Abstract
Background:
Excessive alcohol consumption leads to liver disease. Interorgan crosstalk contributes to ethanol (EtOH)-induced liver injury. EtOH exposure causes gut dysbiosis resulting in negative alterations in intestinal fermentation byproducts, particularly decreased luminal butyrate concentrations. Therefore, in the present work, we investigated the effect of butyrate supplementation, in the form of trybutyrin, as a prophylactic treatment against EtOH-induced gut injury.
Methods:
C57BL/6J mice were treated with 3 different EtOH feeding protocols: chronic feeding (25 days, 32% of kcal), short-term (2 days, 32%), or acute single gavage (5 g/kg). Tributyrin (0.83 to 10 mM) was supplemented either into the liquid diet or by oral gavage. Intestinal expression of tight junction (TJ) proteins and a butyrate receptor and transporter were evaluated, as well as liver enzymes and inflammatory markers.
Results:
All 3 EtOH exposure protocols reduced the expression and co-localization of TJ proteins (ZO-1, occludin) and the expression of a butyrate receptor (GPR109A) and transporter (SLC5A8) in the ileum and proximal colon. Importantly, tributyrin supplementation protected against these effects. Protection of the intestine with tributyrin supplementation was accompanied by mitigation of EtOH-induced increases in aspartate aminotransferase and inflammatory measures in the short-term and acute EtOH exposure protocols, but not after chronic EtOH feeding.
Conclusions:
These findings suggest that tributyrin supplementation could serve as a prophylactic treatment against gut injury caused by short-term EtOH exposure.
Keywords: Butyrate, Tributyrin, Intestine, Liver, Ethanol
EXCESSIVE ALCOHOL CONSUMPTION is associated with liver injury. Alcoholic liver disease is characterized by fatty liver, hepatitis, fibrosis, and cirrhosis. There is growing appreciation that interorgan crosstalk contributes to ethanol (EtOH)-induced liver injury. Impairment of intestinal barrier function during heavy EtOH exposure is associated with progression of EtOH-induced liver injury in humans and rodents (Rao, 2009). The epithelial barrier is controlled by a variety of specific proteins and intercellular junctional complexes (e.g., occludins, zonula occludens, adherens junctions), together forming a complex called the tight junction (TJ; Rao, 2009). Intact TJs prevent diffusion of macromolecules, such as endotoxin, from the intestine into the lymphatic system and blood stream. Mechanisms by which EtOH impairs the epithelial barrier are not completely understood, but involve complex interactions between reactive EtOH metabolites, changes in intestinal epithelial cell function, as well as shifts in the gut microbiota (Bull-Otterson et al., 2013; Mutlu et al., 2009).
EtOH metabolism, via the alcohol dehydrogenase (ADH) and cytochrome P450 2E1 (CYP2E1) pathways, generates acetaldehyde. Both ADH and CYP2E1 are expressed in liver and intestine, and ADH is also expressed in commensal gut microbiota (Bergheim et al., 2005; Visapaa et al., 2002). While ingested EtOH is primarily absorbed in the proximal small intestine, the distal intestine is exposed to EtOH on the basolateral surface via the blood. EtOH concentration in the colonic lumen is equivalent to that in blood (Halsted et al., 1973). Acetaldehyde increases gut permeability to gut-derived endotoxin by altering intestinal epithelial integrity (Visapaa et al., 2002). Acetaldehyde is further metabolized to acetate by aldehyde dehydrogenase (Geokas et al., 1981). In humans, 70 to 80% of oxidized EtOH appears as free acetate in the hepatic vein (Lundquist et al., 1962). EtOH-derived acetate affects peripheral tissues, for example, acetate has marked effects on central nervous system function (Israel et al., 1994), but it is not clear if acetate also impacts intestinal epithelial integrity.
The gut microbiota is comprised of trillions of commensal bacteria dominated by nearly 800 different species (Hooper et al., 2002). One of the best characterized beneficial functions of gut microbiota is the ability to ferment long-chain polysaccharides that escape human digestion yielding short-chain fatty acids (SCFA). The predominant SCFA produced are acetate, propionate, and butyrate (Canani et al., 2011; Velazquez et al., 1996). Although butyrate is the least abundant SCFA, it serves many biological roles. Butyrate induces epithelial cell proliferation in normal intestinal tissue, but decreases cell proliferation, increases apoptosis, and stimulates cell differentiation in colon cancer cells (Canani et al., 2011). Butyrate stimulates water and electrolyte absorption and inhibits prosecretory action of several cAMP-generating secretagogues, making butyrate beneficial in treating diarrheal disorders (Binder, 2010). Butyrate is the primary fuel source for colonocytes and improves gut barrier function (Canani et al., 2011; Ploger et al., 2012). Butyrate inhibits activation of transcription factor NF-κB, decreasing expression of inflammatory cytokines by colonic epithelial cells (Inan et al., 2000). Absence of intestinal butyrate is associated with apoptosis, inflammation and mucosal atrophy (Hass et al., 1997; Thangaraju et al., 2008, 2009). Higher acetate:butyrate ratios are associated with colonic pathology, including colon polyps and tumors (Weaver et al., 1988).
Multiple concentration-dependent processes regulate intestinal transport of SCFA (Thibault et al., 2010). Mono-carboxylate transporters (MCT), expressed in both apical and basolateral membranes of intestinal epithelial cells, play an important role in absorption of SCFA into the enterocytes from the gut lumen and systemic circulation (Shimoyama et al., 2007). Arterial plasma acetate levels are elevated as much as 20-fold following high-dose EtOH consumption (Lundquist et al., 1962). Therefore, acetate transport from systemic circulation into the gut lumen via MCTs during EtOH consumption is highly likely. Butyrate also interacts with several nutrient sensing G protein–coupled receptors, identified as SCFA receptors (Brown et al., 2003). GPR109A is abundantly expressed in the lumen-facing apical membrane of colonocytes (Thangaraju et al., 2009) and serves as a luminal SCFA sensor (Borthakur et al., 2012). Thus, effects of SCFA are mediated both via activation of these receptors and/or transport into the cell (Brown et al., 2003).
When mice are chronically exposed to high concentrations of EtOH (40 to 60% kcal), gut dysbiosis results (Bull-Otterson et al., 2013; Mutlu et al., 2009; Yan et al., 2011). Acute or binge exposure to EtOH also causes gut dysbiosis, resulting in higher intestinal ratios of Escherichia coli to Lactobacilli, increased plasma endotoxin levels and histological and ultrastructure changes in the intestinal mucosa and epithelia (Zhou et al., 2013). These gut integrity changes are associated with liver injury after acute and chronic EtOH exposure (Bertola et al., 2013; Wang et al., 2012; Zhou et al., 2013). Probiotic supernatant protects against negative effects of acute EtOH exposure on markers of intestinal permeability, endotoxemia, and liver injury (Wang et al., 2012). Similarly, prebiotic and probiotic administration minimizes chronic EtOH-induced changes in gut microbiota (Bull-Otterson et al., 2013; Mutlu et al., 2009) and oral administration of broad-spectrum antibiotics reduces endotoxemia and EtOH-induced liver injury severity in rats (Adachi et al., 1995).
One consequence of gut dysbiosis following EtOH exposure is a skewing of intestinal SCFA concentrations, characterized by higher acetate and lower butyrate concentrations (Xie et al., 2013). Therefore, we hypothesized that the combination of EtOH-induced gut dysbiosis, resulting in lower luminal butyrate concentrations, as well as increased acetate concentrations resulting from EtOH metabolism would negatively alter the acetate:butyrate ratio in the intestinal lumen. Given the important role of butyrate in maintaining gut health and integrity, the purpose of this study was to investigate the effcacy of pharmacologic manipulation of luminal SCFA with the pro-drug tributyrin (glyceryl tributyrate) during EtOH exposure in protecting gut integrity and mitigating EtOH-induced release of liver enzymes, hepatic steatosis, and expression of inflammatory cytokines and chemokines in liver.
MATERIALS AND METHODS
Materials
Glyceryl Tributyrate was purchased from Sigma-Aldrich (St. Louis, MO). Pair-fed control diet and modified Lieber-DeCarli high-fat diet were purchased from Dyets, Inc. (product number 710260; Bethlehem, PA). All primers for quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) were synthesized by Integrated DNA Technologies (Coralville, IA). Primary antibodies were purchased from the following companies: Occludin (Hycult Biotech, Plymouth Meeting, PA); GPR109A (Bioss, Woburn, MA); tumor necrosis factor-alpha (TNFα; R&D Systems, Minneapolis, MN); Zonula Occluden-1 (ZO-1) and SLC5A8 (Abcam, Cambridge, MA).
Animals
Female C57BL/6J mice (8 to 10 weeks old) were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were housed in standard microisolator cages (2 animals per cage) and fed standard laboratory chow (rodent diet #2918; Harlan-Teklad, Madison, WI) prior to initiation of liquid diet feeding. All animal procedures were approved by Cleveland Clinic Institutional Animal Care and Use Committee.
Ethanol Feeding and Tributyrin Provision
Age-matched mice were randomized into EtOH-fed and pair-fed groups and adapted to a control liquid diet for 2 days. EtOH-fed groups were allowed free access to a complete liquid diet containing EtOH. Control mice were pair-fed a control diet that isocalorically substituted maltose dextrins for EtOH over the entire feeding period. The model for chronic EtOH-induced liver injury (25 days, 32%) consisted of increasing concentrations of EtOH (vol/vol) as follows: 1% for 2 days, 2% for 2 days, 4% for 7 days, 5% for 7 days, and finally 6% for 7 days. The 6% (vol/vol) diet provided EtOH as 32% of total calories in the diet.
Two models of acute EtOH-induced liver injury were used to model ad lib consumption and a controlled, acute dose: (i) A short-term EtOH-induced liver injury model (2 days, 32%) consisted of 1% (vol/vol) EtOH for 2 days, to adapt the mice to the liquid diet, followed by 6% (vol/vol) EtOH for 2 more days, and was used as a model of binge EtOH consumption (Roychowdhury et al., 2009). (ii) An acute EtOH-induced liver injury model consisted of a single gavage of EtOH (5 g/kg body weight) or maltose to mice after an overnight fast. Mice were euthanized 6 hours following the acute EtOH challenge. Tributyrin or glycerol (equimolar to serve as a control) was provided to mice at doses ranging from 0 to 10 mM, either by oral gavage or as part of the liquid diet over specific periods of EtOH feeding. Figure captions contain details of tributyrin treatment.
Following EtOH exposure protocols, mice were randomized, weighed and anesthetized. Blood was collected from the posterior vena cava by syringe and expelled into EDTA-containing tubes. Livers were excised and mice were euthanized by exsanguination. Livers were weighed and portions fixed in formalin, frozen in optimal cutting temperature (OCT) medium (Sakura Finetek USA, Torrance, CA), snap frozen in liquid nitrogen, or stored in RNAlater (Ambion, Austin, TX) for further analysis. Plasma was separated from whole blood and stored at −80°C.
Biochemical Assays
Plasma samples were assayed for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using commercially available enzymatic assay kits (Diagnostic Chemicals, Ltd., Oxford, CT) following manufacturer’s instructions. Total hepatic triglycerides were assayed using the Triglyceride Reagent Kit from Pointe Scientific Inc. (Lincoln Park, MI).
Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was isolated from liver and 4 mg of total hepatic RNA was reverse transcribed as previously described (Mandal et al., 2010). Real-time PCR amplification was performed using Brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA) in a M×3000p PCR machine (Stratagene) for primers: TNFα, macrophage inflammatory protein-2 (MIP2), interleukin 1-beta (IL1β), monocyte chemoattractant protein-1 (MCP1), and 18S (Table 1). Relative amount of target mRNA was determined using comparative threshold (Ct) method by normalizing target mRNA Ct values to those of 18S (McMullen et al., 2005).
Table 1.
Primer Sequences
| Gene | Sequences (forward/reverse 5′-3′) | |
|---|---|---|
| TNFα | CCCTCACACTCAGATCATCTTCT | GCTACGACGTGGGCTACAG |
| MCP1 | AGGTCCCTGTCATGCTTCTG | TCTGGACCCATTCCTTCTTG |
| MIP2 | GCGCCCAGACAGAAGTCATAG | AGCCTTGCCTTTGTTCAGTATC |
| IL1β | GACTCTTGCGTCAACTTCAAGG | CAGGCTGTCTTTTGTCAACGA |
| 18S | ACGGAAGGGCACCACCAGGA | CACCACCACCCACGGAATCG |
TNFα, tumor necrosis factor-alpha; MCP1, monocyte chemoattractant protein-1; MIP2, macrophage inflammatory protein-2; IL1β, interleukin 1-beta.
Liver Homogenate and Immunoblotting
Liver homogenates were prepared and protein concentrations were determined for immunoblotting (Mandal et al., 2010). Protein (35 mg) was resolved on 15% polyacrylamide gels and transferred to polyvinylidene fluoride membranes. Membranes were probed with antibodies specific for CYP2E1; HSC70 was used as loading control.
Histology and Immunohistochemistry
Frozen intestinal sections were used for immunostaining of TJ proteins (occludin, ZO-1), butyrate receptor (GPR109A) and butyrate transporter (SLC5A8). Frozen liver sections were used for immunostaining of TNFα. Slides were coded before examination to blind investigators to the treatment groups; a single investigator blinded to the treatment viewed them. All images presented represent at least 3 images per tissue section and 4 to 6 mice per experimental condition. Semi-quantification of positive staining was performed using ImagePro plus software (Media Cybernatics, Silver Spring, MD).
Statistical Analysis
Values shown in all figures represent the mean ± SEM (n = 4 to 6 for pair-fed, n = 6 for EtOH-fed). Analysis of variance was performed using the general linear models procedure (SAS, Cary, NC). Data were log-transformed as necessary to obtain a normal distribution. Follow-up comparisons were made by least square means testing. p-Values of <0.05 were considered significant.
RESULTS
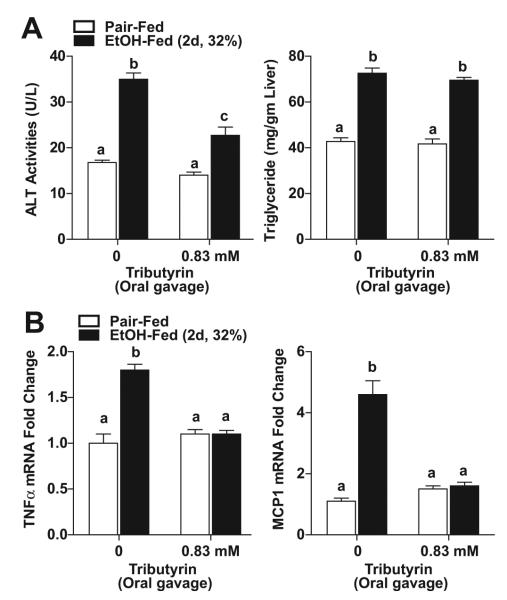
Tributyrin Supplementation via Oral Gavage Protected Tight Junction Protein Expression and Co-Localization in the Proximal Colon During Chronic Ethanol Feeding, but Did Not Protect Mice from Increased Alanine Aminotransferase or Hepatic Triglycerides
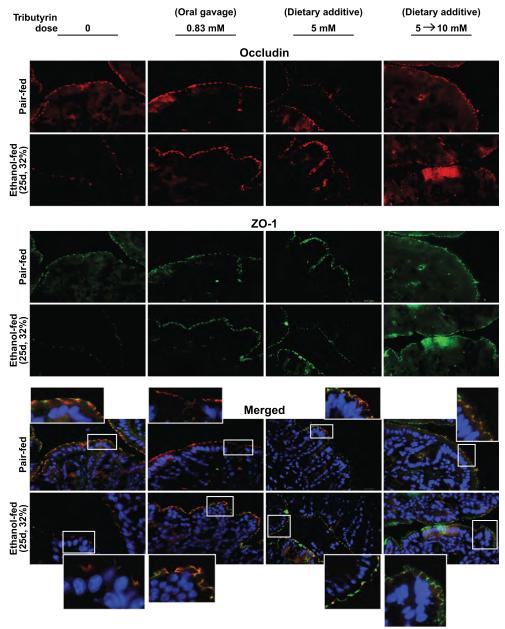
Formation of TJs, visualized via the interaction between ZO-1 and occludin, is critical in maintaining gut integrity. In response to chronic EtOH feeding (25 days, 32%) ZO-1 and occluding expression, as well as co-localization of these 2 proteins in TJs, was reduced in the proximal colon compared to pair-fed mice (Fig. 1). When mice were supplemented with trybutyrin at a concentration of 0.83 mM by oral gavage during the last 21 days of EtOH feeding (3 times weekly), EtOH-induced loss in expression of ZO-1 and occludin, as well as TJ formation, was prevented (Fig. 1). Chronic EtOH feeding (25 days, 32%) increased ALT in plasma and hepatic triglycerides (Table 2). Despite protecting TJ formation, supplementation with tributyrin via gavage did not protect the liver from chronic EtOH-induced increases in ALT and hepatic triglycerides (Table 2).
Fig. 1.
Tributyrin supplementation protected tight junction protein expression and co-localization in the proximal colon during chronic ethanol (EtOH) feeding. Mice were allowed free access to EtOH (25 days, 32%) or pair-fed control diets as described in Materials and Methods. Doses of glycerol (control) or tributyrin were provided as follows: tributyrin (0.83 mM) was delivered by oral gavage 3 times per week beginning at 4% EtOH (total 9 doses); tributyrin (5 mM) was added to liquid diet for the entire EtOH feeding period (25 days); or tributyrin (5 mM) was added to liquid diet the first 11 days (1 to 4% EtOH) followed by tributyrin (10 mM) for days 12 to 25 (5 and 6% EtOH; 5 to 10 mM). Occludin (red) and ZO-1 (green) were visualized by immunohistochemistry in sections of proximal colon frozen in OCT. Nuclei were counterstained with DAPI (blue). A selected area was cropped and enlarged. All images were acquired using a 40× objective. Images are representative of at least replicate images captured per mouse in 4 to 6 mice (pair-fed) or 6 mice (EtOH-fed).
Table 2.
Liver Tests in Chronic Ethanol Feeding
| ALT (U/l) |
Triglyceride (mg/gm liver) |
|||
|---|---|---|---|---|
| Tributyrin dose | Pair-fed | EtOH-fed (25 days, 32%) |
Pair-fed | EtOH-fed (25 days, 32%) |
| Oral gavagea | ||||
| 0 | 17.4 ± 1.6 | 74.1 ± 15.1* | 54.7 ± 3.8 | 126 ± 13.7* |
| N = 5 | N = 6 | N = 6 | N = 6 | |
| 0.83 mM | 17.4 ± 1.9 | 112 ± 7.7* | 54.4 ± 4.4 | 98.1 ± 12.7* |
| N = 6 | N = 6 | N = 6 | N = 6 | |
| Dietary additiveb | ||||
| 0 | 18.1 ± 2 | 47.1 ± 6.4* | 32.2 ± 3.3 | 71.8 ± 3.8* |
| N = 4 | N = 5 | N = 4 | N = 5 | |
| 5 mM | 20.6 ± 3.5 | 52.1 ± 3.9* | 33.9 ± 5.4 | 63.9 ± 4.1* |
| N = 4 | N = 6 | N = 4 | N = 6 | |
| Dietary additivec | ||||
| 0 | 20.8 ± 4.0 | 32.5 ± 9.6* | 90.6 ± 14.6 | 97.6 ± 17.1 |
| N = 4 | N = 4 | N = 4 | N = 5 | |
| 5 to 10 mM | 25.9 ± 5.2 | 49.9 ± 5.5* | 71.7 ± 6.7 | 126 ± 11.9* |
| N = 6 | N = 6 | N = 6 | N = 6 | |
ALT, alanine aminotransferase.
Pair-fed versus EtOH-fed, p < 0.05.
Tributyrin (0.83 mM) orally gavaged 3 d/wk for 25 days.
Tributyrin (5 mM) added to liquid diet for 25 days.
Tributyrin (5 mM) added to liquid diet days 1 to 11, then increased to 10 mM days 12 to 25.
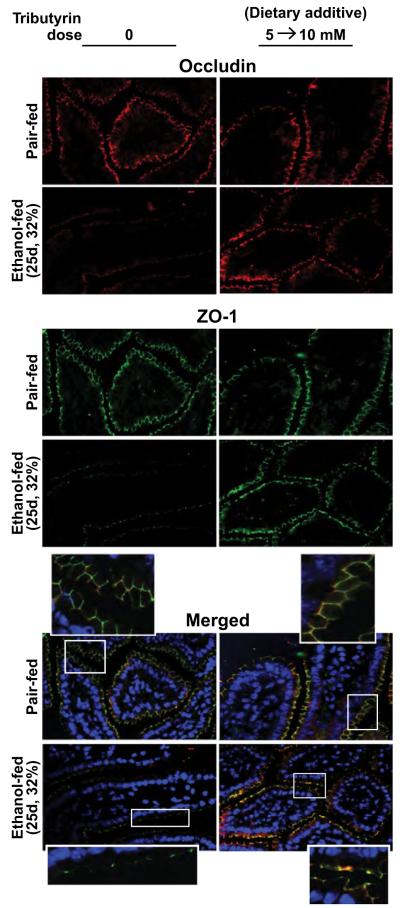
Tributyrin Supplementation to the Liquid Diet Protected Tight Junction Protein Expression and Co-Localization in the Proximal Colon and Ileum During Chronic Ethanol Feeding
To provide a more constant tributyrin supplementation and minimize handling stress to mice, liquid diets were supplemented with glycerol or tributyrin (5 mM) during the entire chronic feeding protocol, or 5 mM for the first 11 days and then increased to 10 mM for the last 14 days (5 to 10 mM). Both dosing regimens completely protected mice from chronic EtOH-induced loss in expression of ZO-1 and occludin, as well as TJ formation, in the proximal colon (Fig. 1). Because the ileum is also the primary target of chronic EtOH exposure (Kirpich et al., 2012), we investigated the role of tributyrin supplementation in TJs in the ileum. Similarly to proximal colon (Fig. 1), chronic EtOH feeding decreased expression of immunoreactive ZO-1 and occludin protein expression, as well as TJ protein complex formation, in the ileum compared with pair-fed mice (Fig. 2). Dietary supplementation with 5 to 10 mM tributyrin during the chronic EtOH feeding protocol protected both ZO-1 and occludin protein expression and TJ protein complex formation (Fig. 2).
Fig. 2.
Tributyrin supplementation sustains tight junction protein expression and co-localization in the ileum during chronic ethanol (EtOH) feeding. Mice were allowed free access to EtOH (25 days, 32%) or pair-fed control diets as described in Materials and Methods. Tributyrin (5 mM) was added to liquid diet for the first 11 days (1 to 4% EtOH) and then increased to 10 mM for days 12 to 25 (5 and 6% EtOH; 5 to 10 mM). Occludin (red) and ZO-1 (green) were visualized by immunohistochemistry in sections of ileum frozen in OCT. Nuclei were counterstained with DAPI (blue). A selected area was cropped and enlarged. All images were acquired using a 40× objective. Images are representative of at least replicate images captured per mouse in 4 to 6 mice (pair-fed) or 6 mice (EtOH-fed).
Tributyrin Supplementation in the Diet Mitigated Decreased Butyrate Transporter Expression in the Ileum and Proximal Colon During Chronic Ethanol Feeding
Gut microbiota presence is a prerequisite for the expression of a butyrate transporter (SLC5A8) at the luminal surface in the intestine (Cresci et al., 2010). Further, tributyrin supplementation supports the expression of SLC5A8 during gut dysbiosis (Cresci et al., 2013). Because chronic EtOH feeding causes both gut dysbiosis (Yan et al., 2011; Bull-Otterson et al., 2013) and decreased luminal butyrate levels (Xie et al., 2013), we investigated whether EtOH feeding also influenced expression of a butyrate transporter and whether tributyrin supplementation mitigates this effect. In both ileum and proximal colon, chronic EtOH feeding reduced SLC5A8 expression (Fig. 3). Tributyrin supplementation in the EtOH diet abated losses of SLC5A8 protein expression in ileum (5 to 10 mM) and proximal colon (5 mM) during chronic EtOH feeding (Fig. 3).
Fig. 3.
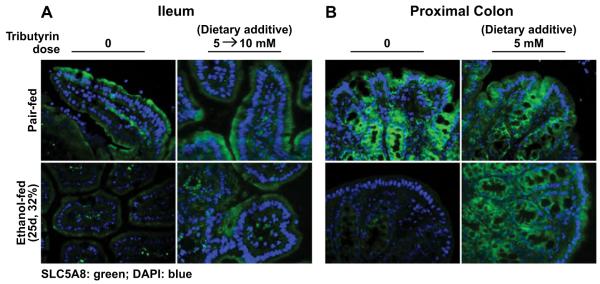
Tributyrin supplementation mitigates decreased butyrate transporter expression in the ileum and proximal colon during chronic ethanol (EtOH) feeding. Mice were allowed free access to EtOH (25 days, 32%) or pair-fed control diets as described in Materials and Methods. Doses of glycerol (control) or tributyrin were provided as follows: tributyrin (5 mM) added to liquid diet for the entire EtOH feeding period (25 days); or tributyrin (5 mM) added to liquid diet the first 11 days (1 to 4% EtOH) followed by tributyrin (10 mM) for days 12 to 25 (5 and 6% EtOH; 5 to 10 mM). SLC5A8 (green) was visualized by immunohistochemistry in sections of (A) ileum and (B) proximal colon frozen in OCT. Nuclei were counterstained with DAPI (blue). All images were acquired using a 409 objective. Images are representative of at least replicate images captured per mouse in 4 to 6 mice (pair-fed) or 6 mice (EtOH-fed).
Effects of Tributyrin Supplementation in the Diet on Release of Liver Enzymes and Steatosis During Chronic Ethanol Feeding
Chronic EtOH feeding is associated with hepatocyte injury, indicated by the release of liver enzymes into the circulation and hepatic steatosis. Plasma ALT and liver triglycerides were increased in mice after chronic EtOH feeding (Table 2). Despite TJ protection in the intestine, dietary supplementation with tributyrin at either 5 or 5 to 10 mM did not prevent increases in ALT or triglycerides after chronic EtOH feeding (25 days, 32%). Chronic EtOH feeding also increased expression of liver TNFα mRNA compared with pair-fed mice, but tributyrin supplementation (5 mM) did not prevent this effect of EtOH (Fig. 4A). CYP2E1 protein expression was increased in response to chronic EtOH feeding both in the presence and absence of tributyrin (5 to 10 mM; Fig. 4B).
Fig. 4.
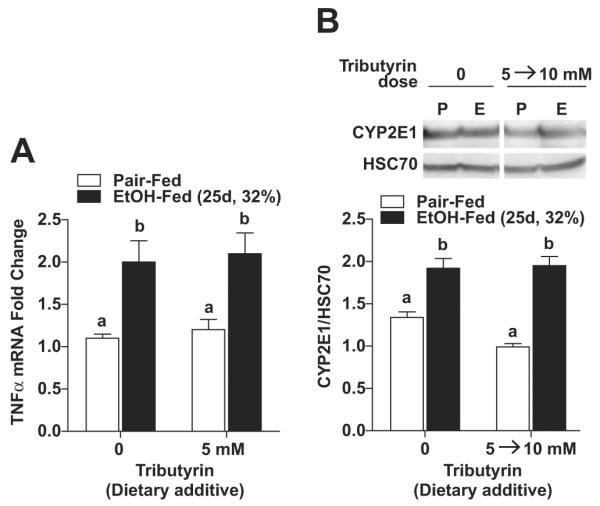
Effect of tributyrin treatment on expression of tumor necrosis factor-alpha (TNFα) and CYP2E1 in mouse liver during chronic ethanol (EtOH) feeding. Mice were allowed free access to EtOH (25 days, 32%) or pair-fed control diets as described in Materials and Methods. Doses of glycerol (control) or tributyrin were provided as follows: tributyrin (5 mM) added to liquid diet for the entire EtOH feeding period (25 days); or tributyrin (5 mM) added to liquid diet the first 11 days (1 to 4% EtOH) followed by tributyrin (10 mM) for days 12 to 25 (5 and 6% EtOH; 5 to 10 mM). (A) Expression of TNFα mRNA was detected in mouse livers using quantitative reverse transcription polymerase chain reaction. Normalized to 18S. (B) Immunoreactive CYP2E1protein was assessed by Western blot using whole liver extracts. HSC70 was used as loading control. Insets show representative image of CYP2E1 Western blots. Values represent means ± SEM. n = 4 to 6 in pair-fed, n = 6 in EtOH-fed. Values with different alphabetical superscripts were significantly different from each other, p < 0.05.
Tributyrin Delivery Stabilized Tight Junction Protein Expression and Co-Localization in Proximal Colon During Short-Term Ethanol Feeding
Short-term EtOH exposure alters gut permeability (Wang et al., 2012; Zhou et al., 2013) and reduces commensal gut microbiota (Zhou et al., 2013), but effects on TJ complex formation is unknown. Therefore, we investigated whether tributyrin treatment would also stabilize TJ complexes during short-term EtOH feeding (2 days, 32%). Immunoreactive occludin and ZO-1 protein expression was lower in EtOH-fed mice (2 days, 32%) compared with pair-fed mice (Fig. 5). When mice were treated with 0.83 mM tributyrin via oral gavage during EtOH feeding, occludin and ZO-1 expression was maintained and TJ protein complexes stabilized; TJ complexes in EtOH-fed mice supplemented with tributyrin were indistinguishable from those in pair-fed mice (Fig. 5).
Fig. 5.
Tributyrin delivery stabilized tight junction protein expression and co-localization in proximal colon during short-term ethanol (EtOH) feeding. Mice were allowed free access to EtOH (2 days, 32%) or pair-fed control diets as described in Materials and Methods. Doses of glycerol or tributyrin (0.83 mM) were provided via oral gavage at 1% (1 day) and 6% (2 days) of EtOH feeding (total 3 doses). Occludin (red) and ZO-1 (green) were visualized by immunohistochemistry in sections of proximal colon frozen in OCT. Nuclei were counterstained with DAPI (blue). A selected area was cropped and enlarged. All images were acquired using a 40× objective. Images are representative of at least replicate images captured per mouse in 4 to 6 mice (pair-fed) or 6 mice (EtOH-fed).
Effect of Tributyrin Treatment on Expression of a Butyrate Receptor and Butyrate Transporter in Proximal Colon During Short-Term Ethanol Feeding
Butyrate-dependent responses in the colon are dependent on butyrate receptors and transporters on the apical surface (Borthakur et al., 2012). Expression of immunoreactive GPR109A (butyrate receptor; Fig. 6A) and SLC5A8 (SCFA transporter; Fig. 6B) in proximal colon was decreased following short-term EtOH feeding (2 days, 32%) compared with pair-fed mice. In contrast, mice treated with tributyrin during short-term EtOH feeding had similar receptor and transporter expression as pair-fed mice, indicating tributyrin protected mice from this effect of short-term EtOH feeding (2 days, 32%).
Fig. 6.
Tributyrin delivery protected expression of a butyrate receptor and butyrate transporter in proximal colon during short-term ethanol (EtOH) feeding. Mice were allowed free access to EtOH (2 days, 32%) or pair-fed control diets as described in Materials and Methods. Tributyrin (0.83 mM) was delivered via oral gavage at 1% (1 day) and 6% (2 days) of EtOH feeding (total 3 doses). A butyrate receptor, GPR109A (green) (A) and a butyrate transporter SLC5A8 (green) (B) were visualized by immunohistochemistry in sections of proximal colon frozen in OCT. Nuclei were counterstained with DAPI (blue). All images were acquired using a 40× objective. Images are representative of at least replicate images captured per mouse in 4 to 6 mice (pair-fed) or 6 mice (EtOH-fed).
Tributyrin Delivery Prevented Short-Term Ethanol -Induced Increases in Alanine Aminotransferase and Hepatic Proinflammatory Cytokine and Chemokine Expression in Mice
Short-term EtOH feeding (2 days, 32%) increased plasma ALT activity and liver triglycerides in mice (Fig. 7A), as well as expression of TNFα and MCP1 mRNA in the liver (Fig. 7B). In contrast to the chronic EtOH feeding model, tributyrin treatment blunted short-term EtOH-induced increases in plasma ALT, as well as increases expression of TNFα and MCP1 mRNA expression in liver (Fig. 7B). However, tributyrin treatment had no effect on short-term EtOH-induced increases in hepatic triglycerides (Fig. 7A).
Fig. 7.
Tributyrin delivery prevented short-term ethanol (EtOH)-induced increases in alanine aminotransferase (ALT) and hepatic proinflammatory cytokine and chemokine expression in mice. Mice were allowed free access to EtOH (2 days, 32%) or pair-fed control diets as described in Materials and Methods. Tributyrin (0.83 mM) was delivered via oral gavage at 1% (1 day) and 6% (2 days) of EtOH feeding (total 3 doses). (A) Activity of ALT was measured in plasma. Hepatic triglyceride content was measured in whole liver homogenates. (B) Expression of tumor necrosis factor-alpha (TNFα) and monocyte chemoattractant protein-1 (MCP1) mRNA was detected in mouse livers using quantitative reverse transcription polymerase chain reaction. Values represent means ± SEM. n = 4 to 6 in pair-fed, n = 6 to 10 in EtOH-fed. Values with different alphabetical superscripts were significantly different from each other, p < 0.05.
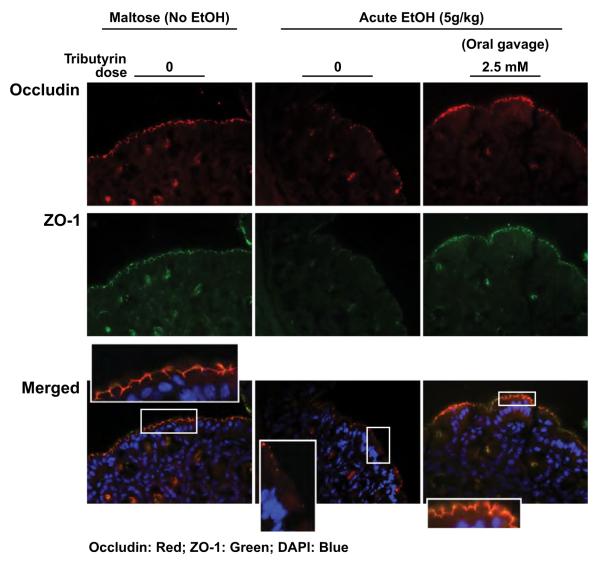
Tributyrin Pretreatment Supports Tight Junction Protein Expression and Co-Localization in Proximal Colon Following Acute Ethanol Exposure
As tributyrin co-treatment protected TJ complexes during short-term EtOH feeding, we also tested the impact of tributyrin pretreatment in response to a single exposure to high-dose EtOH delivered via gavage. Immunoreactive occludin and ZO-1 protein expression in the proximal colon was lower in EtOH-challenged mice compared with control mice (Fig. 8). Co-localization of TJ complexes was also disrupted after even this single gavage with EtOH (Fig. 8). Mice pretreated with tributyrin prior to the single gavage with EtOH had sustained TJ protein expression and co-localization in proximal colon. These data demonstrate tributyrin-pretreated mice were more resistant to the acute effects of EtOH compared with controls not treated with tributyrin.
Fig. 8.
Tributyrin pretreatment supports tight junction protein expression and co-localization in proximal colon following acute ethanol (EtOH) exposure. Mice were given an oral gavage daily of tributyrin (2.5 mM) or glycerol while fed chow food for 3 days. On the third day, 5 g/kg maltose or EtOH were mixed with glycerol or tributyrin (2.5 mM) and the mice were gavaged and euthanized 6 hours later. Occludin (red) and ZO-1 (green) were visualized by immunohistochemistry in sections of proximal colon frozen in OCT. Nuclei were counterstained with DAPI (blue). A selected area was cropped and enlarged. All images were acquired using a 40× objective. Images are representative of at least replicate images captured per mouse in 6 mice per treatment group.
Tributyrin Pretreatment Prevented Acute Ethanol-Induced Increases in Aspartate Aminotransferase and Hepatic Proinflammatory Cytokine and Chemokine Expression in Mice
Plasma AST activity and liver triglycerides were elevated 6 hours after a single EtOH gavage in control mice (Fig. 9A). Single high-dose EtOH gavage also induced proinflammatory mediators in liver, assessed by the expression of TNFα, IL1β, and MIP2 mRNA (Fig. 9B). TNFα protein, assessed by immunohistochemistry, also increased with EtOH challenge (Fig. 9C). Tributyrin pretreatment attenuated increases in AST (Fig. 9A) and proinflammatory mediators (Fig. 9B,C), but not liver triglycerides (Fig. 9A). Taken together, these results indicate mice pretreated with tributyrin prior to a single EtOH gavage were protected against release of liver enzymes and inflammation.
Fig. 9.
Tributyrin pretreatment prevented acute ethanol (EtOH)-induced increases in aspartate aminotransferase (AST) and hepatic proinflammatory cytokine and chemokine expression in mice. Mice were given an oral gavage daily of tributyrin (2.5 mM) or glycerol while fed chow food for 3 days. On the third day, 5 g/kg maltose or EtOH were mixed with glycerol or tributyrin (2.5 mM) and the mice were gavaged and euthanized 6 hours later. (A) Activity of AST was measured in plasma. Hepatic triglyceride content was measured in whole liver homogenates. (B) Expression of tumor necrosis factoralpha (TNFα), interleukin 1-beta (IL1β), and macrophage inflammatory protein-2 (MIP2) mRNA was detected in mouse livers using quantitative reverse transcription polymerase chain reaction. (C) Immunoreactive TNFα protein was evaluated by immunohistochemistry in liver frozen in OCT. All images were acquired using a 40× objective. TNF-positive areas were quantified using Image-Pro Plus software and analyzed. Values represent means ± SEM. n = 6 mice per treatment group. Values with different alphabetical superscripts were significantly different from each other, p < 0.05. *p = 0.07
DISCUSSION
The present study investigated the role of tributyrin supplementation on chronic and acute EtOH-induced changes in expression of TJ protein complexes, a butyrate receptor and transporter in the intestine, as well as markers of early stages of EtOH-induced steatosis and inflammatory cytokine/chemokine expression. The data presented demonstrate for the first time that supplementation of mice with tributyrin, either as part of a liquid diet or as an oral gavage, prevented EtOH-induced down-regulation of proteins involved with maintaining intestinal epithelial barrier, transporting butyrate across the intestinal epithelium and butyrate-sensing signaling receptors.
When the gut microbiota is provided desirable substrates (e.g., fermentable fibers), the resultant SCFA ratio persists in a nearly constant molar ratio of acetate:propionate:butyrate (Canani et al., 2011). However, alterations in commensal gut microbiota can impair the concentration and distribution of SCFAs (Canani et al., 2011). Indeed, a vital role of commensal gut microbiota is to yield optimal butyrate to support gut homeostasis. EtOH exposure depletes commensal bacteria, allowing overgrowth of pathogenic bacteria (Xie et al., 2013; Zhou et al., 2013) and results in decreased luminal butyrate concentrations (Xie et al., 2013). This effect is associated with increased gut permeability, endotoxin translocation, and liver injury (Bull-Otterson et al., 2013; Mutlu et al., 2009; Yan et al., 2011). While select prebiotic and probiotic mitigate EtOH-induced oxidative stress to gut epithelium in animal models of EtOH exposure (Tang et al., 2009; Wang et al., 2012), the ability of these pre- and probiotic treatments to maintain a healthy profile of microbiota fermentation byproducts is unknown. Not all fermentable fibers yield butyrate to the same extent (Cummings et al., 2001). Additionally, probiotic supplementation has a transient effect in the colon and fermentation endproducts of most probiotics is unknown. Taken together, long-term clinical effectiveness of probiotic therapy to sustain intestinal health during continued EtOH exposure is questionable (Canani et al., 2011). Tributyrin (glyceryl tributyrate) is a triglyceride with glycerol esterified with butyrate at the 1, 2, and 3 positions and is neutral, chemically stable, and rapidly hydrolyzed by pancreatic and gastric lipases to glycerol and 3 butyrate molecules (Wachtershauser and Stein, 2000). Tributyrin provision protects gut integrity and intestinal expression of a butyrate receptor and transporter (Cresci et al., 2013).
Supplementing mice with tributyrin during chronic and short-term EtOH exposure maintained expression levels of proteins involved with gut barrier function (occludin, ZO-1) to that of non-EtOH exposed mice. Additionally, co-localization of these proteins forming TJ complexes remained intact in the distal intestine (ileum and proximal colon) with tributyrin provision. The presence of both proteins is important for gut barrier protection, as ZO-1 acts as the scaffold to organize occludin at the junction (Mitic and Anderson, 1998). Butyrate’s ability to exert beneficial cellular effects is concentration-dependent. Therefore, several factors were considered in the design of tributyrin supplementation protocols used in the present study: (i) Doses previously reported to exert beneficial effects (0.83 to 10 mM/l); (ii) in vivo physiologic concentrations of butyrate (10 to 15 mM/l); (iii) butyrate yield from trybutyrin metabolism; and (iv) safety data regarding butyrate cytotoxicity (Newmark et al., 1994; Thangaraju et al., 2009; Velazquez et al. 1996). Tributyrin was provided as either as an oral gavage or as part of a liquid diet. Tributyrin delivered orally in animals has a plasma half-life of 40 minutes with peak plasma butyrate concentrations occurring between 0.25 and 3 hours after dose (Newmark et al., 1994). Dietary supplementation with tributyrin was just as effective as oral gavage at preventing chronic EtOH-induced loss of TJs in the proximal colon (Fig. 1).
Luminal butyrate absorption is critical for the health benefits it confers to the intestinal mucosa (Thibault et al., 2007, 2010). Butyrate and other monocarboxylate absorption occurs via transporters localized to both apical and basolateral membranes in the intestinal epithelial cell (Shimoyama et al., 2007). Regulation of these transporters significantly impacts colonic epithelial health. Exposure of intestinal epithelial cells to SCFA enhances transporter expression and function via NF-κB-dependent transcriptional mechanisms (Borthakur et al., 2008). SLC5A8 transports butyrate via a Na+-dependent electrogenic process and GPR109A is a Gαi protein–coupled receptor activated by butyrate (Thangaraju et al., 2009). While both SLC5A8 and GPR109A are expressed in the lumen-facing apical membrane throughout the intestine, they are most abundant in the ileum and colon (Thangaraju et al., 2008, 2009). Their expression is reduced markedly when luminal bacteria is depleted, as in germ-free or antibiotic-treated mice (Cresci et al., 2010, 2013). Other monocarboxylate transporters (MCT1-8) are also present throughout the intestine (Gill et al., 2005).
Luminal butyrate induction of butyrate transporter surface expression and function involves a nutrient sensing mechanism with GPR109A as an SCFA sensor; this orchestration ultimately increases butyrate absorption when more butyrate is available (Borthakur et al., 2012). As a major consequence of reduced intracellular SCFA oxidation is metabolic starvation of the colonocyte and mucosal atrophy (Gassull, 2006), we predicted that EtOH-induced deterioration of intestinal TJs was associated with depleted butyrate transporter and receptor expression. Interestingly, both chronic and acute EtOH exposure decreased protein expression of SLC5A8 in the ileum and proximal colon and GPR109A protein expression in the proximal colon during acute EtOH exposure. Importantly, tributyrin provision preserved the expression of both these proteins.
Tributyrin supplementation did not protect against elevated hepatic triglyceride levels (Figs 7 and 9). While the mechanism for this sustained triglyceride increase is not clear, it is unlikely that the provision of extra butyrate, which could serve as a substrate for lipid metabolism, contributed to higher triglycerides as there is no difference in hepatic triglycerides between pair-fed groups supplemented with tributyrin compared with glycerol.
Acute EtOH exposure induces hepatic neutrophil infiltration which contributes to hepatocellular damage by generating reactive oxygen species and cytotoxic mediators (Bertola et al., 2013; Wang et al., 2012). Results from the current study reveal tributyrin supplementation prevented both short-term and single gavage EtOH-induced elevations of cytokines and chemokines implicated to play a role in the acute phase response following acute EtOH exposure (TNFα, IL1β, and MIP2; Shukla et al., 2013). These data suggest that tributyrin treatment provided as an oral gavage may act in a pharmacologic manner providing beneficial effects of butyrate locally in the intestinal epithelium preventing increased expression of inflammatory cytokines and chemokines in the liver.
In summary, our data demonstrate tributyrin provision protects intestinal TJ protein expression and co-localization, as well as the presence of a butyrate sensing receptor and transporter in the ileum and proximal colon during chronic and short-term EtOH exposure. Additionally, tributyrin pretreatment provided as a gavage was protective against deleterious effects of short-term EtOH exposure on EtOH-induced increases in liver enzymes and inflammation. Frequency, dose, and duration of EtOH intake influences resultant negative effects of EtOH. Binge drinking is defined by the National Institute on Alcohol Abuse and Alcoholism (2004) as a pattern of drinking that brings a person’s blood alcohol concentration more than 0.08% (80 mg/dl). Typically this is consumption of 5 or more drinks (males) or 4 or more drinks (females) in approximately 2 hours. Periods of binge drinking (several consecutive days, weeks, or months) are typically followed by periods of abstinence or, in some cases, significantly lower levels of consumption (Connors et al., 1986). Binge drinking incidence is increasing with a 17.1% prevalence reported in 2010 among adults, being most common and with highest intensity among persons aged 18 to 24 years (28%, 9.3 drinks on occasion) and 25 to 34 years (27.9%, 8.4 drinks on occasion; CDC, 2012). Finding an effective treatment to prophylactically protect the intestine and liver against the harmful effects of acute EtOH exposure is clearly needed. These data suggest oral supplementation of tributyrin as a protective therapy against binge drinking-induced gut injury and warrants further investigation. The oral availability of butyrate is likely of clinical importance as previous studies delivering sodium butyrate via enema or colonoscopy, while effective in improving negative clinical outcomes in patients with inflammatory bowel disease, were not well accepted by patients, and therefore not utilized in clinical practice (Breuer et al., 1997). Further investigation regarding overall acceptability and effcacy of tributyrin supplementation as a preventative therapy against EtOH-induced gut injury in humans is warranted. Characterization of molecular mechanisms of tributyrin actions in the intestine during EtOH exposure will enhance our understanding regarding beneficial effects of butyrate and advance developments of new therapeutic strategies for EtOH-induced organ injury.
ACKNOWLEDGMENTS
We want to thank Katie Pollard, Jazmine Danner, and Megan McMullen for their expert technical assistance and Manoa Hui for her assistance in preparation of the manuscript.
REFERENCES
- Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Bergheim I, Bode C, Parlesak A. Distribution of cytochrome P450 2C, 2E1, 3A4, and 3A5 in human colon mucosa. BMC Clin Pharmacol. 2005;5:4. doi: 10.1186/1472-6904-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology. 2013;58:1814–1823. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder HJ. Role of colonic short-chain fatty acid transport and diarrhea. Annu Rev Physiol. 2010;72:297–313. doi: 10.1146/annurev-physiol-021909-135817. [DOI] [PubMed] [Google Scholar]
- Borthakur A, Priyamvada S, Kumar A, Natrajan AA, Gill RK, Alrefai WA, Dudeja PK. A novel nutrient sensing mechanism underlies sub-strate-induced regulation of monocarboxylate transporter-1. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1126–G1133. doi: 10.1152/ajpgi.00308.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthakur A, Saksena S, Gill RK, Alrefai WA, Ramaswamy K, Dudeja PK. Regulation of monocarboxylate transporter 1 (MCT1) promoter by butyrate in human intestinal epithelial cells: involvement of NF-κB pathway. J Cell Biochem. 2008;103:1452–1463. doi: 10.1002/jcb.21532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer RI, Lashner BA, Christ ML, Hanauer SB, Vanaqunas A, Harig JM, Keshavarzian A, Robinson M, Sellin JH, Weinberg D, Vidican DE, Flemal KL, Rademaker AW. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: a randomised, placebo controlled trial. Gut. 1997;40:485–489. doi: 10.1136/gut.40.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wiae A, Dowell SJ. The orphan G protein-coupled receptors GPR41 and GPR43 are active by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Bull-Otterson L, Feng W, Kirpich I, Wang Y, Qin X, Liu Y, Gobejishvili L, Joshi-Barve S, Ayvaz T, Petrosino J, Kong M, Barker D, McClain C, Barve S. Metagenomic analysis of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8:e53028. doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–1528. doi: 10.3748/wjg.v17.i12.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Vital signs: binge drinking prevalence, frequency, and intensity among adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:14–19. [PubMed] [Google Scholar]
- Connors GJ, Tarbox AR, McLaughlin EF. Contrasting binge and continuous alcoholic drinkers using demographic and drinking history variables. Alcohol Alcohol. 1986;21:105–110. [PubMed] [Google Scholar]
- Cresci G, Nagy L, Ganapathy V. Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. J Parenter Enteral Nutr. 2013;37:763–774. doi: 10.1177/0148607113486809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresci G, Thangaraju M, Mellinger JD, Liu K, Ganapathy V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg. 2010;14:449–461. doi: 10.1007/s11605-009-1045-x. [DOI] [PubMed] [Google Scholar]
- Cummings JH, MacFarlane GT, Englyst HN. Prebiotic digestion and fermentation. Am J Clin Nutr. 2001;73(Suppl 2):415S–420S. doi: 10.1093/ajcn/73.2.415s. [DOI] [PubMed] [Google Scholar]
- Gassull MA. The intestinal lumen as a therapeutic target in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24(Suppl 3):90–95. doi: 10.1111/j.1365-2036.2006.03067.x. [DOI] [PubMed] [Google Scholar]
- Geokas MC, Leber CS, French S, Halsted C. Ethanol, the liver, and the gastrointestinal tract. Ann Intern Med. 1981;95:198–211. doi: 10.7326/0003-4819-95-2-198. [DOI] [PubMed] [Google Scholar]
- Gill RK, Saksena S, Alrefai WA, Sarwar Z, Goldstein JL, Carroll RE, Ramaswamy K, Dudeja PK. Expression and membrane localization of MCT isoforms along the length of the human intestine. Am J Physiol Cell Physiol. 2005;289:C846–C852. doi: 10.1152/ajpcell.00112.2005. [DOI] [PubMed] [Google Scholar]
- Halsted CH, Robles EA, Mezey E. Distribution of ethanol in the human gastrointestinal tract. Am J Clin Nutr. 1973;26:831–834. doi: 10.1093/ajcn/26.8.831. [DOI] [PubMed] [Google Scholar]
- Hass R, Busche R, Luciano L, Reale E, Engelhardt WV. Lack of butyrate is associated with induction of Bax and subsequent apoptosis in the proximal colon of guinea pig. Gastroenterology. 1997;112:875–881. doi: 10.1053/gast.1997.v112.pm9041249. [DOI] [PubMed] [Google Scholar]
- Hooper L, Midtvedt T, Gordon J. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- Inan MS, Rasoulpour RJ, Yin L, Hubbard AK, Rosenberg DW, Giardina C. The luminal short-chain fatty acid butyrate modulates NF-κB activity in a human colonic epithelial cell line. Gastroenterology. 2000;118:724–734. doi: 10.1016/s0016-5085(00)70142-9. [DOI] [PubMed] [Google Scholar]
- Israel Y, Orrego H, Carmichael FJ. Acetate-mediated effects of ethanol. Alcohol Clin Exp Res. 1994;18:144–148. doi: 10.1111/j.1530-0277.1994.tb00894.x. [DOI] [PubMed] [Google Scholar]
- Kirpich IA, Feng W, Wang Y, Liu Y, Barker DF, Barve SS, McClain CJ. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic Toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol Clin Exp Res. 2012;35:835–846. doi: 10.1111/j.1530-0277.2011.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist F, Tugstrup N, Winkler K, Mellemgaard K, Munck-Petersen S. Ethanol metabolism and the production of free acetate in human liver. J Clin Invest. 1962;41:955–961. doi: 10.1172/JCI104574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P, Roychowdhury S, Park PH, Pratt BT, Roger T, Nagy LE. Adiponectin and heme oxygenase-1 suppress TLR4/MyD88-independent signaling in rat Kupffer cells and in mice after chronic ethanol exposure. J Immunol. 2010;185:4928–4937. doi: 10.4049/jimmunol.1002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen MR, Pritchard MT, Wang Q, Millward CA, Croniger CM, Nagy LE. Early growth response-1 transcription factor is essential for ethanol induced fatty liver injury in mice. Gastroenterology. 2005;128:2066–2076. doi: 10.1053/j.gastro.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitic L, Anderson J. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Mutlu E, Keshavarzian A, Engen P, Forsyth C, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009;33:1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism NIAAA council approves definition of binge drinking. 2004 NIAAA Newsletter No. 3, p. 3. Available at: http://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/ Newsletter_Number3.pdf. Accessed December 12, 2013.
- Newmark HL, Lupton JR, Young CW. Butyrate as a differentiating agent: pharmacokinetics, analogues and current status. Cancer Lett. 1994;78:1–5. doi: 10.1016/0304-3835(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Ploger S, Stumpff F, Penner G, Schulzke JD, Gabel G, Martens H, Shen Z, Gunzel D, Aschenbach JR. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychowdhury S, McMullen MR, Pritchard MT, Li W, Salomon RG, Nagy LE. Formation of gamma-ketoaldehyde-protein adducts during ethanol-induced liver injury in mice. Free Radic Biol Med. 2009;47:1526–1538. doi: 10.1016/j.freeradbiomed.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoyama Y, Kirat D, Akihara Y, Kawasako K, Komine M, Hirayama K, Matsuda K, Okamoto M, Iwano H, Kato S, Taniyama H. Expression of monocarboylate transporter 1 (MCT1) in the dog intestine. J Vet Med Sci. 2007;69:599–604. doi: 10.1292/jvms.69.599. [DOI] [PubMed] [Google Scholar]
- Shukla SD, Pruett SB, Szabo G, Arteel GE. Binge ethanol and liver: new molecular developments. Alcohol Clin Exp Res. 2013;37:550–557. doi: 10.1111/acer.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Forsyth C, Banan A, Fields J, Keshavarzian A. Oats supplementation prevents alcohol-induced gut leakiness in rats by preventing alcohol-induced oxidative tissue damage. J Pharmacol Exp Ther. 2009;329:952–958. doi: 10.1124/jpet.108.148643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaraju M, Cresci G, Anath S, Digby G, Lambert N, Mellinger J, Ganapathy V. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaraju M, Cresci G, Itagaki S, Mellinger J, Browning D, Berger F, Prasad P, Ganapathy V. Sodium-coupled transport of the short chain fatty acid butyrate by SLC5A8 and its relevance to colon cancer. J Gastrointest Surg. 2008;12:1773–1782. doi: 10.1007/s11605-008-0573-0. [DOI] [PubMed] [Google Scholar]
- Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis. 2010;16:684–695. doi: 10.1002/ibd.21108. [DOI] [PubMed] [Google Scholar]
- Thibault R, De Coppet P, Daly K, Bourreille A, Cuff M, Bonnet C, Mosnier JF, Galmiche JP, Shirazi-Beechey S, Segain JP. Downregulation of the monocarboxylate transporter 1 is involved in butyrate deficiency during intestinal inflammation. Gastroenterology. 2007;133:1916–1927. doi: 10.1053/j.gastro.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Velazquez OC, Lederer HM, Rombeau JL. Butyrate and the colonocyte: implications in neoplasm. Dig Dis Sci. 1996;41:727–739. doi: 10.1007/BF02213129. [DOI] [PubMed] [Google Scholar]
- Visapaa JP, Tillonen J, Salaspuro M. Microbes and mucosa in the regulation of intracolonic acetaldehyde concentration during ethanol challenge. Alcohol Alcohol. 2002;37:322–326. doi: 10.1093/alcalc/37.4.322. [DOI] [PubMed] [Google Scholar]
- Wachtershauser A, Stein J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur J Nutr. 2000;39:164–171. doi: 10.1007/s003940070020. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu Y, Sidhu A, Ma Z, McClain C, Feng W. Lactobacillus rhamnosus GG culture supernatant ameliorates acute alcohol-induced intestinal permeability and liver injury. Am J Physiol Gastrointest Liver Physiol. 2012;303:G32–G41. doi: 10.1152/ajpgi.00024.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver GA, Miller TL, Wolin MJ. Short chain fatty acid distributions of enema samples from a sigmoidoscopy population: an association of high acetate and low butyrate ratios with adenomatous polyps and colon cancer. Gut. 1988;29:1539–1543. doi: 10.1136/gut.29.11.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie G, Zhong W, Zheng X, Li Q, Qiu Y, Li H, Chen H, Zhou Z, Jia W. Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. J Proteome Res. 2013;12:3297–3306. doi: 10.1021/pr400362z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, Tsukamoto H, Nelson KE, Brenner DA, Schnabl B. Enteric dysiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Zhao J, Li J, Wang H, Tang C. Acute ethanol administration inhibits Toll-like receptor 4 signaling pathway in rat intestinal epithelia. Alcohol. 2013;47:231–239. doi: 10.1016/j.alcohol.2013.01.003. [DOI] [PubMed] [Google Scholar]