Abstract
Past studies in songbirds highlight a central role for the medial preoptic nucleus (mPOA) in context-appropriate vocal communication. During the breeding season, male songbirds sing primarily to attract females (sexually-motivated song) and to repel competitors (agonistically-motivated song). Past data link dopamine and D1 dopamine receptors in the mPOA to sexually-motivated but not agonistically-motivated song; however, direct effects of dopamine receptor manipulations in the mPOA on song have not been experimentally tested. Here we tested the hypothesis that D1 receptor stimulation in the mPOA selectively influences sexually-motivated male song and the possibility that the effects of D1 agonism differ at low and high doses. In a first study, breeding condition male European starlings received infusions of saline or a single dose of the D1 receptor agonist SKF 38393 on separate test days into mPOA or hypothalamic control areas. Stimulation of D1 receptors in mPOA triggered sexually-motivated but not agonistically-motivated song. A second study showed inverted-U shaped dose-response effects of the agonist such that low levels of sexually-motivated song were observed at low and high levels of D1 receptor activation. A third study showed that effects of the D1 agonist were blocked by the D1 receptor antagonist SCH 23390. These findings suggest that an optimal level of D1 dopamine receptor stimulation in mPOA is needed to facilitate sexually-motivated vocal production. The results support a central, context-specific role for the mPOA in vocal communication and more broadly demonstrate a complex, modulatory influence of D1 receptors in mPOA on sexually-motivated behavior.
Keywords: Sturnus vulgaris, social context, communication, birdsong, motivation
Vocal signals mediate social interactions across vertebrates (Bradbury & Vehrencamp, 2011), yet relatively little is known about the neural regulation of the motivation to communicate or how the brain adjusts communication to match a specific social context. Songbirds provide a unique and highly tractable system for addressing these topics. Male songbirds are highly motivated to communicate. They produce songs and calls in multiple social contexts, with songs functioning primarily to attract females (sexually-motivated) or repel male competitors (agonistically-motivated) (Catchpole & Slater, 2008).
Across vertebrates, the medial preoptic nucleus (mPOA) is central to the regulation of sexually-motivated male behaviors (Crews, 2005; Hull & Dominguez, 2006; Balthazart & Ball, 2007; Stolzenberg & Numan, 2011). In songbirds, studies show the mPOA to stimulate sexually-motivated song, to inhibit nonsexually-relevant forms of song, and so far to play no role in agonistically-motivated song (Riters & Ball, 1999; Riters et al., 2000; Riters et al., 2004; Heimovics & Riters, 2005; Alger & Riters, 2006; Alger et al., 2009; Riters, 2010; 2011; 2012). The mPOA contains dopamine, dopamine synthetic enzymes, and receptors (Heimovics & Riters, 2008; Heimovics et al., 2009; Kubikova et al., 2010), and studies link sexually-motivated singing behavior to dopamine markers in mPOA (i.e., tyrosine hydroxylase and D1 dopamine receptors). Furthermore, data suggest that D1 receptors in mPOA may influence sexually- but not agonistically-motivated song (Heimovics & Riters, 2008; Heimovics et al., 2009).
It is generally accepted that stimulation of D1 receptors in mPOA facilitates male sexual behaviors (Markowski et al., 1994; Stolzenberg & Numan, 2011); however, in some studies D1 agonists in mPOA inhibit (e.g., (Kleitz-Nelson et al., 2010a)) or appear to have no effect (e.g., (Hull et al., 1989)) on such behaviors. These inconsistencies have lead researchers to question the importance (or existence) of a role for D1 receptors in mPOA in male sexual motivation (reviewed in (Paredes & Agmo, 2004)). In songbirds, systemic pharmacological manipulations reveal a stimulatory role for D1 receptors in sexually-motivated song (Schroeder & Riters, 2006; Rauceo et al., 2008). Whether this effect is mediated by receptors in the mPOA is not known, and correlations linking dopamine markers in the mPOA to song (i.e., tyrosine hydroxylase and D1 dopamine receptors described in the paragraph above) are negative and difficult to interpret. For example the negative correlation found between sexually-motivated song and D1 dopamine receptor densities in mPOA may be interpreted as evidence that D1 receptors inhibit singing behavior or alternatively that high levels of dopamine release in mPOA stimulate song and also cause D1 receptors to down-regulate (for further discussion of interpretational issues see (Heimovics & Riters, 2008; Heimovics et al., 2009)). Site-specific manipulations are now needed to precisely define the role of D1 receptors in mPOA in song.
D1 receptor stimulation can have excitatory or inhibitory effects that depend on current receptor occupancy (reviewed in (Williams & Castner, 2006)). Dopamine acting at D1 receptors in the prefrontal cortex induces dose-dependent, inverted-U shaped effects, such that both low and high levels of D1 receptor stimulation inhibit behavior; whereas, intermediate levels of stimulation facilitate behavior (reviewed in (Seamans & Yang, 2004; Williams & Castner, 2006; Berridge & Arnsten, 2012)). This suggests that behavior is facilitated by an optimal level of D1 receptor activity. Whether this is the case for the mPOA, sexually-motivated singing behavior or sexually-motivated behaviors in general, is not known. The present set of experiments was designed to explore the extent to which D1 receptor stimulation in mPOA selectively and dose-dependently modifies sexually-motivated song.
Experiment 1: Effects of a single dose of D1 agonist in mPOA on sexually- and agonistically-motivated singing behavior
Materials and Methods
Animals and housing
Procedures followed a protocol approved by the University of Wisconsin Institutional Animal Care and Use Committee and adhered to the National Institutes of Health Guidelines for the use of animals in research. Nineteen photosensitive (Dawson et al., 2001) adult experimental male, 8 stimulus male, and 8 stimulus female European starlings were trapped and housed in single sex groups of 5 in cages (47cm H × 47cm W × 91cm L) on an 8h light: 16h dark photoperiod. All birds were in captivity for at least one year prior to the start of the experiment.
Hormone treatment
The endocrine state of starlings is sensitive to day length such that exposure to more than 11 h light per day (as occurs naturally in the spring breeding season) first stimulates breeding physiology and behavior (including sexually-motivated song) but then induces a state of photorefractoriness (Dawson & Goldsmith, 1983). Photorefractoriness is characterized by regressed gonads and an absence of courtship singing behavior. To avoid inducing photorefractoriness, males in this study were housed on an 11h light: 13h dark photoperiod. Males on 11 h light display little courtship behavior; however, treating them with testosterone induces the full suite of breeding season typical sexual behaviors, including high rates of song, without inducing photorefractoriness (e.g., (Heimovics & Riters, 2008; Heimovics et al., 2009)). Therefore all males in the present study were housed on 11h light: 13h dark and received implants of testosterone. Under isoflurane anesthesia, all males were implanted subcutaneously over the breast muscle with 2, silastic implants (14mm length, i.d. = 1.47mm, o.d. = 1.96mm; Dow Corning, Midland, MI, USA) filled with testosterone proprionate (Sigma-Aldrich, T1500). Stimulus females received 1 implant of 17β-estradiol (Sigma-Aldrich, T1875; 18mm length). Groups of approximately 10 males were then housed in indoor aviaries for screening and stimulus males and females returned to single sex cages.
Screening procedure
In our laboratory we find that there are some males that never sing during our observation periods (over periods of several months). Because the site-specific pharmacological manipulations reported here are labor and time intensive, and because we are interested specifically in the effects of dopamine receptor manipulations on singing behavior, we screened males (as in past studies (e.g., (Schroeder & Riters, 2006)) to ensure that they exhibited at least some level of consistent singing. Each experimental bird spent between 8 and 10 days (mean = 9.6, sd = 1.12) in a screening aviary (3.5 × 2.25 × 2m). The screening aviary contained perches, 4 nest boxes, food, and water. Approximately 4 days after being placed into an aviary, behavioral responses of males to stimulus birds were observed for 3 – 4 days (mean = 3.6, sd = 0.51). Each day, a female or male was released into the aviary (15min), followed by a male or female (15min), whichever had not been introduced the first time. Males that sang to the female on at least 3 screening days were included in the experiment.
Singing males were placed individually into cages (61cm × 91cm × 56cm) located inside a larger aviary (one cage / aviary (same dimensions as the screening aviary)). Cages contained a nest box, food, and water. Experimental males habituated for 3 days. On the fourth day a novel stimulus female was released into the room, followed by a stimulus male, for 15 min. This was continued daily until males resumed singing (i.e., sang at least once when presented with a male or female), which occurred for all birds within 3 days. For this study we housed the experimental male in a smaller cage within the aviary because in past studies we found this arrangement to facilitate singing behavior (Riters & Ball, 1999; Schroeder & Riters, 2006).
Cannula surgery
Experimental males were anesthetized with isoflurane and placed into a stereotaxic apparatus (Kopf, Tujunga, CA, USA) with the beak approximately 45° below the horizontal plane of the ear bars. The anterior-posterior target coordinate was 0.8mm posterior to zero (taken from the ear bar). A 26 gauge stainless-steel indwelling permanent cannula guide (PlasticsOne, C315G-5UP/SP, Roanoke, VA, USA) was inserted at a 2° angle 0.6mm to the right or left of the midvein zero (subjects were alternated right and left). The ventral target was -5.5mm from the skull. Misses were included as controls. Cannulae were secured using 2 screws and dental cement (Plastics One, Roanoke, VA, USA). Males were returned to test cages.
Drug injection procedures
Four days after surgery, each male was anesthetized with isoflurane, and given a single mock injection, which involved insertion of a cannula into the guide for 8 min to simulate test day injection conditions. The male was placed back into his cage and 15 min after injection, a stimulus female was released into the test room for 15 min. The female was removed and 5 min later a stimulus male was released for 15 min.
The next day, 4 consecutive days of experimental testing began. On alternating days males received either 0.3 μl vehicle (0.9% sterile saline) or the D1 receptor agonist SKF 38393 (0.20 μg; Sigma-Aldrich, S102). This dose was selected based on pilot studies (Alger et al., 2007) which demonstrated that a dose of 0.20 μg affected song behavior without affecting motor activity or inducing stereotypic behavior. Treatment order was counterbalanced across males.
Each test day, males were anesthetized with isoflurane and infused over a 3 min period with drug or vehicle as in (Pawlisch et al., 2011) using a cannula that extended 2mm past the end of the guide. The injection cannula remained in place for 5 min after injection. Males were returned to home cages in the aviary and became alert and active within 2 min. A researcher unaware of the drug treatments or sites of injection observed males from behind a one-way window beginning 15 min after males returned to their home cages.
Behavioral testing
In starlings, the same song that is attractive to females is avoided by males (Mountjoy & Lemon, 1991). Unlike some songbird species, male starlings do not accompany courtship song with distinct postural displays; therefore, differences in song structure or singing posture could not be used to distinguish between differences in the function of song. We assumed here that a larger proportion of song induced by the presence of a female is likely to be sexually-motivated and that a larger proportion of song induced by the presence of an unfamiliar male is likely to be agonistically-motivated (as in (Eens et al., 1993)). Therefore, to examine effects of D1 receptor manipulations on sexually- and agonistically-motivated song, each test day a handful of green nest materials (i.e., green leaves and grasses) was placed into an experimental male's test cage, and a stimulus female or male (counterbalanced across treatments) was released into the area outside the test cage. The experimental males were then observed for 15 min. Stimulus males and females were selected randomly from birds housed in single sex cages in our animal facilities, with the qualification that no experimental male ever saw the same stimulus bird more than once. Other than noting that none of the stimulus birds sang during testing, we did not record stimulus bird behavior. It is possible that differences in the stimulus birds influenced results; however, despite this potential source of variability, results were consistent and significant (see Results below). Five min after removal of the stimulus bird, a second stimulus bird (male or female, whichever had not been introduced during the first 15 min) was released and experimental males were again observed for 15 min. The orders of stimulus presentation and drug treatment conditions were counterbalanced. Behaviors were observed in real time. Measures included the duration of time spent singing, the latency to first song, and the number of times males gathered nest materials and entered a nest box. Bouts of feeding, beak wiping, and preening (with bouts separated by 2 sec) were also recorded.
Histology
After the last test, Chicago Blue dye (0.3 μl, 10% solution; Fisher Scientific, 2610-05-1) was injected into cannula and birds euthanized. Brains were collected, frozen on dry ice, stored at -80° C, cut into 50 μm coronal sections using a cryostat and thaw mounted onto microscope slides. Alternating sections were Nissl stained to aid in the identification of landmarks and unstained to enhance visibility of the blue dye. Cannula placement was determined using a microscope by an observer blind to experimental results. Males for which the cannula tip was centered within the mPOA were assigned to the experimental group, and males for which the cannula tip was centered outside the mPOA were assigned to the control group.
Analyses
Behaviors were summed for the two saline test days and the two drug test days. A multivariate analysis of variance (MANOVA) was run with duration of time spent singing (in secs) and the latency to first song (in secs) as repeated measures dependent variables, treatment (saline and SKF 38393) and social stimulus (female and male) as repeated within subjects variables, and the site of the cannula tip (outside or in mPOA) as a categorical between subjects variable. To examine non-specific behaviors, a second MANOVA was run that was identical to the first except bouts of preening, beak wiping, and feeding were entered as repeated measures dependent variables. Assumptions of normality and homogeneity of variance were tested using Lilliefors tests and Levene's tests, respectively. For both analyses Levene's tests revealed that data violated assumptions of homogeneity of variance. Square root transformation (SQRT (x+ 1)) corrected this problem.
Significant MANOVAs were followed by Fisher's posthoc LSD tests to compare males treated with saline versus SKF with 1) the cannula tip located outside mPOA with a female present, 2) the tip located outside mPOA with a male present, 3) the tip located inside mPOA with a female present, and 4) the tip located inside mPOA with a male present. Comparisons were also made between birds treated with 5) saline and 6) SKF with cannula located in versus outside mPOA when a female was present and when a male was present.
Only 3 of the 7 birds for which the cannula was centered outside mPOA consistently showed nesting behavior, and variability in this group was extreme rendering parametric statistical analysis inappropriate. Thus, for each group (cannula outside mPOA, cannula inside mPOA) 2 separate McNemar Chi-square tests (one to compare behavior in saline versus SKF 38393-treated males presented with a female and one to compare behavior in saline versus SKF 38393-treated males presented with a male) were run to analyze the numbers of males treated with saline versus drug demonstrating nesting-related behaviors in the presence and absence of a female. p < 0.05 was considered significant for all analyses.
Results: Experiment 1
Cannula placement
The location of blue dye and identification of the cannula path in tissue revealed that for 8 birds the cannula tip was centered in mPOA (experimental group; Figure 1). For 7 birds the cannula tip was centered outside mPOA (control group; Figure 1). Four birds were dropped from the analyses because tissue was damaged and the precise location of the cannula tip could not be identified.
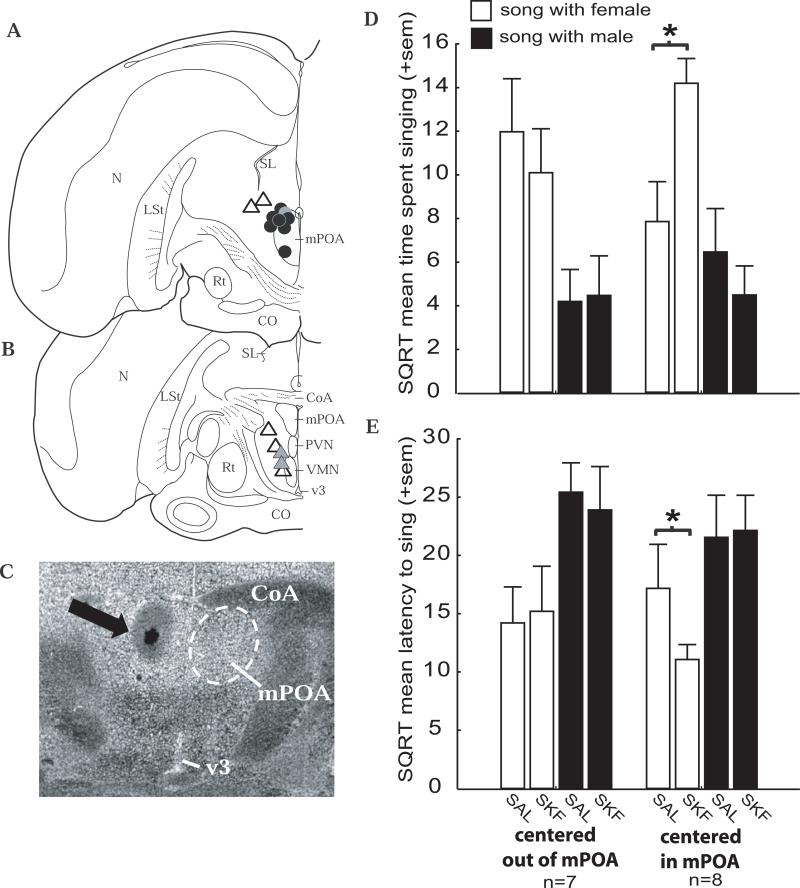
Figure 1.
Stimulation of D1 receptors in mPOA affects sexually-motivated song. A and B) Locations of the cannula tip for each bird in coronal sections of one hemisphere of the starling brain. Circles represent experimental animals for which the site of injection was centered in mPOA. Triangles represent control animals for which the site of injection was centered outside of mPOA. The site of injection for the gray circle was located approximately 50 microns rostral to the dark circles, in the mPOA at the level of the tractus septomesencephalicus. The site of injection for the two gray triangles was located approximately 50 microns caudal to the anterior commissure (CoA). C) Photomicrograph showing an injection on the left side of the brain in mPOA. The cannula tip was located in the dark spot in the center of the area indicated by the black arrow, and the surrounding cloud illustrates the diffusion of blue dye (see text for details). Approximate borders of the mPOA are indicated by the dashed circle in the right hemisphere just ventral to the CoA. Other abbreviations: N = nidopallium; SL = lateral septum; LSt = lateral striatum; mPOA = medial preoptic nucleus; Rt = nucleus rotundus; CO = optic chiasm; PVN = paraventricular nucleus; VMN = ventromedial nucleus of the hypothalamus; v3 = 3rd ventricle. D - E) Bar graphs illustrating the effects of saline (SAL) and the D1 receptor agonist SKF 38393 (SKF; 0.20 μg) on sexually-motivated (open bars) and agonistically-motivated (filled bars) measures of male song for control males for which the cannula tip was centered outside mPOA (left side of each figure) and experimental males for which the cannula tip was centered in mPOA (right side of each figure). Sample sizes are indicated for control and experimental groups at the bottom of the figure. * indicates significant (p < 0.05) results of planned posthoc analyses (see text for details).
Effects of SKF 38393 on singing behavior
A MANOVA examining the effects of a single dose of D1 agonist on singing behavior revealed main effects for behavior and stimulus and several significant interactions (Table 1; Figure 1). Most relevant to the hypothesis tested here, a significant behavior × stimulus × treatment × site of injection interaction (F1,13= 6.80, p = 0.022) was detected. Post hoc analysis of this result indicates that compared to saline treatment, D1 agonist treatment increased the time spent singing (p = 0.028) and decreased the latency to initiate singing behavior (p = 0.034) during presentation of a female compared to a male and that these effects were only observed in males with cannulae targeting the mPOA (Figure 1; See Table 2 for untransformed means). Furthermore, a significant behavior × stimulus interaction indicated that males spent more time singing (p = 0.028) and initiated singing behavior more quickly (p = 0.004) in the presence of a female compared to a male (Figure 1, Table 1).
Table 1.
MANOVA results for analysis of D1 agonist effects on measures of singing behavior
| SS | df | MS | F | p | |
|---|---|---|---|---|---|
| Site of injection | 9.65 | 1 | 9.65 | 0.3068 | 0.589035 |
| error | 408.65 | 13 | 31.43 | ||
| Behavior | 3557.40 | 1 | 3557.40 | 19.7500 | 0.000662 |
| Behavior × site of injection | 36.98 | 1 | 36.98 | 0.2053 | 0.657955 |
| error | 2341.57 | 13 | 180.12 | ||
| Stimulus | 53.52 | 1 | 53.52 | 3.5374 | 0.082587 |
| Stimulus × site of injection | 1.68 | 1 | 1.68 | 0.1114 | 0.743933 |
| error | 196.70 | 13 | 15.13 | ||
| Treatment | 5.47 | 1 | 5.47 | 0.7938 | 0.389151 |
| Treatment × site of injection | 0.52 | 1 | 0.52 | 0.0757 | 0.787474 |
| error | 89.66 | 13 | 6.90 | ||
| Behavior × stimulus | 1671.06 | 1 | 1671.06 | 18.2139 | 0.000916 |
| Behavior × stimulus × site of injection | 20.81 | 1 | 20.81 | 0.2268 | 0.641806 |
| error | 1192.70 | 13 | 91.75 | ||
| Behavior × treatment | 37.48 | 1 | 37.48 | 1.1740 | 0.298274 |
| Behavior × treatment × site of injection | 56.39 | 1 | 56.39 | 1.7659 | 0.206740 |
| error | 415.09 | 13 | 31.93 | ||
| Stimulus × treatment | 1.79 | 1 | 1.79 | 0.2101 | 0.654267 |
| Stimulus × treatment × site of injection | 0.96 | 1 | 0.96 | 0.1126 | 0.742515 |
| error | 110.96 | 13 | 8.54 | ||
| Behavior × stimulus × treatment | 50.47 | 1 | 50.47 | 1.8852 | 0.192969 |
| Behavior × stimulus × treatment × site of injection | 181.98 | 1 | 181.98 | 6.7974 | 0.021708 |
| error | 348.05 | 13 | 26.77 |
Table 2.
Untransformed means (sem) for measures of behavior
| Singing behaviors | Cannula tip out of mPOA | Cannula tip in mPOA | ||
|---|---|---|---|---|
| Time spent singing (secs) | SAL | SKF | SAL | SKF |
| sexually-motivated (with female) | 177.75 (53.03) | 126.12 (36.45) | 84.97 (49.61) | 210.41 (34.10) |
| agonistically-motivated (with male) | 30.40 (24.78) | 39.46 (20.29) | 69.21 (23.18) | 31.75 (18.98) |
| Latency to sing (secs) | ||||
| sexually-motivated (with female) | 257.86 (112.68) | 318.93 (85.70) | 393.94 (105.40) | 134.00 (80.16) |
| agonistically-motivated (with male) | 684.36 (124.03) | 651.86 (134.32) | 554.25 (116.02) | 554.13 (125.64) |
| Nesting behaviors | Cannula tip out of mPOA | Cannula tip in mPOA | ||
|---|---|---|---|---|
| Gathering nest material | ||||
| sexually-motivated (with female) | 4.14 (3.43) | 3.86 (2.62) | 10.75 (3.21) | 6.50 (2.46) |
| agonistically-motivated (with male) | 4.71 (2.82) | 3.14 (2.09) | 2.63 (2.64) | 4.38 (1.96) |
| Entering nest box | ||||
| sexually-motivated (with female) | 7.86 (2.22) | 5.14 (1.48) | 4.25 (2.08) | 6.25 (1.38) |
| agonistically-motivated (with male) | 2.00 (1.42) | 3.00 (1.63) | 2.00 (1.33) | 1.88 (1.52) |
| Nonspecific behaviors | Cannula tip out of mPOA | Cannula tip in mPOA | ||
|---|---|---|---|---|
| Preening | SAL | SKF | SAL | SKF |
| sexually-motivated (with female) | 11.00 (6.53) | 10.86 (3.63) | 12.13 (6.10) | 4.25 (3.39) |
| agonistically-motivated (with male) | 14.86 (4.89) | 15.29 (5.53) | 17.75 (4.58) | 13.25 (5.18) |
| Beak wiping | ||||
| sexually-motivated (with female) | 27.29 (8.18) | 44.43 (10.33) | 33.88 (7.66) | 28.25 (9.66) |
| agonistically-motivated (with male) | 57.57 (10.89) | 48.86 (7.97) | 42.38 (10.18) | 34.88 (7.45) |
| Feeding | ||||
| sexually-motivated (with female) | 1.86 (1.42) | 2.86 (1.27) | 6.38 (1.33) | 4.50 (1.19) |
| agonistically-motivated (with male) | 4.29 (1.47) | 3.71 (1.60) | 6.75 (1.38) | 4.00 (1.50) |
Effects of SKF 38393 on other behaviors
Separate McNemar Chi-square tests on the numbers of birds observed gathering nest material and on the numbers entering a nest box did not reveal any significant differences (all p values were > 0.182; see Table 2 for means). A MANOVA examining preening, beak wiping, and feeding revealed a significant main effect for stimulus (p = 0.001), which reflected a general increase in the production of nonspecific behaviors in the presence of a male compared to a female stimulus (Tables 2 and 3). A significant behavior × stimulus interaction also indicated that males preened (p = 0.0008) and beak wiped (p = 0.0002) significantly more in the presence of a male compared to a female. A significant treatment × site of injection interaction revealed that D1 agonist generally decreased production of nonspecific behaviors when injected into the mPOA (p = 0.026; Tables 2 and 3). An observer could not identify which birds received drug or saline based on subjective behavioral observations.
Table 3.
MANOVA results for analysis of D1 agonist effects on preening, beak wiping, and feeding
| SS | df | MS | F | p | |
|---|---|---|---|---|---|
| Site of injection | 0.035 | 1 | 0.035 | 0.0035 | 0.953610 |
| error | 128.738 | 13 | 9.903 | ||
| Behavior | 492.854 | 2 | 246.427 | 50.1504 | 0.000000 |
| Behavior × site of injection | 8.460 | 2 | 4.230 | 0.8609 | 0.434487 |
| error | 127.758 | 26 | 4.914 | ||
| Stimulus | 24.099 | 1 | 24.099 | 18.1767 | 0.000924 |
| Stimulus × site of injection | 1.117 | 1 | 1.117 | 0.8427 | 0.375333 |
| error | 17.236 | 13 | 1.326 | ||
| Treatment | 2.799 | 1 | 2.799 | 2.4433 | 0.142033 |
| Treatment × site of injection | 7.253 | 1 | 7.253 | 6.3323 | 0.025778 |
| error | 14.891 | 13 | 1.145 | ||
| Behavior × stimulus | 7.113 | 2 | 3.556 | 3.9116 | 0.032723 |
| Behavior × stimulus × site of injection | 1.557 | 2 | 0.778 | 0.8562 | 0.436425 |
| error | 23.639 | 26 | 0.909 | ||
| Behavior × treatment | 0.869 | 2 | 0.435 | 0.3041 | 0.740371 |
| Behavior × treatment × site of injection | 0.479 | 2 | 0.240 | 0.1676 | 0.846607 |
| error | 37.168 | 26 | 1.430 | ||
| Stimulus × treatment | 2.688 | 1 | 2.688 | 1.9789 | 0.182965 |
| Stimulus × treatment × site of injection | 2.414 | 1 | 2.414 | 1.7777 | 0.205327 |
| error | 17.657 | 13 | 1.358 | ||
| Behavior × stimulus × treatment | 1.491 | 2 | 0.746 | 0.9384 | 0.404093 |
| Behavior × stimulus × treatment × site of injection | 1.306 | 2 | 0.653 | 0.8219 | 0.450696 |
| error | 20.655 | 26 | 0.794 |
Experiment 2: Dose-dependent effects of D1 receptor stimulation in mPOA on sexually-motivated singing behavior
Rationale
The first experiment showed that D1 agonist injection into the mPOA selectively stimulated sexually-motivated singing behavior; however, as reviewed in the introduction, D1 receptor stimulation can have different, including opposing, effects depending on the level of stimulation. The goal of this study was to gain insight into the extent to which lower and higher levels of D1 receptor stimulation in the mPOA differentially regulate sexually-motivated singing behavior.
Materials and Methods: Experiment 2
This study involved 26 adult experimental male and 16 stimulus female European starlings. An additional 24 males served as partners for experimental males (details below). The birds were trapped, treated with hormones, screened, and implanted with cannula targeting the mPOA following the same procedures described for Experiment 1. However, in this study experimental males were housed with a second male conspecific in indoor aviaries containing a single nest box for the duration of the study. We first planned Experiments 2 and 3 to be part of a separate paper (i.e., separate from Experiment 1). However, we found the combined results of the 3 studies formed a cohesive data set. The methodological change between Experiment 1 and 2 was part of ongoing efforts to facilitate male singing behavior in the laboratory. We address the possibility that this change may have influenced results of pharmacological manipulations in the discussion section.
Housing males in pairs allows one to establish clear dominance over the other which facilitates singing behavior in the dominant male (Sartor & Ball, 2005; Kelm et al., 2011). This situation also provides social contact for the experimental male. In each aviary, one male occupied the nest box and sang at high rates in response to a female. This male was assigned to the experimental condition and received a cannula targeting mPOA. The other male in each aviary did not sing in response to a female. (Two non-singing males served twice as partners. All other non-singing birds served as partners for only a single experimental male.) Other than singing behavior, the behavior of subordinate partners was not recorded, thus contributions that differences in partners may have on behavior of the experimental birds are not known. This may introduce noise in the data set; however, even with this source of variability results of drug treatment were consistent and significant (as reported below). During surgery to implant the cannula into the mPOA of the experimental male, both males were removed from the aviary so that the subordinate male would not take over the nest box while the experimental male was in surgery.
Experimental males received a mock injection to habituate them to the experimental procedures as in Experiment 1. They were tested on 4 days. Each test day they received injections of either 0.4 μl vehicle (0.9% sterile saline), a low (0.02 μg), intermediate (0.20 μg), or high (2.0 μg) dose of the D1 receptor agonist SKF 38393 in a counterbalanced order. Prior to each injection the experimental male and his partner were both removed from the observation aviary. After injection the experimental male was returned alone to the aviary for 15 min to allow drugs to take effect and to allow him to recover fully from anesthesia. The male's partner was then returned to the aviary for 15 min. Only 3 experimental males were observed to sing during this time (one produced one song, one produced two, and one produced three songs), indicating that reintroduction of the familiar male partner was not a potent stimulus for song induction. A novel stimulus female was then released into the aviary for 15 min and behavioral observations were recorded as detailed for Experiment 1. In contrast to reintroduction of the familiar male partner, introduction of a female elicited high levels of singing behavior (reported below), indicating that singing behavior recorded in this study was likely to be sexually and not agonistically motivated. The female was then removed and returned to her home cage. After the last test, Chicago Blue dye was injected into cannulae to identify the location in which the tip was centered, birds were euthanized, and brain tissue was processed as described in Experiment 1.
Analyses
A MANOVA was run with duration of time spent singing (in secs) and the latency to first song (in secs) as repeated measures dependent variables and treatment (saline and the 3 doses of SKF 38393) entered as the independent variable. Two additional MANOVAs were run to examine effects of treatment on nesting behaviors (with gathering nest material and entering the nest box entered as dependent variables) and on other nonspecific behaviors (with preening, beak wiping, and feeding entered as dependent variables). Lilliefors tests were run to test the assumption of normality. These tests indicated that the latency data and the nesting data violated assumptions of normality. These problems were corrected using a Log10 (x+1) transformation. For each analysis significant main effects were followed by Fisher's posthoc LSD tests to compare males treated with saline versus each dose of SKF 38393. For all analyses p < 0.05 was considered significant.
Results: Experiment 2
Cannula placement
Given natural variation in skull and brain morphology typical of wild caught starlings, using the same stereotaxic coordinates we typically miss mPOA in roughly 50% of our birds (as in Experiment 1). However, in this study, the cannula tip was centered in mPOA in 23 birds and missed in only 3 birds (misses shown in Figure 2). Two birds with cannula in mPOA failed to sing on any of the test days and were dropped from analyses, resulting in n = 21 birds with cannula targeting mPOA.
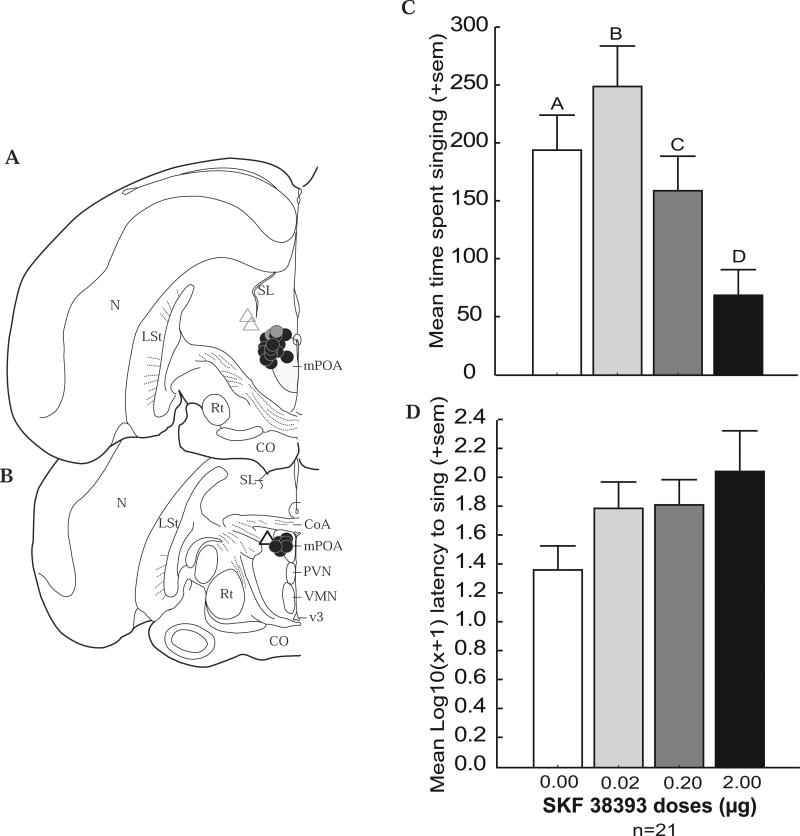
Figure 2.
Inverted-U shaped dose-dependent effects of D1 receptor agonist treatment on singing behavior. A and B) Locations of the cannula tip for each bird in coronal sections of one hemisphere of the starling brain. Circles represent experimental animals for which the site of injection was centered in mPOA. Triangles represent control animals for which the site of injection was centered outside of mPOA. The site of injection for the gray circle and the triangles with gray outlines was located approximately 50 microns rostral to the dark circles at the level of the tractus septomesencephalicus. See Figure 1 for abbreviations. C - D) Bar graphs illustrating effects of saline (SAL) and 3 doses (0.2 μg; 0.20 μg; 2.0 μg) of the D1 receptor agonist SKF 38393 on measures of sexually-motivated male song. Sample size is indicated at the bottom of the figure. Different letters above bars indicate significant differences resulting from post hoc comparisons (p < 0.05).
Dose-response effects of SKF 38393 in mPOA on singing behavior
A first MANOVA was run to examine the time spent singing and log transformed latency to initiate song including only males for which the cannula tip was centered in mPOA. This analysis revealed a significant main effect for behavior (F1,20 = 37.78, p = 0.000005), a main effect for drug dose (F3,60 = 30.38, p = 0.045-10), and a behavior × drug dose interaction (F3,60 = 30.50, p = 0.042-10; Figure 2; Table 4 for means). Post hoc analyses revealed that relative to control injections the amount of time spent singing was significantly higher after treatment with the low dose of SKF 38393 (p = 0.0002) but significantly lower after treatment with the intermediate (p = 0.014) and high dose of SKF 38393 (p = 0.0050-10 ; Figure 2, Table 4). No significant effects of drug treatment were observed for the latency to initiate singing behavior Figure 2, Table 4).
Table 4.
Untransformed means (sem) for measures of behavior after agonist treatment
| Singing behaviors | SAL | SKF (0.02 μg) | SKF (0.20 μg) | SKF (2.00 μg) |
|---|---|---|---|---|
| Time spent singing (secs) | 195.14 (29.80) | 249.38 (35.14) | 160.24 (29.29) | 68.81 (22.38) |
| Latency to sing (secs) | 101.00 (45.49) | 237.00 (71.46) | 235.29 (74.87) | 444.29 (92.07) |
| Nesting behaviors | SAL | SKF (0.02 μg) | SKF (0.20 μg) | SKF (2.00 μg) |
|---|---|---|---|---|
| Gathering nest material | 9.81 (1.57) | 7.86 (1.77) | 11.24 (2.78) | 4.52 (1.33) |
| Entering nest box | 6.14 (1.23) | 5.81 (1.24) | 6.14 (1.46) | 3.19 (1.06) |
| Nonspecific behaviors | SAL | SKF (0.02 μg) | SKF (0.20 μg) | SKF (2.00 μg) |
|---|---|---|---|---|
| Preening | 0.86 (0.31) | 1.24 (0.44) | 1.05 (0.36) | 2.19 (0.62) |
| Beak wiping | 15.14 (2.51) | 17.29 (2.40) | 16.10 (2.92) | 18.95 (2.83) |
| Feeding | 1.10 (0.25) | 1.29 (0.34) | 1.62 (0.40) | 1.62 (0.37) |
The data for birds for which cannula were located outside the mPOA were not analyzed as part of the MANOVA for birds with cannula located in the mPOA because in this study cannula missed mPOA in only 3 individuals. Results of a separate MANOVA run on these individuals showed no significant effects for behavior or dose and no significant interaction (p > 0.49 in all cases).
Dose-response effects of a D1 receptor agonist on other behaviors
In contrast to Experiment 1, the log transformed measures of nesting behavior (gathering of nest material and entering the nest box) were appropriate for parametric statistical analysis. A MANOVA examining the effects of drug dose on these behaviors revealed a significant main effect for behavior (birds gathered nest material more times than they entered nest boxes; F1,20 = 8.28, p = 0.009) and a significant effect for drug dose (F3,60 = 6.55, p = 0.0007). Post hoc analyses revealed that the highest dose of SKF 38393 significantly reduced nesting activities compared to saline treatment (p = 0.0003) the low (p = 0.007) and the intermediate dose of SKF 38393 (p = 0.0004; see Table 4 for means). Finally, the behavior × drug dose interaction was not significant (F3,60 = 0.83, p = 0.48). A MANOVA examining preening, beak wiping, and feeding revealed a significant main effect for behavior (F2,40 = 45.24, p = 0.005-8). (This reflected the fact that birds engaged in significantly more beak wiping than preening (p = 0.004-7 ) or feeding (p = 0.004-7).) There was no significant effect for drug dose (F3,60 = 2.11, p = 0.108) and no behavior × drug dose interaction (F6,120 = 0.78, p = 0.590; Table 4). An observer could not identify which birds received drug or saline based on subjective behavioral observations.
Experiment 3: Selectivity of behavioral effects of SKF 38393 to D1 receptor stimulation
Rationale
The goal of this study was to confirm that the effects of SKF 38393 on singing behavior were mediated by selective activation of the D1 receptor. To do this, we initially tested the birds in Experiment 2 again following a protocol identical to that described above for Experiment 2 after injections of saline, a low (0.02 μg), intermediate (0.20 μg), and high dose (2.0 μg) of the selective D1 receptor antagonist SCH 23390. None of the doses had any significant effect on behavior and no trends were observed (see Table 5 for means). We suggest that this reflects the fact that injections were made unilaterally and that singing behavior was not affected by treatment because dopamine activity in the untreated hemisphere was capable of supporting normal singing behavior. Therefore, we next attempted to address this issue by determining whether SCH 23390 was able to block the potent suppression of singing behavior observed in response to the highest dose (2.0 μg) of the agonist SKF 38393 used in Experiment 2.
Table 5.
Untransformed means (sem) for measures of behavior after antagonist treatment
| Singing behaviors | SAL | SCH (0.02 μg) | SCH (0.20 μg) | SCH (2.00 μg) |
|---|---|---|---|---|
| Time spent singing (secs) | 185.90 (31.46) | 192.57 (32.53) | 176.62 (28.86) | 192.71 (30.86) |
| Latency to sing (secs) | 187.19 (59.95) | 194.86 (72.87) | 231.14 (63.36) | 183.10 (65.63) |
| Nesting behaviors | SAL | SCH (0.02 μg) | SCH (0.20 μg) | SCH (2.00 μg) |
|---|---|---|---|---|
| Gathering nest material | 11.57 (1.87) | 10.81 (2.51) | 10.00 (1.89) | 10.24 (2.29) |
| Entering nest box | 6.48 (1.29) | 5.57 (1.32) | 5.29 (1.18) | 5.86 (1.29) |
| Nonspecific behaviors | SAL | SCH (0.02 μg) | SCH (0.20 μg) | SCH (2.00 μg) |
|---|---|---|---|---|
| Preening | 1.86 (0.42) | 1.10 (0.44) | 0.52 (0.25) | 1.10 (0.32) |
| Beak wiping | 16.33 (3.06) | 18.14 (2.52) | 18.57 (3.04) | 19.67 (2.77) |
| Feeding | 1.48 (0.41) | 1.10 (0.28) | 1.57 (0.34) | 1.29 (0.31) |
Materials and Methods: Experiment 3
This study involved 9 adult experimental male and 5 stimulus female European starlings. An additional 9 males served as partners for the experimental males. These birds were trapped, treated with hormones, screened, and implanted with cannula targeting the mPOA following the same procedures described for Experiment 2. For each pair of males, the male that occupied the nest box in each aviary was assigned to the experimental group as in Experiment 2. Males received a mock injection to habituate them to experimental procedures as in Experiments 1 and 2. They were then tested on 3 days. On each test day each male received 2 injections of a 0.4 μl solution with injections separated by 3 min. The first group received 2 injections of 0.4 μl vehicle (0.9% sterile saline). The second received 0.4 μl vehicle followed by 0.4 μl vehicle containing the high (2.0 μg) dose of the D1 receptor agonist SKF 38393. The third group received a 0.4 μl injection of vehicle containing 2.0 μg of the D1 receptor antagonist SCH 23390 (Sigma-Aldrich, D-054) followed by a 0.4 μl injection of the high dose of the D1 receptor agonist. Each experimental male received one of the 3 treatments on each of the 3 observation days, with order counterbalanced across individuals and test days. The experimental observations were identical to Experiment 2. After the last test, Chicago Blue dye was injected into cannulae to identify the location in which the tip was centered, birds were euthanized, and brain tissue was processed as in Experiments 1 and 2.
Analyses
A repeated measures ANOVA was run with duration of time spent singing (in secs) as a repeated measures dependent variable and treatment (saline, SKF 38393, and SCH 23390 + SKF 38393) entered as the independent variable. A separate repeated measures ANOVA was used to examine effects of treatment on the latency to first song (in secs) because effects of SKF 38393 on this behavior were extreme and its inclusion in an overall MANOVA masked all other effects. As in Experiment 2, two additional MANOVAs were run to examine effects of treatment on nesting behaviors (with gathering nest material and entering the nest box entered as dependent variables) and on other nonspecific behaviors (with preening, beak wiping, and feeding entered as dependent variables). For each analysis significant main effects were followed by Fisher's posthoc LSD tests to compare males treated with saline versus each dose of SKF 38393. For all analyses p < 0.05 was considered significant. Lilliefors tests of normality showed that measures of nesting behavior and non-specific behaviors were not normally distributed. Data were log10 + 1 transformed for these analyses.
Results: Experiment 3
Cannula placement
The location of blue dye and identification of the cannula path in tissue revealed that for 6 birds the cannula tip was centered in mPOA and for 3 birds the tip was centered outside mPOA (Figure 3).
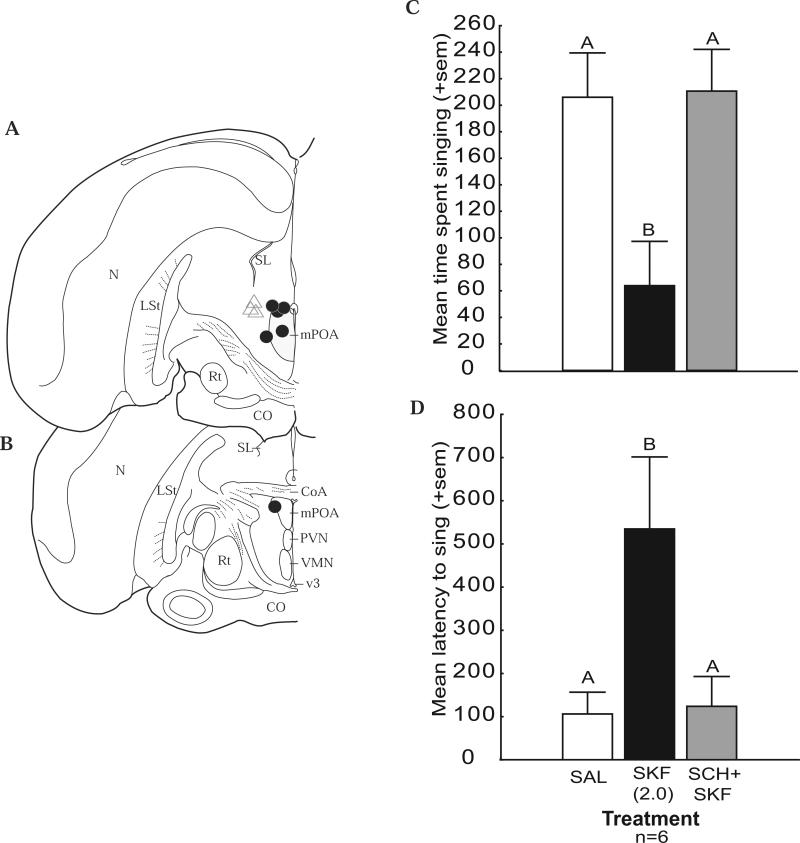
Figure 3.
Effects on singing behavior induced by a D1 agonist are blocked by a D1 antagonist. A and B) Locations of the cannula tip for each bird in coronal sections of one hemisphere of the starling brain. Circles represent experimental animals for which the site of injection was centered in mPOA. Triangles represent control animals for which the site of injection was centered outside of mPOA and approximately 50 microns rostral to the dark at the level of the tractus septomesencephalicus. See Figure 1 for abbreviations. C - D) Bar graphs illustrating effects of saline (SAL), the D1 receptor agonist SKF 38393 (SKF; 2.0 μg), and co-administration of the D1 selective antagonist SCH 23390 (SCH; 2.0 μg) plus SKF on measures of sexually-motivated male song. Sample size is indicated at the bottom of the figure. Different letters above bars indicate significant differences resulting from post hoc comparisons (p < 0.05).
Effects of SKF 38393 + SCH 23390 treatment in mPOA on singing behavior
An ANOVA examining the time spent singing revealed a significant main effect of drug treatment (F2,10 = 38.91, p = 0.00002; Figure 3; Table 6 for means). Relevant to the prediction that SCH 23390 would block suppressive effects of the high dose of SKF 38393, post hoc analyses revealed that the time spent singing after treatment with SKF 38393 was significantly lower than after treatment with saline (p = 0.000021) or SCH 23390 + SKF 38393 (p = 0.000015). A repeated measures ANOVA also revealed a significant effect for drug treatment on the latency to initiate singing behavior (F2,10 = 9.26, p = 0.005; Figure 3, Table 6). Posthoc comparisons reveal that birds took significantly longer to initiate singing behavior after treatment with SKF 38393 compared to either saline (p = 0.003) or SCH 23390 + SKF 38393 (p = 0.005; Figure 3, Table 6).
Table 6.
Untransformed means (sem) for measures of behavior
| Singing behaviors | SAL | SKF | SCH + SKF |
|---|---|---|---|
| Time spent singing (secs) | 205.83 (34.05) | 64.83 (32.57) | 211.00 (31.83) |
| Latency to sing (secs) | 106.5 (54.62) | 536.83 (167.18) | 126.83 (68.31) |
| Nesting behaviors | SAL | SKF | SCH + SKF |
|---|---|---|---|
| Gathering nest material | 5.17 (1.96) | 5.00 (3.13) | 3.33 (1.54) |
| Entering nest box | 2.83 (0.87) | 3.00 (1.29) | 2.00 (0.73) |
| Nonspecific behaviors | SAL | SKF | SCH + SKF |
|---|---|---|---|
| Preening | 2.00 (0.89) | 0.67 (0.42) | 2.17 (1.14) |
| Beak wiping | 19.00 (6.01) | 25.33 (4.89) | 14.67 (6.67) |
| Feeding | 0.33 (0.21) | 0.67 (0.42) | 0.83 (0.40) |
A MANOVA was run to examine effects of drug treatment on both the time spent singing and latency to initiate singing behavior for birds in which the cannula was located outside mPOA. No significant effects were identified (behavior: F1,2= 3.01, p = 0.225; drug treatment: F2,4 = 0.44, p = 0.675; behavior × treatment: F2,4 = 2.27, p = 0.219).
Effects of D1 SKF 38393 + SCH 23390 treatment in mPOA on other behaviors
A MANOVA examining the effects of drug dose on log transformed nesting behaviors (gathering nest material and entering the nest box) revealed no significant main effects for behavior (F1,5 = 0.276, p = 0.622) or drug treatment (F2,10 = 0.807, p = 0.473) and no significant behavior × drug treatment interaction (F2,10 = 0.612, p = 0.561; see Table 6 for means). A MANOVA examining log transformed preening, beak wiping, and feeding also revealed a significant main effect for behavior (F2,10 = 42.03, p = 0.00001). (As in Experiment 2 this reflected the fact that birds engaged in more beak wiping than preening (p = 0.00003) or feeding (p = 0.000007)). However, there was no significant effect for drug dose (F2,10 = 0.15, p = 0.890) and no significant behavior × drug treatment interaction (F4,20 = 2.38, p = 0.086; Table 6). Furthermore, an observer could not identify which birds received drug or saline based on subjective behavioral observations.
Discussion
The present studies are the first to demonstrate experimentally a role for D1 dopamine receptors in the mPOA in the regulation of vocal communication. The results support the hypothesis that an optimal level of D1 receptor stimulation in mPOA is needed to facilitate sexually-motivated vocal production. Furthermore, data indicate that D1 receptors in mPOA modify sexually- but not agonistically-motivated communication (at least at the doses tested) and that effects are region specific.
Intra-mPOA D1 receptor stimulation facilitates sexually-motivated male song
In Experiment 1 we found that administration of a single dose of the D1 receptor agonist SKF 38393 stimulated song when males were presented with females but not when they were presented with unfamiliar males. As described in the methods, we assumed that a larger proportion of song induced by the presence of a female was likely to be sexually-motivated and that a larger proportion of song induced by the presence of an unfamiliar male was likely to be agonistically-motivated (as in (Eens et al., 1993)). Past studies in rats and quail implicate D1 receptors in mPOA in the regulation of male sexual motivation (e.g., (Markowski et al., 1994; Kleitz-Nelson et al., 2010a)). In songbirds, the mPOA is reciprocally connected to multiple brain regions implicated in social behavior and motivation and projects directly to at least one vocal control region and indirectly to several others (Lewis et al., 1981; Appeltants et al., 2000; Appeltants et al., 2002; Riters & Alger, 2004). We suggest that D1 receptor stimulation in mPOA induces a state of sexual motivation that, in the presence of a female, is conveyed directly or indirectly via projections from mPOA to brain regions involved in vocal production to induce sexually-motivated male song.
The results of the present study did not reveal a role for mPOA D1 receptor stimulation in agonistically-motivated song. This experiment was limited in that we examined only a single dose of agonist, thus it is possible that effects on agonistically-motivated song would be observed at lower or higher doses. This remains to be tested; however, the lack of evidence for D1 receptor involvement in agonistically-motivated singing observed here is consistent with a past study in which no links were identified between D1 receptor density in mPOA and song in the presence of other males (Heimovics et al., 2009). In contrast, past work does show a strong correlation between singing in the presence of other males and D1 receptors in the lateral septum (LS; (Heimovics et al., 2009)), a region previously implicated in agonistically-motivated song (Goodson, 1998; Goodson et al., 1999). Future studies are now needed to explore the possibility that dopamine acts at D1 receptors in mPOA to modulate sexually-motivated song and in LS to regulate agonistically-motivated song.
Regional and behavioral specificity of D1 receptor agonist effects
In Experiment 1, cannulae that missed mPOA tended to target hypothalamic regions ventrolateral to caudal mPOA. In Experiments 2 and 3 cannulae that missed mPOA tended to be centered dorsal to the most rostral portions of mPOA. Injection of the D1 agonist in these regions had no effect on singing behavior, indicating that effects of D1 stimulation on sexually-motivated male song are not caused by stimulation of D1 receptors in areas just outside of the mPOA and that D1 stimulation of the lateral hypothalamic zone or areas dorsal to rostral portions of mPOA is not sufficient to stimulate sexually- or agonistically-motivated song. Even in cases in which the cannula tip was located equidistant from the third ventricle (and thus if drug were to leak into the ventricle, it should do so at similar concentrations), only birds with cannulae centered in mPOA displayed increases in sexually-motivated song production. This further supports regional specificity and indicates that effects on song were not caused by diffusion of the D1 agonist into the ventricular system.
The data also suggest that the effects of D1 manipulations on song were not a consequence of drug effects on general motor behavior. In Experiments 2 and 3 general motor behaviors (preening, beak wiping, and feeding) were unaffected by agonist treatment, and in Experiment 1 the same dose of D1 agonist that increased singing behavior suppressed general motor behaviors. It is possible that these behaviors were reduced because D1 receptor stimulation directly inhibits these behaviors; however, we suggest that this likely occurred because the D1 agonist caused birds to spend more time singing which prevented them from spending time on other behaviors. In any case, the data indicate that the agonist was not having its effects on song by stimulating general motor behaviors.
An optimal level of intra-mPOA D1 receptor stimulation facilitates sexually-motivated song
In Experiment 2 we found that stimulation of D1 receptors in mPOA induced dose-dependent inverted-U shaped effects on sexually-motivated singing behavior. This suggests that an optimal level of D1 receptor activity is needed to facilitate sexually-motivated singing behavior. This may also be the case for other sexually-motivated male behaviors given that the highest dose of agonist also suppressed nesting behaviors, which can be considered a reflection of sexual motivation. These findings are consistent with a past study that showed that intraventricular injections of a D1 receptor agonist (injected near the mPOA) also exerted inverted-U shaped effects on sexually-motivated behavior in male Japanese quail (Kleitz-Nelson et al., 2010a). These findings suggest that individual differences in dopamine activity in mPOA due to genetic or environmental influences may in part explain individual differences in the propensity for males to sing to females or to engage in other sexually-motivated behaviors. As reviewed in the introduction, results of studies of D1 receptor involvement in male sexually-motivated behavior are inconsistent (Paredes & Agmo, 2004), and the results of Experiments 1 and 2 reported here are no exception. Specifically, we found that the same dose of SKF 38393 (dose of 0.20 μg) that stimulated sexually-motivated singing behavior in Experiment 1 had no effect on singing behavior in Experiment 2. In past work inconsistent effects of a same dose of D1 receptor agonist appeared to depend on already existing levels of dopamine (Floresco & Phillips, 2001). Specifically, D1 receptor agonist injection into the prefrontal cortex in rats with elevated dopamine inhibited performance on a working memory task; whereas the same treatment in rats with low levels of dopamine stimulated performance. Similar findings have also been reported more recently in pigeons (Herold et al., 2008). Although untested, it is possible that baseline dopamine concentrations in mPOA were lower in birds in Experiment 1 compared to Experiment 2 due to methodological differences between the two experiments. Dopamine levels are known to rise in the mPOA of rats and quail in association with the production of sexually-motivated behaviors (Hull et al., 1995; Kleitz-Nelson et al., 2010b). Compared to males in Experiment 2 (which were housed with subordinate male partners), males in Experiment 1 (which were housed alone) produced lower levels of sexually-motivated behavior (i.e., they took substantially longer to initiate singing behavior and spent less time singing in response to a female after saline treatment; see Tables 2 and 4). It is thus possible that dopamine release in mPOA in the males in Experiment 1 was suboptimal which may explain why a dose of agonist that had no effect on singing behavior in males in Experiment 2, facilitated singing behavior in these males. Future studies are now needed to test this possibility.
Related to this, in the present study we included only males that were observed to sing in response to a female on at least three days. In our laboratory we find that some males are never observed to sing, no matter how many times they are presented with females (unpublished observations). We did not wish to include these males in the study because our interest was in the effects of dopamine manipulations on singing behavior. However, our screening procedure may have resulted in the selection of males that had relatively similar levels of dopamine and dopamine receptors. It is possible that males that failed to sing consistently during screening differ in terms of dopamine levels or D1 receptors in the mPOA. Future studies are needed to explore this possibility.
Effects are specific to D1 receptors
To provide insight into the extent to which effects of SKF 38393 were caused by selective stimulation of D1 receptors, in Experiment 3 we tested whether effects of the highest dose of D1 agonist used in Experiment 2 (2.0 μg; which strongly suppressed singing behavior) could be blocked by co-administration of the agonist SKF 38393 and the D1 selective antagonist (SCH 23390). The results showed that this treatment entirely blocked the suppressive effects of the high dose of SKF 38393. This finding is consistent with selective involvement of intra-mPOA D1 receptors in sexually-motivated male song.
Future directions and conclusions
The present findings support a converging body of evidence that suggests a central, integrative role for the mPOA in the adjustment of communication to match a specific social context (reviewed in (Riters, 2011; 2012)), and they reveal a complex modulatory role for D1 receptors in the mPOA in this process. Dopamine in mPOA binds to families of D1-like and D2-like receptors as well as noradrenergic receptors (Hull et al., 1989; Cornil et al., 2002; Kleitz et al., 2009) and can induce either stimulatory or inhibitory effects depending upon receptor occupancy and the second messenger systems activated (Missale et al., 1998). It has been suggested that to understand the role of dopamine in mPOA in sexual behavior the ratio of D2/D1 receptors is necessary to consider and that D2 receptor activation can reverse D1 receptor facilitation of behavior depending on the level of dopamine release (e.g., (Hull et al., 1989; Kleitz-Nelson et al., 2010a)). Dopamine within midbrain and striatal regions is also linked to measures of song in male songbirds (e.g., (Sasaki et al., 2006; Hara et al., 2007; Huang & Hessler, 2008; Kubikova et al., 2010; Leblois et al., 2010; Heimovics et al., 2011; Bosikova et al., 2012; Leblois & Perkel, 2012)). Future studies on the role of dopamine in the mPOA in vocal behavior must include manipulations and measures of D1, D2, and norepinephrine receptors in mPOA and other brain regions. However, the results of the present study suggest that different levels of intra-mPOA D1 receptor stimulation alone, independent of D2 and norepinephrine receptor activation by dopamine, can also have stimulatory or inhibitory effects on sexually-motivated behavior.
Acknowledgments
This work was supported by NIMH grant R01 MH080225 to LVR. We thank Bill Feeny for illustrations and Chris Elliot and Kate Skogen for animal care.
Abbreviations
- CO
optic chiasm
- CoA
anterior commissure
- LSt
lateral striatum
- MANOVA
multivariate analysis of variance
- mPOA
medial preoptic nucleus
- N
nidopallium
- PVN
paraventricular nucleus
- Rt
nucleus rotundus
- SAL
saline
- SCH
SCH 23390
- SKF
SKF 38393
- SL
lateral septum
- v3
3rd ventricle
- VMN
ventromedial nucleus of the hypothalamus
References
- Alger SJ, Maasch SN, Riters LV. Lesions to the medial preoptic nucleus affect immediate early gene immunolabeling in brain regions involved in song control and social behavior in male European starlings. European Journal of Neuroscience. 2009;29:970–982. doi: 10.1111/j.1460-9568.2009.06637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger SJ, Pawlisch BA, Riters LV. D1 dopamine receptor activation within the medial preoptic nucleus stimulates sexually motivated song in male European starlings. Society for Neuroscience Abstracts; 2007. [Google Scholar]
- Alger SJ, Riters LV. Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris). Behav Neurosci. 2006;120:1326–1336. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002;13:649–653. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Topography in the preoptic region: differential regulation of appetitive and consummatory male sexual behaviors. Front Neuroendocrinol. 2007;28:161–178. doi: 10.1016/j.yfrne.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Arnsten AF. Psychostimulants and motivated behavior: Arousal and cognition. Neurosci Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Bosikova E, Kostal L, Cvikova M, Bilcik B, Niederova-Kubikova L. Song-related dopamine receptor regulation in Area X of zebra finch male. Gen Physiol Biophys. 2012;31:291–298. doi: 10.4149/gpb_2012_034. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of Animal Communication. Sinauer Associates; Sunderland, MA.: 2011. [Google Scholar]
- Catchpole CK, Slater PJB. Bird song : biological themes and variations. Cambridge University Press; Cambridge, [England]: 2008. [Google Scholar]
- Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V. Dopamine activates noradrenergic receptors in the preoptic area. J Neurosci. 2002;22:9320–9330. doi: 10.1523/JNEUROSCI.22-21-09320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D. Evolution of neuroendocrine mechanisms that regulate sexual behavior. Trends Endocrinol Metab. 2005;16:354–361. doi: 10.1016/j.tem.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Dawson A, Goldsmith AR. Plasma prolactin and gonadotrophins during gonadal development and the onset of photorefractoriness in male and female starlings (Sturnus vulgaris) on artificial photoperiods. J Endocrinol. 1983;97:253–260. doi: 10.1677/joe.0.0970253. [DOI] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Function of the song and song repertoire in the European starling (Sturnus vulgaris): An aviary experiment. Behaviour. 1993;125:51–66. [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla). Horm Behav. 1998;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Eibach R, Sakata J, Adkins-Regan E. Effect of septal lesions on male song and aggression in the colonial zebra finch (Taeniopygia guttata) and the territorial field sparrow (Spizella pusilla). Behav Brain Res. 1999;98:167–180. [PubMed] [Google Scholar]
- Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur J Neurosci. 2007;25:3406–3416. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Cornil CA, Ball GF, Riters LV. D1-like dopamine receptor density in nuclei involved in social behavior correlates with song in a context-dependent fashion in male European starlings. Neuroscience. 2009:962–973. doi: 10.1016/j.neuroscience.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Evidence that dopamine within motivation and song control brain regions regulates birdsong context-dependently. Physiol Behav. 2008;95:258–266. doi: 10.1016/j.physbeh.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimovics SA, Salvante KG, Sockman KW, Riters LV. Individual differences in the motivation to communicate relate to levels of midbrain and striatal catecholamine markers in male European starlings. Horm Behav. 2011;60:529–539. doi: 10.1016/j.yhbeh.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold C, Diekamp B, Gunturkun O. Stimulation of dopamine D1 receptors in the avian fronto-striatal system adjusts daily cognitive fluctuations. Behav Brain Res. 2008;194:223–229. doi: 10.1016/j.bbr.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Huang YC, Hessler NA. Social modulation during songbird courtship potentiates midbrain dopaminergic neurons. PLoS ONE. 2008;3:e3281. doi: 10.1371/journal.pone.0003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Dominguez JM. Getting his act together: roles of glutamate, nitric oxide, and dopamine in the medial preoptic area. Brain Res. 2006;1126:66–75. doi: 10.1016/j.brainres.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Hull EM, Du J, Lorrain DS, Matuszewich L. Extracellular dopamine in the medial preoptic area: implications for sexual motivation and hormonal control of copulation. J Neurosci. 1995;15:7465–7471. doi: 10.1523/JNEUROSCI.15-11-07465.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull EM, Warner RK, Bazzett TJ, Eaton RC, Thompson JT, Scaletta LL. D2/D1 ratio in the medial preoptic area affects copulation of male rats. J Pharmacol Exp Ther. 1989;251:422–427. [PubMed] [Google Scholar]
- Kelm CA, Forbes-Lorman RM, Auger CJ, Riters LV. Mu-opioid receptor densities are depleted in regions implicated in agonistic and sexual behavior in male European starlings (Sturnus vulgaris) defending nest sites and courting females. Behav Brain Res. 2011;219:15–22. doi: 10.1016/j.bbr.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitz-Nelson HK, Cornil CA, Balthazart J, Ball GF. Differential effects of central injections of D1 and D2 receptor agonists and antagonists on male sexual behavior in Japanese quail. European Journal of Neuroscience. 2010a;32:118–129. doi: 10.1111/j.1460-9568.2010.07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitz-Nelson HK, Dominguez JM, Cornil CA, Ball GF. Is sexual motivational state linked to dopamine release in the medial preoptic area? Behavioral Neuroscience. 2010b;124:300–304. doi: 10.1037/a0018767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleitz HK, Cornil CA, Balthazart J, Ball GF. Species differences in the relative densities of D1- and D2-like dopamine receptor subtypes in the Japanese quail and rats: an in vitro quantitative receptor autoradiography study. Brain Behav Evol. 2009;73:81–90. doi: 10.1159/000209864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubikova L, Wada K, Jarvis ED. Dopamine receptors in a songbird brain. J Comp Neurol. 2010;518:741–769. doi: 10.1002/cne.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Perkel DJ. Striatal dopamine modulates song spectral but not temporal features through D1 receptors. Eur J Neurosci. 2012;35:1771–1781. doi: 10.1111/j.1460-9568.2012.08095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Wendel BJ, Perkel DJ. Striatal dopamine modulates basal ganglia output and regulates social context-dependent behavioral variability through D1 receptors. J Neurosci. 2010;30:5730–5743. doi: 10.1523/JNEUROSCI.5974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. J Comp Neurol. 1981;196:347–354. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- Markowski VP, Eaton RC, Lumley LA, Moses J, Hull EM. A D1 agonist in the MPOA facilitates copulation in male rats. Pharmacol Biochem Behav. 1994;47:483–486. doi: 10.1016/0091-3057(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Mountjoy DJ, Lemon RE. Song as an attractant for male and female European starlings, and the influence of song complexity on their response. Behavioral Ecology and Sociobiology. 1991;28:97–100. [Google Scholar]
- Paredes RG, Agmo A. Has dopamine a physiological role in the control of sexual behavior? A critical review of the evidence. Prog Neurobiol. 2004;73:179–226. doi: 10.1016/j.pneurobio.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Pawlisch BA, Stevenson SA, Riters LV. alpha(1)-Noradrenegic receptor antagonism disrupts female songbird responses to male song. Neurosci Lett. 2011;496:20–24. doi: 10.1016/j.neulet.2011.03.078. [DOI] [PubMed] [Google Scholar]
- Rauceo S, Harding CF, Maldonado A, Gaysinkaya L, Tulloch I, Rodriguez E. Dopaminergic modulation of reproductive behavior and activity in male zebra finches. Behav Brain Res. 2008;187:133–139. doi: 10.1016/j.bbr.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV. Evidence for opioid involvement in the motivation to sing. J Chem Neuroanat. 2010;39:141–150. doi: 10.1016/j.jchemneu.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV. Pleasure seeking and birdsong. Neurosci Biobehav Rev. 2011;35:1837–1845. doi: 10.1016/j.neubiorev.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV. The role of motivation and reward neural systems in vocal communication in songbirds. Front Neuroendocrinol. 2012;33:194–209. doi: 10.1016/j.yfrne.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris). Horm Behav. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris). Horm Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav Brain Res. 2004;155:307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris). Behav Neurosci. 2005;119:233–244. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MB, Riters LV. Pharmacological manipulations of dopamine and opioids have differential effects on sexually motivated song production in male European starlings. Physiology and Behavior. 2006;88:575–584. doi: 10.1016/j.physbeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Numan M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci Biobehav Rev. 2011;35:826–847. doi: 10.1016/j.neubiorev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Williams GV, Castner SA. Under the curve: critical issues for elucidating D1 receptor function in working memory. Neuroscience. 2006;139:263–276. doi: 10.1016/j.neuroscience.2005.09.028. [DOI] [PubMed] [Google Scholar]