Abstract
Objective
Infectious spinal disease is regarded as an infection by a specific organism that affects the vertebral body, intervertebral disc and adjacent perivertebral soft tissue. Its incidence seems to be increasing as a result of larger proportion of the older patients with chronic debilitating disease, the rise of intravenous drug abuser, and the increase in spinal procedure and surgery. In Korea, studies assessing infectious spinal disease are rare and have not been addressed in recent times. The objectives of this study are to describe the epidemiology of all kind of spinal infectious disease and their clinical and microbiological characteristics as well as to assess the diagnostic methodology and the parameters related to the outcomes.
Methods
A retrospective study was performed in all infectious spinal disease cases presenting from January 2005 to April 2010 to three tertiary teaching hospitals within a city of 1.5 million in Korea. Patient demographics, risk factors, clinical features, and outcomes were assessed. Risk factors entailed the presence of diabetes, chronic renal failure, liver cirrhosis, immunosuppressants, remote infection, underlying malignancy and previous spinal surgery or procedure. We comparatively analyzed the results between the groups of pyogenic and tuberculous spinal infection. SPSS version 14 statistical software was used to perform the analyses of the data. The threshold for statistical significance was established at p<0.05.
Results
Ninety-two cases fulfilled the inclusion criteria and were reviewed. Overall, patients of tuberculous spinal infection (TSI) and pyogenic spinal infection (PSI) entailed 20 (21.7%) and 72 (78.3%) cases, respectively. A previous spinal surgery or procedure was the most commonly noted risk factor (39.1%), followed by diabetes (15.2%). The occurrence of both pyogenic and tuberculous spondylitis was predominant in the lumbar spine. Discs are more easily invaded in PSI. At initial presentation, white cell blood count and C-reactive protein levels were higher in PSI compared to TSI (p<0.05). Etiological agents were identified in 53.3%, and the most effective method for identification of etiological agents was tissue culture (50.0%). Staphyococcus aureus was the most commonly isolated infective agent associated with pyogenic spondylitis, followed by E. coli. Surgical treatment was performed in 31.5% of pyogenic spondylitis and in 35.0% of tuberculous spondylitis cases.
Conclusion
Many previous studies in Korea usually reported that tuberculous spondylitis is the predominant infection. However, in our study, the number of pyogenic infection was 3 times greater than that of tuberculous spinal disease. Etiological agents were identified in a half of all infectious spinal disease. For better outcomes, we should try to identify the causative microorganism before antibiotic therapy and make every effort to improve the result of culture and biopsy.
Keywords: Spondylitis, Osteomyelitis, Pyogenic, Tuberculosis, Spinal infection
INTRODUCTION
Infectious spinal disease is regarded as an infection by a specific organism that affects the vertebral body, intervertebral disc and adjacent perivertebral soft tissue27). In spite of medical development, its incidence seems to be increasing as a result of larger proportion of the older patients with chronic debilitating disease, the rise of intravenous drug abuser, and the increase in spinal procedure, instrumentation and surgery25,27).
In Korea, studies assessing infectious spinal disease are rare, and most of the researches have been focused to pyogenic spondylitis or tuberculous spondylitis, or radiological differentiation of them4,14,15,18,19,20,24,28).
The objectives of this study are to describe the epidemiology of all types of spinal infectious disease and their clinical and microbiological characteristics as well as to access the diagnostic methodology, and parameters related to the outcomes.
MATERIALS AND METHODS
A retrospective study was performed in all infectious spinal disease cases presenting from January 2005 to April 2010 to three tertiary teaching hospitals within a city of 1.5 million in Korea. Cases fulfilling the following criteria were enrolled on the basis of clinical, radiological, laboratory, pathologic, and microbiological data.
1) Clinical symptoms suggestive of infectious spinal disease : fever or chill, axial pain, limb pain or numbness, neurologic deficit.
2) Laboratory abnormalities : white cell blood count (WCC) >10000×106/L, erythrocyte sedimentation rate (ESR) >20 mm/h, C-reactive protein (CRP) >5 mg/dL.
3) Radiologic abnormalities : spondylitis, discitis, epidural abscess, perispinal abscess and/or pyomyositis on magnetic resonance images (MRI).
4) Pathologic findings : granuloma formation in case of tuberculous spondylitis.
5) Microbiological results : from blood cultures, percutaneous fine needle aspiration, percutaneous bone biopsy or open surgery.
The patients who refused any evaluation or treatment or the cases of superficial wound infection were excluded.
Patients without any positive results from the microbial studies were regarded as cases of pyogenic infection, if they showed characteristic radiologic findings consistent with spinal infection, clinical response to antimicrobial therapy, and their histology not showing granulomatous finding.
Patient demographics, risk factors, clinical features and outcomes were assessed. Risk factors entailed the presence of diabetes, chronic renal failure, liver cirrhosis, intake history of immunosuppressaant, remote infection, and underlying malignancy. Previous spinal surgery or procedure as well as the other types of treatment they received were documented.
SPSS version 21 was used for statistical analyses of the data. t-test was used to compare the continuous variables, and χ2 tests and Mann-Whitney test were used to compare the non-parametric variables. The threshold for statistical significance was established at p<0.05.
RESULTS
Demographic findings
Ninety-two cases fulfilled the inclusion criteria and were reviewed. There were 53 (57.6%) males and 39 (42.4%) females, with a mean age of 59.8 years. Pyogenic spinal infection (PSI) and tuberculous spinal infection (TSI) entailed 72 (78.3%) and 20 (21.7%) cases, respectively. Of the PSI group, 40 (55.6%) were male, and the mean age was 59.7 years. Of the TSI group, 13 (65.0%) were male, and the mean age was 59.9 years. Gender and age did not differ between PSI and TSI (p>0.05) (Table 1).
Table 1.
Comparison of demographics, predisposing factors, clinical manifestations in patients with pyogenic spinal infection vs. tuberculous spinal infection
PSI : pyogenic spinal infection, TSI : tuberculous spinal infection
Predisposing factors
Among the predisposing factors those we had investigated, previous spinal surgery or procedure was the most commonly noted risk factor (39.1%), followed by diabetes (15.2%). Previous spinal surgeries or procedures include percutaneous procedures, such as perispinal acupuncture, spinal nerve block, discography, vertebroplasty and endoscopic discectomy, and open surgery such as discectomy, laminectomy and spinal arthrodesis. Patients who had undergone previous spinal surgery or procedure were much more frequent in PSI group (44.4%) than TSI group (20.0%), and it showed statistically significant difference (p=0.048) (Table 1).
Clinical manifestations
Back or neck pain was by far the most common presenting symptom in both groups, occurring in 88.9% of PSI and 85.0% of TSI. The number of patients with fever (axillary temperature >37.5℃) was 26 (36.1%) in the PSI and 4 (25.0%) in the TSI patients. Neurologic deficits at presentation, such as limb weakness, paralysis, or sensory loss were documented in 9 (12.5%) in the PSI and 3 (15%) in TSI. There was no significant difference in both groups (Table 1).
Radiologic findings
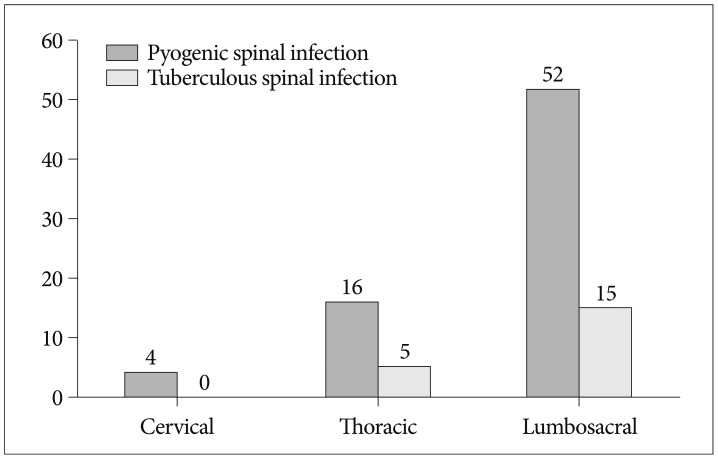
The lumbosacral spine was the most frequently affected region in both groups (p<0.001). 72.2% of PSI had involvement of the lumbar or lumbosacral spine, and the other hand, all of TSI were located in thoracic or lumbar spine (Fig. 1). To know the influence of the spinal procedures to distribution of spinal infections, we additionally investigated the distribution of infections in patients of PSI group who had not taken spinal procedures. Of the 41 patients, 29 patients (70.7%) had involvement of lumbar or lumbosacral spine, 11 patients (26.8%) had thoracic spine infections and one patient (2.4%) had cervical spine infection. There was no significant difference, compared with group of PSI patients who had taken spinal procedures (p=0.907).
Fig. 1.
Distribution of spinal in fection by spine region.
Table 2 presents the type of infection based on the initial MRI findings. The most common type of infection was spondylitis, and second was perispinal abscess. Perispinal abscesses occurred more frequently in TSI group than PSI group (p=0.046). We mixed and combined the each type of infection and re-analyzed by dividing each groups as a group only showing a form of spondylitis (S), a group showing spodylodiscitis (SD), a group showing all patterns of infection, that is, spondylodiscitis with abscess (SDA), a group showing the forms of spondylitis and epidural/perispinal abscess without discitis (SA), and a group showing epidural or paraspinal abscess only (A). Table 3 shows the results that SD group was more frequently in PSI group (12.5%) than in TSI group (0.0%) (p=0.003), SDA group was also frequent in PSI group (p<0.001), and SA group was more frequent in TSI group (45%), but did not show the statistical significance (p=0.117).
Table 2.
Type of spinal Infection based on the initial radiologic findings
PSI : pyogenic spinal infection, TSI : tuberculous spinal infection
Table 3.
Type of spinal infection based on the re-formatted distribution of infection with initial radiologic findings
S : spondylitis only, SD : spondyodiscitis, SDA : spondylodiscitis with epidural or paraspinal abscess, SA : spondylitis with epidural or paraspinal abscess, A : epidural or paraspinal abscess only, PSI : pyogenic spinal infection, TSI : tuberculous spinal infection
Laboratory data
We measured initial and peak value of the ESR, CRP, and WCC (Table 4). All values of inflammatory markers were higher in PSI group than those in TSI group. Mean value of WCC checked initially were 9.5 103/µL and 7.0 103/µL for PSI and TSI, respectively (p=0.007). Mean value of initial CRP were 8.1 mg/dL for PSI and 3.5 mg/dL for TSI, respectively (p=0.006). The other results did not show the significant difference statistically.
Table 4.
Value of inflammatory markers at presentation and peak in patients of PSI and TSI
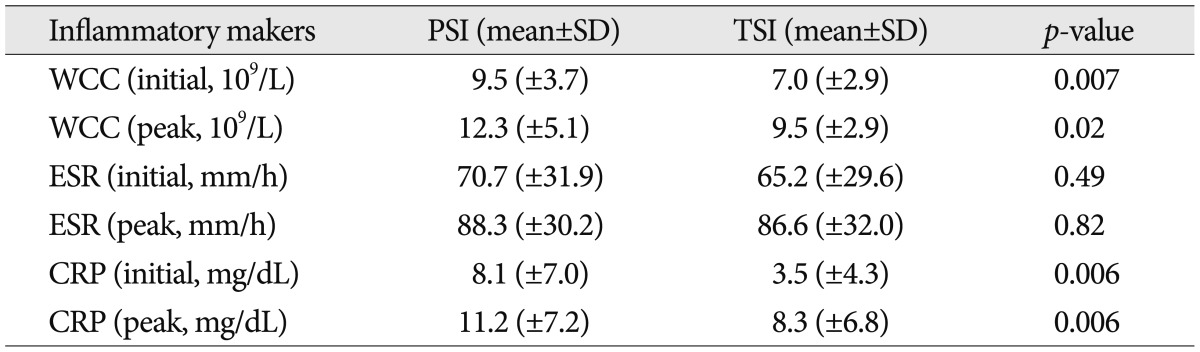
PSI : pyogenic spinal infection, TSI : tuberculous spinal infection, WCC : white cell blood count, ESR : erythrocyte sedimentation rate, CRP : C-reactive protein
Microbiological data
We performed various procedures to find out the etiologic organism, including blood culture, percutaneous soft tissue or pus aspiration, percutaneous bone biopsy or open surgery (Table 5). Blood cultures were obtained in 84 patients (91.3%) of total spinal infection, and 23 (34.3%) of PSI were positive. Tissue cultures and biopsies were carried out for 77 patients (83.7%) of total spinal infection, among them, 40 (52.6%) cases showed positive result. Consequently, causative organism was disclosed in 55.4% of all spinal infectious disease. In PSI group, positive culture rate of percutaneous biopsy and open surgery were 51.1% and 38.9%, respectively. On the other hand, in TSI group, positive culture rate of percutaneous biopsy and open surgery were 46.2% and 83.3%, respectively (Table 6).
Table 5.
Positive ratio of culture studies in PSI and TSI
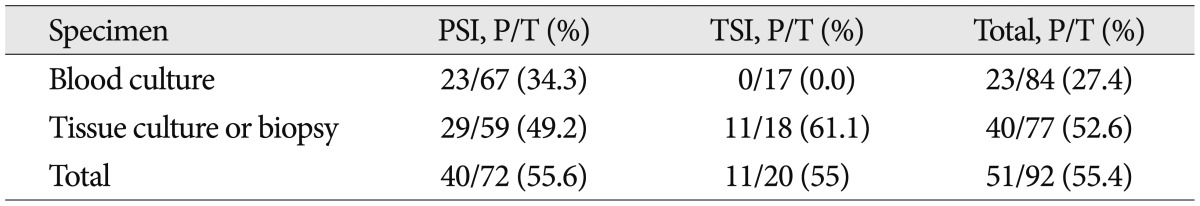
PSI : pyogenic spinal infection, TSI : tuberculous spinal infection, P : the number of patients with positive result, T : total number of patients performed culture studies
Table 6.
Positive ratio of tissue culture/biopsy based on type of method in PSI and TSI
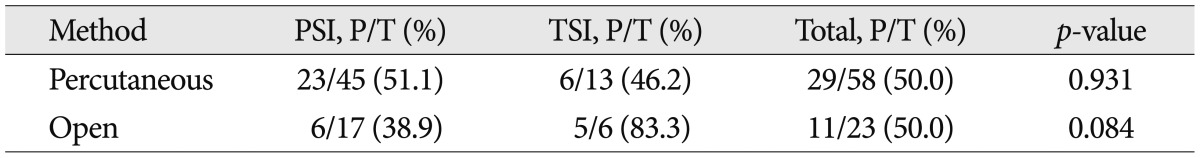
PSI : pyogenic spinal infection, TSI : tuberculous spinal infection, P : the number of patients with positive result, T : total number of culture performed
Table 7 illustrates the etiologic microorganism in PSI cases. Staphylococcus aureus was the most commonly found etiologic microorganism, accounting for 16 cases (22.2%), followed by Escherichia coli for 8 cases (11.1%).
Table 7.
Etiologic microorganism cultured from blood and/or tissue in patiens with pyogenic spinal infection
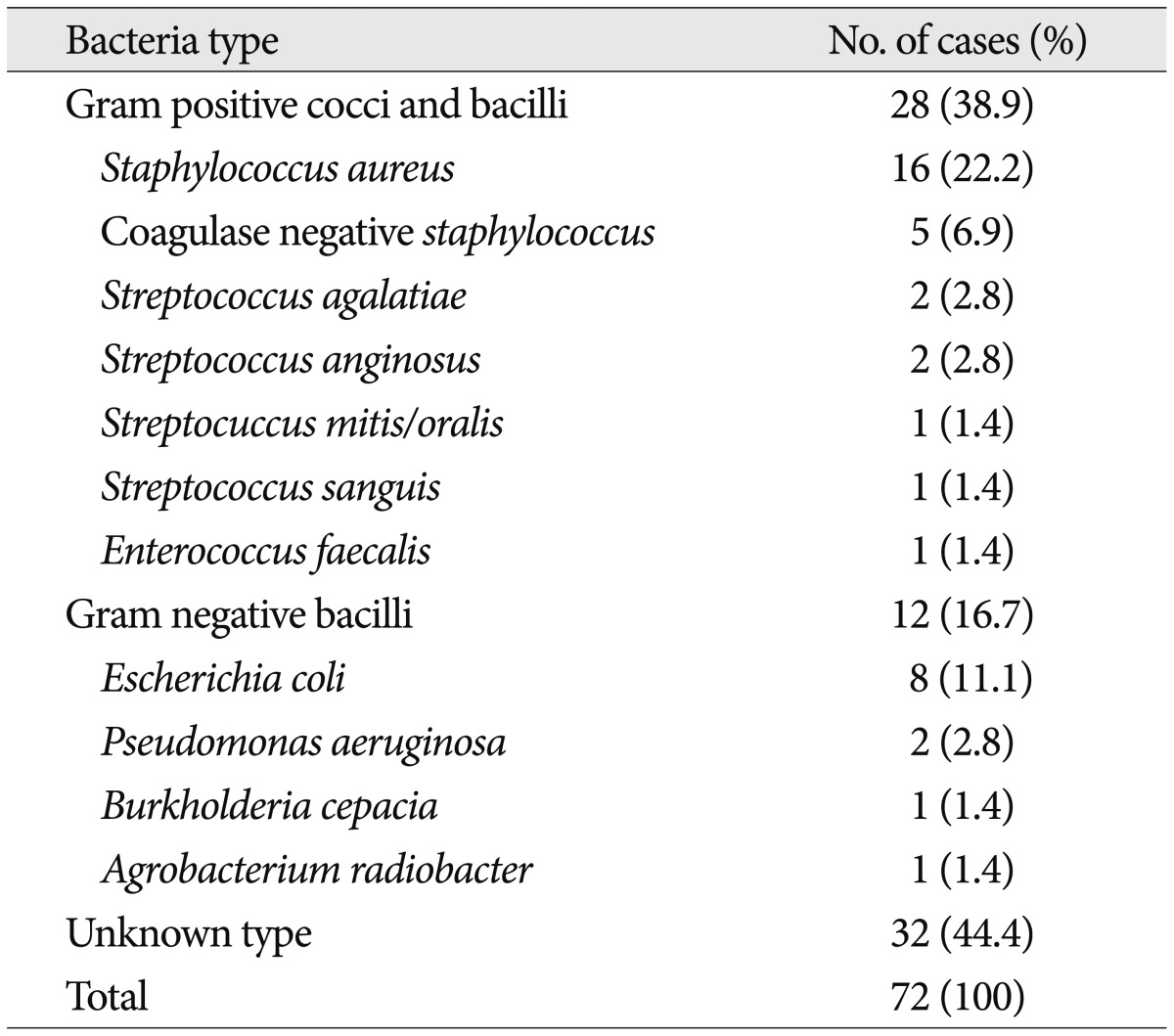
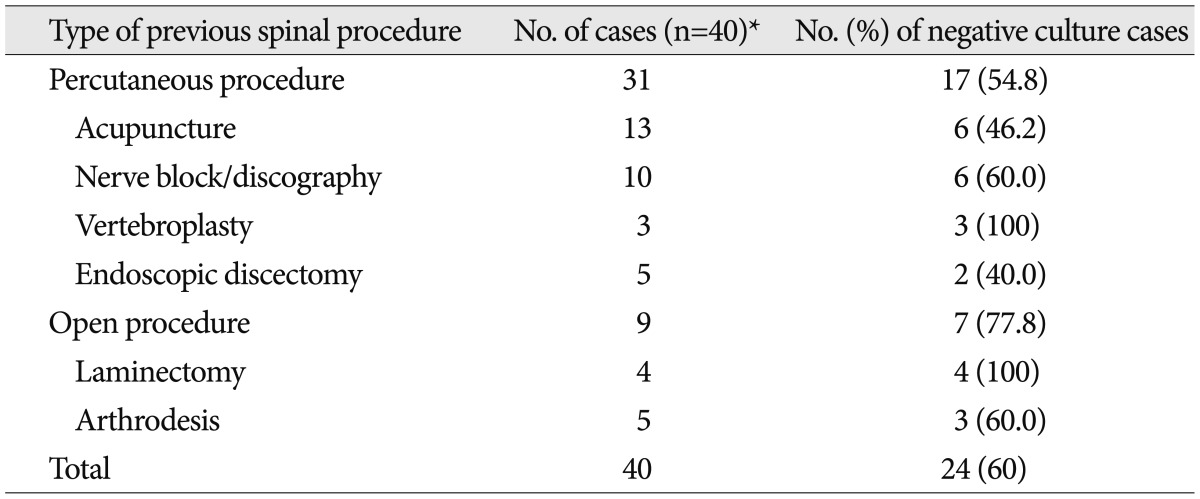
We investigated the prevalent etiologic microorganisms in two groups of patients having more frequent predisposing factors; previous spinal surgery or procedure and diabetes (Table 8). The etiologic microorganisms unknown were significantly more frequent in group of patients who had undergone previous spinal surgery or procedures (52.7%). We additionally analyzed individual negative culture rate according to the type of procedure. Table 9 shows various types of spinal procedure received previously in patients with spinal infection and negative culture rate according to the type of procedure. Negative culture rate in patients received open surgery (77.8%) was slightly higher than that in patients received percutaneous procedures (54.8%) (p=0.051). The most frequent causative microorganism among the known etiologic agents was also Staphylococcus in both two groups (Table 8).
Table 8.
Prevalent etiologic microorganisms in two groups of patients having more frequent predisposing factor
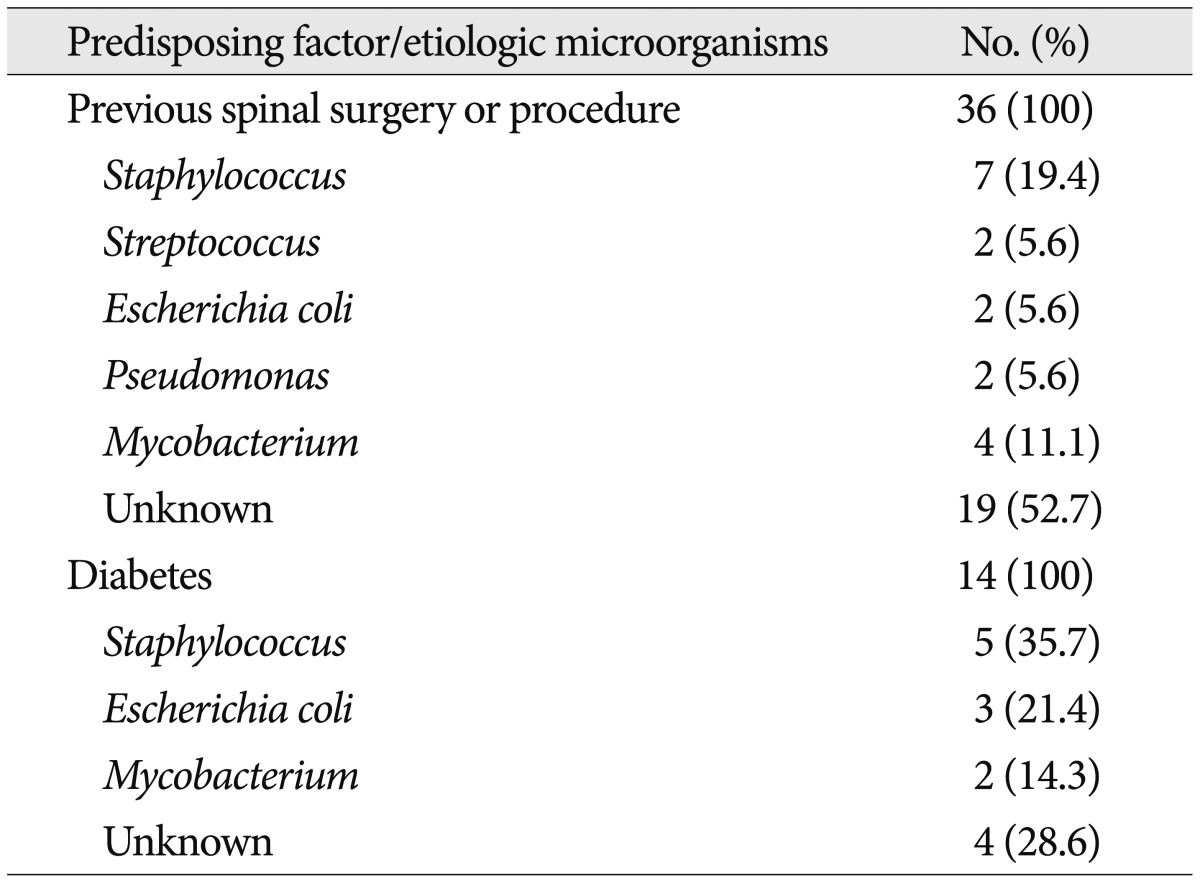
Table 9.
Various types of spinal procedure received previously and result of culture according to them
*Some patients received multiple procedures
Treatment and outcome
The mean duration of hospitalization was 53.3 days (±SD : 28.0; range : 16-151) and 48.2 days (±SD : 37.2, range : 9-134) for PSI and TSI group, respectively. There is no significant difference statistically (p=0.506).
In all cases, antibiotics were administered. The antibiotic drugs for PSI were chosen according to the culture results, but in culture-negative cases, we treated empirically. Combination anti-tuberculosis chemotherapy was administered for TSI.
Open surgery was performed in following cases; 1) progressive neurologic deficits, 2) progressive deformity or fracture result from infection, 3) intractable pain despite conservative treatment, 4) failure with antibiotics-only treatment. Among all patients, 29 patients (31.5%) underwent open surgery. Twenty-two patients (30.6%) of PSI, 7 (35.0%) of TSI underwent open surgery, respectively.
In 6 cases among them, open surgery was performed during the course of antibiotic treatment, because antibiotics administered were revealed as ineffective, all of which were PSI.
Unfortunately, two (2.8%) patients in PSI died of uncontrolled infection, but none of TSI died during the follow-up period.
DISCUSSION
Infectious spinal disease is not a common disease, but its incidence seems to be increasing. In spite of advanced medical science including surgical techniques making operations shorter and its wound smaller and various newly developed antibiotics, the incidence of infectious spinal disease is getting higher as a result of larger proportion of the older patients with chronic debilitating disease, the rise of intravenous drug abuser, and the increase in spinal surgery and instrumentation25,27). In Korea, like other eastern developing country, it has been thought that tuberculous disease is frequent and the incidence of TSI is higher than PSI. Lee et al.19) reported 85% of primary spinal infection were TSI in 1996, and another Lee et al.20) reported 71.0% of spondylitis were TSI in 1999. In 2001, Park and Kim24) reported that 13 patients of the 19 spondylitis patients who had been managed surgically were diagnosed as tuberculous spine infection. However, the incidence of TSI tends to decrease owing to vaccination of tuberculosis and anti-tuberculosis medication and the incidence of PSI seems to rise as results from increase of spinal procedures or surgeries and misuse of antibiotics12,16,27). Recently, in 2010, Kim et al.15) reported pyogenic spondylodiscitis (62.7%) is more frequent than tuberculous spondylodiscitis (37.3%). In our study, PSI (78.3%) had occurred three times more often than TSI (21.7%). Despite many spine-special hospitals manage patients with spinal diseases, the patients of infectious spinal disease tend to be transferred to tertiary hospitals because of the availability of various antibiotics. We think our study is more objective and reliable than any other studies, because we gathered and analyzed the data from three tertiary hospitals in one city.
The known risk factors of the infectious spinal disease are diabetes, liver cirrhosis, underlying malignant disease, end stage of renal disease, intravenous drug abuser, remote infection and any other immune compromised conditions7,11,29). We focused whether the previous spinal surgeries or procedures would be a risk factor of infection. Recently, it has been increasing not only the number of spinal surgeries and procedures, but also the incidence of spinal infectious disease. We included the history of previous spinal surgeries or procedures in the investigation lists to find out their correlation. In our study, 39.1% of all infectious patients have had spine surgery or invasive procedure which was proven as the most predisposing factor. We cannot state that all these surgeries and procedures affected infection, and cannot conclude that previous spinal surgery or procedure become a definite risk factor, because this study was retrospective design and we could not exclude the patients whose infectious condition had been prior to their spinal surgery or procedures. In comparison of both groups, however, the ratio of presence of previous spinal surgery or procedure in PSI is more than twice as high as that in TSI and it showed statistically significant difference (p=0.048). The previous spinal surgery and procedure can cause spinal infection by direct inoculation of bacteria. If we suppose that it is very difficult to inoculate Mycobacterium tuberculosis directly to the back when performing the procedures, and in addition, the ratio of presence of previous spinal surgery or procedure in PSI is significantly higher than that in TSI, it can be suggested that previous spinal surgeries and invasive procedures is one of the crucial risk factors in PSI.
WCC, ESR, and CRP are the important screening markers of infectious diseases in both making the diagnosis and assessing the treatment response. In most studies, values of inflammatory markers are higher in pyogenic infection than tuberculous infection3,15,29). These patterns help in differentiation of pyogenic and tuberculous spinal infection. Furthermore, decreasing pattern of CRP which had elevated initially helps us make decisions of the accurate diagnosis and the effectiveness of treatment, because CRP responses appropriately for effective treatment. In our study, all inflammatory markers were elevated in both groups, and all inflammatory markers were increased higher in PSI group than TSI group (Table 4). Among them, the differences between both groups were significant in initial WCC and initial CRP which are entirely consistent with the report of Yee et al.29).
There is consensus that the most common lesion of pyogenic spinal infection is lumbar spine1,7,15,16,21,29). According to the studies about tuberculous spinal infection, the frequency of involvement varies widely, with the peak levels being at the thoracolumbar junction and with decreasing frequencies at more rostral and caudal levels27). Some authors reported tuberculous spondylitis occurred more frequently in thoracic spine5,8,13,24,29). Meanwhile, much more studies investigated in Korea, including this study, showed that TSI occurred more frequently in lumbar spine4,14,17,18,28).
Infectious spinal disease may involve various skeletal structures such as vertebral body, intervertebral disc, epidural space and perispinal soft tissue. Among them, most common type of infection is spondylitis in both PSI and TSI. However the preference of coincidence is different according to the etiologic microorganisms. Generally, disc involvement of infection is occurred more frequently and earlier in PSI, in the other hand, the discs are often preserved in TSI6,26). We might conclude that discs are more easily invaded in PSI from the results of our analysis. Table 2 and 3 showed that the frequency of disc involvement is just slightly higher in PSI which did not meet statistically significance (p=0.197), but the number of patients with pyogenic spondylitis invading disc space without abscess (SD) is 9 (12.5%) in PSI, whereas no case in TSI (p=0.003). In addition, the proportion of spondylitis making the abscess without discitis (SA) in PSI is about a half of that of TSI (p=0.117).
The most important point for the treatment of spinal infection is the identification of etiologic microorganism. The procedures for this step include blood culture, percutaneous tissue biopsy and culture, and open biopsy and culture. Nowadays real-time polymerase chain reaction (RT-PCR) is used as one of the definite diagnostic tools for tuberculosis and this method has improved the diagnostic results9,23). The results of RT-PCR were not analyzed here, because we had performed it just in some cases in our study. When a spinal infection is suspicious, the blood culture is firstly performed, if neurologically stable, because it is the simplest. But it shows lower positive rates than any other procedures. When the result of blood culture is negative, the procedures for obtaining infected tissue should be considered before antibiotic treatment start6). Positive rate of open biopsy and culture is the highest and percutaneous culture and blood culture follows21). The reason of higher positive rate of open biopsy and culture is that accurate and enough specimens can be obtained as much as needed from the infected tissue. In our study, however, positive culture rate of open biopsy was lower than that of percutaneous culture. We thought these findings might come from the many cases in which antibiotic therapy started before open surgery. Seven cases of 11 showing the negative culture results were administered antibiotics within a month before open culture. We thought this also affected our overall positive culture rate, which was lower than the other reports. The other cause of low positive culture rate is thought to be the techniques obtaining the specimen. Percutaneous biopsies were usually fine needle bone marrow biopsies based on fluoroscopic technique. In order to get enough tissue more accurately from the pathologic area, percutaneous CT-guided needle biopsy is a good option to enhance the culture result6,10,25). The most common isolated etiologic microorganism causing PSI in this study is Staphylococcus aureus followed by E. coli, like in most of the reports1,7,15,16,21,22,24,27,29).
When we investigated the prevalent etiologic microorganisms in two groups of patients having predisposing factor of infection; previous spine procedures or diabetes mellitus (Table 8), we had expected that Staphylococcus aureus would be more frequent in group with medical history of spinal procedures, but unknown etiologic organism was the most. When the negative culture rate was respectively analyzed according to the type of previous spinal procedures to verify the reason, we found that negative rate was higher in the group of open surgeries than the group of percutaneous procedures (Table 9), which was thought to result also from use of prophylactic parenteral antibiotics we always uses for about a week in cases of open surgeries, that is, prior use of antibiotics makes identification of the etiologic organism difficult2).
This study has both advantages and limitations. We collected a lot of clinical data of spinal infectious diseases from three tertiary hospitals in one city, and expect this is the latest data representing South Korea. However, this study lacks the consistency of diagnostic tools and therapeutic options, because this was retrospective and the diagnosis and management were different between the hospitals.
CONCLUSION
The incidence of PSI has shown three times greater than that of TSI. Previous invasive spinal procedure is the most commonly noted risk factor, and its numerical increment is also thought to be associated with increasing incidence of PSI. Even though the lumbar spine is the most involved segments in both types of spinal infection, disc is more susceptible to PSI unlike abscess to TSI. Initial higher WCC and CRP could be one strong indicator of PSI rather than TSI, especially in early phase of infection in which etiologic microorganism has not yet been proven.
References
- 1.Ahmed M, Modic MT. Degenerative disease and infection : role of imaging. In: Benzel EC, Francis TB, editors. Spine Surgery : Techniques, Complication Avoidance, and Management. ed 3. Philadelphia, PA: Elsevier/Saunders; 2012. pp. 1623–1646. [Google Scholar]
- 2.Bhagat S, Mathieson C, Jandhyala R, Johnston R. Spondylodiscitis (disc space infection) associated with negative microbiological tests : comparison of outcome of suspected disc space infections to documented non-tuberculous pyogenic discitis. Br J Neurosurg. 2007;21:473–477. doi: 10.1080/02688690701546155. [DOI] [PubMed] [Google Scholar]
- 3.Capelo J, Carragoso A, Albuquerque C, Mocho ML, Canto-Moreira N. [Infectious spondylodiscitis : a study of forty-one cases] Acta Reumatol Port. 2007;32:255–262. [PubMed] [Google Scholar]
- 4.Chang HG. Tuberculous infection of the spine. J Korean Soc Spine Surg. 1999;6:237–246. [Google Scholar]
- 5.Colmenero JD, Reguera JM, Fernández-Nebro A, Cabrera-Franquelo F. Osteoarticular complications of brucellosis. Ann Rheum Dis. 1991;50:23–26. doi: 10.1136/ard.50.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cottle L, Riordan T. Infectious spondylodiscitis. J Infect. 2008;56:401–412. doi: 10.1016/j.jinf.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 7.D'Agostino C, Scorzolini L, Massetti AP, Carnevalini M, d'Ettorre G, Venditti M, et al. A seven-year prospective study on spondylodiscitis : epidemiological and microbiological features. Infection. 2010;38:102–107. doi: 10.1007/s15010-009-9340-8. [DOI] [PubMed] [Google Scholar]
- 8.Fuentes Ferrer M, Gutiérrez Torres L, Ayala Ramírez O, Rumayor Zarzuelo M, del Prado González N. Tuberculosis of the spine. A systematic review of case series. Int Orthop. 2012;36:221–231. doi: 10.1007/s00264-011-1414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuursted K, Arpi M, Lindblad BE, Pedersen LN. Broad-range PCR as a supplement to culture for detection of bacterial pathogens in patients with a clinically diagnosed spinal infection. Scand J Infect Dis. 2008;40:772–777. doi: 10.1080/00365540802119994. [DOI] [PubMed] [Google Scholar]
- 10.Gasbarrini A, Boriani L, Salvadori C, Mobarec S, Kreshak J, Nanni C, et al. Biopsy for suspected spondylodiscitis. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 2):26–34. [PubMed] [Google Scholar]
- 11.Helewa RM, Embil JM, Boughen CG, Cheang M, Goytan M, Zacharias JM, et al. Risk factors for infectious spondylodiscitis in patients receiving hemodialysis. Infect Control Hosp Epidemiol. 2008;29:567–571. doi: 10.1086/588202. [DOI] [PubMed] [Google Scholar]
- 12.Hong YP, Kim SJ, Lew WJ, Lee EK, Han YC. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis. 1998;2:27–36. [PubMed] [Google Scholar]
- 13.Kayani I, Syed I, Saifuddin A, Green R, MacSweeney F. Vertebral osteomyelitis without disc involvement. Clin Radiol. 2004;59:881–891. doi: 10.1016/j.crad.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Kim BJ, Ko HS, Lim Y, Seo JG, Zoo SK, Jeon TH. The clinical study of the tuberculous spondylitis. J Korean Orthop Assoc. 1993;28:2221–2232. [Google Scholar]
- 15.Kim CJ, Song KH, Jeon JH, Park WB, Park SW, Kim HB, et al. A comparative study of pyogenic and tuberculous spondylodiscitis. Spine (Phila Pa 1976) 2010;35:E1096–E1100. doi: 10.1097/BRS.0b013e3181e04dd3. [DOI] [PubMed] [Google Scholar]
- 16.Kim CW, Currier BL, Eismont FJ. Infections of the spine. In: Herkowitz HN, Balderston RA, editors. Rothman-Simeone the Spine. ed 5. Philadelphia: Saunders/Elsevier; 2011. pp. 1513–1570. [Google Scholar]
- 17.Kim SW, Lee SM, Shin H. Preoperative gadolinium-enhanced magneticresonance images on infectious spondylitis. J Korean Neurosurg Soc. 2005;38:355–358. [Google Scholar]
- 18.Kim YH, Song JK, Shin H. A clinical analysis of surgically managed tuberculous spondylitis. J Korean Neurosurg Soc. 1997;26:223–234. [Google Scholar]
- 19.Lee KS, Doh JW, Bae HG, Yun IG. Primary infections disorders of the spine : report of 40 cases. J Korean Neurosurg Soc. 1996;25:1655–1660. [Google Scholar]
- 20.Lee KY, Sohn SK, Hwang KS. Comparison of pyogenic and tuberculous spondylitis. J Korean Soc Spine Surg. 1999;6:443–450. [Google Scholar]
- 21.Luzzati R, Giacomazzi D, Danzi MC, Tacconi L, Concia E, Vento S. Diagnosis, management and outcome of clinically-suspected spinal infection. J Infect. 2009;58:259–265. doi: 10.1016/j.jinf.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 22.McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis : long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34:1342–1350. doi: 10.1086/340102. [DOI] [PubMed] [Google Scholar]
- 23.Merino P, Candel FJ, Gestoso I, Baos E, Picazo J. Microbiological diagnosis of spinal tuberculosis. Int Orthop. 2012;36:233–238. doi: 10.1007/s00264-011-1461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JH, Kim KH. A clinical analysis of surgically managed primary spondylitis. J Korean Neurosurg Soc. 2001;30:1163–1169. [Google Scholar]
- 25.Pintado-García V. [Infectious spondylitis] Enferm Infecc Microbiol Clin. 2008;26:510–517. [PubMed] [Google Scholar]
- 26.Rivas-Garcia A, Sarria-Estrada S, Torrents-Odin C, Casas-Gomila L, Franquet E. Imaging findings of Pott's disease. Eur Spine J. 2013;22(Suppl 4):567–578. doi: 10.1007/s00586-012-2333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vollmer DG, Tandon N. Infection of the spine. In: Winn HR, editor. Youmans Neurological Surgical. ed 6. Philadelphia, PA: Saunders/Elsevier; 2011. pp. 3216–3232. [Google Scholar]
- 28.Whee SM, Eoh W, Nam DH, Lee JI, Kim JS, Hong SC, et al. Clinical evaluation of surgical treatments for ten cases of tuberculous spondylitis. J Korean Neurosurg Soc. 2001;30:1314–1319. [Google Scholar]
- 29.Yee DK, Samartzis D, Wong YW, Luk KD, Cheung KM. Infective spondylitis in Southern Chinese : a descriptive and comparative study of ninety-one cases. Spine (Phila Pa 1976) 2010;35:635–641. doi: 10.1097/BRS.0b013e3181cff4f6. [DOI] [PubMed] [Google Scholar]