Abstract
Ultraviolet radiation (UVR) is a major risk factor for melanoma development, but it has been unclear exactly how UVR leads to melanomagenesis. In a recent publication in Nature, Viros et al. identify TP53/Trp53 as a UVR-target gene in melanoma and show that UVR-induced TP53/Trp53 mutations accelerate BRAF(V600E)-driven melanomagenesis.
Melanoma is the deadliest skin cancer, and its incidence has relentlessly increased over recent decades. According to the American Cancer Society's estimates for melanoma in the United States for 2014, about 76 100 new melanomas will be diagnosed and about 9 710 people are expected to die from melanoma. It is well known that UVR is the major environmental factor contributing to melanomagenesis1. This suggests that there is at least a component of melanoma risk which may be preventable through UVR protective strategies — an issue of immense public health importance due to the availability of sunblocks and sun-safe behaviors. Importantly, while many studies have been conducted to elucidate the link between UVR and melanoma, the precise molecular mechanism(s) by which UVR triggers melanoma formation have remained incompletely understood.
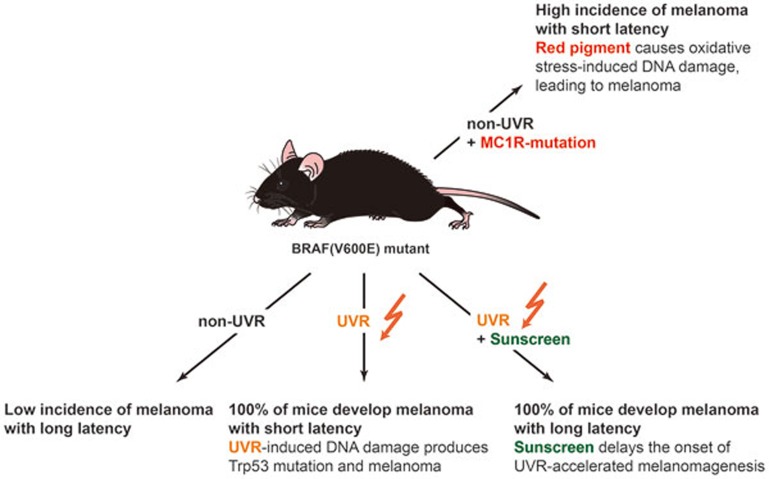
Recently, a powerful UVR-induced skin inflammatory response has been shown to provoke metastasis of melanoma2 and the presence of UV signature mutations has also been reported throughout the melanoma exome, including recurrent melanoma genes such as RAC1, PPP6C, and STK193,4. Previous mouse models for UVR-induced melanoma revealed that UVR-induced inflammation promoted melanomagenesis in neonatal mice5,6,7. These studies underlined UVR's significant contribution to melanoma formation. In a recent study published in Nature, Viros et al.8 address the role of UVR in previously established BRAF(V600E)-expressing melanocytes in vivo, and demonstrate that significantly accelerated melanoma formation often associated with mutations in TP53/Trp53. To mimic both somatic mutation acquisition and mild sunburn in humans, BRAF(V600E) was expressed at physiological levels in adult mice which were subsequently exposed to repeated low doses of UVR. In addition, certain mice were partially covered with UVR-proof cloth or topically treated with SunSense Milk Sunscreen SPF50 (2.2 mg/cm2) 30 min before UVR exposure, to assess the impact of these protective strategies.
UVR was seen to significantly accelerate melanoma formation in mice whose melanocytes express BRAF(V600E), but not in BRAF wild-type mice (which unlike BRAF(V600E)-expressing mice do not develop long latency melanomas independently of UVR). Application of UVR-proof cloth or sunscreen delayed the onset of UVR-driven melanoma and partially prevented acceleration of BRAF(V600E)-driven melanomagenesis by UVR, and sunscreen-protected UVR-exposed BRAF(V600E) mice developed a reduced number of melanomas compared with unprotected UVR-exposed BRAF(V600E) mice (Figure 1). More somatic single nucleotide variants and a significantly higher proportion of C-to-T transitions at the 3′ end of pyrimidine dimers were observed in UVR-exposed melanomas, providing direct evidence of UVR-induced DNA damage. In addition, Trp53 mutations (H39Y, S124F, R245C, R270C, C272G) were detected in UVR-exposed BRAF(V600E) mouse melanomas, indicating a direct role of UVR in the induction of Trp53 mutations in melanoma. The mutated corresponding residues (S127F/S124F, R248C/R245C, R273C/R270C, C275G/C272G) were also identified in TP53 mutations in human melanoma, suggesting that TP53 mutations are linked to evidence of UVR-induced DNA damage in human melanoma. These results are consistent with previous reports that p53 deletion accelerates BRAF(V600E)-driven melanomagenesis both in mice9 and in zebrafish10, but demonstrate the ability of UVR to inflict UV signature mutations within the gene as has been widely observed in non-melanoma skin cancers and also in human melanomas.
Figure 1.
A diagram depicting feasible routes of BRAF(V600E)-driven melanomagenesis.
This elegant study by Viros et al. clearly helps to establish key roles of UVR in melanomagenesis, and further validates the functional importance of TP53 as a UVR-targeted tumor suppressor gene in a fraction of melanomas. The study also raises several intriguing questions worthy of follow-up analysis. For example, through which mechanism(s) did sunscreen or sunshielding delay but not prevent UVR-induced melanoma? Induction of cutaneous inflammatory changes that are less anatomically restricted to UV irradiated fields, would seem to be an attractive mechanism. This may help to explain the known risk of melanoma in both sun-exposed and less-exposed skin of lightly pigmented people. It is also valuable to better understand the role of UVB vs UVA wavelengths in melanomagenesis. Mechanistically, these distinct regions of the UV spectrum inflict largely distinctive chemical alterations on the genome. Efforts to block UVA as well as UVB in commercial sunscreen products are currently being promoted by the US Food and Drug Administration, a welcome improvement to sun protection strategies. Still, the precise role(s) of UVA in melanomagenesis remain incompletely understood and may involve both cell-autonomous and non-cell-autonomous targets. In addition to the acceleration of BRAF(V600E)-driven melanoma formation by UVR, red pigment (pheomelanin) has also been observed to accelerate BRAF(V600E)-driven melanomagenesis even in the absence of UVR11. Pheomelanin has been identified as an intrinsic risk factor for melanoma with the red pigment itself producing reactive oxygen species that cause DNA damage in the skin, and consequently promote melanomagenesis independently of UVR. UVR likely exacerbates red pigment-induced BRAF(V600E)-driven melanoma, and still remains as a major contributor to melanomagenesis. Therefore, along with UV shielding by sunscreens, further preventative strategies should be investigated to diminish UVR-independent melanoma risk mechanisms.
Viros et al. provide intriguing answers to several controversial questions regarding melanomagenesis: Does UVR really trigger melanoma? And can sunscreen actually prevent melanoma? The studies by Viros et al. provide experimental evidence for acceleration of BRAF(V600E)-driven melanoma by UVR-induced TP53/Trp53 mutation and demonstrate that sunscreen delayed but did not completely block UVR-driven melanoma. The current study clearly shows that UVR boosts melanoma and sunscreens may provide partial UVR protection against melanoma — evidence which matches human epidemiologic data. Nevertheless, to protect the public from melanoma, Viros et al. advise that sunscreen should be utilized in combination with additional sun avoidance strategies. In addition, measures that may prevent UV-independent melanoma formation will require additional research and may also be needed in order to optimally battle the incidence of this life-threatening malignancy.
References
- Garibyan L, Fisher DE. Curr Oncol Rep. 2010. pp. 319–326. [DOI] [PubMed]
- Bald T, Quast T, Landsberg J, et al. Nature. 2014. pp. 109–113. [DOI] [PubMed]
- Hodis E, Watson IR, Kryukov GV, et al. Cell. 2012. pp. 251–263. [DOI] [PMC free article] [PubMed]
- Krauthammer M, Kong Y, Ha BH, et al. Nat Genet. 2012. pp. 1006–1014. [DOI] [PMC free article] [PubMed]
- Noonan FP, Recio JA, Takayama H, et al. Nature. 2001. pp. 271–272. [DOI] [PubMed]
- Luo C, Sheng J, Hu MG, et al. Cancer Res. 2013. pp. 4337–4348. [DOI] [PMC free article] [PubMed]
- Zaidi MR, Davis S, Noonan FP, et al. Nature. 2011. pp. 548–553. [DOI] [PMC free article] [PubMed]
- Viros A, Sanchez-Laorden B, Pedersen M, et al. Nature. 2014. pp. 478–482. [DOI] [PMC free article] [PubMed]
- Goel VK, Ibrahim N, Jiang G, et al. Oncogene. 2009. pp. 2289–2298. [DOI] [PMC free article] [PubMed]
- Patton EE, Widlund HR, Kutok JL, et al. Curr Biol. 2005. pp. 249–254. [DOI] [PubMed]
- Mitra D, Luo X, Morgan A, et al. Nature. 2012. pp. 449–453. [DOI] [PMC free article] [PubMed]