Abstract
Objective
The aims of our study are to evaluate the effect of curcumin on spinal cord neural progenitor cell (SC-NPC) proliferation and to clarify the mechanisms of mitogen-activated protein (MAP) kinase signaling pathways in SC-NPCs.
Methods
We established cultures of SC-NPCs, extracted from the spinal cord of Sprague-Dawley rats weighing 250 g to 350 g. We measured proliferation rates of SC-NPCs after curcumin treatment at different dosage. The immuno-blotting method was used to evaluate the MAP kinase signaling protein that contains extracellular signal-regulated kinases (ERKs), p38, c-Jun NH2-terminal kinases (JNKs) and β-actin as the control group.
Results
Curcumin has a biphasic effect on SC-NPC proliferation. Lower dosage (0.1, 0.5, 1 µM) of curcumin increased SC-NPC proliferation. However, higher dosage decreased SC-NPC proliferation. Also, curcumin stimulates proliferation of SC-NPCs via the MAP kinase signaling pathway, especially involving the p-ERK and p-38 protein. The p-ERK protein and p38 protein levels varied depending on curcumin dosage (0.5 and 1 µM, p<0.05).
Conclusion
Curcumin can stimulate proliferation of SC-NPCs via ERKs and the p38 signaling pathway in low concentrations.
Keywords: Curcumin, Spinal cord neural progenitor cell, Mitogen activated protein kinase
INTRODUCTION
A neural progenitor cell (NPC) has the capability to divide, migrate and differentiate into neurons, and NPC proliferation is an important pathway in functional recovery after spinal cord injury (SCI)1,2). Cellular and molecular mechanisms have been studied to understand NPC proliferation31). Recently, mitogen-activated protein (MAP) kinase signaling pathways are among the most important mechanisms of NPC proliferation, and extracellular signal-regulated kinases (ERKs), p38 kinases and c-Jun NH2-terminal kinases (JNKs) are well known MAP kinases5,8,15,29).
Curcumin (diferuloylmethane) is found in the rhizomes of the plant Curcuma longa Linn (tumeric). Traditionally, tumeric has been used as a dietary spice and as a treatment of diseases associated with injury and inflammation9,10). Curcumin has a diverse set of biologic effects, including anti-inflammatory and antioxidanteffects3,11,12,22). Recently, some studies suggest the possibility that curcumin could increase the proliferation of subventricular zone (SVZ) originated NPCs via a MAP kinase signaling pathway1,23,32).
However, SVZ-NPCs and spinal cord NPCs (SC-NPCs) have some differences involving gene expression and proliferation characteristics. Liu et al.19) reported different electrophysiological properties of mitogen-expansion between adult rat SC-NPCs and SVZ-NPCs. Also, Pfenninger et al.27) reported different gene expressions and proliferations between adult spinal cord ependymal cells and SVZ originated neural precursor cells.
Therefore, the objectives of this study are to evaluate the effect of curcumin on SC-NPC proliferation and to clarify the mechanisms of the MAP kinase signaling pathways in SC-NPCs.
MATERIALS AND METHODS
NPC cultures
To establish cultures of SC-NPCs, Sprague-Dawley rats weighting 250 g to 350 g were sacrificed and their spinal cords were extracted. The spinal cords were chopped using microscissors and incubated in a cocktail containing papain (0.1%, Worthington Biochemical Corp., Lakewood Township, NJ, USA), dispase (0.1%), DNase (0.01%), and MgSO4 (12.4%) in a Hanks balanced salt solution with glucose (0.45%) for 30 minutes at 37℃. The dissociated cells were cultured to form neurospheres in a neurobasal medium containing B-27, glutamin (2 mmol/L), penicillin/streptomycin (0.1 g/mL), fibroblast growth factor-2 (20 µg/mL), and heparin (2 µg/mL). Every 5 days, the neurospheres were dissociated with accutase and NPCs were plated into 96-well plates or culture dishes containing culture medium. The NPCs between 5 and 25 passages were used for the experiments.
NPC proliferation assay
The NPCs were suspended in 100 uL of a neurobasal medium containing curcumin and cultured in a 96-well plate. In different experiments, various concentrations of curcumin (0.1-50 µmol/L) were used. A 20-uL cell proliferation assay solution containing the tetrazolium compound MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfo-phenyl)-2H-tetrazolium), (CellTiter 96 Aqueous One Solution, Promega®, Fitchburg, WI, USA) was added to a well containing cultured cells and incubated for 2.5 hours (hrs) at 37℃. The optical density was measured at 490 nm with a microplate spectrophotometer. The same volume of culture medium with the assay solution was used as a blank. The optical density at 490 nm after reaction with MTS showed a proportional correlation to the number of cells. To study the effect of the duration of curcumin, cells were seeded at a density of 1×104 cells in 96-well plates. After 48 hrs, the cells were treated with different concentrations of curcumin (0.1, 0.5, 1, 10, 20, and 50 µM).
MAP kinase signaling pathway analysis
We used an immunoblotting method for evaluating the MAP kinase signaling protein. The protein concentration was mixed with a RIPA buffer (25 mM Tris-Cl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing a protease inhibitor cocktail (Roche®, Mannheim, Germany) and immediately homogenized. The homogenate was centrifuged at 13000 rpm for 30 min at 4℃ and the supernatants were determined using the BCA protein assay (Sigma®, St. Louis, MO, USA). Proteins were separated by a 10% SDS-PAGE gel and transferred to a nitrocellulose membrane. Incubation was in a blocking solution of 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween 20 for 1 hr at room temperature. The membrane was incubated with a 1 : 1000 dilution of mouse monoclonal phospho-ERK, phospho-p38, phospho-JNK (Santa Cruz®, CA, USA), β-actin (Sigma®, St. Louis, MO, USA) and mouse monoclonal anti-β-actin (Sigma Aldrich®, Saint Louis, MO, USA) overnight at 4℃, and then with a horseradish peroxidase-conjugated secondary antibody for 1 hr at room temperature. The proteins were detected with chemiluminescence reagents. Immune-positive bands used an image J, version 1.46 r, computer-assisted image analyzer (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All statistical comparisons were computed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as mean±standard error of the means. Repeated measure analysis of variance was used to compare groups. Null hypotheses of no difference were rejected if p-values were less than 0.05.
RESULTS
Curcumin increases the proliferation of SC-NPCs
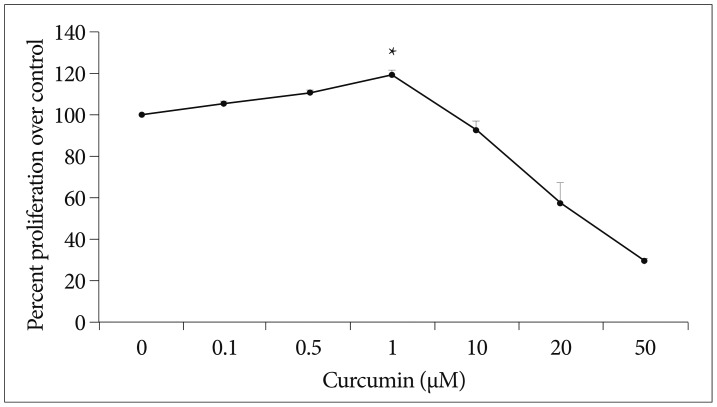
To study that the effect of curcumin on the proliferation of SC-NPCs, SC-NPCs growing in 96-well plates were maintained in medium lacking or containing increased concentrations of curcumin, and the cell proliferation rate was quantified at different time points. After 48 hours of curcumin treatment, lower dosage (0.1, 0.5, 1 µM) of curcumin increased NPC proliferation, whereas high dosage (≥10 µM) caused a decrease in NPC proliferation (Fig. 1). The result shows a few percent improvement compared to each control group.
Fig. 1.
Curcumin has biphasic effects on SC-NPC proliferation. The SC-NPC proliferation rate was quantified at different time points. After 48 hrs of curcumin treatment, lower curcumindosage (0.1, 0.5, 1 µM) increased NPC proliferation. But higher dosage (10, 20, 50 µM) of curcumin decreased the NPC proliferation rate. *Significantly increased compared with corresponding value for control group (p<0.05). SC-NPC : spinal cord neural progenitor cell.
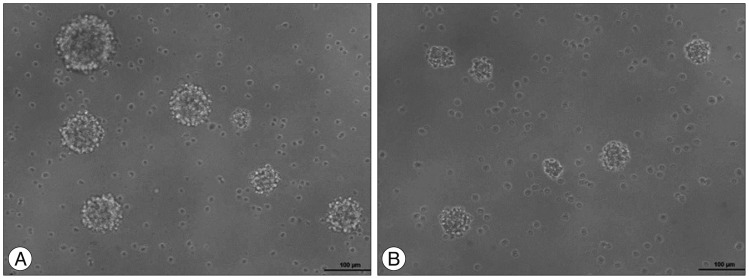
To evaluate the neurosphere formation, dissociated SC-NPCs were diluted in a culture medium and plated into 96-well plate dishes and cultured for 48 hrs. The cells were treated with 1 µM curcumin for 24 hrs. NPC proliferation and neurosphere formation of the curcumin treated group (Fig. 2A) were increased compared to the control group (Fig. 2B).
Fig. 2.
The images show that different cell proliferation between the control group and curcumin treated group. Microscopically, the curcumin treated group (A) had increased cell proliferation and neurosphere formation compared with the control group (B).
The proliferation of NPC via the MAP kinase pathway
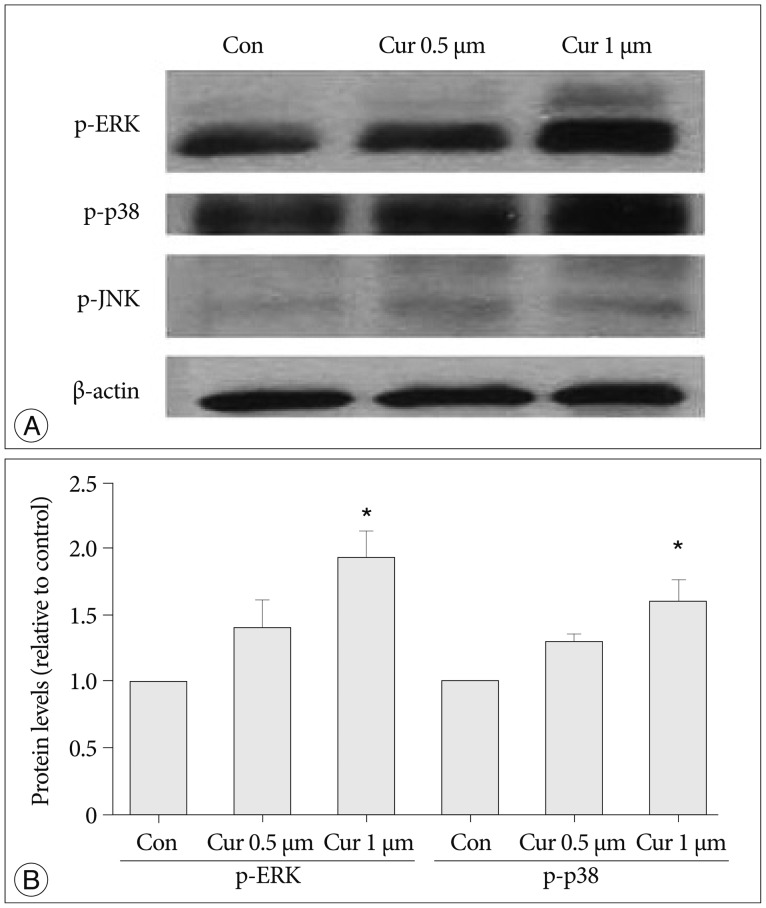
We investigated curcumin treatment associated with MAP kinase signaling pathways, especially involving ERK, p38, and JNK proteins related to NPC proliferation19,29). Immunoblot analysis was used on antibodies against phospho-ERK (p-ERK), phospo-P38 (p-p38), and phosph-o-JNK (p-JNK), and levels of β-actin were determined as a control against possible protein loading variability. The p-ERK protein and p38 protein levels show increases depending on curcumin dosage (0.5, 1 µM, p<0.05), whereas p-JNK and β-actin protein levels show marginal differences without depending on curcumin dosage (Fig. 3).
Fig. 3.
Curcumin stimulates proliferation of NPCs via the MAP kinase signaling pathway, and ERK and p38 proteins are connected with this MAP pathway. A : Immunoblot analysis was used by antibodies against phospho-ERK, phospo-p38, phospho-JNK. The phospho-ERK protein level increased with curcumin dosage of 0.5, 1 µM and the phospho-p38 protein level also showedanincrease. But phospho-JNK and β-actin protein levels show a marginal difference with curcumin dosage. Levels of β-actin were determined as a control against possible protein loading variability. B : Phospho-ERK and phospho-p38 protein levels increased via curcumin dosage-dependence (p<0.05). *Significant increased protein level compared with control group. NPC : neural progenitor cell, MAP : mitogen-activated protein, ERK : extracellular signal-regulated kinase, JNK : c-Jun NH2-terminal kinase.
DISCUSSION
SCI has been thought to be irreversible and the cause of critical sequela. Many studies have reported a desire to minimize secondary injury and increase functional recovery from neurological deficit16,17,21,30). Recently, NPC proliferation after SCI plays an important role in functional recovery from neurological dys-function18,26).
This study finds that curcumin stimulates NPCs proliferation through a MAP kinase signaling pathway, and especially involves the ERK protein and the p38 protein. Also, the effect is biphasic in that low concentrations stimulated cell proliferation, while high concentrations inhibited it15,24,28,29).
Previous studies have documented that curcumin could affect proliferation of stem cells through diverse pathways. Kim et al.13) found that a low concentration of curcumin stimulates proliferation and stemness acting signals of pre-adipocytes by the phosphoinositide 3-kinase pathway, whereas it inactivates p38 kinases and JNK protein. Mujoo et al.25) reported that curcumin induces differentiation of embryonic stem cells through nitric oxide-cyclic signaling. Kim et al.15) documented that curcumin stimulates proliferation of embryonic NPCs and neurogenesis in the adult hippocampus. The study suggested that a MAP kinase signaling pathway is connected to NPC proliferation through ERKs and the p38 protein.
In the present study, we investigated the effect of curcumin on SC-NPCs and analyzed MAP kinase signaling. According to the results, SC-NPC proliferation increased with lower dosage (0.1, 0.5, 1 µM) of curcumin. But, higher dosage (10, 20, 50 µM) of curcumin decreased the NPC proliferation rate. These results are comparable with previous studies showing that curcumin stimulates NPC proliferation relative to the dosage15,28,29). Consequently, for effective clinical applications, these results suggest that proper dosage is important. Therefore, in future studies, we expect that setting the appropriate curcumin-dosage with human cells will be very important.
We found that curcumin affects the proliferation of SC-NPCs, and likewise it affects SVZ-NPCs via the MAP kinase signaling pathway, as we analyzed MAP kinase proteins in this study. Especially, the MAP kinase signaling pathway was revealed as an important mechanism for most stem cell proliferation. There are many different types of MAP kinase and stem cells originated each organization was founded to have slightly different mechanism of MAP kinases5,8,15). Our findings are the first study to show that SC-NPCs are more relevant to ERK and p38 protein associated MAP kinases. The ERK protein has been reported to be concerned with cell proliferation, survival and apoptosis7), and the p38 protein played a vital role in cellular responses to external stress signals like anti-inflammatory reactions6). Anti-inflammatory effects might promote the proliferation of NPCs. Our finding suggests these effects are relevant to curcumin effect NPC proliferation. We consider that this result would be helpful to more selective agonists for NPC proliferation.
However, this study has some limitations. First, this study involves only in-vitro experiments. The spinal cord is a complicated environment, so we need further in-vivo study to evaluate the curcumin effect in live tissue. Second, we used rat SC-NPCs in this experiment. The rat SC-NPC is different from the human SC-NPC, so the proper dosage could be different in humans.
CONCLUSION
This study suggests that curcumin stimulates NPCs proliferation through a MAP kinase signaling pathway, and especially involves the ERK protein and the p38 protein. Also, the effect is biphasic in that low concentrations stimulated cell proliferation, while high concentrations inhibited it. In future studies, we should evaluate the effect of curcumin and MAP kinase signaling in a SCI model.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Ministry of Education, Science and Technology (2012R1A1A1014361).
References
- 1.Alvarez-Buylla A, García-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- 2.Arias-Carrión O, Olivares-Buñuelos T, Drucker-Colín R. [Neurogenesis in the adult brain] Rev Neurol. 2007;44:541–550. [PubMed] [Google Scholar]
- 3.Basnet P, Skalko-Basnet N. Curcumin : an anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabrese V, Butterfield DA, Stella AM. Nutritional antioxidants and the heme oxygenase pathway of stress tolerance : novel targets for neuroprotection in Alzheimer's disease. Ital J Biochem. 2003;52:177–181. [PubMed] [Google Scholar]
- 5.Chen CW, Liu CS, Chiu IM, Shen SC, Pan HC, Lee KH, et al. The signals of FGFs on the neurogenesis of embryonic stem cells. J Biomed Sci. 2010;17:33. doi: 10.1186/1423-0127-17-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dent P. Crosstalk between ERK, AKT, and cell survival. Cancer Biol Ther. 2014;15:245–246. doi: 10.4161/cbt.27541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gangwal RP, Bhadauriya A, Damre MV, Dhoke GV, Sangamwar AT. p38 Mitogen-activated protein kinase inhibitors : a review on pharmacophore mapping and QSAR studies. Curr Top Med Chem. 2013;13:1015–1035. doi: 10.2174/1568026611313090005. [DOI] [PubMed] [Google Scholar]
- 8.Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol. 2009;86:237–250. doi: 10.1189/jlb.0209097. [DOI] [PubMed] [Google Scholar]
- 9.Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric : from farm to pharmacy. Biofactors. 2013;39:2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- 10.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol. 2012;39:283–299. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joe B, Vijaykumar M, Lokesh BR. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit Rev Food Sci Nutr. 2004;44:97–111. doi: 10.1080/10408690490424702. [DOI] [PubMed] [Google Scholar]
- 12.Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa : a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–153. [PubMed] [Google Scholar]
- 13.Kim JH, Park SH, Nam SW, Kwon HJ, Kim BW, Kim WJ, et al. Curcumin stimulates proliferation, stemness acting signals and migration of 3T3-L1 preadipocytes. Int J Mol Med. 2011;28:429–435. doi: 10.3892/ijmm.2011.680. [DOI] [PubMed] [Google Scholar]
- 14.Kim KT, Kim MJ, Cho DC, Park SH, Hwang JH, Sung JK, et al. The neuroprotective effect of treatment with curcumin in acute spinal cord injury : laboratory investigation. Neurol Med Chir (Tokyo) 2014;54:387–394. doi: 10.2176/nmc.oa.2013-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, et al. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;283:14497–14505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon BK, Fisher CG, Dvorak MF, Tetzlaff W. Strategies to promote neural repair and regeneration after spinal cord injury. Spine (Phila Pa 1976) 2005;30(17 Suppl):S3–S13. doi: 10.1097/01.brs.0000175186.17923.87. [DOI] [PubMed] [Google Scholar]
- 17.Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451–464. doi: 10.1016/j.spinee.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Lepski G. Cell transplantation for spinal cord injury : a systematic review. Biomed Res Int. 2013;2013:786475. doi: 10.1155/2013/786475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu RH, Morassutti DJ, Whittemore SR, Sosnowski JS, Magnuson DS. Electrophysiological properties of mitogen-expanded adult rat spinal cord and subventricular zone neural precursor cells. Exp Neurol. 1999;158:143–154. doi: 10.1006/exnr.1999.7078. [DOI] [PubMed] [Google Scholar]
- 20.Liu ZQ, Xing SS, Zhang W. Neuroprotective effect of curcumin on spinal cord in rabbit model with ischemia/reperfusion. J Spinal Cord Med. 2013;36:147–152. doi: 10.1179/2045772312Y.0000000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo V, Esquenazi Y, Han MK, Lee K. Critical care management of patients with acute spinal cord injury. J Neurosurg Sci. 2013;57:281–292. [PubMed] [Google Scholar]
- 22.Lodha R, Bagga A. Traditional Indian systems of medicine. Ann Acad Med Singapore. 2000;29:37–41. [PubMed] [Google Scholar]
- 23.McNally SJ, Harrison EM, Ross JA, Garden OJ, Wigmore SJ. Curcumin induces heme oxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int J Mol Med. 2007;19:165–172. [PubMed] [Google Scholar]
- 24.Ménard C, Hein P, Paquin A, Savelson A, Yang XM, Lederfein D, et al. An essential role for a MEK-C/EBP pathway during growth factor-regulated cortical neurogenesis. Neuron. 2002;36:597–610. doi: 10.1016/s0896-6273(02)01026-7. [DOI] [PubMed] [Google Scholar]
- 25.Mujoo K, Nikonoff LE, Sharin VG, Bryan NS, Kots AY, Murad F. Curcumin induces differentiation of embryonic stem cells through possible modulation of nitric oxide-cyclic GMP pathway. Protein Cell. 2012;3:535–544. doi: 10.1007/s13238-012-2053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura M, Okano H. Cell transplantation therapies for spinal cord injury focusing on induced pluripotent stem cells. Cell Res. 2013;23:70–80. doi: 10.1038/cr.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfenninger CV, Steinhoff C, Hertwig F, Nuber UA. Prospectively isolated CD133/CD24-positive ependymal cells from the adult spinal cord and lateral ventricle wall differ in their long-term in vitro self-renewal and in vivo gene expression. Glia. 2011;59:68–81. doi: 10.1002/glia.21077. [DOI] [PubMed] [Google Scholar]
- 28.Shehzad A, Lee YS. Molecular mechanisms of curcumin action : signal transduction. Biofactors. 2013;39:27–36. doi: 10.1002/biof.1065. [DOI] [PubMed] [Google Scholar]
- 29.Suh HW, Kang S, Kwon KS. Curcumin attenuates glutamate-induced HT22 cell death by suppressing MAP kinase signaling. Mol Cell Biochem. 2007;298:187–194. doi: 10.1007/s11010-006-9365-6. [DOI] [PubMed] [Google Scholar]
- 30.Tederko P, Krasuski M, Kiwerski J, Nyka I, Białoszewski D. Repair therapies in spinal cord injuries. Ortop Traumatol Rehabil. 2009;11:199–208. [PubMed] [Google Scholar]
- 31.Wade A, McKinney A, Phillips JJ. Matrix regulators in neural stem cell functions. Biochim Biophys Acta. 2014;1840:2520–2525. doi: 10.1016/j.bbagen.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Yin WK, Shi XD, Li Y. Curcumin activates Wnt/β-catenin signaling pathway through inhibiting the activity of GSK-3β in APPswe transfected SY5Y cells. Eur J Pharm Sci. 2011;42:540–546. doi: 10.1016/j.ejps.2011.02.009. [DOI] [PubMed] [Google Scholar]