Abstract
Objectives
To evaluate the feasibility of a randomized-controlled trial (RCT) and to obtain estimates of the effects of combined cognitive behavioral therapy (CBT) and milnacipran for the treatment of fibromyalgia (FM).
Methods
Fifty eight patients with FM were randomized to one of the 3 treatment arms: (1) combination therapy (n=20), (2) milnacipran + education (n=19), and (3) placebo + CBT (n=19). Subjects received either milnacipran (100 mg/day) or placebo. Subjects also received 8 sessions of phone-delivered CBT or educational instructions, but only from baseline to week 9. Assessments were conducted at baseline, week 9 and 21. The primary endpoints were baseline to week 21 changes in weekly average pain intensity and physical function (SF-36 physical function scale).
Results
Compared to milnacipran, combination therapy demonstrated a moderate effect on improving SF-36 physical function (mean difference (SE) = 9.42 (5.48), p=0.09, effect size (ES) =0.60) and in reducing weekly average pain intensity (mean difference (SE) = −1.18 (0.62), p=0.07, ES=0.67). Compared to milnacipran, CBT had a moderate to large effect in improving SF-36 physical function (mean difference (SE) = 11.0 (5.66), p=0.06, ES=0.70). Despite the presence of concomitant centrally-acting therapies, dropout rate was lower than anticipated (15% at week 21). Importantly, at least 6 out of the 8 phone-based therapy sessions were successfully completed by 89% of the subjects; and adherence to the treatment protocols was >95%.
Conclusions
In this pilot study, a therapeutic approach that combines phone-based CBT and milnacipran was feasible and acceptable. Moreover, the preliminary data supports conducting a fully powered RCT.
Keywords: Fibromyalgia, Cognitive Behavioral Therapy, Milnacipran, Pain, Physical Function
INTRODUCTION
Fibromyalgia (FM) is a common chronic pain condition that affects 2% – 4% of the population(1). Apart from the enormous societal burden of FM, the pain and disability related to FM result in significantly reduced quality of life for patients with this illness. FM, as yet incurable, must be managed as a chronic illness. The two treatment approaches that have well established efficacy for FM are cognitive behavioral therapy (CBT) and drug therapy. Specifically, research literature supports the usefulness of drug therapy in improving the symptoms associated with FM (e.g., pain, sleep disturbance and depression)(2;3), and CBT in reducing pain-related disability(4). Despite the presence of 3 currently approved drugs for FM however, the multifaceted nature of FM and the modest benefit demonstrated in mono-therapy trials supports the need to begin testing combination treatments.
The main goal of the study was to test the feasibility of a randomized-controlled trial (RCT) to investigate the effectiveness of the combination of CBT and milnacipran compared to CBT and milnacipran alone. In the current trial, the use of CBT and milnacipran as adjunct to the medication regimen at study entry has the advantage of reflecting “real world” clinical practice. To help us design for a future fully powered RCT, our feasibility study had the following specific aims: (1) determine recruitment and retention rate; (2) assess the tolerability of milnacipran when added to the usual FM medication regimen; (3) determine the mean number of successfully completed therapy sessions per treatment arm; and (4) obtain preliminary estimates of the treatment effects of the combination of CBT and milnacipan on our two co-primary endpoints: reduction in weekly average pain intensity and improvement in physical function, as measured by SF-36 physical function. We hypothesized that subjects receiving the combination therapy would evidence larger pre-treatment to week 21 benefits in the co-primary outcome measures than subjects who received either milnacipran or CBT monotherapy.
MATERIALS AND METHODS
Eligibility
All potential participants were referred from rheumatology clinics and met the following entry criteria: (1) American College of Rheumatology (ACR) classification criteria for FM(5); (2) weekly average pain intensity score ≥ 4 (as recorded from the wrist watch monitor); (3) on stable doses of medications for FM ≥ 4 weeks; and (4) between 18–65 years old.
Excluded were individuals with (1) current use of selective serotonin reuptake inhibitor (e.g., fluoxetine), venlafaxine, duloxetine or mirtazapine; (2) past or current use of milnacipran; (3) current use of tricyclics ≥100 mg per day; (4) uncontrolled hypertension; (5) active suicidal ideation; (6) planned elective surgery during the study period; (7) ongoing unresolved disability claims; (8) inflammatory rheumatic conditions (e.g., rheumatoid arthritis); (9) active psychosis; (10) pregnancy; and (11) previous CBT for pain-related issues.
Study Design and Procedures
This study was a 21-week randomized double blind placebo controlled trial in which participants were randomized to one of the 3 groups: (1) combination treatment (CBT and milnacipran), (2) CBT (CBT and placebo), and (3) milnacipran (milnacipran and education). The study design involved 4 phases: 1-week screening, baseline assessment, 1-week dose escalation, and 20-week stable-dose phase. During the 1-week screening phase, subjects entered their daily pain scores on a wrist watch monitor (ActiWatch). At the end of the 1-week screening and after completing baseline assessment, eligible subjects were randomized to one of the three treatment arms. During the 1-week dose escalation, subjects received milnacipran or an identical placebo with the following dosing regimen: 12.5 mg on day 1, 12.5 mg twice a day on day 2, and 25 mg twice a day on day 4 to 7. Thereafter, subjects received milnacipran or an identical placebo at 50 mg twice a day throughout the 20-week stable-dose phase.
From week 2 to 9, the combination and CBT groups received eight telephone-delivered CBT sessions, while the milnacipran group received an equal number of telephone-delivered educational instructions. Outcome assessments (including thumb pressure pain sensitivity test) were conducted at baseline, week 9 (immediately after the psycho-educational treatment component) and week 21.
Importantly, subjects were allowed to continue with all their centrally-acting therapies commonly used for FM, including anticonvulsants, opiates, muscle relaxants; and other analgesics such as acetaminophen, aspirin, and nonsteroidal anti-inflammatory agents (NSAID). To reduce co-intervention effects, subjects were instructed to stay on the same baseline medication regimen (including dose and frequency) and to avoid participating in any formal physical therapy or exercise program throughout the 21-week study period. To avoid drug effects on pressure pain sensitivity subjects were asked to avoid any as needed medications (e.g., hydrocodone, acetaminophen, etc.) for at least 6 hours, and any NSAID for 48 hours prior to each outcome assessment.
Medication safety assessments were conducted at the end of the 1-week escalation phase and once weekly thereafter for 8 weeks.
The study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki for experiments involving humans. Study procedures, including written informed consent, were approved by Indiana University-Purdue University Indianapolis Institutional Review Board.
Psycho-educational Therapy
Cognitive behavioral therapy (CBT)
Participants in the combination and CBT groups had 8 telephone-delivered therapy sessions from baseline to week 9. Treatment was provided by two psychology graduate students under the supervision of a clinical psychologist with a great deal of experience providing CBT treatment (MPJ), using a treatment manual adapted from an Otis’s CBT workbook(6). Specifically, the CBT intervention included (1) education on chronic pain including theories of pain; (2) training in progressive muscle relaxation and visual imagery; (3) education on the relationship between automatic thoughts and pain; (4) cognitive restructuring; (5) stress management; (6) time-based pacing and pleasant activity scheduling; (7) anger management and sleep hygiene; and (8) relapse prevention and flare-up planning. Participants had a companion handbook to guide home practice and facilitate learning for each session. Each CBT session lasted for 35 minutes on the average.
Education
Participants in the milnacipran group also received the same number of telephone contacts with an average duration of 30 minutes for each session. The same study clinician (JLS) delivered the educational instructions(7). One topic area was covered during each phone session, and included the following: (1) cost of chronic pain, (2) acute versus chronic pain, (3) sleep (4) depression and other mood changes, (5) pain and communication-part 1, (6) pain and communication–part 2, (7) working with health care providers, and (8) how to make changes. On the subject of mood and sleep, the education treatment protocol only provided general knowledge on the topics while being neutral with respect to actual problem solving.
To assess treatment fidelity, one of the co-authors (MPJ) oversaw the coding of 20% of the all the audiotaped sessions to assess the adherence to the CBT and education protocols. We created fidelity assessment sheets that were based on the contents of each manualized session, which were then used by coders (no study clinician coded her own sessions) to indicate whether or not the components of the session (as described in the manual) were included in that session. A fidelity score was then computed for each session that represented the percent of essential components of that session that were successfully completed by the interventionists. The fidelity scores for each of the audited sessions were than averaged to create an average percent of adherence to the intervention as a whole, across different points during the duration of study.
To evaluate the feasibility of phone-based psycho-educational treatment intervention, completion of at least 6 out of the 8 therapy sessions was considered acceptable.
Primary Outcome Measures
Given that the two main goals of chronic pain management are to reduce pain intensity and improve physical function, our co-primary outcome measures assessed each of these two domains:
Weekly average pain intensity
Using the wrist watch pain monitor (ActiWatch) subjects entered their daily pain scores twice a day for one week at baseline, week 9 and week 21. Subjects rated their pain intensity on a 0-to-10 numerical rating scale, with 0 = no pain sensation and 10 = the most intense pain sensation imaginable. The weekly average pain intensity was the average of the 14 pain intensity scores obtained during the 7-day assessment window. This composite measure of weekly average pain intensity had shown sensitivity to change in chronic pain intervention studies(7–9). The baseline to week 21 change in the weekly average pain intensity was one of the 2 primary endpoints.
Physical functioning
The Medical Outcome Study Short-Form Health Survey (SF-36) Physical Functioning (PF) scale was used to assess physical functioning. The 10-item PF scale inquires about the subject’s perception of their limitations in the performance of various types of physical activities. Scale scores can range from 0 to 100, with higher scores indicating better functioning. The psychometric properties of PF scale are well established(10–12) even in the chronic pain population(13). Moreover, the normative data for the United States general population of women age 45–54 years [mean score (standard deviation): 82.9 (21.7)] is known(12). Despite being a generic assessment of physical function, PF has demonstrated responsiveness to both medical (14–17)and non-medical interventions(18–20) in the FM population. The baseline to week 21 change in the PF scores was the second primary endpoint in this study.
Secondary Outcome Measures
Disease impact
The Fibromyalgia Impact Questionnaire (FIQ) is a reliable, validated self- assessment questionnaire that measures the disease impact in patients with FM. FIQ contains a physical impairment subscale and 6 visual analog scales (VAS) for measuring sleep, pain, anxiety, morning stiffness and depression. It also measures work status, and overall well-being(14;19;21–23).
Depression
The Patient Health Questionnaire 8-item Depression Scale (PHQ-8) was used to assess depression symptoms. PHQ-8 is a brief self-administered scale that assesses major depressive disorder core symptoms and allows a score (possible range: 0 to 24) based on the total number and severity of depressive symptoms noted over the previous two-week period. Its validity and capacity to detect changes of depressive symptoms over time are well established (24–27).
Pain sensitivity
The Thumb Pressure Pain Sensitivity Test was used to assess pain sensitivity. The test uses random direct scaling method to deliver stimuli of varying pressure (5 pressure levels with 3 repetitions at each level) in a random sequence(28–30). With this method, the subject is unaware of this random sequence and must, therefore, attend to, and rate, the stimulus-evoked sensations to produce more meaningful (less prone to bias) data(31). In this study, discrete 5-second pressure stimuli were applied to the left thumb by a 1-cm2 hard rubber probe. Using a numeric verbally anchored (0–20) pain scale(29), subjects rated the intensity of evoked pressure-pain sensations. During the screening visit the 5 stimulus pressures were determined based on pain threshold and tolerance. A random sequence of these stimulus pressures was applied to the left thumb at baseline, week 9 and week 21. Higher pain scores on the numeric (0–20) pain scale represent greater sensitivity to pressure pain stimuli.
We also collected data on the FM-related medications, number of comorbid illnesses, and body mass index at baseline.
Statistical analyses
Baseline continuous variables are summarized in mean (SD) and differences among the three treatment groups at baseline were evaluated by the nonparametric Kruskal-Wallis test. Categorical variables are summarized in percentage and treatment groups were compared using Fishers Exact test. A mixed-model with unstructured covariance was used to examine the effects of interventions on the two primary outcomes assessed at weeks 9 and 21. Treatment effects were adjusted for the baseline value of the response variable, body mass index, and number of comorbidity at baseline. Pain sensitivity was assessed based on self report evoked pain scores corresponding to 15 random pressure stimuli resulting from 5 pressure levels each repeated three times. Pressure levels varied from subject to subject. To analyze this outcome, mean pain score for each subject at weeks 9 and 21 was computed at each pressure level. Repeated measure ANOVA was used to model this outcome at week 21 and treatment comparisons were adjusted for baseline value of the mean pain score, pressure level, body mass index and number of comorbidity at baseline.
Results
Over a period of 14 months (January 2010 to February 2011), 67 subjects were screened for potential participation in the study. Of these, 58 (86%) met the inclusion criteria, enrolled and were randomized to combination (n=20), milnacipran (n=19) and CBT (n=19). The majority of screening failures were due to low pain intensity scores [7 (77%)] (Figure 1). Nine subjects (15%) discontinued the study and did not complete the week 21 assessment: 3 (15%) subjects in the combination, 2 (10%) in the milnacipran, and 4 (21%) in the CBT group. Compared to the 49 subjects who completed the study, the 9 subjects who discontinued the study with no follow-up data had slightly worse mean (standard deviation) FIQ score [70.66 (11.05) vs. 63.75 (11.53), p=0.09] and were more sensitive to pressure stimuli [9.38 (1.54) vs. 8.75 (0.64), p=0.07] at study entry.
Figure 1.
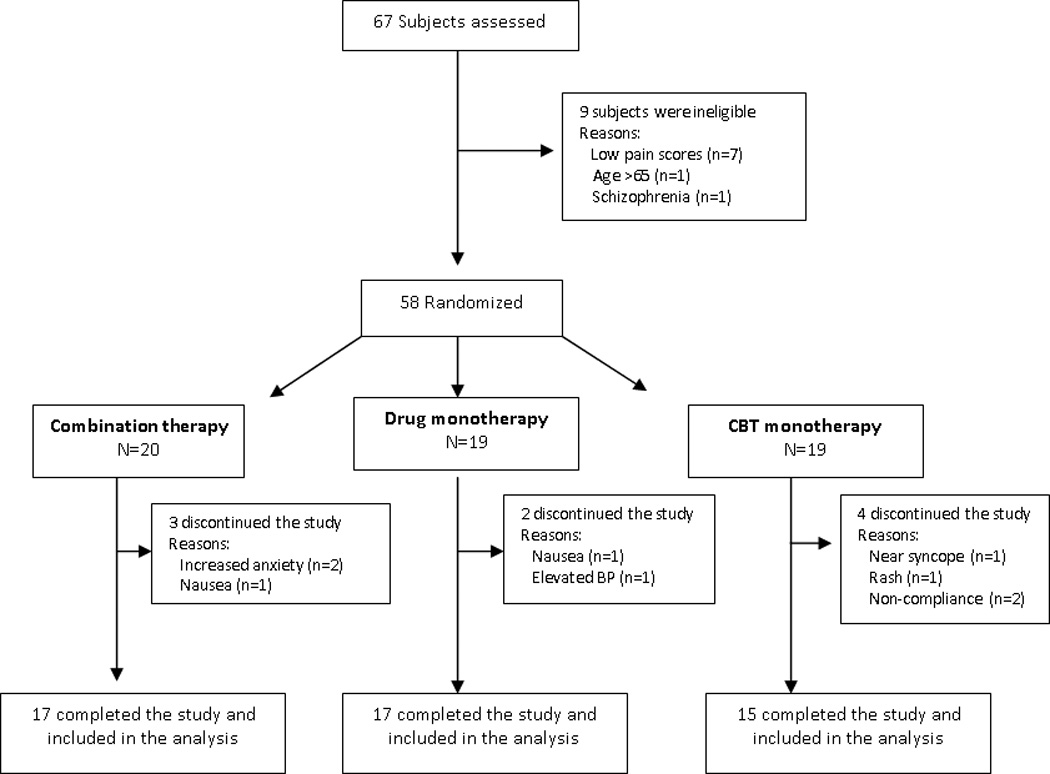
Flow of Participants in the Trial
Baseline characteristics were similar across treatment groups except for body mass index (Table 1). Subjects in the milnacipran group were heavier compared to subjects in the other two groups (p=0.03).
Table 1.
Baseline Characteristics
| Combination N=20 |
Milnacipran N=19 |
CBT N=19 |
All Subjects N=58 |
|
|---|---|---|---|---|
| Demographics | ||||
| Age in years | 44.85 (8.80) | 47.89 (10.61) | 47.11 (11.93) | 46.59 (10.39) |
| Gender, % female | 95% | 100% | 84% | 93% |
| Race, % white¶ | 65% | 95% | 84% | 81% |
| Education, % > high school | 70% | 58% | 84% | 71% |
| Marital status, % married | 60% | 42% | 42% | 48% |
| Employment, % employed | 55% | 33% | 67% | 52% |
| Clinical Variables | ||||
| Weekly average pain score | 6.24 (1.28) | 6.33 (1.30) | 6.36 (1.30) | 6.31 (1.27) |
| SF-36 physical function | 49.75 (22.15) | 41.84 (23.35) | 43.95 (22.15) | 45.26 (22.41) |
| FIQ (range 0–100) | 66.56 (11.27) | 66.35 (10.92) | 61.62 (12.59) | 64.84 (11.64) |
| PHQ-8 depression (range 0–24) | 9.15 (5.23) | 11.37 (4.23) | 11.21 (4.74) | 10.55 (4.79) |
| Years with fibromyalgia diagnosis | 11.01 (9.77) | 13.37 (10.70) | 11.89 (10.03) | 12.07 (10.04) |
| Body mass index (kg/m2)* | 30.94 (7.27) | 36.60 (8.57) | 30.52 (6.78) | 32.66 (7.94) |
| Number of comorbidity† | 0.67 (0.77) | 1.50 (1.20) | 1.05 (1.18) | 1.07 (1.10) |
| Medications, % prescribed | ||||
| Tricyclics | 6 (30%) | 8 (42%) | 5 (26%) | 19 (33%) |
| Anticonvulsants | 6 (30%) | 7 (37%) | 6 (32%) | 19 (33%) |
| Opioid analgesics | 8 (40%) | 6 (32%) | 11 (58%) | 25 (43%) |
| NSAID | 15 (75%) | 13 (68%) | 13 (68%) | 41 (70%) |
| Muscle relaxant | 1 (5%) | 0 (0%) | 2 (10%) | 3 (5%) |
| Pain Sensitivity | ||||
| Evoked pain score (range 0–20)Ψ | 9.2 (1.01) | 8.9 (1.02) | 8.5 (1.02) | 8.8 (0.59) |
Weekly average pain score was based on real time (ActiWatch) daily recording of pain scores. (_) standard deviation
Group difference p value=0.05;
Group difference p value=0.03;
Group difference p value=0.08
Self-report pain in response to 15 random varying pressure stimuli (5 pressure levels with 3 repetitions at each level); mean (SE) was adjusted for baseline pressure stimuli.
CBT: Cognitive behavioral therapy; FIQ: Fibromyalgia impact questionnaire; NSAID: Non steroidal anti-inflammatory drug
Clinical Outcomes
Compared to milnacipran alone, combination therapy demonstrated a moderate effect on improving SF-36 physical function (mean difference (SE) = 9.42 (5.48), p=0.09, effect size=0.60) and in reducing weekly average pain intensity (mean difference (SE) = −1.18 (0.62), p=0.07, effect size=0.67) (See Table 2). The magnitude of change in the weekly average pain intensity score in the combination group was more than twice the magnitude of change in the milnacipran group. In addition, the improvement in physical function in the combination group was more than three times of the improvement in the milnacipran group.
Table 2.
Efficacy outcomes
| Combination N=17 |
Milnacipran N=17 |
CBT N=15 |
P values Combination vs. milnacipran Combination vs. CBT Milnacipran vs. CBT |
|
|---|---|---|---|---|
| Primary Outcomes | ||||
| Δ Weekly average pain intensity score¶ |
−2.15 (0.43)† | −0.97 (0.43)† | −1.67 (0.45)† | 0.07 |
| 0.44 | ||||
| 0.28 | ||||
| Δ SF-36 physical function score | 13.47 (3.74)† | 4.05 (3.84) | 15.04 (4.01)† | 0.09 |
| 0.77 | ||||
| 0.06 | ||||
| Secondary Outcomes | ||||
| Δ FIQ score | −15.20 (3.89)† | −12.70 (3.89)† | −11.16 (4.05)† | 0.66 |
| 0.47 | ||||
| 0.78 | ||||
| Δ PHQ-8 depression score | −2.65 (1.06)† | −2.93 (1.07)† | −3.19 (1.11)† | 0.86 |
| 0.72 | ||||
| 0.86 | ||||
| Δ Evoked pain scores | −0.76 (1.20) | −0.41 (1.22) | 0.78 (1.27) | 0.83 |
| 0.37 | ||||
| 0.50 | ||||
Δ: Baseline to week 21 change in the specified variable; Values represent means and standard error ()
Real time (ActiWatch) daily recording of pain intensity scores
Significant within group difference (p<0.04)
Above analyses were adjusted for baseline response outcome, body mass index, and number of comorbidity
Number of subjects with ≥ 30% reduction in weekly average pain intensity score: combination (n=8) vs. Milnacipran (n=7) and CBT (n=4)
CBT: Cognitive behavioral therapy; FIQ: Fibromyalgia impact questionnaire; PHQ-8: Patient health questionnaire-8
In comparing combination therapy vs. CBT, there were no statistically significant differences in either the weekly average pain intensity, and the effect sizes were small (mean difference (SE) = −0.49 (0.62), p=0.44, effect size=0.27) or the SF-36 physical function (mean difference (SE) = −1.58 (5.50), p=0.77, effect size=0.10).
Compared to milnacipran, CBT was marginally efficacious in improving SF-36 physical function (mean difference (SE) = 11.0 (5.66), p=0.06, effect size=0.70). However, in terms of improvement in weekly average pain intensity, no significant difference was noted between CBT and milnacipran (mean difference (SE) = 0.69 (0.64), p=0.28, effect size=0.40).
Further, in terms of the improvement in disease impact (FIQ), depression severity (PHQ8) and changes in pain sensitivity, the effect sizes for all the pair-wise group comparisons were <0.30 (very small). Interestingly, subjects in the combination and milnacipran became less sensitive to pressure stimuli compared to subjects in the CBT group, albeit this difference was not statistically significant.
Sample Size in the Main Trial
To test the superiority of combination therapy over milnacipran for the co-primary outcome measures of changes in weekly average pain intensity and SF-36 physical function, a sample size of 54 per group has at least 80% power (α=0.05 and Bonferroni adjustment) to detect mean group differences similar to what we have observed in this pilot study. On the other hand, to test the superiority of CBT over milnacipran for the improvement in SF-36 physical function, a sample size of 33 per group has 80% power (α=0.05) to detect a mean group difference of 11 would be needed.
Adverse Events
Adverse events occurring in at least 5% of subjects in the milnacipran-treated subjects, and at an incidence of at least 2 times that of CBT (placebo) subjects included nausea, constipation, insomnia, blood pressure elevation, anxiety, hot flushes and increase sweating (Table 3). Consistent with the most common side effect reported in the literature(17;32), the most common adverse event in the milnacipran-treated subjects was nausea that typically resolved after 1 to 2 weeks of being on stable dose of milnacipran. As seen in Figure 1, adverse events resulted in the premature discontinuation of 15%, 10%, and 21% of combination, drug and CBT subjects, respectively. One subject in the CBT group discontinued the study at week 5 due to near syncope/syncope that required hospital admission. After extensive in-hospital evaluation the discharge diagnosis was psychogenic syncope.
Table 3.
Adverse effects
| Combination therapy N=20 |
Milnacipran N=19 |
CBT N=19 |
|
|---|---|---|---|
| Nausea | 9 (45%) | 8 (42%) | 3 (16%) |
| Vomiting | 2 (10%) | 0 (0%) | 1 (5%) |
| Headache | 4 (20%) | 4 (21%) | 4 (21%) |
| Constipation | 5 (25%) | 5 (26%) | 2 (11%) |
| Diarrhea | 4 (20%) | 2 (11%) | 3 (16%) |
| Insomnia | 3 (15%) | 6 (32%) | 3 (16%) |
| Palpitations | 1 (5%) | 0 (0%) | 0 (0%) |
| Blood pressure elevation | 2 (10%) | 1 (5%) | 0 (0%) |
| Rash | 0 (0%) | 0 (0%) | 1 (5%) |
| Depression | 2 (10%) | 0 (0%) | 2 (11%) |
| Anxiety | 3 (15%) | 4 (21%) | 0 (0%) |
| Hot flush | 2 (10%) | 5 (26%) | 0 (0%) |
| Fatigue | 3 (15%) | 0 (0%) | 0 (0%) |
| Increased sweating | 3 (15%) | 5 (26%) | 0 (0%) |
| Near syncope | 0 (0%) | 0 (0%) | 1 (5%) |
CBT: Cognitive behavioral therapy
Psycho-educational Treatment Dose and Fidelity
The mean (SD) number of successfully completed phone therapy sessions were about the same in all the treatment groups [7.5 (1.6) for combination vs. 7.6 (1.0) for milnacipran vs. 7.3 (1.2) for CBT, p=0.64)]. Similarly, there were no group differences in the proportion of subjects who completed ≥6 therapy sessions (90% for combination vs. 89% for milnacipran vs. 89% for CBT).
As can be seen in Table 4, adherence to both the CBT and the education manualized treatment protocol was consistently strong. Over the course of the study, the adherence to protocol for the education intervention improved slightly. Adherence to the CBT protocol fluctuated slightly over time; however the variations in adherence were minimal and overall percentage of adherence was high (97%).
Table 4.
Rates of Treatment Protocol Adherence for the CBT and Education Interventions
| Fidelity to the Intervention (Percent) | |||||
|---|---|---|---|---|---|
| 1st 4 months | 2nd 4 months | 3rd 4 months | 4th 3 months | Overall | |
| CBT | 98.6 | 97.7 | 95.4 | 97.4 | 97.3 |
| Education | 98.7 | 100 | 100 | 100 | 99.4 |
CBT: Cognitive behavioral therapy
Discussion
The primary goal of the study was to test the feasibility of conducting an RCT and to obtain preliminary estimates of the effects of combining CBT and milnacipran for the treatment of moderately severe FM. In support of a future adequately powered RCT, our results showed the following: (1) combination therapy was marginally efficacious than either milnacipran or CBT in reducing pain and improving physical function; (2) milnacipran, as an adjunctive treatment, was well tolerated despite the presence of concurrent centrally-acting therapies; (3) adequate recruitment rate (4 subjects per month) was achieved through physicians referrals, and high retention rate (84%) was observed almost six months post-randomization; (4) our interventionists achieved >95% adherence to the manualized treatment protocols, and (5) at least 6 of the 8 phone-based psycho-educational treatment sessions was successfully completed by 89% of study participants.
Given that mono-therapies (drug or psychologically-based therapies) produce only modest reductions in symptoms (15;16;33;34), it is time to consider more careful study of treatment components that are designed to complement each other. The clinical benefits of combined therapy may be additive (i.e., both therapies contribute independently to an overall positive outcome) or synergistic (i.e., the presence of one enhances the efficacy of the other). For instance, if pain is reduced modestly by a medication, patients may then be better able to process the information provided and take advantage of CBT skills included in a treatment program. Unfortunately, the absence of a fourth group (i.e., placebo-education control group) in our study precluded us from assessing the interaction of milnacipran and CBT. However, we purposely designed our study to be a 3-arm RCT because of the established efficacy of milnacipran (17;32;35) or CBT (4;36;37) as monotherapy in the management of FM; thus, to add a fourth arm (“double placebo”) is neither scientifically necessary nor ethically justified.
In contrast to the typical drug trial in FM, our study protocol allowed subjects to continue their centrally-acting therapies throughout the entire length of the study. We decided to use milnacipran as an adjunctive treatment (rather than as a standalone drug) to reflect real world clinic practice, and also to assess tolerability of milnacipran for patients on multiple concomitant medications. If the study participants were washed off of their medications larger effect sizes of combination therapy (vs. monotherapy) might have been observed.
Under the guidance of a CBT-trained clinical psychologist (MPJ), our study interventionists, two psychology graduate students, successfully delivered and completed the therapy sessions. Using manual-based treatment protocols, the interventionists undertook 1 day of face-to-face training, four weekly, and six biweekly one-hour phone supervisions. A number of studies have shown that the extent of therapists’ clinical experience is of little value in predicting outcome in patients with low back pain(38) and various psychiatric diagnoses(39;40). In fact, treatment outcome of CBT appears to be more strongly associated with whether the treatments are manual-based or not(41). Perhaps, future larger RCT should consider training clinical nurses or other health care providers (e.g., physical therapists) as interventionists to provide the manual-based CBT. In real world clinic setting, the greater availability of nurses over clinical psychologist may make CBT more accessible to the greater majority of FM patients. In addition, providing CBT over the phone may also improve access to CBT. In Mohr et al depression study(42), the lesser long term effects of phone-based CBT (compared to face-to-face CBT) after treatment cessation suggests the importance of maintenance CBT intervention to sustain clinical improvement over the long term.
As this was a feasibility study, two primary outcome measures were piloted. In support of the latter decision, the intensity of pain and impairment in physical function are two important therapeutic targets in the management of chronic pain. In the future larger RCT, a composite measure of improvement in pain intensity and physical function may be warranted to reduce the chance of false positives. For example, the primary efficacy end point could be a composite responder rate(43) in which a subject is classified as responder if she/he has ≥30% improvement from baseline in the weekly average pain intensity(44), and ≥10-point improvement from baseline in the SF-36 physical function score(45). In our study, the proportions of responders defined in this way in each arm were as follows: 41% for combination, 24% for milnacipran and 7% for CBT (p=0.10). In the future larger RCT, we may assume the following responder rates as a basis for determining sample size in a power analysis: 40% for combination, 20% for milnacipran and 10% for CBT. Based on these assumptions, the sample size needed to compare the combination and the milnacipran arms would be 82 per group (80% power and 0.05 ά level). For comparing the combination versus the CBT arms, the sample size needed would be 36 per group.
Importantly, while the magnitude of changes in the main outcomes was larger in the CBT compared to the milnacipran group (Table 2), a smaller proportion of subjects in the CBT compared to the milnacipran group (7% vs. 24%) met our proposed definition of response (i.e., composite responder rate). There are several explanations for these seemingly contradictory results. First, while more CBT than milnacipran subjects (73% vs. 41%) had improvement in either pain intensity or physical function, CBT subjects rarely reported improvement in both measures (7% vs. 24%). Second, among subjects who reported increased pain intensity (2 subjects in the milnacipran and 3 subjects in the CBT groups), the magnitude of increase was much more in the milnacipran group than in the CBT group, which resulted in smaller mean change score in the milnacipran group. Finally, once we use a certain cut point to define a dichotomous outcome we are no longer able to consider the differences in the distributions of the continuous outcome for values above the cut point and for values below the cut point. Thus, depending on the distributions of the outcome variables, the use of continuous versus categorical (based on cut-points) outcomes can lead to different conclusions. This makes it imperative to make a priori decisions about the primary outcome variable (s).
Indeed, the findings from this pilot study support the need to test the efficacy of combination treatment versus medication mono-therapy. The substantial additive benefits of CBT, if replicated in a larger study, could potentially convince clinicians to consider CBT as an add-on therapy prior to prescribing another medication. In addition, our findings suggest that the additive benefits of milnacipran over and above CBT may be minimal. Some readers might conclude that this finding argues against the inclusion of a third arm (i.e., CBT alone) in a larger trial. However, the inclusion of a third (CBT) arm is necessary in our view because the clinical implications of replicating these findings in a larger more definitive trial are potentially profound; they might suggest, for example, that CBT should be used to replace medication management, and that medications (at least the medications studied) may not contribute to positive outcomes over and above CBT alone. Without the inclusion of a CBT (alone) arm, however, it would not be possible to test this important hypothesis.
Clearly, high quality RCTs that test a combination of proven biological and psychological therapies are necessary to enhance treatment of FM. In this pilot study, a therapeutic approach that combines phone-based CBT and milnacipran was feasible and acceptable, and that our preliminary outcome data supports conducting a fully powered RCT.
ACKNOWLEDGEMENTS
Funding: National Institute of Arthritis and Musculoskeletal and Skin Diseases (Grant number: 1R21AR056046-01A2). The funding institution did not play any role in the collection, analysis, data interpretation, and writing of the manuscript. The authors thank Forest Research Institute for providing the active drug and placebo. The authors also thank Chen-Ping Lin, PhD who helped in the treatment fidelity assessment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest to declare
Reference List
- 1.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38(1):19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 2.Hauser W, Bernardy K, Uceyler N, Sommer C. Treatment of fibromyalgia syndrome with antidepressants: a meta-analysis. JAMA. 2009;301(2):198–209. doi: 10.1001/jama.2008.944. [DOI] [PubMed] [Google Scholar]
- 3.Hauser W, Bernardy K, Uceyler N, Sommer C. Treatment of fibromyalgia syndrome with gabapentin and pregabalin--a meta-analysis of randomized controlled trials. Pain. 2009;145(1–2):69–81. doi: 10.1016/j.pain.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Hassett AL, Williams DA. Non-pharmacological treatment of chronic widespread musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25(2):299–309. doi: 10.1016/j.berh.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 6.Otis J. Managing Chronic Pain: A cognitive behavioral therapy approach (Therapist Guide) New York City: Oxford University Press; 2007. [Google Scholar]
- 7.Ehde DM, Jensen MP. Feasibility of a cognitive restructuring intervention for treatment of chronic pain in persons with disabilities. Rehabilitation Psychology. 2004;49:254–258. [Google Scholar]
- 8.Cardenas DD, Warms CA, Turner JA, Marshall H, Brooke MM, Loeser JD. Efficacy of amitriptyline for relief of pain in spinal cord injury: results of a randomized controlled trial. Pain. 2002;96(3):365–373. doi: 10.1016/S0304-3959(01)00483-3. [DOI] [PubMed] [Google Scholar]
- 9.Jensen MP, Hanley MA, Engel JM, Romano JM, Barber J, Cardenas DD, et al. Hypnotic analgesia for chronic pain in persons with disabilities: a case series. Int J Clin Exp Hypn. 2005;53(2):198–228. doi: 10.1080/00207140590927545. [DOI] [PubMed] [Google Scholar]
- 10.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 11.Neumann L, Berzak A, Buskila D. Measuring health status in Israeli patients with fibromyalgia syndrome and widespread pain and healthy individuals: utility of the short form 36-item health survey (SF-36) Semin Arthritis Rheum. 2000;29(6):400–408. doi: 10.1053/sarh.2000.7171. [DOI] [PubMed] [Google Scholar]
- 12.Ware JE, Snow KK, Kosinski M, et al. SF-36 Health Survey: Manual and Interpretation Guide. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 13.Bergman S, Jacobsson LT, Herrstrom P, Petersson IF. Health status as measured by SF-36 reflects changes and predicts outcome in chronic musculoskeletal pain: a 3-year follow up study in the general population. Pain. 2004;108(1–2):115–123. doi: 10.1016/j.pain.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Bennett RM, Kamin M, Karim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study. Am J Med. 2003;114(7):537–545. doi: 10.1016/s0002-9343(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 15.Crofford LJ, Rowbotham MC, Mease PJ, Russell IJ, Dworkin RH, Corbin AE, et al. Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52(4):1264–1273. doi: 10.1002/art.20983. [DOI] [PubMed] [Google Scholar]
- 16.Arnold LM, Lu Y, Crofford LJ, Wohlreich M, Detke MJ, Iyengar S, et al. A double-blind, multicenter trial comparing duloxetine with placebo in the treatment of fibromyalgia patients with or without major depressive disorder. Arthritis Rheum. 2004;50(9):2974–2984. doi: 10.1002/art.20485. [DOI] [PubMed] [Google Scholar]
- 17.Mease PJ, Clauw DJ, Gendreau RM, Rao SG, Kranzler J, Chen W, et al. The efficacy and safety of milnacipran for treatment of fibromyalgia. a randomized, double-blind, placebo-controlled trial. J Rheumatol. 2009;36(2):398–409. doi: 10.3899/jrheum.080734. [DOI] [PubMed] [Google Scholar]
- 18.Mannerkorpi K, Nyberg B, Ahlmen M, Ekdahl C. Pool exercise combined with an education program for patients with fibromyalgia syndrome. A prospective, randomized study. J Rheumatol. 2000;27(10):2473–2481. [PubMed] [Google Scholar]
- 19.Mannerkorpi K, Ahlmen M, Ekdahl C. Six- and 24-month follow-up of pool exercise therapy and education for patients with fibromyalgia. Scand J Rheumatol. 2002;31(5):306–310. doi: 10.1080/030097402760375223. [DOI] [PubMed] [Google Scholar]
- 20.Valim V, Oliveira L, Suda A, Silva L, de AM, Barros NT, et al. Aerobic fitness effects in fibromyalgia. J Rheumatol. 2003;30(5):1060–1069. [PubMed] [Google Scholar]
- 21.Goldenberg D, Mayskiy M, Mossey C, Ruthazer R, Schmid C. A randomized, double-blind crossover trial of fluoxetine and amitriptyline in the treatment of fibromyalgia. Arthritis Rheum. 1996;39(11):1852–1859. doi: 10.1002/art.1780391111. [DOI] [PubMed] [Google Scholar]
- 22.Burckhardt CS, Mannerkorpi K, Hedenberg L, Bjelle A. A randomized, controlled clinical trial of education and physical training for women with fibromyalgia. J Rheumatol. 1994;21(4):714–720. [PubMed] [Google Scholar]
- 23.Bennett RM, Burckhardt CS, Clark SR, O'Reilly CA, Wiens AN, Campbell SM. Group treatment of fibromyalgia: a 6 month outpatient program. J Rheumatol. 1996;23(3):521–528. [PubMed] [Google Scholar]
- 24.Lowe B, Unutzer J, Callahan CM, Perkins AJ, Kroenke K. Monitoring depression treatment outcomes with the patient health questionnaire-9. Med Care. 2004;42(12):1194–1201. doi: 10.1097/00005650-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Lowe B, Kroenke K, Herzog W, Grafe K. Measuring depression outcome with a brief self-report instrument: sensitivity to change of the Patient Health Questionnaire (PHQ-9) J Affect Disord. 2004;81(1):61–66. doi: 10.1016/S0165-0327(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe B, Spitzer RL, Grafe K, Kroenke K, Quenter A, Zipfel S, et al. Comparative validity of three screening questionnaires for DSM-IV depressive disorders and physicians' diagnoses. J Affect Disord. 2004;78(2):131–140. doi: 10.1016/s0165-0327(02)00237-9. [DOI] [PubMed] [Google Scholar]
- 28.Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, et al. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144(1–2):95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Gracely RH, Dubner R, McGrath PA. Narcotic analgesia: fentanyl reduces the intensity but not the unpleasantness of painful tooth pulp sensations. Science. 1979;203(4386):1261–1263. doi: 10.1126/science.424753. [DOI] [PubMed] [Google Scholar]
- 30.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105(3):403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 31.Gracely RH. Studies of Experimental Human Pain. In: McMahon S, Koltzenburg M, editors. Wall and Melzack's Textbook of Pain. 5th edition. Philadelphia, PA: Elsevier, Health Sciences Division, Churchill Livingstone; 2005. pp. 267–289. [Google Scholar]
- 32.Clauw DJ, Mease P, Palmer RH, Gendreau RM, Wang Y. Milnacipran for the treatment of fibromyalgia in adults: a 15-week, multicenter, randomized, double-blind, placebo-controlled, multiple-dose clinical trial. Clin Ther. 2008;30(11):1988–2004. doi: 10.1016/j.clinthera.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Holman AJ, Myers RR. A randomized, double-blind, placebo-controlled trial of pramipexole, a dopamine agonist, in patients with fibromyalgia receiving concomitant medications. Arthritis Rheum. 2005;52(8):2495–2505. doi: 10.1002/art.21191. [DOI] [PubMed] [Google Scholar]
- 34.Williams DA, Cary MA, Groner KH, Chaplin W, Glazer LJ, Rodriguez AM, et al. Improving physical functional status in patients with fibromyalgia: a brief cognitive behavioral intervention. J Rheumatol. 2002;29(6):1280–1286. [PubMed] [Google Scholar]
- 35.Vitton O, Gendreau M, Gendreau J, Kranzler J, Rao SG. A double-blind placebo-controlled trial of milnacipran in the treatment of fibromyalgia. Hum Psychopharmacol. 2004;19(Suppl 1):S27–S35. doi: 10.1002/hup.622. [DOI] [PubMed] [Google Scholar]
- 36.Thieme K, Gracely RH. Are psychological treatments effective for fibromyalgia pain? Curr Rheumatol Rep. 2009;11(6):443–450. doi: 10.1007/s11926-009-0065-6. [DOI] [PubMed] [Google Scholar]
- 37.Eccleston C, Williams AC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2009;(2):CD007407. doi: 10.1002/14651858.CD007407.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Resnik L, Hart DL. Using clinical outcomes to identify expert physical therapists. Phys Ther. 2003;83(11):990–1002. [PubMed] [Google Scholar]
- 39.Propst A, Paris J, Rosberger Z. Do therapist experience, diagnosis and functional level predict outcome in short term psychotherapy? sCan J Psychiatry. 1994;39(3):168–176. doi: 10.1177/070674379403900309. [DOI] [PubMed] [Google Scholar]
- 40.Nyman SJ, Nafziger MA, Smith TB. Client Outcomes Across Counselor Training Level within a Multitiered Supervision Model. Journal of Counseling and Development. 2010;88(2):204–209. [Google Scholar]
- 41.Wilson GT. Manual-based treatments: the clinical application of research findings. Behav Res Ther. 1996;34(4):295–314. doi: 10.1016/0005-7967(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 42.Mohr DC, Ho J, Duffecy J, Reifler D, Sokol L, Burns MN, et al. Effect of telephone-administered vs face-to-face cognitive behavioral therapy on adherence to therapy and depression outcomes among primary care patients: a randomized trial. JAMA. 2012;307(21):2278–2285. doi: 10.1001/jama.2012.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the Clinical Importance of Treatment Outcomes in Chronic Pain Clinical Trials: IMMPACT Recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 44.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD. A comparison of clinically important differences in health-related quality of life for patients with chronic lung disease, asthma, or heart disease. Health Serv Res. 2005;40(2):577–591. doi: 10.1111/j.1475-6773.2005.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


