Abstract
Background
There have been improvements in combination antiretroviral therapy (cART) over the last 15 years. The aim of this analysis was to assess whether improvements in ART have resulted in improvements in surrogates of HIV outcome.
Methods
Patients in the Australian HIV Observational Database who initiated treatment using mono/duo therapy prior to 1996, or using cART from 1996 onwards, were included in the analysis. Patients were stratified by era of ART initiation. Median changes in CD4+ and the proportion of patients with detectable HIV viral load (>400 copies/ml) were calculated over the first 4 years of treatment. Probabilities of treatment switch were estimated using the Kaplan-Meier method.
Results
2,753 patients were included in the analysis: 28% initiated treatment <1996 using mono/duo therapy; and 72% initiated treatment ≥1996 using cART (30% 1996–99; 12% 2000–03; 11% 2004–07; and 19% ≥2008). Overall CD4 response improved by later era of initiation (p<0.001), although 2000–03 CD4 response was less than that for 1996–99 (p=0.007). The average proportion with detectable viral load from 2 to 4 years post treatment commencement by era was: <1996 mono/duo 0.69 (0.67–0.71); 1996–99 cART 0.29 (0.28–0.30); 2000–03 cART 0.22 (0.20–0.24); 2004–07 cART 0.09 (0.07–0.10); ≥2008 cART 0.04 (0.03–0.05). Probability of treatment switch at 4 years after initiation decreased from 53% in 1996–99 to 29% after 2008 (p<0.001).
Conclusions
Across the five time-periods examined, there have been incremental improvements for patients initiated on cART, as measured by overall response (viral load and CD4 count), and also increased durability of first-line ART regimens.
Introduction
There have been incremental improvements in combination antiretroviral therapy (cART) over the last 15 years, including increased potency, reduced pill burden, combination formulations and new drug classes(1–5). Determining the extent to which these are reflected by improvements in surrogates of HIV outcome, such as median CD4 cell count and the proportion of patients with an undetectable HIV viral load, is important for developing health care management strategy but analysis is challenging at the epidemiological level. Responses to cART can be affected by individual patient factors including baseline clinical status and treatment history, genotypic variation, drug interactions, toxicities and tolerability, as well as lifestyle (6). The complex interaction of these factors is difficult to explicitly model, especially without limiting the scope of results, and can lead to differences between the estimated and actual utility of treatment. Descriptions of population level trends based on large-scale longitudinal studies can produce generalizable results by avoiding confounding inherent in smaller studies of discrete populations.
Conversely, large-scale long-term longitudinal studies (often observational) can be confounded by temporal trends associated with both treatment stage and calendar year. The pattern of surrogate response to cART is strongly characterised by duration of treatment, particularly by the first months after treatment initiation when there is often a rapid decrease in HIV viral load (7, 8). Outcomes are also influenced by broader changes to available ART, contemporary resourcing, guidelines and interventions associated with calendar year (9, 10). For example, Australian cART initiation guidelines have shifted from early stage intervention (“hit hard, hit early”) (1996–99), to delayed intervention (at CD4 cell counts <200 cells/µL in asymptomatic patients) (2000), and back to relatively early stage intervention (at CD4 cell counts ≤350 cells/µL in asymptomatic patients) (2008) (11–13). However, few analyses of population level trends in treatment response simultaneously resolve both of these time scales which may in part be because many studies do not have both long-term follow up and ongoing recruitment necessary to reflect these changes.
In particular, early post-treatment initiation response is strongly indicative of overall treatment utility. At early stages (less than 4 years after treatment initiation), response is most strongly influenced by pre-initiation conditions and baseline disease stage as well as by the potency of implemented ART, and is also predictive of later outcomes (14–17). Generally, significant levels of virological control are reached within a year of treatment (7, 8, 18, 19). Phillips et al have shown attainment of at least one viral load measure less than 500 copies/ml in 85% of patients within 32 weeks after initiating therapy for early cART era patients, but that there were subsequently increasing rates of viral rebound over the duration of that study (up to 192 weeks) (7). Subsequent immunological repletion has been shown to be more gradual (between 4 and 8 years in patients with sustained virological control) and strongly influenced by baseline levels (16).
We investigated trends in response to ART over the first 4 years of treatment by duration of treatment and calendar year of treatment initiation using the Australian HIV Observational database (AHOD). AHOD is a large observational cohort study with extensive treatment and clinical data, and ongoing patient recruitment. The aim of this analysis was to assess whether improvements in ART over calendar time have resulted in improvements in surrogates of HIV outcome.
Methods
Study population
The Australian HIV Observational Database (AHOD) is an observational clinical cohort study of patients with HIV infection seen at 27 clinical sites throughout Australia. AHOD uses methodology which has been described in detail elsewhere (20). Data are transferred electronically to The Kirby Institute, University of New South Wales every 6 months. Prospective data collection commenced in 1999, with retrospective data provided where available.
Ethics approval for the AHOD study was granted by the University of New South Wales Human Research Ethics Committee, and all other relevant institutional review boards. Written informed consent was obtained from participating individuals. All study procedures were developed in accordance with the revised 1975 Helsinki Declaration.
This analysis included all patients who had been recruited to AHOD as part of general AHOD recruitment prior to 31 March 2013 who initiated treatment using mono therapy/dual therapy (mono/duo therapy) prior to 1996, or using cART from 1996 onwards. Patient follow-up was from treatment initiation until censoring at the earlier of death, lost to follow-up, 4 years after treatment commencement, or cohort censoring date (31 March 2013). Patients were defined as lost to follow-up at date of last clinical visit if they had no recorded visit in the year prior to cohort censoring date (31 March 2013). Patients were retained in the analysis until censoring regardless of interruptions or changes to treatment using an intention-to-treat approach. Patients were stratified by time period of treatment initiation as follows: <1996 mono/duo, 1996–99 cART; 2000–03 cART; 2004–07 cART, ≥2008 cART. Patients were excluded if they did not have a record of CD4 or HIV-RNA tests after commencing ART.
Covariates analysed were: sex; age (“<20”, “20–29”, “30–39”, “40–49”, “50–59”, “60–69”, “70–79”); mode of HIV exposure (men who have sex with men (MSM), heterosexual, injecting drug user (IDU), other/unknown); time updated instance of first AIDS defining illness (ADI); HCV antibody (no/not tested, positive); HBV surface antigen (HBVsAg) (no/not tested, positive); CD4 cell count (from the closest test date between 180 days prior to a given analysis time-point and up to 30 days after that time-point – “0–199”,”200–349”, “350–499”, “≥500” cells/ml); viral load (from the closest test date between 180 days prior to a given analysis time-point and up to 30 days after that time-point – “≤400”, “401–1000”, “1001–10,000”, >10000 copies/ml) and drug class (“Nucleoside/Nucleotide Reverse Transcriptase Inhibitors (NRTI)”, “Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI)”, “Protease Inhibitors (PI)”, “Integrase Inhibitors (II)”).
Statistical Analysis
Patient characteristics at start of ART were summarised by era of ART commencement. Median and interquartile range for CD4 cell count and viral load at start of treatment was summarised by era. Kruskal-Wallis rank test of equality of baseline CD4 cell count by era was performed. Median changes in CD4 cell counts for the first 4 years of treatment were calculated using the closest CD4 test result to each 90 day cut point following first ART per patient.
Percentage of patients with detectable viral load (>400 copies/ml) for the first 4 years of treatment was calculated using the closest viral load test result to each 90 day cut point following first ART per patient. The threshold of >400 copies/ml was used to allow comparison of all treatment eras including earlier treatment eras when more sensitive assays were not available. Kruskal-Wallis rank test of equality of the log10 of baseline viral load by era was performed.
Equality of response by era for the first 4 years after cART was assessed using multivariate generalised estimating equation modelling with assumed exchangeable variance structure, and robust calculated variances. Models were adjusted for era, age group, IDU mode of exposure, prior ADI and baseline CD4. For immune response, a normal distribution of change in CD4 cell count per unit time was assumed, while for virological response a binomial distribution of detectable viral load with logistic link was used. Wald tests of the overall significance of era (excluding the mono/duo era) and pairwise comparison of the predicted probability of CD4 cell response conditional on era category levels (excluding the mono/duo era) were also conducted. The mono/duo era was excluded from tests of response by era because mono/duo regimen response has been shown to be minimal in comparison to cART response (21) and would have otherwise masked the differentiation of cART era response using overall tests of the association of era with response.
Rates of first switch of treatment were calculated by era for all switches; for switches with recent virological failure (from the closest viral load test within 180 days prior to switch and up to 30 days after switch >1000 copies/ml); for switches without recent virological failure (closest viral load test within 180 days prior to switch and up to 30 days after switch ≤1000 copies/ml); and for switches without a recent viral load reading (closest viral load test was over 180 days prior to switch and/or over 30 days after switch). A switch was defined as the commencement of 2 or more new antiretroviral drugs and/or the commencement of a new class.
Kaplan-Meier curves by era were generated from treatment start until first change of regimen for up to 4 years after baseline for specific treatment switch types (all switches, switch with recent virological failure, and switch without recent virological failure). A log rank test of equality of curves was conducted but excluding the mono/duo era for reasons discussed above.
Results
Patient characteristics
By March 2013, 3,607 patients had been recruited to AHOD as part of general recruitment, of whom 854 were excluded from the analysis: 327 had no record of treatment initiation; 481 had treatment commencement prior to or later than analysis treatment era censoring dates; and 46 had no record of subsequent CD4 cell count or viral load test. The remaining 2,753 patients were included in the analysis (Table 1): 767 (28%) from the mono/duo treatment era (<1996); 817 (30%) from the early cART era (1996–99); 330 (12%) from the middle cART era (2000–03); 310 (11%) from the middle/late cART era (2004–07); and 529 (19%) from the late cART era (≥2008).
Table 1.
Patient characteristics at start of treatment by treatment era and type
| Era | ||||||
|---|---|---|---|---|---|---|
| Early (Mono/Duo) (<1996) |
Early (1996–99) |
Middle (2000–03) |
Middle/Late (2004–07) |
Late (2008+) |
||
| N (%) | N (%) | N (%) | N (%) | N (%) | ||
| N | 767 (100) | 817 (100) | 330 (100) | 310 (100) | 529 (100) | |
| Gender | ||||||
| F | 41(5) | 39(5) | 21(6) | 26(8) | 42(8) | |
| M | 726(95) | 778(95) | 309(94) | 284(92) | 487(92) | |
| Age (years) | ||||||
| Mean (SD) | 37(8.9) | 38(9.8) | 40(10.4) | 43(9.9) | 42(10.9) | |
| <20 | 7(1) | 7(1) | 1(0) | 1(0) | (0) | |
| 20–29 | 176(23) | 155(19) | 48(15) | 23(7) | 70(13) | |
| 30–39 | 335(44) | 349(43) | 136(41) | 114(37) | 168(32) | |
| 40–49 | 181(24) | 200(24) | 90(27) | 104(34) | 179(34) | |
| 50–59 | 62(8) | 87(11) | 41(12) | 48(15) | 79(15) | |
| 60–69 | 6(1) | 15(2) | 11(3) | 18(6) | 27(5) | |
| 70–79 | 0(0) | 4(0) | 3(1) | 2(1) | 6(1) | |
| Mode of exposure | ||||||
| MSM | 642(84) | 632(77) | 221(67) | 202(65) | 376(71) | |
| Heterosexual | 63(8) | 88(11) | 71(22) | 84(27) | 107(20) | |
| IDU | 38(5) | 67(8) | 21(6) | 14(5) | 22(4) | |
| Other | 24(3) | 30(4) | 17(5) | 10(3) | 24(5) | |
| Prior ADI | ||||||
| No | 706(92) | 718(88) | 278(84) | 270(87) | 491(93) | |
| Yes | 61(8) | 99(12) | 52(16) | 40(13) | 38(7) | |
| HCV ever | ||||||
| No | 585(76) | 654(80) | 263(80) | 251(81) | 421(80) | |
| Yes | 105(14) | 92(11) | 33(10) | 26(8) | 50(9) | |
| Not recorded | 77(10) | 71(9) | 34(10) | 33(11) | 58(11) | |
| HBV ever | ||||||
| No | 653(85) | 670(82) | 257(78) | 239(77) | 415(78) | |
| Yes | 42(5) | 43(5) | 14(4) | 6(2) | 11(2) | |
| Not recorded | 72(9) | 104(13) | 59(18) | 65(21) | 103(19) | |
| CD4 cell count (cells/µl) | ||||||
| Mean (SD) | 330(219.8) | 342(232.1) | 307(257.8) | 298(217.2) | 338(189.0) | |
| Median (IQR) | 294(190–435) | 320(170–475) | 250(120–420) | 250(170–373) | 320(220–430) | |
| Log10 viral load | ||||||
| Mean (SD) | 5.16 (0.78) | 4.75 (0.95) | 4.88 (0.89) | 4.62 (1.14) | 4.53 (0.98) | |
| Median (IQR) | 5.16 (4.61–5.71) | 4.89 (4.28–5.43) | 5.00 (4.60–5.51) | 4.99 (4.26–5.35) | 4.78 (4.13–5.04) | |
| Regimen class1 | ||||||
| NNRTI | 5/767 (1) | 346/817 (42) | 195/330 (59) | 205/310 (66) | 350/529 (66) | |
| NRTI | 766/767 (100) | 815/817 (100) | 330/330 (100) | 310/310 (100) | 526/529 (99) | |
| PI | 4/767 (1) | 472/817 (58) | 113/330 (34) | 104/310 (34) | 138/529 (26) | |
| II | 0/767 (0) | 0/817 (0) | 0/330 (0) | 1/310 (0) | 74/529 (14) | |
NNRTI= Non-Nucleoside Reverse Transcriptase Inhibitors, NRTI=Nucleoside/Nucleotide Reverse Transcriptase Inhibitors, PI=Protease Inhibitors, II=Integrase Inhibitors
Patients were predominantly male (94%), and mode of exposure was predominantly via MSM (75%). The mean age at ART initiation was 39.4 years (standard deviation (SD) 10.08), which increased by later era (38.4 (SD 9.79) 1996–99, 42.1 (SD 10.9) ≥2008). Over cART eras the proportion of patients commencing with NNRTI containing regimens increased from 42% (1996–99) to 66% (≥2008), and the number commencing with PI based regimens fell from 58% (1996–99) to 26% (≥2008).
Immunology
Base line CD4 cell counts were relatively similar by era of treatment commencement but were slightly lower for middle era commencements. Median CD4 cell counts at commencement were: 294 cells/µl (IQR 190–435) for <1996 mono/duo; 320 cells/µl (IQR 170–475) for 1996–99 cART; 250 cells/µl (IQR 120–420) for 2000–03 cART; 250 cells/µl (IQR 170–373) for 2004–07 cART; and 320cells/µl (IQR 220–430) for ≥2008 cART (Table 1). Kruskal-Wallis rank test of baseline CD4 cell count by era (excluding mono/duo) showed significant change over time (p<0.001).
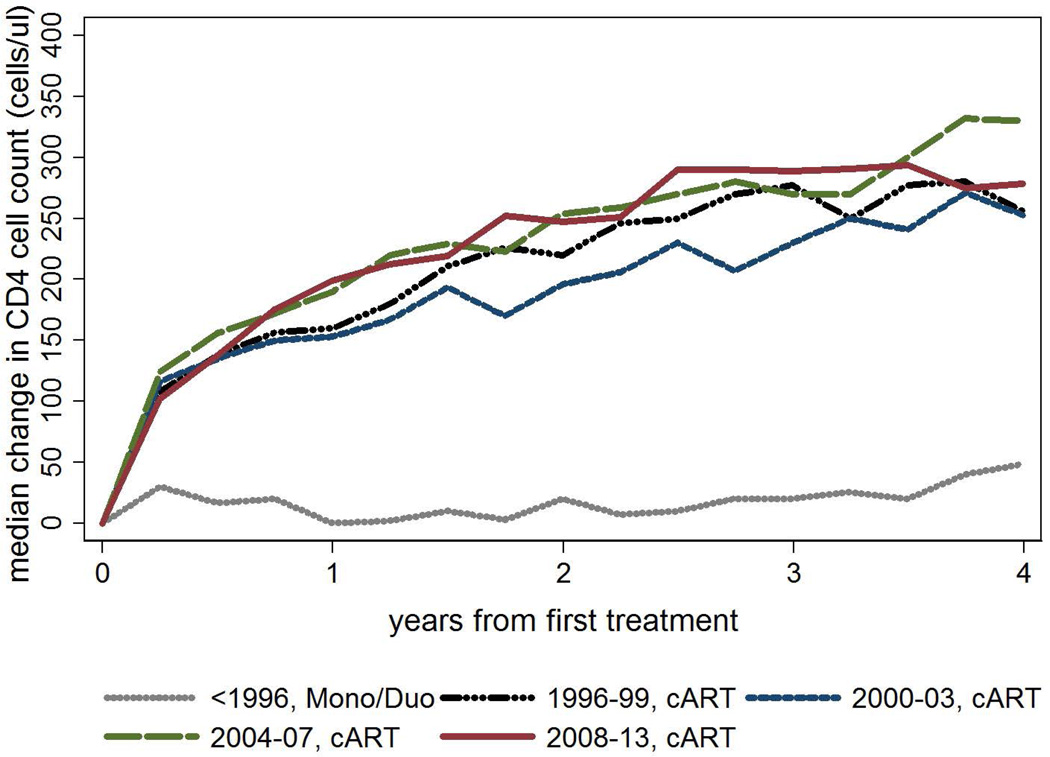
Median CD4 cell counts increased by year from first treatment for cART regimens. Median CD4 cell count change from baseline was greatest in the first year after treatment (Figure 1). A global Wald test indicated that era of commencement was a significant predictor of change from baseline CD4 (p<0.001) in an adjusted model. Pairwise comparison of predicted change in CD4 cell count by different eras of treatment initiation (excluding the mono/duo era) showed difference between 2000–03 cART compared to all other eras (1996–99 cART p=0.007, 2004–07 cART p=0.006, 2008–13 cART p<0.001), and marginal difference between 1996–99 cART compared to 2008–13 cART (p=0.044). There was no difference for other pairwise comparisons (1996–99 cART compared to 2004–07 cART p=0.603; and 2004–07 cART compared to 2008–13 cART p=0.252).
Figure 1.
Median change in CD4 cell count after first treatment by first treatment type and era of treatment commencement1
1Closest patient CD4 cell count test date to each 90 day interval following first treatment; global Wald test for era (excluding mono/duo era) p<0.001 (based on GEE model using normal family, identity link, adjusted for age, IDU exposure, prior ADI and baseline CD4)
Virology
Baseline viral loads were elevated across all eras of treatment commencement. Medians of log10 of viral load copies/ml were similar by era of commencement: 5.16 (IQR 4.61–5.71) for <1996 mono/duo; 4.89 (IQR 4.28–5.43) for 1996–99 cART; 5.00 (IQR 4.60–5.51) for 2000–03 cART; 4.99 (IQR 4.26–5.35) for 2004–07 cART; and 4.78 (IQR 4.13–5.04) for ≥2008 cART. Kruskal-Wallis rank test of log10 of baseline viral load by era (excluding mono/duo era) showed a significant lower viral load at initiation in later eras (p<0.001).
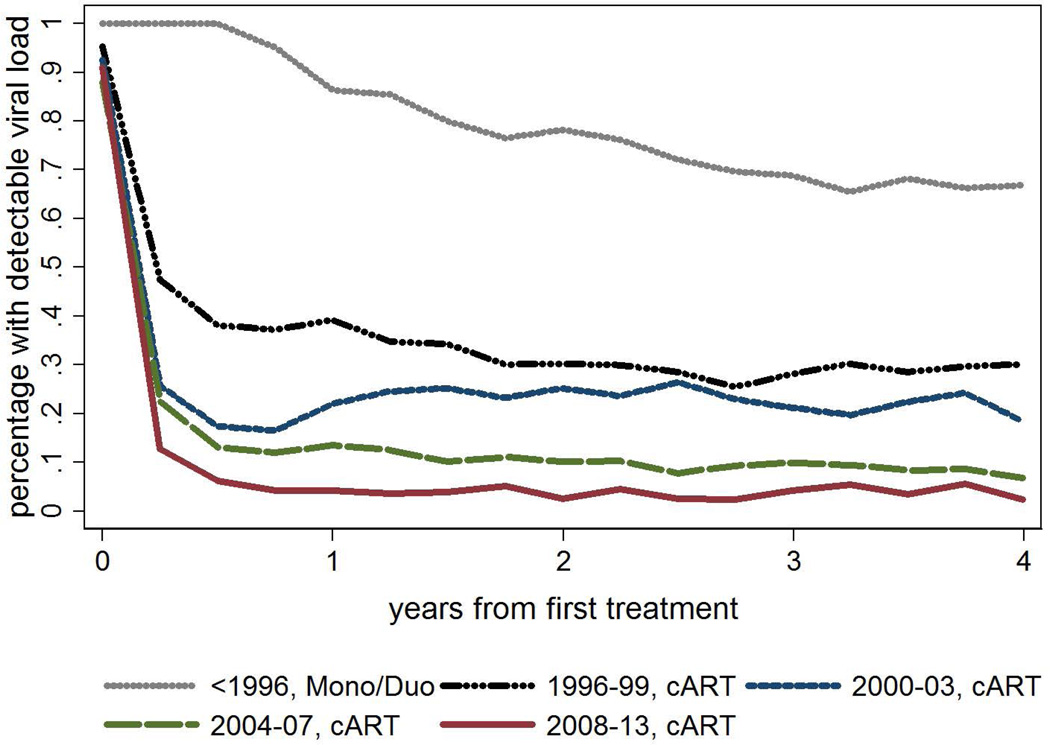
Median viral load dropped to below detectable thresholds after the first year of treatment for patients commencing on cART from relatively similarly high baseline levels. The proportion of patients with detectable viral load more rapidly reached lower thresholds by later era of treatment commencement (Figure 2). The average proportion with detectable viral load per quarter from 2 years to 4 years post treatment commencement by era was: <1996 mono/duo 0.69 (0.67–0.71); 1996–99 cART 0.29 (0.28–0.30); 2000–03 cART 0.22 (0.20–0.24); 2004–07 cART 0.09 (0.07–0.10); ≥2008 cART 0.04 (0.03–0.05).
Figure 2.
Percentage of tests with undetectable viral load by first treatment type and era of treatment commencement1
1Closest patient viral load test date to each 90 day interval following first treatment. Viral loads less than or equal to 400 cells/ul categorised as undetectable; global Wald test p-value for era p<0.001 (based on GEE model using normal family, identity link, adjusted for age, IDU exposure, prior ADI and baseline CD4)
Treatment Switching
The rate of first treatment switches by era and viral measure category is shown in Table 2. The overall rate of switching was 17.3 (95% CI 16.4–18.3) per 100 person years. There were declines by cART era in the rate of switching overall (p<0.001); with recent virological failure (p<0.001); and with unrecorded recent viral record (p<0.001). There was no change in the observed rate of switching without recent viral failure (p=0.428).
Table 2.
Rate of first regimen switch in first 4 years of treatment by switch type and treatment era
| Early Mono/Duo |
Early cART |
Middle cART |
Middle/ Late cART |
Late cART |
p2 | All eras | |
|---|---|---|---|---|---|---|---|
| Switch Category1 |
per 1000 pyrs (95% CI) |
per 1000 pyrs (95% CI) |
per 1000 pyrs (95% CI) |
per 1000 pyrs (95% CI) |
per 1000 pyrs (95% CI) |
per 1000 pyrs (95% CI) |
|
| Without recent virological failure | 0.9 (0.6–1.4) | 7.4 (6.3–8.6) | 7.2 (5.7–9.2) | 8.5 (6.8–10.7) | 6.7 (5.3–8.4) | 0.428 | 5.4 (4.9–5.9) |
| With recent virological failure | 7.2 (6.2–8.4) | 9.9 (8.6–11.3) | 7.4 (5.8–9.4) | 4.2 (3.1–5.9) | 3.5 (2.6–4.8) | <0.001 | 7.1 (6.5–7.7) |
| No recent viral record | 11.4 (10.1–12.9) | 2.8 (2.2–3.6) | 1.3 (0.8–2.3) | 1.0 (0.5–2.0) | 1.0 (0.5–1.8) | <0.001 | 4.8 (4.3–5.3) |
| Overall | 19.5 (17.8–21.5) | 20.1 (18.3–22.1) | 16.0 (13.6–18.8) | 13.8 (11.5–16.5) | 11.2 (9.4–13.3) | <0.001 | 17.3 (16.4–18.3) |
Virological failure defined as viral load >1000 copies/ml at most recent test within 180 days prior to and up to 30 days after switch.
log-rank test (excludes Mono/Duo era)
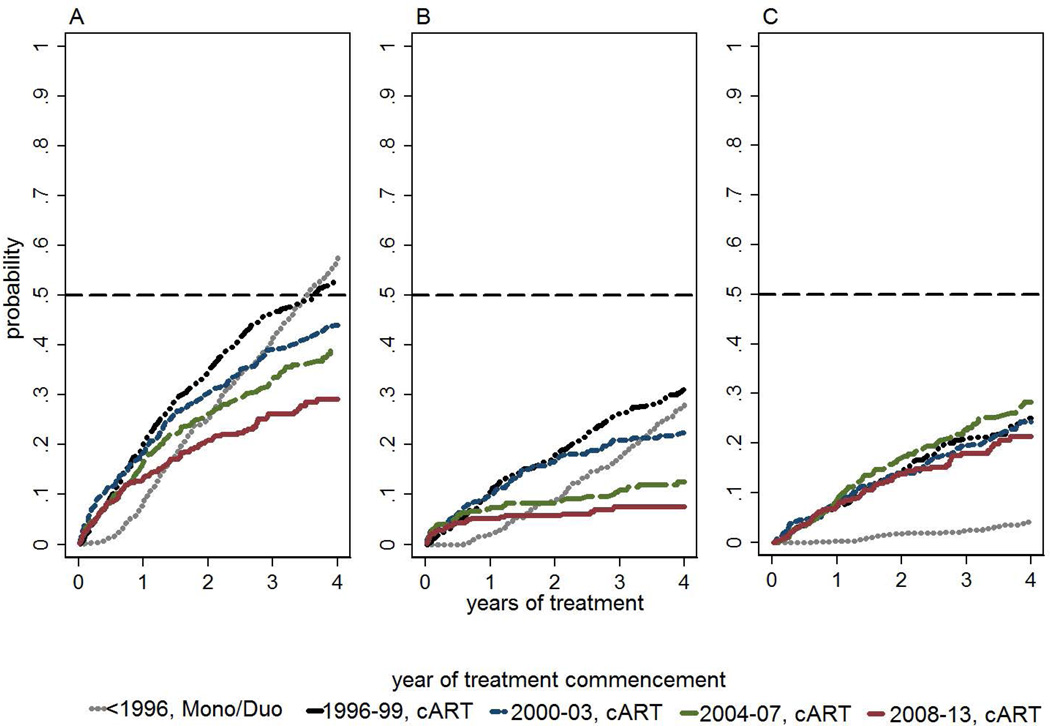
Kaplan Meier probability of having switched treatment (all switches) decreased by later era of cART commencement (Figure 3A). Kaplan Meier probability of having switched treatment (all switches) after 4 years was 0.58 (95% CI 0.54–0.61) for <1996 mono/duo; 0.53 (95% CI 0.50–0.57) for 1996–99 cART; 0.44 (95% CI 0.39–0.50) for 2000–03 cART; 0.39 (95% CI 0.34–0.45) for 2004–07 cART; and 0.29 (95% CI 0.25–0.35) for ≥2008 cART. Log rank test of time to switch by era (excluding the mono duo era) showed significant difference (p<0.001).
Figure 3.
Kaplan-Meier probabilities of time to first switch by era. A, All switches. B, Switches with recent virological failure. C, Switches without recent virological failure1
1Virological failure defined as viral load >1000 copies/ml at most recent test within 180 days prior to and up to 30 days after switch; Log rank test of era for (excluding mono/duo era) A: p<0.001; B: p<0.001; C: p=0.424
Kaplan Meier probability of having switched treatment with recent virological failure also decreased by later era of cART commencement (p<0.001) (Figure 3B) but there was not a difference for switches without recent virological failure (p=0.424) (Figure 3C).
Discussion
We measured trends in early stage response to treatment for a large HIV-positive cohort. Over the first 4 years of treatment CD4 cell count response was marginally improved by later cART era of treatment commencement although 2000–03 cART commencements were seen to have relatively less CD4 cell count recovery. However, there were significantly improved rates of virological suppression by later era of treatment commencement for all eras. We found improving durability of ART over the same period of follow-up.
Median baseline CD4 cell counts were low across all eras of treatment initiation (<350 counts/µL), although levels were relatively lower for middle eras (2000–2007) (p<0.001). There was indication of difference in rates of immunological recovery across different eras of treatment initiation. Plots of median change in CD4 cell count and pairwise comparison of CD4 cell count response by era of treatment initiation suggested that later era responses were marginally improved. However, there was relatively reduced recovery of early middle era counts (2000–03) compared to early era cART (1996–99). Baseline CD4 levels were lower for 2000–03 compared to 1996–99 but were similar to those for 2004–07 (where there was improved CD4 cell count response). This suggests that level of response by era may be influenced by factors other than initiation stage related to era for example, although investigation of these potential causal factors is beyond the scope of this study. The observed trends in baseline CD4 cell count and CD4 cell count response are consistent with findings from similarly resourced countries although baseline levels in this study are generally higher than elsewhere (19). Generally, while there is indication of improved later era CD4 cell count response, we found that CD4 cell count measures alone are likely to be insufficient surrogates to differentiate HIV treatment outcomes. In particular, observed changes in CD4 cell count were not stratified by baseline CD4 cell count, baseline viral load, or rate of attainment of viral suppression or patient age which have been shown to significantly modify immune response (22, 23). In this study the improvement in immune response by era of initiation was less evident than that observed for virological suppression. Changes in proportions with detectable viral load following treatment were clearly different by era despite generally high baseline levels. In particular, we observed increasing rate of on-treatment community return to virological control, and a clear trend towards lower asymptotic proportions with detectable viral loads for later eras. The average proportion with detectable viral load over the period from 2 years (when viral loads had reached asymptotic thresholds for all eras) to 4 years post ART fell from 69% (95% CI 66.7%–71.6%) in the mono/duo era and 29% (95% CI 27.8%–30.0%) in the early cART era to 4% (95% CI 2.8%–4.5%) in the late cART era. This is consistent with increasing effective ART potency from the suppression of HIV-RNA aspect although it should be noted that this study uses an intention-to-treat approach and findings are not stratified by potentially different levels of adherence per era which might also modify rates of suppression. Our results are qualitatively consistent with those of Torian et al who have shown improved rates of virological suppression by year of diagnosis for diagnoses between 2006 and 2009 (17) although that study did not look at time from cART initiation. We have found limited publication of comparable measures for other in-care cohorts.
We observed a clear, measureable decrease in all first line cART switches by later era of commencement (p<0.001). This was attributable to decreased rates of switching with associated virological failure in recent eras (p<0.001). Only moderate changes in rates of switching were observed for switches without associated virological failure (p=0.428) and there were very low rates of observed switching with missing recent viral load tests, particularly after 2000. This suggests improved durability of first line regimens and that later era patients who commence ART early will not be disadvantaged by frequent treatment switches and less treatment choices in the future. While we did not observe any difference in time to first switch without virological failure across cART eras, other studies have shown increased tolerability of many later era regimens (1, 24, 25). It is possible that recent reductions in switching associated with increased tolerability might have been offset by other factors such as increases in relative rates of switching associated with the increased availability of regimens with improved potency, reduced toxicity and reduced pill burden, although this was not examined by this analysis (26).
Generally, while we found improving overall therapeutic utility at the on-treatment community level, there were not consistent trends in observed clinical baselines. Baseline CD4 cell count dropped to a minimum of 247cells/µl in the middle eras (2000–2007) before increasing again in the later era (from 2008). Similarly, baseline viral load did not uniformly fall over calendar time. This is consistent with contemporary Australian treatment guidelines which have shifted from aggressive early stage initiation immediately following the inception of cART in 1996, towards relatively delayed treatment initiation in asymptomatic patients with lower CD4 cell counts (<200 cells/µL) over the middle eras of this study (2000–2007) (11, 27). From 2008 onwards guidelines supported earlier treatment initiation (at ≤350 cells/µL in asymptomatic patients) (11). Given the relatively advanced stage of immunological decline and virological failure observed at baseline across all periods in this study and improved treatment response, then this supports a focus of HIV care on treatment naive populations, including on improved early risk assessment, symptom awareness and diagnosis.
A limitation of this study is the generalizability of findings to the wider on-treatment community, as well as to the wider HIV-positive population. AHOD represents a significant subset (15–20%) of the Australian on-treatment population (28, 29), which has access to publicly funded ART. While AHOD data may be reasonably representative of the Australian HIV-positive population who are in care, key subsets of the broader HIV-positive population are not included in this analysis. In particular trends in off-treatment, as well as of in-care but irregular or chaotic treatment groups with likely significantly increased viral loads are difficult to assess.
The selection bias represented by cohort data in general has been described in detail by other papers (30–32). In particular, likely higher rates of detectable viral load associated with out-of-care patients are not collected in AHOD. Generally, this study focuses on on-treatment community level trends and measures do not incorporate off-treatment subpopulations that are more difficult to reliably capture. Although our results do highlight off-treatment subpopulations as relatively important targets of intervention because of trends towards more highly effective treatment, extrapolation of our results to broader HIV-positive communities should be made with caution.
Secondly, in this study, observations from early treatment eras (<2000) are likely to reflect a healthy survivor bias to an extent. Response in surrogates of HIV outcome for these eras would otherwise be lower than those observed by this study because patients with advanced disease who died or were lost to clinical follow-up before 2000 were not included in AHOD enrolment. However, in this study virological response was found to be significantly improved in later eras regardless of survivor bias and similarly, CD4 response was marginally improved for 2004–13 cART compared to earlier eras (1996–99 and 2000–03).Overall, this study demonstrates continuing improvement in surrogates of HIV treatment outcomes by later era of treatment initiation.
A third limitation of our findings is that community level observation cannot necessarily be attributed to causal effects at the individual level (30). In terms of immunological and virological response in this study, we describe treatment response in broad or net terms, and conversely, have examined a relatively uniform, stratified on-treatment community. However, results are likely also attributable to unaccounted for exogenous factors for any given patient, and or a causal structure of determinants not necessarily described in these analyses (30).
A strength of our study is that the population size, its extended follow up and further recruitment have facilitated estimation of generalizable indicators of treatment response. In particular, our measures resolve both treatment duration and calendar year related effects. Very few studies adjust population level indicators for temporal confounding in this way and therefore may have relatively limited generalizability.
Conclusions
Across the five time-periods examined, there have been incremental improvements for patients initiated on cART, as measured by overall response of surrogates of HIV outcome (viral load and CD4 count) across treatment eras, and also increased durability of first-line regimens. There were inconsistent changes in clinical stage of initiation which might be explained by evolving attitudes of patients and prescribers to the risk/benefit of cART initiation as the era of cART has unfurled. Population level indicators of treatment response should be adjusted for both calendar time and treatment duration to properly assess these outcomes.
Acknowledgements
The Australian HIV Observational Database is funded as part of the Asia Pacific HIV Observational Database, a program of The Foundation for AIDS Research, amfAR, and is supported in part by a grant from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases (NIAID) (Grant No. U01-AI069907) and by unconditional grants from Merck Sharp & Dohme; Gilead; Bristol-Myers Squibb; Boehringer Ingelheim; Roche; Pfizer; GlaxoSmithKline; Janssen-Cilag. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales. The views expressed in this publication do not necessarily represent the position of the Australian Government.
Appendix
AHOD Collaborators
Australian HIV Observational Database contributors
Asterisks indicate steering committee members in 2013.
New South Wales: D Ellis, General Medical Practice, Coffs Harbour; M Bloch, S Agrawal, T Vincent, Holdsworth House Medical Practice, Darlinghurst; D Allen, JL Little, Holden Street Clinic, Gosford; D Smith, C Mincham, Lismore Sexual Health & AIDS Services, Lismore; D Baker*, V Ieroklis, East Sydney Doctors, Surry Hills; DJ Templeton*, CC O’Connor, S Phan, RPA Sexual Health Clinic, Camperdown; E Jackson, K McCallum, Blue Mountains Sexual Health and HIV Clinic, Katoomba; M Grotowski, S Taylor, Tamworth Sexual Health Service, Tamworth; D Cooper, A Carr, F Lee, K Hesse, K Sinn, R Norris, St Vincent’s Hospital, Darlinghurst; R Finlayson, I Prone, A Patel, Taylor Square Private Clinic, Darlinghurst; R Varma, J Shakeshaft, Nepean Sexual Health and HIV Clinic, Penrith; K Brown, C McGrath, V McGrath, S Halligan, Illawarra Sexual Health Service, Warrawong; L Wray, P Read, H Lu, Sydney Sexual Health Centre, Sydney; D Couldwell, Parramatta Sexual Health Clinic; DE Smith*, V Furner, Albion Street Centre; Clinic 16 – Royal North Shore Hospital, S Fernando; Dubbo Sexual Health Centre, Dubbo; Holdsworth House Medical Practice, Byron Bay, J Chuah*; J Watson*, National Association of People living with HIV/AIDS; C Lawrence*, National Aboriginal Community Controlled Health Organisation; B Mulhall*, Department of Public Health and Community Medicine, University of Sydney; M Law*, K Petoumenos*, S Wright*, H McManus*, C Bendall*, M Boyd*, The Kirby Institute, University of NSW. Northern Territory: N Ryder, R Payne, Communicable Disease Centre, Royal Darwin Hospital, Darwin. Queensland: D Russell, S Doyle-Adams, Cairns Sexual Health Service, Cairns; D Sowden, K Taing, K McGill, Clinic 87, Sunshine Coast-Wide Bay Health Service District, Nambour; D Orth, D Youds, Gladstone Road Medical Centre, Highgate Hill; M Kelly, A Gibson, H Magon, Brisbane Sexual Health and HIV Service, Brisbane; B Dickson*, CaraData. South Australia: W Donohue,O’Brien Street General Practice, Adelaide. Victoria: R Moore, S Edwards, R Liddle, P Locke, Northside Clinic, North Fitzroy; NJ Roth*, H Lau, Prahran Market Clinic, South Yarra; T Read, J Silvers*, W Zeng, Melbourne Sexual Health Centre, Melbourne; J Hoy*, K Watson*, M Bryant, S Price, The Alfred Hospital, Melbourne; I Woolley, M Giles*, T Korman, J Williams*, Monash Medical Centre, Clayton. Western Australia: D Nolan, J Robinson, Department of Clinical Immunology, Royal Perth Hospital, Perth.
Coding of Death Form (CoDe) reviewers: D Sowden, J Hoy, L Wray, I Woolley, K Morwood, N Roth, K Choong, CC O'Connor, MA Boyd.
Footnotes
Disclosure statement
The authors declare no competing interests.
References
- 1.Boyd MA. Improvements in antiretroviral therapy outcomes over calendar time. Current opinion in HIV and AIDS. 2009;4(3):194–199. doi: 10.1097/COH.0b013e328329fc8d. Epub 2009/06/18. [DOI] [PubMed] [Google Scholar]
- 2.Willig JH, Abroms S, Westfall AO, Routman J, Adusumilli S, Varshney M, et al. Increased regimen durability in the era of once-daily fixed-dose combination antiretroviral therapy. AIDS. 2008;22(15):1951–1960. doi: 10.1097/QAD.0b013e32830efd79. Epub 2008/09/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parienti JJ, Bangsberg DR, Verdon R, Gardner EM. Better adherence with once-daily antiretroviral regimens: a meta-analysis. Clin Infect Dis. 2009;48(4):484–488. doi: 10.1086/596482. Epub 2009/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Llibre JM, Clotet B. Once-daily single-tablet regimens: a long and winding road to excellence in antiretroviral treatment. AIDS reviews. 2012;14(3):168–178. Epub 2012/07/27. [PubMed] [Google Scholar]
- 5.Bartlett JA, Fath MJ, Demasi R, Hermes A, Quinn J, Mondou E, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006;20(16):2051–2064. doi: 10.1097/01.aids.0000247578.08449.ff. Epub 2006/10/21. [DOI] [PubMed] [Google Scholar]
- 6.Robertson GR, Grant DM, Piquette-Miller M. Pharmacogenetics of pharmacoecology: which route to personalized medicine? Clin Pharmacol Ther. 2009;85(4):343–348. doi: 10.1038/clpt.2009.17. Epub 2009/03/20. [DOI] [PubMed] [Google Scholar]
- 7.Phillips AN, Staszewski S, Weber R, Kirk O, Francioli P, Miller V, et al. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. JAMA. 2001;286(20):2560–2567. doi: 10.1001/jama.286.20.2560. Epub 2001/11/28. [DOI] [PubMed] [Google Scholar]
- 8.Bart PA, Rizzardi GP, Tambussi G, Chave JP, Chapuis AG, Graziosi C, et al. Immunological and virological responses in HIV-1-infected adults at early stage of established infection treated with highly active antiretroviral therapy. AIDS. 2000;14(13):1887–1897. doi: 10.1097/00002030-200009080-00002. Epub 2000/09/21. [DOI] [PubMed] [Google Scholar]
- 9.Frieden TR, Das-Douglas M, Kellerman SE, Henning KJ. Applying public health principles to the HIV epidemic. N Engl J Med. 2005;353(22):2397–2402. doi: 10.1056/NEJMsb053133. Epub 2005/12/02. [DOI] [PubMed] [Google Scholar]
- 10.Marconi VC, Grandits GA, Weintrob AC, Chun H, Landrum ML, Ganesan A, et al. Outcomes of highly active antiretroviral therapy in the context of universal access to healthcare: the U.S. Military HIV Natural History Study. AIDS Res Ther. 2010;7:14. doi: 10.1186/1742-6405-7-14. Epub 2010/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Services DoHaH. 2006. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. editor. [Google Scholar]
- 12.Hammer SM, Saag MS, Schechter M, Montaner JS, Schooley RT, Jacobsen DM, et al. Treatment for adult HIV infection-2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296(7):827–843. doi: 10.1001/jama.296.7.827. Epub 2006/08/15. [DOI] [PubMed] [Google Scholar]
- 13.2013 Antiretroviral Guidelines with Australian Commentary. [cited 2014 11/03/2014];Australasian Society for HIV Medicine. 2014 [updated 2/12/2013]; Available from: http://arv.ashm.org.au/arv-guidelines/initiating-art-in-treatment-naive-patients.
- 14.Wright S, Petoumenos K, Boyd M, Carr A, Downing S, O'Connor C, et al. Ageing and long-term CD4 cell count trends in HIV-positive patients with 5 years or more combination antiretroviral therapy experience. HIV Med. 2013;14(4):208–216. doi: 10.1111/j.1468-1293.2012.01053.x. Epub 2012/10/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–129. doi: 10.1016/s0140-6736(02)09411-4. Epub 2002/07/20. [DOI] [PubMed] [Google Scholar]
- 16.Hughes R, Sterne J, Walsh J, Bansi L, Gilson R, Orkin C, et al. Long-term trends in CD4 cell counts and impact of viral failure in individuals starting antiretroviral therapy: UK Collaborative HIV Cohort (CHIC) study. HIV Med. 2011;12(10):583–593. doi: 10.1111/j.1468-1293.2011.00929.x. Epub 2011/05/17. [DOI] [PubMed] [Google Scholar]
- 17.Torian LV, Xia Q. Achievement and maintenance of viral suppression in persons newly diagnosed with HIV, New York City, 2006–2009: using population surveillance data to measure the treatment part of "test and treat". J Acquir Immune Defic Syndr. 2013;63(3):379–386. doi: 10.1097/QAI.0b013e3182926b02. Epub 2013/03/29. [DOI] [PubMed] [Google Scholar]
- 18.Bartlett JA, Johnson J, Herrera G, Sosa N, Rodriguez A, Liao Q, et al. Long-term results of initial therapy with abacavir and Lamivudine combined with Efavirenz, Amprenavir/Ritonavir, or Stavudine. J Acquir Immune Defic Syndr. 2006;43(3):284–292. doi: 10.1097/01.qai.0000243092.40490.26. Epub 2006/09/13. [DOI] [PubMed] [Google Scholar]
- 19.May MT, Sterne JA, Costagliola D, Sabin CA, Phillips AN, Justice AC, et al. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368(9534):451–458. doi: 10.1016/S0140-6736(06)69152-6. Epub 2006/08/08. [DOI] [PubMed] [Google Scholar]
- 20.The Australian HIV Observational Database. Rates of combination antiretroviral treatment change in Australia, 1997–2000. HIV Med. 2002;3(1):28–36. doi: 10.1046/j.1464-2662.2001.00094.x. Epub 2002/06/13. [DOI] [PubMed] [Google Scholar]
- 21.Fischl MA, Richman DD, Hansen N, Collier AC, Carey JT, Para MF, et al. The safety and efficacy of zidovudine (AZT) in the treatment of subjects with mildly symptomatic human immunodeficiency virus type 1 (HIV) infection. A double-blind, placebo-controlled trial. The AIDS Clinical Trials Group. Ann Intern Med. 1990;112(10):727–737. doi: 10.7326/0003-4819-112-10-727. Epub 1990/05/15. [DOI] [PubMed] [Google Scholar]
- 22.Staszewski S, Miller V, Sabin C, Schlecht C, Gute P, Stamm S, et al. Determinants of sustainable CD4 lymphocyte count increases in response to antiretroviral therapy. AIDS. 1999;13(8):951–956. doi: 10.1097/00002030-199905280-00011. Epub 1999/06/17. [DOI] [PubMed] [Google Scholar]
- 23.Li TS, Tubiana R, Katlama C, Calvez V, Ait Mohand H, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351(9117):1682–1686. doi: 10.1016/s0140-6736(97)10291-4. Epub 1998/09/12. [DOI] [PubMed] [Google Scholar]
- 24.Ortiz R, Dejesus E, Khanlou H, Voronin E, van Lunzen J, Andrade-Villanueva J, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatmentnaive HIV-1-infected patients at week 48. AIDS. 2008;22(12):1389–1397. doi: 10.1097/QAD.0b013e32830285fb. Epub 2008/07/11. [DOI] [PubMed] [Google Scholar]
- 25.Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. New Engl J Med. 2006;354(3):251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 26.Davidson I, Beardsell H, Smith B, Mandalia S, Bower M, Gazzard B, et al. The frequency and reasons for antiretroviral switching with specific antiretroviral associations: the SWITCH study. Antiviral Res. 2010;86(2):227–229. doi: 10.1016/j.antiviral.2010.03.001. Epub 2010/03/10. [DOI] [PubMed] [Google Scholar]
- 27.Hammer SM, Saag MS, Schechter M, Montaner JS, Schooley RT, Jacobsen DM, et al. Treatment for adult HIV infection-2006 recommendations of the International AIDS Society--USA panel. Top HIV Med. 2006;14(3):827–843. Epub 2006/10/05. [PubMed] [Google Scholar]
- 28.Australian HIV Observational Database Annual Report. Institute TK. The University of New South Wales; 2013. editor. [Google Scholar]
- 29.Falster K, Gelgor L, Shaik A, Zablotska I, Prestage G, Grierson J, et al. Trends in antiretroviral treatment use and treatment response in three Australian states in the first decade of combination antiretroviral treatment. Sexual health. 2008;5(2):141–154. doi: 10.1071/sh07082. Epub 2008/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller WC, Powers KA, Smith MK, Cohen MS. Community viral load as a measure for assessment of HIV treatment as prevention. Lancet Infect Dis. 2013;13(5):459–464. doi: 10.1016/S1473-3099(12)70314-6. Epub 2013/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia Q, Wiewel EW, Torian LV. Revisiting the methodology of measuring HIV community viral load. J Acquir Immune Defic Syndr. 2013;63(2):e82–e84. doi: 10.1097/QAI.0b013e318282d2a4. Epub 2013/05/15. [DOI] [PubMed] [Google Scholar]
- 32.Law MG, Woolley I, Templeton DJ, Roth N, Chuah J, Mulhall B, et al. Trends in detectable viral load by calendar year in the Australian HIV observational database. Journal of the International AIDS Society. 2011;14:10. doi: 10.1186/1758-2652-14-10. Epub 2011/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]