligned unequivocally due to the presence of a large number of indels including spliceosomal and group I introns. A total of 7433 sites were analyzed phylogenetically for the 5+4+3+2-gene dataset (Table 1).
Phylogenies derived from the four multigene datasets were generally concordant among each other, including the 1317 OTU data with 54% of missing sequences. The cladogram of the ML optimal tree resulting from the 5+4+3+2-gene analysis is presented in Fig. 1 (the phylogram version of this tree is shown in the Supplemental Fig. S1) and its schematic version in Fig. 2. A few cases of conflict were detected at the level of 70% and higher bootstrap support (red boxes in Fig. 1) and involved: 1) terminal relationships among species (in the genera Sphaerophorus, Peltigera and Trapeliopsis), 2) the sister relationship between Degelia and Parmeliella in Pannariaceae (contrary to the sister relationship between Degelia and Erioderma based on the 5- gene dataset), and 3) the placement of Caloplaca arnoldii in the Xanthorioideae (Teloschistaceae).
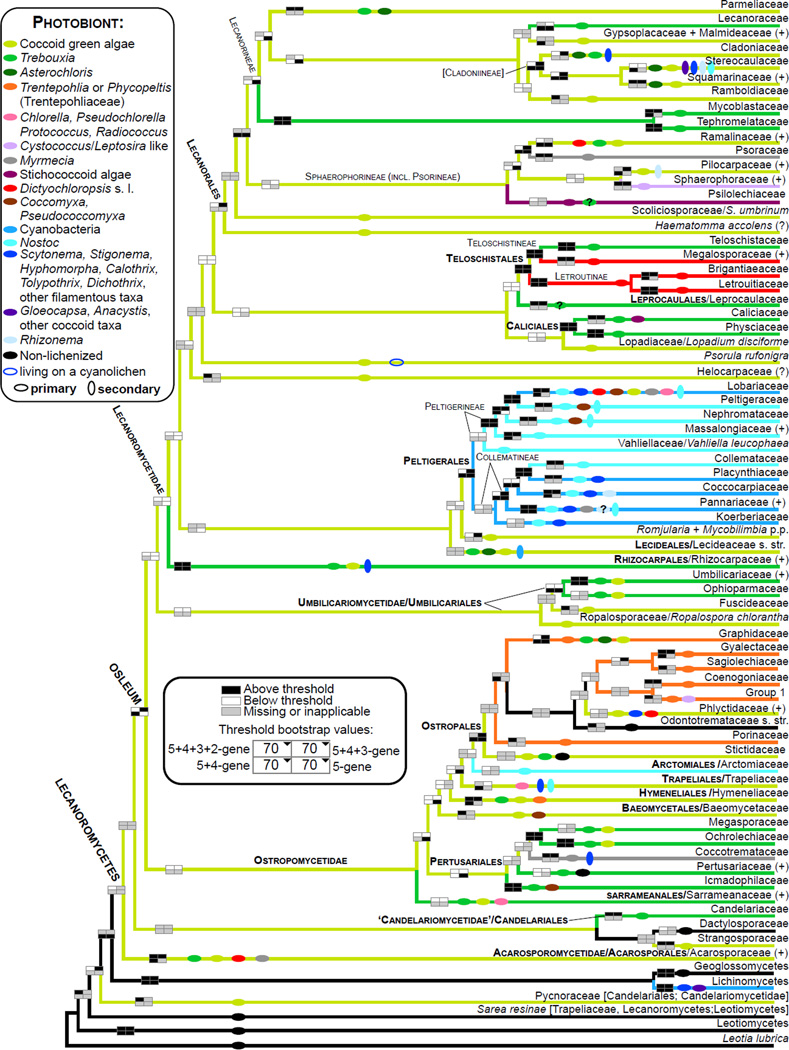
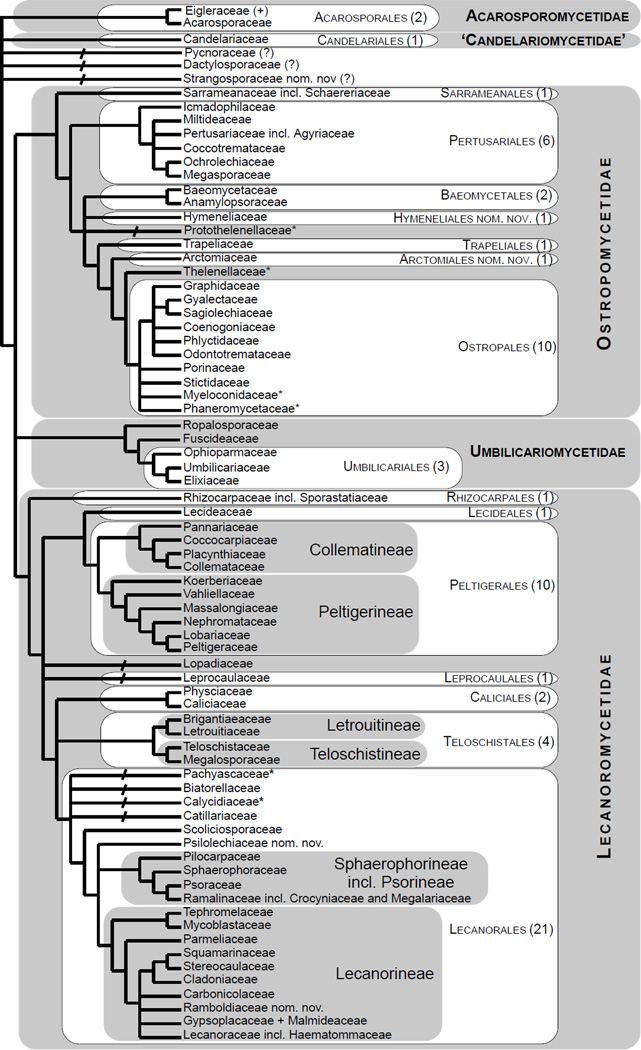
Figure 2.
Schematic cladogram derived from the 5+4+3+2-gene phylogeny (Fig. 1) depicting relationships among major phylogenetic lineages (families and above) reconstructed within the Lecanoromycetes. A plus sign after a taxon name indicates additional species, genera or families included in that lineage (see Fig. 1). Bootstrap support is shown according to the legend and explanation provided for Fig. 1. Colored branches indicate dominant type of primary photobiont and colored horizontal ovals indicate all photobiont groups reported in association with mycobionts within order/family/phylogenetic lineage based on available records for selected members classified in these taxa, even if not present in the tree. Some records of Trebouxia may represent Asterochloris. Presence of secondary photobionts (different genera or families of cyanobacteria) is indicated by vertical ovals. Selected literature used for the photobiont assignment: Backor et al., 2010; Baloch et al., 2010; Beck, 2002; Brodo, 1990; Brodo et al. 2001; Coppins and Purvis, 1987; Dal Grande et al., 2014; Engelen et al., 2010; Fernández-Mendoza et al., 2011; del Hoyo et al, 2011; Friedl and Büdel, 2008; Gueidan et al., 2014; Höggnaba et al., 2009; Kantvilas, 2004; Lücking et al., 2009; Miadlikowska et al., 2006; Muggia et al., 2011; Nash et al. 2002, 2004; Otálora et al., 2010b, 2013c; Peksa and Skaloud, 2011; Purvis et al., 1992; Raggio et al., 2012; Skaloud and Peksa, 2010; Spribille and Muggia, 2012; Tschermak-Woess, 1988; Voytsekhovich, 2013; Voytsekhovich et al., 2011a, b.
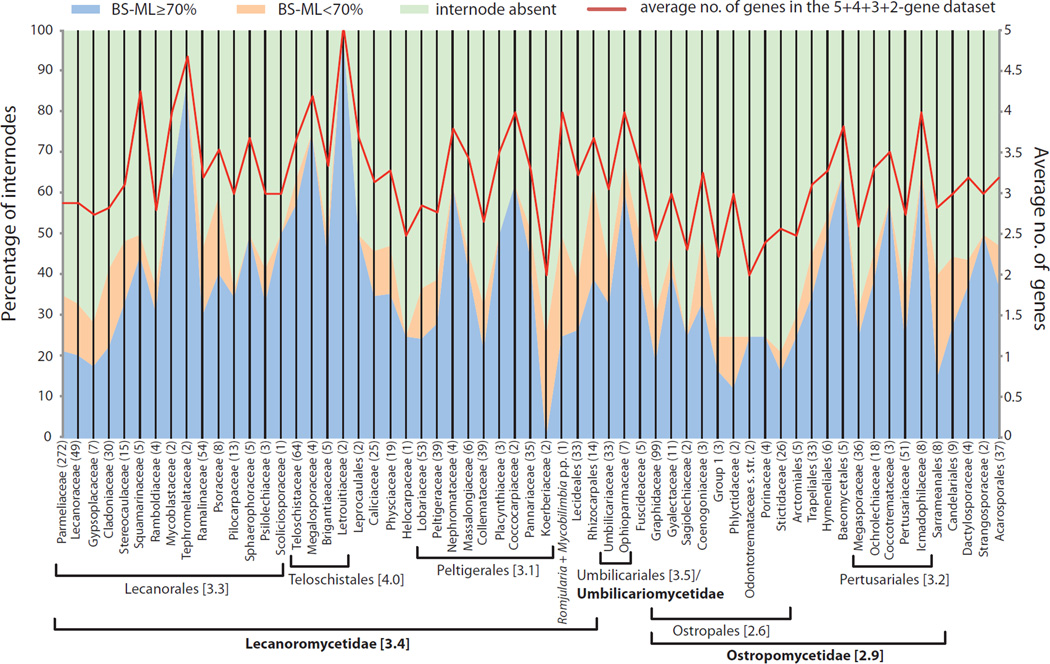
An increasing proportion of missing data, from 0% (in the 5-gene dataset) to 54% (in the 5+4+3+2-gene dataset), affected bootstrap support, which frequently decreased below the significance cutoff (white boxes in Figs. 1 and 2). This trend was more pronounced than in our previous study (Miadlikowska et al., 2006) where we did not include taxa with fewer than three genes, and where the maximum proportion of missing sequences was 37%. However, most relationships remained unchanged, and many of them were highly supported by at least one of the four bootstrap analyses (black boxes in Figs. 1 and 2). Certain portions of the phylogeny were less stable (e.g., Parmeliaceae and Lecanoraceae in the Lecanoromycetidae, and Ostropales in general) than others (e.g., Teloschistales and Peltigerales) due to a higher concentration of taxa with only two (RNA-coding) genes (Figs. 3 and 4; Supplemental Table S5). Instances of highly supported relationships contradicting published multigene phylogenies were very rare and usually involved different combination of molecular markers used in previous studies, e.g., discrepancies in delimitation of new genera in the Collema /Leptogium complex based on MCM7 and beta-tubulin in addition to nucLSU and mitSSU loci (Otálora et al., 2013a, 2013b versus Fig. 1E–F). Nevertheless, adding taxa with only two or three genes sometime improved phylogenetic confidence above the 70% bootstrap threshold (e.g., monophyly of Cladoniaceae, Parmeliaceae, Sphaerophoraceae, and Lecanorales; Fig. 1). However, other relationships at various taxonomic levels were not well supported, even if they involved taxa represented by the complete (5 loci) or almost complete set of genes.
Figure 3.
Comparison between average number of genes (right Y axis and red line) across lineages in the Lecanoromycetes (Fig. 2) and stability of reconstructed phylogenies approximated by the percentage of internodes with BS-ML ≥ 70% (blue shade; corresponding to black boxes in Fig. 1), percentage of internodes with BS-ML < 70% (orange shade; corresponding to white boxes in Fig. 1), and percentage of missing internodes in bootstrap analyses (green shade; corresponding to blue boxes in Fig. 1) (Supplemental Table S2). Numbers in parentheses after taxon names refer to the number of internodes (grids) included in each clade. Average number of sequenced genes are also provided for larger taxonomic entities (orders and subclasses) associated with horizontal brackets.
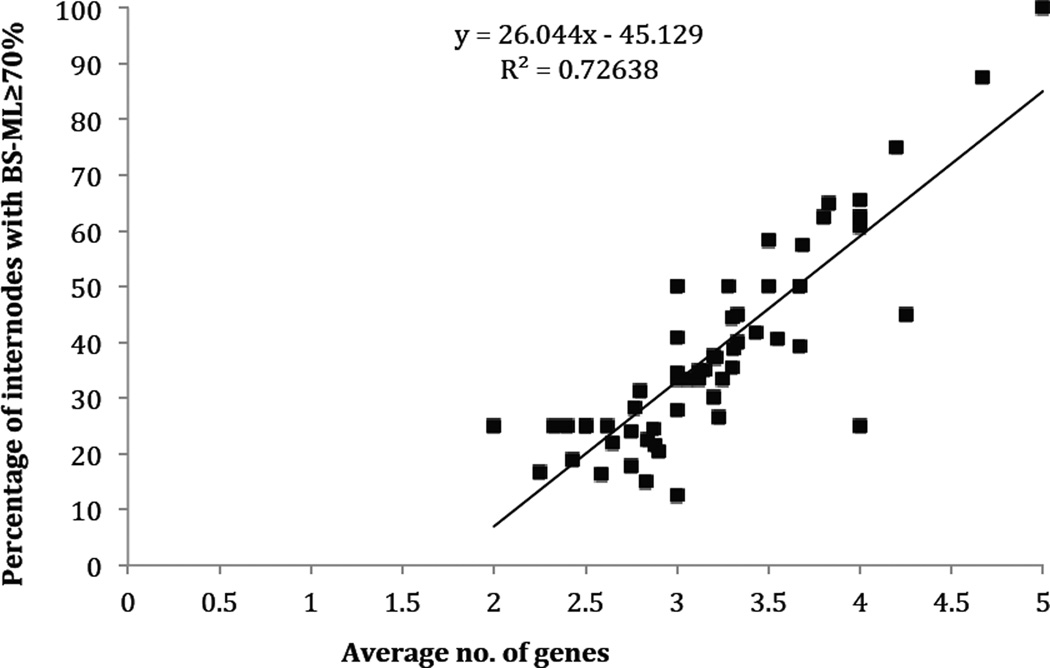
Figure 4.
Strong positive correlation (p < 2.2e-16) between average number of genes included in our supermatrix and the percentage of internodes with BS-ML ≥ 70%, based on results from bootstrap analyses on 5-gene, 5+4-gene, 5+4+3-gene, and 5+4+3+2-gene datasets (i.e. correlation between blue shade and red line in Fig. 3).
3.2 Lecanoromycetes: delimitation and relationships among subclasses (Figs. 1 and 2)
About half (34/65) of the backbone relationships (up to the family level exclusively) received bootstrap support only below the 70% bootstrap level (i.e., no black boxes in the four-cell grid in Fig. 2). However, ten of these 34 internodes were recovered by at least one of the four bootstrap analyses (i.e., at least one white box per grid) and an additional 15 internodes were recovered by at least two of the four bootstrap analyses (i.e., at least two white boxes per grid; Fig. 2). Only nine of the 65 backbone internodes were never recoved by at least one bootstrap analysis (i.e., no white or black boxes in each given grid; Fig. 2). Compared to the previous large-scale phylogeny of the Lecanoromycetes reconstructed for 274 taxa (5+4+3-gene dataset; Fig. 1 in Miadlikowska et al., 2006), we lost support ≥ 70% for the monophyly of the Lecanoromycetidae (with or without Rhizocarpales included), Ostropomycetidae including Sarrameanales (previously referred to as “Loxosporales?”), and Umbilicariomycetidae excluding the Ropalosporaceae (this family was not included in the previous study). However, we did recover highly supported monophyletic Ostropomycetidae-Umbilicariomycetidae-Lecanoromycetidae, Rhizocarpales, Acarosporomycetidae, and Lecanorales. The subclass Lecanoromycetidae, including Rhizocarpales, although not supported with bootstrap values above 70%, was recovered by three of the four bootstrap analyses in this study, and was recovered with significant posterior probability and bootstrap support (ML) by Bendiksby and Timdal (2013). In our phylogeny, the Ostropomycetidae (including Sarrameanales), Lecanoromycetidae, and Umbilicariomycetidae form a strongly supported monophyletic group (here refered to as the OSLEUM clade; Fig. 2). The sister relationship between the Lecanoromycetidae and Umbilicariomycetidae, which is in agreement with Miadlikowska et al. (2006) and Lutzoni et al. (2004; PP = 1.00 based on four genes), was recovered by three of the four bootstrap analyses (Fig. 2), but never with bootstrap support above 70%. Previous large-scale phylogenies for the Lecanoromycetes show support for two contradicting relationships among these three subclasses: a sister relationship between the Ostropomycetidae and Umbilicariomycetideae (PP = 0.98 in Lutzoni et al., 2004, for the phylogeny based on three genes), and between the Lecanoromycetidae and Ostropomycetidae (PP = 1.00 and ML-BP = 80% in Bendiksby and Timdal, 2013; and PP = 0.99 in Prieto et al., 2012). The recognition of the Umbilicariomycetidae as a separate subclass enables the tracking of this phylogenetic uncertainty.
The phylogenetic position of the Acarosporomycetidae and ‘Candelariomycetidae’ within the Lecanoromycetes is mostly inconclusive in this study. However, we recovered the Acarosporomycetidae and ‘Candelariomycetidae’ as derived from the first and second split, respectively, within the Lecanoromycetes. This resolution was also shown in Miadlikowska et al. (2006) with PP > 0.95 and bootstrap support above 70%, and by Reeb et al. (2004) with PP >0.95. Hofstetter et al. (2007), Lumbsch et al. (2007b) and Bendiksby and Timdal (2013) recovered a phylogeny with strong bootstrap support for the ‘Candelariomycetidae’ diverging first, followed by the Acarosporomycetidae. In Wedin et al. (2005) the Acarosporomycetidae and paraphyletic ‘Candelariomycetidae’ (sensu Bendiksby and Timdal, 2013) form a monophyletic group closely related to the outgoup taxa (Eurotiomycetes; however, without support). Most recently, Prieto et al. (2012) presented a Bayesian tree based on four ribosomal and two protein-coding genes (MCM7 and RPB1) where Candelariales is separated from the Lecanoromycetes and placed as an independent lineage among early diverging Leotiomyceta. However, this placement, as well as many other deep phylogenetic relationships associated with the Leotiomyceta radiation, did not receive strong support (Lutzoni et al, 2004).
Using Leotiomycetes, Geoglossomycetes and Lichinomycetes as outgroup taxa, as we did previously (Miadlikowska et al., 2006), the delimitation of the Lecanoromycetes has revealed itself to be somewhat more problematic with the addition of 1043 OTUs. The Dactylosporaceae, a family classified in the Lecanorales based on morphological and anatomical characters (Lumbsch and Huhndorf, 2010) was resolved within the Eurotiomycetes, close to Chaetothyriales and Pyrenulales, by Schoch et al. (2009) and Rossman et al. (2010). Similar results were obtained by Diederich et al. (2013) where Dactylospora was found within the Eurotiomycetes, with the lichenicolous fungus Sclerococcum sphaeriale and an unidentified endolichenic fungus ALr-1. Our phylogenetic analyses support the placement of Dactylospora within the Lecanoromycetes, a result that is more inline with phenotypic traits (e.g., amyloid ascus). Interestingly, Dactylospora was resolved here as sister to Strangospora (without support), a genus involved in a similar discordance with respect to crossing the Lecanoromycetes boundary (Figs. 1 and 2). However, this sister relationship was recovered only by the phylogenetic analysis of the 5+4+3+2-gene dataset. Strangospora pinicola, tentatively classified within Lecanorales (Lumbsch and Huhndorf, 2010), was placed with high support outside all families sampled from the order Lecanorales in Miadlikowska et al. (2006), as the result of the second split within the Lecanoromycetes (with high PP) according to Reeb et al. (2004), and outside all currently recognized subclasses in the phylogeny presented here.
Sarea resinae represents another case of a genus currently classified within the Lecanoromycetes (Trapeliaceae; Lumbsch and Huhndorf, 2010), which has been resolved phylogenetically outside this class, possibly within the Leotiomycetes according to Reeb et al. (2004). Our study supports the placement of this taxon outside Lecanoromycetes (Figs. 1A and 2), but this result is based on only two sequences for the entire genus, which have never been corroborated with additional sequences from other specimens or by more genes.
The phylogenetic placement of Pycnoraceae by Bendiksby and Timdal (2013) (a new family introduced to accommodate Pycnora) in the ‘Candelariomycetidae’ and Lecanoromycetes is questionable based on our phylogenetic analyses. In this study, this family is resolved with high confidence outside Lecanoromycetes, before the split of the Lecanoromycetes from the Geoglossomycetes+Lichinomycetes clade (Figs. 1A and 2). In Bendiksby and Timdal (2013), the ‘Candelariomycetidae’, including the Pycnoraceae, received a low bootstrap value (59%). The inclusion of only one taxon for the outgroup (Geoglossum nigritum), and the use of ribosomal genes only, mtSSU, nucLSU and ITS to infer relationships across the Lecanoromycetes, where positional homology of many sites might be questionable but nevertheless included in the phylogenetic analyses, was probably the cause of this artifactual result.
Biatoridium, represented by the type species (B. monasteriense) and considered currently as a member of the Lecanoromycetes of uncertain affiliation, is placed with high confidence outside of the class, sister to the Lichinomycetes (Fig. 1A). This confirms the results obtained by Reeb et al. (2004), where Biatoridium together with Sarcosagium campestre or Thelocarpon laureri were found to be sister to Lichinomycetes. Biatoridium shares morphological similarities in the apothecium and ascus structure with Sarea and Strangospora (Hafellner, 1995), two other genera of doubtful position within the Lecanoromycetes (Figs. 1A and 2).
The phylogenetic placement of Candelariales, Dactylosporaceae, Strangosporaceae, Pycnoraceae, Biatoridium, and Sarea, as well as a robust delimitation of the Lecanoromycetes must be assessed with more taxa and more than six loci (see Prieto et al., 2012) within the broad phylogenetic context of the Leotiomyceta.
Taxonomic conclusions:
A. Strangospora should be removed from Lecanorales and placed in its own family, Strangosporaceae, which should be classified as Lecanoromycetes incertae sedis.
Family: Strangosporaceae S. Stenroos, Miadl. & Lutzoni fam. nov.
MycoBank no.: MB 808512.
Typus: Strangospora Körb,
Diagnosis – A monogeneric family of uncertain placement in the Lecanoromycetes comprising epiphytic, epixylic or bryophylous lichens with crustose and often poorly developed thalli associated with chlorococcoid photobiont. Apothecia biatorine, but exciple poorly developed, asci clavate, with gelatinous outer layer, a strongly thickened I+blue wall and apical dome, multispored; ascospores aseptate.
Strangospora Körb., Parerga lichenol. (Breslau): 173 (1860).Type species: S. pinicola (A. Massal.) Körb. 1860
B. Dactylosporaceae should be removed from Lecanorales and classified as Leotiomyceta incertae sedis.
3.3 Acarosporomycetidae /Acarosporales/Acarosporaceae (Fig. 1A)
The subclass Acarosporomycetidae consists of a single order and family of nine genera that are mostly saxicolous (Harris and Knudsen, 2005; Hibbett et al., 2007; Lumbsch and Huhndorf, 2010; Reeb et al., 2004). We sampled seven of eight recognized genera (Lithoglypha was not included) and more than doubled the number of taxa compared to Miadlikowska et al. (2006; 36 versus 15 taxa). The Acarosporomycetidae is monophyletic and well supported (Fig. 1A). A newly sequenced Eiglera flavida (currently classified in Hymeneliaceae; Ostropomycetidae inc. sed.; Lumbsch and Huhndorf, 2010) is placed with high confidence in Acarosporales. Eiglera flavida represents a monotypic genus, previously classified in the family Eigleraceae, which was erected by Hafellner (1984) to accommodate a species formerly placed in Aspicilia or Lecanora. The genus Eiglera is distinguished from the rest of Hymeneliaceae by its ascus type (I+ uniformly blue tholus versus I+ faint blue or I- tholus typical for Hymeneliaceae), but shares with members of this family morphological, anatomical and isozymic characteristics (Lutzoni and Brodo, 1995), as well as ascoma ontogenic features (weakly hemiangiocarpous, relatively simple, and having paraphysoids; Lumbsch, 1997). If future study confirms the phylogenetic placement of Eiglera (based on multiple individuals) as derived from the first split within Acarosporomycetidae, it would justify the reinstitution of the family Eigleraceae.
We confirm here the polyphyletic delimitation of the genera Acarospora, Sarcogyne, and Pleopsidium (Reeb et al., 2004; Crewe et al., 2006; Miadlikowska et al., 2006). Delimitation of these genera should be redefined in a phylogenetic context. It is very likely that Sarcogyne plicata represents a member of the genus Polysporina (sister relationship with P. simplex in Fig.1) as suggested also by shared morphology and substrate (Magnusson, 1935; Knudsen and Kocurkova, 2011) between the two taxa. Our phylogeny supports the distinctiveness of S. plicata from S. privigna (the latter being nested in the ‘Acarospora ’ + Sarcogyne clade; Fig. 1A) as indicated in Knudsen and Kocurkowa (2011). For future taxonomical changes within the genus Acarospora, the name Myriospora that was recently recircumscribed (Arcadia and Knudsen, 2012) is available to accomodate the A. smaragdula group.
Taxonomic conclusions:
The genera Acarospora, Pleopsidium and Sarcogyne need taxonomic revision within the phylogenetic framework of the Acarosporaceae.
If confirmed, Sarcogyne plicata should be transferred to Polysporina.
Eiglera (Hymeneliaceae, Ostropomycetidae) needs to be sampled for multi-locus sequencing to confirm phylogenetic placement within Acarosporales. If this genus is derived from the first divergence within this order, the family Eigleraceae should be added to the Acarosporales.
3.4 ’Candelariomycetidae’/Candelariales/Candelariaceae (Fig. 1A)
The subclass ‘Candelariomycetidae’ (Miadlikowska et al., 2006) and order Candelariales (Hibbett et al., 2007) includes two families according to Bendiksby and Timdal (2013): Candelariaceae, with four genera (Candelaria, Candelariella, Candelina and Placomaronea ; Lumbsch and Huhndorf, 2010; Westberg et al., 2009), and Pycnoraceae, with a single genus, Pycnora (Bendiksby and Timdal, 2013; formerly the Hypocenomyce xanthococca group; Hafellner and Türk, 2001). Our phylogeny supports the Candelariales as accepted in Lumbsch and Huhndorf (2010), with a single family, Candelariaceae. The newly established family Pycnoraceae (represented in our tree by two species with two loci each) was resolved here as monophyletic and highly supported but not as a sister group to Candelariaceae. Instead, this new family was placed, with high phylogenetic confidence (ML bootstrap support), outside of the Lecanoromycetes (Fig. 1A). The alternative sister relationship of Pycnoraceae and Candelariaceae received sometimes high posterior probabilities based on ribosomal loci (0.98 in Wedin et al., 2005, but see Bendiksby and Timdal, 2013). It was already demonstrated, based on morphological and molecular data, that the current delimitation of genera within Candelariaceae does not reflect monophyletic grouping (Westberg et al., 2007, 2009). Our phylogeny confirms the polyphyly of Candelariella and the necessity to revise generic circumscriptions to reflect the inferred phylogenetic structure for the family Candelariaceae. As the number of spores per ascus and thallus habit varies within and between closely related species, and the chemistry is uniform throughout the family, a new set of characters is needed to circumscribe monophyletic genera in Candelariaceae.
Taxonomic conclusions:
Candelariella is polyphyletic and needs systematic revision within the phylogenetic framework of the Candelariaceae.
Inclusion of Pycnoraceae within Candelariales remains uncertain, however, its taxonomic placement outside Lecanoromycetes is not supported by morpho-anatomical characters and should be confirmed within a more comprehensive phylogenetic framework, particularly with respect to the Leotiomycetes.
3.5 Ostropomycetidae: relationships within the subclass (Fig. 1A–D)
Phylogenetic relationships within Ostropomycetidae are highly unsettled, mostly because fewer genes have been sequenced for many families within this subclass compared to the rest of the Lecanoromycetes (Figs. 3 and 4; Supplemental Table S5). Adding more taxa without sequencing more loci would not improve this situation (Figs. 3 and 4 and Supplemental Fig. S2; e.g., Parnmen et al., 2012; Rivas Plata and Lumbsch, 2011; Rivas Plata et al., 2012b), Consequently, delimitations of families and orders are unstable in this subclass (Baloch et al., 2010; Bendiksby and Timdal, 2013; Hofstetter et al., 2007; Lumbsch et al., 2007b; Miadlikowska et al., 2006; Schmull et al., 2011). Currently, six families are not assigned to any order (incertae sedis) in Ostropomycetidae (Lendemer and Hodkinson, 2011; but see Gueidan et al., 2014). The general delimitation of the Ostropomycetidae (Figs. 1A–D and 2) is in agreement with the recent phylogenies; however, the addition of the Sarrameanales (Loxospora) and Schaereriaceae resulted in a decrease of phylogenetic support for the monophyly of the subclass, including this study (e.g., Bendiksby and Timdal, 2013; Lumbsch et al., 2007b; Schmull et al., 2011, but see Miadlikowska et al., 2006).
The results of our phylogenetic analyses support recognition at the ordinal level of two currently acepted families, the Hymeneliaceae and Arctomiaceae, in order to retain existing orders in this subclass. The phylogenetic placement of the Ostropales within the Lecanoromycetes has long been unclear due to the unstable backbone of the Lecanoromycetes phylogeny (Lumbsch et al., 2007b). The Ostropales has been found sister to many different lineages in Lecanoromycetes (Grube et al., 2004; Lücking et al., 2004; Lumbsch et al., 2004): sister to the Trapeliales and Hymeneliaceae (Kauff and Lutzoni, 2002; Miadlikowska and Lutzoni, 2004) or to a lineage including the Trapeliales and Baeomycetales (Miadlikowska et al., 2006); sister to the Fuscideaceae, an incertae sedis family within Lecanoromycetes (Reeb et al., 2004); and sister to a lineage including Anzina and Arthrorhaphis (Wedin et al., 2005). None of these relationships were strongly supported. Here the order Ostropales (Thelenellaceae, Protothelenellaceae, Myeloconidaceae, and Phaneromycetaceae not sampled), forms a highly supported monophyletic group with Arctomiaceae and Trapeliales (Figs. 1B–D and 2). However, the relationships among these three lineages are not supported. Our study confirms that Hymeneliaceae and Baeomycetales, together with this trio, form a well-supported monophyletic assemblage (Miadlikowska et al., 2006; Schmull et al., 2011). However, Arthrorhaphidaceae is not part of this major group (which was recovered in the Lecanoromycetideae, but without bootstrap support). The orders Sarrameanales (including Schaereriaceae) and Pertusariales are derived from the first and second divergences within Ostropomycetidae, as previously reported (Miadlikowska et al., 2006; Schmull et al., 2011).
3.5.1 Arctomiales/Arctomiaceae (Fig. 1C)
The order Arctomiales is proposed here to accommodate a single family, the Arctomiaceae (Fries, 1860), which occupies an independent lineage outside the recognized orders in the Ostropomycetidae (Figs.1C and 2). This small family consists of three genera, all of which are associated with the cyanobacterium Nostoc as the main photobiont: Arctomia (six recognized species and four newly added from the former Collema fasciculare group; Otálora and Wedin, 2013), the monospecific Gregorella, and Wawea (Magain and Sérusiaux, 2012). This family was always strongly supported as monophyletic in previous work (e.g., Lumbsch et al., 2005; Magain and Sérusiaux, 2012), although its placement in the Ostropomycetidae was never recovered with high confidence (incertae sedis in Lumbsch and Huhndorf, 2010). Our phylogeny confirms the monophyletic circumscription of the family, but with bootstrap support below the significance threshold because of the placement of Arctomia interfixa outside the Arctomia clade derived from the first divergence within Arctomiaceae (Fig. 1C). A similar position of this taxon (and the non-monophyletic delimitation of the genus Arctomia) was shown recently in Magain and Sérusiaux (2012) and in Otálora and Wedin (2013), but was not revealed earlier in Lumbsch et al. (2005), where the RPB1 locus was not included. Although no single phylogeny incorporates all six Arctomia species, it is possible that A. interfixa (with two ribosomal RNA-coding genes only, in this and previous studies) represents a different, undescribed genus in the family Arctomiaceae. Recent papers (Magain and Sérusiaux 2012; Otálara and Wedin 2013) have demonstrated that diversity in the Arctomiaceae is much greater than previously expected as several species with collematoid thalli actually belong to that family.
Because of the isolated position of the Arctomiaceae between two recognized and well delimited orders, the Ostropales and the Trapeliales in the Ostropomycetidae (see also Lumbsch et al., 2005) and because of its unique characteristics among Ostropomycetidae (e.g., Nostoc as the primary photobiont and gymnocarpous ascomata development; Henssen, 1969, Lumbsch et al., 2005) we propose to recognize Arctomiaceae at the order level.
Taxonomic conclusions:
Order: Arctomiales S. Stenroos, Miadl. & Lutzoni ord. nov.
MycoBank no.: MB 808508.
Typus: Arctomiaceae Th. Fr (1860) based on Arctomia Th. Fr. (1860)
Diagnosis. – Closely related to Ostropales and Trapeliales in the Ostropomycetidae, this order contains a single family Arctomiaceae comprizing bryophilous crustose or fruticose, gelatinized lichens associated with the cyanobacterium Nostoc. Ascomata apothecia with gymnocarpous development, often with poorly developed outer wall, cylindrical asci with a well-developed apical cap (but no ocular chamber) and a I+ mucous outer layer; 8 hyaline ascospores, elongated and transversely septated, often with attenuated apices.
Examplar genera: Arctomia Th. Fr. 1860, Wawea Henssen & Kantvilas 1985.
3.5.2 Baeomycetales/Baeomycetaceae (Fig. 1B)
The order Baeomycetales (Kauff and Lutzoni, 2002; Reeb et al., 2004; Hibbett et al., 2007) currently includes two families, Baeomycetaceae and the monotypic Anamylopsoraceae (Hodkinson and Lendemer, 2011; Lumbsch et al., 1995). The family Baeomycetaceae, which comprises three genera, Baeomyces, Phyllobaeis and the recently added Ainoa (Lumbsch et al., 2007b, c; Lumbsch and Huhndorf, 2010), is well supported as a monophyletic group resulting from a cladogenic event distinct from the great majority of divergences that took place during the evolution of the Ostropomycetidae (Figs. 1B and 2). The classification of Anamylopsoraceae in Baeomycetales (Hodkinson and Lendemer, 2011; Lumbsch et al., 2007b; Lumbsch and Huhndorf, 2010) should be confirmed because Anamylopsora (not included in this study) was never shown with high confidence to be closely affiliated with members of the family Baeomycetaceae (Lumbsch et al., 2001).
3.5.3 Hymeneliales/Hymeneliaceae (Fig. 1B)
The family Hymeneliaceae (Körber 1855), including Ionaspis, Hymenelia and Tremolecia (Melanolecia was not sampled; Lumbsch and Huhndorf, 2010), but without Eiglera (E. flavida is sister to the Acarosporaceae), was reconstructed with strong support as a monophyletic and distinct lineage in the subclass Ostropomycetidae (Figs. 1B and 2). This is the only group outside the order Ostropales in which some species form symbiotic associations with Trentepohlia (Lutzoni and Brodo, 1995). The family is derived from the divergence following the evolutionary split of the Baeomycetales and preceding the split of Trapeliales; therefore, it is flanked by two well-supported internodes. In agreement with the currently accepted orders in the subclass Ostropomycetidae, the Hymeneliaceae should be recognized at the order level. The phylogenetic placement of Eiglera outside of the Hymeneliaceae, sister to Acarosporaceae, needs to be confirmed.
Taxonomic conclusions:
Order: Hymeneliales S. Stenroos, Miadl. & Lutzoni ord. nov.
MycoBank no.: MB 808509.
Typus: Hymeneliaceae Körb. (1855) based on Hymenelia Kremp. (1852)
Diagnosis. – This order of uncertain placement in the Ostropomycetidae, consisting of a single family (Hymeneliaceae), contains saxicolous lichens with mostly crustose, sometimes evanescent thalli associated with chlorococcoid algae or Trentepohlia. Apothecia deeply immersed, asci with a well-developed I+ blue or I− apical cap, usually without an ocular chamber, with an outer I+ blue gelatinous layer; ascospores large, aseptate, hyaline.
Exemplar genera: Hymenelia Kremp. 1852, Ionaspis Th. Fr. 1871.
3.5.4 Ostropales (Fig. 1C–D)
Ostropales is the largest order within the class Ostropomycetidae (131 genera according to Kirk et al., 2008). It includes mostly crustose lichenized and nonlichenized taxa, with high species diversity in the tropics. It hosts one of the major losses of lichenization detected by Lutzoni et al. (2001; Stictidaceae). Currently, ten families are recognized within this order: Coenogoniaceae, Graphidaceae (including Gomphillaceae and Thelotremataceae), Gyalectaceae, Myeloconidaceae, Odontotremataceae, Phaneromycetaceae, Phlyctidaceae, Porinaceae, Sagiolechiaceae, and Stictidaceae (Baloch et al., 2010; Lumbsch and Huhndorf, 2010; Rivas Plata et al., 2012a). Relationships among these families remain highly unsettled (e.g., see Baloch et al., 2010) with the exception of the sister relationship between Gyalectaceae and Sagiolechiaceae as well as the monophyly of all families excluding Porinaceae and Stictidaceae, which are branching outside this major clade (Figs. 1C–D and 2).
The latest classification of the Graphidaceae (Rivas Plata et al., 2012a) comprises three major clades which are delimited as subfamilies: Fissurinoideae, Gomphilloideae, and Graphidoideae, the latter being composed of three major clades which are recognized as tribes Graphideae, Ocellularieae, and Thelotremateae. All three recently circumscribed subfamilies of mostly undetermined placement in Rivas Plata et al. (2012a), received strong support in our study, except for the monophyly of the Thelotremateae and the Gomphilloideae (Fig. 1C–D). The subfamilies Fissurinoideae and Gomphilloideae together form a monophyletic group (but without significant support) derived from the first divergence within the Graphidaceae (in agreement with Rivas Plata et al., 2012a). Considering morphological and phylogenetic distinctness of the recently proposed taxa (subfamilies and tribes), five families could be recognized instead of a broadly delimited single family Graphidaceae.
As reported by Rivas Plata et al. (2013), phylogenetic relationships among genera within the recognized subfamilies are mostly unsupported and unsettled. The genus Thelotrema is highly polyphyletic with its members distributed across the Graphidaceae, as shown also by Rivas Plata et al. (2013), and is currently undergoing a taxonomical treatment by the same authors. Diploschistes ocellatus, which has lecanoroid ascomata and norstictic acid as the main secondary compound in its thallus, is separated from the other members of the genus (Rivas Plata et al., 2013), but its phylogenetic placement did not receive significant support in our study (Fig. 1D) as a result of the first divergence within a clade composed of Diploschistes s. str., Wirthiotrema, Topeliopsis and two ‘Thelotrema’ species (see also Fernández-Brime et al., 2013). The genera Ocellularia and Myriotrema (Ocellularieae) form a monophyletic group, which was strongly supported by one of the four bootstrap analyses. The inclusion of Myriotrema within Ocellularia s. l. or the expansion of Myriotrema to include Ocelluaria species outside Ocellularia s. str. (Fig. 1C) would prevent the proliferation of new generic names (see Rivas Plata et al., 2012b). However, the sequencing of more loci is urgently needed for a meaningful and stable taxonomic revision of the Graphidaceae (see Rivas Plata et al., 2013). Members of the former genus Chapsa s. l. (e.g., Astrochapsa and Pseudochapsa ; Parnmen et al., 2012) belong to the Thelotremateae (Rivas Plata et al., 2013). Contrary to the recent study by Rivas Plata et al. (2013), Chroodiscus coccineus is resolved here outside the core of the genus (which is monophyletic and well supported in our 5+4+3+2-locus phylogeny; Fig. 1D) perhaps due to an erroneous nucLSU sequence obtained from GenBank (Supplemental Table S1). Dyplolabia afzelii is here nested within a well-supported Fissurina clade in agreement with Rivas Plata et al. (2013). Based on an extensive taxon (mostly single-locus) sampling, Fissurina s. l. encompasses several morphologically unrecognized genera (Rivas Plata et al., 2013), which would favor a broad circumscription of this genus.
The families Coenogoniaceae, Gyalectaceae, Porinaceae, and Sagiolechiaceae (here represented by their respective type genus) are all monophyletic with high confidence (Fig. 1C). If future studies confirm the sister relationship of Gyalectaceae and monogeneric Sagiolechiaceae, recovered with high support using our 5+4+3-locus dataset (unsettled in Baloch et al., 2010), both families could be merged, considering the phenotypical similarities between Gyalecta and Sagiolechia. Petractis clausa, classified currently in Stictidaceae (Lumbsch and Huhndorf, 2010), is affiliated with Phlyctis, however, its inclusion in Phlyctidaceae is not well supported. In agreement with a previous study (Baloch et al., 2010), the Odontotremataceae are divided into two unrelated well-supported clades: the Odontotrema s. str. clade (with the type species included), and a second clade currently recognized as the genus Sphaeropezia (Baloch et al., 2012), which is nested within Stictidaceae. As anticipated in Baloch et al. (2012), Coccomycetella richardsonii is nested within Odontotrema (O. phacidiellum and O. phacidioides) with high support (Fig. 1C). Because this phylogenetic affiliation is corroborated by similarities in habit (except by its sigmoid spores) and ecology among these taxa, Coccomycetella richardsonii should be considered as a member of Odontotrema. The genus Stictis is confirmed here to be highly polyphyletic. A new well-supported assembly of potentially unrelated taxa classified in various families (Graphidaceae, Gyalectaceae and Stictidaceae) was discovered in the Ostropales (Group 1; Fig. 1C). This clade of uncertain placement in the Ostropales was partially represented in Baloch at al. (2010; Gyalidea praetermissa and Petractis luetkemuelleri). If justified phenotypically, this new lineage may be circumscribed as a new family in the Ostropales or merged to an existing family once its affiliation is resolved with high phylogenetic confidence.
Taxonomic conclusions:
Coccomycetella richardsonii should be accepted as Odontotrema species (the name Odontotrema richardsonii Leight. is available).
The distinction of Sagiolechiaceae from the Gyalectaceae needs re-evaluation.
Absconditella, Petractis, Gyalidea, Stictis, and Thelotrema need systematic revisions within the phylogenetic framework of the Ostropales.
Petractis nodispora and P. luetkemuelleri (Stictidaceae), Gyalidea praetermissa (Graphidaceae) and Ramonia sp. (Gyalectaceae) should be accommodated in different genera outside of their respective families.
The generic placements of Hemithecium implicatum, Absconditella lignicola, and Cryptodiscus rhopaloides need re-evaluation with the potential of introducing new genera.
3.5.5 Pertusariales (Fig. 1A–B)
The order Pertusariales, recently retained over Agyriales (Hodkinson and Lendemer, 2011), includes currently seven families: Agyriaceae (monotypic family), Coccotremataceae, Icmadophilaceae, Megasporaceae, Miltideaceae (not sampled in this study), Ochrolechiaceae, and Pertusariaceae (Hodkinson and Lendemer, 2011; Lumbsch and Huhndorf, 2010; Schmitt et al., 2010; Widhelm and Lumbsch, 2011). The families Megasporaceae, Coccotremataceae, and Icmadophilaceae, as currently defined, are recovered here as monophyletic and highly supported (Figs. 1A–B and 2). Relationships among families within the Pertusariales clade remain unstable, with the exception of the sister relationship of Megasporaceae with Ochrolechiaceae, and the early divergence of the family Icmadophilaceae from the rest of the Pertusariales (not supported here, but see Miadlikowska et al., 2006).
The family Ochrolechiaceae (including Pertusaria dactylina, a specimen which may represent a sterile isidiate Ochrolechia ; see Schmitt and Lumbsch, 2004) is delimited as monophyletic and well supported, without the genera Variolaria, which was formally reinstated to accommodate Loxospora pustulata and two Pertusaria species not sampled in this study (Lendemer et al., 2013), and Varicellaria (Pertusariaceae; Lumbsch and Huhndorf, 2010, Schmitt et al., 2006, 2012), which together form a monophyletic group sister to the rest of Pertusariaceae (relationships that were recovered in almost all phylogenetic analyses conducted here; Fig. 1A). Varicellaria and Variolaria were segregated from Pertusaria s. l. based on chemical and morphological characters shared between the two genera (e.g., the presence of lecanoric acid as a major secondary compound, disciform apothecia, strongly amyloid asci, non21 amyloid hymenia, 1–2 spored asci, and 1- or 2- celled ascospores with thick, 1-layered walls). However, their close affiliation and suggested alternative placement outside of Pertusariaceae (in Ochrolechiaceae, sister to Ochrolechia) was poorly supported most of the time or not reconstructed, regardless of the markers used (Nordin et al., 2010; Schmitt et al., 2006; Schmitt et al., 2010; Schmitt et al. 2012). The only exception where these three genera form a highly supported monophyletic group was in the study by Schmitt and Lumbsch (2004), where the ingroup was restricted to Pertusariaceae and Ochrolechiaceae; i.e., in the absence of representative species from the families Megasporaceae, Icmadophilaceae, Coccotremataceae, and Agyriaceae.
Here the family Agyriaceae is well supported as sister to the monophyletic genus Pertusaria s.str. + Loxosporopsis (Schmitt and Lumbsch, 2004) as part of a monophyletic Pertusariaceae (but without bootstrap values ≥ 70%; Fig. 1A–B). To retain the family Agyriaceae (and perhaps the Miltideaceae; sister to Agyriaceae, e.g. Lendemer and Hodkinson, 2012; Schmitt et al., 2012; Widhelm and Lumbsch, 2011), a new family should be created to accommodate the monophyletic assembly of Varicellaria and Variolaria (Schmitt et al., 2010) or otherwise the Agyriaceae should be simply subsumed within Pertusariaceae, as suggested in Lendemer and Hodkinson (2012). To account for future alternative relationships among members of Pertusaria s. l. and the remaining families in Pertusariales, two distinct families Variolaceae and Varicellariacea could be recognized as proposed in Lendemer and Hodkinson (2012). However, this would result in more monogeneric families, which is not an informative taxonomic practice.
In the Megasporaceae clade (Fig. 1B), Sagedia is nested within Aspicilia with significant support, questioning the validity of this genus. The genus Sagedia was previously reintroduced by Nordin et al. (2010), although the monophyly of Aspicilia without Sagedia was not strongly supported in that study (significant posterior probability only, but see Alfaro et al., 2003). Based on the same two loci (nucLSU and mitSSU), Sohrabi et al. (2013) confirmed a distinct relationship between Circinaria and Aspicilia s.str. (with strong support), but the segregation of Sagedia remained uncertain because the monophyly of Aspicilia was not supported and both genera shared a most recent common ancestor. The family affiliation of the genus Aspilidea in the Pertusariales (Nordin et al., 2010) is not resolved, although its close relationship with the Megasporaceae + Ochrolechiaceae clade was significantly supported (Fig. 1B). Interestingly, both Ochrolechia and Aspilidea host lichenicolous fungi from the genus Sagediopsis (Verrucariales). In the Icmadophilaceae, the genus Siphula was not recovered as monophyletic.
Taxonomic conclusions:
Sagedia should be included in Aspicilia.
Agyrium should be transferred to the Pertusariaceae and the Agyriaceae should be synonymized with the Pertusariaceae.
Siphula needs a systematic revision within the framework of the Icmadophilaceae.
3.5.6 Sarrameanales/Sarrameanaceae (Fig. 1A)
The recently introduced order Sarrameanales, resulting from the first divergence within the Ostropomycetidae clade (Lumbsch et al., 2007b; Miadlikowska et al., 2006; Schoch et al., 2009), which includes a single family – Sarrameanaceae (= Loxosporaceae; Kantvilas, 2004; Lumbsch et al., 2007a), is monophyletic and well supported (Fig. 1A). In our phylogeny, the Sarrameanaceae, exemplified by the genus Loxospora, forms a clade with the non-monophyletic genus Schaereria as demonstrated earlier by Schmull et al. (2011), because no sequence data are available for Sarrameana.. It is very likely that S. corticola, which seems to be more closely related to Loxospora than to the remaining members of its own genus, represents a distinct genus outside Schaereriaceae (highly supported in Schmull et al., 2011).. However, its close morphological affinity to S. parasemella (currently Hafellnera ; Houmeau and Roux, 1984) may indicate that S. corticola shares a more recent common ancestor with members of the genus Hafellnera. The subclass Ostropomycetidae is not well supported when the Sarrameanaceae and Schaereriaceae are included (Figs. 1B and 2, but see Miadlikowska et al., 2006). Although it is difficult to explain the sister relationship of Schaereria and Loxospora due to substantial anatomical and chemical differences between these two genera (Hafellner, 1984; Wirth, 1995; see discussion in Schmull et al., 2011), delimitation of the order Sarrameanales may be extended to include Schaereria within its own family or as part of the Sarrameanaceae, depending on the results of future phylogenetic studies.
Taxonomic conclusions:
Schaereria corticola should be transferred to a separate genus (perhaps closely related to Hafellnera).
The Schaereriaceae and Sarrameanaceae need to be subjected to a systematic revision within the phylogenetic framework of the Lecanoromycetes.
3.5.7 Trapeliales/Trapeliaceae (Fig. B–C)
As currently delimited, the order Trapeliales includes a single family, Trapeliaceae (Hodkinson and Lendemer, 2011), formerly placed in the Agyriales (Lumbsch et al., 2001) and the Baeomycetales (Lumbsch and Huhndorf, 2010). The monophyly of the Trapeliales, previously demonstrated in several studies (e.g., as Agyriaceae in Miadlikowska et al., 2006), received significant support in the 5+4-gene dataset analysis. The order forms a highly supported monophyletic group together with the Arctomiales and Ostropales (Figs. 1B–D and 2). In addition to the four genera traditionally classified in the Trapeliaceae (Orceolina, Placopsis, Trapelia and Trapeliopsis ; Hertel, 1970), our phylogeny confirms that Aspiciliopsis, Placynthiella, Ptychographa, Rimularia and Xylographa belong to this family (Lumbsch et al., 2001; Schmitt et al., 2003). All genera with multiple representative species, except Placopsis (the only tri-membered genus within this family), are strongly supported as monophyletic, at least based on two of the four datasets analyzed in this study. Based on our phylogeny, the genus Sarea (S. resinae), which was provisionally placed in the Trapeliaceae (Hodkinson and Lendemer, 2011), does not belong to this family and the Lecanoromycetes in general (Figs. 1A and 2), as demonstrated earlier by Reeb et al. (2004). However, this result needs to be confirmed with the sequencing of at least one additional specimen.
Taxonomic conclusions:
More specimens of Sarea need to be sequenced to confirm their current phylogenetic placement outside Lecanoromycetes.
3.6 Umbilicariomycetidae/Umbilicariales (Fig. 1D)
As suggested by Miadlikowska et al. (2006), a new order, Umbilicariales (Hibbett et al., 2007; Zhou and Wei, 2007), and recently a subclass, Umbilicariomycetidae (Bendiksby and Timdal, 2013) were established. The current classification for Umbilicariales includes the family Umbilicariaceae (Lumbsch and Huhndorf, 2010) or the families Umbilicariaceae, Elixiaceae, Fuscideaceae, and Ophioparmaceae (Kirk et al., 2008). Depending on the taxon sampling, the following families are included in the Umbilicariomycetidae clade: Umbilicariaceae, Elixiaceae, and Fuscideaceae in Tehler and Wedin (2008); Umbilicariaceae, Ophioparmaceae, and Fuscideaceae in Miadlikowska et al. (2006); and Umbilicariaceae, Elixiaceae, and Ophioparmaceae in Bendiksby and Timdal (2013), a study where the subclass was formally described. An additional family, the Ropalosporaceae (part of Fuscideaceae s.l.), although without support, was also shown to be part of this order (Bylin et al., 2007).
Here Umbilicariomycetidae is monophyletic with all five families included; however, the first well-supported clade (corresponding to Umbilicariales sensu Bendiksby and Timdal [2013]) excludes Fuscideaceae and Ropalosporaceae (Fig. 1D). Strongly supported as monophyletic, the Umbilicariaceae contains two umbilicate and intermixed, genera – Umbilicaria and Lasallia. The independent generic status of Lasallia separated from Umbilicaria has often been questioned; for example Frey (1933) considered Lasallia as a subgenus. A recent molecular study (Hestmark et al., 2011) suggested that selected species of Lasallia represent a well defined group recognizable molecularly and morphologically (based on the average thallus size and color rather than features associated with the anatomy or reproductive structures) at the same level as several other distinct groups within Umbilicaria, e.g. the vellea group, the cylindrica group or the hyperborea group. Overall, interspecific relationships among Umbilicaria species are not well established based on existing phylogenies; however, no discrepancy was detected for significantly supported clades across phylogenetic studies (Fig.1D; Hestmark et al., 2011; McCune and Curtis, 2012). Our analyses revealed two well-supported clades representing the main division within this family – Umbilicaria s. str. and a clade including a monophyletic Lasallia and remaining Umbilicaria species (i.e., Lasallia + ‘Umbilicaria’ pro parte) (Fig. 1D). Because our phylogeny includes approximately one-third of all recognized Umbilicaria species, and half of Lasallia species only, a broader taxon sampling is necessary before taxonomic conclusions can be made for these two genera. Based on our most comprehensive supermatrix (5+4+3+2-gene) and resulting bootstrap support ≥ 70%, Umbilicariaceae includes also the epiphytic genus Xylopsora (Hypocenomyce friesii group; Bendiksby and Timdal, 2013). The placement of Elixia flexella (Elixiaceae) is well supported within the Umbilicariaceae + Ophioparmaceae clade and was shown to be sister to Umbilicariaceae based on an extensive taxon sampling in Bendiksby and Timdal (2013). The genus Fuscidea (Fuscideaceae) may contain multiple genera (not monophyletic in this phylogeny; Fig. 1D) if Maronea (Fuscideaceae, see also Reeb et al., 2004 and Miadlikowska et al., 2006) is recognized as a separate genus.
Taxonomic conclusions:
In addition to the Umbilicariaceae, Ophioparmaceae, and Elixiaceae, the families Fuscideaceae and Ropalosporaceae should be considered as members of the Umbilicariales or as separate orders in the Umbilicariomycetidae.
Lasallia should be synonymized with Umbilicaria or additional genera should be recognized in the Umbilicaria/Lasallia complex based on a more extensive taxon sampling.
Maronea was confirmed as belonging to the family Fuscideaceae.
Fuscideaceae and the genus Fuscidea require a phylogenetic revision within the phylogenetic framework of Umbilicariomycetidae. Fuscidea mollis may represent a separate genus in the family Fuscideaceae.
3.7 Lecanoromycetidae: relationships among orders (Fig. 1D–K)
None of the phylogenetic relationships among orders of this subclass was supported by bootstrap support ≥ 70%. This is due, in large part, to the addition of 1043 OTUs to the supermatrix used by Miadlikowska et al. (2006), most of which are members of the Lecanoromycetidae clade and are represented by only two of the five genes. However, most of these deep internodes were consistently reconstructed based on different subsets of our supermatrix (Figs. 1D–K and 2), and some of them were highly supported in previous studies. For example, the sister group relationship of the orders Teloschistales and Caliciales (as circumscribed in Gaya et al., 2012) and the close affiliation with the Lecanorales received significant support in Miadlikowska et al. (2006), but was not supported in this study. Different relationships among these orders were reconstructed (with significant support) by Gaya et al. (2012). In that study, which was based on the same five markers but used drastically different taxon sampling (focussing on Teloschistales), Teloschistales was sister to Lecanorales and both formed a monophyletic clade with the Caliciales. We lost support for the sister relationships between the orders Lecideales and Peltigerales (recovered from one of the four datasets) which was highly supported in Miadlikowska et al. (2006; but see Gaya et al., 2012). The recently introduced order Leprocaulales (Fig. 1G), with a single family Leprocaulaceae, seems to be more closely related to the Teloschistales (sister to Teloschistales in all analyses, but without significant support) than to the Caliciales as suggested by Lendemer and Hodkinson (2013).
In agreement with Miadlikowska et al. (2006), the Rhizocarpales (including Sporastatiaceae; Fig 1D–E) is monophyletic and is derived from the first split in the Lecanoromycetideae clade. Within Lecanoromycetidae, a few lineages (represented mostly by a single species) were found to be distantly related to all recognized families: Haematomma accolens, Psorula rufonigra, and Helocarpon crassipes together with Lecidea diapensiae.
The genus Scoliciosporum (Scoliciosporaceae) was found to be polyphyletic (Fig. 1H), with S. schadeanum nested in the Ramalinaceae clade, S. intrusum in the Pilocarpaceae clade, and the type species, S. umbrinum, resolved as a distinct lineage in the Lecanorales. Phenotypical traits including, in part, the presence of lobaric acid, suggest that Scoliciosporum schadeanum forms a distinct morphological group in the Ramalinaceae, together with other taxa not sampled in this study: S. pruinosum, S. pennsylvanica, Bacidia lobarica, members of the ‘Bacidia lutescens group’ sensu Ekman (1996), the Australian genus Jarmania, and Myrionora (Harris, 2009; Palice et al., 2013). Although poorly supported, Scoliciosporum intrusum is shown to be closely affiliated with members of Micarea (in the Pilocarpaceae), a relationship confirming its former classification in this genus as Micarea intrusa (Th.Fr.) Coppins & H. Kilias based on phenotypic similarities (Coppins, 1983).
The genus Haematomma (monogeneric family Haematommaceae) was revealed here as a heterogenous assembly of unrelated taxa based on available sequences (Fig. 1H–I). The type species, H. ochroleucum, is nested in Lecanora (Lecanoraceae, see also Ekman et al., 2008). Haematomma persoonii falls within the Lecanoraceae clade but without support for its exact phylogenetic position. The resolution of H. accolens, depicted as the result from the first divergence within the Lecanorales clade, is possibly due to an erroneous nucSSU sequence (see Supplemental Table S1). Contrary to the results presented here, the genus was shown previously to be monophyletic based on nucLSU and mitSSU data (Lumbsch et al., 2008) and seems to be well defined morphologically and chemically. The individual we used to represent Haematomma ochroleucum (collection from Norway; Ekman 3184) might have been confused with the overlooked species Lecanora thysanophora R. C. Harris. Harris et al. (2000) report that both species look similar when sterile (thick, sorediate, yellow thallus occasionally sourrounded by a distinct prothallus) and often share a similar chemistry (presence of atranorin, usnic acid, zeorin and porphyrylic acid).
The monotypic Psorula rufonigra (a lichenized fungus forming a unique lichenicolous association with a filamentous cyanolichen Spilonema revertens; Timdal, 2002) is separated from the remaining members of Psoraceae (eight individuals representing seven species) where it is currently classified (Lumbsch and Huhndorf, 2010) and may represent an undescribed family in the Lecanoromycetidae (Figs. 1F–H, 2; but see Ekman et al., 2008).
The type species of the genus Helocarpon (H. crassipes, Lecanoromycetes inc. sed. according to Lumbsch and Huhndorf, 2010) forms, together with Lecidea diapensiae (Group 3 in Schmull et al., 2011), a distinct lineage within Lecanoromycetidae (Figs. 1F, 2), to which the family name Helocarpaceae (Hafellner, 1984) can be applied (see also Andersen and Ekman, 2005). However, it was indicated (by an anonymous reviewer) that the specimen representing H. crassipes, the only collection for which sequence data is available in GenBank, was misidentified. To fully circumscribe the family Helocarpaceae, reliable collections of Helocarpon, Micareopsis (Lendemer et al., 2013) and other potentially affiliated taxa (Ekman et al., 2008; Schmull et al., 2011) should be included in a comprehensive multilocus phylogenetic analysis.
Taxonomic conclusions:
Scoliciosporum (Scoliciosporaceae) and Psorula (Psoraceae) need taxonomic revisions within the phylogenetic framework of the Lecanoromycetidae. Scoliciosporum intrusum should be reverted to Micarea intrusa.
Haematomma (Haematommaceae) phylogenetic relationships should be revisited within the phylogenetic framework of the Lecanoromycetidae using more genes.
The resurrection of Helocarpaceae (Lecanoromycetidae inc. sed.) should be revisited based on a more exhaustive taxon and locus sampling.
3.7.1 Caliciales and Teloschistales (Fig. 1F–H)
The recently resurrected order Caliciales (Gaya et al., 2012; i.e., Physciinae sensu Miadlikowska et al., 2006) includes two families: Caliciaceae (with two subfamilies, Calicioideae and Buellioideae) and Physciaceae. The monophyly of the families and subfamilies as delimited by Gaya et al. (2012) is highly supported here (Figs. 1F–H, 2). Lopadium disciforme was resolved in a sister position to the Caliciales by all phylogenetic analyses that included this species, but never with bootstrap support values ≥ 70%. This phylogenetic uncertainty and uniqueness is reflected by its tentative classification within the Ectolechiaceae (Kirk et al., 2008) and Pilocarpaceae (Lumbsch and Huhndorf, 2010). See section 3.7.2.5 for the proposed classification of Lopadium disciforme within the context of its current classification in the family Pilocarpaceae.
In the family Caliciaceae, the genera Buellia and Cyphelium are not monophyletic. Buellia dispersa and C. inquinans appear as lineages distinct from their respective genera (anchored by their type species). Dimelaena radiata is closely related to Buellia tesserata (Gaya et al., 2012) as corroborated by their shared chemical (3-chlorodivaricatic acid), morphological (except crustose versus placodioid thallus growth), ecological and biogeographical characteristics (Rico et al., 2003). Both taxa should be recognized under the same generic name (Buellia or Dimelaena) depending on the future taxonomic treatments (Rico et al., 2003) and the phylogenetic position of the type species D. oreina.
In the Physciaceae, the genus Physconia is reconstructed as paraphyletic with high support (P. enteroxantha is placed outside of the Anaptychia+Physconia clade). Gaya et al. (2012) reported that Physconia enteroxantha (and its close relative P. venusta) occupies an uncertain position within this clade. The monophyly of Physconia will be retained if Anaptychia is included (Helms et al., 2003), or if a separate genus is introduced to accommodate P. enteroxantha and its relatives. A more comprehensive phylogeny of the Physciaceae is needed before a decision can be made.
The recently redelimited order Teloschistales (Physciinae excluded) is restricted to four families (Brigantiaeaceae, Letrouitiaceae, Megalosporaceae, and Teloschistaceae) classified in two suborders, Teloschistineae and Letrouitineae (Gaya et al., 2012). The Teloschistaceae includes three subfamilies: Caloplacoideae, Teloschistoideae, and Xanthorioideae (Gaya et al., 2012; Arup et al., 2013). Monophyly of these taxa and their phylogenetic relationships received high bootstrap support from nearly all analyses (Fig. 1G–H) and are in agreement with Gaya et al. (2012). We confirm that a comprehensive taxonomic treatment is urgently needed for the genera Xanthoria and Caloplaca, reconstructed as highly polyphyletic (divided into two and five distinct clades, respectively) across the Teloschistaceae (Gaya et al., 2012). The recently proposed 31 new and resurrected genera as a result of a phylogenetic study centered on the family Teloschistaceae using ribosomal RNA-coding genes (Arup et al., 2013) requires a complete reassessment using more genes (protein-coding), as some of these genera, and several relationships among genera, were not strongly supported as monophyletic.
Several species groups previously included within Xanthoria have been recently transferred to new genera (e.g., Fedorenko et al. 2009, 2012). Delimitation of the South African genus Xanthodactylon is problematic in our tree because X. turbinatum is affiliated with the Rusavskia clade (former Xanthoria elegans group; Kondratyuk and Kärnefelt 2003; Fig. 1G). Contrary to Gaya et al. (2012), the genus Fulgensia is not monophyletic here (C. xanthostigmoidea was not included in Gaya et al., 2012) and together with Seirophora, these two genera are nested within the Caloplaca s. str. clade. If C. xanthostigmoidea represents C. epiphyta, which shows phenotypic similarities with Fulgensia its phylogenetic placement in the Fulgensia clade would support its transfer to that genus. The Caloplacoideae clade is in need of a thorough revision and with the inclusion of more taxa, in combination with multiple loci, it is very likely that several well-delimited groups will be transferred to new genera (e.g., C. carphinea group; see Arup et al., 2013, for their proposal). If this is the case, the taxonomic delimitation of Fulgensia and Seirophora will have to be reassessed.
Similarly, the clades labeled ‘Caloplaca’ II (sister to Teloschistes in the subfamily Teloschistoideae and comprising some taxa from the Southern Hemisphere), ‘Caloplaca’ III (including mostly species with reduced thalli), ‘Caloplaca’ IV (delimiting mainly the C. saxicola group), and ‘Caloplaca’ V (including mostly maritime species or such with parasitic preferences), might become subject to new generic delimitations in upcoming studies. Conversely, the genera Teloschistes and Xanthomendoza are recovered as monophyletic in accordance to previous phylogenies (Gaya et al. 2012, Arup et al. 2013). We accept a broad delimitation of Xanthomendoza including the recently introduced generic names within this genus (Fedorenko et al. 2012) because the morphological traits used to circumscribe these new entities do not reflect evolutionary relationships within the Xanthomendoza clade. Our phylogeny strongly supports the expansion of the family Megalosporaceae to include the genus Sipmaniella (formerly Lecanoraceae) as demonstrated earlier (Gaya et. al., 2012).
Taxonomic conclusions:
The genera Buellia s.l., Dimelaena, and Physconia need taxonomic revisions within the phylogenetic framework of the Caliciales (sensu Gaya et al., 2012).
The genera Caloplaca, Fulgensia, and Seirophora need taxonomic revision within the phylogenetic framework of the Teloschistaceae (sensu Gaya et al., 2012).
Caloplaca verruculifera should be transferred to the genus Massjukiella.
Fulgensia and Seirophora should be synonymized with Caloplaca or smaller generic units should be described to accommodate these taxa.
‘Caloplaca’ II, III, IV, and V clades should be transferred to new genera if a narrower concept for Caloplaca is applied, or to new subgenera.
Sipmaniella should be transferred from the Lecanoraceae to the Megalosporaceae.
Brigantiaeaceae should be classified within the Teloschistales.
3.7.2 Lecanorales (Fig. 1H–K)
The Lecanorales is the largest and most diverse order of the class Lecanoromycetes (Kirk et al., 2008; Lumbsch and Huhndorf, 2010). From a total of 20 families currently recognized in this order, members of three families (Pachyascaceae, Calycidiaceae and the recently introduced Carbonicolaceae [Bendiksby and Timdal, 2013]) were not sampled in our study because DNA sequences for these taxa were not available when we assembled our supermatrix. Here we report that the order Lecanorales encompasses all families classified in this order, with high bootstrap support, except Dactylosporaceae and Strangosporaceae, which are resolved outside of the OSLEUM clade (Figs. 1A and 2).
Although most families in the Lecanorales were confirmed with high confidence as monophyletic by our phylogenetic analyses, several families should be re-circumscribed to accommodate taxa that were erroneously classified based on false interpretation of phenotypic trait similarity/dissimilarity. Our results confirm the polyphyletic nature of the families Lecideaceae, Pilocarpaceae, Psoraceae, and Ramalinaceae as presently delimited. A few independent lineages emerged that deserve to be recognized at the family level, e.g., Ramboldiaceae and Psilolechiaceae (both introduced here).
All recognized families in the Lecanorales are grouped within two main sister clades: a well-supported clade corresponding to the suborder Lecanorineae and a poorly supported clade encompassing families classified in the suborders Psorineae + Sphaerophorineae (Tehler and Wedin, 2008; Fig. 2). Scoliciosporum umbrinum (Scoliciosporaceae) and Haematomma accolens both appear outside the two major clades. Both genera and their respective monogeneric families are polyphyletic, with the remaining members placed in various families (highly supported in some analyses) in the Lecanorales: S. intrusum in the Pilocarpaceae, Sphaerophorineae (possible artifact; see Ekman et al., 2008); S. schadeanum in the Ramalinaceae, Sphaerophorineae; and Haematomma ochroleucum (perhaps misidentified with Lecanora thysanophora) and H. persoonii in the Lecanoraceae, Lecanorineae. This phylogenetic split of the Lecanorales into two major groups was previously recovered in Ekman et al. (2008), but the Lecanorineae was poorly supported in that study. The suborder Lecanorineae consists of the monophyletic Cladoniineae sensu Poelt (1974), with Stereocaulaceae and Squamarinaceae sister to each other (but see Ekman et al., 2008) and their close relative, Cladoniaceae (Miadlikowska et al., 2006; Myllys et al., 2005; Wedin et al., 2000).
The family Squamarinaceae was introduced by Hafellner (1984) to accommodate a single genus, Squamarina, which was later transferred to Stereocaulaceae (Lumbsch and Huhndorf, 2010). A re-circumscription of the Squamarinaceae, to include the genus Herteliana (currently in the Ramalinaceae), should be considered, based on previous results (H. taylorii sister to S. lentigera in Ekman et al., 2008), and on our study (Fig. 1H).
The Cladoniineae, together with Lecanoraceae, Gypsoplacaceae, Malmideaceae (and other potentially affiliated taxa), and Ramboldia (Kantvilas and Elix, 1994; Ramboldiaceae introduced below), form a well-supported monophyletic clade, sister to the Parmeliaceae (based on the 5-gene dataset; Figs. 1H–K and 2). This large monophyletic entity is sister to a lineage comprising two monogeneric families, Mycoblastaceae and Tephromelataceae, currently in synonymy (Lumbsch and Huhndorf, 2010). Although the Lecanorineae was also recovered in Ekman et al. (2008), a few internal relationships differ from our phylogeny, e.g., a sister relationship of Parmeliaceae with Mycoblastaceae, as well as the Cladoniaceae and Stereocaulaceae forming a monophyletic group.
The Sphaerophorineae clade includes the family Sphaerophoraceae (Wedin and Döring, 1999) and the non-monophyletic Psorineae (Tehler and Wedin, 2008), where Ramalinaceae and Psoraceae form a well-supported clade and Pilocarpaceae are sister to Sphaerophoraceae (but without bootstrap values ≥ 70%). Similar clades were reconstructed with high PP by Ekman et al. (2008) and Miadlikowska et al. (2006), although the Pilocarpaceae were not included in the latter study. The genus Psilolechia (including the type species) is part of a lineage distinct from the Pilocarpaceae, where it was tentatively classified (Lumbsch and Huhndorf, 2010). Psilolechia seems to be derived from the first split within Sphaerophorineae (based on our taxon sampling) and is here recognized at the family level (Psilolechiaceae; Fig 1H). A few highly supported relationships within the order Lecanorales obtained here contradict results from former studies, such as the reported sister relationship between Parmeliaceae and Gypsoplacaceae by Arup et al. (2007) that received 76% ML bootstrap support based on combined nucLSU and nucSSU analyses.
Taxonomic conclusions:
Family: Ramboldiaceae S. Stenroos, Miadl. & Lutzoni fam. nov.
MycoBank no.: MB 808510.
Typus: Ramboldia Kantvilas & Elix
Diagnosis – A monogeneric family in the suborder Lecanorineae (Lecanorales, Lecanoromycetidae) comprising species with lecideoid apothecia with an unpigmented (in some species pigmented apothecia occur due to the presence of russulone and related anthraquinones) prosoplectenchymatous exciple, a Lecanora-type ascus, sparsely branched and anastomosing paraphyses, and simple, hyaline, non-halonate ascospores.
Ramboldia Kantvilas & Elix, Bryologist 97: 296 (1994)
Type species: R. stuartii (Hampe) Kantvilas & Elix 1994
Family: Psilolechiaceae S. Stenroos, Miadl. & Lutzoni fam. nov.
Mycobank no.: MB 808511.
Typus: Psilolechia A. Massal.
Diagnosis – A monogeneric family classified in the order Lecanorales (Lecanoromycetidae, Lecanoromycetes) comprising crustose lichens with effuse, ecorticate, leprose thalli formed by goniocysts containing Trebouxia or stichococcoid algae. Apothecia immarginate, asci 8-spored, cylindrical-clavate, with a central, elongate K/I+ dark blue tube-like structure ('Röhrenstrukturen'; see Hafellner, 1984), non-amyloid ascus wall surrounded by a thin, K/I+dark blue outer layer. Ascospores are oblong-ovoid to dacryoid, simple (rarely 1-septate in P. leprosa), hyaline. All species of Psilolechia grow in shaded, humid places.
Psilolechia A. Massal., Atti Reale Ist. Veneto Sci. Lett. Arti, Sér. 3 5: 264 (1860)
Type species: P. lucida (Ach.) M. Choisy 1949
The suborder Lecanorineae includes: Cladoniaceae, Gypsoplacaceae, Lecanoraceae, Mycoblastaceae, Parmeliaceae, Ramboldiaceae, Squamarinaceae, Stereocaulaceae, and Tephromelataceae.
The suborder Sphaerophorineae is re-delimited to include the former Psorineae (Pilocarpaceae, Psoraceae and Ramalinaceae) and Psilolechiaceae.
Scoliciosporum and Haematomma are in need of a taxonomic revision within the broad phylogenetic context of the Lecanorales.
3.7.2.1 Lecanoraceae (Fig. 1I)
In addition to the genera currently classified in the Lecanoraceae (Lecanora, Lecidella, Protoparmeliopsis, and Rhizoplaca; Lumbsch and Huhndorf, 2010), the family contains other taxa that were anticipated to belong to different families based on morphological and anatomical characters, e.g., Japewia tornoënsis and Frutidella currently classified in the Ramalinaceae, Haematomma persoonii in the Haematommaceae, Glyphopeltis ligustica in the Psoraceae, and Lecidea turgidula in the Lecideaceae. The placement of Japewia, Frutidella and Lecidea turgidula has been shown to be problematic by Schmull et al. (2011), who also proposed that Japewia and Frutidella should be recognized as members of Lecanoraceae. This result is confirmed here, with high bootstrap support (Fig. 1I). The genera Lecidella and Protoparmeliopsis (which are in needs of a conserved name status and representative type; see Knudsen and Lendemer [2009]) are monophyletic. However, only the latter is highly supported.
The genus Lecanora is highly polyphyletic with its members spread across the family. Here the most inclusive monophyletic entity for this genus includes the L. subfusca group, considered as Lecanora s.str. (L. allophana included; Brodo and Vitikainen, 1984), as well as the L. symmicta and L. rupicola groups, intermixed with other species of Lecanora and the following unrelated taxa: Haematomma ochroleucum (possibly a misidentified sterile Lecanora thysanophora), Pyrrhospora quernea (Lumbsch et al., 2004) and Ramboldia insidiosa (Kalb et al., 2008; Fig. 1I). Lecanora glabrata, a potential member of the L. subfusca group (Arup et al., 2007), came out distantly related to taxa from that group. Two other groups of Lecanora, the L. dispersa and L. polytropa groups, are placed outside the monophyletic genus Lecanora as circumscribed here (Fig. 1I). The well-supported monophyly of Protoparmeliopsis and its phylogenetic placement within Lecanoraceae supports its re-instoration as a distinct genus from Lecanora (see Lumbsch and Huhndorf, 2010). This genus, with additional Lecanora species s. l., Rhizoplaca and two members of Micarea (M. incrassata and M. assimilata) together form a well-supported clade. Delimitation of genera and phylogenetic relationships within Lecanoraceae are currently very unstable and a substantial improvement in resolution and support must be achieved by sequencing a minimum of four to five loci before formal changes can be made.
Taxonomic conclusions:
The Lecanoraceae and the genus Lecanora need systematic revision within the phylogenetic context of the Lecanorales.
Frutidella and Japewia need to be transferred from Ramalinaceae to Lecanoraceae as recommended by Schmull et al. (2011).
Glyphopeltis should be transferred from Psoraceae to Lecanoraceae if its phylogenetic placement in Lecanoraceae is confirmed.
Pyrrhospora quernea and Ramboldia insidiosa should be transferred to Lecanora within the broad circumscription of that genus proposed here, once their phylogenetic placement is confirmed with additional specimens.
3.7.2.2 Parmeliaceae (Fig. 1I–K)
The overall delimitation of genera and major clades in the speciose family Parmeliaceae presented here is in agreement with the large scale and collaborative study by Crespo et al. (2010). This congruence and stability might reflect the benefit of adopting a strategy combining a comprehensive taxon sampling with sequencing a high number of the same genes used throughout the Lecanoromycetes (Figs. 3 and 4). In some cases, our Lecanoromycetes-wide study provided more resolution and support for the Parmeliaceae (Fig. 1I–K). In the parmelioid group (Fig. 1J–K), the Parmotrema clade is sister to the Xanthoparmelia clade in both studies. However, the genus Canoparmelia does not represent the first split in the Parmotrema clade as shown in Crespo et al. (2010), but appears to be rather sister to Xanthoparmelia (with high support from the 5+4+3-gene dataset). Punctelia seems to be more closely related to Flavopunctelia (a former subgenus of Punctelia containing usnic acid instead of atranorin in the cortex) than to lichenicolous Nesolechia, contrary to the alternative placement in Crespo et al. (2010). In our trees, Punctelia subrudecta, which corresponds to P. subrudecta 2 in Crespo et al. (2010), is nested (for an unknown reason) in Parmotrema with high support, rather than with members of its own genus as previously reported.
The genus Cetrelia (Cetrelia clade in Crespo et al., 2010) is nested in a highly supported Parmelina clade (uncertain placement in Crespo et al., 2010). The Parmelia clade, excluding Relicina, is monophyletic and highly supported. It was resolved as sister to Parmeliopsis (highly supported by our 5+4-gene analysis), a genus recognized as a separate clade in Crespo et al. (2010). Relicina was nested in the Parmelia clade in Crespo et al. (2010), but occupies a separate uncertain position in our phylogeny.
Everniastrum lipidiferum does not cluster with the remaining specimens from this genus (see also Divakar et al., 2006) and is possibly affiliated with Cetrariastrum as shown in Crespo et al. (2010). The newly introduced genus Montanelia, to accommodate the former Melanelia disjuncta group (Divakar et al., 2012), is sister to Pleurosticta and part of the extended, highly supported Melanohalea clade (Crespo et al., 2010), which holds an uncertain placement outside the delimited parmelioid clades sensu Divakar et al. (2006).
Similar to previously published phylogenies (Crespo et al., 2007, 2010; Thell et al., 2009, 2012), the backbone structure among the “other non-parmelioid group” of Parmeliaceae generally did not receive bootstrap support above 70%. However, many genera represented by at least two species are shown to be monophyletic with high confidence (e.g., Alectoria, Arctoparmelia, Asahinea, Brodoa, Cetraria, Evernia, Menegazzia, Platismatia, Pseudevernia, Vulpicida, and Usnea) and their sister relationships are well established. For example, the alectorioid group, as defined in Crespo et al. (2007; Alectoria, Pseudephebe and Sulcaria), should be expanded to include also the closely related monophyletic genus Bryoria (together with Bryocaulon divergens), which occupied an uncertain position in the Parmeliaceae in past studies. The genus Gowardia forms a sister group relationship to Alectoria (well supported) and can be kept as a separate genus following the Halonen et al. (2009) circumscription, or can be treated as a member of Alectoria following Lumbsch and Huhndorf (2010). The distant relationship between Usnea and Protousnea, where the latter is sister to Pannoparmelia (although not highly supported), was also recovered in Crespo et al. (2010). The paraphyletic relationship (without support in previous studies) between Platismatia and Imshaugia was replaced here by a well-supported monophyletic relationship (Fig. 1I–J).
The cetrarioid core group was reconstructed as monophyletic (but not strongly supported, if Melanelia is included) and in general agreement with Thell et al. (2009) and Nelsen et al. (2011). Our study confirms the monophyly of the hypogymnioid group as previously circumscribed (Crespo et al., 2007), possibly with the genus Evernia resulting from the first phylogenetic split in this clade. Contrary to Crespo et al. (2007), we did not recover the letharioid clade. Here, Letharia is resolved sister to Asahina (not supported) within a very unstable part of the Parmeliaceae tree (Fig. 1J).
Taxonomic conclusions:
Everniastrum lipidiferum does not seem to belong to this genus if phylogenetic analyses of sequences from other individuals of this species confirm our and previous results.
Bryocaulon divergens needs to be transferred to the genus Bryoria if phylogenetic analyses of sequences from other individuals of this species confirm our results.
3.7.2.3 Remaining families in the Lecanorineae (Figs. 1I and 2)
Initially the family Gypsoplacaceae was established for a single species –Gypsoplaca macrophylla (Timdal, 1990). Here, this genus is nested within a monophyletic group (highly supported by the 5+4-gene analysis) encompassing several mostly corticolous members of ‘Lecidea’ and the saxicolous Miriquidica garovaglii (Lecanoraceae). In Schmull et al. (2011), these ‘Lecidea’ species were reconstructed with a very low confidence as part of Group 1 in the Lecanoraceae and Group 2 in the Pilocarpaceae. Recently the family Malmideaceae has been recognized to accommodate mostly corticolous species from the tropics previously classified in the Lecidea piperis- and Lecanora granifera-groups (Kalb et al., 2011) and a very distinctive new genus, Savoromala from Madagascar (Ertz et al., 2013). In our phylogeny, Lecidea floridensis, a member of the new genus Malmidea together with L. cyrtidia (see Ertz et al. 2013), and another unidentified species of the genus Lecidea represent a highly supported Malmideaceae (Fig. 1I). A sister relationship between Malmideaceae and Gypsoplacaceae, revealed in this study (uncertain placement in the Lecanorales in Ertz et al. [2013]), did not receive strong support. Incomplete sampling within the Malmideaceae and lack of obvious morphological similarities with Gypsoplaca and other members of Lecideaceae s.l. (e.g., Lecidea nylanderi) do not support a formal expansion of the family Gypsoplacaceae.
Within the Cladoniaceae, Metus and Pycnothelia form a sister group closely related to Cladonia, whereas the remaining genera Cladia (including Ramalinora glaucolivida) and Pilophorus show unsettled placements in the family. The phylogenetic placement of the genus Hertelidea in the Stereocaulaceae (Lumbsch and Huhndorf, 2010) is confirmed for the first time based on molecular data.
Taxonomic conclusions:
The taxonomy of, members of the families Malmideaceae and Gypsoplacaceae, and their close relatives, needs to be reassessed within a more comprehensive phylogenetic context.
3.7.2.4 Ramalinaceae (Fig. 1H)
In its current circumscription, the family Ramalinaceae is not monophyletic. The following genera should be excluded from the family and placed elsewhere in the Lecanorales: Herteliana (type species placed in the Squamarinaceae), Schadonia (one representative placed in the Pilocarpaceae), Sipmaniella (one representative placed in the Megalosporaceae), Frutidella, and Japewia (type species placed in the Lecanoraceae; Schmull et al., 2011). The remaining genera classified in the Ramalinaceae form a well-supported monophyletic group, including the monogeneric Crocyniaceae and the family Megalariaceae (type species sampled) as well as selected members of the Catillariaceae, Lecideaceae, Pilocarpaceae and Scoliciosporaceae. A similar phylogenetic delimitation of the family, but with different taxon samplings was shown in previous studies (Andersen and Ekman, 2005; Ekman et al., 2008; Reese Næsborg et al., 2007; Miadlikowska et al., 2006; Schmull et al., 2011), with the exception of Byssolecania variabilis, which was resolved within Pilocarpaceae (where it is currently classified) based on mitSSU and nucLSU sequences (Nelsen et al., 2008; Andersen and Ekman, 2005), and Scoliciosporum schadeanum (Scoliciosporaceae), which was never sampled before. In light of this and previously published phylogenies, the family Crocyniaceae should be synonymized with Ramalinaceae. It is uncertain if the entire family Megalariaceae is nested within Ramalinaceae because the genus Tasmidella was not sampled (Catillochroma was recently synonymized with Megalaria by Fryday and Lendemer, 2010; but see Lücking et al., 2011).
Our study revealed a non-monophyletic delimitation of the genera Bacidina, Lecania, Mycobilimbia (Reese Naesborg et al., 2007), and Toninia, although high support was received only for the placement of L. chloritiza outside the genus Lecania s.str., previously reported in Sérusiaux et al. (2012). There are many validly published genera within Ramalinaceae that were never sequenced that could potentially accommodate L. chlorotiza, L. naegelii and L. falcata (Sérusiaux et al., 2012) outside of the genus Lecania s.str. (Fig.1H). The genus Bilimbia (Lecanoromycetes inc. sed.) belongs to the Ramalinaceae and should retain its distinct status from Mycobilimbia. Our data revealed two main lineages within the Ramalinaceae s.l., corresponding to the Ramalinaceae s.str. (poorly supported) and the Bacidiaceae (highly supported), as circumscribed in many earlier papers (the most recent being Sérusiaux et al. 2012).
Taxonomic conclusions:
Herteliana should be transferred from the Ramalinaceae to the resurrected Squamarinaceae.
Japewia and Frutidella should be removed from the Ramalinaceae and classified within Lecanoraceae.
Mycobilimbia needs a systematic revision within the phylogenetic context of the Lecanoromycetidae, and should be transferred from the Lecideaceae to the Ramalinaceae.
Bilimbia can now be classified within the the Ramalinaceae.
Bacidina, Toninia and Lecania need systematic revisions within the context of the family Ramalinaceae.
Crocynia should be transferred to the Ramalinaceae and the family Crocyniaceae synonymized with Ramalinaceae.
The genus Megalaria should be transferred from the Megalariaceae to the Ramalinaceae and the Megalariaceae should be synonymized with the latter family.
The phylogenetic placement of Byssolecania should be revisited within the context of the Lecanorales and based on a more extensive sampling of this genus.
3.7.2.5 Remaining families in the Sphaerophorineae + Psorineae clade (Figs. 1H and 2)
The well-delimited Psoraceae contains two monophyletic genera, Psora and Protoblastenia (poorly supported), as well as Micarea sylvicola (Ekman et al., 2008), a current member of the Pilocarpaceae. The genus Micarea should be subjected to a phylogenetically based taxonomic revision, as it consists of unrelated taxa placed in the Lecanoraceae (Fig. 1I) and Psoraceae (Fig. 1H), whereas the core of this genus (the former Micareaceae) belongs to the Pilocarpaceae (Ekman et al., 2008). Protomicarea limosa (Psoraceae) belongs to Sphaerophoraceae as already demonstrated by Ekman et al. (2008).
Here we report that the family Pilocarpaceae (highly supported based on two of four datasets; Fig. 1H) also includes members of the Ramalinaceae (Schadonia fecunda; see also Ekman et al., 2008) and Scoliciosporaceae (Scoliciosporum intrusum; lacking morphological justification for this phylogenetic placement). The Pilocarpaceae is revealed as highly heterogeneous, with many members resolved outside this family, closely affiliated with families in the Lecanorales (Psoraceae, Ramalinaceae, and Lecanoraceae) and with Lecideaceae, or as separate lineages. For example, the genus Psilolechia, classified tentatively in the Pilocarpaceae (Lumbsch and Huhndorf, 2010) is placed outside of recognized families in Sphaerophorineae (with high support in some analyses) as the first evolutionary split within this clade (but not supported). It is recognized here in its own family (Psilolechiaceae; Fig. 1H). The inclusion of Catillaria erysiboides (Catillariaceae) in Psilolechiaceae did not receive significant support, and therefore should be revisited in future studies. Another putative member of the Pilocarpaceae (Lumbsch and Huhndorf, 2010), the genus Lopadium (represented here by L. disciforme), occupies an uncertain position in the Lecanoromycetidae outside the Pilocarpaceae and perhaps the Lecanorales in general (appears closely related to the Caliciales but not supported; Figs. 1F and 2). Its isolated position within the Lecanoromycetidae may justify resurrecting the family Lopadiaceae (Hafellner, 1984).
Taxonomic conclusions:
The family Lopadiaceae (Lecanoromycetidae inc. sed.) should be resurrected to accommodate Lopadium s.str. if our results are confirmed with additional sequences from additional species.
Micarea is not monophyletic and needs a phylogenetically-based revision within the Lecanorales framework.
The Catillariaceae and Catillaria are not monophyletic and need a systematic revision within the Lecanoromycetidae framework. New genera should be created to accommodate C. scotinoides in the Ramalinaceae, C. erysiboides in the Psilolechiaceae, and C. modesta in the Lecideaceae if these results are confirmed with additional specimens from these species.
A new genus should be created to accommodate Micarea sylvicola in the Psoraceae if further studies confirm this result.
Psilolechia should be removed from the Pilocarpaceae and classified together with Catillaria erysiboides (under a new genus name, if confirmed with additional data) in Psilolechiaceae (Lecanorales).
Protomicarea should be transferred from the Psoraceae to the Sphaerophoraceae.
More individuals of Miriquidica should be included in phylogenetic analyses before transferring the genus from the Lecanoraceae to the Gypsoplacaceae.
Glyphopeltis may belong to the Lecanoraceae, not the Psoraceae, but additional data are needed, including from the type species.
3.7.3 Lecideales (Fig. 1E)
The order Lecideales was recently resurrected to accommodate a single family, Lecideaceae, now restricted to saxicolous species from the genera Lecidea s.str. (sensu Hertel) including the type species (L. fuscoatra), and Porpidia (Schmull et al., 2011). Our phylogeny confirms with high support the monophyletic delimitation of Lecideaceae sensu Schmull et al., (2011). Several newly added saxicolous species of Lecidea (e.g., L. plana) and members of Porpidia (e.g., P. carlottiana, P. macrocarpa, P. melinodes, P. contraponenda, P. crustulata), as well as the genus Bellemerea (including B. alpina, the type species) and Lecidoma demissum (part of unsupported Group 3 in Schmull et al., 2011) cluster without support within the Lecideaceae (Lumbsch and Huhndorf, 2010). Moreover, the genus Arthrorhaphis (Arthrorhaphidaceae) considered a member of uncertain placement in the Ostropomycetidae (Lumbsch and Huhndorf, 2010) and shown as such in previous phylogenies (e.g., Miadlikowska et al., 2006; Lumbsch et al., 2007b), is unexpectedly found here to be affiliated with the Lecideaceae; however, this is not supported. Surprisingly, two other morphologically distinct taxa, Leimonis erratica (classified and placed in the Pilocarpaceae, but not supported in Schmull et al., 2011) and Catillaria modesta (Catillariaceae) are also part of the extended Lecideaceae, but without support. This and previous studies have already demonstrated that the genera Porpidia and Lecidea s.str. are not monophyletic and therefore must be redefined based on a more comprehensive and stable phylogeny for this group. Contrary to the phylogenetic studies focusing on the former family Porpidiaceae (Buschbom and Mueller, 2004; Buschbom and Barker, 2006), an individual of Porpidia speirea (different specimen and markers used) is nested in the Lecidea s.str. clade (Miadlikowska et al., 2006; Schmull et al., 2011), indicating a misidentification or heterogeneity for this species. Two potential members of the Lecideaceae, the monotypic genus Romjularia and its closest relative Mycobilimbia berengeriana (possibly Romjularia) are separated from the remaining members of Lecideaceae, but without high confidence.
Taxonomic conclusions:
Lecidea, Mycobilimbia, and Porpidia are not monophyletic and need systematic revisions within the phylogenetic framework of the Lecanoromycetidae.
The order Lecideales should be accepted to accommodate the family Lecideaceae s.str., although its final delimitation must be assessed with more loci to improve phylogenetic confidence.
3.7.4 Leprocaulales (Fig. 1G)
Leprocaulaceae was originally created for two genera, Halecania (not represented in this study) and Leprocaulon (Lendemer and Hodkinson, 2013). It comprised morphologically diverse, primarily sterile, asexually reproducing lichens that produce pannarin, argopsin, and usnic acid. Fertile taxa have Halecania-type asci, lecanorine apothecia, and hyaline ascospores that resemble those of Lecania. Based on our results, the circumscription of the Leprocaulaceae could be extended to include the monotypic genus Speerschneidera (S. euploca; Lendemer and Hodkinson, 2013; Nelsen et al., 2008) and Solenopsora candicans (Catillariaceae), two taxa forming a robust monophyletic group with Leprocaulon quisquiliare (Fig. 1G), as well as Catinaria atropurpurea (not included in our study, but see Ekman et al., 2008).
Although included in the study by Lendemer and Hodkinson (2013), S. euploca was excluded from their taxonomic conclusions because of its nucLSU sequence (AY300862) which blasts with 100% identity to a sequence of Catinaria atropurpurea from the Ramalinaceae (AY756347), the family in which S. euploca has traditionally been classified (but currently classified in the Lecanorales inc. sed.; Lumbsch and Huhndorf, 2010). The high similarity of the nucLSU sequences between these two genera can be explained because C. atropurpurea does not cluster with the Ramalinaceae but instead forms a sister relationship with Halecania alpivaga, currently a member of the Leprocaulaceae (Ekman et al., 2008).
The genus Solenopsora comprises approximately 19 published species (Kirk et al., 2008) of diverse morphology ranging from crustose with immersed to emergent apothecia, as for example in S. candicans, to squamulose or small foliose with substipitate apothecia, as seen in S. holophaea (the type species of the genus). Despite the broad range of gross morphology, all species of Solenopsora are united in having eight-spored, Catillaria type asci, simple paraphyses with internally brown pigmented, clavate apices, and hyaline, one-septate, non-halonate ascospores. On the basis of these anatomical characters, the genus is classified in the family Catillariaceae (Lumbsch and Huhndorf, 2010; Verdon and Rambold, 1998).
Taxonomic conclusions:
Speerschneidera should be transferred from the Lecanorales inc. sed. to the Leprocaulaceae (Leprocaulales).
Proper delimitations of the genera Solenopsora, Halecania, and Catinaria require further taxonomic revisionary studies.
3.7.5 Peltigerales (Fig. 1E–F)
Currently, Peltigerales includes ten families (Spribille and Muggia, 2012) grouped in two suborders, Collematineae and Peltigerineae (Miadlikowska and Lutzoni, 2004). The Collematineae consists of four families (Coccocarpiaceae, Collemataceae, Pannariaceae and Placynthiaceae), whereas the Peltigerineae includes six families (Lobariaceae, Massalongiaceae, Nephromataceae, Peltigeraceae, and the most recently added Koerberiaceae and Vahliellaceae; Miadlikowska and Lutzoni, 2004; Muggia et al., 2011; Spribille and Muggia, 2012; Wedin et al., 2007, 2011). The first four families listed above for Peltigerineae form a well-supported clade (Fig. 1F), to which Vahliellaceae is sister (but without support values ≥ 70%), whereas Koerberiaceae is resolved here as sister to Collematineae (a relationship also not well supported). The monophyly of Peltigerineae with Vahliellaceae and Koerberiaceae was supported only by posterior probabilities in Spribille and Muggia (2012; see Alfaro et al. 2003). Therefore, these two families hold an uncertain phylogenetic placement in the order Peltigerales. However, the family Koerberiaceae seems to be more closely related to the suborder Collematineae than Peltigerineae (Fig. 1E; see also Muggia et al., 2011).
Within Peltigerineae, the sister relationship between Peltigeraceae and Lobariaceae, as well as their close affiliation with the Nephromataceae and Massalongiaceae are confirmed (Miadlikowska et al., 2006) with strong bootstrap support (Fig. 1F). These phylogenetic relationships contradict (sometimes with conflicting high support values) the results from other studies on the Peltigerales based on a different taxon sampling (Muggia et al., 2011; Wedin et al., 2009; Wedin et al., 2007; Wiklund and Wedin, 2003; Spribille and Muggia, 2012) and on a different selection of loci (exclusively ribosomal except RPB1 in Wedin et al., 2009). Here for the first time, the monophyly of the Collematineae, including all families traditionally included in this suborder, received high bootstrap support. We recovered a stable sister relationship between the Collemataceae and the Placynthiaceae. This clade is closely related to the Coccocarpiaceae (significantly supported based on two of four datasets), a family of unresolved (e.g., Muggia et al., 2011; Spribille and Muggia, 2012) or unsupported (Wedin et al., 2009) phylogenetic placement in past studies.
Except for Lobaria and Pseudocyphellaria, all genera in the Lobariaceae, including newly described genera (Moncada et al., 2013) are monophyletic and for the most part received at least one bootstrap value ≥ 70%. Our results do not support the recognition of Anomalobaria as a separate genus (nested in Lobaria; Fig. 1F). In the study by Moncada et al. (2013) based on combined ribosomal loci, the monophyly of Lobaria without Anomalobaria (former Pseudocyphellaria anomala group) received only 47% of ML bootstrap support, suggesting that the split between these two genera may be artificial. Three representatives of the former Lobaria s.l. (L. hallii, L. meridionalis, L. pseudoglaberrima) and Pseudocyphellaria rainierensis – none of which was included in Moncada et al. (2013) – cannot be assigned to newly circumscribed genera with certainty based on this phylogeny. A close relationship of P. rainierensis with members of Lobarina was shown in Miadlikowska and Lutzoni (2004), but without support. Reading errors within the conserved parts of the nucLSU sequence of P. rainieriensis (AF401963) identified in Moncada et al. (2013), did not effect the phylogenetic placement of this taxon because they were part of ambiguous regions excluded from all analyses. To avoid the recognition of additional genera (for example to accommodate L. meridionalis) and to allow the classification of existing species of Lobaria, a broad delimitation of this genus (Lobaria s. l. in Fig. 1F) to reinclude newly proposed Ricasolia, Lobariella, Lobarina and perhaps Pseudocyphellaria rainierensis should be reconsidered.
An unexpectedly high level of support was obtained for intrageneric relationships within the genus Peltigera (Peltigeraceae). The latest global phylogeny for Peltigera based on concatenated nucLSU sequences and phenotypic characters (54 individuals from 40 putative species) allowed the circumscription, with high confidence, of eight sections within the genus; however, their relationships were largely unsettled (Miadlikowska and Lutzoni, 2000). Our 5+4+3+2-gene dataset contains fewer individuals and fewer taxa than sampled in 2000 (33 individuals from 30 putative species), but half of them are represented by more than three genes including part of RPB1 or RPB2. All sections, except section Peltigera (the P. canina group), are monophyletic, although one (section Polydactylon) is not well supported and three others (sections Chloropeltigera, Phlebia and Retifoveatae) are represented by only one species. Contrary to Miadlikowska and Lutzoni (2000), all sorediate members from the P. canina group (section Peltigera I in Fig. 1F) are separated from the remaining species of that section (section Peltigera II in Fig. 1F) by sections Retifoveatae and Horizontales (sister to Peltigera II clade in Fig. 1F). This phylogenetic relationship was reconstructed and supported based only on the 5+4+3-gene dataset. All tri-membered Peltigera species (part of three sections: Chloropeltigera, Phlebia and Peltidea) share a most recent common ancestor (high support in the 5+4-gene dataset), suggesting a single, or two at the most (depending on the placement of the bi-membered species P. malacea and P. frippi in the section Peltidea) independent acquisitions of the green photobiont Coccomyxa during the evolutionary history of the genus (Miadlikowska and Lutzoni, 2004). Section Polydactylon forms a well supported monophyletic clade with this tri-membered sections. The aquatic species P. hydrothyria is part of this clade, but its phylogenetic placement remains uncertain. Another aquatic Peltigera species (Peltigera sp. nov. from Chile), which will be formally described as part of a revisionary work on the section Peltigera in a separate publication, is nested in the P. canina section (Peltigera II clade; Fig. 1F).
Our results highly support the recent recircumscription of the genera Collema s. str. and Leptogium s. str (Otálora et al., 2013a, 2013b) and their sister relationship in the absence of Paracollema (not sampled in this study; Fig. 1E–F). Strongly supported monophyletic circumscription of Scytinium, Lathagrium, and Enchylium (based on the combined nucLSU, mitSSU, MCM7, and beta-tubulin in Otálora et al., 2013a, 2013b) was not revealed in this study. Moreover, members of these three newly delimited genera of the former Collema/Leptogium complex are intermixed with high bootstrap support (mostly from the 5+4+3+2-gene dataset). Placement of Pseudoleptogium as the first split in Collemataceae is in agreement with Otálora et al., 2013a, b.
Our results support the exclusion of Physma and Staurolemma from Collemataceae (despite their homiomerous thallus and lack of detectable secondary metabolites shared with members of Collema and Leptogium), as well as their transfer to the Pannariaceae (Otálora et al., 2010a; Spribille and Muggia, 2012; Wedin et al., 2009). A single member of the genus Ramalodium included in our analyses is nested within the genus Staurolemma (Wedin et al., 2009). Both genera share asci without apical amyloid structure, but the phylogenetic identity of Ramalodium needs to be confirmed with a broader sampling. Degelia durietzii and D. gayana (type species) are more closely related to Leioderma and Erioderma than to D. plumbea, which is placed sister to Parmeliella (Muggia et al., 2011).
Taxonomic conclusions:
Anomalobaria, Lobariella, Lobarina and Ricasolia could be re-included in the genus Lobaria to prevent the erection of more new genus names to accommodate the newly proposed classification within Lobaria s.l.
The identity and phylogenetic placement of the specimen representing Pseudocyphellaria rainierensis should be reassessed based on additional samples.
Degelia needs to be subjected to a systematic revision within the phylogenetic framework of Pannariaceae.
Leptochidium should be transferred from Placynthiaceae to Massalongiaceae (see also Muggia et al., 2011).
The identity and phylogenetic placement of Ramalodium succulentum within Staurolemma should be reassessed based on additional samples.
Steinera should be removed from Coccocarpiaceae and Koerberia from Placynthiaceae to be classified, together with Vestergrenopsis, in the family Koerberiaceae (Spribille and Muggia, 2012), a family that should be recognized as an incertae sedis within Peltigerales.
3.7.6 Rhizocarpales (Fig. 1D–E)
The order Rhizocarpales, introduced by Miadlikowska et al. (2006), includes a single family (Rhizocarpaceae) and the genus Sporastatia (formerly in the Catillariaceae) recently classified in a separate family, Sporastatiaceae, outside of the Rhizocarpales by Bendiksby and Timdal (2013). In the past, the sister relationship between the genera Sporastatia and Rhizocarpon (Rhizocarpaceae) was demonstrated in several phylogenies (Buschbom and Mueller, 2004; Lutzoni et al., 2004; Miadlikowska et al., 2006; Reeb et al., 2004); however, none of them included the genus Toensbergia (formerly Hypocenomyce or Pycnora leucococca). Here we confirm, with strong support from all four analyses, the close relationship between Rhizocarpon and Sporastatia (Fig. 1D). Overall we also recovered the same phylogenetic placement of this order within Lecanoromycetes, but without support values ≥ 70% (Fig. 2). The phylogenetic position of the Rhizocarpales as the first split within the Lecanoromycetidae (as defined here), although often reconstructed, has rarely been supported (Miadlikowska et al., 2006). This is the second time that we show that Catolechia wahlenbergii (the type species of the genus) is nested within Rhizocarpon (see Miadlikowska et al., 2006).
Taxonomic conclusions:
The monotypic genus Catolechia should be subsumed within Rhizocarpon.
The genus Sporastatia should be transferred from the Catillariaceae to the Rhizocarpaceae or, alternatively, be accommodated in Sporastatiaceae (together with Toensbergia; Bendiksby and Timdal, 2013) as the second family in the Rhizocarpales.
3.8 Photobiont distribution across Lecanoromycetes
A comprehensive overview on the photobionts associated with members of Lecanoromycetes (Fig. 2), based on available data, is included in Gueidan et al. (2014), Miadlikowska et al. (2006), Voytsekhovich et al. (2011b) and literature cited therein. The most frequently reported photobionts across the Lecanoromycetes are coccoid green algae (Chlorophyta) representing the following genera: Trebouxia, Asterochloris, Dictyochloropsis s.l., Coccomyxa, Myrmecia, Pseudococcomyxa, Stichococcus and Chlorella. For many lichens, the photobiont is reported as an unidentified unicellular alga (e.g., in the Pilocarpaceae, Gypsoplacaceae, Rhizocarpaceae, and Acarosporaceae), reflecting the difficulty to identify these algae and generally the state of the systematic classification of lichen photobionts (Fig. 2). In the case of Trebouxia, which is one of the most common chlorobionts reported for the Lecanoromycetidae, Umbilicariomycetidae, ‘Candelariomycetidae’ and selected lineages in Ostropomycetidae (e.g., Graphidaceae, Pertusariales), its identity needs to be verified in many groups as it may represent the closely related genus Asterochloris or other unidentified chlorococcoid genera. Filamentous green algae such as Trentepohlia and Phycopeltis (Trentepohliaceae) are restricted to most lineages in the Ostropales and family Hymeneliaceae, all in the subclass Ostropomycetidae. These lichens are usually common in humid and shaded habitats, on trees predominantly in tropics (members of the Ostropales) or on rocks (Hymeneliaceae).
Many lineages across the Lecanoromycetes are associated with one photobiont type, a trend especially visible in trentepohlioid lichens in the Ostropales, where only a few have switched to the coccoid type, (e.g., Phlyctidaceae or Graphidaceae), or have lost their photobiont completely (Stictidaceae and Odontotremataceae s.str.; Lutzoni et al., 2001; Wedin et al., 2005, 2006; Baloch et al., 2012). The same applies to the large lineage comprising the Parmeliaceae and affiliated families in the Lecanorineae or in the Umbilicariales, which are strictly associated with Trebouxia/Asterochloris as the main photobiont according to published records. The multilocus evolutionary study by Dal Grande et al. (2014) demonstrated that the algal genus Dictyochloropsis associated with macrolichens from the family Lobariaceae (Peltigerales) is polyphyletic and that members of a single clade (a putative new genus) are involved in symbiotic associations with mycobionts from this family and other lichen genera in Lecanoromycetes (Brigantaea and Megalospora [Teloschistales], Phlyctis [Ostropales], and Biatora [Ramalinaceae, Lecanorales]. Usually a single photobiont has been reported to be associated with a mycobiont in bi-membered symbioses (e.g., Dal Grande et al, 2014); however, recently multiple green algae in addition to their main photobiont were isolated from the thalli of selected species of Micarea and Placynthiella in Ukraine (del Hoyo et al., 2011, Voytsekhovich et al., 2011c).
Cyanobacteria as primary photobionts are restricted to the order Peltigerales in the Lecanoromycetes, and two unrelated lineages in the Ostropomycetidae: Arctomiaceae (most of which grow on bryophytes in Arctic and subarctic areas) and in Petractis clausa, the only cyanobacteria-associating member of this genus as currently circumscribed (a genus which is primarily associated with Trentepohlia, as are most chlorolichens in the order Ostropales). Nostoc is present in all families of the order Peltigerales, but only in a few of them as the exclusive primary cyanobiont (for example in the Collemataceae, Massalongiaceae, Nephromataceae, Peltigeraceae, and Vahliellaceae). In addition to Nostoc, other filamentous cyanobacteria are associated with mycobionts in all lineages of Collematineae (except Collemataceae) and a single family (Lobariaceae) in the suborder Peltigerineae. The only other lichen lineage with cyanobacteria as the primary photobiont (the class Lichinomycetes) is associated with various filamentous and coccoid cyanobacteria (e.g., Gleocapsa and Anacystis) other than Nostoc, except for Lemphollema, which is associated with the latter.
Tri-membered associations with cyanobacteria as secondary photobionts (within cephalodia) in addition to green algae, are very rare outside of the Peltigerales suborder Peltigerineae (Lobariaceae, Peltigeraceae, and Nephromataceae) and the Pannariaceae (Collematineae). They can be found in other genera within the Lecanoromycetidae such as in the genera Pilophorus (Cladoniaceae), Stereocaulon (Stereocaulaceae), Amygdalaria, and Rhizocarpon (R. hensseniae; not sampled in this study), as well as in several genera in the Pilocarpaceae. Cephalodia can also be found in the subclass Ostropomycetidae, for example in the genera Coccotrema (Coccotremataceae, Pertusariales) and Placopsis (Trapeliaceae, Trapeliales). Micarea assimilata and M. incrassata, two of the cephalodiate members of the Pilocarpaceae, are shown with high confidence to belong to the Lecanoraceae based on two out of the four datasets analyzed (Fig. 1I). All tri-membered lichens in the Peltigerales contain exclusively Nostoc as the secondary photobiont despite the presence of other types of cyanobacteria as the primary photobionts in members of many families in this order. With a few exceptions, for example Placopsis from Trapeliaceae (Raggio et al., 2012) and Stereocaulon from Sterocaulaceae (Voytsekhovich et al, 2011b), Nostoc was rarely reported to be an accessory photobiont outside of the Peltigerales (instead, filamentous cyanobacteria are present). According to the mycobiont phylogeny (Fig. 2), cyanobacteria were acquired independently several times as the secondary photobiont in the predominantly chlorococcoid lichen lineages outside of the Peltigerales, twice in the Lecanorales (Lecanoromycetidae), and twice in the Ostropomycetidae (Trapeliaceae and Coccotremataceae). With the exception of tripartite Pannariaceae having Myrmecia as the primary photobiont (Oksner, 1974; Spribille and Muggia, 2012), none of the other members of the Collematineae form symbiotic associations with green algae, whereas in the Peltigerineae, Coccomyxa/Pseudococcomyxa are the primary photobiont in several lineages within a clade comprising the Nephromataceae, Peltigeraceae and Lobariaceae. In the latter family, Dictyochloropsis and other green algae are also involved as primary photobionts (Fig. 2). In the genus Peltigera, the acquisition of Coccomyxa/Pseudococcomyxa took place only once, leading to the diversification of tri-membered species within a single well supported clade (sections Chloropeltigera, Peltidea, and Phlebia) together with two bi15 membered taxa, P. malacea and P. frippii; often expressing a high level of specificity toward Nostoc (O’Brien et al., 2013). If the recent discovery that some bi-membered cyanobacterial lichens (Pseudocyphellaria, Sticta and Peltigera) are cryptic tri-membered symbioses in which the mycobiont has two ‘co-primary photobionts’, a cyanobacterium (dominant) and a green alga (Henskens et al, 2013), is confirmed, and the identity of the cryptic algae revealed, this might change profoundly our understanding of the evolution of symbiotic associations in Peltigerales and other groups of lichens.
It is very likely that Scytonema, reported from many lichens as a primary cyanobiont based on visual observations, represents another genus including a novel symbiotic lineage, Rhizonema, which was identified in Stereocaulon, Coccocarpia and basidiolichens (Dictyonema and Acantholichen) collected in the tropics. Rhizonema was predicted to be also associated with members of the Pannariaceae, Bacidina spp., and selected genera in the Pilocarpaceae as an accessory photobiont (Lücking et al., 2009).
In corroboration with phenotypic synapomorphies, photobionts can be helpful in the delimitation of taxa at the family and higher ranks. For example the Teloschistales, Letrouitineae and Megalosporaceae are associated with Dictyochloropsis (Gaya pers. comm.), contrary to the members of the Teloschistaceae, which are associated with Trebouxia. In the order Lecanorales, members of the family Psilolechiaceae have a strong preference for stichococcoid algae, a group of photobionts most probably corresponding to the green algal genus Diplosphaera (see Thüs et al., 2011). Photobiont-mycobiont associations can also explain in part relationships and evolutionary history of Lecanoromycetes as suggested earlier by Rambold et al. (1998). However, since 2006 no significant progress has been made, with the exception of Lücking et al. (2009), Otalora et al. (2010), O’Brien et al. (2013) and Dal Grande et al. (2014) toward an inventory of chloro- and cyanobionts in the Lecanoromycetes using molecular markers. A good example to follow is the survey of photobionts across Verrucariales (Eurotiomycetes) completed recently by Thüs et al. (2011).
4. Conclusions
Figure 5 represents a revised classification based on published phylogenies and our results. Our broad scale phylogeny (Fig. 1) demonstrated that monophyly of taxa (genera, families, and orders) in the Lecanoromycetes should be assessed phylogenetically within a broader taxonomic framework than is assumed based on their current classifications. Examples include remnants of the Zahlbruckner’s families waiting for comprehensive taxonomical treatments such as Catillariaceae and Lecideaceae, which are spread across the Lecanoromycetidae; members of Ramalinaceae and Pilocarpaceae placed in different families in the Lecanorales; and genera with members scattered in the Lecanoromycetidae. To reach this goal, a drastic improvement in the robustness of future phylogenies on the Lecanoromycetes is needed.
Figure 5.
Schematic representation of phylogeny and classification of the class Lecanoromycetes based on Gueidan et al. (2014) and modified according to relationships resulting from this study and other most recent studies. Phylogenetic relationships among families, orders and subclasses were compiled based on published phylogenies and are shown as resolved if reported with posterior probability ≥ 95% and/or maximum likelihood bootstrap ≥70% in multiple studies (in most cases). The number of recognized families in each order is provided in parentheses after the order name. Bars across branches indicate families with unknown placement (not sampled, not monophyletic, not supported) in the Lecanoromycetes, Lecanoromycetidae and Ostropomycetidae. Stars after family names indicate that they are not represented in our datasets due to lack of DNA sequences (Pachyascaceae and Calycidiaceae) or are represented by a single locus in GenBank. Question marks indicate that Dactylosporaceae and Strangosporaceae may be placed outside of the Lecanoromycetes (as an independent lineage in the Eurotiomycetes; according to Diederich et al., 2013; Schoch et al., 2009; Rossman et al., 2010), as well as Pycnoraceae (according to this study). A plus sign indicates that the family Eigleraceae might need to be resurrected. Sarea resinae (Trapeliales, Lecanoromycetidae) and Biatoridium (Lecanoromycetes inc. sed.) are not depicted here.
We predict, that expanding our datasets by adding taxa with a single gene will reduce the stability of phylogenetic relationships (Fig. 4) due to the lack of characters for these taxa (see Wiens and Morrill, 2011) paired with an increasing number of very short internodes (Supplemental Fig. S1). This was already demonstrated in this study by adding taxa with two genes to the 5+4+3-gene dataset used in Miadlikowska et al. (2006), as well as in the study by Schmull et al. (2011). Although previous studies reported a poor relationship between intermodal support and the amount of missing data when adding taxa under certain conditions (if the remaining characters are invariant or saturated and/or when obvious rate heterogeneity is ignored, e.g., Pyron and Wiens, 2011; Wiens and Morrill, 2011), we noticed that in the case of Lecanoromycetes, bootstrap support for the clades containing mainly taxa with two loci only was generally lower compare to clades with more genes. This difference can be explained in part by the fact that our datasets contained a maximum of five loci (versus 12 in Pyron and Wiens, 2011) and only two of them were single copy protein coding genes (versus 10 in Pyron and Wiens, 2011). Most individuals in our dataset (except the complete 5-gene) were represented exclusively by ribosomal loci, mainly nucLSU and mitSSU (RPB1 and RPB2 were absent in 59% and 67%, respectively, of all OTUs included in this study), which provided only 20% of the complete set of the targeted 7,000 bp (5-gene dataset; Table 1) versus 12,712 bp in Pyron and Wiens (2011). Ribosomal loci, although commonly used for reconstrucing phylogenies of various groups in Lecanoromycetes and fungi in general, have relatively low phylogenetic informativeness (sensu Townsend 2007), and therefore provide less phylogenetic resolving power for resolving relationships across the Lecanoromycetes and Ascomycota compared to protein-coding genes (Hofstetter et al., 2007; Schoch et al., 2009). By using a cumulative supermatrix approach (Miadlikowska et al., 2006; Gaya et al., 2012) we were able to monitor the joint impact of the increasing number of taxa and missing sequences on the stability of the nodes (Fig. 1).
Many of the clades that were weakly supported in this study (including the most complete dataset) were also poorly supported in other studies focusing on specific groups witin the Lecanoromycetes, and thus potentially with less missing data. The same is true for strongly supported clades. Sequencing efforts for phylogenetic studies of Lecanoromycetes and fungi in general cannot be limited anymore to the easily amplified ribosomal RNA-coding genes, but should be extended to include protein-coding genes such as MCM7, RPB1 and RPB2 for at least one representative from each genus. In systematic biology, new molecular markers, such as MCM7 (e.g., for the Ostropomycetidae in Schmitt et al., 2010; Collemataceae in Otálora et al., 2013a, 2013b) and markers derived from the AFToL 2 project (aftol.org), are rarely developed and sequenced as part of studies on Lecanoromycetes. Yet, protein-coding genes have been shown to have a major positive impact on phylogenetic resolution and confidence for Lecanoromycetes (Reeb et al., 2004; Hofstetter et al., 2007), but can also contribute to highly supported conflicting relationships (see Otálora et al, 2013a, 2013b versus this study).
Currently, the need for more genes (characters) is higher than for more taxa for large31 scale phylogenetic studies within Lecanoromycetes (Fig. 4 and Supplemental Fig. S2). This issue will be addressed, increasingly, with genomic sequencing directly from (environmental) genomic DNA obtained from lichen thalli (the feasibility of which has been demonstrated by McDonald et al., 2013). Nevertheless, in parallel to sequencing more genes, taxon sampling should be increased to include species (with type species preferably) from genera and families, which were never subjected to phylogenetic analyses (e.g., Biatorellaceae) or are underrepresented in existing phylogenies (e.g., Catillariaceae). Phylogenetic studies of lichens within the broader context of Leotiomyceta, including newly described classes (e.g., Xylonomycetes in Gazis et al., 2012 and Coniocybomycetes in Prieto et al., 2012), are also needed for a stable and meaningful delimitation of the Lecanoromycetes and the accurate phylogenetic placement of several lichenized lineages that do not belong to this class (Figs. 1 and 2). It is also within the broader context of the Leotiomyceta that a better understanding of the origin of lichen symbiosis can be achieved.
In large-scale phylogenetic studies (i.e., high number of taxa and genes) the only viable support values currently available to estimate phylogenetic confidence seems to come from non15 parametric bootstrap datasets, especially when analyzed with maximum likelihood as the optimization criterion. Conflicts that receive high bootstrap values with ML are rarely observed across phylogenetic studies of the Lecanoromycetes. However, this is not the case for internodes with high posterior probabilities, where most well supported conflicts are observed. Phylogenetic trees including large numbers of taxa have high numbers of very short internodes. Simulation studies have shown that current Bayesian methods can give high posterior probabilities for wrong relationships (Alfaro et al. 2003, Lewis et al. 2005), especially for short internodes. Moreover, current implementations of Bayesian phylogenetic methods cannot be completed on datasets with large number of taxa, due to a lack of convergence by Monte Carlo Markov chains. As shown here with our cumulative supermatrix approach (Miadlikowska et al. 2006, Gaya et al. 2012), different combinations of taxa and genes can yield different levels of supports (Figs. 1 and 2). Therefore, phylogenetic studies can be more inclusive, in terms of taxa with fewer genes, and more conclusive, if multiple data sets (such as the four data sets used here; i.e., 5-gene, 5+4- gene, 5+4+3-gene and 5+4+3+2-gene datasets) are used for bootstrap analyses instead of a single ML bootstrap analysis.
Supplementary Material
Phylogram representing phylogenetic relationships among 1307 putative members of the Lecanoromycetes based on maximum likelihood analyses of the combined mitSSU, nucLSU, nucSSU, RPB1 and RPB2 sequences (5+4+3+2-locus dataset) and 10 species used as outgroup (Geoglossomycetes, Lichinomycetes and Leotiomycetes). Numbers (0, 1, 2) after taxon names indicate presence of multiple specimens from the same taxon. The four-box grids associated with each internode indicate maximum likelihood bootstrap support based on different datasets. Black boxes indicate bootstrap support ≥70%; white boxes indicate bootstrap support <70%, blue boxes indicate cases where internodal support is not applicable due to at least one of the (usually two) immediately downstream branches being absent (due to missing taxa) or the node was not recovered by the bootstrap analysis (not present in the majority-rule consensus tree with all compatible groupings included; 1% treshold); and red boxes indicate conflicting relationships at bootstrap support ≥70%.
Non-significant correlation (p = 0.055) between the number of internodes within each recognized lineage (Fig. 2) and the percentage of internodes with BS-ML ≥ 70%, based on results from bootstrap analyses on 5-gene, 5+4-gene, 5+4+3-gene, and 5+4+3+2-gene datasets.
Large-scale molecular phylogenetic synthesis of the third largest class of fungi.
Based on a cumulative supermatrix approach of > 1100 species representing all orders.
Up to ca. 8 kb from four nuclear and one mitochondrial genes were sequenced per species.
Comprehensive revision of classification including 74 taxonomic conclusions.
A new module “Hypha” of the Mesquite software is introduced.
Acknowledgements
We thank T. Milledge and J. Pormann for their assistance with the Duke DSCR computer cluster, L. Bukovnik (Duke IGSP) for assistance with DNA sequencing, members of the Lutzoni lab for helpful comments and suggestions, M. McMullen (Duke University Herbarium) for curating lichen specimens and proofreading the manuscript, W. R. Buck and collaborators in the Assembling the Fungal Tree of Life (AFToL) project for providing lichen specimens, R. Harris and T. Ahti for verifying identifications of selected specimens, and reviewers for helpful comments on the manuscript. This publication was supported by NSF awards DEB-0228668, DEB-0640956 and DEB-0919455 to FL, DEB-0732984 to FL and JM, DEB-0640996 to AEA and the Academy of Finland (grant 211171) to SS. Additional financial support comes from NSF award DEB- 1025930 to JM and FL, DEB-11455511 to JL, and the Intramural Research Program of the NIH, National Library of Medicine to CLS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alfaro M, Zoller S, Lutzoni F. Bayes or bootstrap? A simulation study comparing the performance of Bayesian Markov chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Mol. Biol. Evol. 2003;20:255–266. doi: 10.1093/molbev/msg028. [DOI] [PubMed] [Google Scholar]
- Andersen HL, Ekman S. Disintegration of the Micareaceae (lichenized Ascomycota): a molecular phylogeny based on mitochondrial rDNA sequences. Mycol. Res. 2005;109:21–30. doi: 10.1017/s0953756204001625. [DOI] [PubMed] [Google Scholar]
- Arcadia LI, Knudsen K. The name Myriospora is available for the Acarospora smaragdula group. Opuscula Philolichenum. 2012;11:19–25. [Google Scholar]
- Arnold AE, Miadlikowska J, Higgins KL, Sarvate SD, Gugger P, Way A, Hofstetter V, Kauff F, Lutzoni F. A phylogenetic estimation of trophic transition networks for ascomycetous fungi: are lichens cradles of symbiotrophic fungal diversification? Syst. Biol. 2009;58:283–297. doi: 10.1093/sysbio/syp001. [DOI] [PubMed] [Google Scholar]
- Arup U, Ekman S, Grube M, Mattsson JE, Wedin M. The sister group relation of Parmeliaceae (Lecanorales, Ascomycota) Mycologia. 2007;99:42–49. doi: 10.3852/mycologia.99.1.42. [DOI] [PubMed] [Google Scholar]
- Arup U, Søchting U, Frödén P. A new taxonomy of the family Teloschistaceae. Nord. J. Bot. 2013;31:016–083. [Google Scholar]
- Backor M, Peksa O, Skaloud P, Backorova M. Photobiont diversity in lichens from metal-rich substrata based on ITS rDNA sequences. Ecotoxicology and Environmental Safety. 2010;73:603–612. doi: 10.1016/j.ecoenv.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Baloch E, Gilenstam G, Wedin M. The relationships of Odontotrema (Odontotremataceae) and the resurrected Sphaeropezia (Stictidaceae) - new combinations and three new Sphaeropezia species. Mycologia. 2012 doi: 10.3852/12-134. [DOI] [PubMed] [Google Scholar]
- Baloch E, Lücking R, Lumbsch TH, Wedin M. Major clades and phylogenetic relationships between lichenized and non-lichenized lineages in Ostropales (Ascomycota: Lecanoromycetes) Taxon. 2010;59:1483–1494. [Google Scholar]
- Beck A. Univ. München. Germany: München; 2002. Selektivität der Symbionten schwermetalltoleranter Flechten [doctoral dissertation] [Google Scholar]
- Bendiksby M, Timdal E. Molecular phylogenetics and taxonomy of Hypocenomyce sensu lato (Ascomycota, Lecanoromycetes) — extreme polyphyly and morphological/ecological convergence. Taxon. 2013;62:940–956. [Google Scholar]
- Brodo IM. Rhizocarpon hensseniae, a cephalodiate lichen from the Northwest coast of North America. Biblioth. Lichenol. 1990;38:29–35. [Google Scholar]
- Brodo I, Sharnoff SD, Sharnoff S. Lichens of North America. New Haven, London: Yale Univ Press; 2001. [Google Scholar]
- Brodo IM, Vitikainen O. The typification of Lecanora subfusca (L.) Ach., its varieties, and some of its related taxa published before 1850. Mycotaxon. 1984;21:281–298. [Google Scholar]
- Buschbom J, Mueller G. Resolving evolutionary relationships in the lichen-forming genus Porpidia and related allies (Porpidiaceae, Ascomycota) Mol. Phylogenet. Evol. 2004;32:66–82. doi: 10.1016/j.ympev.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Buschbom J, Barker Evolutionary history of vegetative reproduction in Porpidia s.l. (Lichen-forming ascomycota) Syst. Bio. 2006;55:471–484. doi: 10.1080/10635150600697465. [DOI] [PubMed] [Google Scholar]
- Bylin A, Arnerup J, Högberg N, Thor G. A phylogenetic study of Fuscideaceae using mtSSU rDNA. In: Frisch A, Lange U, Staiger B, editors. Lichenologische Nebenstunden. Contributions to lichen taxonomy and ecology in honour of Klaus Kalb. Biblioth. Lichenol. Vol. 96. 2007. pp. 49–60. [Google Scholar]
- Cock PJA, Antao T, Chang JT, Chapman BA, Cox CJ, Dalke A, Friedberg I, Hamelryck T, Kauff F, Wilczynski B, de Hoon MJL. Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics. 2009;25:1422–1423. doi: 10.1093/bioinformatics/btp163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppins BJ. A taxonomic study of the lichen genus Micarea in Europe. Bull. Br. Mus. Nat. Hist. (Bot.) 1983;11:17–314. [Google Scholar]
- Coppins BJ, Purvis OW. A review of Psilolechia. Lichenologist. 1987;19:29–42. [Google Scholar]
- Crespo A, Kauff F, Divakar PK, del Prado R, Pérez-Ortega S, de Paz GA, Ferencova Z, Blanco O, Roca-Valiente B, Núñez-Zapata J, Cubas P, Argüello A, Elix JA, Esslinger TL, Hawksworth DL, Millanes AM, Molina MC, Wedin M, Ahti T, Aptroot A, Barreno E, Bungartz F, Calvelo S, Candan M, Cole MJ, Ertz D, Goffinet B, Lindblom L, Lücking R, Lutzoni F, Mattsson JE, Messuti MI, Miadlikowska J, Piercey-Normore MD, Rico VJ, Sipman H, Schmitt I, Spribille T, Thell A, Thor G, Upreti DK, Lumbsch HT. Phylogenetic classification of parmelioid lichens (Parmeliaceae, Ascomycota) based on molecular, morphological and chemical evidence. Taxon. 2010;59:1735–1753. [Google Scholar]
- Crespo A, Lumbsch HT, Mattsson JE, Blanco O, Divakar PK, Articus K, Wiklund E, Bawingan PA, Wedin M. Testing morphology-based hypotheses of phylogenetic relationships in Parmeliaceae (Ascomycota) using three ribosomal markers and the nuclear RPB1 gene. Mol. Phylogenet. Evol. 2007;44:812–824. doi: 10.1016/j.ympev.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Crewe AT, Purvis OW, Wedin M. Molecular phylogeny of Acarosporaceae (Ascomycota) with focus on the proposed genus Polysporinopsis. Mycol. Research. 2006;110:521–526. doi: 10.1016/j.mycres.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Culberson WL, Culberson CF. Secondary metabolites as a tool in ascomycete systematics: lichenized fungi. In: Hawksworth DL, editor. Ascomycete Systematics: problems and perspective in the nineties. New York: Plenum Press; 1994. pp. 155–163. [Google Scholar]
- Dal Grande F, Beck A, Cornejo C, Singh G, Cheenacharoen S, Nelsen M, Scheidegger C. Molecular phylogeny and symbiotic selectivity of the green algal genus Dictyochloropsis s.l. (Trebouxiophyceaceae): a polyphyletic and widespread group forming photobiont-mediated guilds in the lichen family Lobariaceae. New Phytologist. 2014 doi: 10.1111/nph.12678. [DOI] [PubMed] [Google Scholar]
- Diederich P, Ertz D, Lawrey JD, Sikaroodi M, Untereiner WA. Molecular data place the hyphomycetous lichenicolous genus Sclerococcum close to Dactylospora (Eurotiomycetes) and S. parmeliae in Cladophialophora (Chaetothyriales) Fungal Divers. 2013 [Google Scholar]
- Divakar PK, Crespo A, Blanco O, Lumbsch HT. Phylogenetic significance of morphological characters in the tropical Hypotrachyna clade of parmelioid lichens (Parmeliaceae, Ascomycota) Mol. Phylogen. Evol. 2006;40:448–458. doi: 10.1016/j.ympev.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Divakar PK, Del-Prado R, Lumbsch HT, Wedin M, Esslinger TL, Leavitt SD, Crespo A. Diversification of the newly recognized lichen-forming fungal lineage Montanelia (Parmeliaceae, Ascomycota) and its relation to key geological and climatic events. Am. J. Bot. 2012;99:2014–2016. doi: 10.3732/ajb.1200258. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman S. The corticolous and lignicolous species of Bacidia and Bacidina in North America. Opera Botanica. 1996;127:1–148. [Google Scholar]
- Ekman S, Andersen HL, Wedin M. The limitations of ancestral state reconstruction and the evolution of the ascus in the Lecanorales (lichenized Ascomycota) Syst. Biol. 2008;57:141–156. doi: 10.1080/10635150801910451. [DOI] [PubMed] [Google Scholar]
- Elix JA, Stocker-Wörgötter E. Biochemistry and secondary metabolites. In: Nash TH III, editor. Lichen biology. Cambridge: Cambridge University Press; 2008. pp. 104–133. [Google Scholar]
- Engelen A, Convey P, Ott S. Life history of Lepraria borealis at an Antarctic inland site, Coal Nunatak. Lichenologist. 2010;42:339–346. [Google Scholar]
- Eriksson OE. Outline of Ascomycota - 2006. Myconet. 2006;12:1–82. [Google Scholar]
- Eriksson OE, Winka K. Supraordinal taxa of Ascomycota. Myconet. 1997;1:1–16. [Google Scholar]
- Ertz D, Fischer E, Killmann D, Razafindrahaja T, Sérusiaux E. Savoronala, a new genus of Malmideaceae (Lecanorales) from Madagascar with stipes producing sporodochia. Mycol. Progress. 2013;12:645–656. [Google Scholar]
- Fedorenko NM, Stenroos S, Thell A, Kärnefelt I, Kondratyuk SY. A phylogenetic analysis of xanthorioid lichens (Teloschistaceae, Ascomycota) based on ITS and mtSSU sequences. Bibl. Lichen. 2009;100:49–84. [Google Scholar]
- Fedorenko NM, Stenroos S, Thell A, Kärnefelt I, Elix JA, Hur J–S, Kondratyuk SY. Molecular phylogeny of xanthorioid lichens (Teloschistaceae, Ascomycota), with notes on their morphology. In: Kärnefelt I, Seaward MRD, Thell A, editors. Systematics, biodiversity and ecology of lichens. Bibloth. Lichenol. Vol. 108. 2012. pp. 58–76. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. J. Mol. Evol. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fernández-Brime S, Llimona X, Lutzoni F, Gaya E. Phylogenetic study of Diploschistes (lichen-forming Ascomycota: Ostropales: Graphidaceae), based on morphological, chemical, and molecular data. Taxon. 2013;62:267–280. [Google Scholar]
- Fernández-Mendoza F, Domaschke S, García MA, Jordan P, Martín MP, Printzen C. Population structure of mycobionts and photobionts of the widespread lichen Cetraria aculeata. Mol. Ecol. 2011;20:1208–1232. doi: 10.1111/j.1365-294X.2010.04993.x. [DOI] [PubMed] [Google Scholar]
- Frey E. Familie Umbilicariaceae. Dr L Rabenhorst’s Kryptogamen-Flora von Deutschland, Österreich und der Schweiz. (2 ed.) 1933:203–426. Leipzig, Bd. 9, Abt. IV/1, Pl. 4–8. [Google Scholar]
- Friedl T, Büdel B. Photobionts. In: THI II, editor. Lichen biology. Cambridge: Cambridge University Press; 2008. pp. 9–28. [Google Scholar]
- Fries TM. Lichenes arctoi Europae Groenlandiaeque, hactenus cogniti. Nova Acta Reg. Soc. Sci. Upsal. 3 ser. 1860;3:103–398. [Google Scholar]
- Fryday AM, Lendemer JC. Reassessment of the genus Catillochroma (lichenized Ascomycota, Ramalinaceae) Lichenologist. 2010;42:587–600. [Google Scholar]
- Galtier N, Gouy M, Gautier C. SeaView and Phylo_win, two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 1996;12:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- Gaya E, Högnabba F, Holguin Á, Molnar K, Fernández-Brime S, Stenroos S, Arup U, Søchting U, van den Boom P, Lücking R, Sipman HJM, Lutzoni F. Implementing a cumulative supermatrix approach for a comprehensive phylogenetic study of the Teloschistales (Pezizomycotina, Ascomycota) Mol. Phylogenet. Evol. 2012;63:374–387. doi: 10.1016/j.ympev.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Gazis R, Miadlikowska J, Lutzoni F, Arnold AE, Chaverri P. Culture-based study of endophytes associated with rubber trees in Peru reveals a new class of Pezizomycotina: Xylonomycetes. Mol. Phylogenet. Evol. 2012;65:294–304. doi: 10.1016/j.ympev.2012.06.019. [DOI] [PubMed] [Google Scholar]
- Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- Grube M, Baloch E, Lumbsch HT. The phylogeny of Porinaceae (Ostropomycetidae) suggests a neotenic origin of perithecia in Lecanoromycetes. Mycol. Res. 2004;108:1111–1118. doi: 10.1017/s0953756204000826. [DOI] [PubMed] [Google Scholar]
- Gueidan C, Hill DJ, Miadlikowska J, Lutzoni F. Pezizomycotina: Lecanoromycetes. In: McLaughlin DJ, Spatafora JW, editors. Volume VII of The Mycota Part B. Berlin, Germany: Springer Verlag; 2014. pp. XX–XX. In press. [Google Scholar]
- Hafellner J. Studien in Richtung einer natürlicheren Gliederung der Sammelfamilien Lecanoraceae und Lecideaceae. In: Hertel H, Oberwinkler F, editors. Beiträge zur Lichenologie. Festschrift J. Poelt. Beiheft zur Nova Hedwigia 79. J. Cramer, Vaduz. 1984. pp. 241–371. [Google Scholar]
- Hafellner J. Towards a better circumscription of the Acarosporaceae (lichenized Ascomycotina, Lecanorales) Crypt. Bot. 1995;5:99–104. [Google Scholar]
- Hafellner J, Türk R. Die lichenisierten Pilze Österreichs - eine Checkliste der bisher nachgewiesenen Arten mit Verbreitungsangaben. Stapfia. 2001;76:1–167. [Google Scholar]
- Halonen P, Myllys L, Velmala S, Hyvärinen H. Gowardia (Parmeliaceae) - a new alectorioid genus with two species. Bryologist. 2009;112:138–146. [Google Scholar]
- Harris RC. Four novel lichen taxa in the lichen biota of eastern North America. Opuscula Philolichenum. 2009;6:149–156. [Google Scholar]
- Harris RC, Brodo IM, Tønsberg T. Lecanora thysanophora, a common leprose lichen in Eastern North America. Bryologist. 2000;103:790–793. [Google Scholar]
- Harris RC, Knudsen K. The genus Myriospora. Opuscula Philolichenum. 2005;3:1–4. [Google Scholar]
- Helms G, Friedl T, Rambold G. Phylogenetic relationships of the Physciaceae inferred from rDNA sequence data and selected phenotypic characters. Mycologia. 2003;95:1078–1099. doi: 10.1080/15572536.2004.11833022. [DOI] [PubMed] [Google Scholar]
- Henskens FL, Green TGA, Wilkins A. Cyanolichens can have both cyanobacteria and green algae in a common layer as major contributors to photosynthesis. Annals of Botany. 2013 doi: 10.1093/aob/mcs108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henssen A. Eine Studie über die Gattung Arctomia. Svensk Bot. Tidskr. 1969;63:126–138. [Google Scholar]
- Hertel H. Trapeliaceae--eine neue Flechtenfamilie. Deutsch. Bot. Ges. N.F. 1970;4:171–185. [Google Scholar]
- Hestmark G, Miadlikowska J, Kauff F, Fraker E, Molnar K, Lutzoni F. Single origin and subsequent diversification of central Andean endemic Umbilicaria species. Mycologia. 2011;103:45–56. doi: 10.3852/10-012. [DOI] [PubMed] [Google Scholar]
- Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, Lumbsch HT, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Kõljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schüßler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao YJ, Zhang N. A higher-level phylogenetic classification of the fungi. Mycol. Res. 2007;111:509–547. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Hodkinson BP, Lendemer JC. The orders of Ostropomycetidae (Lecanoromycetes, Ascomycota): Recognition of Sarrameanales and Trapeliales with a request to retain Pertusariales over Agyriales. Phytologia. 2011;93:407–412. [Google Scholar]
- Hofstetter V, Miadlikowska J, Kauff F, Lutzoni F. Phylogenetic comparison of protein-coding versus ribosomal RNA-coding sequence data: a case study of the Lecanoromycetes (Ascomycota) Mol. Phylogenet. Evol. 2007;44:412–426. doi: 10.1016/j.ympev.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Houmeau JM, Roux C. Hafellnera Houmeau et Roux gen. nov. genre nouveau de lichen. Bull. Soc. Bot. Centre-Ouest, Nouv. Ser. 1984;15:142. [Google Scholar]
- Högnabba F, Stenroos S, Thell A. Phylogenetic relationships and evolution of photobiont associations in the Lobariaceae (Peltigerales, Lecanoromycetes, Ascomycota) Bibl. Lichen. 2009;100:157–187. [Google Scholar]
- del Hoyo A, Álvarez R, del Campo EM, Gasulla F, Barreno E, Casano LM. Oxidative stress induces distinct physiological responses in the two Trebouxia phycobionts of the lichen Ramalina farinacea. Annals of Botany. 2011;107:109–118. doi: 10.1093/aob/mcq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huneck S, Yoshimura I. Identification of lichen substances. Springer-Verlag: Berlin, Heidelberg; 1996. [Google Scholar]
- James TY, Kauff F, Schoch C, Matheny PB, Hofstetter V, Cox CJ, Celio G, Gueidan C, Fraker E, Miadlikowska J, Lumbsch T, Rauhut A, Reeb V, Arnold AE, Amtoft A, Stajich JE, Hosaka K, Sung G-H, Johnson D, O’Rourke B, Binder M, Curtis JM, Slot JC, Wang Z, Wilson AW, Schüßler A, Longcore JE, O’Donnell K, Mozley–Standridge S, Porter D, Letcher PM, Powell MJ, Taylor JW, White MM, Griffith GW, Davies DR, Sugiyama J, Rossman AY, Rogers JD, Pfister DH, Hewitt D, Hansen K, Hambleton S, Shoemaker RA, Kohlmeyer J, Volkmann–Kohlmeyer B, Spotts RA, Serdani M, Crous PW, Hughes KH, Matsuura K, Langer E, Langer G, Untereiner WA, Lücking R, Büdel B, Geiser DM, Aptroot A, Buck WR, Cole MS, Diederich P, Printzen Ch, Schmitt I, Schultz M, Yahr R, Zavarzin A, Hibbett DS, Lutzoni F, McLaughlin DJ, Spatafora JW, Vilgalys R. Reconstructing the early evolution of the fungi using a six–gene phylogeny. Nature. 2006;443:818–822. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- Kalb K, Rivas Plata E, Lücking R, Lumbsch HT. The phylogenetic position of Malmidea, a new genus for the Lecidea piperis- and Lecanora granifera-groups (Lecanorales, Malmideaceae), inferred from nuclear and mitochondrial ribosomal DNA sequences, with special reference to Thai species. Bibl. Lichen. 2011;106:143–168. [Google Scholar]
- Kalb K, Staiger B, Elix JA, Lange U, Lumbsch HT. A new circumscription of the genus Ramboldia (Lecanoraceae, Ascomycota) based on morphological and molecular evidence. Nova Hedwigia. 2008;86:23–42. [Google Scholar]
- Kantvilas G. Sarrameanaceae. In: McCarthy PM, Mallett K, editors. Flora of Australia, vol 56A. Lichens 4. Australia, Melbourne: ABRS/CSIRO; 2004. pp. 74–77. [Google Scholar]
- Kantvilas G, Elix JA. Ramboldia, a new genus in the lichen family Lecanoraceae. Bryologist. 1994;97:296–304. [Google Scholar]
- Kauff F, Cox CJ, Lutzoni F. WASABI: An automated sequence processing system for multigene phylogenies. Syst. Biol. 2007;56:523–531. doi: 10.1080/10635150701395340. [DOI] [PubMed] [Google Scholar]
- Kauff F, Lutzoni F. Phylogeny of the Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships. Mol. Phylogenet. Evol. 2002;25:138–156. doi: 10.1016/s1055-7903(02)00214-2. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. Ainsworth & Bisby's dictionary of the fungi. 10th ed. Oxon, UK: CABI, Wallingford; 2008. [Google Scholar]
- Knudsen K, Kocourkova Lichenological notes 3: Sarcogyne plicata in California. Mycotaxon. 2011;118:423–431. [Google Scholar]
- Knudsen K, Lendemenr J. Two new species of Lecanora with gyrophoric acid from North America. Opuscula Philolichenum. 2009;7:21–28. [Google Scholar]
- Kondratyuk SY, Kärnefelt I. Revision of three natural groups of xanthorioid lichens (Teloschistaceae, Ascomycota) Ukr. Bot. J. 2003;60:427–437. [Google Scholar]
- Körber GW. Systema lichenum Germaniae. Systema Lichenum Germaniae. Die Flechten Deutschlands (insbesondere Schlesiens) mikroskopisch geprüft, kritisch gesichtet, characteristisch beschrieben und systematisch geordnet. Breslau. 1854–1855:1–192. 1854; p 193–459, I–XXXV, tab. 1–4 1855. [Google Scholar]
- Körber GW. Parerga lichenologica. Breslau. 1860:97–192. [Google Scholar]
- Lendemer JC, Harris RC, Tripp EA. The lichens and allied fungi of Great Smoky Mountains National Park. An annotated checklist with comprehensive keys. The New York Botanical Garden Press. Memoirs of the New York Botanical Garden. 2013;104:1–152. [Google Scholar]
- Lendemer JC, Hodkinson BP. Chirleja buckii, a new genus and species of lichenized fungi from Tierra del Fuego, southern South America. New Zeal. J. Bot. 2012;50:449–456. [Google Scholar]
- Lendemer JC, Hodkinson BP. A radical shift in the taxonomy of Lepraria s.l.: molecular and morphological studies shed new light on the evolution of asexuality and lichen growth form diversification. Mycologia. 2013;105:994–1018. doi: 10.3852/12-338. [DOI] [PubMed] [Google Scholar]
- Lewis PO, Holder MT, Holsinger KE. Polytomies and Bayesian phylogenetic inference. Systematic Biology. 2005;54:241–253. doi: 10.1080/10635150590924208. [DOI] [PubMed] [Google Scholar]
- Lücking R, Lawrey JD, Sikaroodi M, Gillevet PM, Chaves JL, Sipman HJM, Bungartz F. Do lichens domesticate photobionts like farmers domesticate crops? Evidence from a previously unrecognized lineage of filamentous cyanobacteria. Am. J. Bot. 2009;96:1409–1418. doi: 10.3732/ajb.0800258. [DOI] [PubMed] [Google Scholar]
- Lücking R, Seavey F, Common R, Beeching SQ, Breuss O, Buck WR, Crane L, Hodges M, Hodkinson BP, Lay E, Lendemer JC, McMullin RT, Mercado-Díaz JA, Nelsen MP, Rivas Plata E, Safranek W, Sanders WB, Schaefer HP, Jr, Seavey J. The lichens of Fakahatchee Strand Preserve State Park, Florida: Proceedings from the 18th Tuckerman Workshop. Bull. Florida Mus. Nat. Hist. 2011;49:127–186. [Google Scholar]
- Lücking R, Stuart BL, Lumbsch HT. Phylogenetic relationships of Gomphillaceae and Asterothyriaceae: evidence from a combined Bayesian analysis of nuclear and mitochondrial sequences. Mycologia. 2004;96:283–294. doi: 10.1080/15572536.2005.11832978. [DOI] [PubMed] [Google Scholar]
- Lumbsch HT. A comparison of ascoma ontogeny supports the inclusion of the Eigleraceae in the Hymeneliaceae (Lecanorales) Bryologist. 1997;100:180–192. [Google Scholar]
- Lumbsch HT, Archer AW, Elix JA. A new species of Loxospora (lichenized Ascomycota: Sarrameanaceae) from Australia. Lichenologist. 2007a;39:509–517. [Google Scholar]
- Lumbsch HT, Huhndorf SM. Myconet Volume 14. Part One. Outline of Ascomycota - 2009 Part Two. Notes on Ascomycete Systematics. Nos. 4751–5113. Fieldiana Life Earth Sci. 2010;1:1–64. [Google Scholar]
- Lumbsch HT, Lunke T, Feige GB, Huneck S. Anamylopsoraceae - a new family of lichenized ascomycetes with stipitate apothecia (Lecanorales: Agyriineae) Plant Syst. Evol. 1995;198:275–286. [Google Scholar]
- Lumbsch HT, Nelsen MP, Lücking R. The phylogenetic position of Haematommataceae (Lecanorales, Ascomycota), with notes on secondary chemistry and species delimitation. Nova Hedwigia. 2008;86:105–114. [Google Scholar]
- Lumbsch HT, del Prado R, Kantvilas G. Gregorella, a new genus to accommodate Moelleropsis humida and a molecular phylogeny of Arctomiaceae. Lichenologist. 2005;37:291–302. [Google Scholar]
- Lumbsch HT, Schmitt I, Döring H, Wedin M. Molecular systematics supports the recognition of an additional order of Ascomycota: the Agyriales (Ascomycota) Mycol. Res. 2001;105:16–23. [Google Scholar]
- Lumbsch HT, Schmitt I, Lücking R, Wiklund E, Wedin M. The phylogenetic placement of Ostropales within Lecanoromycetes (Ascomycota) revisited. Mycol. Res. 2007b;111:257–267. doi: 10.1016/j.mycres.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Lumbsch HT, Schmitt I, Mangold A, Wedin M. Ascus types are phylogenetically misleading in Trapeliaceae and Agyriaceae (Ostropomycetidae, Ascomycota) Mycol. Res. 2007c;111:1133–1141. doi: 10.1016/j.mycres.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Lumbsch HT, Schmitt I, Palice Z, Wiklund E, Ekman S, Wedin M. Supraordinal phylogenetic relationships of Lecanoromycetes based on a Bayesian analysis of combined nuclear and mitochondrial sequences. Mol. Phylogenet. Evol. 2004;31:822–832. doi: 10.1016/j.ympev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Lutzoni F, Brodo IM. A generic redelimitation of the Ionaspis-Hymenelia complex (lichenized Ascomycotina) Syst. Bot. 1995;20:224–258. [Google Scholar]
- Lutzoni F, Kauff F, Cox C, McLaughlin D, Celio G, Dentinger B, Padamsee M, Hibbett D, James TY, Baloch E, Grube M, Reeb V, Hofstetter V, Schoch C, Arnold AE, Miadlikowska J, Spatafora J, Johnson D, Hambleton S, Crockett M, Shoemaker R, Sung GH, Lücking R, Lumbsch T, O'Donnell K, Binder M, Diederich P, Ertz D, Gueidan C, Hansen K, Harris RC, Hosaka K, Lim YW, Matheny B, Nishida H, Pfister D, Rogers J, Rossman A, Schmitt I, Sipman H, Stone J, Sugiyama J, Yahr R, Vilgalys R. Assembling the fungal tree of life: progress, classification, and evolution of subcellular traits. Am. J. Bot. 2004;91:1446–1480. doi: 10.3732/ajb.91.10.1446. [DOI] [PubMed] [Google Scholar]
- Lutzoni F, Pagel M, Reeb V. Major fungal lineages are derived from lichen symbiotic ancestors. Nature. 2001;411:937–940. doi: 10.1038/35082053. [DOI] [PubMed] [Google Scholar]
- Lutzoni F, Wagner P, Reeb V, Zoller S. Integrating ambiguously aligned regions of DNA sequences in phylogenetic analyses without violating positional homology. Syst. Biol. 2000;49:628–651. doi: 10.1080/106351500750049743. [DOI] [PubMed] [Google Scholar]
- Maddison DR, Maddison WP. McClade: analysis of phylogeny and character evolution. 4.07. Sunderland: Massachusetts: Sinauer Associates Inc; 2003. [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 2.75. 2011 http://mesquiteproject.org. [Google Scholar]
- Magain N, Sérusiaux E. A further new species in the lichen genus Arctomia: A borbonica from Reunion (Mascarene archipelago) MycoKeys. 2012;4:9–21. [Google Scholar]
- Magnusson AH. On the species of Biatorella and Sarcogyne in America. Ann. Crytog. Exotiq. 1935;7:115–146. [Google Scholar]
- Mason-Gamer R, Kellogg E. Testing for phylogenetic conflict among molecular datasets in the tribe Triticeae (Gramineae) Syst. Biol. 1996;45:524–545. [Google Scholar]
- McCune B, Curtis MJ. Umbilicaria semitensis (lichenized fungi: Umbilicariaceae) resurrected. Bryologist. 2012;115:255–264. [Google Scholar]
- McDonald TR, Mueller O, Dietrich FS, Lutzoni F. High-throughput genome sequencing of lichenizing fungi to assess gene loss in the ammonium transporter/ammonia permease gene family. BMC Genomics. 2013;14:225. doi: 10.1186/1471-2164-14-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miadlikowska J, Kauff F, Hofstetter V, Fraker E, Grube M, Hafellner J, Reeb V, Hodkinson BP, Kukwa M, Lücking R, Hestmark G, Otálora MG, Rauhut A, Büdel B, Scheidegger C, Timdal E, Stenroos S, Brodo IM, Perlmutter GB, Ertz D, Diederich P, Lendemer JC, May PF, Schoch C, Arnold AE, Gueidan C, Tripp E, Yahr R, Robertson C, Lutzoni F. New insights into classification and evolution of the Lecanoromycetes (Pezizomycotina, Ascomycota) from phylogenetic analyses of three ribosomal RNA- and two protein-coding genes. Mycologia. 2006;98:1088–1103. [PubMed] [Google Scholar]
- Miadlikowska J, Lutzoni F. Phylogenetic revision of the genus Peltigera (lichen-forming Ascomycota) based on morphological, chemical, and large subunit nuclear ribosomal DNA data. Int. J. Plant Sci. 2000;161:925–958. [Google Scholar]
- Miadlikowska J, Lutzoni F. Phylogenetic classification of Peltigeralean fungi (Peltigerales, Ascomycota) based on ribosomal RNA small and large subunits. Am. J. Bot. 2004;91:449–464. doi: 10.3732/ajb.91.3.449. [DOI] [PubMed] [Google Scholar]
- Moncada B, Lücking R, Betancourt L. Phylogeny of the Lobariaceae (lichenized Ascomycota: Peltigerales), with a reappraisal of the genus Lobariella. Lichenologist. 2013;45:203–263. [Google Scholar]
- Muggia L, Nelson P, Wheeler T, Yakovchenko LS, Tønsberg T, Spribille T. Convergent evolution of a symbiotic duet: The case of the lichen genus Polychidium (Peltigerales, Ascomycota) Am. J. Bot. 2011;98:1647–1656. doi: 10.3732/ajb.1100046. [DOI] [PubMed] [Google Scholar]
- Myllys L, Högnabba F, Lohtander K, Thell A, Stenroos S, Hyvönen J. Phylogenetic relationships of Stereocaulaceae based on simultaneous analysis of beta-tubulin, GAPDH and SSU rDNA sequences. Taxon. 2005;54:605–618. [Google Scholar]
- Nash TH III, Ryan BD, Diederich P, Gries C, Bungartz F, editors. Flora of the Greater Sonoran Desert Region I. Lichens Unlimited. Tempe, Arizona: Arizona State University; 2002. [Google Scholar]
- Nash TH, III, Ryan BD, Diederich P, Gries C, Bungartz F. Flora of the Greater Sonoran Desert Region. II. Lichens Unlimited. Tempe, Arizona: Arizona State University; 2004. [Google Scholar]
- Nelsen PM, Chavez N, Sackett-Hermann E, Thell A, Randlane T, Divakar P, Rico VJ, Lumbsch T. The cetrarioid core group revisited (Lecanorales: Parmeliaceae) The Lichenologist. 2011;43:537–551. [Google Scholar]
- Nelsen MP, Lumbsch HT, Lücking R, Elix JA. Further evidence for the polyphyly of Lepraria (Lecanorales: Stereocaulaceae) Nova Hedwigia. 2008;87:361–371. [Google Scholar]
- Nordin A, Savic S, Tibell L. Phylogeny and taxonomy of Aspicilia and Megasporaceae. Mycologia. 2010;102:1339–1349. doi: 10.3852/09-266. [DOI] [PubMed] [Google Scholar]
- O’Brien HE, Miadlikowska J, Lutzoni F. Assessing population structure and host specialization in lichenized cyanobacteria. New Phytologist. 2013;198:557–566. doi: 10.1111/nph.12165. [DOI] [PubMed] [Google Scholar]
- Oksner AN. Handbook of the Lichens of the USSR 2. Morphology, systematic and geographical distribution. Nauka, Leningrad, USSR. 1974:284. [Google Scholar]
- Oliver JC, Miadlikowska J, Arnold AE, Maddison DR, Lutzoni F. Hypha: a Mesquite package for support value integration. Version 1.0. 2013 http://www.mesquiteproject.org/packages/hypha. [Google Scholar]
- Otálora MAG, Aragón G, Martínez I, Wedin M. Cardinal characters on a slippery slope—a re-evaluation of phylogeny, character evolution, and evolutionary rates in the jelly lichens (Collemataceae s. str.) Mol. Phylogenet. Evol. 2013a;68:185–198. doi: 10.1016/j.ympev.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Otálora MAG, Aragón G, Molina MC, Martínez I, Lutzoni F. Disentangling the Collema-Leptogium complex through a molecular phylogenetic study of the Collemataceae (Peltigerales, lichen-forming Ascomycota) Mycologia. 2010a;102:279–290. doi: 10.3852/09-114. [DOI] [PubMed] [Google Scholar]
- Otálora MAG, Jørgensen PM, Wedin M. A revised classification of the jelly lichens, Collemataceae. Fungal Diversity. 2013b [Google Scholar]
- Otálora MAG, Martínez I, O’Brien H, Molina MC, Aragón G, Lutzoni F. Multiple origins of high reciprocal symbiotic specificity at an intercontinental spatial scale among gelatinous lichens (Collemataceae, Lecanoromycetes) Mol. Phylogenet. Evol. 2010b;56:1098–1095. doi: 10.1016/j.ympev.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Otálora MAG, Salvador C, Martínez I, Aragón G. Does the reproductive strategy affect the transmission and genetic diversity of bionts in cyanolichens? A case study uing two closely related species. Microb. Ecol. 2013c;65:517–530. doi: 10.1007/s00248-012-0136-5. [DOI] [PubMed] [Google Scholar]
- Otálora MAG, Wedin M. Collema fasciculare belongs in Arctomiaceae. Lichenologist. 2013;45:295–304. [Google Scholar]
- Palice Z, Printzen C, Spribille T, Svensson M, Tønsberg T, Urbanavichene I, Yakonchenko LS, Ekman S. Taxonomy of the genus Myrionora, with a second species from South America. Lichenologist. 2013;45:159–167. [Google Scholar]
- Parnmen S, Lücking R, Lumbsch HT. Phylogenetic classification at generic level in the absence of distinct phylogenetic patterns of phenotypical variation: A case study in Graphidaceae (Ascomycota) PLoS ONE. 2012;7:e51392. doi: 10.1371/journal.pone.0051392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peksa O, Skaloud P. Do photobionts influence the ecology of lichens? A case study of environmental preferences in symbiotic green alga Asterochloris (Trebouxiophyceae) Mol. Ecol. 2011;20:3946–3948. doi: 10.1111/j.1365-294X.2011.05168.x. [DOI] [PubMed] [Google Scholar]
- Poelt J. Classification. In: Ahmadjian V, Hale ME, editors. The lichens. New York, London: Academic Press; 1974. pp. 599–632. [Google Scholar]
- Prieto M, Baloch E, Tehler A, Wedin M. Mazaedium evolution in the Ascomycota (Fungi) and the classification of mazaediate groups of formerly unclear relationship. Cladistics. 2012 doi: 10.1111/j.1096-0031.2012.00429.x. [DOI] [PubMed] [Google Scholar]
- Printzen C. Lichen systematics: The role of morphological and molecular datat o reconstruct phylogenetic relationships. In: Lüttge U, Beysch W, Büdel B, Francis D, editors. Progress in Botany. Vol. 71. 2010. pp. 233–279. [Google Scholar]
- Purvis OW, Coppins BJ, Hawksworth DL, James PW, Moore DM, editors. The lichen flora of Great Britain and Ireland. London, UK: Natural History Museum; 1992. [Google Scholar]
- Pyron RA, Wiens JJ. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. 2011;61:543–583. doi: 10.1016/j.ympev.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Raggio J, Green TGA, Crittenden PD, Pintado A, Vivas M, Pérez-Ortega S, De los Ríos A, Sáncho LG. Comparative ecophysiology of three Placopsis species, pioneer lichens in recently exposed Chilean glacial forelands. Symbiosis. 2012;56:55–66. [Google Scholar]
- Rambold G, Friedl T, Beck A. Photobionts in lichens: possible indicators of phylogenetic relationships? Bryologist. 1998;101:392–397. [Google Scholar]
- Reeb V, Lutzoni F, Roux C. Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol. Phylogenet. Evol. 2004;32:1036–1060. doi: 10.1016/j.ympev.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Reese Næsborg R, Ekman S, Tibell L. Molecular phylogeny of the genus Lecania (Ramalinaceae, lichenized Ascomycota) Mycol. Res. 2007;111:581–591. doi: 10.1016/j.mycres.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Rico V, Calatayud V, Giralt M. Buellia tesserata and Dimelaena radiata, two closely related species. Lichenologist. 2003;35:117–124. [Google Scholar]
- Rivas Plata E, Lumbsch HT. Parallel evolution and phenotypic divergence in lichenized fungi: a case study in the lichen-forming fungal family Graphidaceae (Ascomycota: Lecanoromycetes: Ostropales) Mol. Phylogenet. Evol. 2011;61:45–63. doi: 10.1016/j.ympev.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Rivas Plata E, Lücking R, Lumbsch HT. A new classification for the family Graphidaceae (Ascomycota: lecanoromycetes: Ostropales) Fungal Divers. 2012a;52:107–121. [Google Scholar]
- Rivas Plata E, Lücking R, Lumbsch HT. Molecular phylogeny and systematics of the Ocellularia clade (Ascomycota: Ostropales: Graphidaceae) Taxon. 2012b;61:1161–1179. [Google Scholar]
- Rivas Plata E, Parnmen S, Staiger B, Mangold A, Frisch A, Weerakoon G, Hernández JE, Cáceres MES, Kalb K, Sipman HJM, Common RS, Nelsen MP, Lücking R, Lumbsch HT. A molecular phylogeny of Graphidaceae (Ascomycota, Lecanoromycetes, Ostropales) including 428 species. Mycokeys. 2013 [Google Scholar]
- Rossman AY, Schoch CL, Farr DF, Nishijima K, Keith L, Goenaga R. Dolabra nepheliae on rambutan and lychee represents a novel lineage of phytopathogenic Eurotiomycetes. Mycoscience. 2010;51:300–309. doi: 10.1007/s10267-010-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt I, Frankhauser JD, Sweeney K, Spribille T, Kalb K, Lumbsch HT. Gyalectoid Pertusaria species form a sister-clade to Coccotrema (Ostropomycetidae, Ascomycota) and comprise the new lichen genus Gyalectaria. Mycology. 2010;1:75–83. [Google Scholar]
- Schmitt I, Lumbsch HT. Molecular phylogeny of the Pertusariaceae supports secondary chemistry as an important systematic character set in lichen-forming ascomycetes. Mol. Phylogenet. Evol. 2004;33:43–55. doi: 10.1016/j.ympev.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Schmitt I, Lumbsch HT, Søchting U. Phylogeny of the lichen genus Placopsis and its allies based on Bayesian analyses of nuclear and mitochondrial sequences. Mycologia. 2003;95:827–835. [PubMed] [Google Scholar]
- Schmitt I, Otte J, Parnmen S, Sadowska-Des AD, Lücking R, Lumbsch HT. A new circumscription of the genus Varicellaria (Pertusariales, Ascomycota) MycoKeys. 2012;4:23–36. [Google Scholar]
- Schmitt I, Yamamoto Y, Lumbsch HT. Phylogeny of Pertusariales (Ascomycotina): resurrection of Ochrolechiaceae and new circumscription of Megasporaceae. J. Hattori Bot. Lab. 2006;100:753–764. [Google Scholar]
- Schmull M, Miadlikowska J, Pelzer M, Stocker-Wörgötter E, Hofstetter V, Fraker E, Hodkinson BP, Reeb V, Kukwa M, Lumbsch HT, Kauff F, Lutzoni F. Phylogenetic affiliations of members of the heterogeneous lichen-forming fungi of the genus Lecidea sensu Zahlbruckner (Lecanoromycetes, Ascomycota) Mycologia. 2011;103:983–1003. doi: 10.3852/10-234. [DOI] [PubMed] [Google Scholar]
- Schoch C, Sung GH, López-Giráldez F, Townsend JP, Miadlikowska J, Hofstetter V, Robbertse B, Matheny B, Kauff F, Wang Z, Gueidan C, Andrie RM, Trippe K, Ciufetti LM, Wynns A, Fraker E, Hodkinson BP, Bonito G, Groenewald JZ, Arzanlou M, De Hoog GS, Crous PW, Hewitt D, Pfister D, Peterson K, Gryzenhout M, Wingfield MJ, Aptroot A, Suh SO, Blackwell M, Hillis DM, Griffith GW, Castlebury LA, Rossman A, Lumbsch HT, Lücking R, Büdel B, Rauhut A, Diederich P, Ertz D, Geiser DM, Hosaka K, Inderbitzin P, Kohlmeyer J, Volkmann-Kohlmeyer B, Mostert L, O'Donnell K, Sipman H, Rogers JD, Shoemaker R, Sugiyama J, Summerbell RC, Untereiner WA, Johnston PR, Stenroos S, Zuccaro A, Dyer PS, Crittenden PD, Cole MS, Hansen K, Trappe JM, Yahr R, Lutzoni F, Spatafora JW. The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst. Biol. 2009;58:224–239. doi: 10.1093/sysbio/syp020. [DOI] [PubMed] [Google Scholar]
- Sérusiaux E, van den Boom PPG, Brand MA, Coppins BJ, Magain N. Lecania falcata, a new species from Spain, the Canary Islands and the Azores, close to Lecania chlorotiza. Lichenologist. 2012;44:577–590. [Google Scholar]
- Skaloud P, Peksa O. Evolutionary inferences based on ITS rDNA and actin sequences reveal extensive diversity of the common lichen alga Asterochloris (Trebouxiophyceae, Chlorophyta) Mol. Phylogenet. Evol. 2010;54:36–46. doi: 10.1016/j.ympev.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Sohrabi M, Stenroos S, Myllys L, Søchting U, Ahti T, Hyvönen J. Phylogeny and taxonomy of the 'manna lichens'. Mycol. Progress. 2013 [Google Scholar]
- Spribille T, Muggia L. Expanded taxon sampling disentangles evolutionary relationships and reveals a new family in Peltigerales (Lecanoromycetidae, Ascomycota) Fungal Divers. 2012 [Google Scholar]
- Stamatakis A. RAxML-VI-HPC: Maximum Likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008;57:758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Tehler A, Wedin M. Systematics of lichenized fungi. In: Nash TH III, editor. Lichen biology. Cambridge: Cambridge University Press; 2008. pp. 338–354. [Google Scholar]
- Thell A, Högnabba F, Elix J, Feurere T, Kärnefelt I, Myllys L, Randlane T, Saag A, Stenroos S, Ahti T, Seaward MRD. Phylogeny of the cetrarioid core (Parmeliaceae) based on five genetic markers. Lichenologist. 2009;41:489–511. [Google Scholar]
- Thell A, Crespo A, Divakar PK, Kärnefelt I, Leavitt SD, Lumbsch HT, Seaward MRD. A review of the lichen family Parmeliaceae – history, phylogeny and current taxonomy. Nord. J. Bot. 2012;30:1–24. [Google Scholar]
- Thüs H, Muggia L, Pérez–Ortega S, Favero–Longo SE, Joneson S, O’Brien H, Nelsen MP, Duque–Thüs R, Grube M, Friedl T, Brodie J, Andrew CJ, Lücking R, Lutzoni F, Gueidan C. Revisiting photobiont diversity in the lichen family Verrucariaceae (Ascomycota) Europ. J. Phycol. 2011;46:399–415. [Google Scholar]
- Timdal E. Gypsoplacaceae and Gypsoplaca, a new family and genus of squamiform lichens. In: Jahns HM, editor. Contributions to lichenology in honour of A. Henssen. Bibl. Lichen. Vol. 38. 1990. pp. 419–427. [Google Scholar]
- Timdal E. Psorula. In: Nash TH III, Ryan BD, Diederich P, Gries C, Bungartz F, editors. Flora of the Greater Sonoran Desert Region. I. Lichens Unlimited. Tempe, Arizona: Arizona State University; 2002. pp. 434–435. [Google Scholar]
- Townsend JP. Profiling phylogenetic informativeness. Syst. Biol. 2007;56:222–231. doi: 10.1080/10635150701311362. [DOI] [PubMed] [Google Scholar]
- Tschermak-Woess E. The algal partner. In: Galun M, editor. CRC Handbook of lichenology. I. Boca Raton: CRC Press, Inc; 1988. pp. 39–92. [Google Scholar]
- Verdon D, Rambold G. A new species in the genus Solenopsora (Catillariaceae, Lecanorales) Mycotaxon. 1998;69:399–408. [Google Scholar]
- Voytsekhovich A. LAP LAMBERT. Germany: Academic Publishing, Saarbrucken; 2013. Photobionty lishaynikov. Raznoobraziye, ekologiya i vzaimootnosheniya s mikobiontom. [Google Scholar]
- Voytsekhovich A, Mikhailyuk TI, Darienko TM. Lichen photobionts. 1: Biodiversity, ecophysiology and co-evolution with the mycobiont. Algologia. 2011a;21:3–26. [Google Scholar]
- Voytsekhovich A, Mikhailyuk TI, Darienko TM. Lichen photobionts. 2: origin and correlation with mycobiont. Algologia. 2011b;21:151–177. [Google Scholar]
- Voytsekhovich A, Dymytrova L, Nadyeina O. Photobiont composition of some taxa of the genera Micarea and Placynthiella (Lecanoromycetes, lichenized Ascomycota) from Ukraine. Folia Cryptog. Estonica. 2011c;48:135–148. [Google Scholar]
- Wedin M, Döring H. The phylogenetic relationship of the Sphaerophoraceae, Austropeltum and Neophyllis (lichenized Ascomycota) inferred by SSU rDNA sequences. Myc. Res. 1999;109:1131–1137. [Google Scholar]
- Wedin M, Döring H, Ekman S. Molecular phylogeny of the lichen families Cladoniaceae, Sphaerophoraceae, and Stereocaulaceae (Lecanorales, Ascomycotina) Lichenologist. 2000;32:171–187. [Google Scholar]
- Wedin M, Döring H, Gilenstam G. Stictis s. lat. (Ostropales, Ascomycota) in northern Scandinavia, with a key and notes on morphological variation in relation to lifestyle. Mycol. Res. 2006;110:773–789. doi: 10.1016/j.mycres.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Wedin M, Jørgensen PM, Wiklund E. Massalongiaceae fam. nov., an overlooked monophyletic group among the cyanobacterial lichens (Peltigerales, Lecanoromycetes, Ascomycota) Lichenologist. 2007;39:61–67. [Google Scholar]
- Wedin M, Jørgensen PM, Ekman S. Vahliellaceae, a new family of cyanobacterial lichens (Peltigerales, Ascomycetes) Lichenologist. 2011;43:67–72. [Google Scholar]
- Wedin M, Wiklund E, Jørgensen PM, Ekman S. Slippery when wet: phylogeny and character evolution in the gelatinous cyanobacterial lichens (Peltigerales, Ascomycetes) Mol. Phylogenet. Evol. 2009;53:862–871. doi: 10.1016/j.ympev.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Wedin M, Wiklund E, Crewe A, Döring H, Ekman S, Nyberg Å, Schmitt I, Lumbsch HT. Phylogenetic relationships of Lecanoromycetes (Ascomycota) as revealed by analyses of mtSSU and nLSU rDNA sequence data. Mycol. Res. 2005;109:159–172. doi: 10.1017/s0953756204002102. [DOI] [PubMed] [Google Scholar]
- Westberg M, Arup U, Kärnefelt I. Phylogenetic studies in the Candelariaceae (lichenized Ascomycota) based on nuclear ITS DNA sequence data. Mycol. Res. 2007;111:1277–1284. doi: 10.1016/j.mycres.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Westberg M, Frödén P, Wedin M. A monograph of the genus Placomaronea (Ascomycota, Candelariales) Lichenologist. 2009;41:513–527. [Google Scholar]
- Widhelm T, Lumbsch HT. The phylogenetic placement of Miltideaceae inferred from ribosomal DNA sequence data. In: Bates ST, Bungartz F, Lücking R, Herrera-Campos MA, Zambrano A, editors. Biomonitoring, ecology, and systematics of lichens. Festschrift Thomas H. Nash III. Biblioth. Lichenol. Vol. 106. 2011. pp. 365–373. [Google Scholar]
- Wiens JJ, Morrill MC. Missing data in phylogenetic analysis: reconciling results from simulations and empirical data. Syst. Biol. 2011 doi: 10.1093/sysbio/syr025. [DOI] [PubMed] [Google Scholar]
- Wiklund E, Wedin M. The phylogenetic relationships of the cyanobacterial lichens in the Lecanorales suborder Peltigerineae. Cladistics. 2003;19:419–431. doi: 10.1111/j.1096-0031.2003.tb00312.x. [DOI] [PubMed] [Google Scholar]
- Wirth V. Die Flechten Baden-Württembergs II. Germany: Stuttgart, Eugen Ulmer Verlag; 1995. [Google Scholar]
- Zhou QM, Wei JC. A new order Umbilicariales J. C. Wei & Q. M. Zhou (Ascomycota) Mycosystema. 2007;26:40–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogram representing phylogenetic relationships among 1307 putative members of the Lecanoromycetes based on maximum likelihood analyses of the combined mitSSU, nucLSU, nucSSU, RPB1 and RPB2 sequences (5+4+3+2-locus dataset) and 10 species used as outgroup (Geoglossomycetes, Lichinomycetes and Leotiomycetes). Numbers (0, 1, 2) after taxon names indicate presence of multiple specimens from the same taxon. The four-box grids associated with each internode indicate maximum likelihood bootstrap support based on different datasets. Black boxes indicate bootstrap support ≥70%; white boxes indicate bootstrap support <70%, blue boxes indicate cases where internodal support is not applicable due to at least one of the (usually two) immediately downstream branches being absent (due to missing taxa) or the node was not recovered by the bootstrap analysis (not present in the majority-rule consensus tree with all compatible groupings included; 1% treshold); and red boxes indicate conflicting relationships at bootstrap support ≥70%.
Non-significant correlation (p = 0.055) between the number of internodes within each recognized lineage (Fig. 2) and the percentage of internodes with BS-ML ≥ 70%, based on results from bootstrap analyses on 5-gene, 5+4-gene, 5+4+3-gene, and 5+4+3+2-gene datasets.