Abstract
Objective
This study investigated the roles of the H1 and H2 histamine receptors, nitric oxide (NO) synthase, and soluble guanylate (sGC) cyclase in histamine-induced modulation of rat mesenteric collecting lymphatic pumping.
Methods
Isolated rat mesenteric collecting lymphatics were treated with 1–100 μM histamine. Histamine receptors were blocked with either the H1 antagonist mepyramine or the H2 antagonist cimetidine. The role of NO/sGC signaling was tested using the arginine analog L-NAME, the sGC inhibitor ODQ, and sodium nitroprusside (SNP) as a positive control.
Results
Histamine applied at 100 μM decreased tone and contraction frequency (CF) of isolated rat mesenteric collecting lymphatics. Pharmacologic blockade of either H1 or H2 histamine receptors significantly inhibited the response to histamine. Pretreatment with ODQ, but not L-NAME, completely inhibited the histamine-induced decrease in tone. ODQ pretreatment also significantly inhibited SNP-induced lymphatic relaxation.
Conclusions
H1 and H2 histamine receptors are both involved in histamine-induced relaxation of rat mesenteric collecting lymphatics. NO synthesis does not appear to contribute to the histamine-induced response. However, sGC is critical for the histamine-induced decrease in tone and contributes to the drop in CF.
Keywords: lymph flow, lymphatic pump, endothelium, signal transduction, Calcium (Ca2+), Contractility, Histamine, Lymphatic Vessel
INTRODUCTION
The lymphatic system has key roles in tissue fluid homeostasis, lipid absorption, and immunity. Lymphatic dysfunction has been linked to both acute and chronic inflammation [1]. Histamine, one of the principal mediators of inflammation, is found within lymphatic vessels after tissue injury and modulates the intrinsic pumping activity of these vessels that contribute to lymph flow [7,26,34,35]. Mast cells are abundant in the vicinity of mesenteric lymphatics, and are poised to release their stores of histamine upon activation during an inflammatory response [5,26].
Histamine increases blood flow and microvascular permeability [4], promoting the elevated interstitial pressure that drives lymph formation [34]. Ferguson and colleagues observed that topical application of histamine to the exteriorized rat mesentery caused increased lymph flow, with collecting lymphatics displaying elevated diameter, stronger phasic contractions, and no change in contraction frequency [9]. Likewise, Dobbins and colleagues observed that histamine increased lymphatic perfusion pressure in the canine forelimb [6]. While these studies highlighted how collecting lymphatics respond to histamine in vivo, other reports by Ohhashi et al and Johnston et al indicated that high concentrations of histamine increased rhythmic contractions of isolated bovine mesenteric lymphatics [17,24]. Additional observations revealed that histamine increased contraction frequency and tone of isolated bovine mesenteric lymphatic smooth muscle when applied at concentrations greater than 5 μM, while at lower concentrations (50 nM – 1 μM) histamine reduced contraction frequency [37]. Pharmacologic studies attributed the acceleration of lymphatic phasic contractions to the histamine H1 receptor subtype and the deceleration to the H2 receptor subtype [37]. Similar observations in guinea pig mesenteric lymphatics were later reported by Fox and von der Weid [11].
Ferguson later showed that acetylcholine or bradykinin could relax porcine tracheobronchial lymphatic vessel rings preconstricted with histamine in an endothelium-dependent manner [10]. Shortly thereafter, Ferguson and colleagues, and Ohhashi and colleagues independently demonstrated NO as the endothelium-derived relaxing factor in lymphatics [8,16,28]. In addition, studies utilizing canine thoracic duct revealed that although histamine could increase constriction on its own, it also had the ability to cause relaxation following norepinephrine-induced preconstriction [33]. In contrast to many of the previous studies using bovine, porcine and guinea pig lymphatics, Petunov and colleagues showed that low concentrations of histamine (10−9 – 10−8 M) increased contraction frequency and amplitude, while higher concentrations (10−6 – 10−4 M) decreased lymphatic contraction frequency and amplitude in an endothelium-dependent manner [25].
The latter observation provokes questions about the mechanism of relaxation of rat mesenteric lymphatic vessels caused by the higher concentrations of histamine. We addressed this by investigating the expression, localization, and function of the H1 and H2 histamine receptors in rat mesenteric collecting lymphatics. We also investigated the potential involvement of NO-mediated relaxation. Several reports indicate that lymphatic endothelial-derived NO inhibits lymphatic smooth muscle tone and phasic contraction frequency and amplitude [13,15,22,29]. We also studied the potential involvement of sGC in histamine-induced lymphatic relaxation because of its established role in NO-mediated reductions in vascular smooth muscle tone [3,12].
MATERIALS AND METHODS
Materials
Histamine dichloride (cat. no. H7250), NG-nitro-L-arginine methyl ester HCl (L-NAME, cat. no. N5751), and sodium nitroprusside(III) dihydrate (SNP; cat. no. 228710) were purchased from Sigma-Aldrich (St. Louis, MO). Mepyramine maleate (cat. no. 0660) and cimetidine (cat. no. 0902) were obtained from Tocris Bioscience (Bristol, UK). ODQ (cat. no. 81410) was purchased from Cayman Chemical (Ann Arbor, MI). Crystallized bovine albumin (purified BSA; cat. no. 10856) used for preparing physiological salt solutions was purchased from Affymetrix (Santa Clara, CA). For blocking solutions, BSA (cat. no. A3059) was purchased from Sigma-Aldrich. Rabbit anti-H1 (cat. no. GTX70501) and rabbit anti-H2 (cat. no. GTX108152) were purchased from GeneTex (Irvine, TX). Mouse anti-α/γ-smooth muscle actin (cat. no. Mab1522) was purchased from Millipore (Billerica, MA). Goat anti-VE-cadherin (cat. no. sc-6458) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Donkey anti-rabbit IgG-HRP (cat. no. ab97064) was obtained from Abcam (Cambridge, MA). Alexa-488-donkey anti-rabbit IgG (cat. no. A21206), Alexa-555-donkey-anti-goat IgG (cat. no. A21432) and Alexa-647-donkey anti-mouse IgG (cat. no. A31571) were obtained from Life Technologies (Grand Island, NY). All other chemicals and reagents, unless otherwise specified, were purchased from Sigma-Aldrich.
Animals
All procedures were approved by the Institutional Animal Care and Use Committees (IACUC) at the Louisiana State University Health Sciences Center and the University of South Florida, and were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (8th Edition, 2011). Male Sprague-Dawley rats (275–350 g body weight) were housed in a controlled temperature (22 °C) and controlled illumination (12:12 h light dark cycle) environment. After arrival, the rats were submitted to a one-week acclimation period and were provided standard rat chow (2018 Teklad Global 18% Protein Rodent Diet, Harlan) and water ad libitum.
Collecting Lymphatic Isolation
Rats were anesthetized (i.p. ketamine and xylazine at 90 and 9 mg/kg, respectively), a midline laparotomy was performed, and the small intestine and mesentery were exteriorized, excised, and placed in chilled (4 °C) albumin physiological salt solution (APSS: NaCl, 120 mM; KCl, 4.7 mM; CaCl2·2H2O, 2 mM; MgSO4·7H2O, 1.2 mM; NaH2PO4, 1.2 mM; Na pyruvate, 2 mM; glucose, 5 mM; EDTA, 0.02 mM; MOPS, 3 mM and purified BSA 1 g/100ml). Rats were euthanized with Euthasol/Somnasol (0.1 ml/450 g body weight, i.m.) followed by opening of the chest to confirm death. In each experiment, a section of mesentery was pinned in a dissection chamber containing chilled APSS, and with the aid of a stereomicroscope, a collecting lymphatic vessel (60–200 μm internal diameter and 0.5–1 cm of length) was carefully dissected from surrounding adipose and connective tissue. The isolated lymphatic was transferred to an isolated vessel chamber (Living Systems Instrumentation, Burlington, VT) and was mounted onto two resistance-matched glass micropipettes and secured with nylon thread. The chamber was transferred to a Nikon Eclipse TS100 inverted microscope, equipped with a halogen lamp, 10X objective, and CCD camera for video image acquisition. The intraluminal pressure was controlled by a servo-controlled feedback pressure system (Living Systems). All experiments were performed with the vessel bathed in 37°C APSS with the intraluminal pressure set at 2 cm H2O. This intraluminal pressure is within the normal physiological range for lymphatics [39]. Only lymphatic vessels that displayed phasic contractions that reduced internal diameter during systole by at least 25% of the diastolic diameter were used for these studies.
Experimental Protocols
After cannulation, the vessels were allowed to equilibrate for 30–45 minutes in order to establish baseline contractions. Upon achieving a steady baseline pumping, the lymphatic response to 1–100 μM histamine was evaluated. To test the roles of the H1 and H2 histamine receptors, lymphatics were pretreated for 20 min with either the selective H1 antagonist mepyramine (100 μM) or the selective H2 antagonist cimetidine (100 μM) prior to the addition of 100 μM histamine. In order to establish whether histamine effects were due to NO, lymphatic vessels were pretreated for 20 min with 100 μM L-NAME then subjected to 100 μM histamine. The NO donor sodium nitroprusside (SNP) was used to confirm NO-mediated lymphatic relaxation, and was added directly to the bath at 1 μM concentration. The role of sGC was tested by pretreating the vessels with ODQ for 10 minutes, then subjecting the vessels to either 100 μM histamine or 1 μM SNP. Vessel diameters were tracked throughout each experiment and the bath solution was changed to Ca2+-free APSS at the end of each experiment to determine the maximal passive diameter (MaxD).
The following parameters were determined from the lymphatic intraluminal diameter measurements [30–32]: Contraction frequency (CF), end diastolic diameter (EDD), end systolic diameter (ESD), amplitude of contraction (AMP = EDD−ESD), tone = 100 * (MaxD − EDD)/MaxD, and ejection fraction (EF) = (EDD2−ESD2)/(EDD2). EDD, ESD, and AMP were normalized to MaxD to account for variability in the resting diameter of lymphatics. For baseline data, the means presented for each parameter represent the averages for the 5-min period just prior to the addition of inhibitors or histamine. For each concentration of histamine, the 5-min increment after the first 2 min post addition was used to calculate the mean data (i.e., means represent 3–7 min after addition of each concentration). When inhibitors were added, we utilized the last 5-min period just prior to the addition of histamine to calculate the mean effect of the inhibitor.
Immunoblotting Protocol
Approximately 10 lymphangions, freshly isolated from rat mesentery, were placed in 200 μl 1X RIPA (4 °C) containing HALT Protease and Phosphatase Inhibitor Cocktail (Pierce, Rockford, IL). The mixture was sonicated at 4 °C for 5 s twice using a Fisher Sonic Dismembranator, model FB-120 (Fisher Scientific, Asheville, NC). Protein concentrations were determined using the BCA protein assay (Pierce) to equilibrate samples. Lysate was mixed with 4X NuPage LDS sample buffer containing reducing agent (Invitrogen, Grand Island, NY). Samples containing 40 μg of protein were loaded into 4–20% Novex Bis-Tris gels (Invitrogen) for SDS-PAGE to separate proteins. The NexusPointer prestained protein ladder (BioNexus, Oakland, CA) was used to determine molecular weight vs. mobility. The proteins were transferred from the gels to Immobilon-P PVDF membranes (Millipore) by wet transfer. Membranes were blocked with 5% BSA in TBST. Primary antibodies were 1:200 rabbit anti-H1 (GTX70501) or 1:200 rabbit anti-H2 (GTX108152). The secondary antibody was 1:3000 donkey anti-rabbit IgG-HRP (ab97064). Bands were visualized using WestPico Supersignal reagent (Pierce; 5 min incubation) and a BioRad Chemi Doc XRS+ System with Quantity 1-D Analysis Software (5 min exposure).
Immunofluorescence Labeling and Confocal Microscopy
Rat mesenteric lymphatics were isolated and one end was mounted onto a glass micropipette filled with APSS in a custom chamber containing APSS for fixation and labeling. The other end of the lymphatic was left free to float in the bath, to allow for bath solution to be pushed into the vessel lumen by gently applying positive pressure on the micropipette via a 1 ml syringe. Sometimes a lymphatic valve restricted flow and in this case the vessel had to be removed and remounted onto the cannula between certain steps. Also, a small amount of luminal pressure was applied from time to time to prevent vessel collapse during the labeling protocol, with solutions appropriate to the various steps. Each vessel was fixed with 4% paraformaldehyde for 10–15 min at room temperature, followed by two 5-min washes with 100 mM glycine buffer and one 5-min wash with Ca2+/Mg2+-free Dulbecco’s PBS (CMF-DPBS). Ice-cold acetone (−10 to −20 °C) was applied for 5 min to permeabilize the cell membranes, followed by three 5-min washes with CMF-DPBS. A blocking solution consisting of 5% normal donkey serum in CMF-DPBS was applied for 30 min at room temperature, followed by overnight incubation with primary antibodies in antibody dilution buffer (151 mM NaCl, 17 mM trisodium citrate, 2% donkey serum, 1% BSA, 0.05% Triton X-100, 0.02% NaN3) at 4 °C: 1:6000 mouse anti-α/γ-smooth muscle actin (Mab1522); 1:50 goat anti-VE-cadherin (sc-6458); and either 1:200 rabbit anti-H1 or anti-H2 (GTX70501 or GTX108152). Labeling controls received antibody dilution buffer containing no primary antibody. After overnight incubation, three 10-min rinses with antibody wash solution (151 mM NaCl, 17 mM trisodium citrate, 0.05% Triton X-100) were performed. The vessels were incubated for 30–60 minutes at room temperature with antibody dilution buffer containing secondary antibodies: 1:400 Alexa-488-donkey anti-rabbit IgG (A21206), 1:400 Alexa-555-donkey-anti-goat IgG (A21432) and 1:400 Alexa-647-donkey anti-mouse IgG (A31571). Three 10-min rinses with antibody wash solution were performed, and each vessel was removed from its cannula, placed on a glass slide with a Secure-Seal imaging spacer in 10 μl of Prolong Gold Anti-Fade reagent containing DAPI (Life Technologies), and covered with a #1 glass coverslip. Care was taken to keep the lymphatic vessel lumen patent. Confocal z-stack images of the vessels were obtained with an Olympus FV1000 MPE multiphoton laser-scanning microscope, using single-photon mode, and a 40X objective (UPLFLN, NA 0.75), at the Lisa Muma Weitz Advanced Microscopy and Cell Imaging Core at the University of South Florida. Confocal images were obtained with four different excitation channels (405, 488, 568, and 635 nm). The depth of field (z-section thickness) was estimated at 1.58 μm, using the longest excitation wavelength (635 nm) multiplied by a diffraction index of 1.4 for living cells, divided by the square of the numerical aperture (0.75). FIJI/ImageJ open source imaging software (http://fiji.sc) was used to process confocal image stacks into montage figures and movie files [27].
Data Analysis
Representative tracings are shown for the histamine and inhibitor treatment protocols. Summarized data are presented as mean ± SE. The responses of lymphatic vessels over time to various treatments were evaluated with repeated measures ANOVA, followed by Dunnett’s test to compare individual time points to baseline or Tukey’s test to compare all time points to each other when appropriate. When comparing two or more time-matched groups, two-way repeated measures ANOVA was used, followed by Šidak multiple comparisons tests when appropriate to compare a particular group at two different time points or two groups at the same time point. For a single comparison between baseline and treatment, a paired t-test was used. Significance was accepted at P<0.05.
RESULTS
Histamine decreases tone and CF of isolated rat mesenteric lymphatic vessels
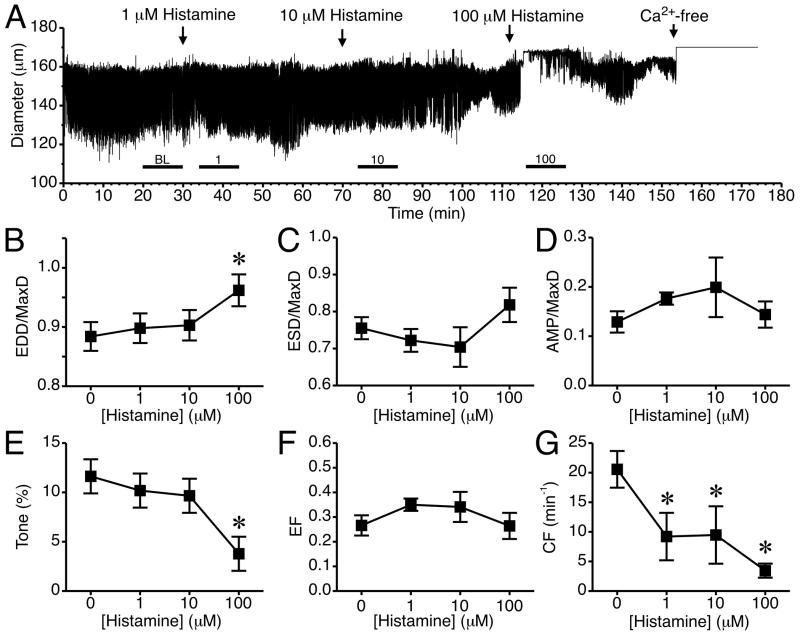
The time course of changes in luminal diameter from a representative isolated lymphatic vessel treated with histamine at concentrations of 1, 10, and 100 μM is shown in Fig. 1A. Addition of 1 and 10 μM histamine caused no consistent noticeable changes in pumping compared to baseline. However, after 100 μM histamine was added, the vessel diameter shifted upward and phasic contractions stopped for 1–2 minutes. The summarized data from five lymphatics treated with 1, 10, and 100 μM histamine are shown in Fig. 1, panels B–G. With the addition of 100 μM histamine, the mean EDD/MaxD increased significantly (Fig. 1B). Mean ESD/MaxD did not change significantly (Fig. 1C), and there was no change in AMP/MaxD (Fig 1D). Histamine at 100 μM significantly decreased tone (Fig. 1E), but did not significantly affect mean EF at any of the concentrations tested (Fig. 1F). Histamine (1–100 μM) also significantly decreased mean CF (Fig. 1G). It is important to note that during the 5-minute time period used to calculate CF, in most vessels histamine caused a brief cessation in phasic contractions, which contributed to the lower average CF.
Fig. 1.
Histamine and rat lymphatic mesenteric vessel pumping. (A) Time course of the changes in diameter after the addition of 1, 10, or 100 μM histamine to the isolated lymphatic vessel bath for a representative lymphatic vessel. The bath solution was changed to Ca2+-free APSS at the end of the experiment to determine MaxD. The subsequent panels (B–G) show the changes in mean values for (B) EDD/MaxD, (C) ESD/MaxD, (D) AMP/MaxD, (E) Tone, (F) EF, and (G) CF. The bars just above the x-axis show the time periods used for baseline (BL) and each concentration of histamine to determine the summarized data in panels B–G. (*P<0.05 versus baseline, prior to the addition of histamine; N=5 lymphatic vessels studied).
H1 and H2 receptors are present on rat mesenteric collecting lymphatic vessels
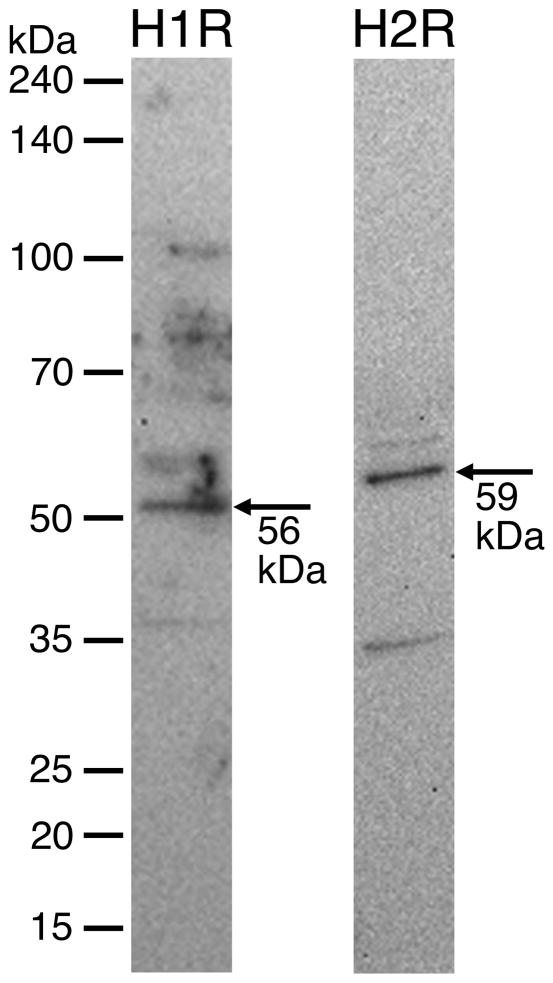
Western blot analysis revealed that both the H1 and H2 histamine receptors are present in protein lysate obtained from isolated rat mesenteric lymphatic vessels (Fig. 2). The H1 and H2 receptors were also visualized using immunofluorescence labeling and confocal microscopy of isolated lymphatic vessels (Figs. 3 and 4, Movies 1 and 2).
Fig. 2.
Western blotting of H1 and H2 receptors from isolated rat mesenteric lymphatic protein lysate. Each lane was loaded with 40 μg protein. Blots are representative of three separate experiments for each antibody. Control blots (no primary antibody) showed no bands (data not shown).
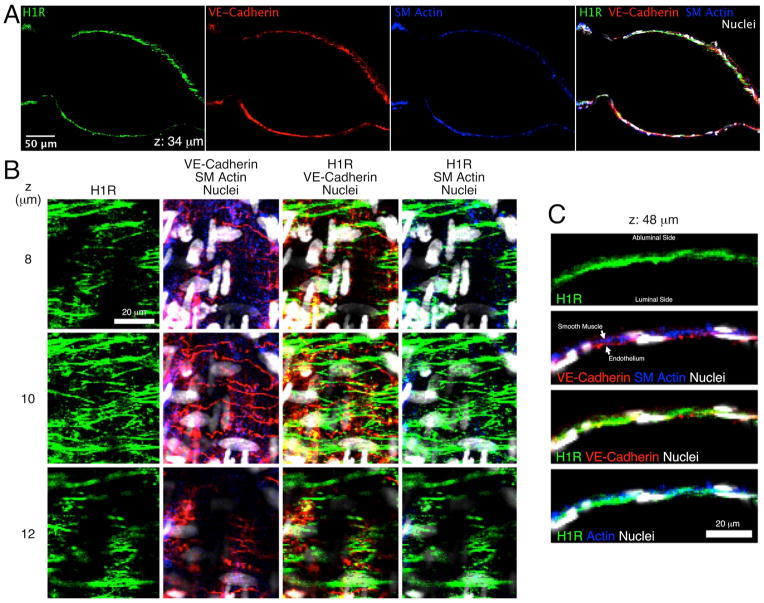
Fig. 3.
Confocal images of H1 histamine receptors in an isolated rat mesenteric lymphatic vessel. A. Images from a single confocal plane show labeling of the H1 receptor (H1R; green), VE-cadherin (red), and smooth muscle (SM) actin (blue) in the lymphatic walls. An overlay of these three channels, plus nuclei (white) is also shown. B. Close up images taken at 8, 10, and 12 μm from the start of the z-stack, featuring an en face view of the lymphatic wall. C. Close up images from a single z-section taken at 48 μm from the start of the z-stack, featuring a cross-sectional view of the lymphatic wall. Representative of three separate labeling experiments.
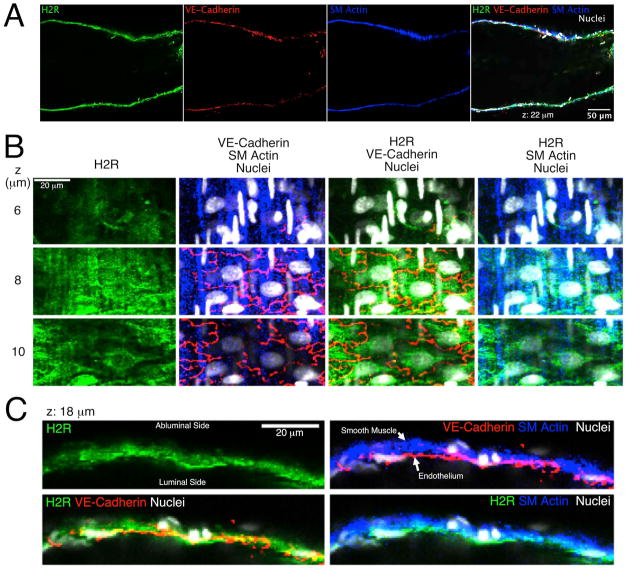
Fig. 4.
Confocal images of H2 histamine receptors in an isolated rat mesenteric lymphatic vessel. A. Images from a single confocal plane show labeling of the H2 receptor (H2R; green), VE-cadherin (red), and smooth muscle (SM) actin (blue) in the lymphatic walls. An overlay of these three channels, plus nuclei (white) is also shown. B. Close up view of z-sections obtained at 6, 8, and 10 μm from the start of the series, featuring an en face view of the lymphatic wall. C. Close up images from a single confocal plane taken at 18 μm from the start of the z-stack, featuring a cross-sectional view of the lymphatic wall. Representative of three separate labeling experiments.
Fig. 3A shows labeling of the H1 receptor, VE-cadherin (to label the outline of endothelial cells), smooth muscle actin, and cell nuclei overlayed with these three channels, in a single confocal z-section of an isolated rat mesenteric lymphatic vessel. The vessel is oriented horizontally, and the z-section is near the center longitudinal axis of the vessel, so that the lumen is in the field of view, with the vessel wall oriented above and below. The entire confocal z-stack, in which images were obtained every 2 μm in the z plane, is also provided in Movie 1. H1 receptor labeling was organized in longitudinal structures that appeared in both the smooth muscle and endothelial layers (Fig 3B). Upon closer inspection, the most intense H2 labeling was in the vicinity of VE-cadherin labeling. The z-sections in Fig. 3B show a region where the lymphatic wall was oriented en face, with the abluminal side closest to the microscope objective. In the first row of images, obtained at z=8 μm, H1 labeling can be detected. In this z-plane, the smooth muscle layer was identified by the presence of smooth muscle actin and narrow smooth muscle cell nuclei oriented perpendicular to the axis of the lymphatic lumen (vertical in these images). At z=10 μm, the H1 labeling is more intense, the smooth muscle actin labeling and smooth muscle nuclei fade, and VE-cadherin labeling showing the outlines of endothelial cells becomes apparent. Endothelial nuclei are also visible in the centers of these outlined cells. At z=12 μm, H1 labeling fades in some parts of the image. In the areas of this plane where H1 labeling is still visible, evidence of endothelial cells also remains visible. One smooth muscle cell remains visible on the left side in this plane as well. Fig. 3C shows a confocal cross section of the lymphatic wall taken at z=48 μm (Fig. 3C). H1R labeling is present almost continuously along the length of the lymphatic wall. The smooth muscle layer is again identified by smooth muscle actin labeling and by the cross sections of smooth muscle cell nuclei, which appear circular and small (<5 μm diameter). VE-cadherin in endothelial cell junctions is also visible, and cross sections of endothelial cell nuclei typically appear long and thin on the luminal side of the wall. Overlays of H1R labeling with the smooth muscle actin and VE-cadherin labeling showed that some H1R localizes within smooth muscle cells, but much more lies in the endothelial layer.
H2 receptor labeling was also observed in confocal sections of rat collecting mesenteric lymphatics (Fig. 4). The labeling of the H2 receptor, VE-cadherin, smooth muscle actin, and an overlay in a single confocal z-section is shown in Fig. 4A, and the entire z-stack is provided in Movie 2. Fig. 4B displays a montage of z-sections with the lymphatic wall positioned en face, with the abluminal side closer to the microscope objective. Labeling of the H2 receptor was strongest at the tight interface between the endothelium and smooth muscle layer, observed at the 8 μm z-section by the presence of both smooth muscle actin and VE-cadherin, and the presence of both smooth muscle cell and endothelial cell nuclei (Fig. 4B). At the preceding z-section (6 μm), where predominantly smooth muscle actin labeling was observed, little H2 receptor labeling was apparent. In contrast, at the subsequent z-section (10 μm), in areas where there was little smooth muscle actin labeling, H2 receptor labeling was observed in perinuclear areas of endothelial cells, identified by the outline of VE-cadherin. In Fig. 4C, showing a cross-sectional view of the lymphatic wall, H2 receptor labeling was strong in the inner surface in the vicinity of VE-cadherin, but also present in the outer layer where smooth muscle actin was present. H2 receptor labeling was also observed in what appear to be neurons, judged by the long, thin axon-like processes, and attached cell bodies. These cells were attached to the outer surface of the lymphatics, visible in the 4 and 6 μm z-sections of movie 2. Images from two different isolated lymphatics are shown in Supplemental Figure S1.
Antagonists of either the H1 or H2 histamine receptor block histamine-induced lymphatic relaxation
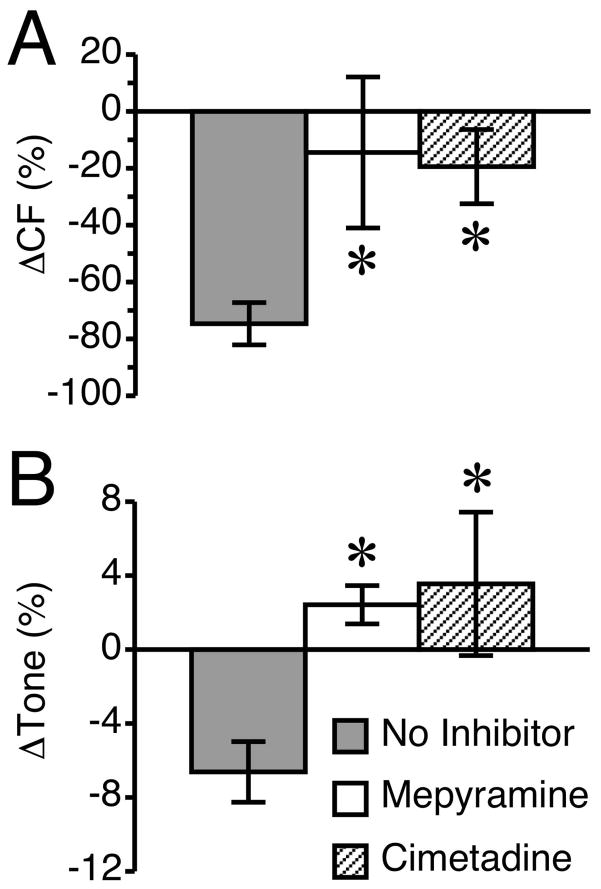
Because both the H1 and H2 histamine receptors were present on lymphatic vessels, we tested the role of each of these receptors with the specific pharmacologic antagonists, mepyramine and cimetidine, respectively. Neither histamine receptor antagonist significantly altered any of the lymphatic parameters measured during the period prior to the addition of histamine (data not shown). However, both significantly inhibited the histamine-induced relaxation of lymphatics (Fig. 5). The histamine-induced reduction in CF was significantly inhibited by either H1 or H2 blockade (Fig. 5A), as was the histamine-induced reduction in lymphatic tone (Fig. 5B). These findings suggest involvement of both the H1 and H2 receptors in histamine-induced lymphatic relaxation.
Fig. 5.
Antagonists of either the H1 or H2 receptor block histamine-induced lymphatic relaxation. The H1 receptor blocker, mepyramine (100 μM) and the selective H2 blocker cimetidine (100 μM) were each applied 20 min prior to the application of 100 μM histamine. A time-matched “no inhibitor” group was used for comparison. Both blockers significantly inhibited the histamine-induced relaxation of lymphatics. (A) The histamine-induced reduction in CF was significantly inhibited by either H1 or H2 blockade. (B) The histamine-induced reduction in tone was significantly inhibited by both H1 and H2 receptor blockers. *P<0.05 versus no inhibitor, post addition of histamine. N=7 lymphatics studied for the “no inhibitor” group and N=5 lymphatic vessels each for the mepyramine and cimetidine groups.
NO/sGC and the histamine-induced lymphatic relaxation
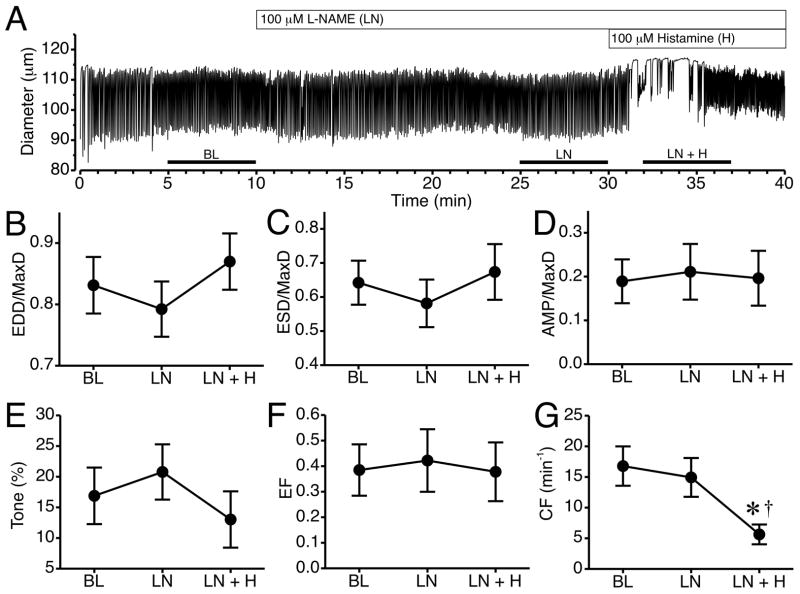
Because we observed a greater degree of labeling of both H1 and H2 receptors on lymphatic endothelial cells, and their joint involvement in histamine-induced lymphatic relaxation, we investigated the potential role of a known endothelial-derived relaxation pathway: NO/sGC signaling. L-NAME (100 μM) was used to inhibit NO synthase (NOS) prior to addition of histamine (Fig. 6). The time course of changes in luminal diameter from a representative isolated lymphatic vessel pretreated with L-NAME for 20 min with addition of histamine is shown in Fig. 6A. Addition of L-NAME caused no noticeable changes in pumping compared to baseline. After 100 μM histamine was added, the vessel diameter shifted slightly upward and phasic contractions were erratic with brief cessations for several minutes. The summarized data from six lymphatics subjected to this protocol are shown in Fig. 6B–G. When L-NAME was present, histamine did not significantly elevate normalized EDD or ESD (Fig. 6B, C). Mean normalized AMP, tone, and EF also did not change significantly (Fig. 6D–F). However, histamine did decrease mean CF after L-NAME pretreatment, causing a significant decrease compared to both baseline and L-NAME alone (Fig. 6G). It is important to note that during the 5-minute time period used to calculate CF, in most vessels histamine caused a brief cessation in phasic contractions, which contributed to the lower average CF.
Fig. 6.
Impact of NOS inhibition on histamine-induced collecting lymphatic relaxation. A. Representative time course of changes in lymphatic luminal diameter in response to the NOS inhibitor L-NAME (LN) and subsequent addition of histamine (LN+H). Changes in mean EDD/MaxD (B), ESD/MaxD (C), AMP/MaxD (D), Tone (E), EF (F), and CF (G) are also shown. The bars just above the x-axis show the time periods used to calculate the means in panels B–G. *P<0.05 versus baseline. †P<0.05 versus L-NAME alone. N=6 lymphatic vessels studied.
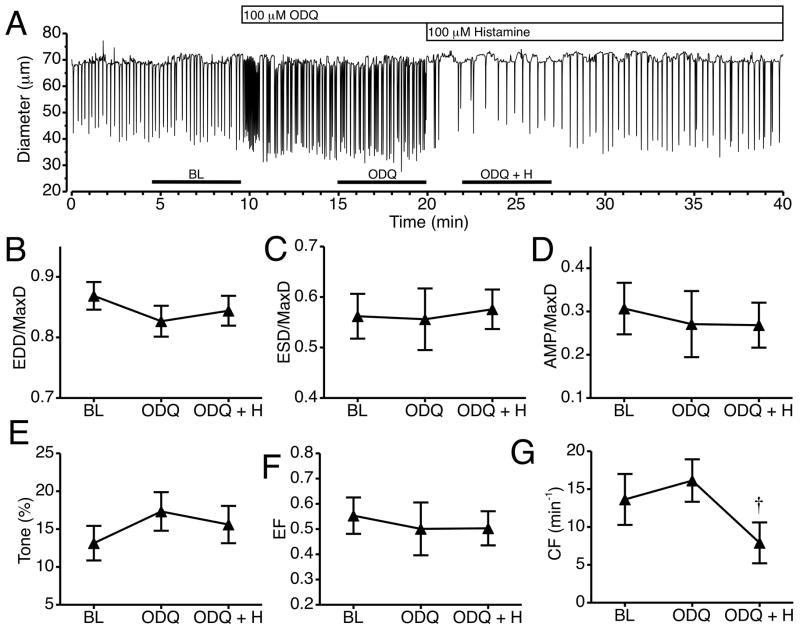
We also tested whether histamine-induced collecting lymphatic relaxation requires soluble guanylate cyclase (sGC) with the sGC inhibitor ODQ. An example of the changes in lymphatic luminal diameter in response to 100 μM ODQ and subsequent addition of 100 μM histamine is shown in Fig. 7A. ODQ caused a noticeable increase in the CF immediately after its addition that lasted about a minute, followed by an noticeable increase in CF until histamine was added 10 min later, when there was a decrease in CF. Fig. 7B–G shows the summarized data from six lymphatics, representing means calculated for 5-minute periods just prior to the addition of ODQ, just prior to histamine, and just after histamine addition. Treatment with ODQ did not significantly change any of the diameter-related parameters (Fig. 7B–G). Addition of histamine after ODQ pretreatment caused no significant changes in normalized EDD, ESD, or AMP, tone, or EF (Fig. 7B–E). In addition, upon histamine treatment, mean CF decreased significantly compared to the mean CF during ODQ treatment alone.
Fig. 7.
Impact of sGC inhibition on histamine-induced collecting lymphatic relaxation. A. Representative time course of changes in lymphatic luminal diameter after the addition of the sGC inhibitor ODQ and subsequent addition of histamine. Changes in mean EDD/MaxD (B), ESD/MaxD (C), AMP/MaxD (D), Tone (E), EF (F), and CF (G) are also shown. The bars just above the x-axis show the time periods used to calculate the means in panels B–G. †P<0.05 versus ODQ alone. N=6 lymphatic vessels studied.
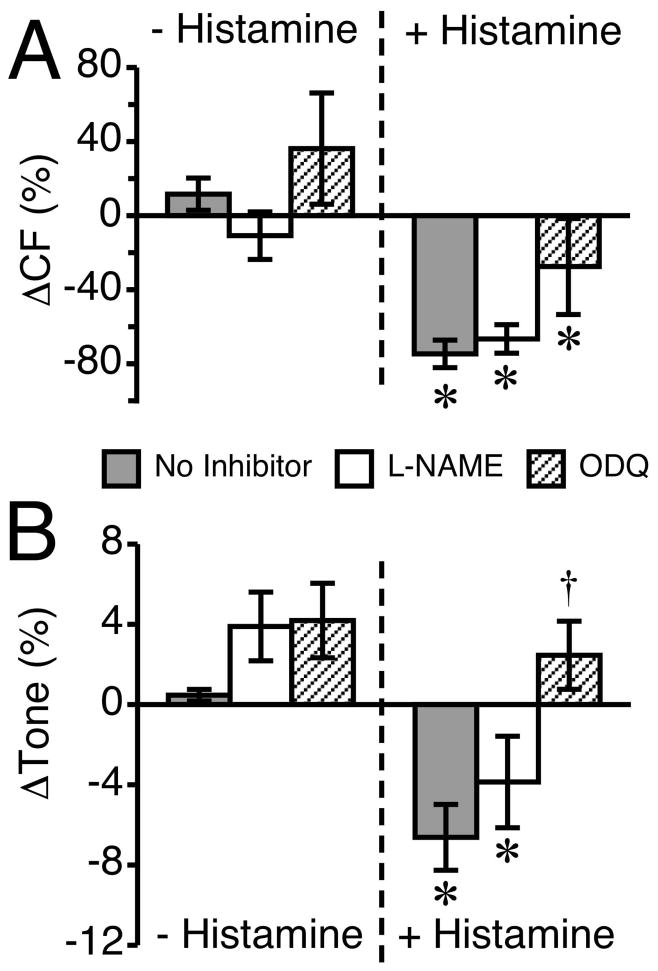
A comparison of lymphatics treated with histamine in the absence of inhibitors to those that were pretreated with the NO/sGC pathway inhibitors is shown in Fig. 8. In both the absence and presence of NO/sGC inhibitors, histamine caused a significant change in CF (Fig. 8A). It is worth noting that in the ODQ-treated lymphatics, although the histamine-induced decrease in CF may appear weaker, the mean CF during ODQ pretreatment was shifted upward from baseline (insignificantly), and the overall decrease from this point was equivalent to the other two groups (Fig. 8A). The impact of histamine on lymphatic tone, in the absence and presence of the NO/sGC inhibitors, is shown in Fig. 8B. An approximately 4% increase in tone occurred after application of either 100 μM L-NAME or 100 μM ODQ, but this was not significant when compared to a time-matched untreated control. When no inhibitor was present, histamine significantly decreased tone, by approximately 7%. In the presence of 100 μM L-NAME, histamine significantly reduced tone to nearly 4% below baseline, from the 4% above baseline tone with inhibitor alone, for an overall net decrease of approximately 8%. In contrast, with 100 μM ODQ pretreatment, which by itself also had a 4% increase in tone, the mean tone after histamine remained elevated, at approximately 2% higher than baseline.
Fig. 8.
Inhibition of the NO/sGC pathway attenuates histamine-induced reductions in lymphatic CF and tone. This figure compares the same data from the lymphatics that received pretreatment with L-NAME or ODQ with lymphatics that had no inhibitors applied prior to the addition of 100 μM histamine. (A) The change in CF is shown for 100 μM L-NAME or 100 μM ODQ versus no treatment (left set of bars), and histamine alone versus histamine treatment in the presence of L-NAME or ODQ (right set of bars). (B) The change in tone is shown in response to the same inhibitors before and after histamine treatment. The “no inhibitor” group baseline measurement was taken during a 5-min period ending 20 min before the addition of histamine, and the change from baseline prior to histamine represents a 5-min period just before the addition of histamine. *P<0.05 versus baseline, same group. †P<0.05 versus no inhibitor + histamine. N=7 for the “no inhibitor” group and N=6 for the L-NAME and ODQ groups.
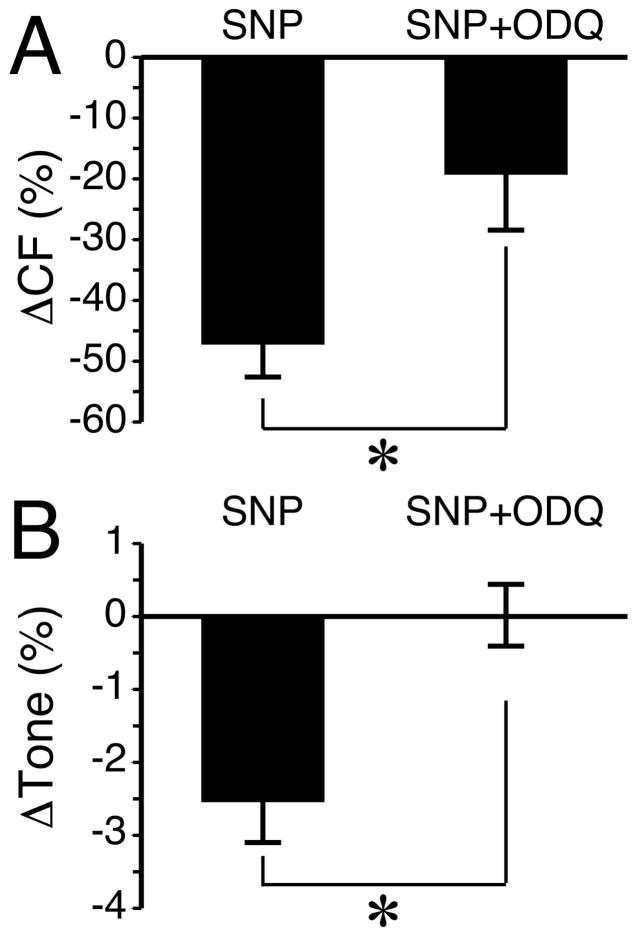
It has previously been shown that NO produced by the exogenous application of the NO donor sodium nitroprusside (SNP), was able to inhibit the phasic constrictions that occur spontaneously or during perfusion in lymphatic vessels of the guinea pig mesentery [36]. In addition, blockade of sGC has been shown to inhibit NO-mediated relaxation of the thoracic duct [14]. To confirm this functionality in isolated rat mesenteric collecting lymphatics, we applied SNP and tested whether blockade of sGC with ODQ would inhibit SNP-mediated lymphatic relaxation. The change in CF caused by 1 μM SNP was significantly attenuated in the presence of ODQ (Fig. 9A). In addition, EDD/MaxD was increased in four out of four vessels treated with SNP (data not shown). This translated into in a mean reduction in tone of approximately 2.5% that was completely abolished during inhibition of sGC with ODQ (Fig. 9B). These data confirmed NO-mediated relaxation in our model, and that sGC plays a role in the NO-mediated decrease in CF and is critical for the NO-mediated decrease in lymphatic tone.
Fig. 9.
SNP-mediated percent changes in (A) CF and (B) Tone are inhibited by ODQ. For both variables, the changes are calculated from the five-minute period before SNP was added and the five-minute period after SNP was added. *P<0.01 between groups. For each treatment, N=4 lymphatic vessels studied.
DISCUSSION
The key findings from this study utilizing isolated rat mesenteric collecting lymphatics are as follows: 1) histamine applied at 100 μM causes lymphatic relaxation, i.e. both tone and phasic contractions are significantly reduced. 2) Both H1 and H2 histamine receptors are detected on rat mesenteric collecting lymphatics, particularly on endothelial cells. 3) Specific blockade of either the H1 or H2 histamine receptors inhibits histamine-induced lymphatic relaxation. 3) Blockade of NO synthesis with L-NAME does not inhibit histamine-induced decreases in lymphatic tone or CF. 4) Inhibition of sGC abrogates the histamine-induced decrease in lymphatic tone. 5) Inhibition of sGC does not block the ability of histamine to decrease CF. 6) Inhibition of sGC attenuates the SNP-induced decrease in CF, and completely blocks the SNP-induced decrease in lymphatic tone. Taken together, these data demonstrate the importance of the H1 and H2 histamine receptors in histamine-induced lymphatic relaxation. In addition, differential mechanisms for histamine-induced decreases in CF and tone are apparent, with the decrease in CF occurring independently of NO/sGC signaling, and the decrease in tone requiring sGC but not NO synthesis.
Alterations in lymphatic contractility may lead to edema, which is also a consequence of increased microvascular permeability during inflammation. Importantly, many inflammatory mediators, which may gain entry to and/or are present within the lymphatic vessels, have been shown to regulate lymphatic pumping activity [26]. Mast cells, located in high amounts in the mesentery where lymphatics are densely located, play an important role in the vascular response during inflammation, and when activated release pro-inflammatory mediators, including histamine [26]. Several studies have shown that histamine modulates pumping of collecting lymphatics, with differential response depending upon the histamine concentration applied, or upon species [34]. In the current study utilizing isolated rat mesenteric lymphatics, 1 and 10 μM histamine did not significantly change lymphatic pump parameters, however 100 μM increased normalized, and reduced tone and mean CF. Our results are in contrast to many of the studies showing histamine-induced increases in CF and tone in bovine, porcine, and guinea pig lymphatics [11,17,24,37]. However, our data are consistent with the reported reduction in force and frequency of phasic contractions of rat mesenteric collecting lymphatics in response to high concentrations of histamine [25].
When compared to the study in which histamine was applied topically to the in vivo rat mesentery, producing increased EDD, larger phasic contractions, and no significant change in CF [9], it is important to note that histamine also relaxes arterioles and increases microvascular permeability, and both of these events increase lymph formation [4,19,20,38]. In turn, this elevation of fluid entering the lymphatics increases both luminal pressure and shear stress. Thus, the direct action of histamine on lymphatics to decrease CF is probably negated in vivo by the increase in luminal pressure, which serves as a stimulus to increase CF. In addition, the amplified histamine-induced increase in lymphatic EDD observed in in vivo is probably due to flow-mediated relaxation [13,15] plus the direct effect of histamine on collecting lymphatics. Although our current data suggest histamine-induced decreases in lymphatic tone do not involve NO synthesis in the isolated lymphatic vessel model, in the in vivo environment where flow-mediated dilation would also be present, NO signaling would be expected to indirectly account for the significant amplification of histamine-induced EDD due to increased lymph formation [9]. In other words, the lack of involvement of NO synthesis in histamine-mediated dilation may allow this mechanism to remain available for flow-mediated dilation.
Several functional studies using various histamine receptor agonists and antagonists have implicated the presence of the H1 and H2 histamine receptors on lymphatic vessels [11,25,28,37]. To our knowledge, this is the first study to confirm expression by Western blot and show detail of the localization of both the H1 and H2 receptors on rat mesenteric lymphatic vessels. The results of our immunofluorescence confocal microscopy study indicated strong labeling of both H1 and H2 receptors in the endothelium, much less labeling of H1 and H2 in the smooth muscle layer, and labeling of H2 receptors in what appear to be neurons on the outer walls of the vessels. The relatively dense labeling of H1 and H2 histamine receptors in the endothelium, combined with our finding that blockade of either H1 or H2 receptors significantly reduces the histamine-induced lymphatic relaxation, implies an endothelial-dependent relaxation mechanism, such as that reported by Petunov and colleagues [25]. Our data is in contrast to results obtained from bovine and guinea pig lymphatics, in which H1 was shown to increase, while H2 was shown to decrease, pump activity [11,37]. Our data also differ in part from those reported by Petunov and colleagues, who measured force changes in rat mesenteric collecting lymphatics using a wire myograph technique [25]. In their study, blockade of the H1 receptor did not inhibit the histamine-induced decrease in CF, while blockade of the H2 receptor did. While they did use a different H1 receptor antagonist (diphenhydramine), this antihistamine has similar properties to mepyramine. Another explanation why we observed inhibition is that we applied mepyramine at a concentration equivalent to our histamine concentration (100 μM), whereas the other study used only 1 μM diphenhydramine [25]. Understanding the causes for these differences, as well as the differential responses between species, represents a key area for future study.
Considering our data showing H1 and H2 receptors on lymphatic endothelium, that blockade of either of these receptors inhibited histamine-induced relaxation, and the finding by Petunov and colleagues that removing the endothelium or applying the NOS inhibitor L-NMMA inhibits histamine-induced relaxation [25], we tested the role of NO/sGC signaling in our model. Surprisingly, in the current study the NOS inhibitor L-NAME did not affect the decrease in CF caused by histamine, in contrast to the inhibition reported by Petunov and colleagues [25]. In addition, inhibition of NOS did not affect the ability of histamine to reduce tone. During blockade of NO synthesis tone was simply shifted slightly higher without affecting the ability of histamine to cause relaxation (Fig. 8B). Several studies have demonstrated NO-dependent relaxation of lymphatics [8,13,15,16,22,28,29]. In our study, we observed consistent constriction (although insignificant in magnitude) in lymphatics treated with L-NAME, and the NO donor SNP caused reductions in tone and CF, showing that NO signaling was functional in the lymphatics we studied. However, the ability of histamine to cause relaxation remained intact, suggesting a NO-independent pathway mediates histamine-induced lymphatic relaxation.
Despite the lack of evidence for the involvement of NO, we found that blockade of sGC inhibited the histamine-induced decrease in tone. Inhibition of sGC did not inhibit the ability of histamine to reduce mean CF. Our finding of slightly elevated CF and tone (consistent response, but not significant in magnitude) with inhibition of sGC is in agreement with similar observations by Mathias and von der Weid in isolated guinea pig collecting lymphatics [21], and suggest a general importance of sGC in regulating CF and lymphatic tone. In addition, our findings suggest histamine decreases CF via a mechanism that does not involve activation of the NO/sGC pathway, and that histamine-induced decreases in lymphatic tone require sGC. NO-independent activation of sGC has been shown with the benzylindazole derivative YC-1 and similar compounds [23]. A future direction will be to identify endogenous, NO-independent activators of sGC.
We should point out that because we used predetermined time frames to collect our summarized data, our findings potentially underestimate some of the effects of histamine and the various inhibitors. The 5-min time frame beginning 2 min after the addition of histamine was used because some lymphatics lagged up to 2 min to produce a noticeable response. The time frames for inhibitors, which were the last 5 min prior to histamine addition, were chosen to allow lymphatics to adjust to a new steady state in the absence of the target receptor or enzyme. However, as a result, any changes in the lymphatic pumping parameters during the initial responses were not included in the means. For example, in Fig. 7A the rapid increase in CF that occurred in the immediate 30 s period following addition of ODQ was not represented in the summarized data. In addition, our study did not evaluate long-term modifications in lymphatic pumping that might be elicited by histamine, which represents an important future topic of investigation.
The four known histamine receptors all belong to the G-protein coupled receptor family. We did not investigate H3 and H4 receptors for a few reasons. First, current evidence suggests these receptors are primarily expressed in the central nervous system and in bone marrow [18]. Second, a previous study showed that the selective H3 receptor agonist R-alpha-methylhistamine did not cause changes in lymphatic pumping [11]. Third, the currently available anti-H4 receptor antibodies have been shown to be nonspecific and thus unreliable [2]. This topic thus represents a potential future area of study once the appropriate tools are available.
In summary, our data show that histamine relaxes isolated perfused collecting lymphatics from the rat mesentery through action on both the H1 and H2 receptors. The evidence supports a relaxing mechanism that is independent of NO synthesis. In addition, the data supports a role for sGC in the histamine-induced decrease in lymphatic tone, but not the histamine-induced decrease in CF. Future studies will be aimed at determining the elusive NO-independent mechanism of relaxation.
Supplementary Material
Fig. S1. H2 histamine receptor immunofluorescence labeling in neuron-like cells present on rat mesenteric collecting lymphatics. A. This image shows the H2 receptor labeling from the 6-μm z-section shown in Movie 2. B. A different rat mesenteric collecting lymphatic also labeled for the H2 histamine receptor. The arrows in both panels indicate axon-like labeling and a suspected neuronal cell body.
Movie 1. Confocal z-stack of H1 histamine receptor labeling in an isolated rat mesenteric collecting lymphatic. Six views of a four-channel image stack, with a total of 51 confocal z-slices, obtained at 2 μm intervals, are shown. The distance in the z-plane is indicated in the top right. In the top row, from left to right, the panels show labeling of VE-cadherin (red), smooth muscle (SM) actin (blue), and the H1 histamine receptor (H1R) (green), with each panel also showing labeled nuclei (white). In the bottom panels, from left to right are the combination of the H1R, VE-cadherin, and nuclei; the H1R, SM Actin, and nuclei; and the combination of all four channels (VE-cadherin, SM actin, H1R, nuclei).
Movie 2. Confocal z-stack of H2 histamine receptor labeling in an isolated rat mesenteric collecting lymphatic. Six views of a four-channel image stack, with a total of 51 confocal z-slices, obtained at 2 μm intervals, are shown. The distance in the z-plane is indicated in the top right. In the top row, from left to right, the panels show labeling of VE-cadherin (red), smooth muscle (SM) actin (blue), and the H2 histamine receptor (H2R) (green), with each panel also showing labeled nuclei (white). In the bottom panels, from left to right are the combination of the H2R, VE-cadherin, and nuclei; the H2R, SM Actin, and nuclei; and the combination of all four channels (VE-cadherin, SM actin, H2R, nuclei).
PERSPECTIVES.
Histamine is a principal inflammatory mediator, and drugs that block histamine receptors are very common in over the counter remedies used for allergies, the common cold, and acid reflux. A better understanding of how histamine impacts lymphatic pump function will provide new insights into the role of lymphatics in inflammation.
Acknowledgments
Research reported in this publication was supported by the National Center for Research Resources and the National Heart, Lung, and Blood Institute under the National Institutes of Health under award numbers P20RR018766 and R01HL098215. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- AMP
amplitude of contraction
- APSS
albumin physiological salt solution
- CF
contraction frequency
- EF
ejection fraction
- EDD
end diastolic diameter
- ESD
end systolic diameter
- L-NAME
NG-nitro-L-arginine methyl ester
- MaxD
maximal passive diameter
- NO
nitric oxide
- NOS
nitric oxide synthase
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- sGC
soluble guanylate cyclase
Footnotes
Microcirculatory Society 2013 August Krogh Young Investigator Award paper
References
- 1.Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- 2.Beermann S, Seifert R, Neumann D. Commercially available antibodies against human and murine histamine H(4)-receptor lack specificity. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:125–135. doi: 10.1007/s00210-011-0700-4. [DOI] [PubMed] [Google Scholar]
- 3.Cary SP, Winger JA, Marletta MA. Tonic and acute nitric oxide signaling through soluble guanylate cyclase is mediated by nonheme nitric oxide, ATP, and GTP. Proc Natl Acad Sci U S A. 2005;102:13064–13069. doi: 10.1073/pnas.0506289102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers R, Zweifach BW. Intercellular cement and capillary permeability. Physiol Rev. 1947;27:436–463. doi: 10.1152/physrev.1947.27.3.436. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee V, Gashev AA. Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol. 2012;303:H693–702. doi: 10.1152/ajpheart.00378.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobbins DE, Buehn MJ, Dabney JM. Constriction of perfused lymphatics by acetylcholine, bradykinin and histamine. Microcirc Endothelium Lymphatics. 1990;6:409–425. [PubMed] [Google Scholar]
- 7.Edery H, Lewis GP. Kinin-Forming Activity and Histamine in Lymph after Tissue Injury. J Physiol. 1963;169:568–583. doi: 10.1113/jphysiol.1963.sp007280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson MK, DeFilippi VJ. Nitric oxide and endothelium-dependent relaxation in tracheobronchial lymph vessels. Microvasc Res. 1994;47:308–317. doi: 10.1006/mvre.1994.1024. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson MK, Shahinian HK, Michelassi F. Lymphatic smooth muscle responses to leukotrienes, histamine and platelet activating factor. J Surg Res. 1988;44:172–177. doi: 10.1016/0022-4804(88)90046-7. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson MK, Tzeng E. Attenuation of histamine-induced lymphatic smooth muscle contractility by arachidonic acid. J Surg Res. 1991;51:500–505. doi: 10.1016/0022-4804(91)90172-i. [DOI] [PubMed] [Google Scholar]
- 11.Fox JL, von der Weid PY. Effects of histamine on the contractile and electrical activity in isolated lymphatic vessels of the guinea-pig mesentery. Br J Pharmacol. 2002;136:1210–1218. doi: 10.1038/sj.bjp.0704820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francis SH, Busch JL, Corbin JD, Sibley D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol Rev. 62:525–563. doi: 10.1124/pr.110.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gashev AA, Davis MJ, Zawieja DC. Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol. 2002;540:1023–1037. doi: 10.1113/jphysiol.2001.016642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gasheva OY, Gashev AA, Zawieja DC. Cyclic guanosine monophosphate and the dependent protein kinase regulate lymphatic contractility in rat thoracic duct. J Physiol. 2013;591:4549–4565. doi: 10.1113/jphysiol.2013.258681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gasheva OY, Zawieja DC, Gashev AA. Contraction-initiated NO-dependent lymphatic relaxation: a self-regulatory mechanism in rat thoracic duct. J Physiol. 2006;575:821–832. doi: 10.1113/jphysiol.2006.115212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto S, Kawai Y, Ohhashi T. Effects of vasoactive substances on the pig isolated hepatic lymph vessels. J Pharmacol Exp Ther. 1994;269:482–488. [PubMed] [Google Scholar]
- 17.Johnston MG, Kanalec A, Gordon JL. Effects of arachidonic acid and its cyclo-oxygenase and lipoxygenase products on lymphatic vessel contractility in vitro. Prostaglandins. 1983;25:85–98. doi: 10.1016/0090-6980(83)90138-7. [DOI] [PubMed] [Google Scholar]
- 18.Jutel M, Blaser K, Akdis CA. Histamine in allergic inflammation and immune modulation. Int Arch Allergy Immunol. 2005;137:82–92. doi: 10.1159/000085108. [DOI] [PubMed] [Google Scholar]
- 19.Kaley G, Rodenburg JM, Messina EJ, Wolin MS. Endothelium-associated vasodilators in rat skeletal muscle microcirculation. Am J Physiol. 1989;256:H720–725. doi: 10.1152/ajpheart.1989.256.3.H720. [DOI] [PubMed] [Google Scholar]
- 20.Kuo L, Davis MJ, Cannon MS, Chilian WM. Pathophysiological consequences of atherosclerosis extend into the coronary microcirculation. Restoration of endothelium-dependent responses by L-arginine. Circ Res. 1992;70:465–476. doi: 10.1161/01.res.70.3.465. [DOI] [PubMed] [Google Scholar]
- 21.Mathias R, von der Weid PY. Involvement of the NO-cGMP-K(ATP) channel pathway in the mesenteric lymphatic pump dysfunction observed in the guinea pig model of TNBS-induced ileitis. Am J Physiol Gastrointest Liver Physiol. 2013;304:G623–634. doi: 10.1152/ajpgi.00392.2012. [DOI] [PubMed] [Google Scholar]
- 22.Mizuno R, Koller A, Kaley G. Regulation of the vasomotor activity of lymph microvessels by nitric oxide and prostaglandins. Am J Physiol. 1998;274:R790–796. doi: 10.1152/ajpregu.1998.274.3.R790. [DOI] [PubMed] [Google Scholar]
- 23.Nossaman B, Pankey E, Kadowitz P. Stimulators and activators of soluble guanylate cyclase: review and potential therapeutic indications. Crit Care Res Pract. 2012;2012:290805. doi: 10.1155/2012/290805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohhashi T, Kawai Y, Azuma T. The response of lymphatic smooth muscles to vasoactive substances. Pflugers Arch. 1978;375:183–188. doi: 10.1007/BF00584242. [DOI] [PubMed] [Google Scholar]
- 25.Petunov SG, Egorova AA, Orlov RS, Nikitina ER. Effect of histamine on spontaneous contractions of mesenteric lymphatic vessels and lymph nodes of white rats: endothelium-dependent responses. Dokl Biol Sci. 2010;432:176–180. doi: 10.1134/S0012496610030038. [DOI] [PubMed] [Google Scholar]
- 26.Plaku KJ, von der Weid PY. Mast cell degranulation alters lymphatic contractile activity through action of histamine. Microcirculation. 2006;13:219–227. doi: 10.1080/10739680600556902. [DOI] [PubMed] [Google Scholar]
- 27.Rasband WS. ImageJ. U.S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2012. http://rsb.info.nih.gov/ij/ [Google Scholar]
- 28.Reeder LB, DeFilippi VJ, Ferguson MK. Characterization of the effects of histamine in porcine tracheobronchial lymph vessels. Am J Physiol. 1996;271:H2501–2507. doi: 10.1152/ajpheart.1996.271.6.H2501. [DOI] [PubMed] [Google Scholar]
- 29.Shirasawa Y, Benoit JN. Stretch-induced calcium sensitization of rat lymphatic smooth muscle. Am J Physiol Heart Circ Physiol. 2003;285:H2573–2577. doi: 10.1152/ajpheart.00002.2003. [DOI] [PubMed] [Google Scholar]
- 30.Souza-Smith FM, Kurtz KM, Breslin JW. Measurement of cytosolic Ca2+ in isolated contractile lymphatics. J Vis Exp. 2011;58:3438. doi: 10.3791/3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Souza-Smith FM, Kurtz KM, Molina PE, Breslin JW. Adaptation of mesenteric collecting lymphatic pump function following acute alcohol intoxication. Microcirculation. 2010;17:514–524. doi: 10.1111/j.1549-8719.2010.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Souza-Smith FM, Molina PE, Breslin JW. Reduced RhoA activity mediates acute alcohol intoxication-induced inhibition of lymphatic myogenic constriction despite increased cytosolic [Ca(2+)] Microcirculation. 2013;20:377–384. doi: 10.1111/micc.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi N, Kawai Y, Ohhashi T. Effects of vasoconstrictive and vasodilative agents on lymphatic smooth muscles in isolated canine thoracic ducts. J Pharmacol Exp Ther. 1990;254:165–170. [PubMed] [Google Scholar]
- 34.von der Weid PY. Review article: lymphatic vessel pumping and inflammation--the role of spontaneous constrictions and underlying electrical pacemaker potentials. Aliment Pharmacol Ther. 2001;15:1115–1129. doi: 10.1046/j.1365-2036.2001.01037.x. [DOI] [PubMed] [Google Scholar]
- 35.Von Der Weid PY, Rehal S. Lymphatic pump function in the inflamed gut. Ann N Y Acad Sci. 2010;1207 (Suppl 1):E69–74. doi: 10.1111/j.1749-6632.2010.05715.x. [DOI] [PubMed] [Google Scholar]
- 36.von der Weid PY, Zhao J, Van Helden DF. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. Am J Physiol Heart Circ Physiol. 2001;280:H2707–2716. doi: 10.1152/ajpheart.2001.280.6.H2707. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe N, Kawai Y, Ohhashi T. Dual effects of histamine on spontaneous activity in isolated bovine mesenteric lymphatics. Microvasc Res. 1988;36:239–249. doi: 10.1016/0026-2862(88)90025-8. [DOI] [PubMed] [Google Scholar]
- 38.Yuan Y, Granger HJ, Zawieja DC, DeFily DV, Chilian WM. Histamine increases venular permeability via a phospholipase C-NO synthase-guanylate cyclase cascade. Am J Physiol. 1993;264:H1734–1739. doi: 10.1152/ajpheart.1993.264.5.H1734. [DOI] [PubMed] [Google Scholar]
- 39.Zweifach BW, Prather JW. Micromanipulation of pressure in terminal lymphatics in the mesentery. Am J Physiol. 1975;228:1326–1335. doi: 10.1152/ajplegacy.1975.228.5.1326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. H2 histamine receptor immunofluorescence labeling in neuron-like cells present on rat mesenteric collecting lymphatics. A. This image shows the H2 receptor labeling from the 6-μm z-section shown in Movie 2. B. A different rat mesenteric collecting lymphatic also labeled for the H2 histamine receptor. The arrows in both panels indicate axon-like labeling and a suspected neuronal cell body.
Movie 1. Confocal z-stack of H1 histamine receptor labeling in an isolated rat mesenteric collecting lymphatic. Six views of a four-channel image stack, with a total of 51 confocal z-slices, obtained at 2 μm intervals, are shown. The distance in the z-plane is indicated in the top right. In the top row, from left to right, the panels show labeling of VE-cadherin (red), smooth muscle (SM) actin (blue), and the H1 histamine receptor (H1R) (green), with each panel also showing labeled nuclei (white). In the bottom panels, from left to right are the combination of the H1R, VE-cadherin, and nuclei; the H1R, SM Actin, and nuclei; and the combination of all four channels (VE-cadherin, SM actin, H1R, nuclei).
Movie 2. Confocal z-stack of H2 histamine receptor labeling in an isolated rat mesenteric collecting lymphatic. Six views of a four-channel image stack, with a total of 51 confocal z-slices, obtained at 2 μm intervals, are shown. The distance in the z-plane is indicated in the top right. In the top row, from left to right, the panels show labeling of VE-cadherin (red), smooth muscle (SM) actin (blue), and the H2 histamine receptor (H2R) (green), with each panel also showing labeled nuclei (white). In the bottom panels, from left to right are the combination of the H2R, VE-cadherin, and nuclei; the H2R, SM Actin, and nuclei; and the combination of all four channels (VE-cadherin, SM actin, H2R, nuclei).