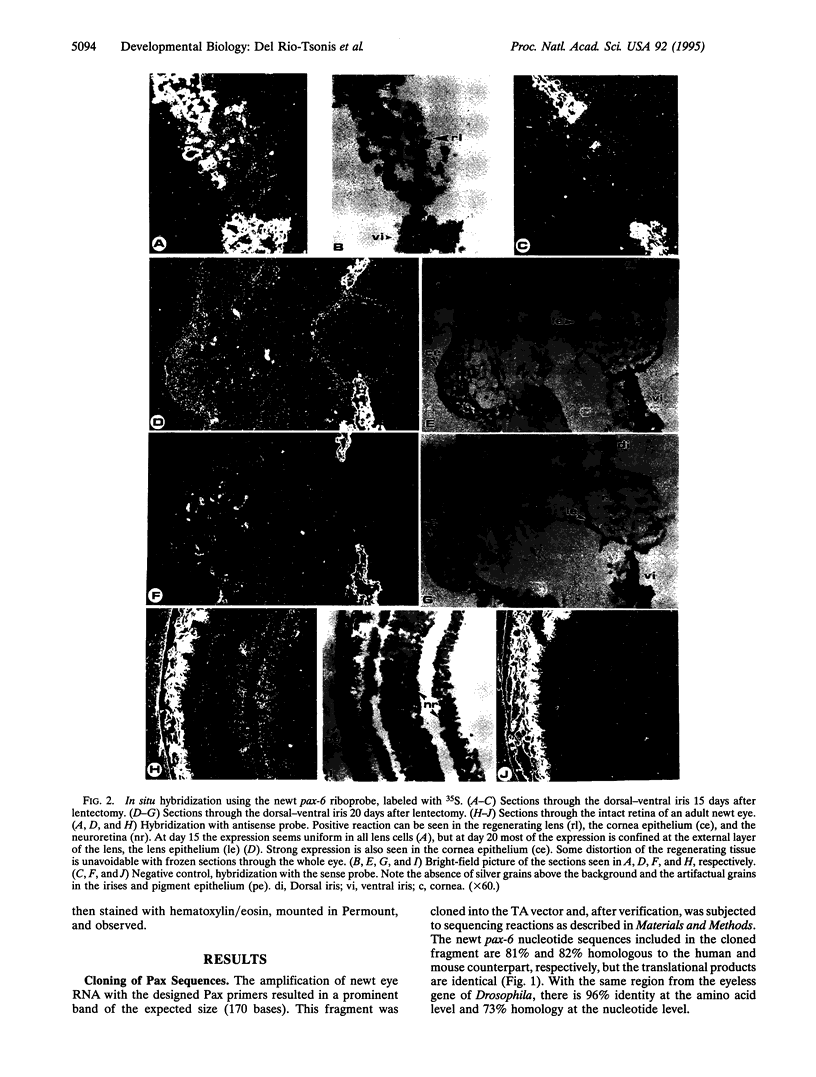
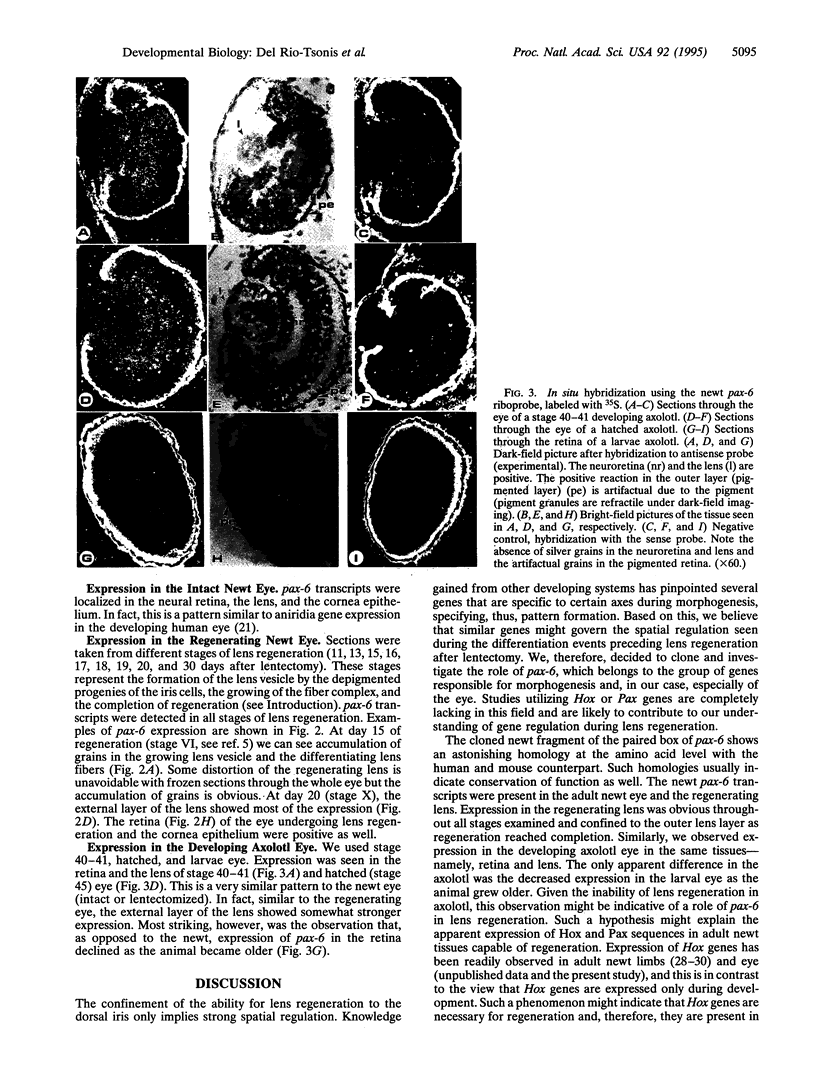
Abstract
Regeneration of eye tissues, such as lens, seen in some urodeles involves dedifferentiation of the dorsal pigmented epithelium and subsequent differentiation to lens cells. Such spatial regulation implies possible action of genes known to be specific for particular cell lineages and/or axis. Hox genes have been the best examples of genes for such actions. We have, therefore, investigated the possibility that such genes are expressed during lens regeneration in the newt. The pax-6 gene (a gene that contains a homeobox and a paired box) has been implicated in the development of the eye and lens determination in various species ranging from Drosophila to human and, because of these properties, could be instrumental in the regeneration of the urodele eye tissues as well. We present data showing that pax-6 transcripts are present in the developing and the regenerating eye tissues. Furthermore, expression in eye tissues, such as in retina, declines when a urodele not capable of lens regeneration (axolotl) surpasses the embryonic stages. Such a decline is not seen in adult newts capable of lens regeneration. This might indicate a vital role of pax-6 in newt lens regeneration.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akam M. The molecular basis for metameric pattern in the Drosophila embryo. Development. 1987 Sep;101(1):1–22. [PubMed] [Google Scholar]
- Beauchemin M., Noiseux N., Tremblay M., Savard P. Expression of Hox A11 in the limb and the regeneration blastema of adult newt. Int J Dev Biol. 1994 Dec;38(4):641–649. [PubMed] [Google Scholar]
- Beauchemin M., Savard P. Two distal-less related homeobox-containing genes expressed in regeneration blastemas of the newt. Dev Biol. 1992 Nov;154(1):55–65. doi: 10.1016/0012-1606(92)90047-k. [DOI] [PubMed] [Google Scholar]
- Belleville S., Beauchemin M., Tremblay M., Noiseux N., Savard P. Homeobox-containing genes in the newt are organized in clusters similar to other vertebrates. Gene. 1992 May 15;114(2):179–186. doi: 10.1016/0378-1119(92)90572-7. [DOI] [PubMed] [Google Scholar]
- Chalepakis G., Fritsch R., Fickenscher H., Deutsch U., Goulding M., Gruss P. The molecular basis of the undulated/Pax-1 mutation. Cell. 1991 Sep 6;66(5):873–884. doi: 10.1016/0092-8674(91)90434-z. [DOI] [PubMed] [Google Scholar]
- Collins J. M. Amplification of ribosomal ribonucleic acid cistrons in the regenerating lens of Triturus. Biochemistry. 1972 Mar 28;11(7):1259–1263. doi: 10.1021/bi00757a022. [DOI] [PubMed] [Google Scholar]
- Collins J. M. Structural changes in deoxyribonucleic acid during early stages of lens regeneration in Triturus. J Biol Chem. 1974 Mar 25;249(6):1839–1847. [PubMed] [Google Scholar]
- Collins J. M. Template ability of activated DNA from the regenerating lens. Biochem Biophys Res Commun. 1974 Mar 25;57(2):359–364. doi: 10.1016/0006-291x(74)90938-3. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M., Blum A. S., Mendez-Otero R., Barnstable C. J. A cell surface molecule distributed in a dorsoventral gradient in the perinatal rat retina. Nature. 1986 Dec 4;324(6096):459–462. doi: 10.1038/324459a0. [DOI] [PubMed] [Google Scholar]
- Dumont J. N., Yamada T. Dedifferentiation of iris epithelial cells. Dev Biol. 1972 Dec;29(4):385–401. doi: 10.1016/0012-1606(72)90079-6. [DOI] [PubMed] [Google Scholar]
- Frigerio G., Burri M., Bopp D., Baumgartner S., Noll M. Structure of the segmentation gene paired and the Drosophila PRD gene set as part of a gene network. Cell. 1986 Dec 5;47(5):735–746. doi: 10.1016/0092-8674(86)90516-7. [DOI] [PubMed] [Google Scholar]
- Funahashi J., Sekido R., Murai K., Kamachi Y., Kondoh H. Delta-crystallin enhancer binding protein delta EF1 is a zinc finger-homeodomain protein implicated in postgastrulation embryogenesis. Development. 1993 Oct;119(2):433–446. doi: 10.1242/dev.119.2.433. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Favor J., Hogan B. L., Ton C. C., Saunders G. F., Hanson I. M., Prosser J., Jordan T., Hastie N. D., van Heyningen V. Mouse small eye results from mutations in a paired-like homeobox-containing gene. Nature. 1991 Dec 19;354(6354):522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- Hill R. E., Hanson I. M. Molecular genetics of the Pax gene family. Curr Opin Cell Biol. 1992 Dec;4(6):967–972. doi: 10.1016/0955-0674(92)90126-w. [DOI] [PubMed] [Google Scholar]
- Hodgkinson C. A., Moore K. J., Nakayama A., Steingrímsson E., Copeland N. G., Jenkins N. A., Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993 Jul 30;74(2):395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- Hyuga M., Kodama R., Eguchi G. Basic fibroblast growth factor as one of the essential factors regulating lens transdifferentiation of pigmented epithelial cells. Int J Dev Biol. 1993 Jun;37(2):319–326. [PubMed] [Google Scholar]
- Imokawa Y., Eguchi G. Expression and distribution of regeneration-responsive molecule during normal development of the newt, Cynops pyrrhogaster. Int J Dev Biol. 1992 Sep;36(3):407–412. [PubMed] [Google Scholar]
- Imokawa Y., Ono S., Takeuchi T., Eguchi G. Analysis of a unique molecule responsible for regeneration and stabilization of differentiated state of tissue cells. Int J Dev Biol. 1992 Sep;36(3):399–405. [PubMed] [Google Scholar]
- Kastner P., Grondona J. M., Mark M., Gansmuller A., LeMeur M., Decimo D., Vonesch J. L., Dollé P., Chambon P. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994 Sep 23;78(6):987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Kissinger C. R., Liu B. S., Martin-Blanco E., Kornberg T. B., Pabo C. O. Crystal structure of an engrailed homeodomain-DNA complex at 2.8 A resolution: a framework for understanding homeodomain-DNA interactions. Cell. 1990 Nov 2;63(3):579–590. doi: 10.1016/0092-8674(90)90453-l. [DOI] [PubMed] [Google Scholar]
- Levine E. M., Schechter N. Homeobox genes are expressed in the retina and brain of adult goldfish. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2729–2733. doi: 10.1073/pnas.90.7.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. S., Yang J. M., Jacobson R. D., Pasko D., Sundin O. Pax-6 is first expressed in a region of ectoderm anterior to the early neural plate: implications for stepwise determination of the lens. Dev Biol. 1994 Mar;162(1):181–194. doi: 10.1006/dbio.1994.1077. [DOI] [PubMed] [Google Scholar]
- McCaffery P., Tempst P., Lara G., Dräger U. C. Aldehyde dehydrogenase is a positional marker in the retina. Development. 1991 Jul;112(3):693–702. doi: 10.1242/dev.112.3.693. [DOI] [PubMed] [Google Scholar]
- McCaffrery P., Posch K. C., Napoli J. L., Gudas L., Dräger U. C. Changing patterns of the retinoic acid system in the developing retina. Dev Biol. 1993 Aug;158(2):390–399. doi: 10.1006/dbio.1993.1197. [DOI] [PubMed] [Google Scholar]
- McDevitt D. S., Meza I., Yamada T. Immunofluorescence localization of the crystallins in amphibial lens development, with special reference to the gamma-crystallins. Dev Biol. 1969 Jun;19(6):581–607. doi: 10.1016/0012-1606(69)90039-6. [DOI] [PubMed] [Google Scholar]
- Mochii M., Agata K., Kobayashi H., Yamamoto T. S., Eguchi G. Expression of gene coding for a melanosomal matrix protein transcriptionally regulated in the transdifferentiation of chick embryo pigmented epithelial cells. Cell Differ. 1988 Jun;24(1):67–74. doi: 10.1016/0045-6039(88)90087-5. [DOI] [PubMed] [Google Scholar]
- Monaghan A. P., Davidson D. R., Sime C., Graham E., Baldock R., Bhattacharya S. S., Hill R. E. The Msh-like homeobox genes define domains in the developing vertebrate eye. Development. 1991 Aug;112(4):1053–1061. doi: 10.1242/dev.112.4.1053. [DOI] [PubMed] [Google Scholar]
- Nornes H. O., Dressler G. R., Knapik E. W., Deutsch U., Gruss P. Spatially and temporally restricted expression of Pax2 during murine neurogenesis. Development. 1990 Aug;109(4):797–809. doi: 10.1242/dev.109.4.797. [DOI] [PubMed] [Google Scholar]
- Onda H., Poulin M. L., Tassava R. A., Chiu I. M. Characterization of a newt tenascin cDNA and localization of tenascin mRNA during newt limb regeneration by in situ hybridization. Dev Biol. 1991 Nov;148(1):219–232. doi: 10.1016/0012-1606(91)90331-v. [DOI] [PubMed] [Google Scholar]
- Ortiz J. R., Vigny M., Courtois Y., Jeanny J. C. Immunocytochemical study of extracellular matrix components during lens and neural retina regeneration in the adult newt. Exp Eye Res. 1992 Jun;54(6):861–870. doi: 10.1016/0014-4835(92)90149-m. [DOI] [PubMed] [Google Scholar]
- Poulin M. L., Patrie K. M., Botelho M. J., Tassava R. A., Chiu I. M. Heterogeneity in the expression of fibroblast growth factor receptors during limb regeneration in newts (Notophthalmus viridescens). Development. 1993 Oct;119(2):353–361. doi: 10.1242/dev.119.2.353. [DOI] [PubMed] [Google Scholar]
- Quiring R., Walldorf U., Kloter U., Gehring W. J. Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science. 1994 Aug 5;265(5173):785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- Rabacchi S. A., Neve R. L., Dräger U. C. A positional marker for the dorsal embryonic retina is homologous to the high-affinity laminin receptor. Development. 1990 Jul;109(3):521–531. doi: 10.1242/dev.109.3.521. [DOI] [PubMed] [Google Scholar]
- Roseman B., Lough J., Houkom E., Herman T. Affinity isolation of transcriptionally active DNA. Biochem Biophys Res Commun. 1986 May 29;137(1):474–479. doi: 10.1016/0006-291x(86)91234-9. [DOI] [PubMed] [Google Scholar]
- STEWART D. S., 'ESPINASSE P. G. Regeneration of the lens of the eye in the rabbit. Nature. 1959 Jun 27;183:1815–1815. doi: 10.1038/1831815a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. P., Weiner A. J. Structural relationships among genes that control development: sequence homology between the Antennapedia, Ultrabithorax, and fushi tarazu loci of Drosophila. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4115–4119. doi: 10.1073/pnas.81.13.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone L. S. An investigation recording all salamanders which can and cannot regenerate a lens from the dorsal iris. J Exp Zool. 1967 Feb;164(1):87–103. doi: 10.1002/jez.1401640109. [DOI] [PubMed] [Google Scholar]
- Ton C. C., Hirvonen H., Miwa H., Weil M. M., Monaghan P., Jordan T., van Heyningen V., Hastie N. D., Meijers-Heijboer H., Drechsler M. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991 Dec 20;67(6):1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- Walther C., Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991 Dec;113(4):1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Wright C. V. Vertebrate homeobox genes. Curr Opin Cell Biol. 1991 Dec;3(6):976–982. doi: 10.1016/0955-0674(91)90116-g. [DOI] [PubMed] [Google Scholar]
- Zalik S. E., Scott V. Sequential disappearance of cell surface components during dedifferentiation in lens regeneration. Nat New Biol. 1973 Aug 15;244(137):212–214. doi: 10.1038/newbio244212a0. [DOI] [PubMed] [Google Scholar]