Abstract
Understanding immunosenescence and changes in antimicrobial immune response with age is of high importance. The association of immunosenescence with gender and persistent infection with human cytomegalovirus (HCMV) is a matter of intensive research. We determined whether replication of another persistent and highly prevalent virus, Torque teno virus (TTV), is related to age, gender, and HCMV IgG serostatus of the host. TTV DNA load in plasma was assessed by real-time PCR in 313 healthy persons: 20–30 years old (young, n = 104), 50–60 years old (middle-aged, n = 101), or >80 years old (elderly, n = 108). TTV DNA loads were further associated with age-groups, gender, and HCMV IgG serostatus. TTV load was significantly higher in the elderly compared to the young group (p < 0.001; Tukey’s honest significant difference (HSD)), and the higher TTV DNA levels over age were found to be gender-specific (p = 0.002; ANOVA), with young women showing the lowest TTV load compared to young men (p = 0.009, t test) and compared to the other female age-groups (middle-aged p = 0.005; elderly p < 0.001; Tukey’s HSD). TTV load of HCMV IgG-seropositive persons was significantly higher than that of the HCMV IgG seronegative in the young (p = 0.005; t test) and middle-aged (p = 0.016; t test) groups. These results indicate that the host’s immune control of TTV replication decreases with age and is gender-specific. Persistent HCMV infection is significantly related to higher TTV DNA loads, especially at a younger age. Therefore, the influence of gender and HCMV on immunosenescence earlier in life should be further explored.
Keywords: TTV, Anellovirus, Aging, Immunosenescence, Gender, Cytomegalovirus
Introduction
Over the past few years, efforts have been undertaken to understand the process of immunosenescence in the healthy population. One main characteristic of immunosenescence, a term which refers to the changes of the immune system that occur with age (Goronzy and Weyand 2013; Pawelec et al. 2010), is the loss of immunological competence to cope with infectious pathogens (Moro-Garcia et al. 2012; Larbi et al. 2008). The aging process of the immune system is influenced by various aspects, one of them being gender. Although the molecular mechanism underlying gender and immunosenescence is poorly understood, there is clear evidence that the immune system of men and women responds differently to infections, in part due to hormonal differences (Sakiani et al. 2013; Klein and Roberts 2010), which are dependent on age (Puchhammer-Stöckl et al. 2012; Gameiro et al. 2010). Another aspect which may potentially affect immunosenescence is infection with human cytomegalovirus (HCMV; Crough and Khanna 2009). Persistent HCMV infection, indicated by a positive HCMV IgG serostatus, is discussed to influence the process of aging of the immune system and to stimulate the progression toward a senescent immune profile, probably by the substantial increase in HCMV-specific immune response (Aberle and Puchhammer-Stöckl 2012; Mekker et al. 2012; Hadrup et al. 2006) and by the expansion of CMV-specific CD8+ cells (Pawelec and Derhovanessian 2011; Colonna-Romano et al. 2007).
The Torque teno virus (TTV) is a small, single-stranded DNA virus which belongs to the family Anelloviridae, genus Alphatorquevirus (Carstens 2010; Biagini and de Micco 2008). It is highly prevalent in the human population, and in about 70 to 90 % of healthy persons, TTV DNA is detectable (Okamoto 2009; Burra et al. 2008). So far, there is no evidence suggesting that TTV is the causative agent of human disease (Okamoto 2009), so that it is most likely a commensal virus (Bernardin et al. 2010; Griffiths 1999). The TTV DNA load in plasma reflects the balance between virus replication and antiviral immune response, and in healthy persons, its level is about 3–6 log10 copies/mL blood (Tyagi et al. 2013; Burra et al. 2008; Maggi et al. 2005b). These levels result from a high daily turnover rate with a daily generation rate of more than 10 log10 virions and a daily host’s clearance rate of over 90 % (Maggi et al. 2001). TTV has been shown to elicit humoral and innate immune responses (Chen et al. 2013; Rocchi et al. 2009) and to influence cytokine production and secretion (Kincaid et al. 2013; Rocchi et al. 2009; Zheng et al. 2007). Studies performed with persons under immunosuppression have revealed that the TTV DNA plasma or serum load mirrors at least, to some extent, the strength of the hosts’ immune response. TTV DNA load substantially increases in patients with immunosuppression after solid organ transplantation (Görzer et al. 2014; Beland et al. 2013; Burra et al. 2008; Moen et al. 2003) and is associated with the reconstitution of the functional immune system after autologous stem cell transplantation (Focosi et al. 2010).
The aim of the present study was to determine whether the extent of chronic replication of a persistent virus increases with age and whether it is associated with the person’s gender or HCMV serostatus. We used TTV and its replication level, defined by the TTV DNA plasma load, as a marker. Our data showed that the TTV load is higher with higher age and that these differences are gender-specific and also provide evidence that the HCMV IgG serostatus is associated with higher TTV loads, especially at a younger age.
Material and methods
Study population and plasma sample selection
The study population consisted of 313 healthy persons, and one plasma sample was used from each subject; these samples had been taken during routine diagnostic blood testing between 2012 and 2013 and frozen at −20 °C at the Department of Virology. Plasma samples from persons were included if their blood was taken due to a previous needlestick injury (for medical staff) or if vaccination antibody titers were taken. Subjects were included who were 20 to 30, 50 to 60, or 80 years of age and older. Subjects with known immunosuppression due to transplantation or autoimmune disease, persons with known malignancies and known acute or chronic infections at the time of blood withdrawal, and pregnant women were excluded. From the 313 persons, 104 persons were 20 to 30 years of age (mean age 25 years; 53 male; 51 female), referred to as the “young” group, 101 persons were 50 to 60 years of age (mean age 55 years; 42 male; 59 female; “middle-aged” group), and 108 persons were >80 years of age (mean age 84 years; maximum 93 years; 43 male; 65 female; “elderly” group).
Determination of TTV DNA plasma loads
TTV DNA was extracted from 200 μL of each plasma sample using the NucliSENS easyMAG platform (bioMerieux, France), as recommended by the manufacturer, and eluted in 50 μL of elution buffer. For the determination and quantification of the TTV DNA plasma load, a TaqMan real-time PCR was used which has been previously described (Maggi et al. 2003). TTV DNA could be quantified within a linear range from 2 to 10 log10 copies/mL, as determined by the use of tenfold dilutions of a plasmid standard. The limit of detection was 2 log10 copies/mL of plasma.
Determination of CRP plasma levels
For testing of C-reactive protein (CRP) plasma levels in all TTV DNA-positive samples, the Quantikine® ELISA system (R&D Systems, Minneapolis, USA) was used.
Determination of HCMV IgG serostatus
For testing HCMV IgG serostatus in all TTV DNA-positive plasma samples, a commercial antibody ELISA system (Anti-CMV-Elisa IgG, Euroimmun, Germany) was used.
Statistical analyses
The chi-squared test was used to analyze the prevalence of TTV DNA positivity across the different age groups. In order to compare the mean TTV DNA plasma levels, the total TTV DNA counts were changed into log10 units. The D’Agostino and Pearson omnibus normality test was used to determine normal distributions within each of the compared groups. The Levene’s test to determine equal variances between the groups was also used, with a p value of lower 0.200 defined as different variances. For analyzing the general effect of the three age groups on TTV DNA plasma load, ANOVA tests were performed, and, to determine the specific changes between the age groups, the Tukey’s honest significant difference (HSD) post hoc test was used. In order to analyze the mean TTV DNA plasma loads within two groups at a time, an unpaired t test was used. For all statistical analyses, PASW statistics version 18.0.0 (IBM, USA) and GraphPad Prism version 5.04 (GraphPad Software Inc., USA) were used. For all tests, p values ≤0.05 were considered significant and p values ≤0.001 were considered highly significant.
Ethical considerations
The study was conducted at the Department of Virology of the Medical University of Vienna (MUW), and the research protocol was approved by the ethics committee of the MUW [EKNR: 1785/2013].
Results
TTV DNA prevalence in the patient groups
We first assessed whether the prevalence of TTV DNA in plasma differs across different age groups. Therefore, plasma samples from all 313 healthy persons included in the study according to the criteria defined in the “Material and methods” section were analyzed with a quantitative TTV assay. In 76 % (238/313) of the plasma samples, TTV DNA could be detected. In the young age group (20–30 years), 70 % of the persons (73/104) were TTV DNA-positive, in the middle-aged group (50–60 years), 78 % (79/101) were TTV DNA-positive, and, in the elderly group, 80 % (86/108) of the persons were TTV DNA-positive. Although TTV prevalence was somewhat higher with higher age, this difference was not significant (p = 0.111; chi-squared test).
In order to exclude patients undergoing current inflammation processes, we further determined the CRP level in the 238 TTV DNA-positive persons. In 21 of the persons, a CRP level of higher 2 mg/dL was detected, and these persons were therefore excluded from the analyses. In further analysis, only the remaining 217 TTV DNA plasma-positive persons were included, 73 of these persons were in the young, 77 were in the middle-aged, and 67 persons were in the elderly group.
TTV DNA plasma load and age
In order to investigate whether a general loss of the immune system’s ability to respond to infectious pathogens in elderly persons is mirrored by TTV, we assessed whether the TTV DNA plasma load, which is supposed to reflect the extent of the immune response to TTV, is associated with a difference in age. The mean TTV DNA values of the TTV DNA-positive plasma samples were 4.28 log10 copies/mL in the young group, 4.62 log10 copies/mL in the middle-aged group, and 4.97 log10 copies/mL in the elderly group. There was a highly significant association between age groups and TTV DNA plasma load (p < 0.001). A post hoc Tukey’s HSD test was performed and, as shown in Fig. 1, revealed that the TTV DNA plasma load in the elderly group was significantly higher compared to the young (p < 0.001) group.
Fig. 1.
TTV DNA log10 plasma loads between three age groups. The ANOVA post hoc Tukey’s HSD test was performed to compare mean values between the age groups. Nonsignificant p values are shown as ns. Thick horizontal bars represent the median value of each group
Association between gender and TTV DNA plasma load
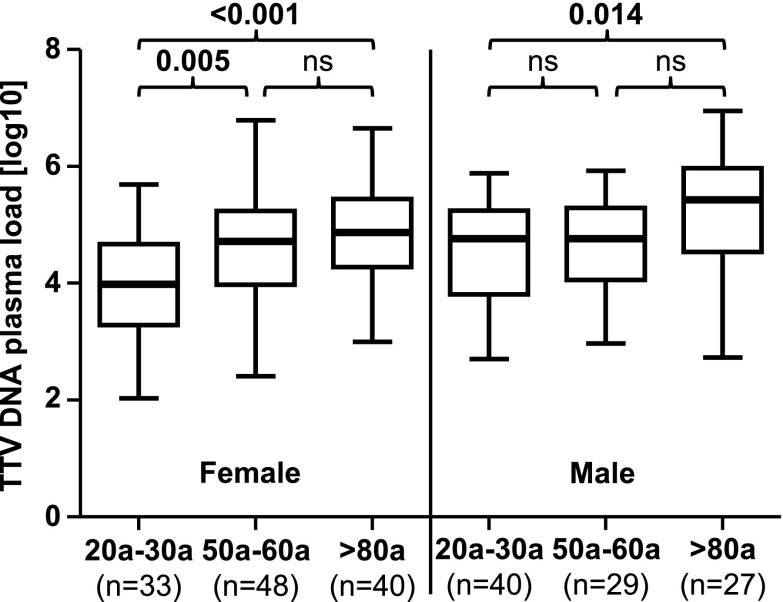
We determined whether the higher TTV DNA levels with higher age were associated with the gender. An ANOVA analysis revealed a significant (p = 0.002) association with TTV DNA levels across the age groups. The male and the female study populations were separately analyzed by ANOVA and post hoc Tukey’s HSD tests. These data are shown in Fig. 2.
Fig. 2.
TTV DNA log10 plasma loads between three age groups divided in a female and a male population. The ANOVA post hoc Tukey’s HSD test was performed to compare mean values between the age groups. Nonsignificant p values are shown as ns. Thick horizontal bars represent the median value of each group
In the female population, a highly significant association was found between age group and TTV DNA plasma load (p < 0.001). Young women had a significantly lower TTV DNA load compared to elderly (p < 0.001) and middle-aged women (p = 0.005). In the male population, a significant association was found between age group and TTV DNA plasma load (p = 0.015). In this population, significantly higher TTV loads were observed in the elderly group compared to those in the younger group (p = 0.014) (Fig. 2).
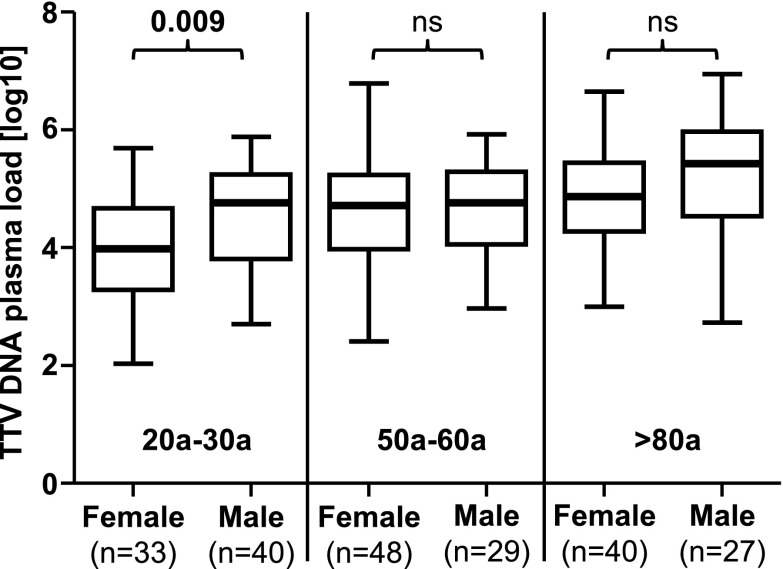
In addition, in order to determine whether gender is differentially associated with TTV DNA plasma loads within the age groups, an unpaired t test was used to compare males and females. As shown in Fig. 3, the young female population showed significantly lower TTV DNA plasma loads compared to males in the same age group (p = 0.009). No significant differences were found in the other two groups.
Fig. 3.
TTV DNA log10 plasma loads between females and males within three age groups. The unpaired t test was performed to compare mean values within the age groups. Nonsignificant p values are shown as ns. Thick horizontal bars represent the median value of each group
Association of HCMV IgG serostatus with TTV DNA plasma load
Since persistent HCMV infection is believed to decrease the immune response against other pathogens, we further investigated whether there is an association between HCMV IgG serostatus and TTV DNA load at different ages. First, we assessed the prevalence of HCMV seropositivity in the healthy TTV DNA-positive plasma samples of our study group by using a commercial IgG-ELISA system. The results indicated a general HCMV IgG seropositivity of 64 % (139/217) and an HCMV IgG seropositivity of 45 % (33/73) in the young group, 78 % (60/77) in the middle-aged group, and 69 % (46/67) in the elderly group. This increase in the prevalence of HCMV status with higher age was significant (p < 0.001; chi-squared test).
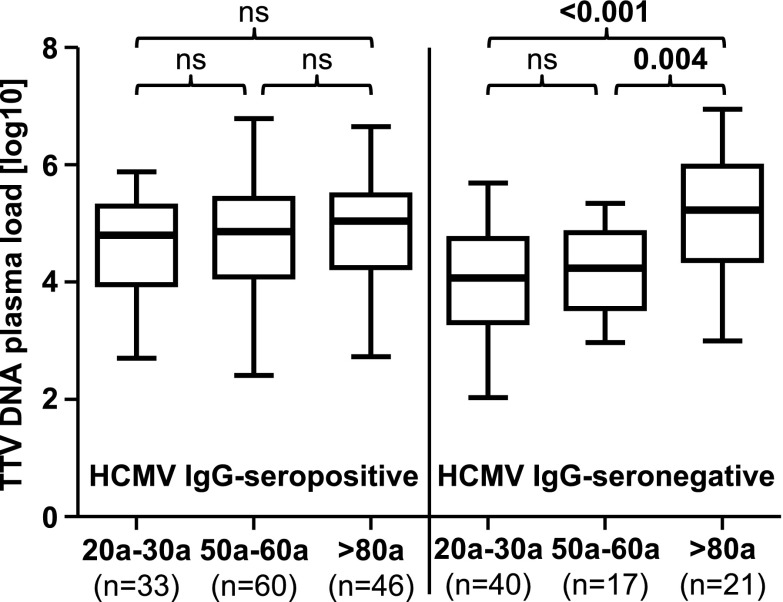
Next, we performed an ANOVA analysis which revealed a significant (p = 0.022) association between higher TTV DNA levels with higher age and HCMV IgG serostatus. We then divided the study population into HCMV IgG-seropositive and HCMV IgG-seronegative persons. As shown in Fig. 4, in the HCMV IgG-seropositive population, no significant association between age groups and the TTV DNA load was found by the ANOVA post hoc Tukey’s HSD tests. In contrast, in the HCMV IgG-seronegative population, a highly significant association of TTV DNA plasma load across the age groups (p < 0.001) was found. Significantly higher TTV loads were observed in the elderly group, compared to those in the young (p < 0.001) and middle-aged groups (p = 0.004; Fig. 4).
Fig. 4.
TTV DNA log10 plasma loads between three age groups divided into two populations according to HCMV IgG serostatus. The ANOVA post hoc Tukey’s HSD test was performed to compare mean values between the age groups. Nonsignificant p values are shown as ns. Thick horizontal bars represent the median value of each group
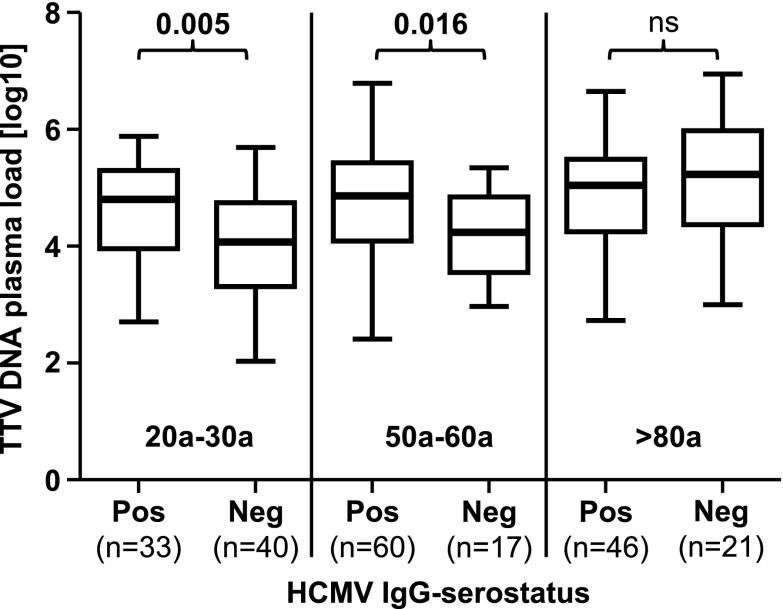
To determine whether HCMV IgG serostatus is associated with TTV DNA plasma load within the same age group, an unpaired t test was used to compare TTV DNA load of HCMV-seropositive and HCMV-seronegative persons. As shown in Fig. 5, significantly higher TTV DNA loads were found in seropositive compared to seronegative people in the young (p = 0.005) and in middle-aged (p = 0.016) groups, whereas no significant difference was found in the elderly population, in which similarly elevated TTV DNA loads in both groups were found.
Fig. 5.
TTV DNA log10 plasma loads between HCMV IgG-seropositive and IgG-seronegative people across three age groups. The unpaired t test was performed to compare mean values within the age groups. Groups are divided in being either HCMV-IgG seropositive (pos) or HCMV-seronegative (neg). Nonsignificant p values are shown as ns. Thick horizontal bars represent the median value of each group
Association of gender and HCMV IgG serostatus with TTV DNA plasma load
Lastly, to determine whether there is an association between the combined effect of gender and HCMV IgG serostatus on the higher TTV DNA plasma loads with higher age, an ANOVA was performed, but no significant association was found (p = 0.054).
Discussion
Over the last few years, the role that aging plays in determining the degree of immune response against microbial infections has become an important area of research. TTV is a persistently infecting virus, which chronically replicates in the healthy host, and is detectable in the majority of the population. TTV plasma DNA level reflects the balance between a high daily virion generation rate and a host’s clearance rate of over 90 % per day (Maggi et al. 2001) and, thus, indirectly reflects the antiviral immune response against TTV (Chen et al. 2013); this finding was confirmed by data of different research groups revealing that after iatrogenic immunosuppression, a significant increase of the TTV load is observed (Görzer et al. 2014; Beland et al. 2013; Focosi et al. 2010; Burra et al. 2008; Moen et al. 2003). Therefore, we selected the TTV DNA level in plasma as a marker to indirectly measure the efficacy of antiviral immune response in persons at different ages. Here, we showed that healthy persons of 80 years and older have significantly higher TTV DNA plasma loads compared to young persons (20–30 years); these findings thus indicate a decreased antiviral immune response against chronic TTV infection with increasing age. This finding is in agreement with previous investigations, showing that immunological changes associated with a lower efficiency of the immune responses occur in the elderly (Goronzy and Weyand 2013; Moro-Garcia et al. 2012; Naylor et al. 2005).
An additional finding of our study was that the differences of the TTV DNA plasma loads across the age groups were significantly different between men and women. We showed that young females had the lowest TTV load of all groups, which was also significantly lower than that of men of the same age. These findings are in agreement with previous data showing that men are more susceptible to infections with various pathogens compared to women (reviewed in Klein and Roberts 2010) and especially compared to women at a younger age (Klein and Huber 2010; Gameiro et al. 2010). This finding, at least to some extent, might be due to the different effects of sex steroid hormones on the host’s immune system (Furman et al. 2014; Sakiani et al. 2013; Ansar Ahmed et al. 2010). We further observed that, in the female population of our study, the young group showed a significant lower TTV load compared to the middle-aged group. These results may support previous data showing that, with the changes of the female sexual hormone status during and after menopause, there might be a decrease of the antiviral immune response (Gameiro et al. 2010). In contrast, in the male population, TTV load was comparable between the young (20–30 years) and the middle-aged (50–60 years) but showed a significant higher TTV load in the elderly group (>80 years) compared to the young, possibly mirroring a difference in efficacy of immune response against TTV replication at different ages over time compared to the female population.
It has previously been shown that persistent infection with HCMV might be associated with decreased immune function and thus contribute to immunosenescence (Pawelec and Derhovanessian 2011), which may result in increased mortality in the persistent HMCV-infected elderly population (Savva et al. 2013; Wang et al. 2010; Roberts et al. 2010). These findings are likely caused, at least partially, by a substantial increase in HCMV-specific T cells with age, which might impair the immune response against other pathogens (Mekker et al. 2012; Colonna-Romano et al. 2007; Hadrup et al. 2006). On the basis of these data, we hypothesized that the efficacy of the antiviral immune response against TTV would also be impaired by a simultaneous persistent HCMV infection leading to a higher TTV load in HCMV-positive persons. In fact, the present data revealed a significant association between a positive HCMV IgG serostatus and higher TTV DNA plasma load; however, an association between persistent HCMV infection and TTV replication was found only in the young (20–30 years) and in the middle-aged (50–60 years) groups, in which the HCMV IgG-seropositive persons had significantly higher TTV DNA plasma loads compared to the HCMV IgG-seronegative persons. These findings support recently published data which demonstrated that healthy, HCMV seropositive young people already may show first signs of HCMV-induced immunosenescence (Turner et al. 2013). In the elderly group, however, no significant difference in TTV DNA plasma loads was found between HMCV-seropositive and HMCV-seronegative people; both groups showed comparable high TTV levels. It therefore appears that, in the elderly population, there might be a general decrease in the defense against TTV replication that occurs regardless of HCMV serostatus. If true, this would signify that the impact of the persistent HCMV infection on the elderly may be lower than believed and would also be in agreement with recently published data showing that the declining immune response to pneumococcal vaccination in the elderly does not depend on persistent HCMV infection (O’Connor et al. 2013). Instead, the present study drives attention to the fact that HCMV seropositivity seems to be associated with decreased antiviral response earlier in life, and further investigations should thus focus on the earlier processes of immunosenescence in the healthy host.
In previous studies, HCMV-associated effects on immunosenescence were investigated by analyzing the defense against newly acquired infections or in response to vaccinations (O’Connor et al. 2013; Moro-Garcia et al. 2012). In contrast, we analyzed the association of HCMV serostatus with another persistent virus infection. While it appears from our data that the HCMV serostatus plays a role in TTV replication, TTV may also have an impact on the aging process. In fact, TTV has the potential ability to increase the production and secretion of proinflammatory cytokines, as has been shown in cell culture experiments (Rocchi et al. 2009). Thus, it is possible that higher TTV replication itself might further contribute to the process of inflammaging, which represents a state of increased inflammatory mediators in the elderly (Shaw et al. 2013; Kim et al. 2012; Larbi et al. 2008; Franceschi et al. 2007). HCMV infection, as shown in cell culture (Wolf et al. 2012), may also contribute to this process; it would be interesting for further research to elucidate whether the combination of persistent HCMV infection and chronic TTV replication might lead to an increased inflammatory status, especially in young or middle-aged persons, compared to TTV DNA-negative populations.
Generally, the prevalence of TTV and its viral load in the presently studied population did not differ significantly from that of other populations in previously published studies. TTV DNA prevalence in this study ranged from 70 % in the young group to 78 % in the middle-aged and 80 % in the elderly group, which is consistent with the known prevalence of TTV DNA in healthy adult populations, which is around 70 to 90 % (Okamoto 2009; Burra et al. 2008; Biagini and de Micco 2008). The mean TTV DNA values of the age groups, which were between 4 and 5 log10 copies/mL, were also comparable to those previously described for healthy immunocompetent persons (Burra et al. 2008). Also, the HCMV IgG seroprevalence, which was in the present population between 45 and 78 %, was comparable to that found by other previously published studies (Crough and Khanna 2009; Hecker et al. 2004).
TTV is a highly variable virus, five genogroups of TTV, as well as about 29 genotypes, exist (Carstens 2010; Biagini and de Micco 2008), and coinfections with different strains occur (Al-Moslih et al. 2004; Niel et al. 2000). Thus, the TTV DNA loads used as a marker probably consisted of the DNA of a number of different TTV strains. Contradictory data have also been published concerning the question of whether the TTV DNA load is associated with the number of genogroups present in clinical samples (Beland et al. 2013; Pinho-Nascimento et al. 2011; Naganuma et al. 2008; Maggi et al. 2005a). We did not find any association between the number of TTV genogroups and age or TTV DNA load in the present population (data not shown).
In conclusion, we have shown that, by using the human TTV infection and the plasma TTV DNA load as markers, the human antiviral defense against TTV replication seems to decrease with age and to change over time in a gender-specific way. In addition, a positive HCMV serostatus seems to be associated with a significant reduction in antiviral defense against TTV, especially at younger age. Further studies will have to elucidate the gender-specific process of immunosenescence and to reveal when and to which degree this process starts during lifetime and which influence persistent HCMV and TTV infections have on this process at a younger age.
Acknowledgments
The authors would like to thank Claudia Kellner, Martin Probst, and Sabine Kreidl for their excellent technical support.
References
- Aberle JH, Puchhammer-Stöckl E. Age-dependent increase of memory B cell response to cytomegalovirus in healthy adults. Exp Gerontol. 2012;47(8):654–657. doi: 10.1016/j.exger.2012.04.008. [DOI] [PubMed] [Google Scholar]
- Al-Moslih MI, Abuodeh RO, Hu YW. Detection and genotyping of TT virus in healthy and subjects with HBV or HCV in different populations in the United Arab Emirates. J Med Virol. 2004;72(3):502–508. doi: 10.1002/jmv.20017. [DOI] [PubMed] [Google Scholar]
- Ansar Ahmed S, Karpuzoglu E, Khan D. Effects of sex steroids on innate and adaptive immunity. In: Klein SL, Roberts C, editors. Sex hormones and immunity to infection. Berlin: Springer; 2010. pp. 19–51. [Google Scholar]
- Beland K, Dore-Nguyen M, Gagne MJ, Patey N, Brassard J, Alvarez F, Halac U. Torque teno virus in children with orthotopic liver transplantation: new insights about a common pathogen. J Infect Dis. 2013 doi: 10.1093/infdis/jit423. [DOI] [PubMed] [Google Scholar]
- Bernardin F, Operskalski E, Busch M, Delwart E. Transfusion transmission of highly prevalent commensal human viruses. Transfusion. 2010;50(11):2474–2483. doi: 10.1111/j.1537-2995.2010.02699.x. [DOI] [PubMed] [Google Scholar]
- Biagini P, de Micco P. Anellovirus. In: Mahy BWJ, Regenmortel MHVV, editors. Encyclopedia of virology. Third. Oxford: Academic; 2008. pp. 104–110. [Google Scholar]
- Burra P, Masier A, Boldrin C, Calistri A, Andreoli E, Senzolo M, Zorzi M, Sgarabotto D, Guido M, Cillo U, Canova D, Bendinelli M, Pistello M, Maggi F, Palu G. Torque teno virus: any pathological role in liver transplanted patients? Transpl Int. 2008;21(10):972–979. doi: 10.1111/j.1432-2277.2008.00714.x. [DOI] [PubMed] [Google Scholar]
- Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009) Arch Virol. 2010;155(1):133–146. doi: 10.1007/s00705-009-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Vaisanen E, Mattila PS, Hedman K, Soderlund-Venermo M. Antigenic diversity and seroprevalences of Torque teno viruses in children and adults by ORF2-based immunoassays. J Gen Virol. 2013;94(Pt 2):409–417. doi: 10.1099/vir.0.046862-0. [DOI] [PubMed] [Google Scholar]
- Colonna-Romano G, Akbar AN, Aquino A, Bulati M, Candore G, Lio D, Ammatuna P, Fletcher JM, Caruso C, Pawelec G. Impact of CMV and EBV seropositivity on CD8 T lymphocytes in an old population from West-Sicily. Exp Gerontol. 2007;42(10):995–1002. doi: 10.1016/j.exger.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22(1):76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focosi D, Maggi F, Albani M, Macera L, Ricci V, Gragnani S, Di Beo S, Ghimenti M, Antonelli G, Bendinelli M, Pistello M, Ceccherini-Nelli L, Petrini M. Torquetenovirus viremia kinetics after autologous stem cell transplantation are predictable and may serve as a surrogate marker of functional immune reconstitution. J Clin Virol. 2010;47(2):189–192. doi: 10.1016/j.jcv.2009.11.027. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiébaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci. 2014;111(2):869–874. doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gameiro CM, Romão F, Castelo-Branco C. Menopause and aging: changes in the immune system—a review. Maturitas. 2010;67(4):316–320. doi: 10.1016/j.maturitas.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14(5):428–436. doi: 10.1038/ni.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görzer I, Haloschan M, Jaksch P, Klepetko W, Puchhammer-Stöckl E. Plasma DNA levels of Torque teno virus and immunosuppression after lung transplantation. J Heart Lung Transpl. 2014;33(3):320–323. doi: 10.1016/j.healun.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Griffiths P. Time to consider the concept of a commensal virus? Rev Med Virol. 1999;9(2):73–74. doi: 10.1002/(SICI)1099-1654(199904/06)9:2<73::AID-RMV254>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hadrup SR, Strindhall J, Køllgaard T, Seremet T, Johansson B, Pawelec G, Thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176(4):2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- Hecker M, Qiu D, Marquardt K, Bein G, Hackstein H. Continuous cytomegalovirus seroconversion in a large group of healthy blood donors. Vox Sang. 2004;86(1):41–44. doi: 10.1111/j.0042-9007.2004.00388.x. [DOI] [PubMed] [Google Scholar]
- Kim OY, Chae JS, Paik JK, Seo HS, Jang Y, Cavaillon JM, Lee JH. Effects of aging and menopause on serum interleukin-6 levels and peripheral blood mononuclear cell cytokine production in healthy nonobese women. Age. 2012;34(2):415–425. doi: 10.1007/s11357-011-9244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid RP, Burke JM, Cox JC, de Villiers E-M, Sullivan CS. A human torque teno virus encodes a microRNA that inhibits interferon signaling. PLoS Pathog. 2013;9(12):e1003818. doi: 10.1371/journal.ppat.1003818. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Klein S, Huber S. Sex differences in susceptibility to viral infection. In: Klein SL, Roberts C, editors. Sex hormones and immunity to infection. Berlin: Springer; 2010. pp. 93–122. [Google Scholar]
- Klein SL, Roberts C, editors. Sex hormones and immunity to infection. Berlin Heidelberg: Springer; 2010. [Google Scholar]
- Larbi A, Franceschi C, Mazzatti D, Solana R, Wikby A, Pawelec G. Aging of the immune system as a prognostic factor for human longevity. Physiology. 2008;23(2):64–74. doi: 10.1152/physiol.00040.2007. [DOI] [PubMed] [Google Scholar]
- Maggi F, Pistello M, Vatteroni M, Presciuttini S, Marchi S, Isola P, Fornai C, Fagnani S, Andreoli E, Antonelli G, Bendinelli M. Dynamics of persistent TT virus infection, as determined in patients treated with alpha interferon for concomitant hepatitis C virus infection. J Virol. 2001;75(24):11999–12004. doi: 10.1128/JVI.75.24.11999-12004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi F, Pifferi M, Fornai C, Andreoli E, Tempestini E, Vatteroni M, Presciuttini S, Marchi S, Pietrobelli A, Boner A, Pistello M, Bendinelli M. TT virus in the nasal secretions of children with acute respiratory diseases: relations to viremia and disease severity. J Virol. 2003;77(4):2418–2425. doi: 10.1128/JVI.77.4.2418-2425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi F, Andreoli E, Lanini L, Fornai C, Vatteroni M, Pistello M, Presciuttini S, Bendinelli M. Relationships between total plasma load of torquetenovirus (TTV) and TTV genogroups carried. J Clin Microbiol. 2005;43(9):4807–4810. doi: 10.1128/JCM.43.9.4807-4810.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi F, Tempestini E, Lanini L, Andreoli E, Fornai C, Giannecchini S, Vatteroni M, Pistello M, Marchi S, Ciccorossi P, Specter S, Bendinelli M. Blood levels of TT virus following immune stimulation with influenza or hepatitis B vaccine. J Med Virol. 2005;75(2):358–365. doi: 10.1002/jmv.20278. [DOI] [PubMed] [Google Scholar]
- Mekker A, Tchang VS, Haeberli L, Oxenius A, Trkola A, Karrer U. Immune senescence: relative contributions of age and cytomegalovirus infection. PLoS Pathog. 2012;8(8):e1002850. doi: 10.1371/journal.ppat.1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen EM, Sagedal S, Bjoro K, Degre M, Opstad PK, Grinde B. Effect of immune modulation on TT virus (TTV) and TTV-like-mini-virus (TLMV) viremia. J Med Virol. 2003;70(1):177–182. doi: 10.1002/jmv.10356. [DOI] [PubMed] [Google Scholar]
- Moro-Garcia MA, Alonso-Arias R, Lopez-Vazquez A, Suarez-Garcia FM, Solano-Jaurrieta JJ, Baltar J, Lopez-Larrea C. Relationship between functional ability in older people, immune system status, and intensity of response to CMV. Age. 2012;34(2):479–495. doi: 10.1007/s11357-011-9240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naganuma M, Tominaga N, Miyamura T, Soda A, Moriuchi M, Moriuchi H. TT virus prevalence, viral loads and genotypic variability in saliva from healthy Japanese children. Acta Paediatr. 2008;97(12):1686–1690. doi: 10.1111/j.1651-2227.2008.00962.x. [DOI] [PubMed] [Google Scholar]
- Naylor K, Li G, Vallejo AN, Lee W-W, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174(11):7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- Niel C, Saback FL, Lampe E. Coinfection with multiple TT virus strains belonging to different genotypes is a common event in healthy Brazilian adults. J Clin Microbiol. 2000;38(5):1926–1930. doi: 10.1128/jcm.38.5.1926-1930.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor D, Trück J, Lazarus R, Clutterbuck EA, Voysey M, Jeffery K, Pollard AJ. The effect of chronic cytomegalovirus infection on pneumococcal vaccine responses. J Infect Dis. 2013 doi: 10.1093/infdis/jit673. [DOI] [PubMed] [Google Scholar]
- Okamoto H. History of discoveries and pathogenicity of TT viruses. Curr Top Microbiol Immunol. 2009;331:1–20. doi: 10.1007/978-3-540-70972-5_1. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res. 2011;157(2):175–179. doi: 10.1016/j.virusres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Larbi A, Derhovanessian E. Senescence of the human immune system. J Comp Pathol. 2010;142(Suppl 1):S39–S44. doi: 10.1016/j.jcpa.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Pinho-Nascimento CA, Leite JP, Niel C, Diniz-Mendes L. Torque teno virus in fecal samples of patients with gastroenteritis: prevalence, genogroups distribution, and viral load. J Med Virol. 2011;83(6):1107–1111. doi: 10.1002/jmv.22024. [DOI] [PubMed] [Google Scholar]
- Puchhammer-Stöckl E, Aberle SW, Heinzl H. Association of age and gender with alphaherpesvirus infections of the central nervous system in the immunocompetent host. J Clin Virol. 2012;53(4):356–359. doi: 10.1016/j.jcv.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172(4):363–371. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocchi J, Ricci V, Albani M, Lanini L, Andreoli E, Macera L, Pistello M, Ceccherini-Nelli L, Bendinelli M, Maggi F. Torquetenovirus DNA drives proinflammatory cytokines production and secretion by immune cells via toll-like receptor 9. Virology. 2009;394(2):235–242. doi: 10.1016/j.virol.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Sakiani S, Olsen NJ, Kovacs WJ. Gonadal steroids and humoral immunity. Nat Rev Endocrinol. 2013;9(1):56–62. doi: 10.1038/nrendo.2012.206. [DOI] [PubMed] [Google Scholar]
- Savva GM, Pachnio A, Kaul B, Morgan K, Huppert FA, Brayne C, Moss PAH, The Medical Research Council Cognitive F. Ageing S. Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell. 2013;12(3):381–387. doi: 10.1111/acel.12059. [DOI] [PubMed] [Google Scholar]
- Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JE, Campbell JP, Edwards KM, Howarth LJ, Pawelec G, Aldred S, Moss P, Drayson MT, Burns VE, Bosch JA. Rudimentary signs of immunosenescence in Cytomegalovirus-seropositive healthy young adults. Age. 2013 doi: 10.1007/s11357-013-9557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi AK, Pradier A, Baumer O, Uppugunduri CR, Huezo-Diaz P, Posfay-Barbe KM, Roosnek E, Ansari M. Validation of SYBR Green based quantification assay for the detection of human Torque Teno virus titers from plasma. Virol J. 2013;10:191. doi: 10.1186/1743-422X-10-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GC, Kao WHL, Murakami P, Xue Q-L, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, Fried LP. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010;171(10):1144–1152. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf J, Weinberger B, Arnold CR, Maier AB, Westendorp RGJ, Grubeck-Loebenstein B. The effect of chronological age on the inflammatory response of human fibroblasts. Exp Gerontol. 2012;47(9):749–753. doi: 10.1016/j.exger.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Ye L, Fang X, Li B, Wang Y, Xiang X, Kong L, Wang W, Zeng Y, Wu Z, She Y, Zhou X. Torque teno virus (SANBAN isolate) ORF2 protein suppresses NF-kappaB pathways via interaction with IkappaB kinases. J Virol. 2007;81(21):11917–11924. doi: 10.1128/JVI.01101-07. [DOI] [PMC free article] [PubMed] [Google Scholar]