Abstract
Objective
To determine if various medical conditions affect the serum concentrations of 3,3′-diiodothyronine (3,3′-T2).
Methods
100 patients, recruited from a group of inpatient and outpatients with a diverse range of medical conditions, donated a single blood sample that was assayed for thyroid hormone derivatives using liquid-chromatography tandem mass spectrometry. The associations between 3,3′-T2 concentrations and physiologic data and medical conditions were assessed.
Results
Higher quartiles of 3,3′-T2 concentrations (quartile 1: 2.01-7.48, quartile 2: 7.74-12.4, quartile 3: 12.5-17, quartile 4: 17.9-45.8 pg/ml) were associated with decreasing occurrence of critical illness (58%, 11%, 0%, 8%,), stroke (29%, 7.7%, 4%, 0%), critical care unit hospitalization (75%, 39%, 8.3 %, 12%), and inpatient status (83%, 42%, 8%, 12%), all p values <0.001. The same quartiles were associated with increasing frequency of thyroidectomy (4%, 12%, 17%, 60%). In multivariate analyses, after adjustment for age and gender, inpatient status was associated with decreasing concentrations of 3,3′-T2 (46% decrease for inpatients with 95% CI 32-57%, p value <0.0001). Thyroidectomy was associated with increasing concentrations of 3,3′-T2 (29% increase (CI 0.5-66%, p value 0.049).
Conclusion
We demonstrate associations between inpatient status and reduced 3,3′-T2 concentrations. This appears to be a global change associated with illness, rather than an association with specific medical conditions. We newly report higher 3,3′-T2 concentrations in athyreotic outpatients receiving TSH suppression therapy. This demonstrates that there is production of 3,3′-T2 from levothyroxine in extrathyroidal tissues. Conversion of T4 to 3,3′T2 via both T3 and rT3 pathways may prevent excessive T3 concentrations in such patients.
Keywords: 3,3′-diiodothyronine; tandem mass spectrometry; illness; thyroidectomy
Introduction
Iodothyronines include thyroxine (T4), triiodothyronine (T3), reverse triiodothyronine (rT3), 3,5- diiodothyronine, and 3,3′-diiodothyronine (3,3′-T2). T4 and T3 have well-established roles and exert the classic effects of thyroid hormones (1). Although an uncontrolled study of 3,5-diiodothyronine administration to humans for 4 weeks by an unspecified route was associated with increased metabolic rate and reduced body weight (2), no specific role of 3,3′-T2 in humans has been demonstrated (3). Animal studies, however, suggest that the 3,5-diiothyronine and 3,3′-T2 increase metabolic rate (3, 4), by acting at the mitochondrial level to increase hepatic cytochrome oxidase activity (5).
3,3′-T2 has been detected in human serum by radioimmunoassay (6) and has also recently been detected by MS (7). When measured by radioimmunoassay, its concentration generally parallels the concentration of other iodothyronines such as T4 and T3. Specifically with regard to thyroid conditions, it decreases with the hypothyroid state, is normal during thyroid hormone replacement, and increases with the hyperthyroid state (6, 8-13). Several studies have documented altered levels during non-thyroidal medical conditions. Variable concentrations have been reported in patients with cirrhosis (9, 14). In one study, patients with myocardial infarction, malignancies, and uremia, were found to have low levels of 3,3′-T2 (9). In another study its concentrations were low in patients with brain injury, normal in patients with sepsis, and elevated in those with either liver disease or brain tumors, compared with healthy controls (12). Individuals with a diagnosis of anorexia nervosa have lower than normal levels of 3,3′-T2 (10), as do those who undergo a period of calorie restriction (15). Serum 3,3′-T2 concentrations have also been noted to decrease with advancing age (10, 16).
We recently developed a MS method to measure T4, T3, rT3, and 3,3′-T2 simultaneously in blood samples (17). We have previously reported reference intervals for 3,3′-T2 in healthy subjects (7). We now use this methodology to document these analytes in a group of patients with a variety of medical conditions.
This was a pilot study designed to examine the concentrations of thyroid hormone derivatives in inpatients and outpatients with a variety of medical conditions. Our primary hypothesis was that various serious illnesses or simply having an inpatient status would be associated with lower 3,3′-T2 levels than were seen in outpatients. A secondary hypothesis was that thyroid disorders would be associated with altered 3,3′-T2 concentrations.
Methods
Patient eligibility and recruitment
One hundred patients were recruited for this pilot study. Patients were recruited from the Medstar outpatient clinics and inpatient services at Georgetown University and Washington Hospital Center between January and June 2011. Individuals of all ages with any medical diagnoses were eligible to be included in order to capture conditions that could potentially affect thyroid hormone derivative concentrations. All patients, or their authorized representatives, signed a written informed consent form. Patients donated a single blood sample. The status of each patient as an outpatient or inpatient, along with the specific unit in which the patient was hospitalized, was recorded. Information on demographic factors, medical history, and medication use were collected by a combination of chart review and personal interview. Height, weight, body mass index (BMI), systolic blood pressure and diastolic blood pressure were abstracted from a current physical examination.
Assay methodology
All samples were stored at −80°C and analyzed in one batch at the completion of the study. The concentrations of the thyroid hormone derivatives (T4, T3, rT3, and 3,3′-T2) were measured by MS, as previously described (7, 17, 18), with methods similar to those used for free thyroid hormones also (19-22). The method for measuring 3,3′-T2 involves a step to separate it chromatographically from 3,5-diiothyronine (17).
The reference interval for TSH was 0.40-4.00 mIU/L. Reference intervals for each of the thyroid analytes are 3,3′-T2 6.7-23 pg/mL; T3 74-168 ng/dl; rT3 7.7-23.1 ng/dL; T4 4.2-10.9 mcg/dL. These latter reference intervals were obtained using samples from 130 healthy females and 130 healthy males within the age range of 20-60 years (7). The 2.5th and 97.5th percentiles were calculated using 3 different methods, including the Percentile, the Gaussian and the Hoffmann approaches. The reference intervals for T4, T3 and rT3 were similar to those found in the literature. The reference interval for 3,3′-T2 was new and not previously known. The narrowest reference intervals were found when employing the Hoffmann approach. Nevertheless the reference intervals obtained using these 3 approaches agreed fairly well.
Statistical Analysis
Descriptive statistics of physiologic data (age, height, weight, body mass index (BMI), systolic blood pressure (SBP), diastolic body pressure (DBP), gender, and race) and medical data (glycosylated hemoglobin (HgbA1C), glucose, creatinine, inpatient/outpatient status, inpatient unit, thyroid cancer, thyroidectomy, levothyroxine (LT4) therapy, hyperthyroidism, Hashimoto’s hypothyroidism, hypertension, taking antihypertensive medications, diabetes, taking oral diabetes medications, taking insulin, critically ill, and stroke) were calculated according to the inpatient or outpatient status and the quartile of thyroid hormone analyte concentrations. Other medications being taken by patients were also documented, but were not included in the analysis as there were many medications, mostly being taken by only a small subset of patients. Data are reported as mean ± standard deviation or median (1st quartile, 3rd quartile) or frequency (proportion). T-test or analysis of variance (ANOVA) was used to compare the means and Wilcoxon rank-sum test was used to compare the distribution. Chi-square test was used to compare the proportions among the groups. Thyroid hormone analyte concentrations were analyzed both as categorical variables within quartiles and continuous variables. Log-transformation was applied to variables that were highly skewed in the analyses as continuous variables.
In order to determine which characteristics were important predictors of thyroid analyte concentrations, the association between potential physiologic or medical factors and the levels of the thyroid hormone analytes was examined with use of a logistic regression model or linear regression model with adjustment for age and gender. Variables with p values ≤0.2 in the univariate model were included in the multivariate model. P values <0.05 were considered statistically significant. In order to describe the relationship between variables, in addition to reporting each parameter estimate as a coefficient, estimates were also back log-transformed. The back-log transformed parameter estimate was then reported as a percentage change in the dependent variable for a one unit increase in the continuous variables, or as compared with the referent group for categorical variables. Data were analyzed using SAS version 9.1 (SAS Institute, Cary, NC). Two-sided P values <0.05 were considered statistically significant.
Results
Population characteristics
Of the 100 study patients, 63 were outpatients and the remaining 37 were inpatients (see table 1). Outpatients were younger (p=0.015) and had higher diastolic blood pressure (p=0.002) than inpatients (see table 1a). Other characteristics such as height, weight, BMI, and systolic blood pressure were similar for the two groups. Ninety five percent of the inpatients were from critical care units, and were fairly equally divided between the medical intensive care unit, the cardiac care unit, and the neurologic intensive care unit (35%, 33%, and 27% respectively) (see table 1b). Sixty seven percent of the outpatients and 49% of the inpatients were of female gender (p=0.077). There were no significant differences in the prevalence of diabetes and hypertension between the inpatients and outpatients (see table 1c). The prevalence of thyroid diseases was higher in the outpatients, compared to the inpatients. Most outpatients with diagnoses of thyroid cancer were taking levothyroxine and had normal or low serum TSH values. With a few exceptions the patients with Hashimoto’s hypothyroidism were a subset of the patients diagnosed with thyroid cancer. The following physiologic data and medical conditions had either few or no correlations with the thyroid derivative means, medians, or quartiles, and, for simplicity, their analysis is not reported further: race, BMI, systolic blood pressure, HgbA1C, glucose, creatinine, hyperthyroidism, taking antihypertensive medications, taking oral diabetes medications, and taking insulin.
Table 1.
Characteristics of the population studied according to inpatient versus outpatient status
| Table 1a | Inpatients (n = 37) | Outpatients (n=63) | Difference | ||
|---|---|---|---|---|---|
| Continuous Variables | Mean | SD | Mean | SD | P value |
| Age (yrs) | 56.7 | 15.8 | 48.8 | 15.1 | 0.015 |
| Height (cms) | 167 | 11.9 | 165 | 9.25 | 0.353 |
| Weight (kg) | 81.9 | 19.5 | 84.6 | 20.1 | 0.514 |
| BMI (kg/m2) | 28.2 | 6.6 | 30.6 | 8.0 | 0.138 |
| SBP (mm Hg) | 128 | 26 | 129 | 21 | 0.834 |
| DBP (mm Hg) | 69 | 15 | 78 | 11 | 0.002 |
| HgbA1C for diabetics (%) | 7.3 | 3.1 | 8.3 | 2.2 | 0.370 |
| Serum Glucose mg/dL | 144 | 67 | 128 | 78 | 0.494 |
| Serum Creatinine (mg/dL) | 1.16 | 0.68 | 1.11 | 0.99 | 0.904 |
| Table 1b | Inpatients (n=37) | Outpatients (n=63) | Difference |
|---|---|---|---|
| Categorical variables | Percentage | Percentage | P value |
| Race | 0.385 | ||
| Caucasian | 43% | 33% | |
| African American | 49% | 52% | |
| Hispanic/ Asian | 8% | 14% | |
| Critical Care Unit | n/a | ||
| Intensive care unit | 35% | n/a | |
| Cardiac care unit | 33% | n/a | |
| Stroke care unit | 27% | n/a | |
| No (Medical floor) | 5% | n/a | |
| Table 1c | Inpatients (n=37) | Outpatients (n=63) | Difference | ||
|---|---|---|---|---|---|
| Categorical variables | Yes | No | Yes | No | P value |
| Male Gender | 51% | 49% | 33% | 67% | 0.077 |
| Thyroid cancer | 3% | 97% | 40% | 60% | <0.0001 |
| Thyroidectomy | 3% | 97% | 35% | 65% | 0.0002 |
| Taking LT4 | 3% | 97% | 37% | 63% | 0.0001 |
| Hyperthyroidism | 0% | 100% | 10% | 90% | 0.082 |
| Hashimoto’s Hypothyroidism | 3% | 97% | 18% | 82% | 0.05 |
| Hypertension | 54% | 38% | 49% | 51% | 0.185 |
| Antihypertensive medications | 51% | 49% | 44% | 56% | 0.427 |
| Diabetes | 27% | 73% | 38% | 62% | 0.614 |
| Oral agents for diabetes | 5% | 95% | 22% | 78% | 0.045 |
| Insulin | 30% | 70% | 22% | 78% | 0.910 |
| Critically ill | 51% | 49% | n/a | n/a | n/a |
| Stroke | 22% | 88% | n/a | n/a | n/a |
Thyroid derivative means or medians
The mean or median analyte concentrations documented in the various medical conditions are shown in table 2 after adjustment for age and gender. The concentrations of 3,3′-T2, T3, and T4 were higher in patients with thyroid cancer, patients who had undergone thyroidectomy, and those who were taking LT4, than patients without. Patients with these conditions also had lower TSH concentrations than those without. Patients who were either critically ill, had sustained a stroke, were hospitalized in a critical care unit, or were inpatients had lower 3,3′-T2, T3, and T4 concentrations than patients without. Those who were critically ill, hospitalized in the critical care unit, or were inpatients also had higher rT3 concentrations had those without each of these conditions.
Table 2.
Thyroid derivative analyte concentration according to the medical condition (only medical conditions with significant differences in analyte concentrations are reported).
| Medical condition |
Analyte | Analyte concentration in patients with medical condition (mean or median) |
Analyte concentration in patients without medical condition (mean or median) |
P value |
|---|---|---|---|---|
| Thyroid cancer (n= 26 with, 74 without) |
3,3′-T2 | 18.4 (12.5-22.3) | 11.0 (6.8-15.0) | 0.0004 |
| T3 | 86.1 ± 18.7 | 69.6 ± 36.3 | 0.0091 | |
| rT3 | 13.6 (11.0-18.4) | 17.2 (11.3-23.4) | 0.0552 | |
| T4 | 9.9 ± 2.5 | 7.6 ± 2.7 | 0.0004 | |
| TSH | 0.54 (0.05-1.21) | 1.30 (0.68-2.43) | 0.0116 | |
| Thyroidectomy (n= 23 with, 77 without) |
3,3′-T2 | 18.9 (13.6-23.6) | 11.1 (6.9-15.0) | 0.0002 |
| T3 | 85.4 ± 19.8 | 70.5 ± 35.9 | 0.0364 | |
| T4 | 10.0 ± 2.6 | 7.6 ± 2.7 | 0.0008 | |
| TSH | 0.33 (0.05-0.76) | 1.37 (0.69-2.42) | 0.0072 | |
| Taking LT4 (n= 24 with, 76 without) |
3,3′-T2 | 18.4 (12.4-23.0) | 11.2 (7.0-15.3) | 0.0014 |
| T3 | 85.0 ± 20.6 | 70.4 ± 35.9 | 0.0371 | |
| rT3 | 13.8 (10.8-19.1) | 16.9 (11.3-24.1) | 0.0702 | |
| T4 | 9.9 ± 2.8 | 7.6 ± 2.7 | 0.0017 | |
| TSH | 0.51 (0.05-0.80) | 1.22 (0.67-2.41) | 0.0282 | |
| Critically ill (n= 19 with, 81 without) |
3,3′-T2 | 6.4 (4.0-9.1) | 14.0 (10.7-18.9) | 0.0003 |
| T3 | 33.4 ± 29.4 | 84.7 ± 25.5 | <.0001 | |
| rT3 | 25.0 (13.9-56.2) | 13.9 (10.9-19.3) | 0.0039 | |
| T4 | 5.6 ± 2.9 | 8.8 ± 2.5 | 0.0004 | |
| Stroke (n=10 with, 90 without) |
3,3′-T2 | 6.4 (4.4-7.8) | 13.3 (9.4-18.8) | 0.0009 |
| T3 | 47.8 ± 13.7 | 77.3 ± 33.5 | <.0001 | |
| T4 | 6.7 ± 1.1 | 8.4 ± 2.9 | 0.0017 | |
| Critical care unit (n= 35 with, 65 without) |
3,3′-T2 | 7.0 (5.2-10.7) | 14.9 (12.0-20.7) | <.0001 |
| T3 | 42.6 ± 26.6 | 91.6 ± 21.9 | <.0001 | |
| rT3 | 22.1 (15.4-35.6) | 13.1 (10.7-18.4) | 0.0002 | |
| T4 | 6.5 ± 2.7 | 9.1 ± 2.5 | 0.0003 | |
| Inpatient (n=37 with, 63 without) |
3,3′-T2 | 7.0 (4.8-10.2) | 15.0 (12.0-20.8) | <.0001 |
| T3 | 45.4 ± 31.9 | 90.6 ± 20.5 | <.0001 | |
| rT3 | 21.3 (15.4-30.5) | 13.1 (10.6-18.4) | 0.0002 | |
| T4 | 6.6 ± 2.9 | 9.1 ± 2.5 | 0.0008 |
Data are mean ± STD or median (1st quartile, 3rd quartile)
*ANOVA test adjusted for age and gender (log-transformation was applied to variables that were highly skewed)
Thyroid derivative concentrations as quartiles
The quartiles used to divide the thyroid derivative concentrations are shown in table 3. These quartiles and categories were then used to examine the effect of physiologic and medical data.
Table 3.
Breakdown of thyroid analytes into quartiles
| Analyte | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 |
|---|---|---|---|---|
| 3,3′-T2 | ||||
| Concentration range (pg/mL) |
2.51-7.48 | 7.74-12.4 | 12.5-17.8 | 17.9-45.8 |
| Number | 24 | 26 | 24 | 25 |
| rT3 | ||||
| Concentration range (ng/dL) |
4.84-11.0 | 11.2-15.4 | 15.5-22.2 | 22.4-109.0 |
| Number | 24 | 26 | 25 | 25 |
| T3 | ||||
| Concentration range (ng/dL) |
5.66-45.3 | 46.1-78.0 | 78.1-92.1 | 92.5-166.0 |
| Number | 25 | 25 | 26 | 24 |
| T4 | ||||
| Concentration range (mcg/dL) |
1.73-6.18 | 6.36-7.80 | 7.85-10.3 | 10.5-14.1 |
| Number | 25 | 25 | 26 | 24 |
| TSH | ||||
| Concentration range (mIU/L) |
0-0.45 | 0.48-0.98 | 0.99-2.16 | 2.23-29.3 |
| Number | 25 | 25 | 25 | 25 |
Reference intervals are as follows: 3,3′-T2 6.7-23 pg/mL;; rT3 7.7-23.1 ng/dL; T3 74-168 ng/dl; T4 4.2-10.9 mcg/dL; TSH 0.40–4.00 mIU/L
Correlation of thyroid hormone derivative concentrations with physiologic data
Using the thyroid analyte concentrations categorized into the quartiles shown in table 3, the following analytes and physiologic data were significantly associated (see table 4a for changes with quartiles, direction of changes, and p values). 3,3′-T2 concentration was negatively associated with age, and positively associated with DBP. T3 concentration was negatively associated with age and height, positively associated with DBP, and positively associated with female gender. T4 concentration was negatively associated with age, height, but positively associated with female gender. TSH concentration was negatively associated with DBP, and positively associated with Caucasian race. rT3 concentration was not associated with any physiological variable.
Table 4a.
Quantification of physiologic data according to the quartiles of thyroid derivative analytes
| Analyte | Physiologic parameter |
Quartiles | Parameter Increases or decreases from Q1 to Q4 |
P value* |
|||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||||
| 3,3′-T2 | Age (years) | 63.4±15.8 | 47.3±15.7 | 50.3±13.7 | 47.1±12.7 | decreases | 0.0007 |
| DBP (mmHg) |
68.5±15.8 | 75.9±11.5 | 78.4±12.6 | 75.7±12.2 | increases | 0.046 | |
| T3 | Age | 62.2±14.6 | 51.2±15.1 | 48.1±14.2 | 45.5±14.7 | decreases | 0.0001 |
| Height | 68.2±4.2 | 65.3±4.5 | 66.6±3.2 | 64.9±4.0 | decreases | 0.031 | |
| DBP | 67±16.56 | 74±10.1 | 80±10.8 | 77±13.1 | increases | 0.0027 | |
| Male Gender (%) |
64 | 32 | 46 | 17 | decreases | 0.0043 | |
| T4 | Age (years) | 61.4±13.9 | 46.6±16.8 | 53.8±13.5 | 44.8±13.8 | decreases | 0.0017 |
| Height (inches) |
67.6±4.3 | 66.9±3.7 | 66.4±3.9 | 64.3±4.1 | decreases | 0.0065 | |
| DBP (mmHg) | 68±15.2 | 77±12.8 | 76±10.7 | 77±13.7 | increases | 0.042 | |
| Male Gender (%) |
60 | 48 | 39 | 12 | decreases | 0.0007 | |
| TSH | DBP | 76±11.6 | 80±14.6 | 76±12.6 | 68±13.4 | decreases | 0.029 |
Data are mean ± STD or percentage
Data only shown for significant relationships Q = quartile
Analysis based on analyte divided into quartiles t-test or ANOVA
Correlation of thyroid hormone derivatives concentrations with medical data
Using the thyroid analyte concentrations categorized into quartiles, the following medical data and analytes were significantly associated (see table 4b). Decreasing 3,3′-T2, T3, and T4 concentrations were associated with a diagnosis of stroke. Decreasing 3,3′-T2, T3, and T4, but increasing rT3 concentrations were associated with being in a critical care unit. Similarly, decreasing 3,3′-T2, T3, and T4, but increasing rT3 concentrations were associated with having a critical illness.
Table 4b.
The prevalence of medical conditions according to the quartiles of the thyroid derivative analytes
| Analyte | Medical condition |
Percentage with condition within Quartile@ |
Percentage with characteristic increases or decreases with increasing quartile |
P value* | |||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||||
| 3,3′-T2 | Thyroid cancer | 4 | 19 | 21 | 60 | increases | <0.0001 |
| Thyroidectomy | 4 | 12 | 17 | 60 | increases | <0.0001 | |
| Taking LT4 | 8 | 15 | 17 | 56 | increases | 0.0002 | |
| Hypothyroidism | 4 | 8 | 4 | 28 | increases | 0.016 | |
| Critically Ill | 58 | 11 | 0 | 8 | decreases | <0.0001 | |
| Stroke | 29 | 8 | 4 | 0 | decreases | 0.001 | |
| Critical Care Unit | 75 | 39 | 8 | 12 | decreases | <0.0001 | |
| Inpatient | 83 | 42 | 8 | 12 | decreases | <0.0001 | |
| rT3 | Critically ill | 8 | 11 | 12 | 48 | increases | 0.0009 |
| Critical Care Unit | 17 | 19 | 36 | 64 | increases | 0.0002 | |
| Inpatient | 17 | 23 | 44 | 64 | increases | 0.0002 | |
| T3 | Thyroid Cancer | 4 | 20 | 54 | 25 | increases | 0.012 |
| Critically ill | 72 | 4 | 0 | 4 | decreases | <0.0001 | |
| Stroke | 20 | 20 | 0 | 0 | decreases | 0.0031 | |
| Critical Care Unit | 92 | 36 | 0 | 8 | decreases | <0.0001 | |
| Inpatient | 100 | 36 | 0 | 12 | decreases | <0.0001 | |
| T4 | Thyroid cancer | 12 | 8 | 35 | 50 | increases | 0.0004 |
| Thyroidectomy | 12 | 4 | 27 | 50 | increases | 0.0004 | |
| Taking LT4 | 12 | 4 | 31 | 50 | increases | 0.0003 | |
| Hypothyroidism | 8 | 0 | 8 | 29 | increases | 0.014 | |
| Critically ill | 48 | 20 | 8 | 4 | decreases | <0.0001 | |
| Stroke | 16 | 16 | 8 | 0 | decreases | 0.039 | |
| Critical Care Unit | 64 | 40 | 19 | 13 | decreases | <0.0001 | |
| Inpatient | 72 | 40 | 19 | 17 | decreases | <0.0001 | |
| TSH | Thyroid cancer | 52 | 28 | 16 | 8 | decreases | 0.0003 |
| Thyroidectomy | 52 | 28 | 4 | 8 | decreases | <0.0001 | |
| Taking LT4 | 52 | 24 | 8 | 12 | decreases | 0.0004 | |
| Hypothyroidism | 24 | 12 | 0 | 8 | decreases | 0.033 | |
Data only shown for significant relationships Analysis based on analyte divided into quartiles Q = quartile
percentage of patients within that analyte quartile that have the particular medical condition (e.g. for Q1 3,3-T2 4% of patients have thyroid cancer and 96% of patients do not have thyroid cancer)
Chi-square test was used
Increasing 3,3′-T2 and T4, but decreasing TSH concentrations were associated with both a diagnosis of thyroid cancer and having had a thyroidectomy. Increasing quartiles of T3 concentration were associated with an increasing percentage of a thyroid cancer diagnosis. No other condition was positively associated with increasing quartiles of T3 concentration. Increasing 3,3′-T2 and T4, but decreasing TSH concentrations were associated with both taking LT4 and having a diagnosis of hypothyroidism.
Multivariate Analysis
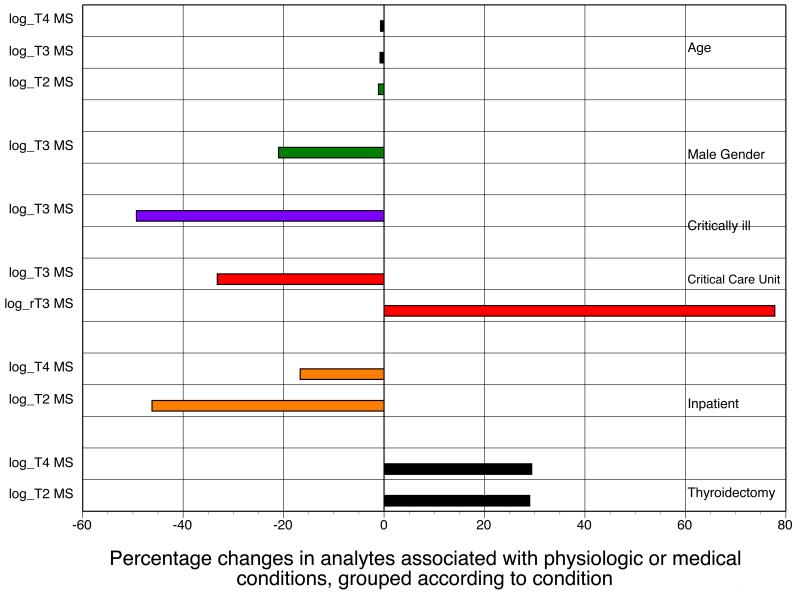
In a multivariate model, the potential independent predictors for the concentration of each thyroid analyte that remained significant after adjustment for age and gender are shown in table 5 and figure 1. (All analytes with p values ≤0.2 were log-transformed for this analysis.)
Table 5.
Significant predictors of thyroid derivative analytes
| Parameter | Parameter estimate as a coefficient |
Standard Error for coefficient |
Parameter estimate as a percentage change* |
95% CI for percentage change |
P |
|---|---|---|---|---|---|
| Log 3,3′-T2 MS | |||||
| Age | −0.011 | 0.0033 | −1.1 | --0.5 – −1.7 | 0.0017 |
| Thyroidectomy | 0.2548 | 0.1276 | 29.0 | 0.5 – 65.7 | 0.049 |
| Inpatient | −0. 6198 | 0.1167 | −46.2 | −32.4 – −57.2 | <0.0001 |
| Log rT3 MS | |||||
| Critical Care Unit | 0.5756 | 0.1234 | 77.8 | 39.6 – 126.5 | <0.0001 |
| Log T3 MS | |||||
| Age | −0.0084 | 0.0023 | −0.8 | −0.4 – −1.3 | 0.0006 |
| Male Gender | −0.2372 | 0.0748 | −21.1 | −8.7 – −31.9 | 0.0021 |
| Critically ill | −0.6793 | 0.1184 | −49.3 | −36.1 – −59.8 | <0.0001 |
| Critical Care Unit | −0.4039 | 0.0998 | −33.2 | −18.8 – −45.1 | 0.0001 |
| Log T4 MS | |||||
| Age | −0.0073 | 0.0021 | −0.7 | −0.3 – −1.1 | 0.0011 |
| Thyroidectomy | 0.2577 | 0.0879 | 29.4 | 8.9 – 53.7 | 0.0042 |
| Inpatient | −0.1825 | 0.0771 | −16.7 | −3.1 – −28.4 | 0.0201 |
| Log TSH | |||||
| Thyroid cancer | −2.2982 | 0.5062 | −90.0 | −72.9 – −96.3 | <0.0001 |
Values are back log-transformed. Parameter estimate as a percentage is the percentage change in dependent variables for one unit increase for continuous variable (age) or as compared with reference group for categorical variables. For example, a one-year increase in age would result in a 1.1% decrease in 3, 3-T2, and an inpatient status was associated with a 46% decrease in 3, 3-T2.
Figure 1.
Multivariate model showing the percentage changes in analytes associated with either physiologic data or medical conditions, grouped according to data or conditions (T2 = 3,3′-T2)
Multivariate model. Percentage is the percentage change in dependent variables for one unit increase for continuous variable (age) or as compared with reference group for categorical variables
As shown in figure 1 in which estimates are grouped by conditions, older age was associated with decreased concentrations of T4, T3, and 3,3′-T2. Male gender was associated with decreased concentration of T3. Being critically ill was associated with decreased concentrations of T3. Being hospitalized in the critical care unit was associated with decreased T3 concentration, but increased concentrations of rT3. Inpatient status was associated with decreased concentrations of T4 and 3,3′-T2. Having had a thyroidectomy was associated with increased concentrations of T4 and 3,3′-T2.
Using back log-transformed data the percentage change in each dependent variable for a unit increase in each continuous variable, or compared with the referent group for categorical variables, is also shown in table 5. As can be seen, for example, 3,3′-T2 concentration was increased by 29% (CI 0.5-65.7%) in athyreotic thyroid hormone-replaced patients and was decreased by 46.2% (CI 32.4-57.2%) in inpatients.
Discussion
These analyses demonstrate the profiles of a panel of thyroid hormone derivatives in patients with various physiologic characteristics and medical conditions with either inpatient or outpatient status at the time of blood sampling.
Multivariate analyses showed a very modest negative effect of age on the concentrations of 3,3′-T2, T3, and T4 concentrations. Other studies of thyroid hormone changes with age from the literature show mixed results, with a decrease in 3,3′-T2 and T3 with age being the most consistent findings. Serum 3,3′-T2 concentrations were seen to decrease with advancing age in 2 studies (10, 16). T3 appears to decrease and rT3 increases with age in general (23), and in the very elderly (24). T4 was shown to decrease significantly with age in the National Health and Nutrition Examination III Survey (25). However, T4 increased with age in a population of elderly men (23).
However, the greatest reductions in thyroid hormone derivatives were associated with being critically ill, being hospitalized in a critical care unit, and having an inpatient status. Both critical illness and being in a critical care unit were associated with decreased T3 concentration. Effects specific to one of these two conditions only were the following: reduced T4 and increased rT3 associated with being hospitalized in a critical care unit. Simply being an inpatient was associated with reduced concentrations of T4, and 3,3′-T2.
While reductions in T3 and increases in rT3 during illness are well-established, the findings with regard to 3,3′-T2 of are less well documented or novel. Prior studies of 3,3′-T2 showed that its levels were differentially affected by various illnesses, but were low in patients with myocardial infarction, malignancies, and uremia, (9), low in patients with brain injury (12), and low during starvation (15) or in individuals with a diagnosis of anorexia nervosa (10). In our analysis 3,3′-T2 concentrations were significantly reduced in individuals who had suffered a stroke in univariate analysis. This could not be confirmed in multivariate analyses, presumably due to the fact that the number of patients who had suffered a stroke was a small subset of all the inpatients. As a novel finding, we observed that 3,3′-T2 concentrations were decreased in patients with an inpatient status, perhaps suggesting a non-specific effect of illness in general or poor nutritional status to reduce 3,3′-T2 concentrations. The observation that 3,3′-T2 concentrations are low in several illnesses that are also associated with increased rT3 and low T3 is interesting. The fact that despite high rT3 levels, high levels of 3,3′-T2 are not being observed, perhaps suggests that while T3 deiodination is occurring, sufficient type 3 deiodinase activity must be present to prevent accumulation of 3,3′-T2.
Most of our patients who were taking thyroid hormone were from the outpatient group, rather than the inpatient group. They were also mostly athyreotic following thyroidectomy, had a diagnosis of thyroid cancer, and were being managed to maintain low serum TSH values. This was demonstrated by the finding that thyroidectomy was associated with high T4 values and low TSH values. We also documented increased concentrations of 3,3′-T2 in these patients. Prior studies have shown that hypothyroidism and hyperthyroidism are associated with lower (6, 8-12) and higher (6, 8-12) than normal concentrations of 3,3′-T2 respectively. Treatment of hypothyroid patients with T3 was observed to increase their 3,3′-T2 levels (6, 8, 10, 11, 13), as was treatment with T4 (11) and rT3 also (8).
Although the treatment of hypothyroid patients with either T4 or T3 has been shown to increase their serum 3,3′-T2 levels, to our knowledge only one study, published in abstract form only has demonstrated this finding of restored 3,3′-T2 levels in athyreotic patients specifically (26). This study therefore confirms the prior abstract finding that 3,3′-T2 is being produced by peripheral conversion from LT4 in extrathyroidal tissues.
3,3′-T2 is produced from T3 by the action of type 1 and 3 deiodinases. 3,3′-T2 is also produced from rT3 by the action of type 1 and 2 deiodinases. As T3 levels were not also increased above the normal range in our LT4-replaced patients, and neither were rT3 concentrations, this might suggest that the higher than normal levels of T4 are being converted to both T3 and rT3 in similar amounts, such that neither of their individual concentrations are elevated, but that the breakdown of both these thyroid derivatives combines to produce higher than normal concentration of 3,3′-T2. LT4-treated patients have been documented to have higher T4 levels than euthyroid patients not receiving treatment and also frequently have lower than normal T3 levels (27-30). Dual use of these two pathways may be a mechanism for preventing excessive T3 levels in those with high T4 levels. This could, paradoxically, also limit the ability to normalize T3 levels in LT4-treated patients.
Our study has several limitations. Our hospitalized patients, as is typical in most inpatient groups, were being administered several different medications. Being given antihypertensive medications, oral diabetes medications, or insulin was not significantly associated with the concentration of any thyroid derivatives. Other medications were being administered to too small a subset of the population to permit meaningful analysis. However, we cannot rule out that use of multiple medications may be contributing to the changes in thyroid hormone derivatives in inpatients. Additionally, because this was a pilot study designed to capture conditions affecting thyroid analytes, our participants had a wide variety of characteristics. Although multivariate analyses identified independent predictors of thyroid analyte concentrations, some of these predictors were associated with each other (e.g. thyroidectomy and thyroid cancer) and others had minimal overlap (e.g. thyroidectomy and inpatient status).
In summary, this pilot study provided preliminary data that supported our primary and secondary hypotheses. We found low 3,3′-T2 concentrations in participants with an inpatient status. The current role of 3,3′-T2 in humans is unknown and explanations for its decreased concentrations in the inpatient situation, and whether it is an adaptive or maladaptive change, can be examined in future larger studies conducted after confirmation of these findings. We also found that 3,3′-T2 levels tended to be high, and in many cases above the normal reference interval, in LT4-replaced athyreotic patients, perhaps shedding light on why such patients may have high T4 levels, but not high T3 levels that exceed the normal reference interval.
Acknowledgements
This project has been funded in part with Federal funds (UL1TR000101 previously UL1RR031975) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise”. Statistical analyses were provided by the Design, Biostatistics, and Population Studies component of the Georgetown-Howard Universities Center for Clinical and Translational Science.
KDB has or has recently had research grants from Pfizer, Eisei, Amgen, Astra Zeneca, and IPE Inc. He is Deputy Editor of the Endocrine Society journal, the Journal of Clinical Endocrinology and Metabolism. He serves on the Food and Drug Administration Endocrine Advisory Committee as an ad hoc member.
Abbreviations
- 3,3′-T2
3,3′-diiodothyronine
- ANOVA
Analysis of variance
- BMI
Body mass index
- CI
Confidence interval
- DBP
Diastolic blood pressure
- HgbA1C
Glycosylated hemoglobin
- LT4
Levothyroxine
- MS
Liquid chromatography tandem mass spectrometry
- Q
Quartile
- rT3
Reverse triiodothyronine
- SBP
Systolic blood pressure
- STD
Standard deviation
- T3
triiodothyronine
- T4
thyroxine
- TSH
Thyroid stimulating hormone
Footnotes
Data presented in part at the 95th Annual Meeting of the Endocrine Society in San Francisco, CA
Conflict of interest: JJ, AS, HW, DF, OS, and SJS have no relevant disclosures.
References
- 1.Piehl S, Hoefig CS, Scanlan TS, Kohrle J. Thyronamines--past, present, and future. Endocr Rev. 2011;32:64–80. doi: 10.1210/er.2009-0040. [DOI] [PubMed] [Google Scholar]
- 2.Antonelli A, Fallahi P, Ferrari SM, et al. 3,5-diiodo-L-thyronine increases resting metabolic rate and reduces body weight without undesirable side effects. J Biol Regul Homeost Agents. 2011;25:655–660. [PubMed] [Google Scholar]
- 3.Moreno M, de Lange P, Lombardi A, Silvestri E, Lanni A, Goglia F. Metabolic effects of thyroid hormone derivatives. Thyroid. 2008;18:239–253. doi: 10.1089/thy.2007.0248. [DOI] [PubMed] [Google Scholar]
- 4.Moreno M, Lombardi A, Beneduce L, et al. Are the effects of T3 on resting metabolic rate in euthyroid rats entirely caused by T3 itself? Endocrinology. 2002;143:504–510. doi: 10.1210/endo.143.2.8613. [DOI] [PubMed] [Google Scholar]
- 5.Lanni A, Moreno M, Cioffi M, Goglia F. Effect of 3,3′-diiodothyronine and 3,5-diiodothyronine on rat liver oxidative capacity. Mol Cell Endocrinol. 1992;86:143–148. doi: 10.1016/0303-7207(92)90138-v. [DOI] [PubMed] [Google Scholar]
- 6.Burman KD, Strum D, Dimond RC, et al. A radioimmunoassay for 3,3′-L-diiodothyronine (3,3′T2) J Clin Endocrinol Metab. 1977;45:339–352. doi: 10.1210/jcem-45-2-339. [DOI] [PubMed] [Google Scholar]
- 7.Soldin S, Nguyen L, Sun K, Greenan G, Hanak M, Soldin O. Adult reference intervals for 3-iodothyronamine, thyroxine, triiodothyronine, reverse T3, and 3,3-diiodothyronine measured by isotop dilution HPLC tandem mass spectrometry in human serum. Abstract E-68; American Association of Clinical Chemists Annual Meeting; 2011. [Google Scholar]
- 8.Wu SY, Chopra IJ, Nakamura Y, Solomon DH, Bennett LR. A radioimmunoassay for measurement of 3,3′-L-diiodothyronine (T2) J Clin Endocrinol Metab. 1976;43:682–685. doi: 10.1210/jcem-43-3-682. [DOI] [PubMed] [Google Scholar]
- 9.Faber J, Kirkegaard C, Lumholtz IB, Siersbaek-Nielsen K, Friis T. Measurements of serum 3′,5′-diiodothyronine and 3,3′-diiodothyronine concentrations in normal subjects and in patients with thyroid and nonthyroid disease: studies of 3′,5′-diiodothyronine metabolism. J Clin Endocrinol Metab. 1979;48:611–617. doi: 10.1210/jcem-48-4-611. [DOI] [PubMed] [Google Scholar]
- 10.Burger A, Sakoloff C. Serum 3,3′-L-diiodothyronine, a direct radioimmunoassay in human serum: method and clinical results. J Clin Endocrinol Metab. 1977;45:384–391. doi: 10.1210/jcem-45-3-384. [DOI] [PubMed] [Google Scholar]
- 11.Gavin LA, Hammond ME, Castle JN, Cavalieri RR. 3,3′-Diiodothyronine production, a major pathway of peripheral iodothyronine metabolism in man. J Clin Invest. 1978;61:1276–1285. doi: 10.1172/JCI109044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pinna G, Hiedra L, Meinhold H, et al. 3,3′-Diiodothyronine concentrations in the sera of patients with nonthyroidal illnesses and brain tumors and of healthy subjects during acute stress. J Clin Endocrinol Metab. 1998;83:3071–3077. doi: 10.1210/jcem.83.9.5080. [DOI] [PubMed] [Google Scholar]
- 13.Nishikawa M, Inada M, Naito K, et al. Serum concentrations of 3, 3′-diiodothyronine, 3′, 5′-diiodothyronine, and 3, 5-diiodothyronine in altered thyroid states. Endocrinol Jpn. 1983;30:167–172. doi: 10.1507/endocrj1954.30.167. [DOI] [PubMed] [Google Scholar]
- 14.Faber J, Thomsen HF, Lumholtz IB, Kirkegaard C, Siersbaek-Nielsen K, Friis T. Kinetic studies of thyroxine, 3,5,3′-triiodothyronine, 3,3,5′-triiodothyronine, 3′,5′-diiodothyronine, 3,3′-diiodothyronine, and 3′-monoiodothyronine in patients with liver cirrhosis. J Clin Endocrinol Metab. 1981;53:978–984. doi: 10.1210/jcem-53-5-978. [DOI] [PubMed] [Google Scholar]
- 15.Jaedig S, Faber J. The effect of starvation and refeeding with oral versus intravenous glucose on serum 3,5-,3,3′-and 3′-5′-diiodothyronine and 3′-monoiodothyronine. Acta Endocrinol (Copenh) 1982;100:388–392. doi: 10.1530/acta.0.1000388. [DOI] [PubMed] [Google Scholar]
- 16.Nishikawa M, Inada M, Naito K, et al. Age-related changes of serum 3,3′-diiodothyronine, 3′,5′-diiodothyronine, and 3,5-diiodothyronine concentrations in man. J Clin Endocrinol Metab. 1981;52:517–522. doi: 10.1210/jcem-52-3-517. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen L, Gu J, Soldin O, Soldin S. Development and validation of an isotope dilution tandem mass spectrometry method for the simultaneous quantification of 3-iodothyronine,thyroxine, triiodothyronine, reverse T3 and 3,3′-diiodo-L-thyronine in human serum. Clin Chem. 2011;57:A82. Abstract B-99. [Google Scholar]
- 18.Soukhova N, Soldin OP, Soldin SJ. Isotope dilution tandem mass spectrometric method for T4/T3. Clin Chim Acta. 2004;343:185–190. doi: 10.1016/j.cccn.2004.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Deventer HE, Mendu DR, Remaley AT, Soldin SJ. Inverse log-linear relationship between thyroid-stimulating hormone and free thyroxine measured by direct analog immunoassay and tandem mass spectrometry. Clin Chem. 2011;57:122–127. doi: 10.1373/clinchem.2010.154088. [DOI] [PubMed] [Google Scholar]
- 20.Soldin SJ, Soukhova N, Janicic N, Jonklaas J, Soldin OP. The measurement of free thyroxine by isotope dilution tandem mass spectrometry. Clin Chim Acta. 2005;358:113–118. doi: 10.1016/j.cccn.2005.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu J, Soldin OP, Soldin SJ. Simultaneous quantification of free triiodothyronine and free thyroxine by isotope dilution tandem mass spectrometry. Clin Biochem. 2007;40:1386–1391. doi: 10.1016/j.clinbiochem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Soldin OP, Jang M, Guo T, Soldin SJ. Pediatric reference intervals for free thyroxine and free triiodothyronine. Thyroid. 2009;19:699–702. doi: 10.1089/thy.2009.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Beld AW, Visser TJ, Feelders RA, Grobbee DE, Lamberts SW. Thyroid hormone concentrations, disease, physical function, and mortality in elderly men. J Clin Endocrinol Metab. 2005;90:6403–6409. doi: 10.1210/jc.2005-0872. [DOI] [PubMed] [Google Scholar]
- 24.Mariotti S, Barbesino G, Caturegli P, et al. Complex alteration of thyroid function in healthy centenarians. J Clin Endocrinol Metab. 1993;77:1130–1134. doi: 10.1210/jcem.77.5.8077303. [DOI] [PubMed] [Google Scholar]
- 25.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 26.Burman KD, Dimond RC, McGuire RA, Earll JM, Strum D, Wartofsky L. The effect of varying serum T4 concentrations on extrathyroidal production of T3, reverse T3, and 3,3′-diiodothyronine. Clin Res. 1976;24:270A. [Google Scholar]
- 27.Jonklaas J, Davidson B, Bhagat S, Soldin SJ. Triiodothyronine levels in athyreotic individuals during levothyroxine therapy. JAMA. 2008;299:769–777. doi: 10.1001/jama.299.7.769. [DOI] [PubMed] [Google Scholar]
- 28.Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One. 2011;6:e22552. doi: 10.1371/journal.pone.0022552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito M, Miyauchi A, Morita S, et al. TSH-suppressive doses of levothyroxine are required to achieve preoperative native serum triiodothyronine levels in patients who have undergone total thyroidectomy. Eur J Endocrinol. 2012;167:373–378. doi: 10.1530/EJE-11-1029. [DOI] [PubMed] [Google Scholar]
- 30.Alevizaki M, Mantzou E, Cimponeriu AT, Alevizaki CC, Koutras DA. TSH may not be a good marker for adequate thyroid hormone replacement therapy. Wien Klin Wochenschr. 2005;117:636–640. doi: 10.1007/s00508-005-0421-0. [DOI] [PubMed] [Google Scholar]