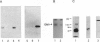Abstract
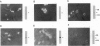
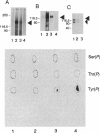
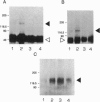
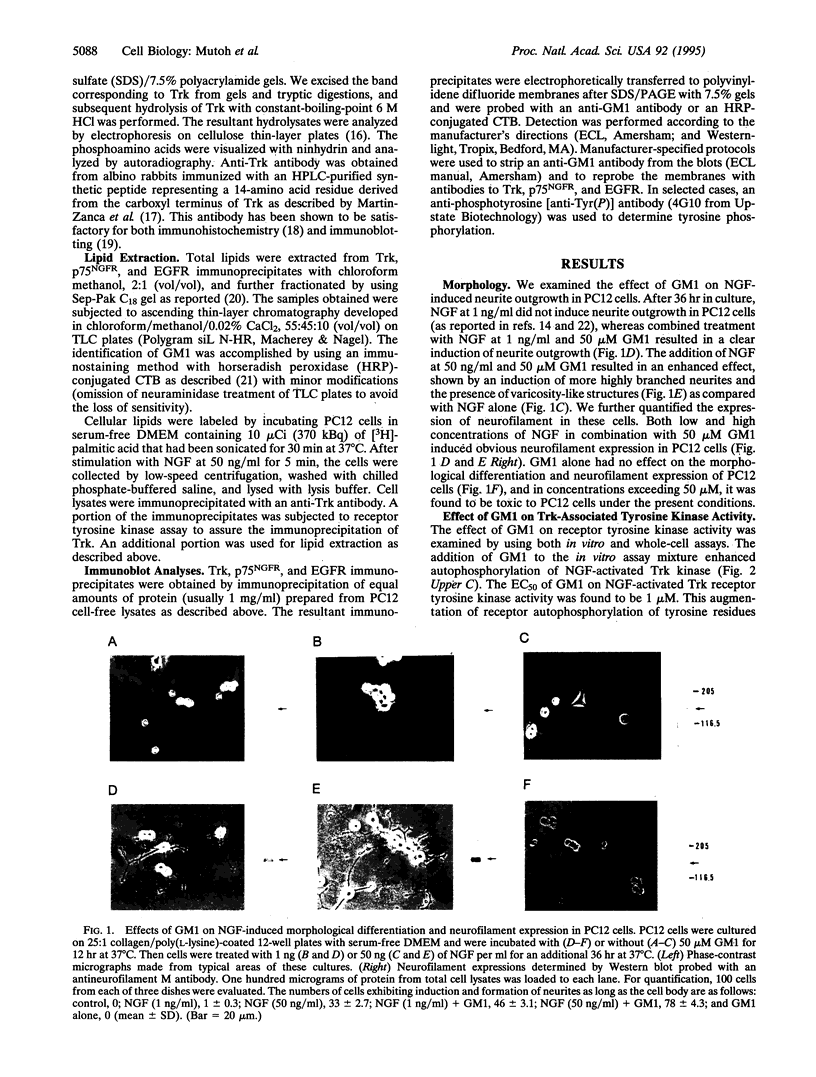
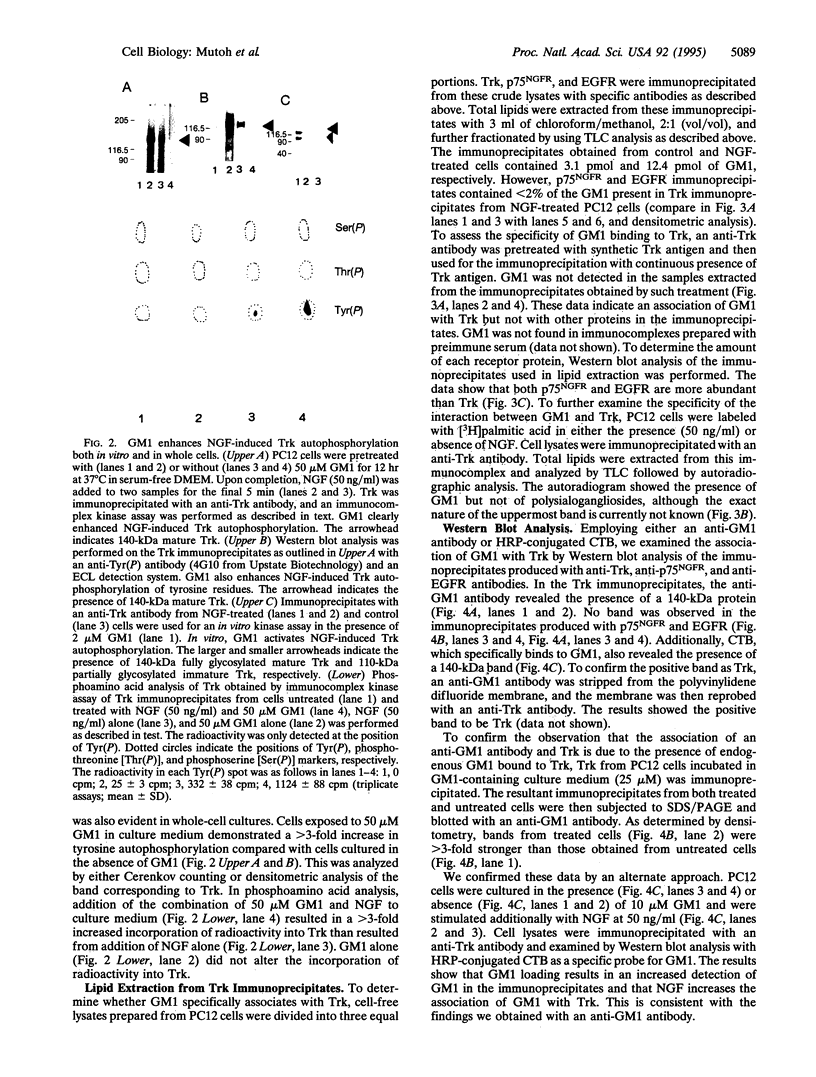
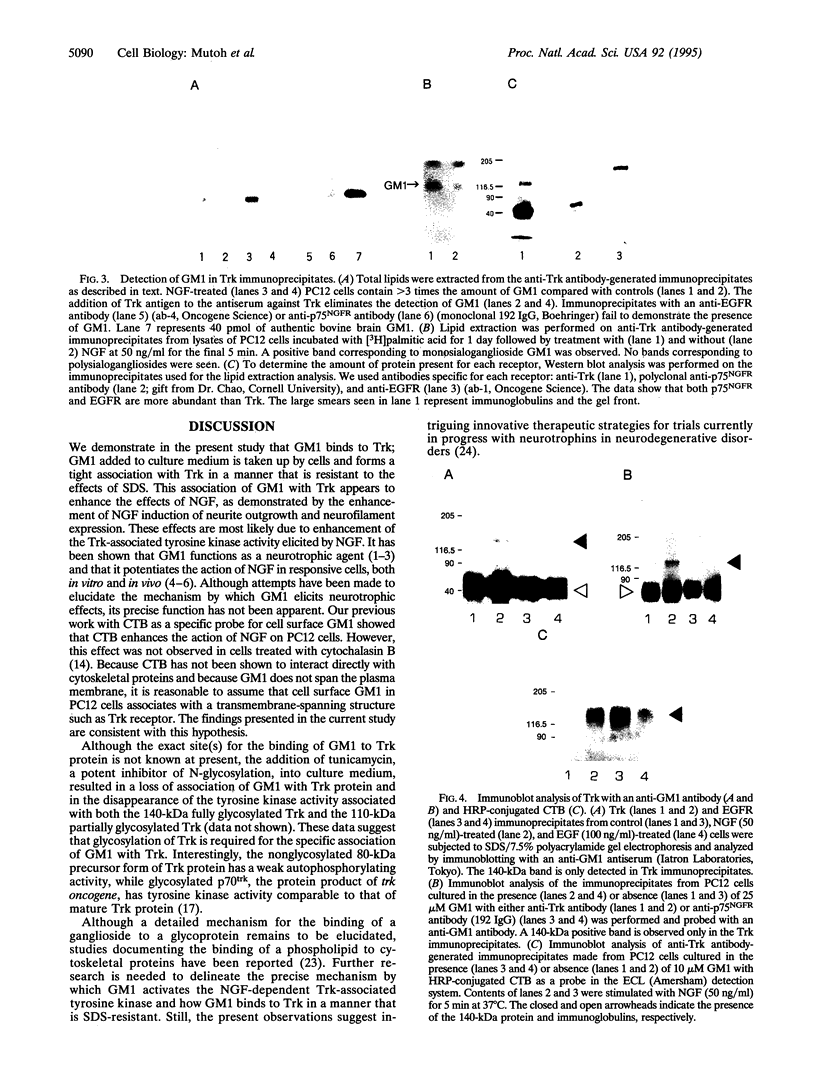
Several lines of evidence have suggested that ganglioside GM1 stimulates neuronal sprouting and enhances the action of nerve growth factor (NGF), but its precise mechanism is yet to be elucidated. We report here that GM1 directly and tightly associates with Trk, the high-affinity tyrosine kinase-type receptor for NGF, and strongly enhances neurite outgrowth and neurofilament expression in rat PC12 cells elicited by a low dose of NGF that alone is insufficient to induce neuronal differentiation. The potentiation of NGF activity by GM1 appears to involve tyrosine-autophosphorylation of Trk, which contains intrinsic tyrosine kinase activity that has been localized to the cytoplasmic domain. In the presence of GM1 in culture medium, there is a > 3-fold increase in NGF-induced autophosphorylation of Trk as compared with NGF alone. We also found that GM1 could directly enhance NGF-activated autophosphorylation of immunoprecipitated Trk in vitro. Monosialoganglioside GM1, but not polysialogangliosides, is tightly associated with immunoprecipitated Trk. Furthermore, such tight association of GM1 with Trk appears to be specific, since a similar association was not observed with other growth factor receptors, such as low-affinity NGF receptor (p75NGR) and epidermal growth factor receptor (EGFR). Thus, these results strongly suggest that GM1 functions as a specific endogenous activator of NGF receptor function, and these enhanced effects appear to be due, at least in part, to tight association of GM1 with Trk.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cannella M. S., Oderfeld-Nowak B., Gradkowska M., Skup M., Garofalo L., Cuello A. C., Ledeen R. W. Derivatives of ganglioside GM1 as neuronotrophic agents: comparison of in vivo and in vitro effects. Brain Res. 1990 Apr 16;513(2):286–294. doi: 10.1016/0006-8993(90)90469-r. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Tapley P., Jing S. Q., Nanduri V., O'Rourke E., Lamballe F., Kovary K., Klein R., Jones K. R., Reichardt L. F. The trk tyrosine protein kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3. Cell. 1991 Jul 12;66(1):173–183. doi: 10.1016/0092-8674(91)90149-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuello A. C., Garofalo L., Kenigsberg R. L., Maysinger D. Gangliosides potentiate in vivo and in vitro effects of nerve growth factor on central cholinergic neurons. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2056–2060. doi: 10.1073/pnas.86.6.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P., Dickson J. G., Flanigan T. P., Walsh F. S. Ganglioside GM1 does not initiate, but enhances neurite regeneration of nerve growth factor-dependent sensory neurones. J Neurochem. 1985 Apr;44(4):1259–1265. doi: 10.1111/j.1471-4159.1985.tb08752.x. [DOI] [PubMed] [Google Scholar]
- Eide F. F., Lowenstein D. H., Reichardt L. F. Neurotrophins and their receptors--current concepts and implications for neurologic disease. Exp Neurol. 1993 Jun;121(2):200–214. doi: 10.1006/exnr.1993.1087. [DOI] [PubMed] [Google Scholar]
- Ferrari G., Fabris M., Gorio A. Gangliosides enhance neurite outgrowth in PC12 cells. Brain Res. 1983 Jun;284(2-3):215–221. doi: 10.1016/0165-3806(83)90006-8. [DOI] [PubMed] [Google Scholar]
- Fukami K., Furuhashi K., Inagaki M., Endo T., Hatano S., Takenawa T. Requirement of phosphatidylinositol 4,5-bisphosphate for alpha-actinin function. Nature. 1992 Sep 10;359(6391):150–152. doi: 10.1038/359150a0. [DOI] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halegoua S., Armstrong R. C., Kremer N. E. Dissecting the mode of action of a neuronal growth factor. Curr Top Microbiol Immunol. 1991;165:119–170. doi: 10.1007/978-3-642-75747-1_7. [DOI] [PubMed] [Google Scholar]
- Iwata H., Ito T., Mutoh T., Ishiguro Y., Xiao H., Hamaguchi M. Abundant but inactive-state gp140proto-trk is expressed in neuroblastomas of patients with good prognosis. Jpn J Cancer Res. 1994 Jan;85(1):32–39. doi: 10.1111/j.1349-7006.1994.tb02883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Hempstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991 Apr 26;252(5005):554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Martin-Zanca D., Parada L. F. Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature. 1991 Mar 14;350(6314):158–160. doi: 10.1038/350158a0. [DOI] [PubMed] [Google Scholar]
- Katoh-Semba R., Skaper S. D., Varon S. Interaction of GM1 ganglioside with PC12 pheochromocytoma cells: serum- and NGF-dependent effects on neuritic growth (and proliferation). J Neurosci Res. 1984;12(2-3):299–310. doi: 10.1002/jnr.490120217. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987 Sep 4;237(4819):1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Loeb D. M., Greene L. A. Transfection with trk restores "slow" NGF binding, efficient NGF uptake, and multiple NGF responses to NGF-nonresponsive PC12 cell mutants. J Neurosci. 1993 Jul;13(7):2919–2929. doi: 10.1523/JNEUROSCI.13-07-02919.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh T., Rudkin B. B., Guroff G. Differential responses of the phosphorylation of ribosomal protein S6 to nerve growth factor and epidermal growth factor in PC12 cells. J Neurochem. 1992 Jan;58(1):175–185. doi: 10.1111/j.1471-4159.1992.tb09293.x. [DOI] [PubMed] [Google Scholar]
- Mutoh T., Rudkin B. B., Koizumi S., Guroff G. Nerve growth factor, a differentiating agent, and epidermal growth factor, a mitogen, increase the activities of different S6 kinases in PC12 cells. J Biol Chem. 1988 Nov 5;263(31):15853–15856. [PubMed] [Google Scholar]
- Mutoh T., Tokuda A., Guroff G., Fujiki N. The effect of the B subunit of cholera toxin on the action of nerve growth factor on PC12 cells. J Neurochem. 1993 Apr;60(4):1540–1547. doi: 10.1111/j.1471-4159.1993.tb03319.x. [DOI] [PubMed] [Google Scholar]
- Taglialatela G., Angelucci L., Ramacci M. T., Werrbach-Perez K., Jackson G. R., Perez-Polo J. R. Acetyl-L-carnitine enhances the response of PC12 cells to nerve growth factor. Brain Res Dev Brain Res. 1991 Apr 24;59(2):221–230. doi: 10.1016/0165-3806(91)90102-o. [DOI] [PubMed] [Google Scholar]
- Toffano G., Savoini G., Moroni F., Lombardi G., Calza L., Agnati L. F. GM1 ganglioside stimulates the regeneration of dopaminergic neurons in the central nervous system. Brain Res. 1983 Feb 14;261(1):163–166. doi: 10.1016/0006-8993(83)91298-2. [DOI] [PubMed] [Google Scholar]
- Wood P. A., McBride M. R., Baker H. J., Christian S. T. Fluorescence polarization analysis, lipid composition, and Na+, K+-ATPase kinetics of synaptosomal membranes in feline GM1 and GM2 gangliosidosis. J Neurochem. 1985 Mar;44(3):947–956. doi: 10.1111/j.1471-4159.1985.tb12909.x. [DOI] [PubMed] [Google Scholar]
- Wu G. S., Ledeen R. Quantification of gangliotetraose gangliosides with cholera toxin. Anal Biochem. 1988 Sep;173(2):368–375. doi: 10.1016/0003-2697(88)90201-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Sobue G., Mutoh T., Li M., Doyu M., Mitsuma T., Kimata K. Gene expression of high- (p140trk) and low-affinity nerve growth factor receptor (LNGFR) in the adult and aged human peripheral nervous system. Neurosci Lett. 1993 Aug 6;158(1):39–43. doi: 10.1016/0304-3940(93)90607-m. [DOI] [PubMed] [Google Scholar]