Abstract
Introduction
In this study, we present a novel classification method for individual assessment of midpalatal suture morphology.
Methods
Cone-beam computed tomography images from 140 subjects (ages, 5.6-58.4 years) were examined to define the radiographic stages of midpalatal suture maturation. Five stages of maturation of the midpalatal suture were identified and defined: stage A, straight high-density sutural line, with no or little interdigitation; stage B, scalloped appearance of the high-density sutural line; stage C, 2 parallel, scalloped, high-density lines that were close to each other, separated in some areas by small low-density spaces; stage D, fusion completed in the palatine bone, with no evidence of a suture; and stage E, fusion anteriorly in the maxilla. Intraexaminer and interexaminer agreements were evaluated by weighted kappa tests.
Results
Stages A and B typically were observed up to 13 years of age, whereas stage C was noted primarily from 11 to 17 years but occasionally in younger and older age groups. Fusion of the palatine (stage D) and maxillary (stage E) regions of the midpalatal suture was completed after 11 years only in girls. From 14 to 17 years, 3 of 13 (23%) boys showed fusion only in the palatine bone (stage D).
Conclusions
This new classification method has the potential to avoid the side effects of rapid maxillary expansion failure or unnecessary surgically assisted rapid maxillary expansion for late adolescents and young adults.
Rapid maxillary expansion (RME) has been used in orthodontic practice for the correction of posterior crossbite and dental crowding1 as well as to facilitate correction of Angle Class II2 and Class III3,4 malocclusions, with the overall objective to widen the maxilla by separating the midpalatal suture and the circummaxillary sutural system.5
Since the pioneering work of Angell6 150 years ago that introduced the concept that the maxilla can be expanded by opening the midpalatal suture, the early orthodontic literature (1860-1930) included controversy as to whether it was possible to widen the hard palate at the midpalatal suture. The landmark work of Haas7 made RME routine in many orthodontic practices, beginning in the 1960s. However, details of the morphology and the maturation of the midpalatal suture have been investigated only in histologic studies,8-12 an autopsy microcomputed tomography study,13 an investigation with occlusal radiographs,14 and an animal study with multislice computed tomography.15
Understanding individual variability in the fusion of the midpalatal suture is essential in identifying prospectively which late adolescent or young adult patient can have RME as a less-invasive alternative to surgically assisted expansion. The midpalatal suture has been described as an end-to-end type of suture16,17 with characteristic changes in its morphology during growth.8,9,11-13,15,16 In the infantile period, Melsen12 reported that the midpalatal suture is broad and Y-shaped in its frontal sections.16,18
The ossification process in the midpalatal suture starts with bone spicules from suture margins along with “islands” (ie, masses of acellular tissue and inconsistently calcified tissue) in the middle of the sutural gap.8,9,13,16 The formation of spicules occurs in many places along the suture, with the number of spicules increasing with maturation9,19 and forming many scalloped areas that are close to each other and separated in some areas by connective tissue.10,11 Concomitantly, interdigitation increases11,12; then fusion occurs earlier in the posterior area of the suture, with progression of ossification taking place from posterior to anterior,9,11 with resorption of cortical bone in the sutural ends and formation of cancellous bone.16,18
The start and the advance of fusion of the midpalatal suture vary greatly with age and sex. Persson and Thilander9 observed fusion of the midpalatal suture in subjects ranging from 15 to 19 years old. On the other hand, patients at ages 27, 32,9 54,11 and even 7113 years have been reported to have no signs of fusion of this suture. Such findings indicate that variability in the developmental stages of fusion of the midpalatal suture is not related directly to chronologic age, particularly in young adults.8-11,13
For this reason, Revelo and Fishman14 proposed individual assessment of the midpalatal suture morphology with occlusal radiographs before RME therapy. However, occlusal radiographs are not reliable for analyzing midpalatal suture morphology because the vomer and the structures of the external nose overlay the midpalatal area and thus might lead to false radiographic interpretations of midpalatal suture fusion.10
Cone-beam computed tomography (CBCT) provides 3-dimensional visualization of the oral and maxillofacial structures at relatively low cost, no superimposition of adjacent structures, easy accessibility, and low radiation exposure compared with multislice medical computed tomography.20 The aim of this study was to present a novel classification method for the individual assessment of midpalatal suture morphology using CBCT images because RME is an unpredictable treatment for late adolescent and young adult patients.
Material and Methods
Baseline diagnostic CBCT images acquired for clinical purposes in 147 subjects were selected initially; however, 7 subjects were excluded because of poor-quality scans (eg, blurry images). CBCT scans from 140 subjects (86 female, 54 male; Table I), with ages from 5.6 to 58.4 years and no history of previous orthodontic treatment, were examined to determine the radiographic stages of midpalatal suture maturation, as described in this study. All CBCT images were taken before orthodontic treatment for clinical reasons to aid in the diagnosis of clinical conditions such as canine impaction or skeletal malocclusion. Institutional review board approval for the study was obtained from the University of Michigan.
Table I. Demographics of the sample for sex and age.
| Sex | 5-<11 y | 11-<14 y | 14-18 y | >18 y | Total |
|---|---|---|---|---|---|
| Female | 24 | 24 | 19 | 19 | 86 |
| Male | 4 | 24 | 13 | 13 | 54 |
| Total | 28 | 48 | 32 | 32 | 140 |
The CBCT scans images were obtained with an iCAT cone-beam 3-dimensional imaging system (Imaging Sciences International, Hatfield, Pa). Each subject was seated in an upright position with the Frankfort horizontal plane (superior aspect of the external auditory canal to infraorbital rim line) parallel to the ground during the scanning process. For all scans, the minimum field of view used was 11 cm, and the scan time ranged from 8.9 to 20 seconds with a resolution of 0.25 to 0.30 mm.
Image analysis was performed using Invivo5 (Anatomage, San Jose, Calif). The following steps were executed for determining and analyzing the maturational stages of the midpalatal suture.
Head orientation. Natural head position in all 3 planes of space was verified or corrected. The cursor (the position indicator) of the image analysis software was positioned at the patient's midsagittal plane in both the coronal and axial views (Fig 1). In the sagittal view, the patient's head was adjusted so that the anteroposterior long axis of the palate was horizontal.
Standardization of the axial cross-sectional slice used for sutural assessment. In the sagittal plane, the midsagittal cross-sectional slice was used to position the palate horizontally, parallel to the software's horizontal orange line (Fig 1, B). After placing the horizontal line along the palate, the central cross-sectional slice in the superoinferior dimension (ie, from the nasal to the oral surface) was used for classification of the maturational stage of the midpalatal suture (Fig 1). For subjects with a curved palate, the palate was evaluated in 2 central cross-sectional axial slices, identifying the posterior and anterior regions of the midpalatal suture separately (Fig 2). For subjects with a thicker palate, the palate was evaluated in the 2 most central axial slices (Fig 3). A curved palate was defined as a palate where the anterior and posterior portions cannot be visualized in the same axial slice, and the sutural staging classification requires 2 slices. A thick palate was defined as a palate where the midpalatal suture can be assessed in more than 3 axial slices (1 oral, 1 central, and 1 nasal); for this reason, a thick palate might have 2 or more central slices.
Definition of the maturational stages of the midpalatal suture. The stages of fusion of the midpalatal suture were described from the analysis of these standardized CBCT cross-sectional images in the axial plane by an orthodontist (F.A.) and an oral and maxillofacial radiologist (E.B.). The definition of each CBCT radiographic appearance of the sutural maturation stage followed the findings of unique morphology in the maturation of the midpalatal suture described in previous histologic studies.8,16,18 The radiographic aspect of the midpalatal suture from early infancy was observed as a high-density line or area even before sutural interdigitation and fusion. The following descriptive stages of midpalatal suture maturation are proposed (Fig 4, A and B).
Fig 1.
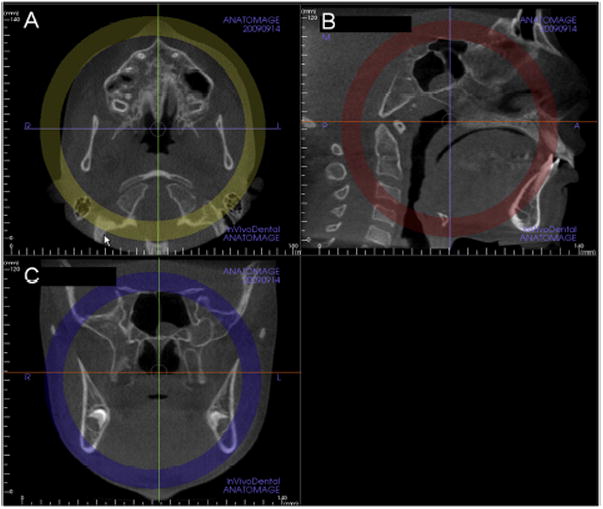
Standardization of head position in the A, axial; B, sagittal, and C, coronal planes to allow consistent assessments of the midpalatal suture. Note that in B, the sagittal view, the orange line that indicates the position of the axial plane view is positioned through the center of the superoinferior dimension of the hard palate (Invivo5).
Fig 2.
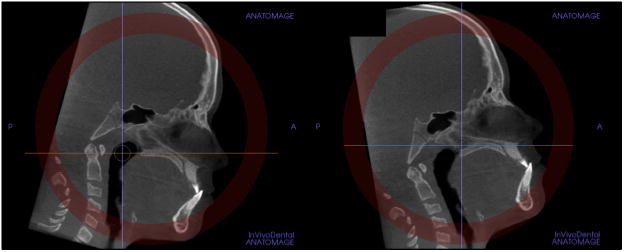
For subjects with a curved palate, 2 axial plane images through the posterior and anterior regions were used. The central cross-sectional slices along the axis of the palate in the anterior and posterior regions were evaluated.
Fig 3.
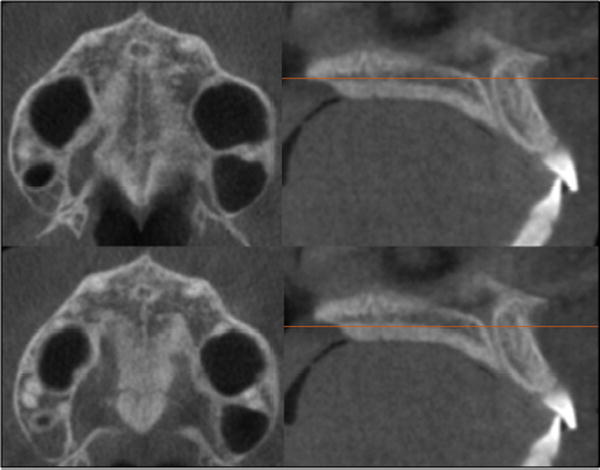
For patients with a thick palate, the 2 most central axial slices were analyzed.
Fig 4.
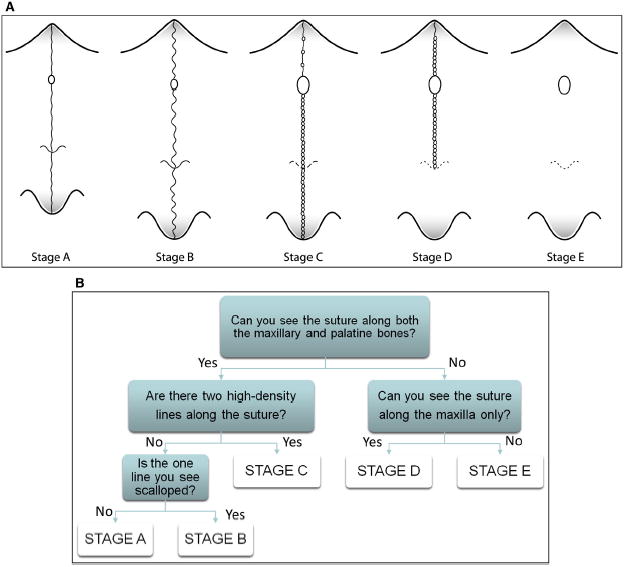
A, Schematic drawing of the maturation stages observed in the midpalatal suture. It is a simplification of the sutural morphology and should not be used for diagnosis. Sutural morphology can vary between stages, and diagnostic criteria are based on the decision tree in B and the definitions of the 5 stages. B, Decision tree for classification of the maturation stages of the midpalatal suture.
In stage A, the midpalatal suture is almost a straight high-density sutural line with no or little interdigitation (Fig 5).12,13,15,16
Fig 5.
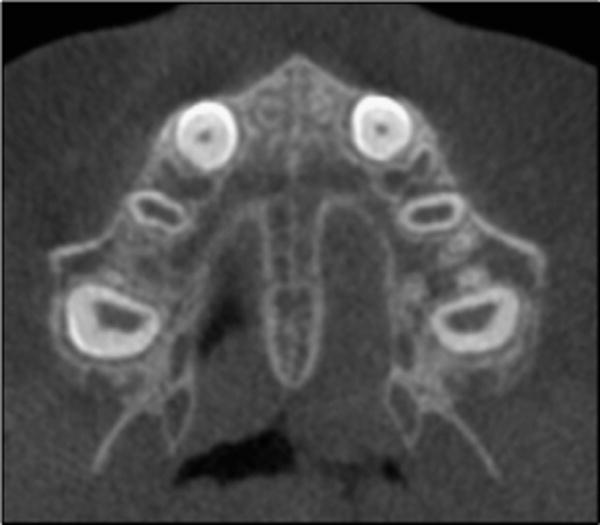
Stage A of maturation of the midpalatal suture is seen in this patient as a relatively straight high-density line at the midline.
In stage B, the midpalatal suture assumes an irregular shape and appears as a scalloped high-density line (Fig 6, A). Patients at stage B can also have some small areas where 2 parallel, scalloped, high-density lines close to each other and separated by small low-density spaces are seen (Fig 6, B).13,15
Fig 6.
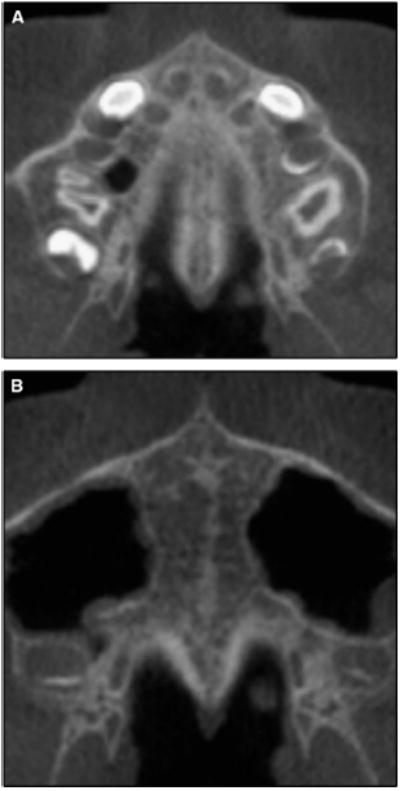
A, Stage B is observed as 1 scalloped, high-density line at the midline. B, Stage B in another subject is characterized by a scalloped high-density line in some areas and, in other areas, as 2 parallel, scalloped, high-density lines close to each other and separated by small low-density spaces.
In stage C, the midpalatal suture appears as 2 parallel, scalloped, high-density lines that are close to each other, separated by small low-density spaces in the maxillary and palatine bones (between the incisive foramen and the palatino-maxillary suture and posterior to the palatino-maxillary suture). The suture can be arranged in either a straight or an irregular pattern (Fig 7, A and B).
Fig 7.
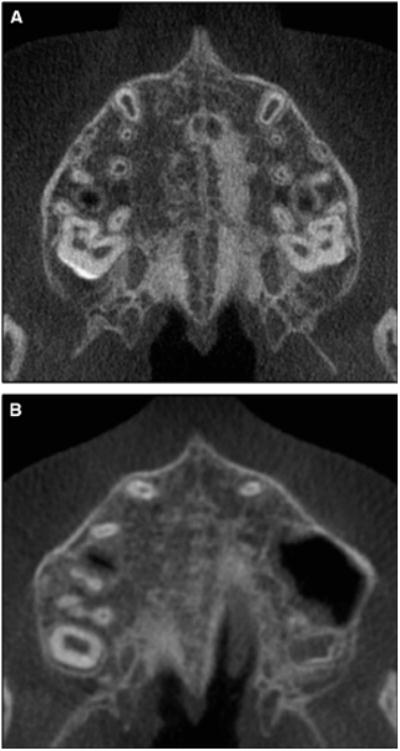
Stage C is visualized as 2 parallel, scalloped, high-density lines that are close to each other and separated in some areas by small low-density spaces. The suture can be arranged in either A, a straight or B, an irregular pattern.
In stage D, the fusion of the midpalatal suture has occurred in the palatine bone, with maturation progressing from posterior to anterior.9,11 In the palatine bone, the midpalatal suture cannot be visualized at this stage, and the parasutural bone density is increased (high-density bone) compared with the density of the maxillary parasutural bone. In the maxillary portion of the suture, fusion has not yet occurred, and the suture still can be seen as 2 high-density lines separated by small low-density spaces (Fig 8).
Fig 8.
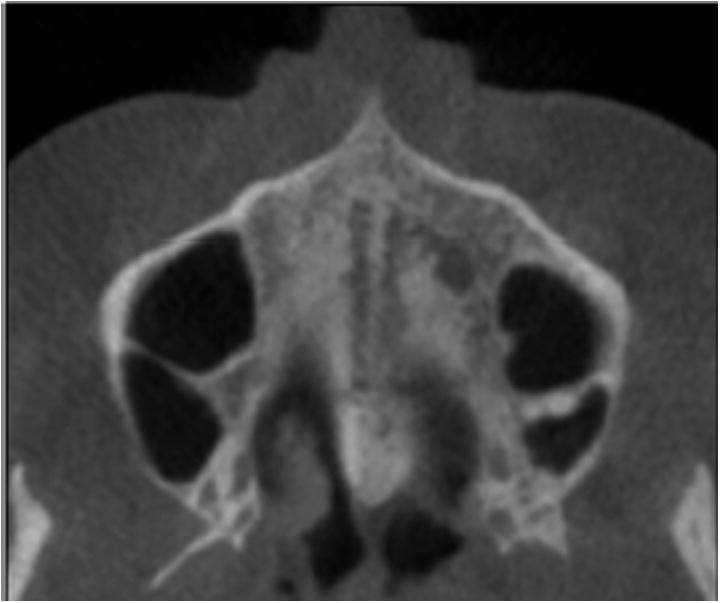
Stage D is visualized as 2 scalloped, high-density lines at the midline on the maxillary portion of the palate. The midpalatal suture cannot be visualized in palatine bone, and the density of the parasutural palatine bone is higher compared with the parasutural maxillary bone.
In stage E, fusion of the midpalatal suture has occurred in the maxilla. The actual suture is not visible in at least a portion of the maxilla.16,18 The bone density is the same as in other regions of the palate (Fig 9).13
Fig 9.
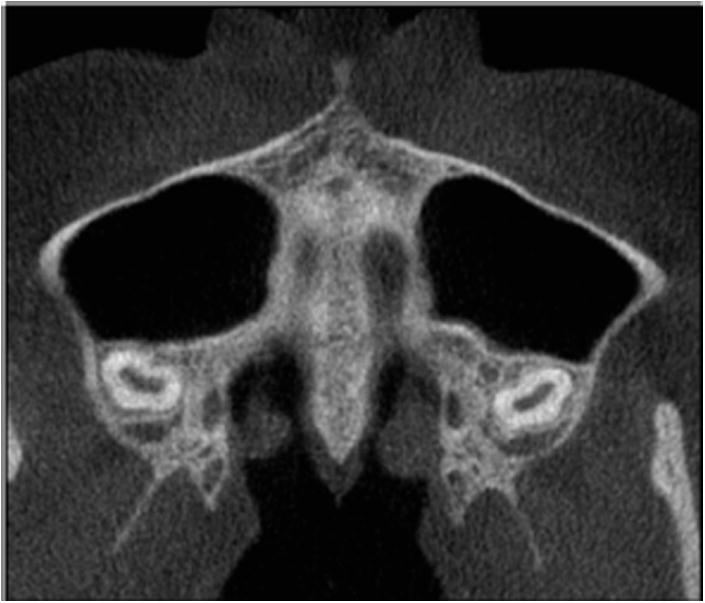
At stage E, sutural fusion has occurred in the maxilla. The midpalatal suture cannot be identified, and the parasutural bone density is the same as in other regions of the palate.
All axial central cross-sectional slices used for assessment of the midpalatal suture were selected after full review of all cross-sectional axial slices by the principal investigator (F.A.). These slices were arranged in a presentation (Microsoft Office PowerPoint 2007; Microsoft, Redmond, Wash) with a black background and codes that were displayed sequentially on a high-definition computer monitor. Each image was classified blindly by the principal investigator in a darkened room. No change in contrast or brightness of these images was undertaken. This evaluation was considered the ground truth. Consensus among radiographic interpretation or more reliable interpretation should not be considered a gold standard because a gold standard would require histologic or microcomputed tomography examination of specimens. The term “ground truth” more frequently is used regarding consensus of radiographic interpretation or reliable interpretations.
A validation study of the proposed maturational stages of the midpalatal suture was performed by 3 experienced orthodontists (F.A., L.H.C., L.F.) who each had over 1 year of experience in interpreting CBCT scans for diagnostic purposes in specific research applications. The definitions and figures (Figs 5-9) of the maturational stages of the midpalatal suture were shown in the Power Point presentation with a black background. Examiner calibration was performed using 10 images, in which all orthodontists openly classified the midpalatal suture, and any questions regarding the different maturation stages were discussed. For the validation study, 30 images were randomly selected to represent all maturational stages of the midpalatal suture. The 3 orthodontists classified all images blindly in the same room under dim light conditions, using the same high-definition monitor. A second viewing session and reclassification by the same orthodontists was done 2 days later in the same way after random rearrangement of the same images.
Statistical analysis
A weighted kappa coefficient was calculated to evaluate intraexaminer and interexaminer agreement, as well as the agreement between the examiners and the ground truth. The statistical software used was MedCalc (version 12.3.0; MedCalc Software, Mariakerke, Belgium). The agreement was defined according to the scale of Landis and Koch.21
Results
The intraexaminer and interexaminer reproducibility values demonstrated substantial agreement, with weighted kappa coefficients from 0.75 (95% confidence interval [CI], 0.57-0.93) to 0.79 (95% CI, 0.60-0.97), and the reproducibility of examiners with the ground truth showed almost perfect agreement, with weighted kappa coefficients from 0.82 (95% CI, 0.64-0.99) to 0.93 (95% CI, 0.86-1.00).
The maturational stages of the midpalatal suture observed in the sample are shown in Table II. Great variability was verified in the distribution of the maturational stages of the midpalatal suture regarding chronologic age. Stage A was noted in the early childhood period from 5 to almost 11 years of age, except for one 13-year-old boy. Stage B was present mainly up to 13 years of age, with 6 of 32 subjects (23% of boys, 15.7% of girls) from 14 to 18 years of age. Stage C was observed mainly from 11 to 18 years of age. However, two 10-year-old girls (8.3% of girls) and 4 of 32 adults (15.7% of girls, 7.7% of boys) were in stage C. No subject from 5 to almost 11 years of age had fusion of the midpalatal suture.
Table II. Distribution of the maturational stages of the midpalatal suture.
| Stage | 5-<11 y | 11-<14 y | 14-18 y | >18 y | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | ||
| A | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 5 |
| B | 19 | 3 | 12 | 16 | 3 | 3 | 1 | 0 | 57 |
| C | 2 | 0 | 6 | 7 | 5 | 7 | 3 | 1 | 31 |
| D | 0 | 0 | 1 | 0 | 3 | 3 | 7 | 3 | 17 |
| E | 0 | 0 | 5 | 0 | 8 | 0 | 8 | 9 | 30 |
| Total | 24 | 4 | 24 | 24 | 19 | 13 | 19 | 13 | 140 |
From 11 to almost 14 years of age, 6 of 24 girls (25%) had fusion of the midpalatal suture in palatine (stage D) or maxillary (stage E) bone. For subjects between 14 and 18 years of age, 11 of 19 girls (57.9%) had fusion of the midpalatal suture in palatine (stage D) or maxillary (stage E) bone; only 3 boys (23%) were in stage D. This variability also was observed in adults, who most frequently had fusion of the midpalatal suture (stages D and E), 4 subjects (12.5%) had no fused suture in stage C, and 1 subject (3.1%) was in stage B.
Discussion
Clinical experience has shown that RME failure is not rare in adolescent and young adult patients.1 Serious pain, mucosal ulceration or necrosis, and accentuated buccal tipping and gingival recession in the posterior teeth have been shown to occur after RME failure.22-26 Although surgical expansion is possible at any time throughout life, maxillary widening through multisegment osteotomies has been reported to be the most unpredictable procedure among all orthognathic surgery modalities.27,28 The unpredictability of the surgical expansion has to do with its relapse potential. Those findings have motivated many surgeons to treat transverse discrepancies in 2 stages with surgically assisted RME. These study findings could elucidate the diagnostic stage of sutural maturation and the indication for surgically assisted RME that increases morbidity and treatment costs.
The treatment choice of whether an adolescent or a young adult patient is a suitable candidate for RME without a surgical assist is a relevant clinical question. Chronologic age is unreliable for determining the developmental status of the suture during growth, as evidenced by our study in which subjects older than 11 years presented at all stages of midpalatal sutural maturation (Table II).29-31 Additionally, histologic studies have shown great variability in the maturation of the midpalatal suture.8-11,13 For these reasons, the development of a method for individual assessment of the maturation of the midpalatal suture has been deemed essential.
The images in Figures 5 through 11 illustrate the stages of sutural fusion; however, the diagnosis of sutural maturation should include a radiographic review of all palate axial cross-sections for adequate staging. After review of all slices, the classification of sutural fusion presented in this study was based on the most central axial CBCT cross-sectional slices through the midportion of the trabecular bone of the hard palate, bordered by the oral cavity and the floor of the nasal cavity in which the suture showed the most advanced signs of maturation. The proposed classification of sutural fusion involved radiographic interpretation of the central axial cross-sectional slice selected from the midsagittal plane view of the palate for subjects with either a relatively straight or a curved palate (Figs 1 and 2). Evaluation of the axial cross-sectional slice through the cortical bone closer to the oral cavity might not be reliable (Fig 10).
Fig 11.
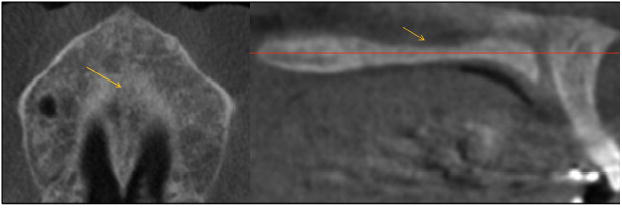
A patient whose palate was thinner (superoinferiorly) in the maxillary region, where the midpalatal suture was fused earlier.
Fig 10.
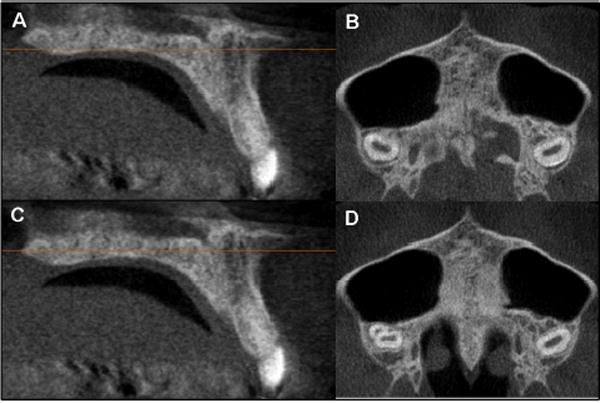
At the axial cross-sectional slice closer to the oral cavity indicated by the orange horizontal line in the sagittal view (A), the midpalatal suture may appear to have a low-density space at the midline (B). However, at the central axial cross-sectional slice indicated by the orange horizontal line in the sagittal view (C), it can be observed that the midpalatal suture is fused (D).
This investigation is the first to evaluate the overall midpalatal suture morphology using CBCT. Histologic and microcomputed tomography analyses are limited to assessments of small sections of the total anteroposterior suture length only, even if several serial sections from 1 area are available. In histologic studies,8-12 only frontal sections have been evaluated; this restricts their clinical application, especially since midpalatal suture maturation occurs from the posterior to the anterior region.9,11
Regarding prediction of RME success or failure, it has been advocated in histology and microcomputed tomography studies that the presence or lack of fusion is not extremely important, whereas the percentage of fusion in each subject is more critical.9,11-13 Persson and Thilander9 have speculated that midpalatal sutures with a fusion index below 5% could be expanded using conventional RME orthopedic forces. Histologic and microcomputed tomography studies found fusion indexes of the midpalatal suture below 5% in subjects from 18 to 38 years,10 14 to 71 years,13 and 18 to 63 years of age.11 However, these histologic and micro-computed tomography data do not explain why it is difficult to open the midpalatal suture clinically with conventional RME in patients older than 25 years of age. Many studies have advocated that most of the resistance to midpalatal suture separation in adults is due to fusion of the circummaxillary sutures.12,13,32-34 The hypothesis that the stage of sutural maturation might be related to the success of orthopedic expansion is a different research question that was not tested in this study.
An interesting finding concerned stage D, in which the fusion of the palatine portion of the midpalatal suture has occurred. Because maturation of the midpalatal suture occurs from the back to the front of the oral cavity, clinical observations in adult and late adolescent patients might be related to sutural fusion in the palatine bone, as verified in this study in which stage D was present in girls after 11 years and in boys after 14 years of age.9,11 These findings have great clinical relevance in that transverse discrepancies are located mostly in the posterior region. Fusion of the midpalatal suture at stage D would prevent sutural opening with RME in the molar region, even though the opening of an anterior diastema could be observed. This scenario would lead to skeletal transverse increase in the anterior maxillary region followed by dental changes only in the posterior region, where side effects could include molar or premolar extrusion and periodontal damage.
In 2 previous histologic investigations, only frontal sections of the midpalatal suture at the maxillary bone were analyzed; the palatine portion of the suture was not evaluated.9,10 In other studies, even though the palatal specimens were between the incisive foramen and the posterior spine of the hard palate, the investigators did not evaluate variations in fusion at different areas of the palate.11,13 Many 11- to 18-year-old girls had a thinner area of the palate in the maxillary bone where the midpalatal suture was fused; for this reason, they were classified as stage E (Fig 11).
Persson and Thilander9 conducted the only histologic study of the posterior maxillary part of the midpalatal suture that reported a sutural fusion index of at least 17% in subjects from 15 to 35 years of age. This percentage of sutural fusion of the posterior part of the palate was considered an indicator that a conventional RME approach would not be a viable treatment option for those patients.
Based our proposed staging methodology, we speculate that at stages A and B a conventional RME approach would have less resistant forces and probably more skeletal effects than at stage C, when there are many initial ossification areas along the midpalatal suture. These areas of initial ossification have been described previously by Melsen19 as “bony islands” throughout the midpalatal suture. Initial diagnosis of stage C might indicate that the timing of RME is critical because the start of fusion of the palatine portion of the suture could be imminent. Patients in stages D and E might be better treated by surgically assisted RME because fusion of the midpalatal suture already has occurred partially or totally, hampering the RME forces from opening the suture.
Interestingly, our results (Table II) might help to elucidate clinical findings in which RME is obtained easily up to 10 years of age, with more skeletal effects than in later circumpubertal ages (11-18 years).35 The resistance to expansion possibly can be explained by the greater percentage of subjects in stage C during puberty or even early fusion of the midpalatal suture (stages D and E) in girls.
Adults had great variability in sutural maturation, as corroborated by other studies.9,11 We found that 53% of the adults were in stage E, 31% were in stage D, 13% were in stage C, and 1 subject (3%) was in stage B. For subjects with fusion of the midpalatal suture only in the palatine bone (stage D), a clinical attempt of RME probably would fail in the posterior region despite the interincisal opening and in the maxillary bone portion of the suture, leading to failure of the RME procedure.
The sutural classification system described and validated in this study has the potential to allow a reliable clinical method for individual assessment of midpalatal suture morphology before RME, mainly for late adolescent and young adult patients in whom this treatment is unpredictable. Moreover, the system of maturation staging of the midpalatal suture described here can be applied to other circummaxillary sutures. Such assessments can aid our understanding of which patients would show more dental than orthopedic effects in RME and provide knowledge of orthopedic effects or resistance at circummaxillary sutures.
Conclusions
The classification of midpalatal sutural fusion using CBCT allows the diagnosis of the overall anteroposterior characteristics of the midpalatal suture, without overlapping of other anatomic structures. This method might provide reliable parameters for the clinical decision between conventional and surgically assisted RME for adolescent and young adult patients.
Acknowledgments
We thank the artist Chris Jung for drawing the stages of midpalatal suture maturation.
Funded in part by Foundation for Research Support of the State of São Paulo (FAPESP); support also was made available through the Thomas M. and Doris Graber Endowed Professorship, the Department of Orthodontics and Pediatric Dentistry, University of Michigan.
Footnotes
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest, and none were reported.
References
- 1.Bishara SE, Staley RN. Maxillary expansion: clinical implications. Am J Orthod Dentofacial Orthop. 1987;91:3–14. doi: 10.1016/0889-5406(87)90202-2. [DOI] [PubMed] [Google Scholar]
- 2.Guest SS, McNamara JA, Jr, Baccetti T, Franchi L. Improving Class II malocclusion as a side-effect of rapid maxillary expansion: a prospective clinical study. Am J Orthod Dentofacial Orthop. 2010;138:582–91. doi: 10.1016/j.ajodo.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Da Silva Filho OG, Magro AC, Capelozza Filho L. Early treatment of the Class III malocclusion with rapid maxillary expansion and maxillary protraction. Am J Orthod Dentofacial Orthop. 1998;113:196–203. doi: 10.1016/s0889-5406(98)70292-6. [DOI] [PubMed] [Google Scholar]
- 4.McNamara JA, Jr, Brudon WL. Orthodontics and dentofacial orthopedics. Ann Arbor Mich: Needham Press; 2001. [Google Scholar]
- 5.McNamara JA., Jr Long-term adaptation to changes in the transverse dimension in children and adolescents: an overview. Am J Orthod Dentofacial Orthop. 2006;129(Suppl):S71–4. doi: 10.1016/j.ajodo.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Angell EC. Treatment of irregularities of the permanent or adult teeth. Dent Cosmos. 1860;1:541–4. 599–600. [Google Scholar]
- 7.Haas AJ. Rapid expansion of the maxillary dental arch and nasal cavity by opening the mid-palatal suture. Angle Orthod. 1961;31:73–90. [Google Scholar]
- 8.Persson M, Magnusson BC, Thilander B. Sutural closure in rabbit and man: a morphological and histochemical study. J Anat. 1978;125:313–21. [PMC free article] [PubMed] [Google Scholar]
- 9.Persson M, Thilander B. Palatal suture closure in man from 15 to 35 years of age. Am J Orthod. 1977;72:42–52. doi: 10.1016/0002-9416(77)90123-3. [DOI] [PubMed] [Google Scholar]
- 10.Wehrbein H, Yildizhan F. The mid-palatal suture in young adults A radiological-histological investigation. Eur J Orthod. 2001;23:105–14. doi: 10.1093/ejo/23.2.105. [DOI] [PubMed] [Google Scholar]
- 11.Knaup B, Yildizhan F, Wehrbein H. Age-related changes in the midpalatal suture. J Orofac Orthop. 2004;65:467–74. doi: 10.1007/s00056-004-0415-y. [DOI] [PubMed] [Google Scholar]
- 12.Melsen B. Palatal growth studied on human autopsy material. Am J Orthod. 1975;68:42–54. doi: 10.1016/0002-9416(75)90158-x. [DOI] [PubMed] [Google Scholar]
- 13.Korbmacher H, Schilling A, Püschel K, Amling M, Kahl-Nieke B. Age-dependent three-dimensional micro-computed tomography analysis of the human midpalatal suture. J Orofac Orthop. 2007;68:364–76. doi: 10.1007/s00056-007-0729-7. [DOI] [PubMed] [Google Scholar]
- 14.Revelo B, Fishman LS. Maturational evaluation of ossification of the midpalatal suture. Am J Orthod Dentofacial Orthop. 1994;105:288–92. doi: 10.1016/S0889-5406(94)70123-7. [DOI] [PubMed] [Google Scholar]
- 15.Hahn W, Fricke-Zech S, Fialka-Fricke J, Dullin C, Zapf A, Gruber R, et al. Imaging of the midpalatal suture in a porcine model: flat-panel volume computed tomography compared with multislice computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:443–9. doi: 10.1016/j.tripleo.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Cohen MM., Jr Sutural biology and the correlates of craniosynostosis. Am J Med Gen. 1993;47:581–616. doi: 10.1002/ajmg.1320470507. [DOI] [PubMed] [Google Scholar]
- 17.Miroue M, Rosenberg L. [thesis] Seattle, Wash: University of Washington; 1975. The human facial sutures: a morphologic and histologic study of age changes from 20 to 95 years. [Google Scholar]
- 18.Sun Z, Lee E, Herring SW. Cranial sutures and bones: growth and fusion in relation to masticatory strain. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:1–22. doi: 10.1002/ar.a.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melsen B. A histological study of the influence of sutural morphology and skeletal maturation on rapid palatal expansion in children. Trans Eur Orthod Soc. 1972;48:499–507. [PubMed] [Google Scholar]
- 20.De Vos W, Casselman J, Swennen GR. Cone-beam computerized tomography (CBCT) imaging of the oral and maxillofacial region: a systematic review of the literature. Int J Oral Maxillofac Surg. 2009;38:609–25. doi: 10.1016/j.ijom.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 21.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 22.Kiliç¸ N, Kiki A, Oktay H. A comparison of dentoalveolar inclination treated by two palatal expanders. Eur J Orthod. 2008;30:67–72. doi: 10.1093/ejo/cjm099. [DOI] [PubMed] [Google Scholar]
- 23.Rungcharassaeng K, Caruso JM, Kan JY, Kim J, Taylor G. Factors affecting buccal bone changes of maxillary posterior teeth after rapid maxillary expansion. Am J Orthod Dentofacial Orthop. 2007;132:428, e1–8. doi: 10.1016/j.ajodo.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 24.Garib DG, Henriques JF, Janson G, Freitas MR, Coelho RA. Rapid maxillary expansion tooth-tissue-borne versus tooth-borne expanders: a computed tomography evaluation of dentoskeletal effects. Angle Orthod. 2005;75:548–57. doi: 10.1043/0003-3219(2005)75[548:RMETVT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Bell WH, Epker BN. Surgical-orthodontic expansion of the maxilla. Am J Orthod. 1976;70:517–28. doi: 10.1016/0002-9416(76)90276-1. [DOI] [PubMed] [Google Scholar]
- 26.Betts NJ, Vanarsdall RL, Barber HD, Higgins-Barber K, Fonseca RJ. Diagnosis and treatment of transverse maxillary deficiency. Int J Adult Orthod Orthognath Surg. 1995;10:75–96. [PubMed] [Google Scholar]
- 27.Proffit WR, Turvey TA, Phillips C. The hierarchy of stability and predictability in orthognathic surgery with rigid fixation: an update and extension. Head Face Med. 2007;3:21. doi: 10.1186/1746-160X-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey LJ, Cevidanes LHS, Proffit WR. Stability and predictability of orthognathic surgery. Am J Orthod Dentofacial Orthop. 2004;126:273–7. doi: 10.1016/S0889540604005207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fishman LS. Radiographic evaluation of skeletal maturation A clinically oriented study based on hand-wrist films. Angle Orthod. 1982;52:88–112. doi: 10.1043/0003-3219(1982)052<0088:REOSM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Baccetti T, Franchi L, McNamara JA., Jr An improved version of the cervical vertebral maturation (CVM) method for the assessment of mandibular growth. Angle Orthod. 2002;72:316–23. doi: 10.1043/0003-3219(2002)072<0316:AIVOTC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Baccetti T, Franchi L, McNamara JA., Jr The cervical vertebral maturation (CVM) method for the assessment of optimal treatment timing in dentofacial orthopedics. Semin Orthod. 2005;11:119–29. [Google Scholar]
- 32.Ghoneima A, Abdel-Fattah E, Hartsfield J, El-Bedwehi A, Kamel A, Kula K. Effects of rapid maxillary expansion on the cranial and circummaxillary sutures. Am J Orthod Dentofacial Orthop. 2011;140:510–9. doi: 10.1016/j.ajodo.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gautam P, Valiathan A, Adhikari R. Stress and displacement patterns in the craniofacial skeleton with rapid maxillary expansion: a finite element method study. Am J Orthod Dentofacial Orthop. 2007;132:5, e1–11. doi: 10.1016/j.ajodo.2006.09.044. [DOI] [PubMed] [Google Scholar]
- 34.Wertz RA. Skeletal and dental changes accompanying rapid midpalatal suture opening. Am J Orthod. 1970;58:41–66. doi: 10.1016/0002-9416(70)90127-2. [DOI] [PubMed] [Google Scholar]
- 35.Baccetti T, Franchi L, Cameron CG, McNamara JA., Jr Treatment timing for rapid maxillary expansion. Angle Orthod. 2001;71:343–50. doi: 10.1043/0003-3219(2001)071<0343:TTFRME>2.0.CO;2. [DOI] [PubMed] [Google Scholar]


