Abstract
Purpose
To assess whether changes in knee cartilage MR-based T2 relaxation times are associated with weight loss in individuals with risk factors for knee osteoarthritis (OA) compared to controls with stable weight.
Materials and Methods
One hundred and twenty-seven individuals with risk factors for knee OA were studied: 62 subjects had a BMI decrease≥10% over 48-months and 65 controls had a BMI change<3%. Cartilage segmentation from 5 knee compartments at baseline and 48-month follow-up was performed, and T2 maps were generated. The association of change in T2 values over 48 months in the weight-loss group versus the control group was assessed using multiple linear regression models.
Results
Weight loss was associated with significantly smaller increases in cartilage T2 in the medial femoral condyle (p=0.035) and overall medial compartment (p=0.006) compared to the controls. In a subgroup analysis comparing weight-loss subjects who were obese (BMI≥30 kg/m2) and overweight (BMI 25-30 kg/m2) at baseline, obesity was associated with smaller increases in cartilage T2 values in the medial femoral condyle (p=0.022), lateral femoral condyle (p=0.015), patella (p=0.002), and globally across all compartments (p=0.002).
Conclusion
A decrease in BMI of ≥ 10% was associated with a slower progression of T2 values in individuals with risk factors for OA, suggesting a beneficial impact of weight loss on cartilage matrix degeneration.
Keywords: Magnetic Resonance Imaging, weight loss, T2 relaxation time, cartilage, osteoarthritis
INTRODUCTION
Osteoarthritis (OA) is the most common joint disorder in the United States, with knee OA being the most prevalent site (1). It has been estimated that almost 28% of adults over age 45 have evidence of radiographic knee OA, with the numbers increasing to approximately 37% of adults over age sixty (2). Body mass index (BMI) is one of the most strongly associated risk factors for knee OA, and individuals with a BMI ≥ 30 kg/m2 are more likely to develop symptomatic knee OA than those with a BMI < 25 kg/m2 (OR: 6.07) (1, 3). The prevalence of knee OA and its association with obesity is of growing concern, as knee OA and obesity are among the most frequent comorbid conditions in older Americans (4, 5). Weight loss significantly reduces knee joint loads during walking (6, 7), and studies suggest that weight loss at any stage in adulthood can halt the accumulation of knee OA risk that can result from a high BMI (4). Recently, a positive association between BMI and Magnetic Resonance (MR)-based patellofemoral cartilage T2 relaxation time measurements was demonstrated, indicating that high BMI has a detrimental impact on the cartilage (8). However, it is not well understood how weight loss specifically impacts cartilage integrity and degeneration. Furthermore, it is unclear whether having a BMI in the obese or overweight range at any point in one’s life precludes improvement in cartilage T2 values with weight loss. Studies looking at cartilage degeneration in obese individuals have shown smaller rates of degeneration (as measured by T2 progression) compared to non-obese individuals, possibly due to extensive damage done prior to the study period (9). Therefore, this raises the question as to whether knee cartilage health in obese persons is beyond the salvage of weight loss, motivating the need to explore the impact of weight loss on knee cartilage health using new in vivo imaging technologies. One such technology is magnetic resonance imaging (MRI) based T2 relaxation time. By measuring markers of cartilage health such as water content and collagen integrity quantitatively, T2 relaxation time enables us to detect and monitor early cartilage degeneration (10-14); most importantly T2 relaxation time can detect changes in cartilage health even before focal morphological changes occur (15).
In terms of determining whether weight loss is beneficial for maintaining cartilage biochemical composition, longitudinal assessment of substantial weight loss and the subsequent impact on cartilage T2 relaxation parameters has yet to be performed. The aims of our present study were therefore to (i) examine the association of weight loss and concurrent change in T2 relaxation time measurements over a period of 4 years by comparing T2 changes in subjects with a BMI decrease of more than 10% with T2 changes in frequency-matched controls who were weight stable over the same period, and (ii) to investigate cartilage T2 changes in individuals who were obese at baseline and controls who were overweight at baseline, when both groups had a BMI decrease of more than 10% over the same time period.
MATERIALS AND METHODS
Subjects
The data used in this study were obtained from the publically available Osteoarthritis Initiative (OAI) database (http://www.oai.ucsf.edu). The OAI is a large, ongoing, nationwide, longitudinal prospective study sponsored by the National Institutes of Health. It combines clinical, serologic, and primarily knee joint imaging data obtained annually from 4,796 patients between 45 and 79 years old, over a period of 8 years. In this study, we analyzed subsets of subjects from the OAI Incidence subcohort. The study was HIPAA compliant and all subjects provided informed consent. The local institutional review boards approved all the study protocol, amendments, and informed consent documentation. Specific OAI datasets used were baseline clinical dataset 0.2.2, baseline imaging dataset 0.E.1, 48 month follow-up clinical dataset 6.2.1, and 48-month follow-up imaging dataset 6.E.1.
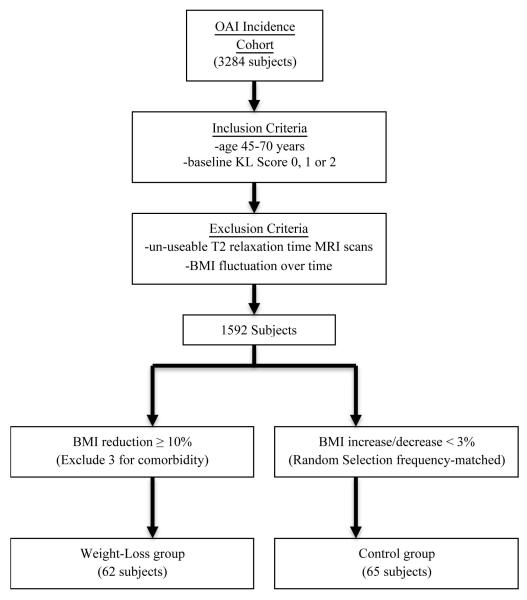
Participants included in the present study were men and women ages 45 to 70 years who did not have symptomatic knee OA in either knee at baseline. Symptomatic knee OA, as outlined in the OAI Study Design Protocol, is defined as a clinical diagnosis of knee osteoarthritis with implications for prevention, requiring both the presence of frequent knee symptoms and radiographic findings reflecting the pathology of osteoarthritis. However, these subjects had characteristic risk factors that placed them at increased risk for developing OA, which included the following: (1) previous knee injury or surgery, (2) family history of total knee replacement, (3) Heberden’s nodes, (4) knee symptoms in the past 12 months (defined as either (a) frequent knee symptoms, (b) frequent use of medication to treat knee symptoms, or (c) infrequent knee symptoms – pain, aching or stiffness in or around the knee at any time in the past 23 months but not on most days for at least one month), (5) overweight, and (6) frequent knee bending activity (9). We only included subjects with a baseline Kellgren-Lawrence (KL) score of 0, 1 or 2 in the right knee and excluded those who did not have technically optimal T2 multiecho sequences of the right knee at baseline and at 48 months. Given our age, BMI, KL score, and image quality criteria, we excluded a total of 1692 subjects from the original pool of 3284 subjects.
From the eligible subjects, we identified two groups defined by BMI change from baseline to 48 months: a weight-loss group who had a BMI reduction ≥ 10% and a weight stable control group with a BMI increase or decrease of < 3% (Figure 1). We considered a BMI reduction of 10% relevant because the beneficial health impact of a weight loss of that magnitude is well documented in a previous study (16). Furthermore, only subjects without substantial BMI fluctuations (< 5% change in BMI between any two 12-month time points) over the four-year period were eligible for this study. We excluded 3 subjects from the weight-loss group who developed cardiac failure, cancer and/or other severe diseases over the course of the 48-month study period that may have been responsible for the weight loss. This assessment was based on annual administration of a comorbidity questionnaire asking about new onset of these conditions at each visit. One subject diagnosed with cancer before initiation of the study, and who was treated and stable at baseline and at 48-month follow-up, was included in the study. A summary of the subject selection criteria is shown in Figure 1.
Figure 1.
Schematic illustration showing creation of the subgroups. Sixty-two weight-loss subgroup subjects and sixty five control subgroup subjects were included in this study based on our inclusion & exclusion criteria and weight change classification. OAI = Osteoarthritis Initiative; BMI = body mass index; KL = Kellgren-Lawrence.
Sixty-two eligible subjects were included in the weight-loss group. Sixty-five weight stable controls were selected from subjects in the OAI Incidence Cohort who met the inclusion criteria but did not exhibit any large change or fluctuations in BMI over the four years of the study. Controls were frequency matched, in a 1 to 1 ratio, to the weight-loss group by randomly selecting controls from five-year age strata. An a priori power analysis was performed to assure an appropriate number of individuals would be included in the study. Using preliminary data gathered in healthy controls (n=36, baseline BMI=22.7±1.5, follow-up BMI=23.4±2.1) (9), the standard deviation of change in mean T2 measurements over 36 months was found to be 1.7 in the medial femoral compartment (Δ mean T2=1.9±1.7). From this data, it was determined that a sample size of 46 patients per group would achieve a power >0.8. Therefore, including 62 subjects in the weight-loss group and 65 in the control group is conservative given the parameters of the power analysis.
MR Imaging
MR images were obtained using one of four identical 3.0 Tesla (Siemens Magnetom Trio, Erlangen, Germany) scanners and quadrature transmit-receive coils (USA Instruments, Aurora, OH, USA) at 4 clinical sites (Ohio State University, Columbus, OH; University of Maryland, School of Medicine, Baltimore, MD; University of Pittsburgh, Pittsburgh, PA; and Memorial Hospital of Rhode Island, Pawtucket, RI). A sagittal two-dimensional (2D) multislice, multiecho (MSME) sequence was acquired and used for measuring T2 relaxation time values. Sequence parameters were: a pulse repetition time of 2700 ms, seven echo times (TEs 10ms, 20ms, 30ms, 40ms, 50ms, 60ms, and 70ms), field-of-view (FOV) = 12 cm, bandwidth of 250 Hz/pixel, in-plane spatial resolution of 0.313mm × 0.446 mm2, slice thickness of 3.0mm, and 0.5mm gap. Detailed information about the sequences is available in the OAI MR protocol (17).
Image Analysis
The sagittal 2D MSME images of the right knee were transferred to a remote workstation (SPARC; Sun Microsystems, Mountain View, Calif). Using in-house spline-based software developed with MATLAB (MathWorks, Natick, Massachusetts) cartilage was semi-automatically segmented at the patella, medial femoral condyle, lateral femoral condyle, medial tibia, and lateral tibia compartments in the first echo images of the MSME sequence. This method has previously been shown to be a highly reproducible way of segmenting cartilage regions of interest (ROI) (18). Each compartment had a range of 8-15 sections, and all sections with well-visualized artifact-free cartilage were segmented.
In each segmented cartilage compartment mean T2 values at baseline and 48 months were calculated using an interactive display language routine; the T2 relaxation time was estimated by fitting an exponential function to the signal intensity at different echo times as follows: SI(TE) ~ exp(−TE/T2), where SI(TE) is the signal intensity as a function of echo time, and T2 is the transverse relaxation time. Previous studies have suggested that dropping the first echo from the MSME sequence minimizes errors in calculated T2 values for cartilage (19). Therefore, a mono-exponential decay model was used (20), and the last six (20, 30, 40, 50, 60, and 70 milliseconds) echo images were used to generate the T2 data.
To maximize reproducibility in segmentation technique, all readers were required to undergo image and data analysis core initial training at the start of the study. Readers on the segmentation team (A.T.S. and T.P.) met on a weekly basis with the project’s lead postdoctoral researcher (H.L.), who had five years of cartilage segmentation experience, to review the segmented sequences. Inter-reader reproducibility was confirmed in baseline T2 maps of randomly selected subjects, on which both readers on the segmentation team segmented all five compartments. Inter- and intra-reader reproducibility errors for T2 measurements of each compartment were calculated on a percentage basis as the root mean square average of the single coefficients of variation per knee, according to Stehling et al (21). Reproducibility results for global T2 measurements indicated an inter-reader reproducibility error for mean T2 of 3.33%, and an intra-reader reproducibility error for mean T2 of 1.11%.
Statistical Analysis
Statistical analysis was performed using STATA version 12.0 software (StataCorp LP, College Station, TX). The differences in subject characteristics (i.e. mean age, BMI, KL Score, etc.) between the control and weight-loss groups were determined using Student’s independent t-tests and chi-square tests. Changes in BMI over the course of the study were analyzed using a regression analysis. Associations between baseline T2 and gender were assessed using Student’s independent t-tests, and associations between baseline T2, baseline BMI, and age were assessed using regression analyses. Changes in mean T2 measurements over 48 months (Δ mean T2) were computed by subtracting baseline T2 measurements from 48-month follow-up T2 measurements. In addition, the relationship between Δ mean T2 and Δ BMI was analyzed using Spearman correlations. Multiple linear regression models were used to assess the association of differences in Δ mean T2 between (a) the weight-loss and control groups, and (b) the overweight and obese subjects within the weight-loss group. Models were adjusted for age, sex, and baseline KL score (1 and 2) and baseline BMI (1). Results were expressed as changes in T2 (ms), with 95% confidence interval (CI) and adjusted p-value. A p-value of less than 0.05 was considered a statistically significant difference.
RESULTS
Subject Characteristics
Mean age, baseline BMI, follow-up BMI, baseline KL score, and gender distribution are listed in Table 1. Sixty-two subjects from the weight-loss group (mean age 56.09 ± 6.89) and sixty-five subjects from the control group (mean age 53.78 ± 4.57) were included in the study with a mean baseline BMI of 27.49 kg/m2 ± 2.98 (BMI range 21.9-34.3 kg/m2) and 24.38 kg/m2 ± 3.39 (BMI range 19.4-37.0 kg/m2), respectively. After 48 months, mean BMI for the weight-loss group was 23.07 kg/m2 ± 2.68, and 24.36 kg/m2 ± 3.34 for the control group. The overall average change in BMI for all subjects was −2.22 kg/m2 ± 2.47. Changes in mean BMI over the 48-month study period were −0.026 kg/m2 ± 0.464 for the control group and −4.35 kg/m2 ± 1.60 for the weight-loss group. The mean percent change in BMI was 15.70% ± 5.00, and 0.07% ± 1.90 for the weight-loss group and control group respectively.
Table 1.
Subject characteristics and descriptive statistics.
| All | Control group (weight change < 3%) |
Weight-loss group (weight loss ≥ 10%) |
P-value** | |
|---|---|---|---|---|
| n | 127 (100%) | 65 (51.18%) | 62 (48.82%) | |
| Age (years)* | 55.15 ± 6.38 | 53.78 ± 4.57 | 56.09 ± 6.89 | 0.156 |
| Sex (females)* | 71 (55.91%) | 38 (58.46%) | 33 (53.23%) | 0.893 |
| Baseline BMI (kg/m2)* | 27.27 ± 3.16 | 24.38 ± 3.39 | 27.49 ± 2.98 | 0.0005 |
| 48-month BMI (kg/m2) | 23.05 ± 2.71 | 24.36 ± 3.34 | 23.07 ± 2.68 | 0.036 |
| BMI Change (kg/m2) | −2.22 ± 2.47 | −0.026 ± 0.464 | −4.35 ± 1.60 | <0.0001 |
| Percent BMI Change (%) | −7.68 ± 8.65 | −0.072 ± 1.90 | −15.704 ± 5.00 | <0.0001 |
| Baseline KL Score* | 0.053 | |||
| KL=0 | 79 (62%) | 45 (69%) | 34 (55%) | |
| KL=1 | 27 (21%) | 15 (23%) | 12 (19%) | |
| KL=2 | 21 (17%) | 5 (8%) | 16 (26%) | |
| Previous Right Knee Injury | 34 (27%) | 18 (28%) | 16 (26%) | 0.619 |
| Previous Right Knee Surgery | 11 (9%) | 3 (5%) | 8 (13%) | 0.103 |
| Frequent Knee Bending Activity |
91 (72%) | 47 (73%) | 44 (71%) | 0.759 |
| Family History (Knee Replacement) |
23 (18%) | 12 (19%) | 11 (18%) | 0.852 |
| Heberden’s Nodes | 35 (28%) | 20 (31%) | 15 (25%) | 0.442 |
| Knee Symptoms (Pain, aching, or stiffness, past 12 months) |
27 (21%) | 9 (14%) | 18 (29%) | 0.030 |
Logistic regression models used for statistical analysis were adjusted for age, gender, baseline BMI, and baseline KL score to account for differences between groups.
P-values <0.05 are in bold.
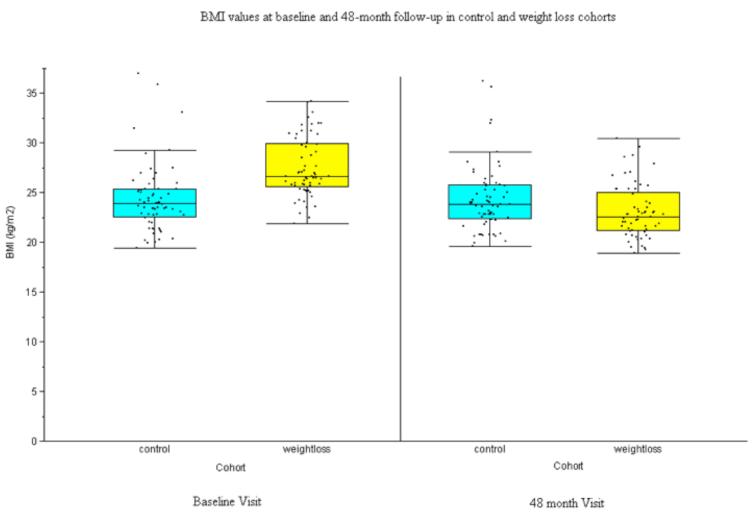
There were no statistically significant differences in mean age or gender distribution between the two study groups (p=0.156 and p=0.893, respectively). As defined by the inclusion criteria, all subjects had KL scores ≤ 2 at baseline in the study knee; while the weight-loss group had more subjects with KL scores of 1 and 2 at baseline, the difference in distribution of scores between the groups did not reach significance (p=0.053). As shown in Figure 2, the weight-loss group had statistically significant higher BMI than the control group at baseline (p<0.001), and statistically significant lower BMI than the controls at 48 months (p=0.036). There was no statistically significant change in the mean BMI of the control group over the 48 months of the study (0.077 kg, 95% CI [−0.078, 0.232], p=0.992).
Figure 2.
The average BMI at baseline and 48-month follow up were analyzed for each group. At baseline, the weight-loss group had a statistically significant higher mean BMI (27.49 ± 3.00 kg/m2) compared to the control group (24.38 ± 3.45 kg/m2) (OR:1.36, p<0.005). However, at 48-month follow up the weight-loss group had a statistically significant lower mean BMI (23.14 ± 2.72 kg/m2) than the control group (24.36 ± 3.39 kg/m2) (OR:0.873, p=0.036), as might be expected since the weight-loss group lost ≥ 10% baseline BMI. No statistically significant changes in weight are noted in the control group.
T2 Relaxation Time Measurements
At baseline, there were no statistically significant differences in T2 relaxation time measurements between the weight-loss and control groups in any of the knee compartments studied (Table 2). Across all subjects, we observed no statistically significant differences in global baseline or Δ mean T2 values by gender (p=0.242 and p=0.540, respectively). We also performed correlations between T2 values and age and found that global T2 values were significantly higher in patients who were older at baseline (coef=0.103, p=0.035, 95% CI [0.007, 0.199]). However there were no statistically significant differences in Δ mean T2 by age (coef=−0.044, p=0.181, 95% CI [−0.108, 0.021]). Within both the weight-loss and weight stable groups there was a similar but weak correlation between global baseline T2 values and BMI, as individuals with higher baseline BMI values also had higher baseline T2 values. However, this was not statistically significant (coef=0.155, p=0.166, 95% CI [−0.066, 0.388] in the control group, and coef=0.233, p=0.088, 95% CI [−0.036, 0.502] in the weight-loss group).
Table 2.
Mean T2 relaxation times at baseline and changes in mean T2 measured in five separate knee compartments comparing weight-loss and control subjects.
| Control group |
Weight-loss group |
Adjusted mean difference* |
95% CI | P-value** | |
|---|---|---|---|---|---|
| Global (All Compartments) | |||||
| Baseline Mean T2 (ms) | 32.80 ± 3.01 | 32.75 ± 3.18 | −0.561 | (−1.760, 0.638) | 0.356 |
| Change in Mean T2 (ms) | 2.52 ± 2.18 | 1.56 ± 1.79 | −0.754 | (−1.540, 0.033) | 0.060 |
| Lateral Femoral Condyle | |||||
| Baseline Mean T2 (ms) | 34.27 ± 2.32 | 34.42 ± 2.23 | −0.182 | (−1.024, 0.660) | 0.669 |
| Change in Mean T2 (ms) | 2.35 ± 2.43 | 1.88 ± 2.30 | −0.094 | (−0.948, 0.760) | 0.828 |
| Lateral Tibia | |||||
| Baseline Mean T2 (ms) | 29.94 ± 2.15 | 29.86 ± 2.35 | −0.357 | (−1.278, 0.563) | 0.443 |
| Change in Mean T2 (ms) | 1.45 ± 2.30 | 0.71 ± 1.84 | −0.641 | (−1.490, 0.208) | 0.137 |
| Lateral Compartment | |||||
| Baseline Mean T2 (ms) | 32.09 ± 2.07 | 32.17 ± 2.01 | −0.165 | (−0.961, 0.632) | 0.683 |
| Change in Mean T2 (ms) | 1.89 ± 2.01 | 1.35 ± 1.93 | −0.454 | (−1.230, 0.321) | 0.248 |
| Medial Femoral Condyle | |||||
| Baseline Mean T2 (ms) | 37.44 ± 2.38 | 37.68 ± 2.55 | −0.287 | (−1.209, 0.636) | 0.539 |
| Change in Mean T2 (ms) | 1.86 ± 1.98 | 0.67 ± 2.06 | −0.866 | (−1.669, −0.063) | 0.035 |
| Medial Tibia | |||||
| Baseline Mean T2 (ms) | 31.60 ± 2.73 | 31.88 ± 2.49 | −0.241 | (−1.289, 0.806) | 0.649 |
| Change in Mean T2 (ms) | 2.23 ± 2.58 | 1.35 ± 2.80 | −1.055 | (−2.151, 0.040) | 0.059 |
| Medial Compartment | |||||
| Baseline Mean T2 (ms) | 34.65 ± 2.28 | 34.25 ± 4.95 | −0.895 | (−2.369, 0.580) | 0.232 |
| Change in Mean T2 (ms) | 2.02 ± 1.95 | 0.95 ± 1.91 | −1.110 | (−1.891, −0.329) | 0.006 |
| Patella | |||||
| Baseline Mean T2 (ms) | 32.78 ± 3.81 | 32.95 ± 3.15 | −0.944 | (−2.169, 0.282) | 0.130 |
| Change in Mean T2 (ms) | 3.81 ± 5.28 | 2.48 ± 3.51 | −0.020 | (−1.340, 1.300) | 0.976 |
The associations between T2 parameters and weight loss of ≥ 10% of baseline BMI over 48 months were assessed using linear regression models (adjusted for age, gender, baseline BMI, and baseline KL scores); reported values represent absolute differences in T2 values.
P-value adjusted for age, gender, baseline BMI, and baseline KL score. P-values <0.05 are in bold.
Changes in T2 Relaxation Time Measurements over 48 months
The results from all knee compartments demonstrate less of an increase in mean T2 values over 48 months in the weight-loss group compared to the control group, suggesting less progression of cartilage degeneration in the weight-loss group (Table 2). Over 48 months, changes in mean cartilage T2 were significantly smaller statistically in the weight-loss group in the medial femoral condyle compartment (−0.866 ms, 95% CI [−1.669, −0.063], p=0.035) and the overall medial compartment (−1.110 ms, 95% CI [−1.891, −0.329], p=0.006), with similar trends observed in the medial tibia compartment (−1.055 ms, 95% CI [−2.151, 0.040], p=0.059) and in the global average across all compartments (−0.754 ms, 95% CI [−1.540, 0.033], p=0.060).
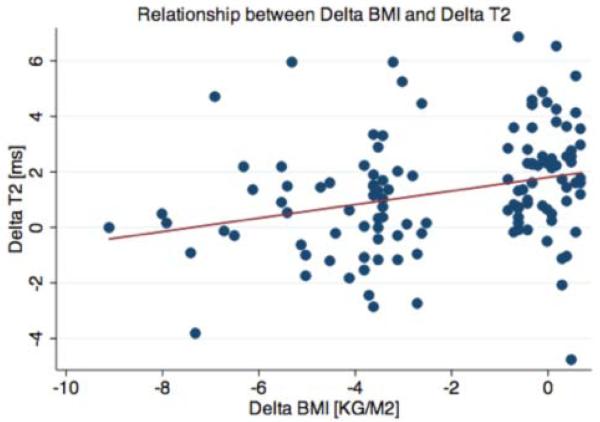
Analysis of BMI change versus Δ mean T2 relaxation times in the medial femoral condyle compartment showed that with greater weight loss, there was significantly less increase in T2 observed over 48 months, (r=0.344, p=0.0001; Figure 3); a similar trend was seen in all compartments, and significance was found in the patella (p=0.03), overall medial compartment (p=0.001), and globally across all compartments (p=0.001).
Figure 3.
Scatterplot of change in mean T2 relaxation times vs. change in BMI over 48 months in medial femoral condyle compartment (r=0.344, p<0.001).
Subgroup Analysis
An additional analysis was performed comparing Δ mean T2 over 48 months in overweight subjects (BMI 25.0 – 29.9 kg/m2) and obese subjects (BMI ≥ 30 kg/m2) within the weight-loss group. A total of 36 overweight subjects and 14 obese subjects were analyzed with a baseline mean BMI of 26.73 and 31.83 kg/m2, respectively. There was no statistically significant difference in percent of BMI loss between the obese and overweight subgroups (16.85% vs. 16.36%, respectively, p=0.105), however, total weight loss in kilograms was higher in the obese cohort compared to the overweight group (5.35 kg vs. 4.39 kg, respectively).
The results from all knee compartments demonstrate a smaller increase in cartilage T2 values over 48 months among those who were obese at baseline compared to those who were overweight at baseline, when both groups lost over 10% of their baseline BMI (Table 3). Smaller increases in cartilage T2 times over 48 months were significantly associated with higher baseline BMI (obese subgroup) globally across all compartments (−1.841 ms, 95% CI [−2.981, −0.702], p=0.002), in the lateral femoral condyle compartment (−1.987 ms, 95% CI [−3.565, −0.409], p=0.015), in the medial femoral condyle compartment (−1.678 ms, 95% CI [−3.096, −0.259], p=0.022), and in the patella compartment (−3.209 ms, 95% CI [−5.201, −1.217], p=0.002). A similar trend was seen in the overall lateral compartment (−1.230 ms, 95% CI [−2.543, 0.083], p=0.066). Interestingly at baseline, mean T2 values were higher in the obese compared to the overweight cohort, but these differences were only statistically significant in the patella 34.67 ms vs. 32.50 ms (p=0.026) and overall medial compartment 35.90 ms vs. 33.63 ms (p=0.044). Differences were not statistically significant in other compartments.
Table 3.
Subgroup analysis comparing overweight (BMI 25-29.9 kg/m2) and obese (BMI ≥ 30 kg/m2) individuals in the weight-loss group.
| Obese (n=14) |
Overweight (n=36) |
Adjusted mean difference* |
95% CI | P-value** | |
|---|---|---|---|---|---|
| Global (All compartments) | |||||
| Baseline Mean T2 (ms) | 33.49 ± 3.74 | 32.53 ± 3.30 | 1.340 | (−0.888, 3.568) | 0.232 |
| Change in Mean T2 (ms) | 0.76 ± 1.90 | 1.92 ± 1.73 | −1.841 | (−2.981, −0.702) | 0.002 |
| Lateral Femoral Condyle | |||||
| Baseline Mean T2 (ms) | 34.49 ± 2.31 | 34.46 ± 2.43 | 0.085 | (−1.459, 1.629) | 0.912 |
| Change in Mean T2 (ms) | 1.04 ± 1.67 | 2.27 ± 2.65 | −1.987 | (−3.565, −0.409) | 0.015 |
| Lateral Tibia | |||||
| Baseline Mean T2 (ms) | 29.84 ± 2.39 | 29.86 ± 2.61 | 0.082 | (−1.853, 2.016) | 0.932 |
| Change in Mean T2 (ms) | 0.87 ± 1.83 | 0.70 ± 1.91 | −0.281 | (−1.679, 1.116) | 0.687 |
| Lateral Compartment | |||||
| Baseline Mean T2 (ms) | 32.27 ± 2.22 | 32.17 ± 2.16 | 0.024 | (−1.457, 1.506) | 0.974 |
| Change in Mean T2 (ms) | 0.98 ± 1.64 | 1.56 ± 2.16 | −1.230 | (−2.543, 0.083) | 0.066 |
| Medial Femoral Condyle | |||||
| Baseline Mean T2 (ms) | 39.03 ± 2.54 | 37.22 ± 2.43 | 1.571 | (−0.152, 3.294) | 0.073 |
| Change in Mean T2 (ms) | −0.11 ± 2.08 | 1.11 ± 1.86 | −1.678 | (−3.096, −0.259) | 0.022 |
| Medial Tibia | |||||
| Baseline Mean T2 (ms) | 32.77 ± 2.90 | 31.72 ± 2.15 | 1.072 | (−0.710, 2.853) | 0.232 |
| Change in Mean T2 (ms) | 1.48 ± 3.54 | 1.16 ± 2.71 | −0.997 | (−3.099, 1.106) | 0.344 |
| Medial Compartment | |||||
| Baseline Mean T2 (ms) | 35.90 ± 2.46 | 33.63 ± 5.94 | 3.758 | (0.108, 7.408) | 0.044 |
| Change in Mean T2 (ms) | 0.68 ± 2.26 | 1.04 ± 1.79 | −1.055 | (−2.420, 0.309) | 0.126 |
| Patella | |||||
| Baseline Mean T2 (ms) | 34.67 ± 3.29 | 32.50 ± 2.91 | 2.406 | (0.296, 4.515) | 0.026 |
| Change in Mean T2 (ms) | −0.12 ± 3.41 | 3.09 ± 2.53 | −3.209 | (−5.201, −1.217) | 0.002 |
The associations between T2 parameters and baseline BMI of subjects in the weight-loss group were assessed using linear regression models (adjusted for age, gender, and baseline KL scores); reported values represent absolute differences in T2 values.
P-value adjusted for age, gender, and baseline KL score. P-values <0.05 are in bold.
DISCUSSION
In conclucion, the results of this study showed an association between changes in mean knee cartilage T2 values and weight loss of greater than 10% of baseline BMI compared to weight stable individuals with risk factors but no OA. Statistically, significantly smaller increases in cartilage mean T2 relaxation times were seen in the medial femoral condyle compartment and overall medial compartments in individuals who lost ≥10% of their baseline BMI over a four year period, as compared to individuals who had < 3% change in BMI over the same time period. Similar changes in mean cartilage T2 relaxation times were observed in all other compartments studied, with trends observed globally across the entire knee and in the medial tibia compartment. These results suggest that weight loss was a contributing factor to these individuals’ slower progression of cartilage T2 relaxation times compared to individuals who did not lose weight, suggesting a slower rate of cartilage degeneration.
Increases in T2 relaxation times are indicative of progressive cartilage degeneration; T2 values increase as water content increases and collagen is degenerating (22), and can therefore serve as surrogate markers to quantify hyaline cartilage water content and collagen integrity. Because the process of cartilage loss is characterized in stages – first by degradation of portions of the cartilage extracellular matrix, followed by loss of proteoglycan content, changes in water content, and finally molecular changes in collagen structure (10) – early cartilage degeneration can be quantified using T2 relaxation time measurements before morphological cartilage defects are found (11). Given that smaller increases in T2 values were associated with being in the weight-loss group, our findings suggest that weight loss is an effective intervention to slow the earliest stages of cartilage degeneration that lead to the development of OA.
Previous studies have shown that in subjects with minimal baseline cartilage damage, obesity is strongly associated with increased cartilage degeneration (23, 24), especially rapid cartilage loss (25). Elevated levels of tibiofemoral articular contact stress have been associated with observed worsening in cartilage morphology (26, 27), and the additional mechanical loading of the knee joint in obese individuals may increase this articular stress (28). To investigate whether weight loss is still of benefit to obese individuals whose cartilage may show more advanced degeneration, a subgroup analysis was performed with obese and overweight subjects within our weight-loss group. Within the obese subcohort, there were statistically significant smaller increases in mean T2 in various knee compartments, suggesting continued benefit of weight loss for obese individuals.
The patterns noted in our findings are consistent with previous studies demonstrating that in the normally aligned ambulating knee, load is disproportionately transmitted to the medial compartment (29). These observed patterns of weight loading are corroborated by radiographic evidence that the medial tibiofemoral compartment has greater prevalence of radiographic osteophytosis and sclerosis compared to the lateral compartments (3). Additionally, recent literature suggests that a mechanical stimulus can induce changes in serum cartilage oligomeric matrix protein associated with cartilage thinning over time, and that these effects are primarily seen in the medial compartments of the knee (30). Similar to previous studies (31), our weight-loss subjects showed the most improvement compared to control subjects in the medial femoral condyle compartment.
From a clinical perspective, using weight loss as an intervention to prevent early OA is supported by other studies, which have suggested that an individual can halt increasing their risk of developing OA by reducing weight at any stage in adulthood (4). We also observed as more weight was lost, mean T2 values increased significantly less, thus suggesting that a greater magnitude of weight loss may be even more beneficial to preserve the cartilage matrix. Given that the higher levels of knee cartilage defects observed with increasing BMI are associated with both objective and self-reported measures of physical disability (32), the potential for improving cartilage health via weight loss could have a profound effect upon the quality of life of heavier individuals at risk for OA. Our data further supports this literature citing the benefits of weight loss as a protective intervention for OA in individuals of any weight.
This study was limited by the fact that we were not able to precisely determine how individuals lost weight. We were able to exclude subjects with severe diseases such as progressive cancer and chronic heart conditions, but were limited in assessing the exact contributions of diet and/or physical exercise to our subjects’ weight loss plans. Further limitations in this study include our small sample size, which prevented us from performing a matched-pairs analysis with subjects who had similar baseline BMI. Therefore, to account for differences in baseline BMI, we instead controlled for the variable in our regression analysis. In our subgroup analysis, we would have ideally compared obese subjects in the weight-loss group to obese subjects in our control group, but our study is limited by the very small number of obese individuals in the control group. In addition the longitudinal changes appear relatively small, on the order of 1 ms difference in T2 relaxation times; however, previous studies have shown differences on this order between patient cohorts with and without disease progression, emphasizing the clinical relevance of those T2 changes (11, 33, 34). Given that the clinically significant changes in T2 values are relatively small, it is important to acknowledge the inherent limitations of the curve-fitting algorithm used in this and other similar studies. However, rigorous quality assurance is performed in the OAI study and excellent reproducibility data for T2 relaxation time measurements in the order of 1.5-5.3% over 8 years have recently been reported (35). In addition it should be noted that the reported T2 changes in this study were pooled averages over the entire compartments under investigation, and consideration was not given to separating superficial and deep layers of cartilage to look at spatial variation in T2 changes. However, previous studies have demonstrated that performing laminar analysis of cartilage provides limited additional information beyond that provided by bulk measurements alone and that reproducibility of laminar measurements is lower than those of bulk measurements (21, 36-37).
In conclusion, our study suggests that weight loss may prevent early cartilage matrix degeneration as measured with MRI based T2 relaxation time. Because T2 relaxation parameters offer non-invasive and safe imaging modalities for evaluating the earliest steps of the cartilage degeneration pathway, they may potentially aid in following the progression of disease and advising patients in a clinical setting before cartilage is lost.
Acknowledgments
Funding Source: This study was funded through National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grants U01-AR059507 and P50-AR060752. The study was made possible through support from the Osteoarthritis Initiative, a public–private partnership comprising 5 NIH contracts (National Institute of Arthritis and Musculoskeletal and Skin Diseases contracts N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262), with research conducted by the Osteoarthritis Initiative Study Investigators. The study was also funded in part by the Intramural Research Program of the National Institute on Aging, NIH. Private funding partners include Merck Research, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer; the private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health.
Footnotes
The authors have no financial or personal relationships to disclose.
REFERENCES
- 1.Lawrence RC, Helmick CG, Arnett FC, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis and rheumatism. 1998;41:778–99. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clinics in geriatric medicine. 2010;26:355–69. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. The Journal of rheumatology. 2006;33:2271–9. [PubMed] [Google Scholar]
- 4.Wills AK, Black S, Cooper R, et al. Life course body mass index and risk of knee osteoarthritis at the age of 53 years: evidence from the 1946 British birth cohort study. Annals of the rheumatic diseases. 2012;71:655–60. doi: 10.1136/ard.2011.154021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boult C, Kane RL, Louis TA, Boult L, McCaffrey D. Chronic conditions that lead to functional limitation in the elderly. Journal of gerontology. 1994;49:M28–36. doi: 10.1093/geronj/49.1.m28. [DOI] [PubMed] [Google Scholar]
- 6.Aaboe J, Bliddal H, Messier SP, Alkjaer T, Henriksen M. Effects of an intensive weight loss program on knee joint loading in obese adults with knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19:822–8. doi: 10.1016/j.joca.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Messier SP, Legault C, Loeser RF, et al. Does high weight loss in older adults with knee osteoarthritis affect bone-on-bone joint loads and muscle forces during walking? Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19:272–80. doi: 10.1016/j.joca.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koff MF, Amrami KK, Kaufman KR. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15:198–204. doi: 10.1016/j.joca.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Baum T, Joseph G, Nardo J, et al. Correlation of magnetic resonance imaging-based knee cartilage T2 measurements and focal knee lesions with body mass index: thirty-six month followup data from a longitudinal, observational multicenter study. Arthritis Care and Research. 2012;65:23–33. doi: 10.1002/acr.21741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dijkgraaf LC, de Bont LG, Boering G, Liem RS. The structure, biochemistry, and metabolism of osteoarthritic cartilage: a review of the literature. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 1995;53:1182–92. doi: 10.1016/0278-2391(95)90632-0. [DOI] [PubMed] [Google Scholar]
- 11.Guermazi A, Zaim S, Taouli B, Miaux Y. Peterfy CG and Genant HG. MR findings in knee osteoarthritis. European radiology. 2003;13:1370–86. doi: 10.1007/s00330-002-1554-4. [DOI] [PubMed] [Google Scholar]
- 12.David-Vaudey E, Ghosh S, Ries M, Majumdar S. T2 relaxation time measurements in osteoarthritis. Magnetic resonance imaging. 2004;22:673–82. doi: 10.1016/j.mri.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 13.Stahl R, Luke A, Li X, et al. T1rho, T2 and focal knee cartilage abnormalities in physically active and sedentary healthy subjects versus early OA patients--a 3.0-Tesla MRI study. European radiology. 2009;19:132–43. doi: 10.1007/s00330-008-1107-6. [DOI] [PubMed] [Google Scholar]
- 14.Bachmann GF, Basad E, Rauber K, Damian MS, Rau WS. Degenerative joint disease on MRI and physical activity: a clinical study of the knee joint in 320 patients. European radiology. 1999;9:145–52. doi: 10.1007/s003300050646. [DOI] [PubMed] [Google Scholar]
- 15.Link TM. Cartilage imaging: Significance, techniques, and new developments. 2011 ed Springer; New York: May 3rd, 2011. p. 256. [Google Scholar]
- 16.Wing RR, Phelan S. Long-term weight loss maintenance. The American journal of clinical nutrition. 2005;82:222S–5S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- 17.Nevitt MC, Felson D, Lester G. The Osteoarthritis Initiative: A knee health study. Protocol for the cohort study. 2006 [Google Scholar]
- 18.Hovis KK, Stehling C, Souza RB, et al. Physical activity is associated with magnetic resonance imaging-based knee cartilage T2 measurements in asymptomatic subjects with and those without osteoarthritis risk factors. Arthritis and rheumatism. 2011;63:2248–56. doi: 10.1002/art.30419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier CF, Tan SG, Hariharan H, Potter HG. T2 quantitation of articular cartilage at 1.5 T. Journal of magnetic resonance imaging : JMRI. 2003;17:358–64. doi: 10.1002/jmri.10263. [DOI] [PubMed] [Google Scholar]
- 20.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–8. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stehling C, Baum T, Mueller-Hoecker C, et al. A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging--data from the Osteoarthritis Initiative. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19:984–9. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moskowitz RWAR, Hochberg MC, Buckwalter JA, Goldberg VM. Osteoarthritis : Diagnosis and Medical/Surgical Management. 4th ed Lippincott Williams & Wilkins; Philadelphia: 2006. Osteoarthritis : Imaging Osteoarthritis with Magnetic Resonance Imaging; p. 528. [Google Scholar]
- 23.Laberge MA, Baum T, Virayavanich W, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects--data from the Osteoarthritis Initiative. Skeletal radiology. 2012;41:633–41. doi: 10.1007/s00256-011-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berry PA, Wluka AE, Davies-Tuck ML, et al. The relationship between body composition and structural changes at the knee. Rheumatology. 2010;49:2362–9. doi: 10.1093/rheumatology/keq255. [DOI] [PubMed] [Google Scholar]
- 25.Roemer FW, Zhang Y, Niu J, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252:772–80. doi: 10.1148/radiol.2523082197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segal NA, Kern AM, Anderson DD, et al. Elevated tibiofemoral articular contact stress predicts risk for bone marrow lesions and cartilage damage at 30 months. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012 doi: 10.1016/j.joca.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segal NA, Anderson DD, Iyer KS, et al. Baseline articular contact stress levels predict incident symptomatic knee osteoarthritis development in the MOST cohort. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27:1562–8. doi: 10.1002/jor.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yusuf E. Metabolic factors in osteoarthritis: obese people do not walk on their hands. Arthritis research & therapy. 2012;14:123. doi: 10.1186/ar3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison JB. The mechanics of the knee joint in relation to normal walking. Journal of biomechanics. 1970;3:51–61. doi: 10.1016/0021-9290(70)90050-3. [DOI] [PubMed] [Google Scholar]
- 30.Erhart-Hledik JC, Favre J, Asay JL, et al. A relationship between mechanically-induced changes in serum cartilage oligomeric matrix protein (COMP) and changes in cartilage thickness after 5 years. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2012 doi: 10.1016/j.joca.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Anandacoomarasamy A, Leibman S, Smith G, et al. Weight loss in obese people has structure-modifying effects on medial but not on lateral knee articular cartilage. Annals of the rheumatic diseases. 2012;71:26–32. doi: 10.1136/ard.2010.144725. [DOI] [PubMed] [Google Scholar]
- 32.Anandacoomarasamy A, Smith G, Leibman S, et al. Cartilage defects are associated with physical disability in obese adults. Rheumatology. 2009;48:1290–3. doi: 10.1093/rheumatology/kep246. [DOI] [PubMed] [Google Scholar]
- 33.Prasad AP, Nardo L, Schooler J, Joseph GB, Link TM. T(1)rho and T(2) relaxation times predict progression of knee osteoarthritis. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2013;21:69–76. doi: 10.1016/j.joca.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joseph AA, Merboldt KD, Voit D, et al. Real-time phase-contrast MRI of cardiovascular blood flow using undersampled radial fast low-angle shot and nonlinear inverse reconstruction. NMR in biomedicine. 2012;25:917–24. doi: 10.1002/nbm.1812. [DOI] [PubMed] [Google Scholar]
- 35.Schneider E, Nessaiver M. The Osteoarthritis Initiative (OAI) magnetic resonance imaging quality assurance update. Osteoarthritis Cartilage. 2013;21:110–116. doi: 10.1016/j.joca.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carballido-Gamio J, Stahl R, Blumenkrantz G, et al. Spatial analysis of magnetic resonance T1rho and T1 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys. 2009;36:4059–67. doi: 10.1118/1.3187228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schooler J, Kumar D, Nardo L, et al. Longitudinal evaluation of T1rho and T2 spatial distribution in osteoarthitis and healthy medial knee cartilage. Osteoarthritis Cartilage. 2014;22:51–62. doi: 10.1016/j.joca.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]