Abstract
The present study investigates whether interepisode mood regulation impairment contributes to disturbances in sleep onset latency (SOL) and rapid eye movement (REM) sleep. Individuals with interepisode bipolar disorder (n = 28) and healthy controls (n = 28) slept in the laboratory for 2 baseline nights, a happy mood induction night, and a sad mood induction night. There was a significant interaction whereby on the happy mood induction night the bipolar group exhibited significantly longer SOL than did the control group, while there was no difference on the baseline nights. In addition, control participants exhibited shorter SOL on the happy mood induction night compared to the baseline nights, a finding that was not observed in the bipolar group. On the sad mood induction night, participants in both groups had shorter SOL and increased REM density when compared to the baseline nights. Bipolar participants exhibited heightened REM density compared to control participants on both nights. These results raise the possibility that regulation of positive stimuli may be a contributor to difficulties with SOL, while hyperactivity may be characteristic of REM sleep.
Keywords: bipolar disorder, interepisode, mood induction, sleep onset latency, REM sleep
Bipolar disorder is ranked in the top 10 leading causes of disability worldwide (Judd, Akiskal, & Schettler, 2002; World Health Organization, 2001). Patients spend 20% of their life in episodes (Angst & Sellaro, 2000), and between manic and depressive episodes—the interepisode period—individuals with bipolar disorder continue to suffer from significant symptomatology (e.g., Harrow, Goldberg, Grossman, & Meltzer, 1990; MacQueen et al., 2003; Robb, Cooke, Devins, Young, & Joffe, 1997). The illness intrusiveness experienced during this period has been calculated to be as severe as it is for those with chronic medical conditions such as multiple sclerosis (Robb et al., 1997). One of the reasons this impairment is of great concern is because it predicts relapse into either mania or depression (MacQueen et al., 2003). The mechanisms that underpin this poor functioning have been minimally researched. Hence, the interepisode period is the focus of the present study.
Bipolar disorder is characterized by significant sleep disturbance. During mania there is a reduced need for sleep. During depression individuals suffer from insomnia or hypersomnia (American Psychiatric Association, 2000; Harvey, 2008). Sleep disturbance is also common in patients during the interepisode period. One study reported that 70% of an interepisode bipolar group had a clinically significant sleep problem, and 55% met diagnostic criteria for insomnia (Harvey, Schmidt, Scarna, Semler, & Goodwin, 2005). Moreover, Jones, Hare, and Evershed (2005) observed more fragmentation and less interdaily stability in the sleep/wake rhythm of interepisode bipolar individuals compared with healthy controls. These findings are significant because even in the absence of a mood disorder, sleep disturbance impairs the quality of life. For example, insomnia has well-established serious consequences for the sufferer including functional impairment, work absenteeism, increased use of medical services (Roth & Ancoli-Israel, 1999), and doubling the risk of accident (Ohayon, Caulet, Philip, Guilleminault, & Priest, 1997). Moreover, there is growing evidence that interepisode sleep disturbance may be a mechanism that contributes to relapse in bipolar disorder. It is a common prodrome of both mania and depression (Jackson, Cavanagh, & Scott, 2003) and is strongly correlated with daily manic symptoms (Barbini, Bertelli, Colombo, & Smeraldi, 1996). Furthermore, experimentally induced sleep deprivation is associated with manic episode onset in a proportion of individuals with bipolar disorder (Jackson et al., 2003; Leibenluft, Albert, Rosenthal, & Wehr, 1996; Wehr, Sack, & Rosenthal, 1987).
Bipolar disorder is fundamentally a disorder of mood regulation impairment (e.g., Kruger, Seminowicz, Goldapple, Kennedy, & Mayberg, 2003). A mood is defined as a slow-moving, stimulus-nonspecific, long-lasting affective state (e.g., Rottenberg, 2005; Watson, 2000). It can be distinguished from an emotion, which is a brief response to salient environmental events that includes coordinated changes in subjective experience, behavior, and physiology (Lazarus, 1991; Rottenberg & Gross, 2003). Mood regulation involves the processes by which individuals influence the duration and intensity of chronic affective states, typically focusing on subjective experiences (Parkinson, Totterdell, Briner, & Reynolds, 1996). Those with bipolar disorder encounter difficulties in mood regulation in the form of oscillations between abnormally and persistently elevated mood and sustained periods of depressed mood (American Psychiatric Association, 2000). There is preliminary evidence that mood regulation impairment is also characteristic of the interepisode period. Bipolar individuals experience significant mood lability and increased reactivity during this period (Fukuda, Etoh, Iwadate, & Ishii, 1983; Keller et al., 1986; Tsuang, Woolson, & Fleming, 1979). They also exhibit strong tendencies to experience excitement and energy in response to goal or reward achievement (Meyer, Johnson, & Winters, 2001). These tendencies have been interpreted as indicative of heightened behavioral activation (Johnson, 2005).
The aim of the present study was to investigate if interepisode mood regulation impairment is one mechanism that contributes to disturbances in sleep onset latency (SOL) and rapid eye movement (REM) sleep in bipolar disorder. Participants spent 4 nights in the laboratory: 2 baseline nights, 1 happy mood induction night, and 1 sad mood induction night. The impact of the happy and sad mood inductions on SOL and selected REM variables, relative to the average of the 2 baseline nights, was assessed.
The rationale for focusing on SOL was that it is the sleep parameter most proximal to the mood induction. Given that mood regulation impairment is a core characteristic of bipolar disorder, a mood induction prior to sleep should adversely impact SOL in these patients, particularly since strong mood states are cognitively and physiologically arousing and are therefore antithetical to sleep onset (Espie, 2002). In contrast, we expected that a mood induction prior to sleep would have less impact on SOL in the control group, relative to the bipolar group, because (a) the healthy controls are presumed to have intact mood regulation abilities and (b) the mood inductions used in this study aimed to induce a mood state akin to those that are naturally experienced in day-to-day life.
The rationale for focusing on selected REM sleep variables was the evidence suggesting a relationship between REM sleep and mood regulation (e.g., Cartwright, Luten, Young, Mercer, & Bears, 1998). Moreover, a meta-analysis of 177 studies showed that altered REM sleep is associated with mood disorders (Benca, Obermeyer, Thisted, & Gillin, 1992), including decreased REM latency (e.g., Kupfer, 1976; Lauer, Riemann, Wiegand, & Berger, 1991), increased REM density (e.g., Riemann, Hohagen, Bahro, & Berger, 1994), and longer first REM period (Benca et al., 1992). However, these variables have primarily been studied in unipolar depression. There has been a relative dearth of research on REM sleep in bipolar disorder, particularly during the interepisode period. Furthermore, to the best of our knowledge this is the first investigation of the effect of mood prior to sleep on REM variables.
Two hypotheses were tested. First, we predicted that, relative to the control group, the bipolar group would exhibit longer SOL and increased REM activity (operationalized as decreased REM latency, increased REM density, and longer first REM period) on the happy mood induction night relative to the baseline nights. Second, we predicted that, relative to the control group, the bipolar group would exhibit longer SOL and increased REM activity on the sad mood induction night relative to the baseline nights.
Method
Participants
Participants were 28 adults (ages 18–65 years) with bipolar I (n = 24) or bipolar II (n = 4) disorder who were currently in the interepisode period and 28 adults with no history of psychiatric or sleep disorders. Participants were recruited through Internet advertisements and flyers distributed to psychiatric clinics in the community. A telephone interview screened for eligibility. Individuals who were considered likely to be eligible on the basis of the initial telephone screen were invited to the laboratory for an extensive interview session.
Individuals in the bipolar group were eligible to participate if they (a) met criteria for a diagnosis of bipolar I or bipolar II disorder according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM–IV–TR; American Psychiatric Association, 2000); (b) did not meet criteria for a diagnosis of current substance or alcohol abuse or dependence; and (c) did not meet criteria for sleep apnea or periodic limb movement disorder on the basis of polysomnography. In addition, bipolar participants were included only if they met criteria for interepisode symptom cutoffs based on prior research (Chengappa et al., 2003; Thompson et al., 2005): a score of less than 7 on the Young Mania Rating Scale (YMRS; Young, Biggs, Ziegler, & Meyer, 1978) and a score of less than 12 on the Inventory of Depressive Symptomatology–Clinician Rating (IDS-C; Rush, Gullion, Basco, Jarrett, & Trivedi, 1996). Eight bipolar participants met criteria for the rapid cycling specifier. All bipolar participants were required to be in the care of a psychiatrist. Information on medications was collected at each visit. All but 1 participant in the bipolar group were taking medications, and the majority of participants were taking three or more. At the first visit, 71% of bipolar patients were taking mood stabilizers, 82% antidepressants, 50% antipsychotics, 21% anxiolytics, and 7% prescription sleep aids. Fifty-six percent remained on the same medication throughout the study.
Participants in the control group were eligible if they (a) did not meet DSM–IV–TR criteria for any past or current Axis I disorder; (b) had scores of less than 7 on the YMRS and less than 12 on the IDS-C; and (c) did not meet criteria for any past or current sleep disorder on the basis of either polysomnography or the Duke Structured Interview for Sleep Disorders (DSISD; Edinger et al., 2004).
Clinical Measures
Participants’ diagnoses were assessed with the Structured Clinical Interview for DSM–IV (SCID; First, Spitzer, Gibbon, & Williams, 1995) and the DSISD (Edinger et al., 2004). The SCID is a semistructured interview designed to assess DSM–IV diagnostic criteria for Axis I disorders. The SCID has shown good reliability for the majority of disorders it covers (Skre, Onstad, Torgersen, & Kringlen, 1991; J. B. Williams et al., 1992). The DSISD is a semistructured interview that assesses research diagnostic criteria for sleep disorders. In addition, the YMRS (Young et al., 1978) and the IDS-C (Rush et al., 1996) were administered to assess interepisode status. The YMRS is an 11-item measure used to assess the severity of manic symptoms, with each item rated on a 5-point scale. The IDS-C is a widely used 30-item instrument assessing depressive symptoms over the past week, with each item rated on a 4-point scale. Both measures have good reliability and validity (Rush et al., 1996; Young et al., 1978).
The assessors had previous experience administering structured clinical interviews. To assess interrater reliability, an independent assessor who was blind to diagnostic group evaluated a sample of SCID and DSISD audiotaped interviews (n = 15). These were randomly selected equally for the bipolar and control participants. Diagnoses matched those made by the original interviewer in all cases (κ = 1.00). This indicates strong interrater reliability, though the use of a “skip-out” strategy may have reduced the number of potential disagreements with the original interviewer.
Mood Inductions
The mood induction procedure combined a well-validated continuous music technique with autobiographical recall. Following the procedures of Eich, Macaulay, and Ryan (1994), participants were told (a) they would soon listen to a selection of music to assist them to develop a happy (or sad) mood; (b) because music alone cannot create the desired state, they should concentrate on ideas and images that make them feel happy (or sad); and (c) they should try hard to develop an intense mood state. Throughout the induction, happy or sad classical music was played. The music for the happy mood induction included the Allegro and the Rondo from Mozart’s Eine Kleine Nachtmusik; the Finale from Mozart’s Serenade no. 9 in D Major (“Posthorn”), K. 320; the Allegro from Bach’s Brandenburg Concerto no. 3; “Waltz of the Flowers,” “Trepak,” and “Dance of the Flutes” from Tchaikovsky’s Nutracker; the Allegro from Dvorak’s Piano Quartet in E-flat Major; the Presto from Dvorak’s Slavonic Dance no. 1 in C Major, op. 46, no. 1; the Allegretto from Dvorak’s Slavonic Dance no. 6 in D Major, op. 46, no. 6; the Allegro from the “Spring” movement of Vivaldi’s Four Seasons; and Brahms’s Hungarian Dance no. 7 in F Major. The music for the sad mood induction included the Adagio from the “Autumn” movement of Vivaldi’s Four Seasons; “Lullaby” from Stravinsky’s Firebird; Chopin’s Prelude in E Minor, op. 28, no. 4; Fauré’s Piano Quintet no. 1 in D Minor, op. 89; Fauré’s Quartet no. 1 in C Minor, op. 15; Rachmaninov’s Vocalise, op. 34, no. 14; Mahler’s Symphony no. 5; Suite no. 1 from Grieg’s Peer Gynt; and Albinoni’s Adagio in G Minor. Five minutes after the induction began and every 5 min thereafter, participants rated their current levels of pleasure and arousal on a computerized version of the affect grid. The affect grid is a self-report rating of pleasure on the horizontal axis (−4 = extremely unpleasant, 0 = neutral, 4 = extremely pleasant) and arousal on the vertical axis (−4 = extremely low arousal, 0 = neutral, 4 = extremely high arousal; Russell, Weiss, & Mendel-sohn, 1989). Following Eich et al. (1994), we included this measure in the current study as a manipulation check (i.e., to check that mood is happier following the happy mood induction and sadder following the sad mood induction). Eich et al. specified mood thresholds on the last rating of at least 3 for the happy induction and at least −3 for the sad induction. When the participant met this threshold, the induction ended. The last mood rating served as the manipulation check.
The mood induction procedure in the current study was adapted from the methodology of Eich et al. (1994) in several ways. Eich et al. specified that if participants had not reached the threshold after 60 min, the procedure was repeated. In our procedure, given that it was important to maintain the participant’s bedtime across the nights, if the participant had not reached the mood threshold by 40 min after the start of the induction, the induction ended and the participant was escorted to bed. As a result of this time cutoff, not all participants reached the threshold specified by Eich et al. for the intended mood. Eich et al.’s procedures have not been used at night, so these cutoffs may not be realistic, and excluding participants who did not reach them would substantially reduce statistical power. In addition to the happy and sad inductions as described by Eich et al., a neutral induction night was added to help prepare participants for the subsequent happy and sad mood inductions. During this induction, participants were asked to focus on neutral topics such as directions to a familiar place or the layout of furniture at home while listening to classical music. Music selections included Fauré’s Ballad for Piano and Orchestra, op. 19, and the second and third movements from Brahms’s Symphony no. 3 in F Major, op. 90. These pieces have previously been used in other neutral mood inductions (Albersnagel, 1988; Stein, Goldman, & Del Boca, 2000; J. M. G. Williams et al., 2002). The neutral mood induction lasted 15 min.
Polysomnography
Considered the gold standard measure of sleep (Thorpy et al., 1995), polysomnography (PSG) involves the continuous and simultaneous recording of electroencephalogram (EEG), electrooculogram (EOG), and electromyogram (EMG) to score sleep. Data were collected with the Compumedics 802 Siesta device (Compumedics, Charlotte, NC) and software. Recording channels included four EEG derivations (C3/A2, C4/A1, O1/A2, O2/A1), two EOG leads, and two submental EMG sensors. On the first baseline night, participants’ heart rate, blood oxygen, nasal and oral air flow, thoracic and abdominal effort, and leg motion were also monitored. Individuals with a respiratory disturbance index greater than 5 for apnea or a periodic limb movement index greater than 15 were excluded (n = 2 from the bipolar group and n = 3 from the control group were excluded on this basis). REM latency was measured as time from sleep onset to the beginning of the first REM episode of the night. Length of first REM period corresponded to time spent in the first REM period. REM density was calculated as the percentage of 5-s REM epochs that contained eye movements (Werth, Dijk, Achermann, & Borbely, 1996). Carefully trained researchers, blind to diagnostic group, scored the PSG data with standard criteria for sleep staging (Rechtschaffen & Kales, 1968). Following the procedures of Perlis et al. (2002), five PSG files were checked for interrater reliability. At least 90% of epochs were scored identically to scoring by Lisa S. Talbot (the first author).
Sleep Diary
Upon waking in the morning, participants completed a sleep diary to assess self-reported SOL. The sleep diary has been shown to be a reliable estimate (Morin & Espie, 2003) and is the recommended subjective measure of sleep (Buysse, Ancoli-Israel, Edinger, Lichstein, & Morin, 2006).
Procedure
All procedures were approved by the University of California, Berkeley, Committee for the Protection of Human Subjects. After signing informed consent, participants met with doctoral student interviewers to complete the SCID, the DSISD, the YMRS, and the IDS-C so that their diagnostic status and symptom severity could be assessed. Eligible participants were then provided an instruction sheet for the day preceding overnight visits; the instructions asked them to (a) avoid caffeine completely or to restrict intake to no more than the equivalent of two cups of coffee before noon and (b) refrain from taking any over-the-counter medications. Participants then visited the lab for an overnight session that assessed for the presence of sleep disorders (i.e., sleep apnea and periodic limb movement disorder). A second night that included a neutral mood induction occurred approximately one month later. Participants then completed two additional overnight visits, again spaced approximately 1 month apart—one a happy mood induction night and the other a sad mood induction night (order counterbalanced). Some individuals did not participate in both the happy and sad mood induction nights (bipolar n = 6; control n = 2). The reasons for attrition were as follows: unable to recontact (bipolar n = 4, control n = 2), moved out of state (bipolar n = 1), and declined to continue participation (bipolar n = 1). In addition, data for some nights were missing due to incomplete sleep diary (bipolar n = 2; control n = 3). The bipolar group and the control group did not differ in the time elapsed between sessions (M = 31.9 days, SD = 15.7 days, for the bipolar group; M = 26.4 days, SD 12.9 days, for the control group). Participants were paid $15 for the initial interview session and $50 for each overnight visit. For all visits, participants arrived at the laboratory 2 hr prior to their typical bedtime. The YMRS and IDS-C were administered each night to confirm interepisode status. If participants exceeded the symptom thresholds for interepisode status, they were assessed for safety, sent home without completing the mood induction, and reassessed in 1 month. This occurred with 4 participants in the bipolar group; in each case the participant’s symptoms had remitted by the second assessment and they then continued study participation. After attaching PSG equipment, participants were told that they would be participating in two different experiments, a music study and a sleep study, in order to disguise the aim of the study. Participants were then seated in a private experimental room where they participated in the mood induction procedure. After the mood induction, the participant was immediately escorted to the nearby laboratory bedroom and asked to go to sleep. Participants were awakened at the time they requested, with the constraint that they could not stay in the lab past 9:00 a.m. due to resource limitations (i.e., the overnight researchers needed to leave the laboratory). Upon awakening in the morning, participants completed the sleep diary. In the morning following the last visit, the participant was debriefed.
Results
Participant Characteristics
There were no significant differences between groups in any of the demographic variables (see Table 1). The groups differed on the YMRS and IDS-C, with the bipolar group exhibiting more depressive and manic symptoms, though they were well below the established clinical cutoffs (Rush et al., 1996; Young et al., 1978).
Table 1.
Characteristics of Participants With Bipolar Disorder and Controls
| Group |
|||
|---|---|---|---|
| Variable | Control (n = 28) | Bipolar (n = 28) | Test statistic |
| Gender | χ2(1, N = 56) = 1.70 | ||
| Male | 8 | 4 | |
| Female | 20 | 24 | |
| Race/ethnicity | χ2(5, N = 56) = 4.31 | ||
| African American | 3 | 2 | |
| Asian American | 5 | 1 | |
| Caucasian | 16 | 20 | |
| Hispanic | 1 | 1 | |
| Native American | 0 | 1 | |
| Other | 3 | 3 | |
| Employment status | χ2(1, N = 56) = 0.13 | ||
| Full-time/part-time/student | 26 | 22 | |
| Unemployed/retired | 2 | 6 | |
| Marital status | χ2(2, N = 56) = 0.66 | ||
| Single | 16 | 14 | |
| Married/partnered | 6 | 9 | |
| Divorced/separated/widowed | 6 | 5 | |
| Mean age (SD) in years | 37.75 (12.87) | 33.46 (10.36) | t(54) = 1.37 |
| Age range in years | 18–61 | 20–61 | |
| Mean age (in years) at bipolar onset (SD) | 19.37 (9.56) | ||
| Mean time (in months) since last episode (SD) | 19.00 (53.47) | ||
| Mean years of education (SD) | 15.07 (2.07) | 15.25 (2.08) | t(54) = 0.32 |
| Mean IDS-C (SD) | 2.67 (2.39) | 7.00 (3.71) | t(47) = 4.84*** |
| Mean YMRS (SD) | 0.88 (1.40) | 2.81 (2.17) | t(51) = 3.87*** |
Note. IDS-C = Inventory of Depressive Symptomatology–Clinician Rating; YMRS = Young Mania Rating Scale.
p < .001.
Manipulation Check
On the happy mood induction night, 58% of the bipolar group and 59% of the control group met the mood rating threshold specified by Eich et al. (1994). On the sad mood induction night, 86% of the bipolar group and 65% of the control group met the threshold. Chi-square tests indicated no significant group differences.
Independent samples t tests indicated no group difference in length of the mood induction procedure on the happy mood induction night (bipolar M = 23.6 min, SD = 15.2 min; control M = 20.8 min, SD = 16.7 min). However, there was a significant difference on the sad mood induction night, t(46) = 2.67, p < .05, with the bipolar group taking less time to reach the sad mood compared with that of the control group (bipolar M = 14.3 min, SD = 11.8 min; control M = 24.8 min, SD = 15.4 min).
Two separate repeated measures analyses of variance (ANOVAs), one for the happy mood induction night and one for the sad mood induction night, were conducted on the mood ratings from the affect grid with group (bipolar, control) as the between-subjects variable and time (before mood induction, after mood induction) as the within-subject variable. For the happy mood induction night, there was no significant main effect of group, F(1, 51) < 1, but there was a significant main effect of time, F(1, 51) = 46.38, p < .001, with all participants reporting more happiness after the induction than before it. The Group × Time interaction was not significant, F(1, 51) < 1. Similarly, for the sad mood induction night there was no significant main effect of group, F(1, 46) <1, but there was a significant main effect of time, F(1, 46) = 117.58, p < .001, with all participants reporting more sadness after the induction than before it. The Group × Time interaction was not significant, F(1, 46) < 1. Thus, the happy and sad mood inductions were effective.
Data Reduction
On the basis that a few outliers in a relatively small sample can distort results (R. B. Klein, 2005) and mask large differences (Wilcox, 2005), we excluded SOL and REM values greater than two standard deviations above the group mean (SOL values retained: 94% in bipolar group, 96% in control group; REM latency values retained: 97% in bipolar group, 97% in control group; REM density values retained: 97% in bipolar group, 96% in control group; duration of first REM period values retained: 95% in bipolar group; 94% in control group).
Baseline Nights
The average of the first two nights (screen for sleep disorders and neutral mood induction) constituted the baseline. These nights were combined because there was no first night effect (Agnew, Webb, & Williams, 1966). Specifically, there were no significant within-group differences in PSG-defined or self-report-defined SOL or in any of the REM variables between these two nights.
Mood Induction Nights
A series of 2 × 2 ANOVAs were conducted to examine differences between the baseline and mood induction nights. This strategy was used in order to maximize statistical power, given that (a) we did not have both happy and sad mood induction night data for every participant (see the Method section) and (b) a repeated measures ANOVA excludes participants with any missing data.
Happy mood induction night
The results for SOL on the happy mood induction night are presented in Figure 1. A repeated measures ANOVA was conducted on PSG-defined SOL with group (bipolar, control) as the between-subjects variable and mood (baseline night average, happy mood induction night) as the within-subject variable. There was no main effect of group, F(1, 43) = 3.09, ns, or mood, F(1, 43) < 1, ns. There was a Group × Mood interaction, F(1, 43) = 6.32, p < .05. Follow-up independent samples t tests indicated no group difference on the baseline nights, t(48) = 0.19, ns. However, there was a significant group difference on the happy mood induction night, t(49) = 2.49, p < .05, with the bipolar group exhibiting longer SOL. In addition, paired samples t tests indicated that the control group displayed significantly shorter SOL on the happy mood induction night compared to the baseline nights, t(23) = 0.341, p < .01. The same difference was not significant in the bipolar group, t(20) = 0.908, ns, though the mean values were in the opposite direction compared with those of the control group.
Figure 1.
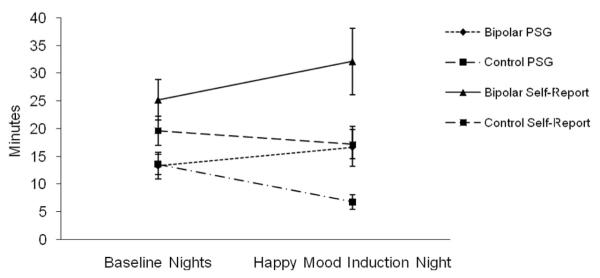
Polysomnography-defined and self-report-defined sleep onset latency in minutes on the happy mood induction night. PSG = polysomnography.
A separate two-way repeated measures ANOVA was conducted on self-reported SOL with group (bipolar, control) as the between-subjects variable and mood (baseline night average, happy mood induction night) as the within-subject variable. There was a group main effect, F(1, 41) = 4.87, p < .05, with the bipolar group reporting longer SOL across the baseline and happy mood induction nights. Neither the mood main effect, F(1, 41) < 1, ns, nor the Group × Mood interaction, F(1, 41) = 2.90, ns, was significant.
Next, we conducted three two-way repeated measures ANOVAs to examine the REM variables on the happy mood induction night. Mean values are presented in Table 2. In each case, group (bipolar, control) was the between-subjects variable and mood (baseline night average, happy mood induction night) was the within-subject variable. For REM latency, neither of the main effects nor the Group × Mood interaction was significant (all Fs < 1). For length of first REM period, there was no significant effect of group, F(1, 39) = 1.84, ns, or mood, F(1, 39) < 1, ns, or a Group × Mood interaction, F(1, 39) < 1, ns. For REM density, there was a significant effect of group, F(1, 41) = 5.08, p < .05, with bipolar participants exhibiting higher REM density. There was no significant effect of mood, F(1, 41) < 1, ns, or a Group × Mood interaction, F(1, 41) < 1, ns.
Table 2.
Mean (SD) REM Sleep Values on the Baseline, Happy Mood Induction, and Sad Mood Induction Nights
| Happy mood analysis |
Sad mood analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline |
Happy mood induction night |
Baseline |
Sad mood induction night |
|||||
| Variable | Control | Bipolar | Control | Bipolar | Control | Bipolar | Control | Bipolar |
| REM-L in minutes | 92.67 (41.16) | 111.92 (67.92) | 97.96 (56.06) | 120.05 (79.03) | 88.94 (41.13) | 108.97 (62.08) | 83.96 (39.44) | 100.91 (47.74) |
| REM-P1 in minutes | 12.47 (5.87) | 15.18 (8.33) | 11.86 (11.91) | 15.90 (15.75) | 11.87 (6.14) | 16.82 (9.35) | 13.32 (10.52) | 11.11 (9.39) |
| REM-D percentage | 8.34 (4.52) | 13.34 (7.97) | 8.91 (6.35) | 12.63 (8.42) | 8.34 (4.42) | 12.82 (8.30) | 10.63 (7.79) | 16.23 (10.79) |
Note. Baseline consists of the average of the first two nights (screen for sleep disorders and neutral mood induction). Baseline night means differ slightly for the two analyses since a few individuals did not participate in both mood nights and a repeated measures analysis of variance excludes a participant if there is missing data on any of the observation points. REM = rapid eye movement; REM-L = REM latency; REM-P1 = length of first REM period; REM-D = REM density.
Sad mood induction night
The mean values for SOL on the sad mood induction night are presented in Figure 2. A two-way repeated measures ANOVA was conducted on PSG-defined SOL with group (bipolar, control) as the between-subjects variable and mood (baseline night average, sad mood induction night) as the within-subject variable. Neither of the main effects nor the Group × Mood interaction was significant (all Fs < 1). The two-way repeated measures ANOVA on self-reported SOL indicated that there was no main effect of group, F(1, 33) = 2.53, ns. There was a significant main effect of mood, F(1, 33) = 9.53, p < .01, such that both bipolar and control participants reported shorter SOL on the sad mood induction night compared to the baseline nights. The Group × Mood interaction was not significant, F(1, 41) < 1, ns.
Figure 2.
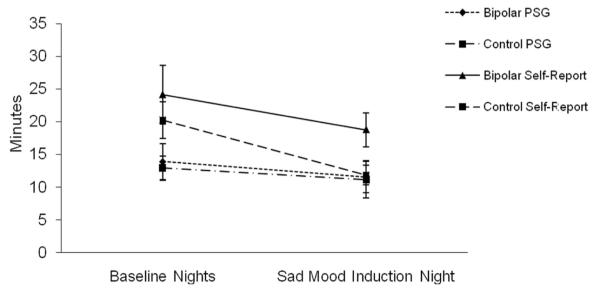
Polysomnography-defined and self-report-defined sleep onset latency in minutes on the sad mood induction night. PSG = polysomnography.
We then conducted three two-way repeated measures ANOVAs to examine the REM variables on the sad mood induction night. Mean values are presented in Table 2. In each case, group (bipolar, control) was the between-subjects variable and mood (baseline night average, sad mood induction night) was the within-subject variable. For REM latency, there was no main effect of group, F(1, 40) = 3.16, ns, or mood, F(1, 40) < 1, ns, or a Group × Mood interaction, F(1, 40) < 1, ns. For length of first REM period, there was no main effect of group, F(1, 38) < 1, ns, or mood, F(1, 38) = 1.20, ns. There was a nonsignificant trend of a Group × Mood interaction, F(1, 38) = 3.38, p = .07. The only follow-up test to approach significance was that the bipolar group tended to spend more time in the first REM period during the baseline, relative to the sad, nights, t(17) = 1.83, ns. For REM density, there was a main effect of group, F(1, 41) = 5.46, p < .05, with the bipolar group exhibiting higher REM density. There was also a main effect of mood, F(1, 41) = 6.26, p < .05, such that both bipolar and control participants demonstrated higher REM density on the sad mood induction night compared with that on the baseline nights. The Group × Mood interaction was not significant, F(1, 41) < 1, ns.
Effect of Medication
Psychotropic medications can have somnolence, insomnia, and/or REM sleep side effects. We elected to not control for these possible medication effects for five reasons. First, nearly all participants were medicated, and they were taking a heterogeneous group of medications. As such, there would be minimal statistical power to examine the effects of individual medications through subgroup analyses. Second, the percentage of individuals who experienced sleep-related side effects was examined on the basis of clinical trials of each medication (GlaxoSmithKline, 2005; Physicians’ Desk Reference Staff, 2007). These data were available for 72% of the medications that participants were taking and indicated that a minority of patients experience sleep-related side effects (between 4% and 37%). Third, numerous medications can have either sedating or alerting side effects (e.g., aripiprazole, venlafaxine, sertraline, zonisamide; Physicians’ Desk Reference Staff, 2007), making it difficult to create sedating-medication and alerting-medication subgroups. Fourth, it is not uncommon for individuals to experience side effects only early in treatment (Ketter & Wang, 2002). Finally, the majority of participants in the bipolar disorder group were taking numerous medications, potentially creating unknown interaction effects (e.g., if a participant is taking one REM-suppressing and one REM-enhancing medication, it is not known whether these effects in fact cancel out).
Effect of Mood Rating Magnitude
To examine the possibility that the magnitude of the mood rating at the end of the mood induction could influence results, we conducted two additional sets of analyses. First, we reran the two-way repeated measures ANOVAs, this time excluding participants who did not reach the mood induction thresholds set by Eich et al. (1994). The results remained the same for SOL. When the REM variable analyses were conducted, the results remained the same for the happy mood induction night. On the sad night, two changes occurred: (1) the length of first REM period interaction, which had been a trend, became significant ( p < .05) and (2) the REM density main effect of mood did not remain. The SOL and REM analyses were not run including only those who did not meet criteria given that the group sample sizes were extremely small.
The second set of analyses examined correlations between mood ratings and each of the outcome variables. These correlations were run for the groups collapsed as well as for each group separately. There were no significant correlations between mood rating and any of the outcome variables in these analyses (all ps > .05).
Effect of Bipolar Disorder Type (I, II)
We reran the analyses excluding bipolar II participants (n = 4). The results remained the same with one exception. For self-reported SOL on the happy mood induction night, the main effect of group was no longer significant.
Discussion
Our first prediction was that relative to the control group, the bipolar group would exhibit longer SOL and increased REM activity on the happy mood induction night relative to the baseline nights. Partial support for the SOL component of the hypothesis was evident for PSG-defined sleep. While the two groups did not differ on the baseline nights, the bipolar group exhibited significantly longer SOL relative to the control group on the happy mood induction night. The reason the first hypothesis was only partially supported was that for the bipolar group the difference between SOL on the happy mood induction night and the baseline nights was not significant (although we note that the means were in the predicted direction). Interestingly, this difference was significant for the control group, albeit in the opposite direction. In other words, the control group fell asleep more quickly on the happy mood induction night, but the bipolar group did not. This intriguing pattern of findings suggests that the control group was able to benefit (by falling asleep more quickly) from a happy presleep mood, whereas the bipolar group was not. This finding points to the broader literature documenting reward sensitivity as characteristic of interepisode bipolar disorder (Johnson, 2005). Affective hyperreactivity to positive stimuli, as indexed by both self-report and psychophysiological measures, has been demonstrated. In particular, it appears that during the interepisode period positive moods can trigger greater goal pursuit (Johnson, 2005). Accordingly, it is possible that while the control group reached a pleasant mood state that facilitated sleep onset, the bipolar group reached a more hyperreactive state with an enhanced focus on rewards and goals. This tendency may have prevented the bipolar group from taking advantage of the mood state in a manner similar to the control group. A related possibility is that the bipolar participants may have experienced elevated rumination in response to the happy mood induction, thus intensifying their mood state (Feldman, Joormann, & Johnson, 2008; Johnson, McKenzie, & McMurrich, 2008). Future research should examine the cognitive content of the mood induction in order to test these hypotheses. A final possibility is that the bipolar participants, compared to controls, may have experienced a longer duration of the happy mood. If so, this would be consistent with a recent report of interepisode bipolar participants exhibiting a more sustained duration of self-reported happiness, compared to controls, following a mood induction (Farmer et al., 2006). Concerning self-reported SOL, while the mean values were in the predicted direction, the results were not significant. It is possible that the large standard deviations precluded the interaction reaching significance. Finally, in regard to the REM variables, the bipolar group exhibited higher REM density compared to the control group. Tentatively, this result is consistent with previous findings of REM hyperactivity in bipolar disorder (Sitaram, Nurnberger, Gershon, & Gillin, 1982).
Our second prediction was that, relative to the control group, the bipolar group would exhibit longer SOL and increased REM activity on the sad mood induction night relative to the baseline nights. This hypothesis was not supported for either PSG-defined or self-report-defined SOL. However, there was a main effect of mood in self-reported SOL, such that both bipolar and control participants had shorter SOL on the sad mood induction night compared to the baseline nights. This result is in contrast to the finding that activating worry prior to sleep impairs sleep onset (Tang & Harvey, 2004). Perhaps worry induces an anxious mood that increases arousal, whereas a sad mood decreases arousal and aids in the process of sleep onset. Consistent with this possibility, arousal ratings from the affect grid for both groups were significantly lower on the sad mood induction night (M = −1.8, SD = 1.7) than on the happy mood induction night (M = −0.27, SD = 2.3). The lack of effects of the sad mood induction, in contrast to the happy mood induction, on SOL in the bipolar group is in line with the emerging pattern of findings in interepisode bipolar disorder of heightened reactivity to positive stimuli (e.g., Johnson, 2005) but inconsistent reactivity to negative stimuli (e.g., Ruggero & Johnson, 2006).
In regard to the REM results for the sad mood induction night, the bipolar group exhibited a group effect of higher REM density. In addition, both groups exhibited higher REM density on the sad, compared to baseline, nights. There was also an interesting nonsignificant trend of a Group × Mood interaction in which the bipolar group’s length of first REM period decreased on the sad, compared to baseline, nights, whereas the control group’s length of first REM period increased on the sad, compared to baseline, nights. These findings are again consistent with previous research (Sitaram et al., 1982) and suggest, in combination with the happy REM density result, that REM density in particular may be heightened in interepisode bipolar disorder. The results also raise the possibility that the bipolar group may have exhibited an adaptive increase in REM density following a sad mood (as did the control group) but that this attempt to regulate mood during sleep was offset by the shorter length of REM in the bipolar group, relative to the control group. There are two reasons why we emphasize that these findings are tentative: (1) the trend for the first REM period length interaction did not reach significance and (2) some REM findings changed when certain participant groups were excluded (see the Results section). Interestingly, we note that the first REM period length interaction reached significance when participants who did not meet the mood thresholds were excluded. Overall, the results suggest that the impact of mood on REM sleep in interepisode bipolar disorder is a worthy focus of future research.
Considering the happy and sad mood induction results together, it could be concluded that the control group benefited in terms of SOL following both mood inductions, whereas the bipolar group only benefited on the sad mood induction night. In controls, perhaps a presleep mood triggers an active regulatory process that influences the duration and intensity of the mood state (Parkinson et al., 1996). In contrast, bipolar disorder is characterized by interepisode mood regulation impairment (e.g., Kruger et al., 2003) and, in particular, appears to involve heightened reactivity to positive affective challenges (e.g., Johnson, 2005). Perhaps this explains why bipolar participants were unable to use such adaptive regulatory processes after the happy mood induction. Interestingly, Silk et al. (2007) reported that children at high risk for depression were less likely to be depressed as adults if they displayed shorter SOL. Silk et al. interpreted the result as shorter SOL possibly being indicative of resilience. The present findings resonate with and extend this observation.
It is interesting to compare the results for self-report-defined SOL and PSG-defined SOL. The pattern of findings were broadly parallel between self-report and PSG when mean values were examined, although sometimes PSG was significant and other times self-report was significant. Previous research has often demonstrated a discrepancy between objective and subjectively estimated sleep both in good sleepers and particularly in patients with psychiatric disorders (Morin, 2000; Schneider-Helmert & Kumar, 1995). This tendency exists among individuals with sleep disturbance across a range of disorders including bipolar disorder (Harvey et al., 2005), unipolar depression (Rotenberg, Indursky, Kayumov, Sirota, & Melamed, 2000), and posttraumatic stress disorder (e.g., Dagan, Zinger, & Lavie, 1997; E. Klein, Koren, Arnon, & Lavie, 2003). At least two possibilities may explain this commonly observed discrepancy. First, the traditional scoring of PSG (Rechtschaffen & Kales, 1968) is based on 30-s epochs. This may be too blunt (Perlis, Merica, Smith, & Giles, 2001). Second, sleep is difficult to perceive, given that the moment of falling asleep is defined by the absence of memory (Ogilvie, 2001) and the context of falling asleep is typically in darkness with few or no time cues (Bonnet, 1990; Ogilvie & Harsh, 1994). Thus, the gold standard in the field is to collect data from both objective and subjective sources and to regard both as important (Buysse et al., 2006). Additionally, future research with more in-depth and detailed tools such as power spectral analysis would be beneficial (Perlis et al., 2001).
Two findings were surprising and thus noteworthy. First, we might have expected baseline SOL and REM differences between groups, but such differences did not emerge. One possibility is that SOL and REM are not always the variables that reflect differences between healthy control and bipolar participants. In our recent review of sleep disturbance in bipolar disorder (Harvey, Talbot, & Gershon, in press), we noted that bipolar and control groups can differ on at least 15 sleep outcome measures and there is a great deal of variation across studies. Second, given previous research that bipolar disorder involves poorer regulation of mood (e.g., Kruger et al., 2003), we might have assumed that those in the bipolar disorder group would be more likely to reach the specified mood induction thresholds and that they would reach these thresholds more quickly. Indeed, Leibenluft, Charney, and Pine (2003) have proposed that individuals with bipolar disorder should have a quicker rise time in response to an affective stimulus and a higher amplitude response. In the present study, we observed a quicker rise time (albeit only to the sad mood induction), but there were no group differences for amplitude of response.
Several limitations are important to consider. First, for the reasons detailed previously, we concluded that the best action was to not control for medication effects. However, we acknowledge that sleep-related side effects of the psychotropic medications are a potential confound. Psychotropic medications for bipolar disorder may have alerting or sedating side effects and may impact REM sleep (e.g., Legros & Bazil, 2003; Physicians’ Desk Reference Staff, 2007). We emphasize, though, that research on severe mental illness (e.g., sleep research, neuroimaging research, neuropsychological research) will be seriously impeded and lack generalizability if it is done on only medication-free samples (Philips, Travis, Fagiolini, & Kupfer, 2008).
Second, the procedures developed by Eich et al. (1994) have not previously been conducted immediately prior to bedtime. We suspect that the lower arousal inherent to the presleep period may have made it more difficult for participants to reach the mood thresholds specified by Eich et al. Hence we did not exclude participants on this basis. While this may have reduced the impact of the inductions on SOL, we note that (a) as a group participants became significantly more happy or sad following the mood inductions; (b) subgroup analyses including only participants who met the Eich et al. criteria did not change the results for SOL (although the results did change for the REM variables); and (c) perhaps because of the limited range of the mood measure, there were no significant correlations between magnitude of the mood rating and SOL according to either PSG or self-report. We also note that past research has emphasized behavioral activation as an important contributor to the course of bipolar disorder (e.g., Depue & Zald, 1993). Accordingly, future research should include measures (e.g., psychophysiology) during the mood induction that allow for ascertaining whether the results are directly attributable to mood state or to behavioral activation levels (e.g., Hayden et al., 2008).
A third limitation concerns the groups’ demographics and baseline symptomatology. The bipolar and control groups did not differ on any demographic variables, including levels of employment. While this matching is helpful for the experimental comparison, it may reduce generalizability given that bipolar patients experience a substantial portion of time with severe disability in work roles (Judd, Schettler, Solomon, et al., 2008), even beyond periods of acute illness (Judd, Schettler, Akiskal, et al., 2003). Moreover, the bipolar and control groups differed on baseline symptomatology, with the bipolar group exhibiting more depressive and manic symptoms (though still below interepisode cutoffs). However, we did not conduct an analysis of covariance to control for these baseline differences on the basis of Miller and Chapman’s (2001) recommendation against this practice. We note, however, that for bipolar participants there were no significant correlations between IDS-C or YMRS scores and response to the mood induction or between IDS-C and YMRS scores and any outcome variable. Nevertheless, the baseline differences remain an important issue. We suggest that future research address them through (a) trying to recruit a larger bipolar disorder group that allows for subgroup analyses with participants who score high and low on the IDS-C and YMRS and (b) adding a unipolar depression control group with symptomatology that is matched with the bipolar disorder group.
A final limitation is that the relatively small sample size may have limited the statistical power of the study. Additionally, a larger sample size would enable a better examination of the differential effects of mood inductions on sleep in bipolar I and bipolar II participants.
In sum, the findings of the current study offer preliminary support for the hypothesis that interepisode mood regulation impairment is one contributor to SOL disturbance in bipolar disorder. In particular, while the control group was able to benefit from a happy presleep mood, the bipolar group was not. The results also suggest that REM hyperactivity may be characteristic of interepisode bipolar disorder. Furthermore, tentative evidence emerged suggesting that REM sleep may be particularly disrupted in interepisode bipolar participants following a sad presleep mood. Future studies investigating presleep mood reactivity and regulation processes are needed as they could yield important insights into the sleep disturbance commonly experienced by bipolar patients.
Acknowledgments
Funding was provided by a grant from NARSAD awarded to Allison G. Harvey. We thank Kerrie Hein and Howard Liu for assistance with data collection and coding.
Contributor Information
Lisa S. Talbot, Department of Psychology, University of California, Berkeley1
Ilana S. Hairston, Psychiatry Department, Addiction Research Center, University of Michigan.
Polina Eidelman, Department of Psychology, University of California, Berkeley.
June Gruber, Department of Psychology, University of California, Berkeley; Department of Psychology, Yale University..
Allison G. Harvey, Department of Psychology, University of California, Berkeley
References
- Agnew HW, Webb WB, Williams RL. The first night effect: An EEG study of sleep. Psychophysiology. 1966;2:263–266. doi: 10.1111/j.1469-8986.1966.tb02650.x. [DOI] [PubMed] [Google Scholar]
- Albersnagel FA. Velten and musical mood induction procedures: A comparison with accessibility of thought associations. Behavior Research and Therapy. 1988;26(1):79–96. doi: 10.1016/0005-7967(88)90035-6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed., text rev Author; Washington, DC: 2000. [Google Scholar]
- Angst J, Sellaro R. Historical perspectives and natural history of bipolar disorder. Biological Psychiatry. 2000;48:445–457. doi: 10.1016/s0006-3223(00)00909-4. [DOI] [PubMed] [Google Scholar]
- Barbini B, Bertelli S, Colombo C, Smeraldi E. Sleep loss, a possible factor in augmenting manic episode. Psychiatry Research. 1996;65:121–125. doi: 10.1016/s0165-1781(96)02909-5. [DOI] [PubMed] [Google Scholar]
- Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: A meta-analysis. Archives of General Psychiatry. 1992;49:651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- Bonnet MH. The perception of sleep onset in insomniacs and normal sleepers. In: Bootzin RR, Kihlstrom JF, Schacter DL, editors. Sleep and cognition. American Psychological Association; Washington, DC: 1990. pp. 148–158. [Google Scholar]
- Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- Cartwright R, Luten A, Young M, Mercer P, Bears M. Role of REM sleep and dream affect in overnight mood regulation: A study of normal volunteers. Psychiatry Research. 1998;81:1–8. doi: 10.1016/s0165-1781(98)00089-4. [DOI] [PubMed] [Google Scholar]
- Chengappa KNR, Kupfer DJ, Frank E, Houck PR, Grochocinski VJ, Cluss PA, et al. Relationship of birth cohort and early age at onset of illness in a bipolar disorder case registry. American Journal of Psychiatry. 2003;60:1636–1642. doi: 10.1176/appi.ajp.160.9.1636. [DOI] [PubMed] [Google Scholar]
- Dagan Y, Zinger Y, Lavie P. Actigraphic sleep monitoring in posttraumatic stress disorder (PTSD) patients. Journal of Psychosomatic Research. 1997;42:577–581. doi: 10.1016/s0022-3999(97)00013-5. [DOI] [PubMed] [Google Scholar]
- Depue RA, Zald DH. Biological and environmental processes in nonpsychotic psychopathology: A neurobehavioral perspective. In: Costello CG, editor. Basic issues in psychopathology. Guilford Press; New York: 1993. pp. 127–237. [Google Scholar]
- Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine work group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- Eich E, Macaulay D, Ryan L. Mood dependent memory for events of the personal past. Journal of Experimental Psychology: General. 1994;123:201–215. doi: 10.1037//0096-3445.123.2.201. [DOI] [PubMed] [Google Scholar]
- Espie CA. Insomnia: Conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annual Review of Psychology. 2002;53:215–243. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- Farmer A, Lam D, Sahakian B, Roiser J, Burke A, O’Neill N, et al. A pilot study of positive mood induction in euthymic bipolar subjects compared with healthy controls. Psychological Medicine. 2006;36:1213–1218. doi: 10.1017/S0033291706007835. [DOI] [PubMed] [Google Scholar]
- Feldman GC, Joormann J, Johnson SL. Responses to positive affect: A self-report measure of rumination and dampening. Cognitive Therapy and Research. 2008;32:507–525. doi: 10.1007/s10608-006-9083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer MB, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders–Patient edition (SCID-I/P, Version 2.0) Biomedics Research Department, New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Fukuda K, Etoh T, Iwadate T, Ishii A. The course and prognosis of manic-depressive psychosis: A quantitative analysis of episodes and intervals. Tohoku Journal of Experimental Medicine. 1983;139:299–307. doi: 10.1620/tjem.139.299. [DOI] [PubMed] [Google Scholar]
- GlaxoSmithKline . Psychotropic prescribing guide. 8th ed Thomson PDR; Montvale, NJ: 2005. [Google Scholar]
- Harrow M, Goldberg JF, Grossman LS, Meltzer HY. Outcome in manic disorders: A naturalistic follow-up study. Archives of General Psychiatry. 1990;47:665–671. doi: 10.1001/archpsyc.1990.01810190065009. [DOI] [PubMed] [Google Scholar]
- Harvey AG. Sleep and circadian rhythms in bipolar disorder: Seeking synchrony, harmony, and regulation. American Journal of Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. American Journal of Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Talbot LS, Gershon AG. Sleep disturbance in bipolar disorder across the lifespan. Clinical Psychology, Science and Practice. doi: 10.1111/j.1468-2850.2009.01164.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden EP, Bodkins M, Brenner C, Anantha S, Nurnberger J, O’Donnell BF, Hetrick WP. A multimethod investigation of the behavioral activation system in bipolar disorder. Journal of Abnormal Psychology. 2008;117:164–170. doi: 10.1037/0021-843X.117.1.164. [DOI] [PubMed] [Google Scholar]
- Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. Journal of Affective Disorders. 2003;74:209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Johnson SL. Mania and dysregulation in goal pursuit: A review. Clinical Psychology Review. 2005;25:241–262. doi: 10.1016/j.cpr.2004.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, McKenzie G, McMurrich S. Ruminative responses to negative and positive affect among students diagnosed with bipolar disorder and major depressive disorder. Cognitive Therapy and Research. 2008;32:702–713. doi: 10.1007/s10608-007-9158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disorders. 2005;7:176–186. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ. The long-term natural history of a weekly symptomatic status of bipolar 1 disorder. Archives of General Psychiatry. 2002;39:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Akiskal HS, Maser J, Coryell W, Solomon D, et al. Long-term symptomatic status of bipolar I vs. bipolar II disorder. International Journal of Neuropsychopharmacology. 2003;6:127–137. doi: 10.1017/S1461145703003341. [DOI] [PubMed] [Google Scholar]
- Judd LL, Schettler PJ, Solomon DA, Maser JD, Coryell W, Endicott J, Akiskal HS. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. Journal of Affective Disorders. 2008;108:49–58. doi: 10.1016/j.jad.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Coryell W, Andreasen NC, Endicott J, Clayton PJ. Differential outcome of pure manic, mixed/cycling, and pure depressive episodes in patients with bipolar illness. Journal of the American Medical Association. 1986;255:3138–3142. [PubMed] [Google Scholar]
- Ketter TA, Wang PW. Psychotropic medications in bipolar disorder: Pharmacodynamics, pharmacokinetics, drug interactions, adverse effects and their management. In: Yatham LM, Kusumakar V, editors. Bipolar disorder: A clinician’s guide to biological treatments. Routledge; London: 2002. [Google Scholar]
- Klein E, Koren D, Arnon I, Lavie P. Sleep complaints are not corroborated by objective sleep measures in post-traumatic stress disorder: A 1-year prospective study in survivors of motor vehicle crashes. Journal of Sleep Research. 2003;12(1):35–41. doi: 10.1046/j.1365-2869.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- Klein RB. Principles and practice of structural equation modeling. 2nd ed Guilford Press; New York: 2005. [Google Scholar]
- Kruger S, Seminowicz D, Goldapple K, Kennedy SH, Mayberg HS. State and trait influences on mood regulation in bipolar disorder: Blood flow differences with an acute mood challenge. Biological Psychiatry. 2003;54:1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- Kupfer DJ. REM latency as a psychobiologic marker for primary depressive disease. Biological Psychiatry. 1976;29:159–174. [PubMed] [Google Scholar]
- Lauer C, Riemann D, Wiegand M, Berger M. From early to late adulthood: Changes in EEG sleep of depressed patients and healthy volunteers. Biological Psychiatry. 1991;29:979–993. doi: 10.1016/0006-3223(91)90355-p. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Emotion and adaptation. Oxford University Press; New York: 1991. [Google Scholar]
- Legros B, Bazil CW. Effects of antiepileptic drugs on sleep architecture: A pilot study. Sleep Medicine. 2003;4:51–55. doi: 10.1016/s1389-9457(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Albert PS, Rosenthal NE, Wehr TA. Relationship between sleep and mood in patients with rapid-cycling bipolar disorder. Psychiatry Research. 1996;63:161–168. doi: 10.1016/0165-1781(96)02854-5. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Charney DS, Pine DS. Researching the pathophysiology of pediatric bipolar disorder. Society of Biological Psychiatry. 2003:1009–1020. doi: 10.1016/s0006-3223(03)00069-6. [DOI] [PubMed] [Google Scholar]
- MacQueen GM, Marriott M, Begin H, Robb J, Joffe RT, Young LT. Subsyndromal symptoms assessed in longitudinal, prospective follow-up of a cohort of patients with bipolar disorder. Bipolar Disorders. 2003;5:349–355. doi: 10.1034/j.1399-5618.2003.00048.x. [DOI] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment. 2001;23:133–143. doi: 10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Morin CM. The nature of insomnia and the need to redefine our diagnostic criteria. Psychosomatic Medicine. 2000;62:483–485. doi: 10.1097/00006842-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Morin CM, Espie CA. Insomnia: A clinical guide to assessment and treatment. Kluwer Academic/Plenum; New York: 2003. [Google Scholar]
- Ogilvie RD. The process of falling asleep. Sleep Medicine Reviews. 2001;5:247–270. doi: 10.1053/smrv.2001.0145. [DOI] [PubMed] [Google Scholar]
- Ogilvie RD, Harsh JR, editors. Sleep onset: Normal and abnormal processes. American Psychological Association; Washington, DC: 1994. [Google Scholar]
- Ohayon MM, Caulet M, Philip P, Guilleminault C, Priest RG. How sleep and mental disorders are related to complaints of daytime sleepiness. Archives of Internal Medicine. 1997;157:2645–2652. [PubMed] [Google Scholar]
- Parkinson B, Totterdell P, Briner RB, Reynolds S. Changing moods: The psychology of moods and mood regulation. Longman; Harlow, United Kingdom: 1996. [Google Scholar]
- Perlis ML, Merica H, Smith MT, Giles DE. Beta EEG activity and insomnia. Sleep Medicine Reviews. 2001;5:365–376. doi: 10.1053/smrv.2001.0151. [DOI] [PubMed] [Google Scholar]
- Perlis ML, Smith MT, Orff HJ, Andrews PJ, Gillin D, Giles DE. The effects of an orally administered cholinergic agonist on REM sleep in major depression. Biological Psychiatry. 2002;51:457–462. doi: 10.1016/s0006-3223(01)01287-2. [DOI] [PubMed] [Google Scholar]
- Philips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. American Journal of Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physicians’ Desk Reference Staff . Physicians’ desk reference 2008: Hospital/library version. 62nd ed Thomson Healthcare; Stamford, CT: 2007. [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stages in human subjects. U.S. Government Printing Office; Washington, DC: 1968. [Google Scholar]
- Riemann D, Hohagen F, Bahro M, Berger M. Sleep in depression: The influence of age, gender, and diagnostic subtype on baseline sleep and the cholinergic REM induction test with RS 86. European Archives of Psychiatry and Clinical Neuroscience. 1994;243:279–290. doi: 10.1007/BF02191586. [DOI] [PubMed] [Google Scholar]
- Robb JC, Cooke RG, Devins GM, Young LT, Joffe RT. Quality of life and lifestyle disruption in euthymic bipolar disorder. Journal of Psychiatric Research. 1997;31:509–517. doi: 10.1016/s0022-3956(97)00030-7. [DOI] [PubMed] [Google Scholar]
- Rotenberg VS, Indursky P, Kayumov L, Sirota P, Melamed Y. The relationship between subjective sleep estimation and objective sleep variables in depressed patients. International Journal of Psychophysiology. 2000;37:291–297. doi: 10.1016/s0167-8760(00)00110-0. [DOI] [PubMed] [Google Scholar]
- Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: Results of the 1991 National Sleep Foundation Survey II. Sleep. 1999;22:S354–S358. [PubMed] [Google Scholar]
- Rottenberg J. Mood and emotion in major depression. Current Directions in Psychological Science. 2005;14:167–170. [Google Scholar]
- Rottenberg J, Gross JJ. When emotion goes wrong: Realizing the promise of affective science. Clinical Psychology: Science and Practice. 2003;10:227–232. [Google Scholar]
- Ruggero CJ, Johnson SL. Reactivity to a laboratory stressor among individuals with bipolar I disorder in full or partial remission. Journal of Abnormal Psychology. 2006;115:539–544. doi: 10.1037/0021-843X.115.3.539. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psycho-metric properties. Psychological Medicine. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Russell JA, Weiss A, Mendelsohn GA. Affect Grid: A single-item scale of pleasure and arousal. Journal of Personality and Social Psychology. 1989;57:493–502. [Google Scholar]
- Schneider-Helmert D, Kumar A. Sleep, its subjective perception, and daytime performance in insomniacs with a pattern of alpha sleep. Biological Psychiatry. 1995;37:99–105. doi: 10.1016/0006-3223(94)00162-V. [DOI] [PubMed] [Google Scholar]
- Silk JS, Vanderbilt-Adriance E, Shaw DS, Forbes EE, Whalen DJ, Ryan ND, Dahl RE. Resilience among children and adolescents at risk for depression: Mediation and moderation across social and neurobiological contexts. Development and Psychopathology. 2007;19:841–865. doi: 10.1017/S0954579407000417. [DOI] [PubMed] [Google Scholar]
- Sitaram N, Nurnberger JI, Gershon ES, Gillin JC. Cholinergic regulation of mood and REM sleep: Potential model and marker of vulnerability of affective disorder. American Journal of Psychiatry. 1982;139:571–575. doi: 10.1176/ajp.139.5.571. [DOI] [PubMed] [Google Scholar]
- Skre I, Onstad S, Torgersen S, Kringlen E. High interrater reliability for the Structured Clinical Interview for DSM-III–R Axis I (SCID-I) Acta Psychiatrica Scandinavica. 1991;84:167–173. doi: 10.1111/j.1600-0447.1991.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Stein KD, Goldman MS, Del Boca FK. The influence of alcohol expectancy priming and mood manipulation on subsequent alcohol consumption. Journal of Abnormal Psychology. 2000;109:106–115. [PubMed] [Google Scholar]
- Tang NK, Harvey AG. Effects of cognitive arousal and physiological arousal on sleep perception. Sleep. 2004;27:69–78. doi: 10.1093/sleep/27.1.69. [DOI] [PubMed] [Google Scholar]
- Thompson JM, Gallagher P, Hughes JH, Watson S, Gray JM, Ferrier IN, et al. Neurocognitive impairment in euthymic patients with bipolar affective disorder. British Journal of Psychiatry. 2005;186:32–40. doi: 10.1192/bjp.186.1.32. [DOI] [PubMed] [Google Scholar]
- Thorpy M, Chesson A, Kader G, Millman R, Potolicchio S, Jr., Reite M, et al. Practice parameters for the use of polysomnography in the evaluation of insomnia. Sleep. 1995;18:55–57. [Google Scholar]
- Tsuang MT, Woolson RF, Fleming JA. Long-term outcome of major psychoses: I. Schizophrenia and affective disorders compared with psychiatrically symptom-free surgical conditions. Archives of General Psychiatry. 1979;36:1295–1301. doi: 10.1001/archpsyc.1979.01780120025002. [DOI] [PubMed] [Google Scholar]
- Watson D. Mood and temperament. Guilford Press; New York: 2000. [Google Scholar]
- Wehr TA, Sack DA, Rosenthal NE. Sleep reduction as a final common pathway in the genesis of mania. American Journal of Psychiatry. 1987;144:201–204. doi: 10.1176/ajp.144.2.201. [DOI] [PubMed] [Google Scholar]
- Werth E, Dijk DJ, Achermann P, Borbely AA. Dynamics of the sleep EEG after an early evening nap: Experimental data and simulations. American Journal of Physiology: Regulatory, Integrative, and Comparative Physiology. 1996;271:501–510. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- Wilcox RR. Outliers. In: Everitt BS, Howell DC, editors. Encyclopedia of statistics in behavioral science. Wiley; Chichester, England: 2005. pp. 1497–1498. [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL, Davis M, Borus J, et al. The Structured Clinical Interview for DSM–III–R (SCID): Multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Healy H, Eade J, Windle G, Cowen PJ, Green MW, et al. Mood, eating behaviour and attention. Psychological Medicine. 2002;32:469–481. doi: 10.1017/s0033291701005177. [DOI] [PubMed] [Google Scholar]
- World Health Organization . World Health Report 2001. Mental health: New understanding, new hope. Author; Geneva, Switzerland: 2001. [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]


