Abstract
Some of the most common and devastating disorders of the brain target the hippocampal formation. The hippocampal formation is a complex circuit of interconnected regions, and it is assumed that clues into the causes of these disorders are embedded within the circuit. Neuroimaging tools have been optimized to interrogate the malfunctioning hippocampal circuit, and by applying these tools to patients in the earliest stages of disease and to animal models, patterns of regional dysfunction have been established for Alzheimer’s disease, schizophrenia and cognitive aging. More recently, studies have begun deciphering the cellular and molecular reasons underlying regional dysfunction. Collectively, this information clarifies the pathophysiology of these disorders and informs on therapeutic strategies.
INTRODUCTION
So deeply ingrained as to seem eternal, ‘anatomical localization’ is a relatively new concept in the annals of Medicine. It is easily forgotten that throughout most of Medicine’s history diseases were thought to reflect diffuse processes caused by imbalances of bodily substances or fluids. The shift toward an anatomical basis of disease began slowly during the late renaissance and was only fully articulated in 1761 when the anatomist Giovanni Morgagni published his textbook, “On the seats and causes and disease through an investigation of anatomy” (Morgagni, 1761). This landmark textbook formulated what subsequently became a first principle in modern Medicine: Localizing the anatomical site of pathology is an important first step towards better understanding, diagnosing and ultimately curing any disease. Cell theory and its medical offshoot ‘cellular pathology’, pioneered by Rudolf Virchow (Virchow, 1863) and others in 19th century, refined the target of disease localization. With the introduction of the idea that ‘sick cells’ are the basic unit of disease, localization needed to scale down, from an organ or structure to the level of afflicted cells.
Besides providing a basis for disease classification and improving diagnostic accuracy, the ultimate promise of disease localization is mechanistic. Answering why one group of cells are afflicted by a disease while a neighboring group is not, promises to shed light on pathogenic causes. Although the logic of disease localization has been validated—zooming in from anatomy to cells and down to molecular mechanisms-- it has only recently been applied to complex disorders that affect the hippocampal formation. Notably, the fact that the hippocampal formation is a circuit made up interconnected regions, each housing a distinct group of neurons, was first suggested by Ramon Y Cajal (Cajal, 1911) (Fig. 1), whose research was performed in the cell theory spirit of the 19th century. (Indeed, compared to other organs, the brain was the last holdout in defiance of cell theory). Subsequent cellular, electrophysiological and molecular studies have further characterized the distinct properties of neurons contained within each region of the hippocampal circuit—the entorhinal cortex, the dentate gyrus, the cornu ammonis (CA) subfields, and the subiculum (Fig. 1). Adding a further level of complexity, the functional and molecular properties of neurons within each region vary across the hippocampal long-axis (as reviewed in (Small et al., 2011)). This diversity within the hippocampal circuit accounts for how the circuit can be a ‘seat’ to a range of mechanistically distinct disorders. Its molecular diversity in particular has led to the hypothesis that different regions of the hippocampal circuit will be preferentially targeted by different disorders (Small, 2001).
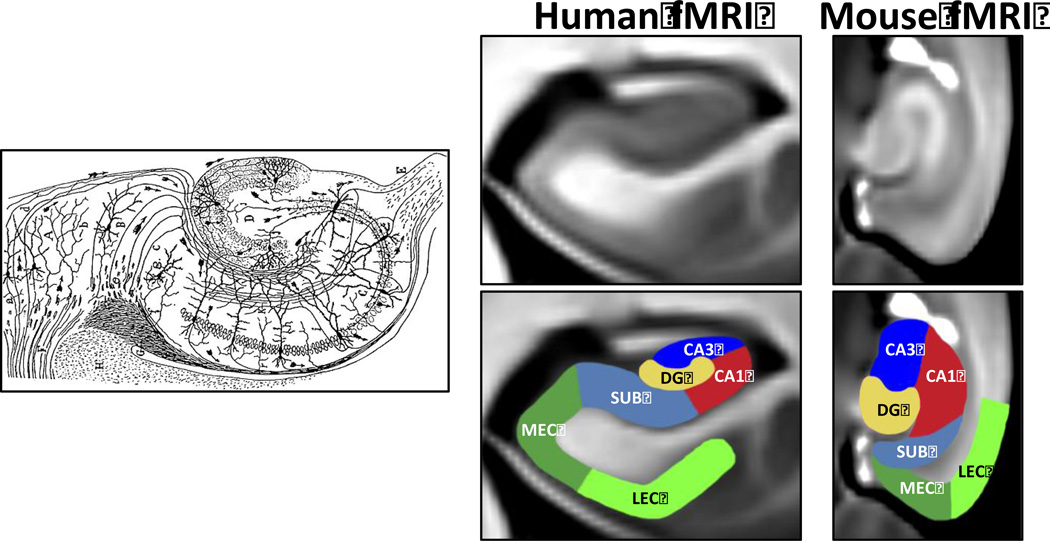
Figure 1. Visualizing the hippocampal circuit.
In 1911, Ramon Y Cajal used the Golg-staining technique applied to the postmortem rabbit (left panel) to first show that regions of the hippocampal formation comprise distinct neurons and that the regions are connected to form a circuit (Cajal, 1911). Approximately a hundred years later, functional imaging techniques were optimized to visualize the regions of the hippocampal circuit in living humans (middle panel) and living mice (right panel). In the examples shown, fMRI images were generated with an exogenous contrast agent allowing submillimeter resolution (Adapted from (Khan et al., 2014). (LEC=lateral entorhinal cortex; MEC=medial entorhinal cortex; DG=dentate gyrus; SUB=subiculum)
While a plausible hypothesis, pinpointing regional dysfunction for many disorders that involve the hippocampal circuit presents a challenge. Most disorders start subtly, in ‘preclinical’ or ‘prodromal’ stages that then worsen and spread over time, and so mapping the anatomy of the earliest stages of disease is often elusive by the time patients come to autopsy. Furthermore, although some disorders develop dramatic cell death and other clear histological findings, nearly all disorders begin in a ‘cell sickness’ stage—that is neuronal dysfunction occurs before neuronal loss. Mapping neuronal dysfunction is tricky using the time-honored approach of postmortem examination.
In principle, in vivo functional imaging is well suited to overcome these challenges, but it was only at the turn of the 21st century that MRI-based techniques began to be optimized, enabling individual regions of the hippocampal circuit to be visualized (Davachi and Wagner, 2002; Gabrieli et al., 1997; Kirwan et al., 2007; Small et al., 2000; Small et al., 2004; Small et al., 1999; Zeineh et al., 2000). Ultimately, by allowing the full circuit to be interrogated during the earliest stages of diseases these tools have been able to localize hippocampal regions preferentially affected by—and, just as importantly, resistant to-- many disorders.
We have previously reviewed how the anatomical organization of the hippocampal circuit provides a framework for characterizing and investigating hippocampal dysfunction, among the surprisingly numerous and diverse range of diseases that target this circuit (Small et al., 2011). The primary purpose of this perspective is to review recent evidence that illustrates how this framework has been successfully applied in isolating pathogenic mechanisms. To date the clearest patterns of regional dysfunction within the hippocampal circuit have been established for Alzheimer’s disease, schizophrenia and cognitive aging, and it is for these disorders that patterns of hippocampal dysfunction have been mechanistically most illuminating.
It should be pointed out that other areas outside the hippocampal circuit are affected in all three disorders, and as previously reviewed (Small et al., 2011) many are directly linked to the circuit (for example, the posterior parietal lobe and the precuneus in Alzheimer’s disease, the ventral striatum in schizophrenia, and the prefrontal cortex in cognitive aging). We have previously reviewed how connectivity with extra-hippocampal areas might account for phenotypic diversity among the range of disorders in which the hippocampal circuit is implicated (Small et al., 2011). In this perspective, with an eye on mechanism, I will focus exclusively on the hippocampal circuit, and demonstrate how the well-characterized organization of the circuit can be used in an effort to follow the first principle of disease localization. As I will discuss, by performing molecular studies designed to address why one region of the circuit is affected and another neighboring region is resistant to a particular disorder, the circuit can be used as an anatomical ‘Rosetta stone’ in trying to decipher pathogenic mechanisms.
Alzheimer’s Disease and endosomal transport
Although Alzheimer’s disease (AD) is a neurodegenerative disorder, it too is thought to begin in a cell sickness stage (Selkoe, 2002), assumed to occur years before cell death. Postmortem maps of neuronal loss have mapped an anatomical pattern of disease, showing that the entorhinal cortex is the hippocampal region most vulnerable to the earliest stages of AD (Gomez-Isla et al., 1996), while suggesting that the neighboring dentate gyrus is the one that appears most resistant to the disease (Braak et al., 2006; Schonheit et al., 2004; West et al., 1994; West et al., 2000). Aside from confirming this pattern of dysfunction in living patients, including the relative resistance of the dentate gyrus (Moreno et al., 2007), the main contribution of neuroimaging is showing that entorhinal cortex dysfunction exists during the earliest preclinical stages of disease (Huijbers et al., 2014; Khan et al., 2014; Miller et al., 2013; Whitwell et al., 2007; Whitwell et al., 2008). Functional neuroimaging has been able to detect entorhinal cortex dysfunction in mouse models of disease (Khan et al., 2014; Moreno et al., 2007), notable for manifesting cell sickness without cell death, thereby validating that these some of these neuroimaging tools can in principle capture the earliest pathophysiological stage of disease
Guided by this pattern of dysfunction, we performed a molecular profiling study on postmortem tissue harvested from the entorhinal cortex as the ‘target’ region and the dentate gyrus as the ‘within-subject control’ region, from brains with and without AD. A statistical model was used to identify molecular correlates that might help explain why the entorhinal cortex is targeted by AD, but the neighboring dentate gyrus is relatively preserved. Molecules linked to the retromer emerged as the primary findings (Small et al., 2005), which were found to be affected in the entorhinal cortex but unaffected in the dentate gyrus. The retromer is an assembly of proteins that work in unison to orchestrate cargo transport out of endosomes (Burd and Cullen, 2014), either to the trans-golgi network or back to the cell surface. As originally suggested (Small et al., 2005), retromer has been found to transport the amyloid-precursor protein (APP) out of endosomes via VPS10-containing receptors, in particular Sorl1 (also called Sorla) (Fjorback et al., 2012). Although this study suggests that retromer defects are a molecular correlate of entorhinal cortex dysfunction, they do not explain why these defects occur in the entorhinal cortex in the first place. This remains an outstanding question that we are currently pursuing.
A pathogenic link to AD has been established by genetic studies led by Richard Mayeux, Peter St. George Hyslop, Lindsay Farrer and their colleagues. Collectively, these studies have identified variants in SORL1 (Rogaeva et al., 2007), genes encoding other VPS10-containing receptors (Reitz et al., 2013), and genes encoding other key elements of retromer (Vardarajan et al., 2012). The importance of SORL1 in particular has been confirmed by a recent large-scale genetic meta-analysis (Lambert et al., 2013). Further support for a pathogenic role has been provided by genetically modified retromer deficient animal models (Muhammad et al., 2008; Wen et al., 2011). It is within endosomes that APP is most likely to be cleaved by BACE1 (beta-site APP cleaving enzyme 1) (Small and Gandy, 2006), a β-secretase that initiates the ‘amyloidogenic’ processing of APP leading to the accumulation of the neurotoxic fragments β-CTF (β C-terminal fragment) and Aβ (amyloid β). Defects in retromer-mediated transport leads to the accumulation of these neurotoxic fragments by increasing the resident time of APP in endosomes (Bhalla et al., 2012; Mecozzi et al., 2014).
The observed defects in the retromer transport pathway should be interpreted in the context of a growing body of evidence that have more broadly implicated endosomal transport defects in the disease. Primary evidence pinpointing the endosome as the intracellular site of dysfunction was described in a seminal series of histological studies performed by Randy Nixon and his colleagues (Cataldo et al., 2000). They showed that endosomes in the entorhinal cortex and the CA subfields of the hippocampal circuit are abnormally enlarged during the earliest stages of disease. Remarkably, the distribution of abnormally enlarged endosomes was nearly diagnostic of the disease, with very little overlap in the size of endosomes observed in age-matched controls. Additionally, Nixon and colleagues showed that carrying the APOE4 gene, the strongest genetic risk factor for late-onset disease, further enhanced endosomal enlargement, and that abnormal enlargement did not occur as part of normal aging. The near uniformity of the abnormality in patients with late-onset ‘sporadic’ AD, suggests a convergence of pathogenic mechanisms leading to endosomal enlargement. Furthermore, they observed enlarged endosomes in brain regions that were relatively free of evidence of Aβ pathology (Cataldo et al., 1997; Cataldo et al., 2000), which agrees with an emerging view that endosomal abnormalities are upstream to Aβ accumulation. The fact that enlarged endosomes represent a cell-biological phenotype of AD has recently been validated in neurons derived via IPSC (induced pluripotent stem cells) from patients (Israel et al., 2012). Furthermore, studies using IPSC-derived neurons have also showed that APP mutations mis-traffic APP to endosomes (Muratore et al., 2014), similar to the effect caused by retromer defects.
Endosomes are a hub of membrane trafficking, and mechanistically it can be assumed that they become enlarged either because of accelerated delivery, via cell-surface endocytosis, or by reducing the transport out of endosomes. Indeed, Nixon and his colleagues provided the first evidence for increased endocytosis in AD (Cataldo et al., 1997), while retromer defects (Bhalla et al., 2012) and an AD-associated deficiency in phosphatidylinositol-3-phosphate (PI3P) (Morel et al., 2013), a lipid that regulates transport of cargo out of endosomes, cause enlarged endosomes. Interestingly, one of many affects of APOE4 is to increase the endocytosis of cargo including APP from the surface membrane (Ye et al., 2005; Yu et al., 2014), which can explain why APOE4 is associated with enlarged endosomes. More generally, additional evidence that endosomal dysfunction plays an upstream role in AD pathology comes from large-scale genetic studies that have identified a group of endosomal transport genes that have emerged as a unified genetic factor linked to AD (Lambert et al., 2013).
Besides accounting for how APP fragments can be misprocessed in late onset AD, recent studies are beginning to link endosomal transport defects to other core features of the disease. For example, retromer defects has been found in microglia of AD patients (Lucin et al., 2013), and this same study showed how these defects reduces the cell surface transport of AD-linked microglia receptors. Of course, besides amyloid accumulation, neurofibrillary tangles, and their constituent abnormal tau species, are the second defining histological feature of AD. While amyloid accumulation, particularly its soluble forms (Lue et al., 1999; Naslund et al., 2000), begins to accumulate in the entorhinal cortex during the early disease stages, it is tau related abnormalities that are very prominent histological features found in this region. Might endosomal dysfunction be linked to tau toxicity? This remains an open question, but recent evidence suggests that it is while tau is translocated to endosomes that the processing to tau pathology is triggered(Michel et al., 2014). Additionally, recent studies have begun finding associations of AD-linked endosomal genes with tau pathology (Ando et al., 2013; Chapuis et al., 2013).
Taken together, these observations establish that endosomal dysfunction is a pathogenic mechanism, and validate endosomal transport as a ‘cell biological’ target for drug discovery. Providing proof-of-principle for this idea, in a collaboration with Greg Petsko and Dagmar Ringe, we has recently isolated a novel class of pharmacological chaperones (Mecozzi et al., 2014) that increases the stability and function of retromer, shunts APP away from endosomes, and decreases the abnormal processing of APP. In principle, there are numerous other pharmacological approaches that can correct endosomal transport defects, and future studies will determine whether these novel classes of drugs will prove efficacious whey applied to early stages of AD.
Schizophrenia and glutamate production or catabolism
In schizophrenia, to contrast with AD, the histological abnormalities observed within the hippocampal circuit are subtler, primarily involving a mild loss of GABAergic interneurons in the CA regions (Benes and Berretta, 2001) (Fig. 2). Neuroimaging has therefore played a more important role in implicating the hippocampal circuit in the disease. This was originally documented using relatively low-resolution techniques, showing dominant hippocampal atrophy in the disease (as reviewed in (Adriano et al., 2012)), and studies suggesting that that the hippocampus is characterized by an abnormal hypermetabolic state (Friston et al., 1992; Heckers et al., 1998; Kawasaki et al., 1992; Malaspina et al., 2004; Medoff et al., 2001; Schobel et al., 2009). A recent study, combining high-resolution functional and structural MRI applied to patients in the earliest stages of the disease, more precisely mapped the spatial and temporal profile of abnormalities within the hippocampal circuit (Schobel et al., 2013). These studies suggest that hypermetabolism occurs first, observed during prodromal stages and is localized to the CA1 region of the hippocampal circuit. With progression to the psychosis stage of disease, hypermetabolism is followed by atrophy, and this too is localized predominantly to the CA1 (Fig. 2).
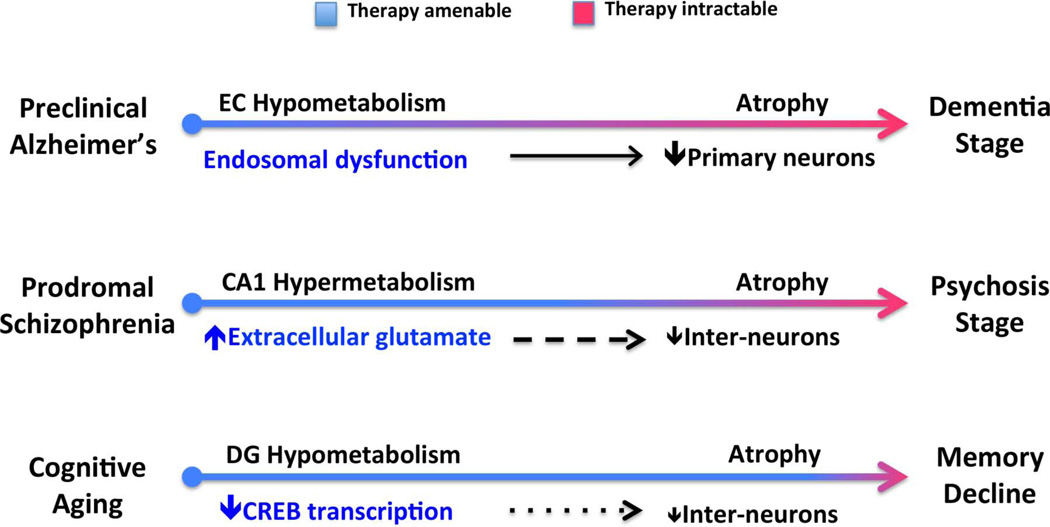
Figure 2. Pathogenic mechanisms and the progression of hippocampal-based disorders.
Alzheimer’s disease starts in a ‘preclinical stage’ before progressing to dementia. Hypometabolism during the preclinical stage has been localized to the entorhinal cortex (EC), in which defects in retromer-mediated endosomal transport have been isolated. Schizophrenia starts in a ‘prodromal stage’ before progressing to psychosis. Hypermetabolism during the prodromal stage has been localized to the CA1 region, and has been linked to increases in extracellular glutamate. Cognitive aging starts in the fourth decade of life and progresses gradually to memory decline. Hypometabolism during normal aging has been localized to the dentate gyrus (DG), in which defects in CREB-dependent histone acetylation have been isolated. During the progression of each entity, a primary ‘cell sickness’ stage is thought to antedate a ‘cell death’ stage. (A dramatic loss of primary neurons in Alzheimer’s disease, a more subtle loss of GABAergic interneurons in schizophrenia, and a subtle loss of hilar interneurons in cognitive aging). Accordingly, the disorders are anticipated to be most amenable to therapeutic interventions during the earliest pathophysiological stage.
The remarkable anatomical concordance suggested that hypermetabolism drives the ensuing atrophy and it was hypothesized that elevations in extracellular glutamate might act as the pathogenic driver of both indicators of hippocampal dysfunction (Schobel et al., 2013). This hypothesis was based on the fact that NMDA-blocking agents, which cause an increase in extracellular glutamate (Moghaddam et al., 1997), recapitulate the disease’s full spectrum of symptoms (Krystal et al., 1994), and because mouse fMRI studies suggested that the CA1 region is differentially sensitive to alterations in glutamate (Gaisler-Salomon et al., 2009). This hypothesis was confirmed when the same high-resolution functional and structural MRI techniques which were originally used to establish the disease’s imaging-phenotype were applied to mice who were administered NMDA-blocking agents (Schobel et al., 2013). These studies established that elevations in extracellular glutamate phenocopied the disease-- first causing selective CA1 hypermetabolism followed more slowly by atrophy. Furthermore, the MRI-detected atrophy was shown to be associated with GABAergic interneuron loss, thereby phenocopying the histological findings of the disease (Schobel et al., 2013). This interpretation is bolstered by a recent study combining magnetic resonance spectroscopy (MRS) and structural MRI (Kraguljac et al., 2013), showing that glutamate elevations in the hippocampus of patients are correlated with CA1 atrophy.
What then causes the elevations in extracellular glutamate? One idea is that there is a relative redistribution of glutamate from the intracellular to the extracellular space. This interpretation might be problematic, however, because the glutamate elevations observed by MRS cannot be caused by a simple redistribution of glutamate. Rather, these neuroimaging findings suggest that there is a net increase in glutamate levels, either by increased production or decreased catabolism. The production and catabolism of glutamate is tightly regulated by a group of enzymes distributed in astrocytes and neurons (Erecinska and Silver, 1990; Maciejewski and Rothman, 2008)—including, glutamate dehydrogenase, glutaminase, glutamine synthetase, glutamic acid decarboxylase, GABA transaminase, and Aspartate & Alanine aminotransferase. It is hypothesized therefore that these enzymes might be defective in schizophrenia. Alterations in this group of enzymes might occur genetically—either directly by genes encoding these enzymes (Jia et al., 2010) or secondarily by genetic links to the glutamatergic system (Walsh et al., 2008; Wilson et al., 2006; Winchester et al., 2014)--or via environmental stressors and risk factors (e.g.(Matrisciano et al., 2012)).
Although this hypothesis awaits further confirmation, the imaging studies summarized above suggest that glutamate elevation itself is a valid drug target and that glutamate-reducing agents might be an effective therapeutic intervention. An important implication of the imaging studies is that these agents should be given during prodromal stages of disease, before the loss of interneurons and its imaging correlate atrophy (Fig. 2). Indeed, recent failures of clinical trials using the glutamate-reducing agent Pomaglumetad (LY2140023) (Adams et al., 2014) might be explained by the fact that they were tested in patients who were already in advanced stages of the disease.
Cognitive aging and CREB-dependent histone acetylation
Perhaps more than any other process that targets the hippocampal circuit, mapping the effects of normal aging is most challenging, as there are a number of diseases that target the aging hippocampal circuit, including AD, vascular disease, and diabetes (Wu et al., 2008). Insofar that it is still difficult to completely exclude these disorders when investigating the hippocampal circuit in aging populations, isolating an anatomical pattern of dysfunction linked to aging per se presents a unique challenge.
One approach in addressing this challenge is to apply high-resolution functional MRI tools in a comparative cross-species manner. The hippocampal circuit is remarkably homologous across mammals (Fig. 1) and all develop age-related hippocampal dysfunction. As non-human mammals do not develop AD, vascular disease or diabetes, it is assumed that mechanisms by which aging affects the hippocampal circuit is preserved from rodents, through nonhuman primates, to humans. If true, then patterns of age-related regional dysfunction should also be preserved. Guided by this reasoning, we have applied high-resolution functional MRI tools first to healthy humans across the life span (Small et al., 2002), and then tested whether these patterns are observed in aging rhesus monkeys (Small et al., 2004), rats, and wildtype mice (Moreno et al., 2007). Together, these results have suggested that the dentate gyrus is the dominant region affected by aging, while the entorhinal cortex is the region that shows the greatest resistance. Notably, dentate gyrus dysfunction appears to begin in the fourth decade of life and to gradually worsen thereafter (Fig. 2).
Other studies using alternative forms of functional (Yassa et al., 2011b) or structural MRI (Wisse et al., 2014) largely agree with this conclusion, and in fact some studies suggest that aging is associated with a mild loss of interneurons selectively in the dentate gyrus (West, 1993). With a greater appreciation of the distinct cognitive operations performed by the dentate gyrus, notably pattern separation (Yassa and Stark, 2011), memory tests have been developed that can assess dentate gyrus function using cognitive measures. By applying these cognitive tools, a growing number of studies have confirmed that the dentate gyrus is a dominant ‘seat’ of age-related hippocampal dysfunction (Gracian et al., 2013; Holden et al., 2012; Yassa et al., 2011a).
Guided by this pattern of dysfunction, we performed a molecular profiling study on postmortem tissue harvested from the dentate gyrus as the ‘target’ region and the entorhinal cortex as the ‘within-subject control’ region, from healthy brains across the adult lifespan. A statistical model was used to identify molecular correlates that might help explain why the dentate gyrus is targeted by aging, but the neighboring entorhinal cortex is relatively preserved. Results implicated the histone-binding protein RbAp48 (Pavlopoulos et al., 2013), which was found to undergo selective age-related deficiency in the dentate gyrus. By interacting with the CREB-Binding Protein (CBP) (Zhang et al., 2000), RbAp48 regulates CREB-dependent histone acetylation and transcription (CREB = cAMP Response Element–Binding Protein). In a collaborative study with Eric Kandel, we first confirmed that RbAp48 is, like in humans, selectively deficient in the dentate gyrus of aging wildtype mice. More importantly, Kandel’s lab developed a mouse model that expressed a dominant-negative inhibitor of RbAp48. These mice were found to phenocopy the cognitive, neuroimaging, and histone acetylation defects characteristics of the aging hippocampal circuit. Providing further causal confirmation, overexpressing RbAp48 selectively in the dentate gyrus of aging wildtype mice rescued age-related memory decline (Pavlopoulos et al., 2013).
Another recent study provided further causal confirmation that the dentate gyrus is the seat of age-related memory decline, and for its underlying molecular mechanism (Villeda et al., 2014). This study used heterochronic parabiosis to transfer the blood of young to old mice. Young blood was found to rescue the electrophysiological and behavior phenotypes of the aging hippocampus, and it did so by increasing spine density selectively in the dentate gyrus. Moreover, the induced increase in dentate gyrus spine density was found to be mediated by the CREB pathway.
Perhaps the strongest pathogenic link between CREB-dependent histone acetylation and cognitive aging in humans comes from a recent genetic study performed by Richard Mayeux and his colleagues (Barral et al., 2014). They tested whether variants in genes encoding RbAp48, CREB1 and CBP are associated with memory performance cognitively healthy elderly. While associations were detected in variants encoding all three proteins, the strongest and most reliable association was for variants encoding CBP (Barral et al., 2014)
These findings suggest interventions for ameliorating age-related memory decline. While age-related memory decline is to some degree universal, and therefore has extremely high prevalence, the symptoms associated with aging are not nearly as devastating as those caused by disease. Nevertheless, with increasing longevity, even mild memory decline has a negative impact on older individuals who are not only living longer but who want to remain engaged in cognitively demanding lifestyles.
There is a pharmacopeia of agents that target the CREB pathway and these might be worth testing in aging populations. Indeed, the FDA has recently opined that age-related memory decline is an approved indication for clinical trials. At the same time, because age-related memory decline is considered a normal process, behavioral or dietary interventions might be more appropriate. Aerobic exercise is one such intervention, as neuroimaging studies have found that it enhances dentate gyrus function (Pereira et al., 2007). Dietary interventions might also work, as Fred Gage and his colleagues have showing that a diet enriched in the flavanol epicatechin improves dentate gyrus function in mice (van Praag et al., 2007). Accordingly, we have initiated a randomized-controlled clinical trial, testing the effect a high flavanol diet has on the hippocampal circuit in healthy aging individuals.
Summary
Giovanni Morgagni was quoted as saying that when presented with a disease one should listen to the “cry of a suffering organ”. With the introduction of ‘cell theory’ and its clinical correlate ‘cellular pathology’, listening needed to be fine-tuned, to detect the cry of suffering cells. High-resolution functional and structural MRI has turned out to be very good listening devices that can isolate the group of neurons preferentially afflicted by, and relatively resistant to, various disorders that target the hippocampal circuit. Moreover, neuroimaging studies have de novo established or re-affirmed that disorders can start in a cell sickness stage before progressing to cell death, a later stage that must be considered more intractable to therapeutic interventions (Fig. 2).
While these neuroimaging tools might be useful in improving diagnostic capabilities, disease localization is most important for isolating pathogenic mechanisms. As reviewed in this perspective, insight into cellular pathophysiology and molecular mechanisms can be used to develop novel interventions. Human genetics or animal models have at least partially validated many of the mechanisms discussed. Nevertheless, the ultimate validation of any hypothesis about disease is showing that an intervention that targets the proposed cellular site or molecular mechanism treats the actual disease. This ultimate validation awaits future studies for all disorders that affect the hippocampal circuit.
Acknowledgements
NIH grants AG034618, AG025161. Richard Mayeux is thanked for discussions on Alzheimer’s disease and cognitive aging, Jeffery Lieberman for discussions on schizophrenia, and Eric Kandel for discussions on cognitive Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams DH, Zhang L, Millen BA, Kinon BJ, Gomez JC. Pomaglumetad Methionil (LY2140023 Monohydrate) and Aripiprazole in Patients with Schizophrenia: A Phase 3, Multicenter, Double-Blind Comparison. Schizophrenia research and treatment. 2014;2014:758212. doi: 10.1155/2014/758212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adriano F, Caltagirone C, Spalletta G. Hippocampal volume reduction in first-episode and chronic schizophrenia: a review and meta-analysis. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2012;18:180–200. doi: 10.1177/1073858410395147. [DOI] [PubMed] [Google Scholar]
- Ando K, Brion JP, Stygelbout V, Suain V, Authelet M, Dedecker R, Chanut A, Lacor P, Lavaur J, Sazdovitch V, et al. Clathrin adaptor CALM/PICALM is associated with neurofibrillary tangles and is cleaved in Alzheimer's brains. Acta neuropathologica. 2013;125:861–878. doi: 10.1007/s00401-013-1111-z. [DOI] [PubMed] [Google Scholar]
- Barral S, Reitz C, Small SA, Mayeux R. Genetic variants in a CREB-dependent histone acetylation pathway influence memory performance in cognitively healthy elderly. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.06.024. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Bhalla A, Vetanovetz CP, Morel E, Chamoun Z, Di Paolo G, Small SA. The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport. Neurobiol Dis. 2012;47:126–134. doi: 10.1016/j.nbd.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta neuropathologica. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C, Cullen PJ. Retromer: a master conductor of endosome sorting. Cold Spring Harbor perspectives in biology. 2014;6 doi: 10.1101/cshperspect.a016774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal RY. Histologie du Systeme Nerveux de l'Homme et des Vertebretes. 1 and 2. Paris: A. Maloine; 1911. 1911. [Google Scholar]
- Cataldo AM, Barnett JL, Pieroni C, Nixon RA. Increased neuronal endocytosis and protease delivery to early endosomes in sporadic Alzheimer's disease: neuropathologic evidence for a mechanism of increased beta-amyloidogenesis. J Neurosci. 1997;17:6142–6151. doi: 10.1523/JNEUROSCI.17-16-06142.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer’s disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. The American journal of pathology. 2000;157:277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis J, Hansmannel F, Gistelinck M, Mounier A, Van Cauwenberghe C, Kolen KV, Geller F, Sottejeau Y, Harold D, Dourlen P, et al. Increased expression of BIN1 mediates Alzheimer genetic risk by modulating tau pathology. Molecular psychiatry. 2013;18:1225–1234. doi: 10.1038/mp.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. Journal of neurophysiology. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Progress in neurobiology. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- Fjorback AW, Seaman M, Gustafsen C, Mehmedbasic A, Gokool S, Wu C, Militz D, Schmidt V, Madsen P, Nyengaard JR, et al. Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing. J Neurosci. 2012;32:1467–1480. doi: 10.1523/JNEUROSCI.2272-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Liddle PF, Frith CD, Hirsch SR, Frackowiak RS. The left medial temporal region and schizophrenia. A PET study. Brain. 1992;115(Pt 2):367–382. doi: 10.1093/brain/115.2.367. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Gaisler-Salomon I, Miller GM, Chuhma N, Lee S, Zhang H, Ghoddoussi F, Lewandowski N, Fairhurst S, Wang Y, Conjard-Duplany A, et al. Glutaminase-deficient mice display hippocampal hypoactivity, insensitivity to pro-psychotic drugs and potentiated latent inhibition: relevance to schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34:2305–2322. doi: 10.1038/npp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracian EI, Shelley LE, Morris AM, Gilbert PE. Age-related changes in place learning for adjacent and separate locations. Neurobiol Aging. 2013;34:2304–2309. doi: 10.1016/j.neurobiolaging.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature neuroscience. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Holden HM, Hoebel C, Loftis K, Gilbert PE. Spatial pattern separation in cognitively normal young and older adults. Hippocampus. 2012;22:1826–1832. doi: 10.1002/hipo.22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Mormino EC, Wigman SE, Ward AM, Vannini P, McLaren DG, Becker JA, Schultz AP, Hedden T, Johnson KA, et al. Amyloid deposition is linked to aberrant entorhinal activity among cognitively normal older adults. J Neurosci. 2014;34:5200–5210. doi: 10.1523/JNEUROSCI.3579-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, et al. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P, Wang L, Meltzer HY, Zhao Z. Common variants conferring risk of schizophrenia: a pathway analysis of GWAS data. Schizophrenia research. 2010;122:38–42. doi: 10.1016/j.schres.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Maeda Y, Urata K, Yamaguchi N, Matsuda H, Hisada K, Suzuki M, Takashima T. Regional cerebral blood flow in patients with schizophrenia. A preliminary report. European archives of psychiatry and clinical neuroscience. 1992;241:195–200. doi: 10.1007/BF02190252. [DOI] [PubMed] [Google Scholar]
- Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, Small SA. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer's disease. Nature neuroscience. 2014;17:304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Jones CK, Miller MI, Stark CE. High-resolution fMRI investigation of the medial temporal lobe. Human brain mapping. 2007;28:959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA psychiatry. 2013;70:1294–1302. doi: 10.1001/jamapsychiatry.2013.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of general psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, O’Brien CE, Bieri G, Czirr E, Mosher KI, Abbey RJ, Mastroeni DF, Rogers J, Spencer B, Masliah E, et al. Microglial beclin 1 regulates retromer trafficking and phagocytosis and is impaired in Alzheimer's disease. Neuron. 2013;79:873–886. doi: 10.1016/j.neuron.2013.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. The American journal of pathology. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewski PK, Rothman DL. Proposed cycles for functional glutamate trafficking in synaptic neurotransmission. Neurochemistry international. 2008;52:809–825. doi: 10.1016/j.neuint.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Harkavy-Friedman J, Corcoran C, Mujica-Parodi L, Printz D, Gorman JM, Van Heertum R. Resting neural activity distinguishes subgroups of schizophrenia patients. Biological psychiatry. 2004;56:931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrisciano F, Tueting P, Maccari S, Nicoletti F, Guidotti A. Pharmacological activation of group-II metabotropic glutamate receptors corrects a schizophrenia-like phenotype induced by prenatal stress in mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:929–938. doi: 10.1038/npp.2011.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecozzi VJ, Berman DE, Simoes S, Vetanovetz C, Awal MR, Patel VM, Schneider RT, Petsko GA, Ringe D, Small SA. Pharmacological chaperones stabilize retromer to limit APP processing. Nat Chem Biol. 2014 doi: 10.1038/nchembio.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Michel CH, Kumar S, Pinotsi D, Tunnacliffe A, St George-Hyslop P, Mandelkow E, Mandelkow EM, Kaminski CF, Kaminski Schierle GS. Extracellular monomeric tau protein is sufficient to initiate the spread of tau protein pathology. J Biol Chem. 2014;289:956–967. doi: 10.1074/jbc.M113.515445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Younes L, Ratnanather JT, Brown T, Trinh H, Postell E, Lee DS, Wang MC, Mori S, O’Brien R, et al. The diffeomorphometry of temporal lobe structures in preclinical Alzheimer’s disease. NeuroImage Clinical. 2013;3:352–360. doi: 10.1016/j.nicl.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel E, Chamoun Z, Lasiecka ZM, Chan RB, Williamson RL, Vetanovetz C, Dall’Armi C, Simoes S, Point Du Jour KS, McCabe BD, et al. Phosphatidylinositol-3-phosphate regulates sorting and processing of amyloid precursor protein through the endosomal system. Nature communications. 2013;4:2250. doi: 10.1038/ncomms3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno H, Wu WE, Lee T, Brickman A, Mayeux R, Brown TR, Small SA. Imaging the abeta-related neurotoxicity of Alzheimer disease. Arch Neurol. 2007;64:1467–1477. doi: 10.1001/archneur.64.10.1467. [DOI] [PubMed] [Google Scholar]
- Morgagni GB. De sedibus, et causis morborum per anatomen indagatis libri quinque. Venice: Typographia Remondini; 1761. [Google Scholar]
- Muhammad A, Flores I, Zhang H, Yu R, Staniszewski A, Planel E, Herman M, Ho L, Kreber R, Honig LS, et al. Retromer deficiency observed in Alzheimer's disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7327–7332. doi: 10.1073/pnas.0802545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratore CR, Rice HC, Srikanth P, Callahan DG, Shin T, Benjamin LN, Walsh DM, Selkoe DJ, Young-Pearse TL. The familial Alzheimer's disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Human molecular genetics. 2014 doi: 10.1093/hmg/ddu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD. Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. Jama. 2000;283:1571–1577. doi: 10.1001/jama.283.12.1571. [DOI] [PubMed] [Google Scholar]
- Pavlopoulos E, Jones S, Kosmidis S, Close M, Kim C, Kovalerchik O, Small SA, Kandel ER. Molecular mechanism for age-related memory loss: the histone-binding protein RbAp48. Science translational medicine. 2013;5:200ra115. doi: 10.1126/scitranslmed.3006373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Sloan R, Gage FH, Brown TR, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C, Tosto G, Vardarajan B, Rogaeva E, Ghani M, Rogers RS, Conrad C, Haines JL, Pericak-Vance MA, Fallin MD, et al. Independent and epistatic effects of variants in VPS10-d receptors on Alzheimer disease risk and processing of the amyloid precursor protein (APP) Translational psychiatry. 2013;3:e256. doi: 10.1038/tp.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007 doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, Inbar BP, Corcoran CM, Lieberman JA, Moore H, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93. doi: 10.1016/j.neuron.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, Small SA. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives of general psychiatry. 2009;66:938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonheit B, Zarski R, Ohm TG. Spatial and temporal relationships between plaques and tangles in Alzheimer-pathology. Neurobiol Aging. 2004;25:697–711. doi: 10.1016/j.neurobiolaging.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Small S, Wu E, Bartsch D, Lacefield C, DeLaPaz R, Mayeux R, Stern Y, Kandel E. Imaging physiologic dysfunction of individual hippocampal subregions in humans and genetically modified mice. Neuron. 2000:653–664. doi: 10.1016/s0896-6273(00)00144-6. [DOI] [PubMed] [Google Scholar]
- Small SA. Age-related memory decline; current concepts and future directions. Archives of Neurology. 2001;58:360–364. doi: 10.1001/archneur.58.3.360. [DOI] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. From The Cover: Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Gandy S. Sorting through the cell biology of Alzheimer’s disease: intracellular pathways to pathogenesis. Neuron. 2006;52:15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Kent K, Pierce A, Leung C, Kang MS, Okada H, Honig L, Vonsattel JP, Kim TW. Model-guided microarray implicates the retromer complex in Alzheimer's disease. Annals of neurology. 2005;58:909–919. doi: 10.1002/ana.20667. [DOI] [PubMed] [Google Scholar]
- Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Annals of neurology. 1999;45:466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nature reviews Neuroscience. 2011;12:585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y. Imaging hippocampal function across the human life span: is memory decline normal or not? Annals of neurology. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- van Praag H, Lucero MJ, Yeo GW, Stecker K, Heivand N, Zhao C, Yip E, Afanador M, Schroeter H, Hammerstone J, et al. Plant-derived flavanol (-)epicatechin enhances angiogenesis and retention of spatial memory in mice. J Neurosci. 2007;27:5869–5878. doi: 10.1523/JNEUROSCI.0914-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardarajan BN, Bruesegem SY, Harbour ME, St George-Hyslop P, Seaman MN, Farrer LA. Identification of Alzheimer disease-associated variants in genes that regulate retromer function. Neurobiol Aging. 2012;33:2231, e2215–e2231, e2230. doi: 10.1016/j.neurobiolaging.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, et al. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nature medicine. 2014 doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow RLK. Cellular pathology as based upon physiological and pathological histology (republished by Dover Publications, 1971) 1863 [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wen L, Tang FL, Hong Y, Luo SW, Wang CL, He W, Shen C, Jung JU, Xiong F, Lee DH, et al. VPS35 haploinsufficiency increases Alzheimer's disease neuropathology. J Cell Biol. 2011;195:765–779. doi: 10.1083/jcb.201105109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiol Aging. 1993;14:287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- West MJ, Kawas CH, Martin LJ, Troncoso JC. The CA1 region of the human hippocampus is a hot spot in Alzheimer's disease. Ann N Y Acad Sci. 2000;908:255–259. doi: 10.1111/j.1749-6632.2000.tb06652.x. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr 3D maps from multiple MRI illustrate changing atrophy patterns as subjects progress from mild cognitive impairment to Alzheimer's disease. Brain. 2007;130:1777–1786. doi: 10.1093/brain/awm112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Shiung MM, Przybelski SA, Weigand SD, Knopman DS, Boeve BF, Petersen RC, Jack CR., Jr MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology. 2008;70:512–520. doi: 10.1212/01.wnl.0000280575.77437.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson GM, Flibotte S, Chopra V, Melnyk BL, Honer WG, Holt RA. DNA copy-number analysis in bipolar disorder and schizophrenia reveals aberrations in genes involved in glutamate signaling. Human molecular genetics. 2006;15:743–749. doi: 10.1093/hmg/ddi489. [DOI] [PubMed] [Google Scholar]
- Winchester CL, Pratt JA, Morris BJ. Risk genes for schizophrenia: Translational opportunities for drug discovery. Pharmacology & therapeutics. 2014;143:34–50. doi: 10.1016/j.pharmthera.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Wisse LE, Biessels GJ, Heringa SM, Kuijf HJ, Koek DH, Luijten PR, Geerlings MI, Utrecht Vascular Cognitive Impairment Study G. Hippocampal subfield volumes at 7T in early Alzheimer's disease and normal aging. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Wu W, Brickman AM, Luchsinger J, Ferrazzano P, Pichiule P, Yoshita M, Brown T, DeCarli C, Barnes CA, Mayeux R, et al. The brain in the age of old: the hippocampal formation is targeted differentially by diseases of late life. Annals of neurology. 2008;64:698–706. doi: 10.1002/ana.21557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011a;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CE. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2011b;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE. Pattern separation in the hippocampus. Trends in neurosciences. 2011;34:515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Huang Y, Mullendorff K, Dong L, Giedt G, Meng EC, Cohen FE, Kuntz ID, Weisgraber KH, Mahley RW. Apolipoprotein (apo) E4 enhances amyloid beta peptide production in cultured neuronal cells: apoE structure as a potential therapeutic target. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:18700–18705. doi: 10.1073/pnas.0508693102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JT, Tan L, Hardy J. Apolipoprotein E in Alzheimer's Disease: An Update. Annual review of neuroscience. 2014 doi: 10.1146/annurev-neuro-071013-014300. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Bookheimer SY. Application of cortical unfolding techniques to functional MRI of the human hippocampal region. NeuroImage. 2000;11:668–683. doi: 10.1006/nimg.2000.0561. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Vo N, Goodman RH. Histone binding protein RbAp48 interacts with a complex of CREB binding protein and phosphorylated CREB. Molecular and cellular biology. 2000;20:4970–4978. doi: 10.1128/mcb.20.14.4970-4978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]