Abstract
CD24 binds to and suppresses inflammation triggered by danger associated molecular patterns (DAMPS) such as heat-shock proteins (HSPs) and HMGB1. Paradoxically, CD24 has been shown to enhance autoimmune disease. Here we attempt to reconcile this paradox by deletion of CD24 (24KO) in a lupus-like disease model driven by forced expression of HSP gp96 at the cell surface (tm). As expected, tm24KO mice showed increased CD11c+ DC activation coupled to a significant increase in DC-specific IL-12 production compared to tm mice. However, tm24KO mice showed less CD4 T cell activation and peripheral inflammatory cytokine production in comparison to tm mice. We characterized an enhanced immune suppressive milieu in tm24KO mice distinguished by increased TGF-β and greater Treg suppressive capacity. We found greater absolute numbers of MDSCs in tm24KO mice and showed that the Ly6C+ MDSC subset had greater suppressive capacity from tm24KO mice. Deletion of CD24 in tm mice led to diminished lupus-like pathology as evidenced by anti-nuclear antibody deposition and glomerulonephritis. Finally, we show that expanded MDSC populations were mediated by increased free HMGB1 in tm24KO mice. Thus, the deletion of CD24 in an HSP-driven model of autoimmunity led to the unexpected development of Treg and MDSC populations that augmented immune tolerance. Further study of these populations as possible negative regulators of inflammation in the context of autoimmunity is warranted.
Keywords: autoimmunity, MDSC, CD24, gp96, HMGB1
Introduction
Autoimmune diseases occur in response to a disruption in immune tolerance triggered by aberrant innate and adaptive immune cell activation resultant in tissue degradation and organ failure (1). Endogenous danger-associated molecular patterns (DAMPs) are released during the course of disease and enhance inflammation (2). The classic DAMP HMGB1 has been shown to trigger inflammation in the context of autoimmunity and to exacerbate diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) (3). DAMP heat shock protein 90 (HSP90) is increased in sera of SLE patients and associated with increased autoantibody production (4). Similarly, auto-antibodies to gp96 have been detected in patients with SLE and RA (5, 6).
The heat stable antigen CD24 is a receptor for HMGB1 as well as heat shock proteins 70 and 90. Ligation of CD24 with HMGB1 or HSPs leads to recruitment of the ITIM Siglec10/G and subsequent abrogation of inflammatory cytokine signals through blockade of NF-κB activation (7). Loss of CD24 in the context of exogenously introduced insult resulted in HMGB1-driven DC inflammatory response. The CD24 inflammatory axis is specific to signaling initiated by DAMPs as pathogen associated molecular pattern (PAMP) lipopolysaccharide (LPS), response did not differ between CD24KO and WT cells (8). It is not known how functional loss of CD24 will affect immune tolerance and subsequent disease progression in an autoimmune context.
Myeloid derived suppressor cells (MDSCs) have a well-defined role as pro-tumorigenic through suppression of host inflammatory mechanisms (9). While the role of MDSCs in autoimmune disease is less defined, reports demonstrate that suppression of host immunity ameliorates disease (3). MDSCs contribute to an immune-suppressive atmosphere through signaling of TGF-β or production of IL-10 to increase Treg cell activity and quell activation of naïve CD4 T cells (10). A mechanism of MDSC development and recruitment may be DAMP HMGB1 as tumor models show (11). HMGB1 may also lead to MDSC expansion in a tumor context to enhance tumor growth and in the context of autoimmune disease to suppress clinical manifestations.
Gp96, or grp94, is an abundant heat shock protein located in the endoplasmic reticulum with homology to HSP90 (12). We previously developed a murine model of forced gp96 expression at the cell surface and shown that the onset of lupus-like disease occurred in these animals and could be abrogated by depletion of T cells (13, 14). CD24 has been shown to be essential for the development of experimental autoimmune encephalomyelitis in the mice (15, 16). Moreover, polymorphism of CD24 is associated with a number of autoimmune diseases including multiple sclerosis, lupus, and rheumatoid arthritis (17–22). However, the role of CD24 in the context of endogenous DAMP driven autoimmune disease has not been investigated. Here we present the unexpected emergence of a population of MDSCs in tm24KO mice that correlated with decreased T cell activation and marks of inflammatory suppression. In the context of autoimmune disease we determined decreased lupus-like clinical manifestations due to the presence of MDSC and the consequent decreased inflammatory atmosphere. Finally, we show that elevated levels of free HMGB1 mediate MDSC infiltration in the absence of CD24 in an autoimmune disease context.
Materials and Methods
Mice
CD24−/− mice, which were produced using ES cells from C57BL/6 mice, have been described (23). Membrane-bound gp96 transgenic mice (tm) (on pure C57BL/6 background) were developed as described previously (13). CD24KO and tm mice were backcrossed for 8 consecutive generations (tm24KO). Mice used for experiments were aged 6–12 months unless otherwise indicated. The Division of Laboratory Animal Resources (DLAR) of Medical University of South Carolina (MUSC) maintained mice according to established guidelines.
Ab and reagents
All flow cytometry and fluorescent microscopy antibodies were purchased from ebioscience unless otherwise indicated. CD11c, CD40, CD44, CD80, CD86, CD69, CD25, CD11b, Gr1, Ly6C, CD19, CD138, CD4 (BD Bioscience), and CD8 (BD Bioscience) were used for extracellular staining. BRDU (BD Biosciences) IFNγ, TNFα, IL-12, IL-10, and Foxp3 were used for intracellular staining and Foxp3 Buffer was used for permeabilization (eBioscience).
Cellular Preparation, Stimulation, and Isolation
Spleens and lymph nodes were harvested in RPMI media supplemented with 10% FBS. Single cell suspensions were obtained by tissue dissociation with glass slides and subsequent lysis of RBCs. For direct flow cytometry cells were kept on ice and immediately placed in PBS 2% FBS for staining with fluorescent conjugated antibodies. For re-stimulation cells were treated with or without PMA/ionomycin (0.2 μg/mL, 1 μg/mL (Sigma) for 4–6 hours with BFA (BD Biosciences) and extracellular/intracellular flow cytometry antibody staining was performed. BrdU staining protocols were followed per manufacturer’s instructions (BD Biosciences). PBMCs were obtained by harvesting blood into heparinized tubes. Blood was stained at RT for 20 minutes with fluorescent conjugated Abs then washed and lysed with ACK. Cells were fixed or permeabilized for intracellular staining. For Western blot cells were lysed with RIPA buffer on ice for 1 hr.
Cell Cultures & Greiss Reaction
Cells were cultured in RPMI supplemented with 10% FBS and antibiotics. For TGF-β ELISA supernatants were collected after 24 hours of culture. For Greiss reaction, cells were plated at 1×105 cells per well in 96 well plates and treated with or without1mg/mL LPS (Sigma) for 24 hours. Supernatants were harvested and Greiss reaction was performed per manufactures’ instructions (Life Technologies).
BrdU & CFSE Assays
Mice were given BrdU (0.8 mg/mL) in drinking water for 3 consecutive days. CFSE was loaded into CD8 T cells per manufactures’ instructions (Invitrogen) and plated at ratios of 1:1 and 10:1 (T: Suppressor). CD8 T and suppressor cell subsets were isolated from single cell splenic suspensions via magnetic bead sort per manufactures’ instructions (Miltenyi: Treg Kit, MDSC Kit). The 1:1 ratios were plated at 1×105 cells in 96 well plates coated with anti-CD3 (ebioscience). On day 3 of co-culture cells were washed and CFSE dilution was read via flow cytometer.
ELISA and Western Blot
ELISA was used to detect TGF- β per manufacturer’s protocol (R&D Sytstems), IL-12p40 (BD Bioscience) and IgM, IgA, IgG1, IgG3, IgG2b, and IgG2c (Southern Biotech). Serum western blot for HMBG1 was performed on diluted sera samples (1:10) and Comassie blue stain of gel was used to detect albumin band as loading control. Lysates and sera were measured by BSA assay (Thermo Fisher). Denatured proteins were run on 10% SDS gel and primary proteins were blotted for overnight at 4C. Primary antibody for HMGB1 was obtained from Cell Signaling, respectively. Anti-rabbit HRP secondary antibody was used for protein detection.
Chemotaxis Assay
Recombinant HMGB1 was obtained from HMGBiotech. HMGB1 was rigorously tested by HMGBiotech and determined to be LPS free. I.P. injection of 100 ng/mouse in sterile PBS was administered and PBMC were assed 1 hr pre and 4 hrs post injection, respectively.
Immunofluorescence detection of glomerulonephritis and clinical evaluation of the severity of kidney disease and Detection of ANA
Kidney sectioning, immunoflourescent staining, H&E, ANA detection and clinical assessments were performed and assessed as previously described (24). For ANA and GN immunoflourescence we used a 5-point scale for assessment of clinical disease where fluorescent intensity was graded 1 (negative staining), 2 (dim staining), 3(dim-moderate staining), 4 (moderate-bright staining), 5 (bright staining).
Statistics
Students’ t test two tailed paired statistics were performed. Paired tests were used where age matched controls were measured unless otherwise specified. Values of p<0.05 were considered significant. Mice analyzed were n = 3 pairs or more for all experiments.
Results
Loss of CD24 in tm mice leads to increased DC activation
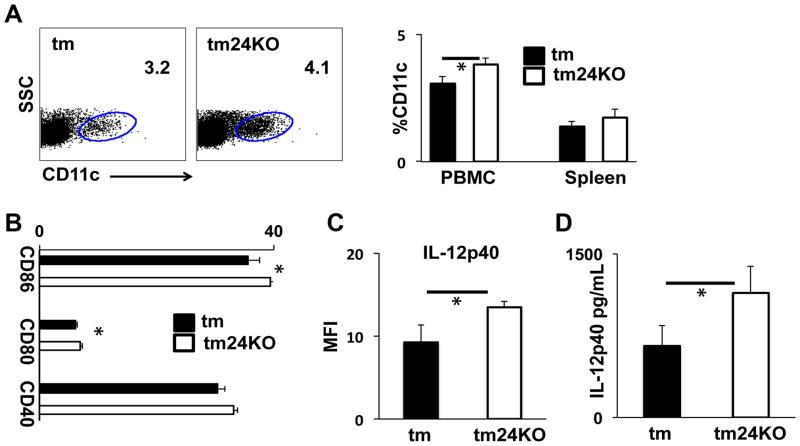
We previously engineered transgenic mice to express gp96 at the cell membrane (tm) and demonstrated that these mice developed lupus-like disease 4 months after birth (13). We further showed that IL-12 and CD11c+ DCs contributed to disease development (14). CD24 is a critical receptor for DAMP HMB1 on DCs and in the absence of CD24 DCs show amplified inflammatory activity to DAMPs upon exogenous stimulation (8). In order to address the role of CD24 in response to DAMPs released due to endogenous inflammation we crossed CD24KO mice to tm mice (tm24KO). At 6 months after birth we assessed CD11c+ cell populations and noted that mice from tm24KO groups consistently showed greater CD11c+ populations as a percentage of peripheral blood mononuclear cells. The increase in CD11c+ cells was not significant in splenocyte populations (Figure 1A). We next assessed DC activation as measured by CD80, CD86, and CD40. We found that PBMC-DC populations from tm24KO had significantly increased expression as measured by MFI of CD80 and CD86 as compared to tm levels (Figure 1B). We next asked whether tm24KO peripheral blood DCs showed enhanced IL-12 production compared to tm mice. Direct ex vivo data show that PB-DCs from tm24KO mice have higher MFI of IL-12 than PB-DCs from tm mice (Figure 1C). We further quantified levels of serum IL-12p40 and noted that enhanced DC activity in tm24KO mice correlated to significantly elevated levels of IL-12p40 in tm24KO mice as compared to tm mice (Figure 1D).
Figure 1. DC activation and IL-12 production in tm and tm24KO mice.
Tm and tm24KO mice were subjected to flow cytometric analysis. (A) Surface staining of peripheral blood CD11c+ single-positive populations gated against SSC. Data of one representative mouse from 3 pairs of mice is shown. Averages of 3 mice per group from PBMC (*=p<0.05) or splenic CD11c+ populations are shown. (B) Single-positive CD11c+ population is gated and surface staining for CD80, CD86, and CD40 populations are gated and represented as average of MFI for 3 pairs of mice, (*=p<0.05). (C) PBMC from tm or tm24KO mice CD11c+ single-positive populations were gated on and intracellular IL-12 production was assessed. Data are graphed as average MFI of n=3 mice per group, (*=p<0.05). (D) Serum level of IL-12p40 at 6 months averaged for 6 pairs of mice, (*=p<0.05).
Decreased inflammatory CD4 T cells in tm24KO mice
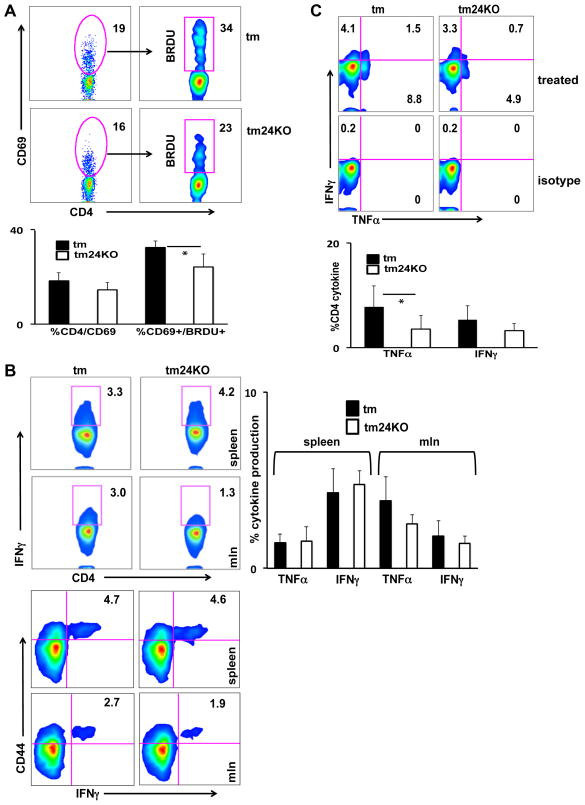
IL-12 is an inducer of Th1 differentiation and leads to enhanced T cell proliferation and IFN-γ production (25). We assessed CD4/CD8 populations in tm and tm24KO mice and did not note a difference between these populations (data not shown). We further investigated CD4 T cells by measurement of early activation marker CD69. We found that splenic tm24KO CD4 T cells expressed less CD69 than tm CD4 T cells. To determine whether tm24KO CD4 T cells were truly “less active” than tm CD4 T cells we fed mice BrdU water and assessed BrdU incorporation after 3 days. We found that CD4/CD69+ populations of tm24KO mice showed decreased BrdU incorporation as compared to tm mice and this effect was significant in splenocytes. These results indicate low CD4 T cell proliferation in tm24KO mice (Figure 2A). To quantify inflammatory potential of T cells we isolated and stimulated (PMA/ionomycin) mixed lymphocytes from tm and tm24KO mice. We found increased IFN-γ (top panels) and TNF-α (data not shown) production from mesenteric lymph nodes (mln) of tm mice as compared to tm24KO mice (Figure 2B). We further assessed CD4 T cell activation in spleens and mlns by analysis of CD44 expression. We determined that IFN-γ (bottom panels) and TNF-α production (data not shown) were produced by CD44high CD4 T cell subsets in tm and tm24KO mice (Figure 2B). Though not significant, tm24KO mice consistently showed less inflammatory cytokine production from CD44high CD4 T cell subsets. Due to enhanced TNF-α and IFN-γ in lymph nodes that approached significance, we focused on T cells in circulation. We performed stimulation of CD4 T cells from peripheral blood of tm and tm24KO mice. Production of TNF-α and IFN-γ were increased in tm CD4 T cells as compared to tm24KO CD4 T cells (Figure 2C). Therefore it is likely that enhanced activation of T cells led to increased peripheral migration and subsequent inflammatory surveillance in tm mice.
Figure 2. Decreased T cell activation, proliferation, and cytokine production in tm24KO mice.
Tm and tm24KO mice were subjected to flow cytometric analysis. CD4+ single-positive populations were gated on. (A) Splenocytes from 6 month tm or tm24KO mice were surface stained for CD69+ and intracellular stained for BrdU after 3 days of BrdU water treatment. Data of one representative pair of mice from 4 pairs of mice is shown. Graph depicts statistical significance for CD4/CD69+ and CD4/CD69/BrdU+ cells, (*=p<0.05) (B) Splenocytes or mesenteric lymph node cells were incubated with PMA/Ionomycin in the presence of brefeldin A for 4–6 hours. Intracellular stain of IFN-γ (shown) and TNF-α were performed. Top flow panels show CD4-specific IFN-γ production. Bottom panels are gated on CD4+ single-positive cells and assessed for CD44/IFN-γ double positive populations. Data shown are one pair of mice, representative of 4 pairs of mice. Graph depicts statistical significance for CD4/TNF-α+ or CD4/IFN-γ+ splenocytes and mesenteric lymph node cells. (C) Peripheral blood mononuclear cells were incubated with PMA/Ionomycin in the presence of brefeldin A for 4 -6 hours. Intracellular stain of IFN-γ (shown) and TNF-α were performed. Data of one representative pair of mice from 5 pairs of mice is shown. Graph depicts statistical significance for CD4/TNF-α+ or CD4/IFN-γ+ PBMCs, (*=p<0.05).
Hallmarks of anti-inflammatory immunity
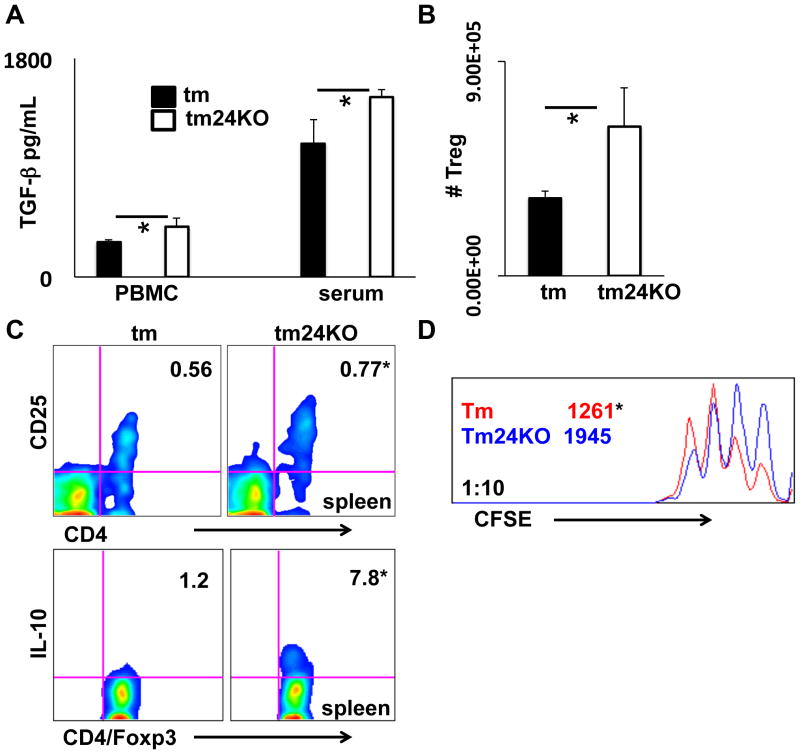
To better understand the cause of decreased CD4 T cell activation in tm24KO mice we assessed parameters of anti-inflammatory immunity. The cytokine TGF-β is tied to activity of immune-suppressive populations such as Treg cells and MDSCs (26, 27). We measured active TGF-β secretion from PBMC cultured 24 hours or from serum. We found significantly increased active TGF-β in culture supernatants and sera from tm24KO mice compared to tm mice (Figure 3A). To further investigate these data we isolated and counted absolute numbers of Tregs from tm or tm24KO mouse spleens and found significantly increased numbers of Tregs in tm24KO mice (Figure 3B). We next directly evaluated the role of Tregs in tm24KO mice as compared to tm mice. IL-10 is a hallmark anti-inflammatory cytokine associated with Treg activation and suppression of inflammatory immunity (28). CD25, the high affinity IL-2 receptor, is a mark of Treg cell activation indicative of active IL-2 signaling and subsequent active Treg cell suppression (29). Compared to tm mice, Tregs from tm24KO mice displayed significantly increased ratios of CD25+ when measured from total Foxp3+/CD4+ Tregs (top panel) (*=p<0.05, tm=0.56±0.03, tm24KO=0.77±0.04). Further, intracellular cytokine stain for IL-10 production showed increased IL-10 from splenic Tregs in tm24KO mice compared to tm mice (bottom panel) (*=p<0.05, tm=1.2±0.7, tm24KO=7.8±2.1). (Figure 3C). CFSE suppression assay at a ratio of 10:1 showed increased suppression from tm24KO Tregs compared to tm Tregs (*=p<0.05, tm=1261±271, tm24KO=1945±174) (Figure 3D). Taken together, increased TGF-β, IL-10, and active Tregs indicate an enhanced regulatory immune axis in tm24KO mice as compared to tm mice.
Figure 3. Measurement of Treg associated suppressive parameters in tm and tm24KO mice.
(A) Averages of active TGF-β measured by ELISA from 24 hour PBMC cultured supernatants (3 pairs of mice) or serum (6 pairs of mice), (*=p<0.05). (B) Tregs from spleens of tm or tm24KO mice were isolated and counted. Graphs are the averages for 3 pairs of mice, (*=p<0.05). (C) Splenocyte or mesenteric lymph node single-cell suspensions were extracellularly stained for CD4/CD25 (top panel). Splenocyte single-cell suspensions were incubated with PMA/Ionomycin in the presence of brefeldin A for 4–6 hours. Cells were extracellularly stained for CD4/CD25 and intracellular staining for Foxp3/IL-10 was performed. Bottom panels are gated on Foxp3+/CD4+ cells (*=p<0.05). Splenic data of one representative pair of mice from 3 pairs of mice is shown D) CD8 T cells and Tregs were isolated from tm or tm24KO spleens. CD8 T cells were labeled with CFSE and plated at ratio of 10:1 with strain specific Tregs on CD3/28 coated plates. CFSE dilution was analyzed by flow cytometry 72 hours later. Representative histograms from 3 separate experiments are shown. Data are presented as average MFI of CFSE (*=p<0.05).
Tm24KO mice have increased MDSC populations
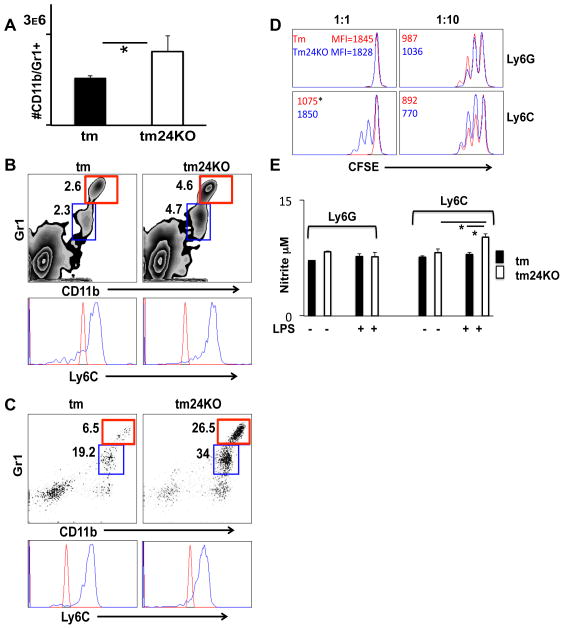
We wished to further understand the driving force behind increased suppressive immunity in tm24KO mice as compared to tm mice. Decreased naïve T cell activation, enhanced Treg cell activity and increased TGF-β signaling are hallmarks of MDSC activation (10, 30). Furthermore, reports demonstrate that HMGB1 has the potential to drive enrichment of granulocytic and myeloid derived suppressor cell populations (11, 31). Due to loss of HMGB1 receptor CD24 in tm24KO mice we wished to assess a potential contribution of MDSC populations in these mice. Absolute numbers of splenocytes indicated a significant increase in the CD11b+ population in tm24KO mice as compared to tm mice (Figure 4A). We used flow cytometry to carefully assess the lineage of increased CD11b+ cells. We gated myeloid and lymphoid cells and looked at CD11b+/Gr1+ status. Both CD11b+/Gr1high and Gr1dim populations were increased in spleens of tm24KO mice when compared to tm mice. Further gating of the CD11b/Gr1 populations distinguished a heterogeneous population of cells defined by Ly6Chigh/Gr1low and Ly6Clow/Gr1high expression. Both populations were increased in tm24KO mice (Figure 4B). We next evaluated these populations in blood taken from tm or tm24KO mice. We found significantly increased percentages of MDSCs in circulation (PBMC) in tm24KO mice as compared to tm mice. Gating of these cells indicated both Ly6Chigh/Gr1low and Ly6Clow/Gr1high were significantly increased in tm24KO mice as compared to tm mice (Figure 4C).
Figure 4. Characterization of MDSC populations in tm and tm24KO mice.
Tm and tm24KO mice were subjected to flow cytometric analysis. Extracellular CD11b+/Gr1+ single-positive populations were gated, except for (D). (A) Spleen cells were counted and total number of Cd11b+/Gr1+ cells was calculated. Graph depicts averages from 3 pairs of mice, (*=p<0.05). (B) Splenic or (C) peripheral blood CD11b+/Gr1high (red) CD11b+/Gr1dim (blue) cells are gated and histogram represents Ly6C+ dim (red) or high (blue) staining of these populations. (D) CD8 T cells, Ly6G+ and Ly6C+ cell fractions were isolated from tm or tm24KO spleens. Effector cells were labeled with CFSE and plated at ratios of 1:1 and 10:1 with strain specific MDSC subsets. CFSE dilution was analyzed by flow cytometry 72 hours later. Representative histograms from 3 separate experiments are shown. Data are presented as average MFI of CFSE (*=p<0.05). (E) Ly6G+ and Ly6C+ cell fractions were isolated from tm or tm24KO spleens and 1×105 cells were plated with or without 1μg/mL LPS for 24 hours. Greiss reaction was performed on cell supernatants (*=p<0.05).
We next measured the suppressive capacity of MDSCs between tm and tm24KO mice. Ratios of 1:1 and 10:1 were plated for Ly6G+ and Ly6C+ MDSC subsets. CFSE dilution of effector cells was measured by MFI. The Ly6C+ subset from tm24KO mice showed greater suppressive capacity of effector cell proliferation than the subset from tm mice (*=p<0.05, tm=1075±109, tm24KO=1850±232) (Figure 4D). To further measure Ly6C+ subset suppression we used Greiss reaction to assess nitrite release in response to LPS stimulation (32). Ly6C+ MDSCs from tm24KO mice showed significantly increased nitrite production in response to LPS compared to subsets from tm mice (Figure 4E).
Presence of MDSC reduce clinical manifestations of lupus-like disease
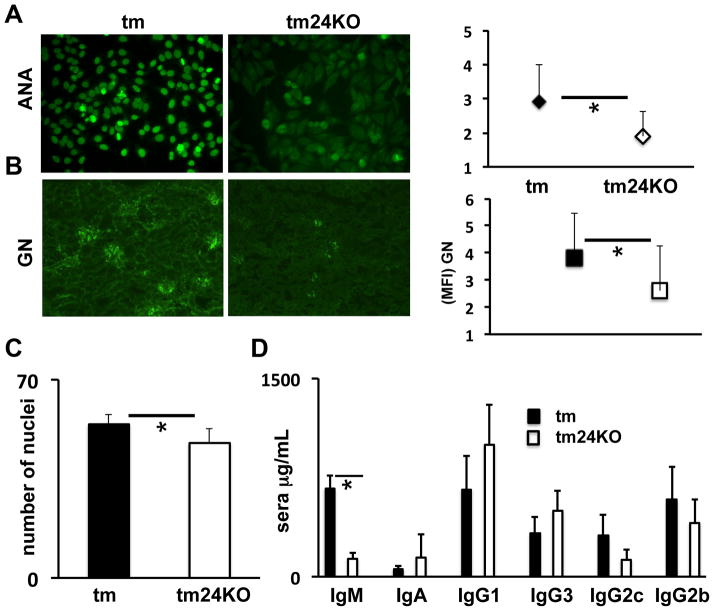
Our previous investigation of tm mice highlighted the development of lupus-like disease marked by anti-nuclear antibody deposition (ANA), glomerulonephritis (GN) and hypergammaglobulinema (13, 14, 24). Due to the suppressive atmosphere in tm24KO mice evidenced by increased MDSCs and Tregs, enhanced TGF-β axis and decreased CD4 T cell activation we wished to assess the manifestation of lupus-like disease in these mice as compared to tm mice. We first categorized ANA with a clinical score between 1 and 5 where 5 is the strongest fluorescent intensity. Among n=10 pairs of mice ANA score was significantly increased in tm mice as compared to tm24KO mice. Representative panels for tm and tm24KO ANA results are shown (Figure 5A). We next assessed glomerulonephritis in kidneys of tm or tm24KO mice. As categorized by fluorescent intensity (score 1–5), tm24KO mice showed decreased GN as compared to tm mice (n=5 pairs) (Figure 5B). Further, we performed H&E staining on kidneys from tm or tm24KO mice and counted nuclei per equal area section via 20X microscopy (13). Tm mice showed significantly more nuclei stain per field of area than tm24KO (Figure 5C). These data indicate higher levels of endothelial and mesangial cells in glomeruli of tm mice. These data indicate significantly less autoimmune disease in tm24KO mice as compared to tm mice. Increased gammaglobulin levels are a hallmark of lupus-like disease. We investigated serum levels of IgM, IgA, IgG1, IgG3, IgG2c, and IgG2b between tm and tm24KO mice (n=5 pairs). Only IgM production was significantly less in tm24KO mice as compared to tm mice (Figure 5C). Further analysis of B cell activation and markers of plasma cell development suggested there were no detectable differences between B cell activity in tm and tm24KO mice (data not shown). These data suggest that B cells were not affected by the enhanced suppressive milieu or increased MDSC populations found in tm24KO mice. Taken together, these results shown a decreased progression of lupus-like disease in tm24KO mice in an immune suppressive atmosphere marked by increased MDSCs, TGF-β axis, activated Tregs and decreased naïve T cell activation.
Figure 5. Decreased autoimmune disease in tm24KO mice as compared to tm mice.
Mean fluorescent staining intensity (MFI) of (A) ANA (B) GN in 6 month old tm or tm24KO mice categorized as clinical score 1–5 for disease severity (5 is sever disease). (C) Quantification of H&E number of nuclei per glomeruli (20X). Data are representative of one pair of 5 or more pairs of mice, (*=p<.05). (C) Serum level of IgM, IgA, IgG1, IgG3, IgG2b, IgG2c are averages of 5 pairs of mice, (*=p<.05).
In the absence of CD24 free HMGB1 recruits MDSCs to impede progression of lupus-like disease
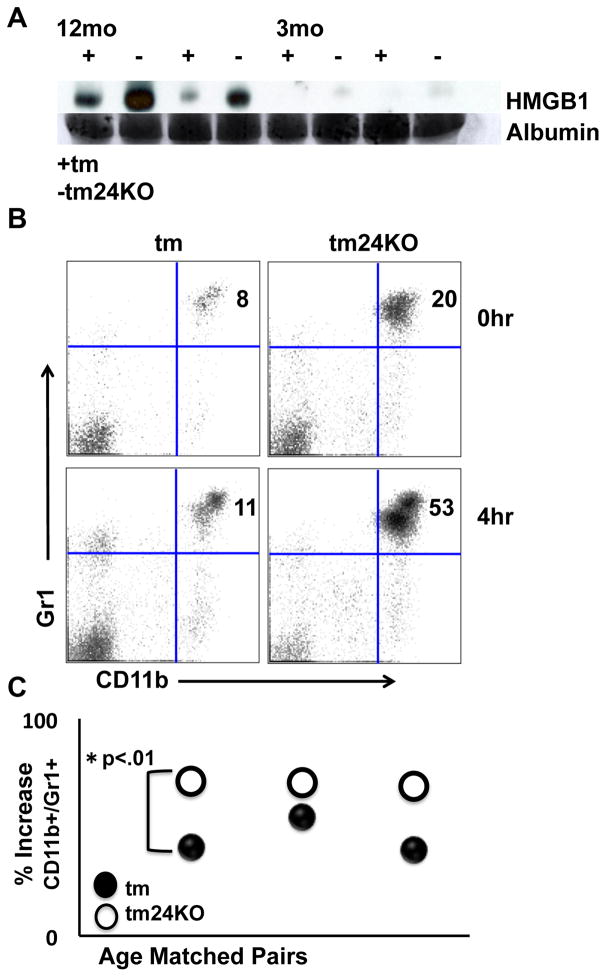
CD24 is a DAMP ligand for HMGB1 and HSPs (8). The role of soluble HMGB1 is not well defined as studies show both a pro and anti-inflammatory role for the cytokine (33). We wished to determine whether loss of CD24 led to altered levels of HMGB1 in the periphery of tm24KO mice. We performed western blot on serum of tm and tm24KO mice and probed for HMGB1. We noted increased HMGB1 in WT and tm24KO sera as mice aged that correlated with progression of autoimmune disease. Along these lines, tm24KO mice showed greater levels of sera HMGB1 at 12 months when compared to WT mice (Figure 6A). Reports demonstrate that HMBG1 has the potential to recruit granulocytic and myeloid derived cells (31, 34). To determine whether HMGB1 acted as a chemo-attractant for myeloid and granulocytic populations in tm and tm24KO mice, we injected mice with low dose (100 ng/mouse) HMGB1 and assessed myeloid cells in circulation prior to and 4 hours post injection. We found that in both tm and tm24KO mice injection of HMGB1 caused significant increases in CD11b+/Gr1+ populations found in blood 4 hours post injection (Figure 6B). Unexpectedly, tm24KO mice showed significantly more recruitment of CD11b+/Gr1+ cells as compared to tm mice. Data are plotted as percentage increase from baseline (Figure 6C). Taken together, enhanced levels of HMGB1 in serum were likely due to loss of CD24 as levels were largely detectable in tm24KO, but not tm mice. In the context of autoimmunity, free-HMGB1 may act as a chemo attractant for MDSCs that led to relative amelioration of lupus-like disease in tm24KO mice.
Figure 6. HMGB1 recruits increased numbers of MDSC in tm24KO mice.
(A) Western blot for HMGB1 on serum from tm (+) or tm24KO (−) mice aged 12 or 3 months. Data are representative of results from 4 pairs of mice. Albumin is used as a loading control from Comassie blue de-stained gel. Tm or tm24KO mice were treated with 100 ng/mouse of rHMGB1 and flow cytometryic analysis was performed on single-suspension PBMC pre (0 hr) and post (4 hr) injection for extracellular stain CD11b/Gr1+. (B) Data of one representative pair of mice from 3 pairs of mice is shown (C) Data are graphed as percentage increase of CD11b/Gr1+ cells from 0 hr to 4 hr time point (*=p<.01).
Discussion
CD24 has been shown to play important but apparently opposite roles in adaptive and innate immunity. On one hand, Chen et al (8) demonstrated CD24 expressed on dendritic cells binds to DAMPs and represses inflammatory responses by innate immune effectors. On the other hand, CD24 has been shown to be a costimulatory molecule on antigen-presenting cells for activation of CD4 and CD8 T cells to antigens (35, 36), especially in the context where the host lacks robust costimulation by CD28 (36, 37). In addition, CD24 on T cells is essential for T cell homeostatic proliferation (38). In the context of autoimmune diseases, CD24-deficiency abrogates experimental autoimmune encephalomyelitis by preventing T cell expansion in CNS (15) and by promoting clonal deletion of autoreactive T cells (39, 40). The apparently opposite function of CD24 in inflammation and autoimmune diseases remain to be reconciled. Here we investigated the role of CD24 in an autoimmune context where endogenous DAMPs are consistently released.
Surprisingly, CD24 deficiency ameliorated clinical manifestations of autoimmune disease in a model of DAMPs-driven autoimmune disease. While CD24 deletion does not affect B cell activation and antibody production (Figure 6), it reduces T-cell activation, proliferation and cytokine production (Figure 2). We have previously shown that depletion of T cells leads to amelioration of the tm driven autoimmune phenotype (14). The T cell proliferation defect that occurs in the absence of CD24 may play a role in decreased inflammatory T cell proliferation and activity in tm24KO mice ultimately lending to decreased Th1 abetted autoimmunity.
We have reported enhanced suppressive capacity of Tregs in tm mice (24). Here we showed that Treg suppressive capacity is further enhanced by CD24 deletion. Our data show an increase in absolute numbers of Tregs and greater IL-10 production in tm24KO mice. Correspondingly, we noted an increase in splenic IL-10 and TGF-β activity in tm24KO mice (Figure 3). Moreover, perhaps because these cytokines are also associated with enhanced MDSC activation (10) we noted a significant increase in the MDSC population found in tm24KO mice. This population was heterogeneous in nature consisting of Ly6Chigh/Gr1low and Ly6Clow/Gr1high populations. Increased numbers of MDSC in tm24KO may account for enhanced overall suppressive of SLE-like symptoms (Figure 5). MDSC expansion was consistent with increased TGF-β and diminished T cell activity in tm24KO mice, respectively (Figure 2–4). Further, Ly6Chigh/Gr1low MDSCs from tm24KO mice have enhanced suppressive capacity as compared to the matched tm subset. Upon ligation with TLR4 ligand LPS, we saw increased nitrite release from Ly6Chigh/Gr1low MDSCs in tm24KO mice. These data point to a role for CD24 in regulation of NO2 release and subsequent MDSC regulation. Taken together, the increased Treg and MDSC suppressive activity in tm24KO mice are causative factors in diminished effector T cell activation and diminished SLE-like symptoms.
Nuclear protein HMGB1 is released from both necrotic and apoptotic cells and promotes inflammatory responses in both cancer and autoimmune diseases (41). The mechanisms through which HMGB1 promotes inflammation are not well defined, and it is important to note that cellular receptors for HMGB1 range from CD24, TLRs, HSPs and CXCL12 (42). In autoimmune contexts HMGB1 has been shown to exacerbate disease through several mechanisms. Nucleosomes containing HMGB1, detected in the serum of SLE patients, led to production of inflammatory cytokines IL-6, IL1β, and TNF-α coupled to DC activation in a TLR-2 dependent manner (43). SLE patients with inflammatory skin lesions release HMGB1 at the lesion site upon exposure to UVB exposure (44). As a whole, reports show that HMGB1 is considered a potent inflammatory mediator in acute settings when associated with autoimmune disease. It is not understood how persistent low-level HMGB1 exposure will effect development of pro and anti-inflammatory immunity in autoimmune diseases. The data presented here show that increased levels of HMGB1 appeared in aged mice and correlated with the appearance of MDSC populations in tm24KO mice (Figures 4 & 6). Loss of CD24 as a potent HMGB1 receptor may explain increased free serum HMGB1 in tm24KO mice. We further show that indeed, HMGB1 acts as a powerful chemo-attractant for myeloid populations in with both tm and tm24KO mice, and tm24KO mice recruited significantly more myeloid cells than tm mice in response to acute HMGB1 (Figure 6). Thus, while CD24 also suppress host response to HMGB1 in DAMPs-driven autoimmune diseases, recruitment of MDSC provided a new mechanism to explain the apparently opposite function of CD24 in adaptive and innate immunity.
Although the role for HMGB1 in MDSC development has not been studied, HMGB1 has been shown to promote regulatory cell activation that enables suppression of host anti-tumor immunity. For example, neutralization of tumor-derived HMGB1 blocks Treg derived IL-10 and enables CD8 T cells and IFN-γ production resultant in tumor rejection (45). Further, in head and neck cancers it has been demonstrated that HMGB1 is a chemo-attractant for Tregs and promotes their suppressive capabilities (46). Promotion of Treg cell suppressive functions is associated with increased MDSC utility at the tumor site (47). In the context of cancer it is well understood that suppressor cells are detrimental to host anti-tumor immunity. However, in inflammatory-mediated autoimmune diseases Treg and MDSC populations may benefit the host. In keeping, cellular therapeutic strategies focus on restoration of immune tolerance through enhancement of these suppressor populations (48–50). The data presented here strongly support a potential role for Tregs and MDSCs in diminishing inflammation mediated autoimmune clinical manifestations. Further, the potential for low-level HMGB1 to drive these populations deserves further study.
Acknowledgments
We would like to thank members of the Li lab and members of Medical University of South Carolina Department of Immunology & Microbiology for continued support.
References
- 1.Liu Z, Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18:871–882. doi: 10.1038/nm.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris HE, Andersson U, Pietsky DS. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol. 2012;31:195–202. doi: 10.1038/nrrheum.2011.222. [DOI] [PubMed] [Google Scholar]
- 4.Shukla HD, Pitha PM. Role of hsp90 in systemic lupus erythematosus and its clinical relevance. Autoimmune Dis 2012. 2012;2012:728605. doi: 10.1155/2012/728605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber CK, Haslbeck M, Englbrecht M, Sehnert B, Mielenz D, Graef D, Distler JH, Mueller RB, Burkhardt H, Schett G, Voll RE, Furnrohr BG. Antibodies to the endoplasmic reticulum-resident chaperones calnexin, BiP and Grp94 in patients with rheumatoid arthritis and systemic lupus erythematosus. Rheumatology. 2010;49:2255–2263. doi: 10.1093/rheumatology/keq272. [DOI] [PubMed] [Google Scholar]
- 6.Boehm J, Orth T, Van Nguyen N, Soling HD. Systemic lupus erythematosus is associated with increased auto-antibody titers against calreticulin and grp94, but calreticulin is not the Ro/SS-A antigen. Eur J Clin Invest. 1994;24:248–257. doi: 10.1111/j.1365-2362.1994.tb01082.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Chen GYG, Zheng P. CD24-Siglec G/10 discriminates danger-from pathogen-associated molecular patterns. Trends Immunol. 2009;30:557–561. doi: 10.1016/j.it.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;15:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W, Wu K, Zhao E, Shi L, Li R, Zhang P, Yin Y, Shuai X, Wang G, Tao K. HMGB1 recruits myeloid derived suppressor cells to promote peritoneal dissemination of colon cancer after resection. Biochem Biophys Res Commun. 2013;436:156–161. doi: 10.1016/j.bbrc.2013.04.109. [DOI] [PubMed] [Google Scholar]
- 12.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3:891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 13.Liu B, Dai J, Zheng H, Stoilova D, Sun S, Li Z. Cell surface expression of an endoplasmic reticulum resident heat shock protein gp96 triggers MyD88-dependent systemic autoimmune diseases. Proc Natl Acad Sci USA. 2003;100:15824–15829. doi: 10.1073/pnas.2635458100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai J, Liu B, Cua DJ, Li Z. Essential roles of IL-12 and dendritic cells but not IL-23 and macrophages in lupus-like diseases initiated by cell surface HSP gp96. Eur J Immunol. 2007;37:706–715. doi: 10.1002/eji.200636643. [DOI] [PubMed] [Google Scholar]
- 15.Bai XF, Li O, Zhou Q, Zhang H, Joshi PS, Zheng X, Liu Y, Wang Y, Zheng P, Liu Y. CD24 Controls Expansion and Persistence of Autoreactive T Cells in the Central Nervous System during Experimental Autoimmune Encephalomyelitis. J Exp Med. 2004;200:447–548. doi: 10.1084/jem.20040131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai XF, Liu JQ, Liu X, Guo Y, Cox K, Wen J, Zheng P, Liu Y. The heat-stable antigen determines pathogenicity of self-reactive T cells in experimental autoimmune encephalomyelitis. J Clin Invest. 2000;105:1227–1232. doi: 10.1172/JCI9012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Lin S, Rammohan KW, Liu Z, Liu JQ, Liu RH, Guinther N, Lima J, Zhou Q, Wang T, Zheng X, Birmingham DJ, Rovin BH, Herbert LA, Wu Y, Lynn DJ, Cooke G, Yu CY, Zheng P, Liu Y. A dinucleotide deletion in CD24 confers protection against autoimmune diseases. PLoS Genet. 2007;3:e49. doi: 10.1371/journal.pgen.0030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Q, Rammohan K, Lin S, Robinson N, Li O, Liu X, Bai XF, Yin L, Scarberry B, Du P, You M, Guan K, Zheng P, Liu Y. CD24 is a genetic modifier for risk and progression of multiple sclerosis. Proc Natl Acad Sci U S A. 2005;100:15041–15046. doi: 10.1073/pnas.2533866100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ronaghi M, Vallian S, Etemadifar M. CD24 gene polymorphism is associated with the disease progression and susceptibility to multiple sclerosis in the Iranian population. Psychiatry Res. 2009;170:271–272. doi: 10.1016/j.psychres.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Piotrowski P, Lianeri M, Wudarski M, Łacki JK, Jagodziński PP. CD24 Ala57Val gene polymorphism and the risk of systemic lupus erythematosus. Tissue Antigens 2010. 2010;75:696–700. doi: 10.1111/j.1399-0039.2010.01447.x. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez E, Abelson AK, Sabio JM, González-Gay MA, Ortego-Centeno N, Jiménez-Alonso J, de Ramón E, Sánchez-Román J, López-Nevot MA, Gunnarsson I, Svenungsson E, Sturfelt G, Truedsson L, Jönsen A, González-Escribano MF, Witte T. Association of a CD24 gene polymorphism with susceptibility to systemic lupus erythematosus. Arthritis Rheum. 2007;56:3080–3086. doi: 10.1002/art.22871. [DOI] [PubMed] [Google Scholar]
- 22.Sánchez E, Fernández-Gutierrez B, González-Gay MA, Balsa A, García A, Rodríguez L, Pascual-Salcedo D, González-Escribano MF, Martin J. Investigating the role of CD24 gene polymorphisms in rheumatoid arthritis. Ann Rheum Dis. 2008;67:1197–1198. doi: 10.1136/ard.2007.084475. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen PJ, Lorenz B, Muller AM, Wenger RH, Brombacher F, Simon M, von der Weid T, Langhorne WJ, Mossmann H, Kohler G. Altered erythrocytes and a leaky block in B-cell development in CD24/HSA-deficient mice. Blood. 1997;89:1058–1067. [PubMed] [Google Scholar]
- 24.Dai J, Liu B, Ngoi SM, Sun S, Vella AT, Li Z. TLR4 hyperresponsiveness via cell surface expression of heat shock protein gp96 potentiates suppressive function of regulatory T cells. J Immunol. 2007;178:3219–3225. doi: 10.4049/jimmunol.178.5.3219. [DOI] [PubMed] [Google Scholar]
- 25.Watford WT, Moriguchi M, Morinobu A, O’Shea JJ. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 26.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 27.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol. 2011;23:424–304. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Scheffold A, Hühn J, Höfer T. Regulation of CD4+CD25+ regulatory T cell activity: it takes (IL−)two to tango. Eur J Immunol. 2005;35:1336–1341. doi: 10.1002/eji.200425887. [DOI] [PubMed] [Google Scholar]
- 30.Lees JR, Azimzadeh AM, Bromberg JS. Myeloid derived suppressor cells in transplantation. Curr Opin Immunol. 2011;23:692–697. doi: 10.1016/j.coi.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med. 2010;16:413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 32.Capietto AH, Kim S, Sanford DE, Linehan DC, Hikida M, Kumosaki T, Novack DV, Faccio R. Down-regulation of PLCg2 B-catenin pathway promotes activation and expansion of myeloid-derived suppressor cells in cancer. J Exp Med. 2013;210:2257–2271. doi: 10.1084/jem.20130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li G, Liang X, Lotze MT. HMGB1: The Central Cytokine for All Lymphoid Cells. Front Immunol. 2013;4:68. doi: 10.3389/fimmu.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penzo M, Molteni R, Suda T, Samaniego S, Raucci A, Habiel DM, Miller F, Jiang HP, Li J, Pardi R, Palumbo R, Olivotto E, Kew RR, Bianchi ME, Marcu KB. Inhibitor of NF-kappa B kinases alpha and beta are both essential for high mobility group box 1-mediated chemotaxis. J Immunol. 2010;184:4497–4509. doi: 10.4049/jimmunol.0903131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Jones B, Aruffo A, Sullivan KM, Linsley PS, Janeway CA., Jr Heat-stable antigen is a costimulatory molecule for CD4 T cell growth. J Exp Med. 1992;175:437–445. doi: 10.1084/jem.175.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Wenger RH, Zhao M, Nielsen PJ. Distinct costimulatory molecules are required for the induction of effector and memory cytotoxic T lymphocytes. J Exp Med. 1997;182:251–262. doi: 10.1084/jem.185.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y, Zhou Q, Zheng P, Liu Y. CD28-independent induction of T helper cells and immunoglobulin class switches requires costimulation by the heat-stable antigen. J Exp Med. 1998;187:1151–1156. doi: 10.1084/jem.187.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li O, Zheng P, Liu Y. CD24 expression on T cells is required for optimal T cell proliferation in lymphopenic host. J Exp Med. 2004;200:1083–1089. doi: 10.1084/jem.20040779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang X, Liu JQ, Shi Y, Reid HH, Boyd RL, Khattabi M, El-Omrani HY, Zheng P, Liu Y, Bai XF. CD24 on thymic APCs regulates negative selection of myelin antigen-specific T lymphocytes. Eur J Immunol. 2012;42:924–935. doi: 10.1002/eji.201142024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carl JW, Jr, Liu JQ, Joshi PS, El-Omrani HY, Yin L, Zheng X, Whitacre CC, Liu Y, Bai XF. Autoreactive T cells escape clonal deletion in the thymus by a CD24-dependent pathway. J Immunol. 2008;181:320–328. doi: 10.4049/jimmunol.181.1.320. [DOI] [PubMed] [Google Scholar]
- 41.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 42.Campana L, Bosurgi L, Bianchi ME, Manfredi AA, Rovere-Querini P. Requirement of HMGB1 for stromal cell-derived factor-1/CXCL12-dependent migration of macrophages and dendritic cells. J Leukoc Biol. 2009;86:609–615. doi: 10.1189/jlb.0908576. [DOI] [PubMed] [Google Scholar]
- 43.Urbonaviciute V, Furnrohr BG, Meister S, Munoz L, Heyder P, De Marchis F, Bianchi ME, Kirschning C, Wagner H, Manfredi AA, Kalden JR, Schett G, Rovere-Querini P, Hermann M, Voll RE. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J Exp Med. 2008;205:3007–3018. doi: 10.1084/jem.20081165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdulahad DA, Westra J, Reefman E, Zuidersma E, Bijzet J, Limburg PC, Kallenberg CG, Bijl M. High mobility group box1 (HMGB1) in relation to cutaneous inflammation in systemic lupus erythematosus (SLE) Lupus. 2013;22:597–606. doi: 10.1177/0961203313483377. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z, Falo LD, Jr, You Z. Knockdown of HMGB1 in tumor cells attenuates their ability to induce regulatory T cells and uncovers naturally acquired CD8 T cell-dependent antitumor immunity. J Immunol. 2011;187:118–125. doi: 10.4049/jimmunol.1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wild CA, Brandau S, Lotfi R, Mattheis S, Gu X, Lang S, Bergmann C. HMGB1 is overexpressed in tumor cells and promotes activity of regulatory T cells in patients with head and neck cancer. Oral Oncol. 2012;48:409–416. doi: 10.1016/j.oraloncology.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology. 2013;138:105–115. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulivieri C, Baldari CT. T-cell-based immunotherapy of autoimmune diseases. Expert Rev Vaccines. 2013;12:297–310. doi: 10.1586/erv.12.146. [DOI] [PubMed] [Google Scholar]
- 49.Moliné-Velázquez V, Cuervo H, Vila-Del Sol V, Ortega MC, Clemente D, de Castro F. Myeloid-derived suppressor cells limit the inflammation by promoting T lymphocyte apoptosis in the spinal cord of a murine model of multiple sclerosis. Brain Pathol. 2011;21:678–691. doi: 10.1111/j.1750-3639.2011.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cripps JG, Gorham JD. MDSC in autoimmunity. Int Immunopharmacol. 2011;11:789–793. doi: 10.1016/j.intimp.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]