Synopsis
Asthma is a complex syndrome that affects an estimated 26 million people in the United States but gaps exist in the recognition and management of asthmatic subgroups. In this manuscript, we propose alternative approaches for future treatments of adult obese asthmatics that do not respond to standard controller therapies of inhaled corticosteroids, bronchodilators, and anti-leukotriene drugs. We draw parallels between seemingly disparate therapeutics through their common signaling pathways. Specifically, we describe how metformin and statins can potentially improve airway inflammation and suggest supplements, for example L-arginine, which can be used in combination with conventional therapies. A move towards more targeted therapies for asthma subgroups is needed. These therapies address asthma and the comorbidities that accompany obesity and metabolic syndrome to provide the greatest therapeutic potential.
Keywords: Severe asthma, L-arginine, Nitric oxide, Metformin, Statins, Obesity
Introduction
The adult obese patient with worsening asthma despite appropriate controller drug therapy is extraordinarily complicated to manage and treat. For example, consider a 40 year old woman with a medical history notable for adult-onset, non-allergic asthma, obesity, diabetes mellitus, and sleep apnea whose course has been punctuated by several emergency department admissions in the past year. She already requires continuous oral prednisone and four drug therapy for her asthma. How should such a patient be evaluated and treated for the foreseeable future? While asthma is a complex syndrome that affects an estimated 26 million people in the United States, there are gaps in the recognition and management of asthmatic subgroups asthmatics. Extrapolating results from short-term, randomized clinical trials to a broad, heterogeneous population of asthmatics treated in community settings is fraught with difficulty and can result in repeated trial-and-error therapeutic interventions. We still lack the ability to recognize different asthma phenotypes, to adapt and integrate care when comorbidities exist, and adopt new treatments. While published guidelines, including the National Asthma Education and Prevention Program (NAEPP) Expert Panel Report-3 (EPR-3) and the World Health Organization (WHO) Global Initiative for Asthma (GINA) present step-wise evaluation and therapeutic recommendations for chronic persistent asthma management, they do not outline coherent plans for the care of adult-onset, obese asthmatics.
In this manuscript, we propose alternative approaches that may prove to be future treatments for adult obese asthmatics that do not respond to the standard controller asthma therapies of inhaled corticosteroids, bronchodilators, and anti-leukotriene drugs. We draw parallels between seemingly disparate therapeutics through their common signaling pathways (Figure 1). Specifically, we describe how metformin and statins potentially improve airway inflammation through activation of adenosine monophosphate activated protein kinase (AMPK), a key regulator of cellular metabolism and energy production, and through their effects on nitric oxide (NO). In addition, we discuss nutritional supplements, such as L-arginine, omega-3 fatty acids, and other minerals and vitamins that are currently studied and may potentially be used in combination with conventional therapies.
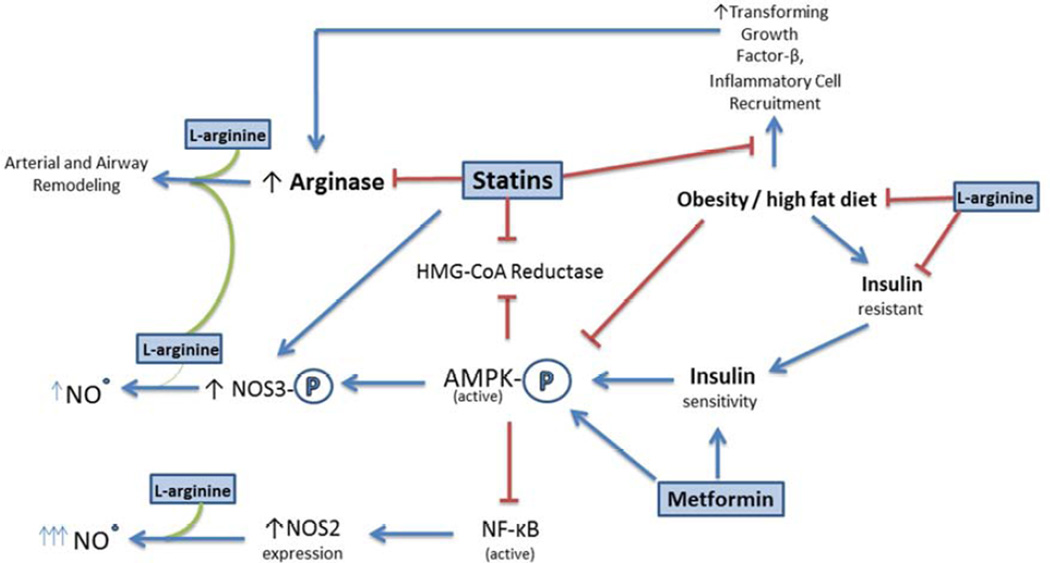
Figure 1.
Potential therapeutics for obese, adult asthmatics described in this review modulate pathways common to several metabolic and nutritional disorders. This allows for the treatment of several comorbidities by targeting their common dysfunction as opposed to individual symptoms. Direct targets include AMPK, which regulates numerous cellular metabolic pathways involved in energy storage and NOS3, which modulates vascular and bronchial smooth muscle tone.
Metformin, insulin resistance, and asthma
The metabolic syndrome with insulin resistance may characterize subsets of asthmatics more than we recognize. The relationship between obesity, insulin resistance and asthma has been clearly established, however the mechanisms by which they influence the pathogenesis of asthma is unclear1. Metformin is a biguanidine class oral anti-diabetic drug used to treat type 2 diabetes and insulin resistance. Although metformin reduces glucose production in the liver through inhibition of gluconeogenesis, the precise mechanisms are unknown and it may have differing modalities in different cell types. Metformin may indirectly activate AMPK by increasing AMP:ATP ratios through mild but specific inhibition of the mitochondrial respiratory chain complex I in hepatocytes, skeletal muscle, endothelial cells, pancreatic B-cells and neurons2. Peroxynitrite, generated by inhibition of complex I, activates AMPK through a c-Src and PI3K-dependent pathway in bovine aortic endothelial cells3. Metformin also directly activates AMPK the inhibitions of AMP deaminase in isolated skeletal muscle4. In the lung, metformin up-regulates AMPK expression and activity and diminishes pro-inflammatory cytokine secretion in human bronchial epithelial cells, downregulating IkK activity and inhibiting NF-κB5.
Obese asthmatics are less responsive to typical asthma controller therapy possibly because of contributing factors such as an increased pro-inflammatory environment that blunt the efficacy of treatment6, yet there have been studies that have shown no difference in induced sputum eosinophils, a biomarker of airway inflammation, between obese and lean asthmatics7,8. However, in a study of obese and lean asthmatics by Desai et al, there were similarities in sputum eosinophil counts between the two groups, but an increase in interleukin-5 (IL5), a mediator of eosinophil activation, in the sputum and increased eosinophil accumulation in the submucosal layer of the obese asthmatic group9.
The results from a study utilizing a high fat-diet-induced obese mouse model (male C57BL6/J) of allergic airway inflammation are in agreement with patient observations. While eosinophil numbers in the bronchoalveolar lavage (BAL) from allergen-challenged, obese mice were decreased compared to their lean counterparts, the levels of infiltrated eosinophils in the lung tissue were higher in the obese mice. Treatment of these allergen-challenged obese mice with metformin reduced tissue eosinophil infiltration and increased the number of cells in the BAL fluid suggest differing modes of regulation for eosinophil migration and function between obese and lean asthmatics, possibly through decreased NF-kB activation10. Another mouse study utilizing lean BALB/C female mice demonstrated that metformin decreases eosinophilic inflammation, peribronchial fibrosis and mucin secretion coupled with increased ratios of activated phospho-AMPK to total AMPK and decreased oxidative stress as measured by the ratio of reduced to oxidized glutathione11.
Metformin can also increase nitric oxide synthase 3 (NOS3, endothelial NOS, eNOS) production of NO and improve endothelial function through AMPK-dependent positive regulation of NOS3 activity and inhibition of NOS3 negative regulators. Treatment of endothelial cell lines and mice with metformin increases AMPK dependent NOS3 phosphorylation at the regulatory site Ser 1177/1179 and NO production12–14. NOS3 activity is negatively regulated through phosphorylation of Thr495 by protein kinase C-β (PKCβ), which is up-regulated in human asthmatics and patients with insulin resistance15. Pharmacologic inhibition of PKCβ in endothelial cells freshly isolated from diabetics decreases basal levels of Thr495 phosphorylated NOS3 and improved insulin-mediated signaling of NOS3. Overall NOS expression and activity is also reduced in murine models of allergic inflammation and human asthmatics16–18. Overexpression of NOS3 in a mouse model of allergic asthma attenuates airway inflammation and airway hyperresponsiveness; possibly acting through increased levels of S-nitrosothiols in the lung or decreased interferon- γ (IFN-γ), IL5 or IL1019. Further studies are necessary to uncover whether NOS3 could be regulated by metformin in models of asthma or asthmatics.
These findings suggest that introducing metformin in conjunction with standard asthma controller therapy could prove beneficial for outcomes in obese asthmatics by modulation of NOS3 activity or other AMPK-dependent metabolic signaling pathways.
Asthma, the mevalonate pathway, and the statin drugs
The statin class of drugs inhibits the enzyme hydroxymethylglutaryl (HMG)-CoA reductase (HMGCR), which is the rate limiting step in cholesterol and isoprenoid biosynthesis in the mevalonate (MVA) pathway20. Isoprenoids include pyrophosphate lipid molecules (e.g. farnesylpyrophosphate (FPP) and geranylgeranylpyrophosphate (GGPP)) that are necessary for post-translational modification of numerous intracellular proteins including small G-proteins, or GTPases (e.g. Rho, Ras, and Rac). GTPases require prenyl tethering to the membrane in order to maintain proximity to the appropriate transmembrane receptors and signaling transduction machinery. Limiting the availability of the isoprenoid substrate reduces the total amount of GTPase available for activation, potentially dampening cytokine and chemokine signal transduction and the responses that lead to cellular hypertrophy and inflammation21.
Statins’ effect on isoprenylated GTPases is a mechanism that may be of particular interest in targeting the obese asthmatic population because translational studies in obese (ob/ob, leptin −/−) mice exposed to allergen have indicated increased activity of the GTPase, RhoA, in airway epithelial cells and airway smooth muscle. Increased RhoA activity has been implicated in airway smooth muscle hyperreactivity and hypertrophy. Geranylgeranylpyrophosphate synthase (GGPPS) expression, the enzyme upstream of FPP and GGPP synthesis, is also increased in obese (ob/ob) mice. Because RhoA requires prenylation by GGPP for activation, the use of statins to manipulate the MVA pathway may have further implications for airway hyperreactivity and remodeling, two key features in asthma22,23.
Statins may also regulate L-arginine metabolism and NO production. In addition to being induced by Th2 cytokines, IL-4 and IL-13, arginase 1 or 2 expression can be induced via a RhoA/RhoKinase (ROCK) mediated pathway. Endothelial cell RhoA/ROCK can be indirectly activated by reactive oxygen and nitrogen species such as H2O2 and ONOO−, via protein kinase C. Insulin resistance and diabetes increases the production of these oxidative species through several sources, including uncoupled mitochondrial complex1, mitochondrial NOS (mtNOS), and NOS3.
RhoA-dependent activation of Rho kinase (ROCK) increases arginase 1 expression in aortas and livers of streptozotocin-induced diabetic rats. Arginase activity depletes intracellular and plasma L-arginine, further reducing endothelium-derived NO production and promoting additional NOS uncoupling. However, this effect on endothelial NO production is reversible with administration of statins, ROCK inhibitor Y-27632, or L-citrulline24–26. The inhibitory effect of simvastatin on arginase expression and activity has also been observed in the airways of mice, in a model of allergic inflammation, though its mechanism of action has not been determined, as statins also inhibit the expression of IL-13 in airway epithelial cells27,28. Statin-dependent reduction in circulating ADMA further aids production of NO by the NOS enzymes and reduces NOS uncoupling. Thus, simvastatin (and potentially other statins) can function to improve dysfunctional NO metabolism in asthmatic, inflamed lungs.
Statins and AMPK collectively engage in crosstalk that regulates NOS3 activity. Treatment of human endothelial cells with statins result in time and dose dependent increases in phosphorylated AMPK, followed by AMPK-dependent phosphorylation of NOS3 at Ser117729. These results were confirmed in the aorta and myocardium of mice orally administered atorvastatin30. Independent of statin administration, AMPK activation also inhibited HMCGR by phosphorylation of the Ser-871/Ser872 site in cultured rat hepatocytes and human endothelial cells31,32.
Statins applied to murine models of allergen-induced airway inflammation reduce inflammatory cell influx, Th2 cytokine production, ADMA levels, airway hyperreactivity, and airway remodeling33–36. Specific inhibition of geranylgeranyltransferase (GGTase) by GGTI-2133 reduces airway hyperreactivity, whereas FTase inhibition increases inflammation and airway hyperreactivity. This indicates that the effect of statins on airway smooth muscle is dependent upon reduced isoprenylation of RhoA by GGPP and the therapeutic effect of statins, at least in allergic airway disease, is through its effects on geranylgeranylation, ie RhoA, not farnesylation37.
In our severe asthma clinics, we found that female-predominant severe asthmatics with a mean body mass index (BMI) of ≥ 30 benefited from statin treatment. Median statin use for one year added to standard inhaler treatment, was associated with a higher asthma control test (ACT) score indicating improved asthma symptoms38. As this is the only study we are aware of that evaluates statin use in obese severe asthmatics, data are limited and clinical application is precluded absent randomized clinical trials to test this observation.
Although data are limited in the obese asthmatic, the above references support the biological plausibility for the role of MVA metabolism and statins in mitigating asthma in this subgroup. The nonatopic adult-onset asthmatic, especially an individual with the confounding characteristics of obesity and metabolic syndrome tend to be more severe and refractory to current therapies. These additional comorbidities contribute to systemic inflammation, insulin resistance and reduced lung function originating from inflammation and mechanical stress derived from increased visceral mass. Statins may address many of these comorbidities; statins reduce pro-inflammatory signaling by RhoA/ROCK, increase NO production and reduce the production of oxidative species by uncoupled NOS enzymes, and activate AMPK, reversing insulin resistance. Therefore, we believe that future experiments in animal models and clinical trials with adult-onset asthmatics with obesity should evaluate the potential therapeutic role of statins and/or statin with L-arginine combination treatment.
L-arginine and asthma
L-Arginine is a substrate in protein synthesis, a molecular component for the conversion of ammonia to urea in the urea cycle and the substrate in a number of diverse enzymatic pathways, including the synthesis of nitric oxide (NO), creatine, agmatine, ornithine, glutamate, proline, polyamine and dimethylarginines, including asymmetric dimethylarginine (ADMA).
Dietary supplementation of amino acid formulations is common practice among healthy individuals and those with chronic illnesses. The semi-essential amino acid, L-arginine has been reported to confer beneficial effects in various small studies of surgery, trauma, and infectious and non-infectious inflammation. Direct infusion of L-arginine to the peripheral or pulmonary arterial beds, has been tested in acute and chronic disease states, including coronary heart disease, pre-eclampsia and myocardial infarction, because of its bronchodilator and vasodilator actions39–41. L-arginine can also be used as a primary therapeutic in sickle cell disease patients with acute chest syndrome, where deficiency in NOS3-derived NO contributes to red cell sickling and vasoconstriction42 augmenting NO production and improving outcomes in this disease.
Factors that regulate the output of NO in the airway, endothelium and adipose tissues of asthmatics include the concentration of L-arginine, the metabolic precursor of NO, and asymmetric dimethylarginine (ADMA), an endogenous NOS inhibitor. NO is primarily derived from the oxidative deamination of L-arginine and O2 into NO and L-citrulline by the NOS family of enzymes. This family includes NOS1 (neuronal NOS, nNOS), NOS2 (inducible NOS, iNOS) and NOS3 (endothelial NOS, eNOS). NOS is also present in mitochondria and referred to as mtNOS but is a variant of NOS143. Depletion of L-arginine can cause the NOS isoforms to produce superoxide (•O2—) in a process referred to as “uncoupling” that results in the transfer of an electron from NADPH to oxygen instead of L-arginine44,45. NO and superoxide (•O2—) can combine to form peroxynitrite (ONOO−). Thus, reduced L-arginine availability to NOS enzymes can have the double effect of reducing NO output and producing oxidative and nitrosative species.
Initial studies using DNA microarray analysis in mouse models of allergen-induced inflammation identified the arginase enzymes as potential “asthma signature genes.” Arginase 1 and arginase 2 catalyze the hydrolysis of L-arginine to ornithine and urea. Increased arginase activity in asthmatics and particularly obese asthmatics is important both, for the downstream products of arginase activity, including proline and polyamines which contribute to airway remodeling and cellular proliferation, and its capacity to regulate the availability of L-arginine to the NOS enzymes by substrate depletion. Analysis of lung tissue of asthmatic subjects confirmed that increased arginase 1 expression correlated with additional effects of increased serum arginase activity and decreased plasma L-arginine compared to controls17,46. Further analysis based on patient subpopulation revealed that serum arginase 1 level in nonatopic asthmatics was further amplified compared to atopic asthmatics.
Animal models of allergic airway inflammation have shown that inhibition of arginase ameliorated lung inflammation, reduced airway and peribronchial eosinophilia, and decreased production of Th2 pro-inflammatory cytokines, IL-4 and IL-13, and eotaxins. Arginase inhibition reduced characteristics of airway remodeling, including goblet cell hyperplasia, airway smooth muscle proliferation, and subepithelial collagen accumulation. Airway hyperreactivity and airway maximal contraction were also reduced47–50. In lieu of arginase inhibition, supplementation with oral or aerosolized L-arginine produced comparable results indicating that the driving force for these improvements was maintaining adequate levels of L-arginine51.
Increased arginase activity in the obese, nonatopic asthmatic subpopulation may be derived from sources beyond the lung. Obese subjects have heightened expression of arginase 1 (4.5 fold) in circulating mononuclear cells compared to normal weight subjects52. Exposure to high levels of insulin causes endothelial cells to have increased arginase II activity that leads to the uncoupling of NOS3 and increased inflammatory cell adhesion via soluble intercellular cellular adhesion molecule 1 (sICAM). Abrogation of these effects can be achieved through arginase inhibition53. Hyperglycemic rats have increased arginase 1 and arginase 2 expression in muscle arteriole beds which inhibit the vasodilation resulting from increased flow. Inhibition of arginase reverses the arteriole flow impedance54. In these models, the beneficial effects of arginase inhibition include mitigation of hypertension, insulin resistance and systemic inflammation.
In an ongoing phase II study (#NCT01841281), we are examining a subset of adult severe asthmatics, who are predominantly overweight/obese and female. The go-to biomarkers that are indicative of asthma do not necessarily apply for this obese, late-onset population, as this subset is nonatopic (low circulating IgE) and exhibit low exhaled NO which inversely correlates with BMI and deceptively low sputum eosinophilia55. Shifts in NO metabolism are indicative of the vascular dysfunction relating to obesity and the changes in exhaled NO may represent corresponding changes in the lung9. We hypothesize that this subset of asthmatics will respond favorably to L-arginine supplementation in addition to their standard therapy because in obese asthmatics, the age of asthma onset appears to dictate how they respond to controller therapies, whether their obesity is causative or a comorbid condition.
ADMA and phosphodiesterase inhibition
NO production by the NOS enzymes can be inhibited by endogenous competitive inhibitor, asymmetric dimethylarginine (ADMA), which can uncouple NOS enzymes, producing superoxide. ADMA is derived from the posttranslational modification of L-arginine by protein-arginine methyltransferase (PRMT) type 156. The lung is a major source of protein bound asymmetrical dimethylarginine with > 3 fold higher levels of bound ADMA compared to heart, kidney or liver tissue, and an estimated 1.4% of arginine residues present in lung proteins asymmetrically dimethylated57. Proteolytic degradation of these proteins releases methylarginases to the free amino acid pool58,59 where they can be enzymatically converted into L-citrulline and dimethylamine by the dimethylarginine dimethylaminohydrolase (DDAH) enzymes or excreted in the urine60.
The lung arginine:ADMA ratio is significantly altered in asthmatics compared to non-asthmatics, with sputum ADMA inversely correlating to exhaled NO61. Increased plasma ADMA has been observed in numerous disease states, including pulmonary arteriole hypertension (PAH), chronic obstructive pulmonary disease (COPD), cardiovascular disease, and diabetes mellitus61–63. The subgroup consisting of obese, nonatopic asthmatics, independent of other confounding metabolic risk factors, may have significantly increased levels of plasma ADMA and reduced L-arginine:ADMA ratio due to obesity alone64. Other potentiating factors such as insulin resistance, even in normotensive individuals, also correlate with increased ADMA65,66.
Mouse models of allergic airway inflammation have given further insight into the role of ADMA metabolism in disease. Allergen exposed mice, like human asthmatics, have significantly increased levels of ADMA in their serum and BALF67 and further examination revealed increased synthesis of ADMA in alveolar epithelial cells and macrophages and reduced expression of ADMA catabolic enzyme, DDAH2 in airway epithelial cells compared to control mice67. Altering ADMA degradation by overexpressing DDAH1 reduced markers of inflammation including eosinophilia, Th2 cytokines IL-4 and IL-13, arginase, and IgE68.
DDAH2 expression is regulated by intracellular cAMP concentration via protein kinase A (PKA)-dependent phosphorylation of the transcription factor, cAMP response element-binding (CREB) protein. PKA activation is mediated by cAMP binding to PKA-regulatory subunits, resulting in the release of active PKA, which can phosphorylate numerous targets, including CREB which require phosphorylation to facilitate binding to the CRE domains upstream of the human DDAH2 gene. Inhibiting cAMP to AMP conversion through the use of phosphodiesterase (PDE) 3/4 inhibitors, increases catabolism of the endogenous NOS inhibitor, ADMA, and increasing NOS3 activity in endothelium and alveolar macrophages69,70. Activation of PKA by PDE4 inhibitors may also target RhoA pathways. PKA-dependent RhoA phosphorylation inhibits RhoA activation and phosphorylation of guanine nucleotide dissociation inhibitor (GDI) by PKA enhances GDI binding to RhoA, deactivating it71. As the Rho/ROCK pathway also regulates expression of arginase, this pathway may further alleviate the depletion of L-arginine and NOS uncoupling26,72.
PDE4 inhibitors tested in murine models of chronic allergic airway inflammation, reduce airway eosinophilia, inflammatory cell recruitment, matrix metalloprotease (MMP)-9 activity, fibroblast migration and hypercontractility and subepithelial collagen deposition73,74. For patient use, second generation PDE4 inhibitors for treatment of COPD have completed multiple randomized clinical trials, noting improvement in prebronchodilator FEV1 with limited side effects including nausea, diarrhea, headaches and weight loss. In combination with other asthma control therapies, the use of PDE4 inhibitors to treat severe, obese asthmatics, especially those with measurably heightened plasma ADMA levels may provide therapeutic benefits.
The potential treatment options reviewed in this section are united by the common principle that decreasing NOS uncoupling has significant clinical benefit in the subgroup of obese, nonatopic asthmatics in which the compounding factors of obesity, endothelial dysfunction, metabolic syndrome, and lung inflammation all contribute to perpetuating NOS dysfunction. These treatments approach the issue of deficient NO production from numerous directions. Supplementation with L-arginine bolsters the L-arginine:ADMA ratio, effectively outcompeting the NOS inhibitor molecule based on enzyme saturation and directly addressing the issue of limited substrate uncoupling NOS enzymes. Arginase inhibition indirectly produces the same effect, increasing both intracellular and plasma L-arginine availability and increasing the L-arginine:ADMA ratio. Finally, inhibition of PDE4 increases ADMA catabolism by decreasing cAMP turnover and subsequently increasing expression of DDAH2. The uncoupling of NOS by ADMA and/or substrate depletion results in the production of oxidative and nitrosative species in the airways, inflammatory cells, endothelial cells and adipose tissue, contributing to the progression of disease.
Vitamins and omega-3 polyunsaturated fatty acids: Other potential consideration in asthma and obesity
The bioavailability of fat soluble vitamins A, D and E in asthmatic patients has not been intensely explored but it can have an impact on obese asthmatic patients as body fat likely acts as a reservoir for storage of fat soluble vitamins. Although more research must be done to evaluate the adequate status of these vitamins in obese asthmatic patients, a summary of these vitamins and omega-3 polyunsaturated fatty acids and their impact on asthma is included.
Recent epidemiological studies point to an inverse relationship between vitamin A levels and severity of asthma75,76. Vitamin A affects a broad array of immune responses through retinoic acid (RA), its major oxidative metabolite. Previous data indicates that vitamin A deficiency can impair immune function, whereas excess RA induces inflammatory disorders. Vitamin A or RA limits the differentiation of naïve T cells to T helper cell (Th)1 by reducing IL-12, INF-γ, and NF-κB signaling, resulting in the increase of Th2 mediated processes. RA can oppose Th17 cell commitment by increasing TGF-β signaling and reducing the expression of IL-6 receptor, while excess vitamin A results in the induction of different subsets of Foxp3+ regulatory T (Treg) cells and their mediated processes77. RA has also been shown to inhibit eosinophilic and basophilic differentiation and regulate IgA and IgG production in response to T cell-dependent antigens. Results from studies in mice and rodents suggest strongly that adequate vitamin A status is the key for maintaining a balance of well-regulated T-cell differentiation and function.
The clinicaltrials.gov website cites more than twenty-five asthma studies that are ongoing with vitamin D. Vitamin D supplementation and asthma has been studied for years and the conflicting findings are likely due to differences in study design, sample size, and method for assessing Vitamin D status through measuring levels of 25-hydroxyvitamin D (25(OH)D)78,79. Potential beneficial effects of 1,25-dihydroxyvitamin D (1,25(OH)2D3 (calcitriol) synthesized by bronchial epithelial cells include increasing antimicrobial peptide production by facilitating toll-like receptor (TLR) signaling, regulation of the inflammatory response, airway remodeling, and respiratory muscle function. Circulating 25(OH)D can suppress IL-17 and IL-4 mediated expression of IL-13 and shift the Th1/Th2 balance towards Th2 dominance. These controversial functions may be due to the direct effect of Vitamin D on CD4+ T-cells by promoting an IL-10 secreting population of Treg cells79. In addition, supplementation of calcitriol has been associated with decreases in body fat mass and improved insulin sensitivity in obese people80. Despite supplementation of Vitamin D, large populations remain vitamin D deficient. This could apply to the adult obese asthmatic population, since the bioavailability seems to be decreased with increased body fat mass.
Whereas vitamin A and D exert most of their affects through the binding of nuclear receptors and regulating of gene transcription, vitamin C and E act as potent antioxidants.. Deficiencies in vitamin C and other plasma antioxidants are associated with lung disease and case-control and cross-sectional studies suggest that vitamin C supplementation may decrease asthma severity and exacerbation frequency through antioxidant mechanisms81. Vitamin E supplementation in asthmatics has been shown to help stabilize lung epithelia membranes and protect against ozone-induced membrane injury by interrupting lipid peroxidation82. Increased levels of vitamin E are associated with less allergic skin sensitization, lower IgE secretion and suppressed neutrophil recruitment. Vitamin E supplementation in animal studies improves vasodilation and decreases oxidation of LDL through increased antioxidants levels in the vasculature. However, randomized controlled clinical trials of supplemental vitamin E in asthmatics have not consistently demonstrated that higher intake of vitamin E reduces asthma events83.
Several clinical studies have been designed to test the hypothesis that diets supplemented with the omega-3 polyunsaturated fatty acids (n-3 PUFA), eicosapentanoic acid (EPA) and docosahexaenoic acid (DHA), the major component of fish oil, ameliorates the development of asthma or improve asthma outcomes84. It is noteworthy in the context of asthma, in the obese patient subgroup, intervention trials with n-3 PUFA show beneficial effects in patients with type 2 diabetes and cardiovascular disease85,86. EPA impacts inflammatory signaling by competitively inhibiting enzymatic pathways that convert arachidonic acid (AA) to potent pro-inflammatory 4-series leukotrienes (LT) and 2-series prostaglandins (PG), mediated by 5-lipoxygenase (5-LO) and cyclo-oxygenase (COX). EPA may also inhibit IgE production through COX87. In addition, EPA is metabolized to less inflammatory 5-series LTs and 3-series PGs and potent anti-inflammatory mediators, resolvins of the E-series (RvE1 and Rv3). The effects of DHA are distinct from EPA; DHA can decrease transcription of AA metabolizing enzymes, such as COX-2, by inhibiting NF-κB activation. DHA can also be converted into anti-inflammatory mediators, including the resolvins of the D-series (RvD1, RvD2, RvD3, and RvD4), docosatrienes, and protectins88. Results of the studies testing effects of n-3 PUFA on asthma pathogenesis over the past twenty years have been conflicting and at least one meta-analysis determined that n-3 PUFA does not affect asthma outcomes84. However, there is a consensus that the total number of subjects in these trials is insufficient to make firm conclusions about the effects of the supplements.
Future Considerations / Summary
Controller drug therapies for asthma may partly or wholly ineffective in adult obese, nonatopic asthma patients, leaving them poorly controlled and exposed to adverse drug side effects, such as relative adrenal insufficiency, osteoporosis, and more frequent respiratory viral infections. One approach to therapy does not fit all and physicians must demonstrate a willingness to try several treatment alternatives. We recommend that asthmatic patients not appropriately responding to controller drug therapy be reevaluated. Adult obese, non-allergic asthmatics may have concomitant conditions that potentiate systemic inflammation; in turn, they may respond to treatment targeted at these associated conditions. Patients with the metabolic syndrome, hypercholesterolemia, and diabetes treated with either metformin or the statin drugs may find benefit for their asthma as well as their metabolic derangements. While firm recommendations cannot be made until further studies are performed, these medications, L-arginine, omega-3 fatty acids and other nutritional supplements are exciting considerations for future treatment of asthma. A move towards more targeted therapies for asthma subgroups is needed, and biologically, these therapies account for and treat the comorbidities associated with the obese, nonatopic asthmatic.
KEY POINTS.
In the future, treatment regimens for obese, adult asthmatics may include several interventions that interfere with pathways common to several metabolic and nutritional disorders.
The diabetic drug, metformin, could decrease inflammatory mediators of asthma by improving insulin sensitivity and altering adenosine monophosphate activated protein kinase (AMPK).
The cholesterol lowering class of medications, statins, could have beneficial effects on both airway inflammation and structural remodeling in asthma.
L-arginine supplementation may benefit a subset of severe asthmatic patients with impaired nitric oxide synthase function in the lung.
Acknowledgments
We appreciate ongoing support from the NIH (T32 HL07013 (JMB), K08 HL076415 (AZ), and HL105573, AI097354 (NJK)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: We have no other disclosures.
References
- 1.Weiss ST. Obesity: insight into the origins of asthma. Nature immunology. 2005 Jun;6(6):537–539. doi: 10.1038/ni0605-537. [DOI] [PubMed] [Google Scholar]
- 2.Viollet B, Guigas B, Sanz Garcia N, Leclerc J, Foretz M, Andreelli F. Cellular and molecular mechanisms of metformin: an overview. Clinical science (London, England: 1979) 2012 Mar;122(6):253–270. doi: 10.1042/CS20110386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou MH, Kirkpatrick SS, Davis BJ, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. The Journal of biological chemistry. 2004 Oct 15;279(42):43940–43951. doi: 10.1074/jbc.M404421200. [DOI] [PubMed] [Google Scholar]
- 4.Ouyang J, Parakhia RA, Ochs RS. Metformin activates AMP kinase through inhibition of AMP deaminase. The Journal of biological chemistry. 2011 Jan 7;286(1):1–11. doi: 10.1074/jbc.M110.121806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myerburg MM, King JD, Jr, Oyster NM, et al. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. American journal of respiratory cell and molecular biology. 2010 Jun;42(6):676–684. doi: 10.1165/rcmb.2009-0147OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark AR. MAP kinase phosphatase 1: a novel mediator of biological effects of glucocorticoids? The Journal of endocrinology. 2003 Jul;178(1):5–12. doi: 10.1677/joe.0.1780005. [DOI] [PubMed] [Google Scholar]
- 7.Todd DC, Armstrong S, D'Silva L, Allen CJ, Hargreave FE, Parameswaran K. Effect of obesity on airway inflammation: a cross-sectional analysis of body mass index and sputum cell counts. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2007 Jul;37(7):1049–1054. doi: 10.1111/j.1365-2222.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 8.McLachlan CR, Poulton R, Car G, et al. Adiposity, asthma, and airway inflammation. The Journal of allergy and clinical immunology. 2007 Mar;119(3):634–639. doi: 10.1016/j.jaci.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Desai D, Newby C, Symon FA, et al. Elevated sputum interleukin-5 and submucosal eosinophilia in obese individuals with severe asthma. American journal of respiratory and critical care medicine. 2013 Sep 15;188(6):657–663. doi: 10.1164/rccm.201208-1470OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calixto MC, Lintomen L, Andre DM, et al. Metformin attenuates the exacerbation of the allergic eosinophilic inflammation in high fat-diet-induced obesity in mice. PloS one. 2013;8(10):e76786. doi: 10.1371/journal.pone.0076786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park CS, Bang BR, Kwon HS, et al. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochemical pharmacology. 2012 Dec 15;84(12):1660–1670. doi: 10.1016/j.bcp.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. The Journal of biological chemistry. 2003 Aug 22;278(34):31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 13.Davis B, Rahman A, Arner A. AMP-activated kinase relaxes agonist induced contractions in the mouse aorta via effects on PKC signaling and inhibits NO-induced relaxation. European journal of pharmacology. 2012 Nov 15;695(1–3):88–95. doi: 10.1016/j.ejphar.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 14.Cheang WS, Tian XY, Wong WT, et al. Metformin Protects Endothelial Function in Diet-Induced Obese Mice by Inhibition of Endoplasmic Reticulum Stress Through 5' Adenosine Monophosphate-Activated Protein Kinase-Peroxisome Proliferator-Activated Receptor delta Pathway. Arteriosclerosis, thrombosis, and vascular biology. 2014 Jan 30; doi: 10.1161/ATVBAHA.113.301938. [DOI] [PubMed] [Google Scholar]
- 15.Tabit CE, Shenouda SM, Holbrook M, et al. Protein kinase C-beta contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013 Jan 1;127(1):86–95. doi: 10.1161/CIRCULATIONAHA.112.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad T, Mabalirajan U, Joseph DA, et al. Exhaled nitric oxide estimation by a simple and efficient noninvasive technique and its utility as a marker of airway inflammation in mice. Journal of applied physiology (Bethesda, Md.: 1985) 2009 Jul;107(1):295–301. doi: 10.1152/japplphysiol.00235.2009. [DOI] [PubMed] [Google Scholar]
- 17.Morris CR, Poljakovic M, Lavrisha L, Machado L, Kuypers FA, Morris SM., Jr Decreased arginine bioavailability and increased serum arginase activity in asthma. American journal of respiratory and critical care medicine. 2004 Jul 15;170(2):148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- 18.Bratt JM, Williams K, Rabowsky MF, et al. Nitric oxide synthase enzymes in the airways of mice exposed to ovalbumin: NOS2 expression is NOS3 dependent. Mediators of inflammation. 2010;2010 doi: 10.1155/2010/321061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ten Broeke R, De Crom R, Van Haperen R, et al. Overexpression of endothelial nitric oxide synthase suppresses features of allergic asthma in mice. Respiratory research. 2006;7:58. doi: 10.1186/1465-9921-7-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeganeh B, Wiechec E, Ande SR, et al. Targeting the mevalonate cascade as a new therapeutic approach in heart disease, cancer and pulmonary disease. Pharmacology & therapeutics. 2014 Feb 26; doi: 10.1016/j.pharmthera.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rikitake Y, Liao JK. Rho GTPases, statins, and nitric oxide. Circulation research. 2005 Dec 9;97(12):1232–1235. doi: 10.1161/01.RES.0000196564.18314.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda N, Kondo M, Ito S, Ito Y, Shimokata K, Kume H. Role of RhoA inactivation in reduced cell proliferation of human airway smooth muscle by simvastatin. American journal of respiratory cell and molecular biology. 2006 Dec;35(6):722–729. doi: 10.1165/rcmb.2006-0034OC. [DOI] [PubMed] [Google Scholar]
- 23.Vigano T, Hernandez A, Corsini A, et al. Mevalonate pathway and isoprenoids regulate human bronchial myocyte proliferation. European journal of pharmacology. 1995 Oct 15;291(2):201–203. doi: 10.1016/0922-4106(95)90143-4. [DOI] [PubMed] [Google Scholar]
- 24.Romero MJ, Platt DH, Tawfik HE, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circulation research. 2008 Jan 4;102(1):95–102. doi: 10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero MJ, Iddings JA, Platt DH, et al. Diabetes-induced vascular dysfunction involves arginase I. American journal of physiology. Heart and circulatory physiology. 2012 Jan 1;302(1):H159–H166. doi: 10.1152/ajpheart.00774.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holowatz LA, Santhanam L, Webb A, Berkowitz DE, Kenney WL. Oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolaemic humans. The Journal of physiology. 2011 Apr 15;589(Pt 8):2093–2103. doi: 10.1113/jphysiol.2010.203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeki AA, Thai P, Kenyon NJ, Wu R. Differential effects of simvastatin on IL-13-induced cytokine gene expression in primary mouse tracheal epithelial cells. Respiratory research. 2012;13:38. doi: 10.1186/1465-9921-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeki AA, Bratt JM, Rabowsky M, Last JA, Kenyon NJ. Simvastatin inhibits goblet cell hyperplasia and lung arginase in a mouse model of allergic asthma: a novel treatment for airway remodeling? Translational research : the journal of laboratory and clinical medicine. 2010 Dec;156(6):335–349. doi: 10.1016/j.trsl.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossoni LV, Wareing M, Wenceslau CF, Al-Abri M, Cobb C, Austin C. Acute simvastatin increases endothelial nitric oxide synthase phosphorylation via AMP-activated protein kinase and reduces contractility of isolated rat mesenteric resistance arteries. Clinical science (London, England: 1979) 2011 Nov;121(10):449–458. doi: 10.1042/CS20110259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun W, Lee TS, Zhu M, et al. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006 Dec 12;114(24):2655–2662. doi: 10.1161/CIRCULATIONAHA.106.630194. [DOI] [PubMed] [Google Scholar]
- 31.Gillespie JG, Hardie DG. Phosphorylation and inactivation of HMG-CoA reductase at the AMP-activated protein kinase site in response to fructose treatment of isolated rat hepatocytes. FEBS letters. 1992 Jul 13;306(1):59–62. doi: 10.1016/0014-5793(92)80837-7. [DOI] [PubMed] [Google Scholar]
- 32.Fisslthaler B, Fleming I, Keseru B, Walsh K, Busse R. Fluid shear stress and NO decrease the activity of the hydroxy-methylglutaryl coenzyme A reductase in endothelial cells via the AMP-activated protein kinase and FoxO1. Circulation research. 2007 Feb 2;100(2):e12–e21. doi: 10.1161/01.RES.0000257747.74358.1c. [DOI] [PubMed] [Google Scholar]
- 33.Chiba Y, Sato S, Misawa M. Lovastatin inhibits antigen-induced airway eosinophilia without affecting the production of inflammatory mediators in mice. Inflammation research : official journal of the European Histamine Research Society … [et al.] 2009 Jul;58(7):363–369. doi: 10.1007/s00011-009-0043-5. [DOI] [PubMed] [Google Scholar]
- 34.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. American journal of respiratory and critical care medicine. 2009 Oct 15;180(8):731–740. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad T, Mabalirajan U, Sharma A, et al. Simvastatin improves epithelial dysfunction and airway hyperresponsiveness: from asymmetric dimethyl-arginine to asthma. American journal of respiratory cell and molecular biology. 2011 Apr;44(4):531–539. doi: 10.1165/rcmb.2010-0041OC. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Dong XW, Shen LL, et al. Simvastatin delivery via inhalation attenuates airway inflammation in a murine model of asthma. International immunopharmacology. 2012 Apr;12(4):556–564. doi: 10.1016/j.intimp.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Chiba Y, Sato S, Hanazaki M, Sakai H, Misawa M. Inhibition of geranylgeranyltransferase inhibits bronchial smooth muscle hyperresponsiveness in mice. American journal of physiology. Lung cellular and molecular physiology. 2009 Nov;297(5):L984–L991. doi: 10.1152/ajplung.00178.2009. [DOI] [PubMed] [Google Scholar]
- 38.Zeki AA, Oldham J, Wilson M, et al. Statin use and asthma control in patients with severe asthma. BMJ open. 2013;3(8) doi: 10.1136/bmjopen-2013-003314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boger RH, Mugge A, Bode-Boger SM, Heinzel D, Hoper MM, Frolich JC. Differential systemic and pulmonary hemodynamic effects of L-arginine in patients with coronary artery disease or primary pulmonary hypertension. International journal of clinical pharmacology and therapeutics. 1996 Aug;34(8):323–328. [PubMed] [Google Scholar]
- 40.Dorniak-Wall T, Grivell RM, Dekker GA, Hague W, Dodd JM. The role of L-arginine in the prevention and treatment of pre-eclampsia: a systematic review of randomised trials. Journal of human hypertension. 2014 Apr;28(4):230–235. doi: 10.1038/jhh.2013.100. [DOI] [PubMed] [Google Scholar]
- 41.Bednarz B, Jaxa-Chamiec T, Maciejewski P, et al. Efficacy and safety of oral l-arginine in acute myocardial infarction. Results of the multicenter, randomized, double-blind, placebo-controlled ARAMI pilot trial. Kardiologia polska. 2005 May;62(5):421–427. [PubMed] [Google Scholar]
- 42.Sullivan KJ, Kissoon N, Sandler E, et al. Effect of oral arginine supplementation on exhaled nitric oxide concentration in sickle cell anemia and acute chest syndrome. Journal of pediatric hematology/oncology. 2010 Oct;32(7):e249–e258. doi: 10.1097/MPH.0b013e3181ec0ae5. [DOI] [PubMed] [Google Scholar]
- 43.Zaobornyj T, Ghafourifar P. Strategic localization of heart mitochondrial NOS: a review of the evidence. American journal of physiology. Heart and circulatory physiology. 2012 Dec 1;303(11):H1283–H1293. doi: 10.1152/ajpheart.00674.2011. [DOI] [PubMed] [Google Scholar]
- 44.Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine-depleted cells leading to peroxynitrite-mediated cellular injury. Proceedings of the National Academy of Sciences of the United States of America. 1996 Jun 25;93(13):6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CA, Wang TY, Varadharaj S, et al. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010 Dec 23;468(7327):1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.North ML, Khanna N, Marsden PA, Grasemann H, Scott JA. Functionally important role for arginase 1 in the airway hyperresponsiveness of asthma. American journal of physiology. Lung cellular and molecular physiology. 2009 Jun;296(6):L911–L920. doi: 10.1152/ajplung.00025.2009. [DOI] [PubMed] [Google Scholar]
- 47.Maarsingh H, Dekkers BG, Zuidhof AB, et al. Increased arginase activity contributes to airway remodelling in chronic allergic asthma. The European respiratory journal. 2011 Aug;38(2):318–328. doi: 10.1183/09031936.00057710. [DOI] [PubMed] [Google Scholar]
- 48.Meurs H, McKay S, Maarsingh H, et al. Increased arginase activity underlies allergen-induced deficiency of cNOS-derived nitric oxide and airway hyperresponsiveness. British journal of pharmacology. 2002 Jun;136(3):391–398. doi: 10.1038/sj.bjp.0704725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takahashi N, Ogino K, Takemoto K, et al. Direct inhibition of arginase attenuated airway allergic reactions and inflammation in a Dermatophagoides farinae-induced NC/Nga mouse model. American journal of physiology. Lung cellular and molecular physiology. 2010 Jul;299(1):L17–L24. doi: 10.1152/ajplung.00216.2009. [DOI] [PubMed] [Google Scholar]
- 50.Bratt JM, Franzi LM, Linderholm AL, O'Roark EM, Kenyon NJ, Last JA. Arginase inhibition in airways from normal and nitric oxide synthase 2-knockout mice exposed to ovalbumin. Toxicology and applied pharmacology. 2010 Jan 1;242(1):1–8. doi: 10.1016/j.taap.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mabalirajan U, Ahmad T, Leishangthem GD, et al. Beneficial effects of high dose of L-arginine on airway hyperresponsiveness and airway inflammation in a murine model of asthma. The Journal of allergy and clinical immunology. 2010 Mar;125(3):626–635. doi: 10.1016/j.jaci.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 52.Kim OY, Lee SM, Chung JH, Do HJ, Moon J, Shin MJ. Arginase I and the very low-density lipoprotein receptor are associated with phenotypic biomarkers for obesity. Nutrition (Burbank, Los Angeles County, Calif.) 2012 Jun;28(6):635–639. doi: 10.1016/j.nut.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Giri H, Muthuramu I, Dhar M, Rathnakumar K, Ram U, Dixit M. Protein tyrosine phosphatase SHP2 mediates chronic insulin-induced endothelial inflammation. Arteriosclerosis, thrombosis, and vascular biology. 2012 Aug;32(8):1943–1950. doi: 10.1161/ATVBAHA.111.239251. [DOI] [PubMed] [Google Scholar]
- 54.Johnson FK, Johnson RA, Peyton KJ, Shebib AR, Durante W. Arginase promotes skeletal muscle arteriolar endothelial dysfunction in diabetic rats. Frontiers in immunology. 2013;4:119. doi: 10.3389/fimmu.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holguin F, Comhair SA, Hazen SL, et al. An association between L-arginine/asymmetric dimethyl arginine balance, obesity, and the age of asthma onset phenotype. American journal of respiratory and critical care medicine. 2013 Jan 15;187(2):153–159. doi: 10.1164/rccm.201207-1270OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Molecular cell. 2005 Apr 29;18(3):263–272. doi: 10.1016/j.molcel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Bulau P, Zakrzewicz D, Kitowska K, et al. Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. American journal of physiology. Lung cellular and molecular physiology. 2007 Jan;292(1):L18–L24. doi: 10.1152/ajplung.00076.2006. [DOI] [PubMed] [Google Scholar]
- 58.Rawal N, Rajpurohit R, Lischwe MA, Williams KR, Paik WK, Kim S. Structural specificity of substrate for S-adenosylmethionine:protein arginine N-methyltransferases. Biochimica et biophysica acta. 1995 Apr 5;1248(1):11–18. doi: 10.1016/0167-4838(94)00213-z. [DOI] [PubMed] [Google Scholar]
- 59.Cantoni GL. Biological methylation: selected aspects. Annual review of biochemistry. 1975;44:435–451. doi: 10.1146/annurev.bi.44.070175.002251. [DOI] [PubMed] [Google Scholar]
- 60.MacAllister RJ, Fickling SA, Whitley GS, Vallance P. Metabolism of methylarginines by human vasculature; implications for the regulation of nitric oxide synthesis. British journal of pharmacology. 1994 May;112(1):43–48. doi: 10.1111/j.1476-5381.1994.tb13026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scott JA, North ML, Rafii M, et al. Asymmetric dimethylarginine is increased in asthma. American journal of respiratory and critical care medicine. 2011 Oct 1;184(7):779–785. doi: 10.1164/rccm.201011-1810OC. [DOI] [PubMed] [Google Scholar]
- 62.Kato GJ, Wang Z, Machado RF, Blackwelder WC, Taylor JGt, Hazen SL. Endogenous nitric oxide synthase inhibitors in sickle cell disease: abnormal levels and correlations with pulmonary hypertension, desaturation, haemolysis, organ dysfunction and death. British journal of haematology. 2009 May;145(4):506–513. doi: 10.1111/j.1365-2141.2009.07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abbasi F, Asagmi T, Cooke JP, et al. Plasma concentrations of asymmetric dimethylarginine are increased in patients with type 2 diabetes mellitus. The American journal of cardiology. 2001 Nov 15;88(10):1201–1203. doi: 10.1016/s0002-9149(01)02063-x. [DOI] [PubMed] [Google Scholar]
- 64.Eid HM, Arnesen H, Hjerkinn EM, Lyberg T, Seljeflot I. Relationship between obesity, smoking, and the endogenous nitric oxide synthase inhibitor, asymmetric dimethylarginine. Metabolism: clinical and experimental. 2004 Dec;53(12):1574–1579. doi: 10.1016/j.metabol.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 65.Stuhlinger MC, Abbasi F, Chu JW, et al. Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA : the journal of the American Medical Association. 2002 Mar 20;287(11):1420–1426. doi: 10.1001/jama.287.11.1420. [DOI] [PubMed] [Google Scholar]
- 66.Sydow K, Mondon CE, Cooke JP. Insulin resistance: potential role of the endogenous nitric oxide synthase inhibitor ADMA. Vascular medicine (London, England) 2005 Jul;10(Suppl 1):S35–S43. doi: 10.1177/1358836X0501000106. [DOI] [PubMed] [Google Scholar]
- 67.Ahmad T, Mabalirajan U, Ghosh B, Agrawal A. Altered asymmetric dimethyl arginine metabolism in allergically inflamed mouse lungs. American journal of respiratory cell and molecular biology. 2010 Jan;42(1):3–8. doi: 10.1165/rcmb.2009-0137RC. [DOI] [PubMed] [Google Scholar]
- 68.Kinker KG, Gibson AM, Bass SA, et al. Overexpression of dimethylarginine dimethylaminohydrolase 1 attenuates airway inflammation in a mouse model of asthma. PloS one. 2014;9(1):e85148. doi: 10.1371/journal.pone.0085148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pullamsetti SS, Savai R, Schaefer MB, et al. cAMP phosphodiesterase inhibitors increases nitric oxide production by modulating dimethylarginine dimethylaminohydrolases. Circulation. 2011 Mar 22;123(11):1194–1204. doi: 10.1161/CIRCULATIONAHA.110.941484. [DOI] [PubMed] [Google Scholar]
- 70.Hwang TL, Tang MC, Kuo LM, et al. YC-1 potentiates cAMP-induced CREB activation and nitric oxide production in alveolar macrophages. Toxicology and applied pharmacology. 2012 Apr 15;260(2):193–200. doi: 10.1016/j.taap.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 71.Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. American journal of physiology. Lung cellular and molecular physiology. 2003 Jun;284(6):L972–L980. doi: 10.1152/ajplung.00429.2002. [DOI] [PubMed] [Google Scholar]
- 72.Ming XF, Barandier C, Viswambharan H, et al. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. 2004 Dec 14;110(24):3708–3714. doi: 10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- 73.Belleguic C, Corbel M, Germain N, Boichot E, Delaval P, Lagente V. Reduction of matrix metalloproteinase-9 activity by the selective phosphodiesterase 4 inhibitor, RP 73–401 in sensitized mice. European journal of pharmacology. 2000 Sep 22;404(3):369–373. doi: 10.1016/s0014-2999(00)00638-5. [DOI] [PubMed] [Google Scholar]
- 74.Kumar RK, Herbert C, Thomas PS, et al. Inhibition of inflammation and remodeling by roflumilast and dexamethasone in murine chronic asthma. The Journal of pharmacology and experimental therapeutics. 2003 Oct;307(1):349–355. doi: 10.1124/jpet.103.053819. [DOI] [PubMed] [Google Scholar]
- 75.Allen S, Britton JR, Leonardi-Bee JA. Association between antioxidant vitamins and asthma outcome measures: systematic review and meta-analysis. Thorax. 2009 Jul;64(7):610–619. doi: 10.1136/thx.2008.101469. [DOI] [PubMed] [Google Scholar]
- 76.Ahmad SM, Haskell MJ, Raqib R, Stephensen CB. Vitamin A status is associated with T-cell responses in Bangladeshi men. The British journal of nutrition. 2009 Sep;102(6):797–802. doi: 10.1017/S0007114509316165. [DOI] [PubMed] [Google Scholar]
- 77.Ross AC. Vitamin A and retinoic acid in T cell-related immunity. The American journal of clinical nutrition. 2012 Nov;96(5):1166S–1172S. doi: 10.3945/ajcn.112.034637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Finklea JD, Grossmann RE, Tangpricha V. Vitamin D and chronic lung disease: a review of molecular mechanisms and clinical studies. Advances in nutrition (Bethesda, Md.) 2011 May;2(3):244–253. doi: 10.3945/an.111.000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paul G, Brehm JM, Alcorn JF, Holguin F, Aujla SJ, Celedon JC. Vitamin D and asthma. American journal of respiratory and critical care medicine. 2012 Jan 15;185(2):124–132. doi: 10.1164/rccm.201108-1502CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pathak K, Soares MJ, Calton EK, Zhao Y, Hallett J. Vitamin D supplementation and body weight status: a systematic review and meta-analysis of randomized controlled trials. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2014 Feb 15; doi: 10.1111/obr.12162. [DOI] [PubMed] [Google Scholar]
- 81.Milan SJ, Hart A, Wilkinson M. Vitamin C for asthma and exercise-induced bronchoconstriction. The Cochrane database of systematic reviews. 2013;10:CD010391. doi: 10.1002/14651858.CD010391.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trenga CA, Koenig JQ, Williams PV. Dietary antioxidants and ozone-induced bronchial hyperresponsiveness in adults with asthma. Archives of environmental health. 2001 May-Jun;56(3):242–249. doi: 10.1080/00039890109604448. [DOI] [PubMed] [Google Scholar]
- 83.Keaney JF, Jr, Gaziano JM, Xu A, et al. Dietary antioxidants preserve endothelium-dependent vessel relaxation in cholesterol-fed rabbits. Proceedings of the National Academy of Sciences of the United States of America. 1993 Dec 15;90(24):11880–11884. doi: 10.1073/pnas.90.24.11880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schuster GU, Kenyon NJ, Stephensen CB. Asthma. In: Caballero B, editor. Encyclopedia of Human Nutrition (Third Edition) Vol 1. Elsevier, Ltd.; 2013. pp. 122–128. [Google Scholar]
- 85.Nakamura T, Azuma A, Kuribayashi T, Sugihara H, Okuda S, Nakagawa M. Serum fatty acid levels, dietary style and coronary heart disease in three neighbouring areas in Japan: the Kumihama study. The British journal of nutrition. 2003 Feb;89(2):267–272. doi: 10.1079/BJN2002747. [DOI] [PubMed] [Google Scholar]
- 86.Bjerregaard P, Pedersen HS, Mulvad G. The associations of a marine diet with plasma lipids, blood glucose, blood pressure and obesity among the inuit in Greenland. European journal of clinical nutrition. 2000 Sep;54(9):732–737. doi: 10.1038/sj.ejcn.1601088. [DOI] [PubMed] [Google Scholar]
- 87.Schmitz G, Ecker J. The opposing effects of n−3 and n−6 fatty acids. Progress in Lipid Research. 2008;47(2):147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 88.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature reviews. Immunology. 2008 May;8(5):349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]