Abstract
The mechanical properties of the extracellular matrix (ECM) play an important role in maintaining cellular function and overall tissue homeostasis. Emerging evidence suggests that biomechanical modifications of the ECM may be initiators and/or drivers of disease, exemplified by increased tissue stiffness. Specific ECM crosslinking enzymes (tissue transglutaminase, lysyl oxidase and lysyl oxidase-like1) are expressed in the trabecular meshwork (TM) and are regulated by transforming growth factor-beta (TGF-β isoforms. Since TGF-β isoforms are elevated in the aqueous humor (AH) of glaucoma patients, TM stiffness mediated by ECM crosslinking may be responsible for increased AH outflow resistance and elevated intraocular pressure.
There is increasing evidence that biomechanical properties of the trabecular meshwork (TM) are altered in glaucoma. Last et al.,1 reported that the mean elastic modulus (e.g. a measure of tissue stiffness) was significantly increased in the glaucomatous human TM when compared to the age-matched control human TM. They suggested that a change in the physical properties of the TM might directly modulate aqueous humor (AH) outflow resistance and intraocular pressure (IOP). In addition the authors presented a mathematical model to demonstrate that increased stiffness of an elastic porous membrane (e.g. the TM) results in a corresponding increase in outflow resistance.
The term “crosslinking” (CXL) is used to describe the formation of chemical bridges between proteins or other molecules. Crosslinking of extracellular matrix (ECM) proteins change the biomechanical properties of the ECM. Crosslinking in connective tissue can occur during aging, as a side effect of diabetes mellitus and in abnormal fibrosis. Cross-linking of ECM proteins prevents proteolytic breakdown and thus results in decreased ECM turnover, excess ECM accumulation and tissue stiffness. Major families of CXL enzymes include transglutaminases and lysyl oxidases.
TRANSGLUTAMINASE FAMILY OF CROSSLINKING ENZYMES
The transglutaminase (TGM) family of CXL enzymes contains 8 members of which tissue transglutaminase (TGM2) is the most ubiquitous and extensively studied.2 TGM2 is a calcium-dependent enzyme involved in specific post-translational modifications via crosslinking ECM proteins. TGM2 modifies ECM proteins by cross-linking epsilon- (gamma-glutamyl) lysine or (gamma-glutamyl) polyamine bonds.3 TGM2 protein and/or enzyme activity is upregulated in a variety of diseases resulting in enhanced accumulation of cross-linked ECM proteins.4 Substrates of TGM2 associated with the TM include fibronectin, collagen, laminin and elastin. TGM2 enzyme CXL activity has been implicated as a causative factor for many fibrotic diseases including pulmonary fibrosis, liver fibrosis, renal fibrosis and atherosclerosis.
Cultured human TM cells and TM tissues express and secrete TGM2, and treatment with transforming growth factor beta 2 (TGF-β2) further induces TGM2 expression.5, 6 Our laboratory further reported elevated TGM2 expression in both isolated glaucomatous TM cells and glaucomatous tissues.6 In addition, we demonstrated increased TGM2 enzymatic activity in glaucomatous TM cells and tissues.6 These results suggest that elevated TGF-β2 levels in the AH and TM leads to an increase in TGM2-mediated CXL of ECM proteins in the TM. A review of TGM2 function and relevance in ocular diseases is available.7
LYSYL OXIDASE FAMILY OF CROSSLINKING ENZYMES
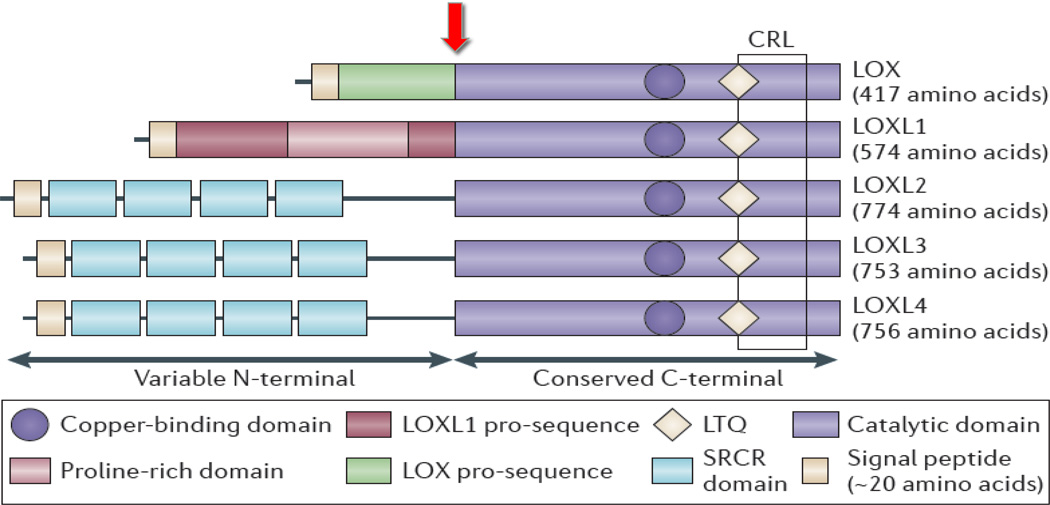
Lysyl oxidase (LOX) is one of five LOX family members (LOX and LOXL1–4) (Figure 1). LOX was initially reported to be expressed and secreted only by fibrogenic cells but is now known to be expressed in several other cell types. LOX oxidizes peptidyl lysine and hydroxylysine residues in collagen and lysine residues in elastin to produce peptidyl alpha-aminoadipic-delta-semi-aldehydes.8 These aldehyde modifications can spontaneously combine with vicinal peptidyl aldehydes or with epsilon-amino groups of peptidyl lysine to form covalent cross-links that stabilize and cause collagen and elastin fibers to be insoluble in the ECM. LOX is synthesized as a 50-kDa pre-protein containing 3 domains; the N-terminal signal peptide sequence, the N-terminal pro-peptide domain and the C-terminal catalytic domain.9 The signal peptide is cleaved and the pro-peptide domain is N-glycosylated in the endoplasmic reticulum and Golgi apparatus yielding a pro-enzyme, which is then secreted from cells as a catalytically inactive protein. The 32-kDa active enzyme, (C-terminal domain) is released by proteolytic cleavage of the pro-peptide by procollagen C-proteinase (bone morphogenetic protein 1; BMP-1).10 (Figure 1). LOX and LOXL enzymes are associated with several abnormalities related to an imbalance in ECM synthesis and/or degradation including fibrotic disorders of the heart (myocardial fibrosis), vasculature (atherosclerosis), lungs (pulmonary fibrosis), skin (hypertrophic scarring), kidney (diabetic nephropathy) and liver (liver fibrosis).9,10
Figure 1. The structural homology of the LOX family members and site of BMP-1 cleavage.
The pro-regions of LOX and LOXL 1 enable extracellular interaction. The four scavenger receptor cysteine-rich domains are thought to be involved in cell-cell or protein-protein interactions. All LOX family members have a highly conserved C-terminal region that contains the catalytic domain. The copper-binding domain is also in the C-terminal region. BMP-1 cleaves the pro-domain of LOX as indicated by arrow. (Modified from Baker et al., Nat. Rev. Cancer. (2012) 12: 540–552.).
We have demonstrated that human TM cells express all members of the LOX family. Significantly with respect to POAG, both mRNA and protein levels are induced by exogenous TGF-β2.11 In addition we reported that TGF-β2 induction of LOX utilized both canonical (e.g. receptor-regulated SMAD) and non-canonical signaling pathways. These results suggest that complex regulation of cross-linking enzymes occurs in the TM. We have also authored a review of LOX function and relevance in ocular diseases.12
LOXL1 AND EXFOLIATION GLAUCOMA
An indication that an alteration in expression or function of cross-linking enzymes in the TM is related to glaucoma comes from studies of exfoliation syndrome (XFS) and exfoliation glaucoma (XFG). The systemic disease XFS presents with significant eye involvement.13,14 Picht et al.,15 reported that TGF-β2 was not elevated in the AH of PXG patients. However, TGF-β1 and TGF-β3 levels are elevated in the AH of XFS and XFG patients.16
Single nucleotide polymorphisms (SNPs) of the LOXL-1 gene are strongly associated risk factors for XFS. Microfibers coated with amorphous material (e.g. exfoliation deposits) coat various structures including the TM.14 The LOXL-1 enzyme is necessary for tropoelastin cross-linking and elastic fiber formation, maintenance and remodeling.17,18 At specific sites for elastic fiber formation, the inactive precursor form of LOXL-1 (e.g. pro-LOXL-1) binds to both fibulin-5 and tropoelastin and targets the formation of elastic microfibrils. At this site, a scaffold is built using fibrillins and microfibril-associated glycoproteins that aids in the alignment of tropoelastin cross-linking domains. Significantly, following LOXL-1 pro-form binding to the scaffold, BMP-1 (e.g. pro-collagen C-terminal proteinase) cleaves the pro-form resulting in LOXL-1 enzymatic activation. As a result of LOXL-1 activation, lysine residues are covalently cross-linked and elastin fibers become highly resistant to degradation or turnover.18 The SNPs associated with XFS are located in exon 1 that codes for an N-terminal domain of the pro-form of LOXL-1.19 It is predicted that this domain is involved in LOXL-1 activation and binding to the scaffold. However, it is not clear how a polymorphism in this region may affect enzyme function and subsequent exfoliation material (XFM) production.
BONE MORPHOGENETIC PROTEIN-1 (PROCOLLAGEN C-PROTEINASE)
BMP-1 was erroneously included as a member of the BMP subfamily but has no homology to other BMP proteins.20 In actuality, BMP-1 is a zinc protease that converts secreted precursor pro-proteins into mature, functional proteins. Both LOX and LOXL-1 are substrates for BMP-1 (Figure 1). As a result of LOXL-1 activation, lysine residues are covalently cross-linked and elastin fibers become highly resistant to degradation or turnover.21 The SNPs associated with XFS are located in exon 1 that codes for an N-terminal domain of the pro-form of LOXL-1. It is predicted that this domain is involved in LOXL-1 activation and binding to the scaffold. However, it is not clear how a polymorphism in this region may affect enzyme function and subsequent XFM production.19
Because BMP-1 is involved in the activation of ECM protein cross-linking enzymes, the regulation of BMP-1 expression and biological activity may be important in understanding TM stiffness and AH outflow resistance. We recently reported that TM cells and tissues express BMP-1 mRNA and protein and that BMP-1 protein is induced by TGF-β2.22 Using a LOX activity assay, we also demonstrated that secreted BMP-1 from human TM cells was biologically active and increased secreted LOX enzyme activity. Significantly, we showed that glaucomatous TM cells secreted higher levels of BMP-1 and that BMP-1 secretion in glaucomatous TM cells was further induced by exogenous TGF-β2 treatment compared to normal TM cells.22
PROPOSED MECHANISM OF LOX AND LOXL1 IN GLAUCOMA
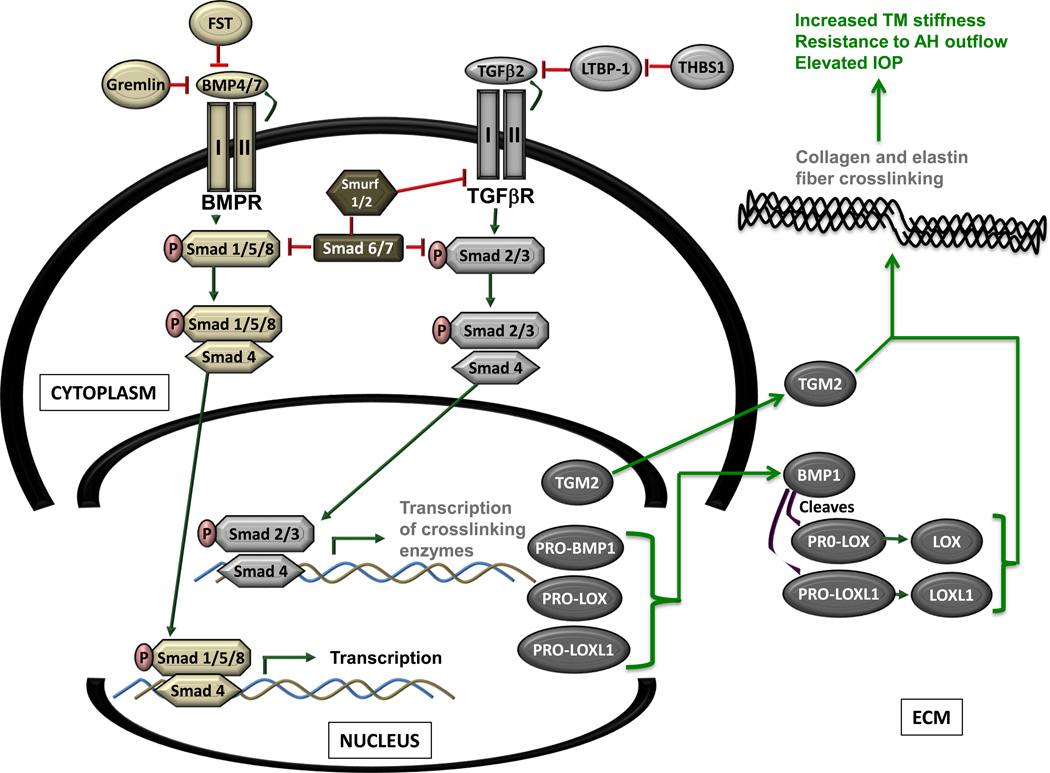
Since exogenous TGF-β2 and gremlin increase cross-linking enzyme expression in cultured human TM cells, we have suggested that in POAG, elevated levels of TGF-β and gremlin in the TM increases expression and secretion of TGM2, LOX and BMP-1. The presence of BMP-1 cleaves the precursor pro-forms of LOX and LOXL-1 to yield enzymatically active molecules. Subsequent covalent cross-linking of collagen/elastic fibers increases stiffness of the TM that may result in AH outflow resistance and elevated IOP (Figure 2). It should be noted that the molecular mechanism for activation of TGM2 has not been clearly defined and warrants examination in the human TM cell. Since all preliminary data have been obtained via cell culture experiments, future studies using animals and/or in situ models are needed to verify that overexpression of cross-linking enzymes in the TM leads to increased TM stiffness, increased AH outflow resistance and elevated IOP.
Figure 2. The proposed mechanism of TGF-β2 regulation of LOX and LOXL 1 in the trabecular meshwork.
Elevated levels of TGF-β2 in the glaucomatous trabecular meshwork (TM), increases the expression and secretion of LOX, LOXL 1 and BMP-1. Increased Localized levels of BMP-1 would result in increased cleavage of precursor forms of LOX and LOXL 1 resulting in increased levels of enzymatically active LOX and LOXL 1 molecules. Subsequent cross-linking of collagen and/or elastic fibers in the TM may increase localized stiffness, aqueous humor outflow resistance and elevate intraocular pressure.
REFERENCES
- 1.Last JA, Pan T, Ding Y, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest. Ophthamol. Vis. Sci. 2011;52:2147–2152. doi: 10.1167/iovs.10-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids. 2008;36:659–670. doi: 10.1007/s00726-008-0190-y. [DOI] [PubMed] [Google Scholar]
- 3.Belkin AM. Extracellular TG2: emerging functions and regulation. FEBS J. 2011;278:4704–4716. doi: 10.1111/j.1742-4658.2011.08346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricotta M, Iannuzzi M, De Vivo G, et al. Physio-pathological roles of transglutaminase-catalyzed reactions. World. J. Biol. Chem. 2010;1:181–187. doi: 10.4331/wjbc.v1.i5.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Welge-Lussen U, May A, Lutjen-Drecoll E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-β1 and TGF-β2. Invest. Ophthamol. Vis. Sci. 2000;41:2229–2238. [PubMed] [Google Scholar]
- 6.Tovar-Vidales T, Roque R, Clark AF, et al. Tissue transglutaminase expression and activity in normal and glaucomatous human trabecular meshwork cells and tissues. Invest. Ophthamol. Vis. Sci. 2008;49:622–628. doi: 10.1167/iovs.07-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tovar-Vidales T, Clark F, Wordinger RJ. Focus on molecules: transglutaminase 2. Exp. Eye Res. 2011;93:2–3. doi: 10.1016/j.exer.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith-Mungo L, Kagan HM. Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol. 1998;16:387–398. doi: 10.1016/s0945-053x(98)90012-9. [DOI] [PubMed] [Google Scholar]
- 9.Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog. Nucleic Acid Res. Mol. Biol. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- 10.Kagan HM, Li W. Lysyl oxidase: properties, specificity and biological roles inside and outside the cell. J. Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 11.Sethi A, Mao W, Wordinger RJ, et al. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest. Ophthamol. Vis. Sci. 2011;52:5240–5250. doi: 10.1167/iovs.11-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethi A, Wordinger RJ, Clark AF. Focus on molecules: lysyl oxidase. Exp. Eye Res. 2012;104:97–98. doi: 10.1016/j.exer.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ritch R, Schlotzer-Schrehardt U. Exfoliation syndrome. Surv. Ophthamol. 2001;45:265–315. doi: 10.1016/s0039-6257(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 14.Schlotzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. Am. J. Ophthamol. 141:921–937. doi: 10.1016/j.ajo.2006.01.047. 200. [DOI] [PubMed] [Google Scholar]
- 15.Picht P, Welge-Lussen U, Grehn F, et al. Transforming growth factor beta 2 in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch. Clin. Exp. Ophthamol. 2001;239:199–207. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- 16.Schlotzer-Schrehardt U, Zenkel M, Küchle M, et al. Role of transforming growth factor-beta1 and its latent form binding protein in pseudoexfoliation syndrome. Exp. Eye Res. 2001;73:765–780. doi: 10.1006/exer.2001.1084. [DOI] [PubMed] [Google Scholar]
- 17.Decitre M, Gleyzal C, Raccurt M, et al. Lysyl oxidase-like protein localizes to sites of de novo fibrin genesis in fibrosis and in the early stromal reaction of ductal breast carcinomas. Lab Invest. 1998;78:143–151. [PubMed] [Google Scholar]
- 18.Liu X, Zhao Y, Gao J, et al. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat. Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 19.Elhawy E, Kamthan G, Dong CQ, et al. Pseudoexfoliation syndrome, a systemic disorder with ocular manifestations. Human Genomics. 2012;6:22–32. doi: 10.1186/1479-7364-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bragdon B, Moseychuk O, Saldanha S, et al. Bone morphogenetic proteins: A critical review. Cellular Signaling. 2011;23:609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins DR, Keles K, et al. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol. 2007;26:508–523. doi: 10.1016/j.matbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tovar-Vidales T, Fitzgerald AM, Clark AF, et al. Transforming growth factor-β2 induces expression of biologically active bone morphogenetic protein-1 in human trabecular meshwork cells. Invest. Ophthamol. Vis. Sci. 2013;54:4741–4748. doi: 10.1167/iovs.13-12203. [DOI] [PMC free article] [PubMed] [Google Scholar]