Abstract
Big-eyed bugs (Geocoris spp. Fallén, Hemiptera: Lygaeidae) are ubiquitous, omnivorous insect predators whose plant feeding behavior raises the question of whether they benefit or harm plants. However, several studies have investigated both the potential of Geocoris spp. to serve as biological control agents in agriculture and their importance as agents of plant indirect defense in nature. These studies have demonstrated that Geocoris spp. effectively reduce herbivore populations and increase plant yield. Previous work has also indicated that Geocoris spp. respond to visual and olfactory cues when foraging and choosing their prey and that associative learning of prey and plant cues informs their foraging strategies. For these reasons, Geocoris spp. have become models for the study of tritrophic plant-herbivore-predator interactions. Here, we present detailed images and ecological observations of G. pallens Stål and G. punctipes (Say) native to the Great Basin Desert of southwestern Utah, including observations of their life histories and color morphs, dynamics of their predatory feeding behavior and prey choice over space and time, and novel aspects of Geocoris spp.’s relationships to their host plants. These observations open up new areas to be explored regarding the behavior of Geocoris spp. and their interactions with plant and herbivore populations.
1. Introduction
Geocoris spp. Fallén (Hemiptera: Lygaeidae), commonly known as big-eyed bugs, are generalist insect omnivores which occur naturally worldwide [1]. Geocoris spp. are well known to prey on a variety of insects, including several economically important agricultural pests [1, 2] but have also been reported to feed on plant material [1–6], particularly seeds [1, 5, 7]. Several studies in laboratories [1, 4, 6, 8–14], agricultural fields [1, 8, 15–21], and natural habitats [22–31] have investigated the potential of multiple Geocoris spp.—including G. bullatus (Say) [1], G. ochropterus (Fieber) [10], G. pallens Stål [1, 20–22, 24, 26–31], G. proteus Distant [32], G. punctipes (Say) [4, 6, 9, 11–18, 20, 21, 33], G. uliginosus (Say) [16, 19, 33], and G. varius (Uhler) [32]—to serve as biological control agents to protect plants against herbivores. These studies have found that individual Geocoris spp. accept a variety of insect prey, and the field studies have also shown that Geocoris spp. reduce herbivore populations [1, 15, 17, 18, 20–24, 26–29] (but see [25]) and increase plant yield [23, 31]. Thus, despite plant feeding, the net effect of Geocoris spp.-plant interactions is usually beneficial to plants [34], and Geocoris spp. can be effective biological control agents in many agricultural systems. The most important consequence of plant feeding by Geocoris spp. may be that it renders them more directly susceptible to agricultural pesticides [6].
Geocoris spp. adults lay their eggs on plants in nature, or on moist cotton or paper cellulose in the laboratory. Life history traits have been characterized in laboratory colonies of G. atricolor Montandon [35], G. bullatus [1], G. lubra Kirkaldy [36], G. pallens [1, 35], and G. punctipes [35, 37]. The speed of development from egg to adult correlates positively with temperature between 21°C and 37°C; outside this range, eggs are not viable [1, 35, 36]. The photoperiod associated with the most rapid development differs among species; the photoperiod for which development is slowest may correspond to the diapause-inducing photoperiod for a species [36]. Eggs hatch after ca. 1–3 weeks depending on temperature (higher temperature = faster development) [1, 36, 37], and nymphs develop through five stages over ca. 1 month before reaching adulthood [36, 37]; nymph viability was found to be higher at 27°C than at 24°C for G. lubra, but higher at 24°C than at 27°C for G. punctipes [35, 36]. Adults can survive from 1 week to nearly 4 months in captivity [37].
Geocoris spp. feed on a combination of insect prey and plant material [1, 2, 4, 5, 7]. They can survive if given a water source and either insect prey or plant seeds, but diets combining insects with seeds or seed pods decrease development time and increase survival rates and fecundity; Geocoris spp. may even require seeds or seed pods in order to complete development [1, 5, 38]. This may be due in part to the fact that Geocoris spp. prey on many different insects of varying nutritional value. Interestingly, although Helicoverpa zea Boddie (Lepidoptera: Noctuidae) eggs are higher quality food for G. punctipes than are Acyrthosiphon pisum Harris (Hemiptera: Aphididae), G. punctipes more often preyed on A. pisum in choice tests [5, 38]. Seed pods and seeds are thus important nutritional resources for Geocoris spp. [1, 2, 5, 7, 38]. However, because leaf feeding has not been shown to increase survival in comparison to a water-only diet, it is thought that leaves serve only as a water source [3, 4].
We study the ecological interactions of the wild tobacco Nicotiana attenuata Torr. ex S. Watson (Solanales: Solanaceae) in its native habitat, the Great Basin Desert of the southwestern USA. The postfire germination behavior of N. attenuata creates large monocultures of plants that host a diverse insect herbivore community [39]. This herbivore community includes several specialists on Solanaceae: Corimelaena extensa Uhler (Hemiptera: Thyreocoridae) [39], Epitrix hirtipennis (Melsheimer) and E. subcrinita LeConte (Coleoptera: Chrysomelidae) [28], Manduca quinquemaculata (Haworth) and M. sexta (Linnaeus) (Lepidoptera: Sphingidae), and Tupiocoris notatus (Distant) (Hemiptera: Miridae) [23]; the generalist herbivores Spodoptera spp. Guenée (Lepidoptera: Noctuidae) and Trimerotropis spp. (Orthoptera: Acrididae) [40]; and opportunistic herbivores which attack only poorly-defended plants, such as Empoasca spp. Walsh (Hemiptera: Cicadellidae) [41, 42] and Heliothis spp. Ochsenheimer (Lepidoptera: Noctuidae) [43]. G. pallens is a common predator of herbivores on N. attenuata, and both G. pallens and G. punctipes can be found on N. attenuata or neighboring plant species during N. attenuata’s growing season [22, 31]. Geocoris spp. respond to volatiles emitted from N. attenuata after herbivory by removing more herbivores from emitting plants [22, 26, 27, 29–31], resulting in a fitness benefit for plants [31].
Here, we present quantitative and qualitative observations and high-resolution images of morphology and behavior for G. pallens and G. punctipes co-occurring with N. attenuata. We have observed aspects of the life history, host plant, and insect prey preferences of both species. For G. pallens, we have also made detailed recordings of feeding behavior with a high-resolution macro lens (courtesy of A. Shillabeer with Merit Motion Pictures); quantified variation in predation activity of subpopulations with respect to the lepidopteran herbivore M. sexta; assayed the inclination of different generations of nymphs and adults from a single wild population to feed on M. sexta over a season; recorded increased occurrence of Geocoris pallens on wilting N. attenuata plants; and demonstrated that G. pallens can, in fact, survive when provided only with water and vegetative plant tissue.
2. Methods
2.1. Study Sites and Insect Collections
Geocoris pallens and G. punctipes were assayed in and collected from Lytle Preserve in the Great Basin Desert of southwestern Utah, USA (latitude 37.146, longitude −114.020), where we have annual field plantations of N. attenuata, and from a nearby location where a native N. attenuata population could be found from 2007 to 2009 after a 2006 burn (latitude 37.077, longitude −113.833). In May and June 2009, we collected adults and nymphs of G. pallens from the native N. attenuata population (four collections of 73 insects (19% adults), 31 insects (71%), 99 insects (58%), and 107 insects (95%)), allowed adults to mate and lay eggs, and observed the eggs through development to adults. These collections, together with ca. 100 insects collected from Lytle and a nearby wash, were used to start a colony of G. pallens at our institute in Jena, Germany. This colony has since received annual inputs from field collections. G. punctipes adults were also collected in June 2009 and used to start a colony in Jena. The colonies are fed a diet of Nutrimac (sterilized Ephestia kuehniella eggs, Biobest N. V.), Manduca sexta and Spodoptera littoralis (Boisduval) eggs and larvae, and N. attenuata green tissue and seeds, with additional water provided using moist dental rolls in microcentrifuge tubes containing tap water. They are kept in 9 L food-quality plastic boxes (Lock & Lock) with two holes in each lid of ca. 8 cm diameter each covered with a fine mesh, and containing paper towels to provide structure for oviposition and hiding places, inside a growth chamber (Snijders Scientific, http://www.snijders-scientific.nl/cooling-and-freezing-systems/) with 16 h D/8 h N (06:00–22:00 D/22:00–06:00 N), 26/22°C, daylight provided by Osram L 36 W/77 fluorescent lamps (http://www.osram.com/) at 50% power, 65% RH, and ventilation by PAPST type 4656 N fans (http://www.ebmpapst.com/en/).
2.2. Images of Geocoris pallens and G. punctipes
Pictures were taken of insects collected from the native N. attenuata population in 2009 (Figures 1, 2, and 3) or from Lytle in 2011 (Figure 4). Images in Figures 1–3 are from an Axiocam HRc connected to a stereomicroscope SV 11 and captured with AxioVision 4.0 software (Zeiss, http://www.zeiss.com/corporate/en_de/home.html; Figure 1, Figure 2 instar 3 and adult), or from a Powershot SD1000 camera (Canon, Inc., http://www.usa.canon.com/cusa/home; Figure 2 instars 1, 2, 4, and 5; Figure 3). Images in Figure 4 were taken with a probe lens (Innovision Optics, http://www.innovision-optics.com/) by A. Shillabeer and kindly provided by Merit Motion Pictures (Winnipeg, MB, Canada). The probe lens permits the capture of HD macro images with an unusually large depth of field.
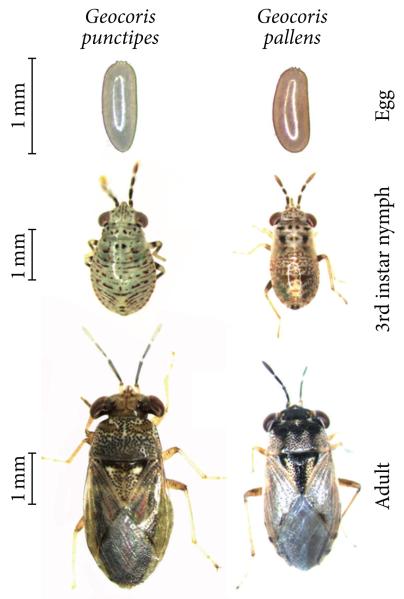
Figure 1. Comparison between Geocoris punctipes and G. pallens collected from the Great Basin Desert in southwestern Utah.
Figure 2. Larval and adult stages of G. pallens from the Great Basin Desert in southwestern Utah. Geocoris spp. have five nymphal instars.
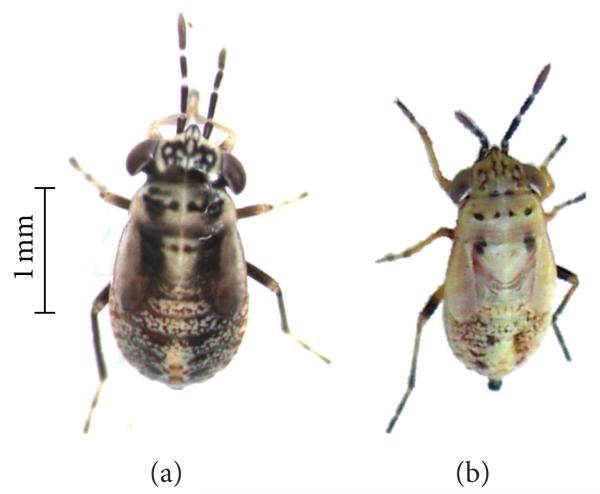
Figure 3. Color morphs of G. pallens nymphs.
The dark (a) and the more common light (b) color morph are shown in the fourth instar. Size differences are not characteristic of the morphs but are rather due to individual differences.
Figure 4. G. pallens feeding on a Manduca sexta egg (a)–(c) or larva (d)–(f).
Note the flexible stylet clearly visible inside the egg in (c). Copyright: Merit Motion Pictures, Winnipeg, MB, Canada.
2.3. Egg Predation Assays
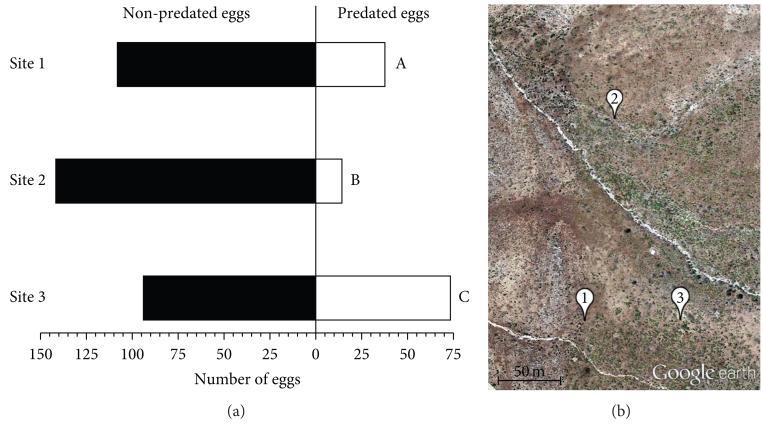
Although M. quinquemaculata and M. sexta moths oviposit in native N. attenuata populations, the number of eggs is usually not sufficient for experiments except in outbreak years. Thus, we use M. sexta eggs and larvae from lab colonies for many field assays. In June 2007, M. sexta eggs purchased from North Carolina State University were frozen to kill developing larvae and thus prevent hatching, then thawed and used to assay native Geocoris spp. predation activity in a wild population of Nicotiana attenuata growing on a recent burn (see Section 2.1). Five eggs were glued with an α-cellulose glue (KVS, Leuna, Germany)—which does not damage plants, induce volatile emission, or prevent egg predation—to the underside of a similarly sized, intact lower stem leaf in a standardized position (as in [22]) on 35 plants per location, in three locations within the native N. attenuata population (Figure 5). After 36 h, empty eggs with intact shells containing visible puncture holes typical of Geocoris feeding (Figure 4(c)) were counted as predated, and intact eggs were counted as non-predated. Missing eggs were not included in counts.
Figure 5. Geocoris spp. predation activity differs among sites within a native N. attenuata population.
(a) The graph shows numbers of M. sexta eggs predated over 36 h by Geocoris spp. Letters indicate significant differences between sites in Bonferroni-corrected pairwise Fisher’s exact tests, P < 0.003. (b) Sites were clusters of plants ca. 50–100 m apart.
2.4. “Feeder/Non-Feeder” Assays
We observed in many years that G. pallens prey on small bugs from invasive stork’s bill ground cover plants (Erodium cicutarium (L.) L’Hér. ex Aiton, Geraniales: Geraniaceae) in April and May and move to N. attenuata plants later in the spring, as E. cicutarium plants are drying up. On N. attenuata, G. pallens prey on flea beetles (Epitrix spp.), which are usually the first herbivores on N. attenuata, and mirids (T. notatus) which arrive on N. attenuata as plants begin to elongate. If Manduca spp. moths oviposit on N. attenuata (which they often do when pollinating flowers), G. pallens will begin to eat Manduca spp. eggs and young larvae [23, 30]. We conducted feeding assays to quantify the tendency of two generations of G. pallens nymphs and adults to feed on M. sexta eggs (Figure 6). On four separate days in May and June 2009, G. pallens were collected from a native N. attenuata population (see Section 2.1); Table 1 shows the distribution of adults and nymphs in each collection. May collections were tested at the field station immediately after collection, and June collections were tested after transportation to the laboratory in Germany, within 48 h after collection (during which G. pallens individuals had access to a variety of field-collected plant and insect food and could adapt to the new conditions). Each individual was put with a piece of damp cotton and a single M. sexta egg into a 30 mL Dixie plastic cup (http://www.dixie.com/) with a lid containing small air holes and left for 72 h; cups were kept by a window in a shaded travel trailer at the field station (May assays) or in a laboratory (June assays), and water from an underground spring (May assays) or from a tap (June assays) was added to the cotton daily. G. pallens individuals which had eaten the egg within 72 h were counted as feeders, and those which had not were counted as nonfeeders. From the June 15th collection, two of the M. sexta eggs hatched and the larvae were eaten; these G. pallens were also counted as feeders. Native Manduca spp. oviposition in the field at this time was not sufficient for feeding assays, and the M. sexta for the May assays in 2009 were kindly provided by C. Miles of the State University of New York at Binghamton; the M. sexta for June assays came from an in-house colony of the same original stock as the Binghamton colony.
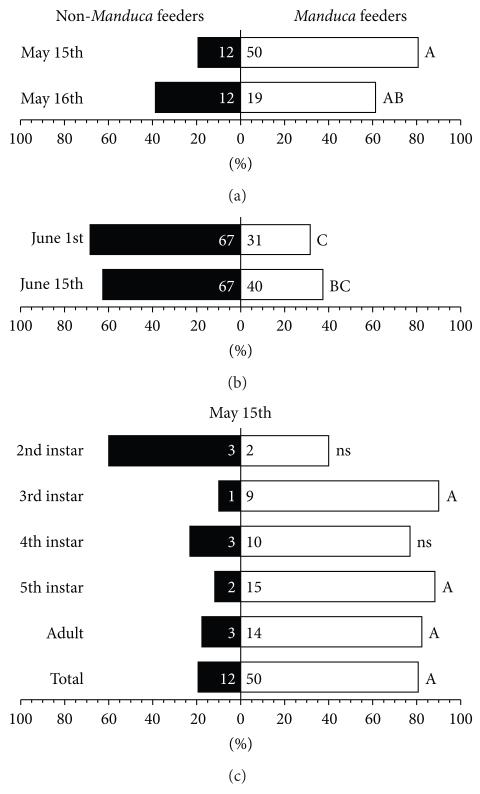
Figure 6. G. pallens collected from a single wild N. attenuata population vary in their tendency to eat M. sexta eggs, but different stages in a single collection do not.
Graphs show percentages of G. pallens in collections which ate M. sexta eggs within 72 h in no-choice assays; counts are in bars. Letters indicate significant differences in Bonferroni-corrected pairwise Fisher’s exact tests across all groups, P < 0.05; ns: no significant difference to any other group. (a) Individuals collected in May and tested immediately after collection show a similar tendency to eat M. sexta eggs (61–81%). (b) Individuals collected at two dates in June and tested 24–48 h later, after transportation to a laboratory and a short adjustment period, also show a similar tendency to eat M. sexta eggs (32–37%), although the tendency is lower than for the May collections. This could be due either to a shift in the population’s tendency to eat M. sexta eggs or to transportation and changed environmental conditions. (c) The May 15th population had a fairly even distribution of different nymphal stages and adults, which did not differ significantly in their tendency to eat M. sexta eggs.
Table 1. Distribution of nymphal and adult stages in G. pallens collections tested for their tendency to eat M. sexta eggs (Figure 6).
| Stage | May 15th | May 16th | June 1st | June 15th |
|---|---|---|---|---|
| Nymphs | ||||
| 1 | — | — | — | — |
| 2 | 8.1% | — | 1.0% | — |
| 3 | 16.1% | — | — | — |
| 4 | 21.0% | — | 9.2% | 1.9% |
| 5 | 27.4% | 29.0% | 21.4% | 2.8% |
| Adults | 27.4% | 71.0% | 68.4% | 95.3% |
| n | 62 | 31 | 98 | 107 |
2.5. G. pallens Populations around Wilting versus Healthy Plants
In several years we observed Geocoris spp. individuals associated with diseased or damaged N. attenuata plants which began to wilt. In 2012, when a massive disease outbreak occurred which killed a huge number of plants, we investigated this phenomenon by counting the presence of Geocoris spp. on dying plants versus the two nearest healthy plants (Figure 7). On two days in May 2012 we searched for wilting plants and directly checked for the presence of Geocoris spp. and then checked the nearest neighboring healthy plant (approximately 1-2 m away) and the second nearest neighboring healthy plant (approximately 2-3 m away) for Geocoris spp. presence. Except for one plant, only single G. pallens individuals were found on plants.
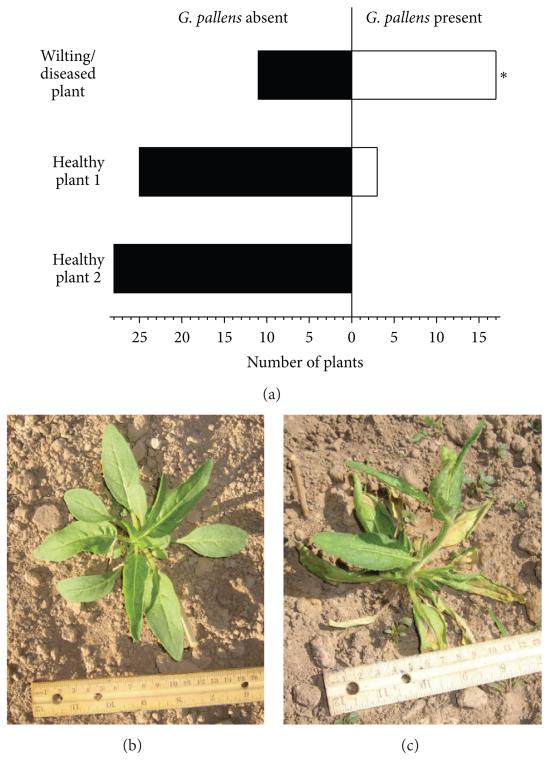
Figure 7. G. pallens is more likely to be found on wilting plants.
The graph shows the number of wilting, diseased, and neighboring healthy plants found to harbor G. pallens individuals (a). Healthy plant 1 (b) was on average ca. 1.5 m, and healthy plant 2 was ca. 2-3 m away from the wilting plant (c). The asterisk indicates significant differences between the presence of G. pallens in both sets of healthy plants and wilting plants in a Fisher’s exact test, P < 0.0001.
2.6. G. pallens Survival on Leaf Tissue and Water versus Water Alone
Geocoris spp. have been reported to feed on seeds and insects. In 2006, to test the potential of G. pallens to survive on leaf tissue, we conducted a feeding assay in which G. pallens adults collected in Lytle were offered either water from an underground spring, or spring water and an N. attenuata leaf (Figure 8). Each individual (n = 12 collected immediately prior to the start of the assay) was caged in a 50 mL food-quality plastic container (Huhtamaki; http://www.huhtamaki.com/) secured with miniature claw-style hair clips and padded on the rim with foam to avoid damaging plant leaves. These “clip cages” contained a cotton ball moistened with spring water or the moist cotton ball and part of an N. attenuata leaf. The leaf was still attached to a living plant and thus did not have to be replaced for the duration of the experiment. The plant did not harbor any insect herbivores. The cotton ball with water was exchanged every second day. Mortality was monitored once a day at noon.
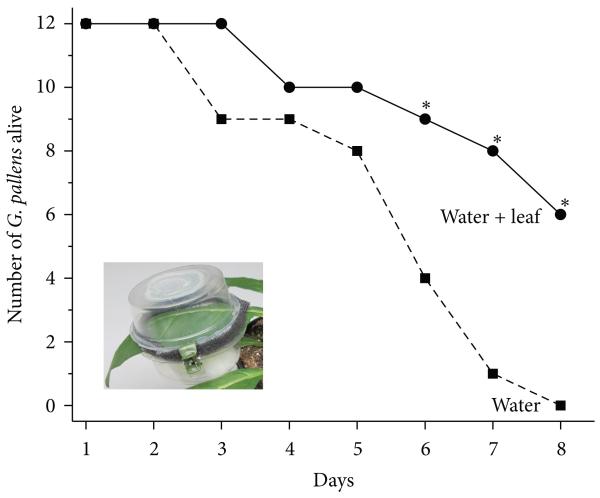
Figure 8. Use of N. attenuata leaf tissue as a food source by G. pallens.
The graph shows the number of G. pallens individuals surviving in clip cages with either a cotton ball soaked with water (water) or water plus an N. attenuata leaf still attached to a living plant (water + leaf). Inset: clip cage on a leaf with a moist cotton ball in the lower right (water + leaf treatment); each half of the cage has a hole covered with netting to permit transpiration (only visible for top half). Asterisks indicate significant differences between treatments obtained by Fisher’s exact tests on indicated days, P < 0.05.
2.7. Statistics
Fisher’s exact tests conducted using a spreadsheet (J. H. Macdonald, http://udel.edu/~mcdonald/statfishers.html) for Excel (Microsoft) [44] were used to compare counts of predated eggs, M. sexta-feeding G. pallens, plants harboring G. pallens individuals, and G. pallens individuals surviving on a water-only versus water and live leaf diet. When necessary, Bonferroni post hoc corrections were calculated using Excel to correct for multiple testing.
3. Results
3.1. Geocoris pallens and G. punctipes Populations at the Study Site
G. pallens and G. punctipes can be easily distinguished by differences in the coloration of their eggs and by the size and coloration of their nymph and adult stages (Figures 1 and 2). In 10 years of field research at Lytle Preserve and in the surrounding areas, we have almost exclusively found G. pallens associated with invasive Erodium cicutarium (L.) L’Hér. ex Aiton (Geraniales: Geraniaceae) plants and alfalfa (Medicago sativa Linnaeus, Fabales: Fabaceae) plantations in the early spring (mid-April to mid-May) and with N. attenuata plants in the late spring to summer (from the end of May). In contrast, we have observed G. punctipes primarily on Cucurbita foetidissima Kunth in Humb. (Cucurbitales: Cucurbitaceae) and Datura wrightii Regel (Solanales: Solanaceae) plants. We have not observed Geocoris spp. in areas where ants are abundant.
Our observations over several years indicate that the main food for G. punctipes on C. foetidissima is Empoasca spp., and on D. wrightii, Lema trilineata (Olivier) (Coleoptera: Chrysomelidae) eggs and Manduca spp. eggs and larvae. The main foods for G. pallens on N. attenuata appear to be Epitrix spp., T. notatus, Manduca spp. eggs and young larvae depending on their abundance, and, when plants are setting seed, C. extensa, the seed-feeding negro bug. On N. attenuata, G. pallens begin by eating primarily flea beetles (Epitrix spp.), which are usually the first herbivores on N. attenuata, and mirids (T. notatus) which arrive on N. attenuata as plants begin to elongate but will switch to eating M. sexta and M. quinquemaculata eggs and young larvae when Manduca spp. are abundant, usually after N. attenuata begins to flower and attract Manduca spp. as pollinators [23, 30]. We have also found G. pallens sheltering in open N. attenuata seed capsules overnight and eating ripe seed (M. C. Schuman and M. Stanton, observation). In 2008–2010 we observed that the number of Manduca spp. eggs preyed on Geocoris spp. increased in locations which received oviposition from native Manduca spp. moths (2008, M. C. Schuman and S. Allmann, observation; 2009, [30]; 2010, I. T. Baldwin and C. Diezel, observation). In 2011, we found that Geocoris spp. began to prey on M. sexta larvae within 24 h after plants were experimentally infested with larvae, in the absence of wild Manduca spp. oviposition [31].
We have observed that Geocoris spp. adults emerge from overwintering sites in March and April and lay eggs which hatch in May, giving rise to a second generation; the adults of this second generation overwinter to the following year. We have generated laboratory colonies of both Geocoris species from field collections. G. punctipes can be easily reared in captivity, and there are multiple other colonies of this species in captivity, primarily for use in biological control [4, 6, 9, 11–18, 20, 21, 33]. The Geocoris spp. in our colonies have similar developmental and survival times as reported in the literature (ca. 1 month for nymph development and 1–3 months survival as adults, see Section 1), and adults reproduce year-round.
3.2. Developmental Stages and Color Morphs of G. pallens
In the 2009 field collections of G. pallens we observed five nymphal stages (instars) (Figure 2) occasionally present as dark morphs (Figure 3(a)) but dominated by a light morph (Figure 3(b)). Adults from dark and light morphs were able to interbreed, and both morphs have since reoccurred in our colony in Jena, which is propagated from annual field collections in Lytle Preserve and the surrounding areas.
3.3. Feeding Behavior of G. pallens
A. Shillabeer with Merit Motion Pictures filmed one of our field-collected G. pallens feeding on M. sexta eggs and larvae in high-resolution macro focus (Figure 4). In these pictures, one can clearly see how the proboscis sheath is used to penetrate prey and then bends at three joints, permitting the flexible stylets to emerge and suck out the prey’s contents.
3.4. Geocoris spp. Predation Activity Varied Significantly within a Single N. attenuata Population
We found that Geocoris spp. predation of M. sexta eggs varied significantly for patches in a single wild N. attenuata population in 2007 (Figure 5, n = 146–167 eggs per site, pairwise Fisher’s exact tests followed by a Bonferroni correction for multiple testing: site 1 versus site 2, P = 0.0002; site 2 versus site 3, P < 0.0001; site 1 versus site 3, P = 0.0027). This difference was driven by total Geocoris spp. predation activity and not necessarily by the attractiveness of plants for Geocoris spp. in each site: between 91% and 100% of plants at each site had at least one egg predated. There were no significant differences among sites in the numbers of plants from which eggs were predated (P > 0.4).
3.5. G. pallens Generations Varied in Their Tendency to Eat M. sexta Eggs and Larvae, but Nymphs and Adults Did Not
We tested field collections of G. pallens from a native N. attenuata population in 2009 for their tendency to eat M. sexta eggs or larvae (Figure 6, Table 1). Between 61 and 81% of G. pallens collected and tested in the field in mid-May (15th or 16th) ate M. sexta. Collections from the same N. attenuata population were tested again in June, within 48 h after collection and transport to the lab in Jena. In a collection from June 1st, 32% of individuals ate M. sexta, and this increased slightly (but not significantly) to 37% in a collection from June 15th (n = 31–107 individuals per collection, pairwise Fisher’s exact tests followed by a Bonferroni correction for multiple testing: P < 0.0001 for the May 15th versus the June 1st collection, P = 0.0163 for the May 16th versus the June 1st collection, P < 0.0001 for the May 15th versus the June 15th collection, but P = 0.0699 [not significant] for the May 16th versus the June 15th collection). G. pallens individuals from collections made within the same month did not significantly differ in their tendency to eat M. sexta (P > 0.2). Mortality over the course of the 72 h assay was less than 15% and did not differ significantly among collections (pairwise Fisher’s exact tests followed by a Bonferroni correction for multiple testing, P > 0.06).
All collections comprised both adults and nymphs, and the May 15th collection had a particularly good representation of most nymphal stages and adults (Table 1). There was no significant difference among different developmental stages in their tendency to eat M. sexta eggs (Figure 6(c), pairwise Fisher’s exact tests followed by a Bonferroni correction for multiple testing, P = 1), although in the case of second-instar nymphs, which tended to eat fewer eggs, this was likely due to low replicate numbers.
3.6. G. pallens Individuals Associated with Wilting and Diseased Plants
When N. attenuata plants wilted in the field due to various stresses, for example, uprooting by wind, cattle damage, or disease, we often observed Geocoris spp. around the dying plants. When a fungal disease outbreak killed a large number of N. attenuata plants in 2012, we found G. pallens more frequently on wilting plants (Figure 7). G. pallens was present on 61% of the wilting plants, but only on 11% of the nearest healthy neighboring plants (1.5 m away), and no Geocoris spp. could be found on the second-nearest healthy plants (approximately 2-3 m away from wilting plants) in any of 28 replicates (Fisher’s exact test of numbers of healthy versus wilting plants harboring G. pallens, P < 0.0001).
3.7. G. pallens Can Use Leaf Tissue as a Food Source
G. pallens adults survived significantly longer if reared on N. attenuata leaves and a water-soaked cotton ball than only on the wet cotton ball. After six days without any insect prey, twice as many G. pallens individuals died if given only water than if given leaf material and water (n = 12 individuals per treatment, Fisher’s exact test, P = 0.0498; Figure 8). This effect lasted until the end of the experiment after eight days, when all G. pallens individuals living only on water had died. After seven days, only four individuals had died if they were allowed to feed on plant diet, while 11 individuals had died in the water-only group (P = 0.0047), and after eight days all animals reared only on water had died, while six individuals given N. attenuata leaves remained alive (P = 0.0069).
4. Discussion
We have observed aspects of the life history, host plant, and insect prey preferences of G. pallens and G. punctipes that co-occur with the native tobacco N. attenuata in the Great Basin Desert of southwestern Utah. For G. pallens, we have also captured images of feeding behavior with a high-resolution macro lens (courtesy of A. Shillabeer and Merit Motion Pictures), quantified variation in the predation of one insect prey species, M. sexta, in space and time, recorded increased occurrence around wilting or sick N. attenuata plants, and demonstrated the ability to survive on only water and vegetative plant tissue, which has not otherwise been demonstrated for any species of Geocoris. Furthermore, we describe how we have maintained laboratory colonies of both Geocoris species from field collections.
4.1. Geocoris pallens and G. punctipes May Have Overlapping Ranges but Separate Niches in Southwestern Utah
G. pallens and G. punctipes can be easily distinguished based on size and morphology at all life stages (Figures 1 and 2). We have found these species feeding on a partially overlapping diet of insect prey but almost always on different plant species: G. pallens is associated with E. cicutarium plants and M. sativa plantations in the early spring and with N. attenuata plants in the late spring and summer; this apparent host shift is likely due to the fact that E. cicutarium, a shallow-rooted ground cover, dries up by the end of May or beginning of June. We have observed G. punctipes primarily on C. foetidissima and D. wrightii plants. Both Geocoris spp. will feed on Manduca sexta and M. quinquemaculata eggs and young larvae, and both eat the same food in our colonies, but in nature their diets may overlap very little except for Manduca spp. and the mirid T. notatus.
Within the native populations of G. pallens, we have observed two color morphs (Figure 3). The light morph seems to be the more prevalent. It would be interesting to know whether the difference in pigmentation is genetically or environmentally based, because dark and light morphs co-occur in the same populations without any obvious differences in microclimate, a genetic basis seems likely. G. pallens nymphs and adults spend most of their time foraging on plants and moving between plants over the sandy ground or sheltering in the shade of plants. The dark morph may be better camouflaged in the shade, while the light morph would be better camouflaged on the sunlit sand. Potential behavioral differences associated with the color morphs, however, remain to be investigated. To our knowledge, such a strong color contrast has not been reported as a morphotype in any other species of Geocoris.
4.2. G. pallens Predation Activity Varied Significantly within an N. attenuata Population
We found that the number of M. sexta eggs predated by G. pallens (Figure 4) varied significantly for N. attenuata plants in different locations within a single population (Figure 5). This might have been due to local variation in G. pallens population density or differences in feeding behavior within a host plant population, perhaps dependent on local Manduca spp. oviposition events or differences in the abundance of other prey. We do not know how far G. pallens individuals travel in search of prey, but the assay sites we chose were ca. 50–100 m apart, and it is possible that G. pallens at the different sites represented local subpopulations with little exchange of individuals between them.
It is also possible that differences in G. pallens predation activity were due to differences in N. attenuata plant phenotypes. N. attenuata plants within a population vary greatly both in neutral genetic markers [45] and in their response to herbivore attack, particularly the volatiles they emit and their degree of induced defense upon herbivore feeding [46]; the variation within populations is as great as the variation between populations in these plant traits [28, 47]. G. pallens and G. punctipes respond to specific herbivore-induced volatiles of N. attenuata [22, 26], but it is not known how quickly or how well they learn to respond to the differing volatile profiles of plants in native populations. The phenomena of associative susceptibility and associative resistance, in which plant traits increase or decrease the herbivore loads of neighboring plants, are widespread in ecological communities [48], and associative susceptibility or resistance due to neighbor volatile emission may contribute to the site-by-site variation in Geocoris predation activity.
4.3. G. pallens Prey Choices May Be Learned Anew with Each Generation but Did Not Differ between Nymphs and Adults Tested Simultaneously
We tested field-collected G. pallens adults and nymphs from the same wild population over a season (Table 1) for their inclination to eat M. sexta eggs in no-choice assays (Figure 6). Based on known emergence and generation times for these insects, the May collections must have been from the first generation of eggs laid in 2009; we found that 61–81% of the individuals consumed M. sexta eggs in no-choice assays. From these collections, we can conclude that there is no significant difference between nymphs and adults in their propensity to eat M. sexta eggs, because the May 15th collection comprised 27% adults, whereas the May 16th collection comprised 71% adults (Table 1), and the two collections did not significantly differ in their tendency to eat M. sexta eggs (Figure 6(a)). Furthermore, different developmental stages within the May 15th collection also did not differ significantly in their tendency to eat M. sexta eggs (Figure 6(c)), although in the case of 2nd instar nymphs this was likely due to low replicate numbers. It should be noted that the composition of nymphs in collections may not accurately reflect the composition of the sampled G. pallens population: later nymphal stages and adults are probably overrepresented, because they are easier to see and catch.
The June 1st collection made two weeks later comprised 68% adults; in this collection, the nymphs were certainly the offspring of the May collections, and the adults may have been a mix of May-nymphs and May-offspring. This collection was transported to the lab in Jena for testing, and although they were allowed to adapt for 24–48 h, transport and laboratory conditions may have negatively affected feeding rates. Only 32% of these G. pallens fed on M. sexta eggs in the same no-choice assay. A final collection made two weeks later (June 15th) and also tested after transport to the laboratory comprised 95% adults, all of which were likely offspring of the May collections. In the June 15th collection, the number of egg feeders had increased slightly to 37%.
The May and June generations may have experienced separate Manduca spp. oviposition events which influenced their propensity to eat M. sexta eggs. There are typically two Manduca spp. oviposition peaks in the Lytle area and surroundings: one at the end of April to the first week of May (mainly on D. wrightii) and one in the middle of June (D. wrightii and N. attenuata). (Manduca spp. oviposition, however, occurs to a minor degree also between those two peaks.) Given that lepidopteran eggs are more nutritious for Geocoris spp. than aphids [5, 38] and likely other hemipteran prey such as T. notatus, it is interesting that G. pallens does not always readily eat M. sexta eggs but might need to learn to prey on them. G. punctipes seem to be strongly influenced by prey mobility rather than nutritional quality [38]; a preference for mobile prey might explain why G. pallens does not always seem to recognize M. sexta eggs as prey [23, 30]. Perhaps G. pallens must first learn to associate Manduca spp. eggs with feeding larvae and the associated herbivore-induced plant volatiles.
4.4. G. pallens May Scavenge from Dying Plants
We found G. pallens individuals to be significantly (5-fold) more abundant on dying, wilting N. attenuata plants than on nearby healthy plants. In fact, in a 2012 plant disease outbreak, G. pallens were not found on healthy plants unless they were next to wilting plants. This could be due to greater foraging success for G. pallens when hunting insects fleeing from dying plants, or it might be that dying plants are a better nutritional supplement to G. pallens’s insect diet, which may also be more nutritious when herbivores feed on dying plants. It has long been known that nutrients, including amino acids, are mobilized from water-stressed and senescing plant tissue, although some reports indicate that the lack of turgor pressure in wilting plants reduces sap flow so that phloem feeders may not be able to access these increased resources [49]. If G. pallens are feeding directly from cells or the apoplastic space, they may be able to harvest the products of cellular senescence and degradation from dying plants. A more speculative hypothesis would be that G. pallens itself transfers disease when feeding on plant tissue, as is known for herbivores (e.g., [42]). If T. notatus is more fecund on diseased plants, as a consequence of impaired host plant resistance, Geocoris could benefit from spreading disease and thus increasing the current population of its prey. However, herbivores likely spread plant disease more efficiently than omnivores such as Geocoris spp.
4.5. G. pallens Feeds on Seeds and Leaves
Although it has been reported that Geocoris spp. feed from vegetative plant tissue, all prior reports indicated that Geocoris individuals could not survive any better on vegetative tissue than on water alone [4]. Here, we show that mortality of G. pallens individuals offered water and living leaf tissue on planta is 50% over 8 d, but for individuals given only water is 100%. The discrepancy between our results and previous results could be due to the appropriateness of the plant tissue for the particular Geocoris spp.; differences in nutritional quality of cut leaves [4] versus leaves left on a plant; or even the increased importance of relative humidity provided by leaf cover in a desert environment, which is unlikely to be a factor in a laboratory. We have seen G. pallens individuals drinking from N. attenuata leaves in wild populations without leaving visible leaf damage (S. Allmann and M. C. Schuman, observation).
5. Conclusions
Wild populations of G. pallens change plant hosts and adapt to changes in host quality and herbivore prey abundance over their lifetimes. G. punctipes, though co-occurring with G. pallens, uses different host plant and herbivore resources than does G. pallens in southwestern Utah. Geocoris spp. are phenotypically plastic generalists which, though omnivorous, benefit plants by reducing their herbivore loads. These insects have become a model system to study the development of plant-herbivore-predator tritrophic interactions, and how predators learn plant cues, and have great promise as effective biological control agents for agriculture.
Acknowledgments
The authors would like to thank A. Shillabeer and Merit Motion Pictures for the film stills of G. pallens (Figure 4), S. Allmann for help with M. sexta feeding screens (Figure 6), the members of the 2009 Utah field research crew for help with Geocoris spp. collections, and Brigham Young University for the use of Lytle Preserve. This work was funded by the International Max Planck Research Schools (MCS), the Max Planck Society (all), and ERC advanced to ITB, number 293926 (all).
References
- [1].Tamaki G, Weeks RE. Biology and Ecology of Two Predators, Geocoris pallens Stål and G. bullatus (Say) Technical Bulletin of the US Department of Agriculture. 1972;1446 [Google Scholar]
- [2].Stone A. Plant feeding by a predacious insect, Geocoris punctipes. Journal of Economic Entomology. 1970;63(6):1911–1915. [Google Scholar]
- [3].Lockwood S. The relation of weeds to insect pests. California Department of Agriculture Monthly Bulletin. 1933;22(6):279–282. [Google Scholar]
- [4].York GT. Food studies of Geocoris spp., predators of the beet leafhopper. Journal of Economic Entomology. 1944;37(1):25–29. [Google Scholar]
- [5].Eubanks MD, Denno RF. The ecological consequences of variation in plants and prey for an omnivorous insect. Ecology. 1999;80(4):1253–1266. [Google Scholar]
- [6].Tillman PG, Mullinix BG. Effect of prey species on plant feeding behavior by the big-eyed bug, Geocoris punctipes (Say) (Heteroptera: Geocoridae), on cotton. Environmental Entomology. 2003;32(6):1399–1403. [Google Scholar]
- [7].Sweet MH. The seed bugs: a contribution to the feeding habits of the Lygaeidae (Hemiptera: Heteroptera) Annals of the Entomological Society of America. 1960;53(3):317–321. [Google Scholar]
- [8].Orphianides GM, Gonzalez D, Bartlett BR. Identification and evaluation of pink bollworm predators in southern California. Journal of Economic Entomology. 1971;64(2):421–425. [Google Scholar]
- [9].Chiravathanapong S, Pitre HN. Effects of Heliothis virescens larval size on predation by Geocoris punctipes. The Florida Entomologist. 1980;63(1):146–151. [Google Scholar]
- [10].Kapadia MN, Puri SN. Biology and comparative predation efficacy of three heteropteran species recorded as predators of Bemisia tabaci in Maharashtra. Entomophaga. 1991;36(4):555–559. [Google Scholar]
- [11].Medal JC, Mueller AJ, Kring TJ, Gbur EE. Predation of Spissistilus festinus (Homoptera: Membracidae) nymphs by hemipteran predators in the presence of alternative prey. Florida Entomologist. 1997;80(4):451–456. [Google Scholar]
- [12].McCutcheon GS. Consumption of tobacco budworm (Lepidoptera: Noctuidae) by Hooded Beetle (Coleoptera: Anthicidae) and bigeyed bug (Hemiptera: Lygaeidae) Journal of Agricultural and Urban Entomology. 2002;19(1):55–61. [Google Scholar]
- [13].Rondon SI, Cantliffe DJ, Price JF. The feeding behavior of the bigeyed bug, minute pirate bug, and pink spotted lady beetle relative to main strawberry pests. Environmental Entomology. 2004;33(4):1014–1019. [Google Scholar]
- [14].Hagler J. Comparative studies of predation among feral, commercially-purchased, and laboratory-reared predators. BioControl. 2009;54(3):351–361. [Google Scholar]
- [15].Bell KO, Whitcomb WH. Field studies on egg predators of the bollworm, Heliothis zea (Boddie) The Florida Entomologist. 1964;47(3):171–180. [Google Scholar]
- [16].Whitcomb WH, Bell K. Predacious insects, spiders, and mites of Arkansas cotton fields. Arkansas Experiment Station Bulletin. 1964;690:1–84. [Google Scholar]
- [17].Lindgren PD, Ridgway RL, Jones SL. Consumption by several common arthropod predators of eggs and larvae of two Heliothis species that attack cotton. Annals of the Entomological Society of America. 1968;61(3):613–618. [Google Scholar]
- [18].Eubanks MD, Denno RF. Host plants mediate omnivore-herbivore interactions and influence prey suppression. Ecology. 2000;81(4):936–947. [Google Scholar]
- [19].Braman SK, Duncan RR, Hanna WW, Engelke MC. Arthropod predator occurrence and performance of Geocoris uliginosus (Say) on pest-resistant and susceptible turfgrasses. Environmental Entomology. 2003;32(4):907–914. [Google Scholar]
- [20].Naranjo SE, Ellsworth PC. Mortality dynamics and population regulation in Bemisia tabaci. Entomologia Experimentalis et Applicata. 2005;116(2):93–108. [Google Scholar]
- [21].Naranjo SE, Ellsworth PC. The contribution of conservation biological control to integrated control of Bemisia tabaci in cotton. Biological Control. 2009;51(3):458–470. [Google Scholar]
- [22].Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291(5511):2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- [23].Kessler A, Baldwin IT. Herbivore-induced plant vaccination. Part I. The orchestration of plant defenses in nature and their fitness consequences in the wild tobacco Nicotiana attenuata. Plant Journal. 2004;38(4):639–649. doi: 10.1111/j.1365-313X.2004.02076.x. [DOI] [PubMed] [Google Scholar]
- [24].Gassmann AJ, Hare JD. Indirect cost of a defensive trait: variation in trichome type affects the natural enemies of herbivorous insects on Datura wrightii. Oecologia. 2005;144(1):62–71. doi: 10.1007/s00442-005-0038-z. [DOI] [PubMed] [Google Scholar]
- [25].Karban R. Damage to sagebrush attracts predators but this does not reduce herbivory. Entomologia Experimentalis et Applicata. 2007;125(1):71–80. [Google Scholar]
- [26].Halitschke R, Stenberg JA, Kessler D, Kessler A, Baldwin IT. Shared signals: ‘alarm calls’ from plants increase apparency to herbivores and their enemies in nature. Ecology Letters. 2008;11(1):24–34. doi: 10.1111/j.1461-0248.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- [27].Skibbe M, Qu N, Galis I, Baldwin IT. Induced plant defenses in the natural environment: Nicotiana attenuata WRKY3 and WRKY6 coordinate responses to herbivory. Plant Cell. 2008;20(7):1984–2000. doi: 10.1105/tpc.108.058594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Steppuhn A, Schuman MC, Baldwin IT. Silencing jasmonate signalling and jasmonate-mediated defences reveals different survival strategies between two Nicotiana attenuata accessions. Molecular Ecology. 2008;17(16):3717–3732. doi: 10.1111/j.1365-294X.2008.03862.x. [DOI] [PubMed] [Google Scholar]
- [29].Meldau S, Wu J, Baldwin IT. Silencing two herbivory-activated MAP kinases, SIPK and WIPK, does not increase Nicotiana attenuata’s susceptibility to herbivores in the glass-house and in nature. New Phytologist. 2009;181(1):161–173. doi: 10.1111/j.1469-8137.2008.02645.x. [DOI] [PubMed] [Google Scholar]
- [30].Allmann S, Baldwin IT. Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science. 2010;329(5995):1075–1078. doi: 10.1126/science.1191634. [DOI] [PubMed] [Google Scholar]
- [31].Schuman MC, Barthel K, Baldwin IT. Herbivory-induced volatiles function as defenses increasing fitness of the native plant Nicotiana attenuata in nature. eLife. 2012;1(1) doi: 10.7554/eLife.00007. Article ID e00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Oida H, Kadono F. Prey consumption by Geocoris varius and G. proteus (Heteroptera: Geocoridae) provided with horticultural major pests in greenhouses. Japanese Journal of Applied Entomology and Zoology. 2011;55(4):217–225. [Google Scholar]
- [33].Joseph SV, Braman SK. Influence of plant parameters on occurrence and abundance of arthropods in residential turfgrass. Journal of Economic Entomology. 2009;102(3):1116–1122. doi: 10.1603/029.102.0333. [DOI] [PubMed] [Google Scholar]
- [34].Hunter MD. Trophic promiscuity, intraguild predation and the problem of omnivores. Agricultural and Forest Entomology. 2009;11(2):125–131. [Google Scholar]
- [35].Dunbar DM, Bacon OG. Influence of temperature on development and reproduction of Geocoris atricolor, G. pallens, and G. punctipes (Heteroptera: Lygaeidae) from California. Environmental Entomology. 1972;1(5):596–599. [Google Scholar]
- [36].Mansfield S, Scholz B, Armitage S, Johnson ML. Effects of diet, temperature and photoperiod on development and survival of the bigeyed bug, Geocoris lubra. BioControl. 2007;52(1):63–74. [Google Scholar]
- [37].Champlain RA, Sholdt LL. Life history of Geocoris tipespunc (Hemiptera: Lygeidae) in the laboratory. Annals of the Entomological Society of America. 1967;60(5):881–885. [Google Scholar]
- [38].Eubanks MD, Denno RF. Health food versus fast food: the effects of prey quality and mobility on prey selection by a generalist predator and indirect interactions among prey species. Ecological Entomology. 2000;25(2):140–146. [Google Scholar]
- [39].Baldwin IT. Jasmonate-induced responses are costly but benefit plants under attack in native populations. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(14):8113–8118. doi: 10.1073/pnas.95.14.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Steppuhn A, Gase K, Krock B, Halitschke R, Baldwin IT. Nicotine’s defensive function in nature. PLoS Biology. 2004;2(8) doi: 10.1371/journal.pbio.0020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kessler A, Halitschke R, Baldwin IT. Silencing the jasmonate cascade: induced plant defenses and insect populations. Science. 2004;305(5684):665–668. doi: 10.1126/science.1096931. [DOI] [PubMed] [Google Scholar]
- [42].Kallenbach M, Bonaventure G, Gilardoni PA, Wissgott A, Baldwin IT. PNAS Plus: Empoasca leafhoppers attack wild tobacco plants in a jasmonate-dependent manner and identify jasmonate mutants in natural populations. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(24):E1548–E1557. doi: 10.1073/pnas.1200363109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kessler D, Gase K, Baldwin IT. Field experiments with transformed plants reveal the sense of floral scents. Science. 2008;321(5893):1200–1202. doi: 10.1126/science.1160072. [DOI] [PubMed] [Google Scholar]
- [44].McDonald JH. Handbook of Biological Statistics. 2nd edition Sparky House Publishing; Baltimore, Md, USA; 2009. [Google Scholar]
- [45].Bahulikar RA, Stanculescu D, Preston CA, Baldwin IT. ISSR and AFLP analysis of the temporal and spatial population structure of the post-fire annual, Nicotiana attenuata, in SW Utah. BMC Ecology. 2004;4 doi: 10.1186/1472-6785-4-12. Article 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Schuman MC, Heinzel N, Gaquerel E, Svatos A, Baldwin IT. Polymorphism in jasmonate signaling partially accounts for the variety of volatiles produced by Nicotiana attenuata plants in a native population. New Phytologist. 2009;183(4):1134–1148. doi: 10.1111/j.1469-8137.2009.02894.x. [DOI] [PubMed] [Google Scholar]
- [47].Wu J, Hettenhausen C, Schuman MC, Baldwin IT. A comparison of two Nicotiana attenuata accessions reveals large differences in signaling induced by oral secretions of the specialist herbivore Manduca sexta. Plant Physiology. 2008;146(3):927–939. doi: 10.1104/pp.107.114785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Barbosa P, Hines J, Kaplan I, Martinson H, Szczepaniec A, Szendrei Z. Associational resistance and associational susceptibility: having right or wrong neighbors. Annual Review of Ecology, Evolution, and Systematics. 2009;40:1–20. [Google Scholar]
- [49].Huberty AF, Denno RF. Plant water stress and its consequences for herbivorous insects: a new synthesis. Ecology. 2004;85(5):1383–1398. [Google Scholar]