Abstract
Rationale
Neutrophil extracellular trap (NET) formation promotes vascular damage, thrombosis, and activation of interferon-α-producing plasmacytoid dendritic cells in diseased arteries. Peptidylarginine deiminase inhibition is a strategy that can decrease in vivo NET formation.
Objective
To test whether peptidylarginine deiminase inhibition, a novel approach to targeting arterial disease, can reduce vascular damage and inhibit innate immune responses in murine models of atherosclerosis.
Methods and Results
Apolipoprotein-E (Apoe)−/− mice demonstrated enhanced NET formation, developed autoantibodies to NETs, and expressed high levels of interferon-α in diseased arteries. Apoe−/− mice were treated for 11 weeks with daily injections of Cl-amidine, a peptidylarginine deiminase inhibitor. Peptidylarginine deiminase inhibition blocked NET formation, reduced atherosclerotic lesion area, and delayed time to carotid artery thrombosis in a photochemical injury model. Decreases in atherosclerosis burden were accompanied by reduced recruitment of netting neutrophils and macrophages to arteries, as well as by reduced arterial interferon-α expression.
Conclusions
Pharmacological interventions that block NET formation can reduce atherosclerosis burden and arterial thrombosis in murine systems. These results support a role for aberrant NET formation in the pathogenesis of atherosclerosis through modulation of innate immune responses.
Keywords: atherosclerosis, immunology, interferon-α, neutrophils, protein-arginine deiminase, thrombosis
Neutrophils, along with other inflammatory cells, infiltrate murine atherosclerotic plaques.1-4 Furthermore, an intriguing role has recently been suggested for neutrophil extracellular trap (NET) formation in this process.5,6 NETs are proinflammatory, antimicrobial structures consisting of extracellular chromatin decorated with granular and cyto-plasmic proteins, such as myeloperoxidase (MPO), neutrophil elastase, and cathelicidin-LL37 (or the murine ortholog cathelicidin-related antimicrobial peptide [CRAMP]).7,8 In addition to causing direct organ and endothelial toxicity,9,10 NETs stimulate plasmacytoid dendritic cells (pDCs) to release interferon-α (IFN-α),11-13 a cytokine with recognized proatherogenic properties.14-18 Through profound effects on platelet and coagulation factor activation, NETs can also promote clotting, as recently recognized in deep vein thrombosis models.19-22
NETs contain deiminated (citrullinated) histones, and there is evidence that histone deimination by peptidylarginine deiminase 4 (PAD4) plays a fundamental role in NET formation. Indeed, PAD4−/− mice do not form NETs, whereas chemical inhibition of PAD4 can also abrogate NET formation by human neutrophils in vitro and mouse neutrophils in vivo.23,24 Cl-amidine is a haloacetamidine-based peptidylarginine deiminase (PAD) inhibitor, which preferentially targets PAD4 over PAD2.25 Previous work from our group and others has suggested that neutrophils are the primary inflammatory/immune cell affected by this highly specific PAD inhibitor.26,27 Indeed, we recently showed that NET formation plays a pathogenic role in a model of murine systemic lupus erythematosus (SLE), where treatment with Cl-amidine can block NET formation and modulate SLE disease activity.26 Furthermore, in that model, Cl-amidine led to a striking abrogation of vascular abnormalities attributable to SLE, including endothelial dysfunction, abnormal vascular repair, and arterial thrombosis.26 Given recent evidence that, similar to SLE, atherosclerosis is exacerbated by both NET formation and IFN-α production,5,6,16 we tested whether PAD inhibition might mitigate atherosclerosis in the apolipoprotein-E (Apoe)−/− murine model. We found a striking improvement in atherosclerosis, which our evidence suggests is at least partially attributable to abrogation of NET formation and local IFN-α production.
Methods
An expanded Materials and Methods section is available in the Online Data Supplement.
Mice and Drug Treatment
C57BL/6 control and Apoe−/− (B6.129P2-Apoetm1Unc/J) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The Apoe−/− Ifnαβr−/− mice, which also have a knockout of the type I IFN receptor, have previously been generated and described by us.18 N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, or Cl-amidine,28 was synthesized as previously described.29 Unless otherwise specified, mice were treated with either Cl-amidine (10 mg/kg/d) or an equal volume of phosphate-buffered saline (PBS) by daily subcutaneous injection, beginning at 7 weeks and through euthanasia at 18 weeks. This dose of Cl-amidine has been published previously.26,27 Mice were fed high-fat chow (42% from fat) beginning at 8 weeks and until euthanasia. For in vitro experiments, Cl-amidine was used at a concentration of 200 μmol/L.
Quantification of Atherosclerosis
Processing and quantification were performed as previously described.30,31 Briefly, arterial trees were stained with Oil Red O to quantify the atherosclerotic surface area occupied in the aortic arch, brachiocephalic trunk, common carotid arteries, and subclavian arteries. Furthermore, paraffin-embedded arteries were sectioned through the aortic sinus and stained with hematoxylin and eosin; the lipid-rich region of the intima (containing foam cells and cholesterol clefts) was quantified in cross-section as a percentage of total intimal area.
Induction of Carotid Artery Thrombosis by Photochemical Injury
This was performed as described previously.18 Briefly, rose bengal dye (Fisher Scientific, Pittsburgh, PA) was injected into the tail vein (50 mg/kg in PBS), and a 1.5-mW green light laser was applied to the carotid artery injury from a distance of 6 cm, and the vessel was monitored until occlusive thrombosis occurred.
Neutrophil Isolation and Neutrophil Assays
Bone marrow neutrophils were isolated as described.32 NET quantification and immunofluorescence microscopy was as previously described by us.13 The generation of H2O2 by neutrophils was quantified as described.33
Quantification of L-Selectin Shedding
In some cases, samples were preincubated with TAPI-0 (100 ug/mL) or Cl-amidine (200 μmol/L) for 30 minutes, before stimulation with phorbol-12-myristate-13-acetate (100 nmol/L) for another 30 minutes. Cells were then stained with fluorochrome-labeled antibodies to L-selectin (BioLegend, San Diego, CA) and Ly-6G (BD Pharmingen, San Jose, CA), and analyzed by flow cytometry. The hydroxamic acid based L-selectin sheddase inhibitor KD-IX-73-4 (TAPI-0) was purchased from Peptides International (Louisville, KY).34,35
Clearance of Lipids From Serum
Lipids were removed by Cleanascite Lipid Removal Reagent (Biotech Support Group, Monmouth Junction, NJ).
Enzyme-Linked Immunosorbent Assay and Multiplex Assay
Commercial enzyme-linked immunosorbent assays for murine anti-double-stranded DNA (dsDNA) antibodies and total immunoglobulin G were performed according to manufacturer instructions (Alpha Diagnostic, San Antonio, TX). In house enzyme-linked immunosorbent assays for anti-NETs and anti-CRAMP are described in Methods in the online Data Supplement. A multiplex assay for 5 cytokines (IFN-γ, IL-2, IL-4, IL-5, and TNF-α) was with a MILLIPLEX MAP Mouse Cytokine/Chemokine Magnetic Bead Panel (EMD Millipore, Billerica, MA).
Quantitative Polymerase Chain Reaction
RNA isolation and quantitative PCR were performed as described.36 RNA Integrity Number (RIN) was >7 for all included samples. Statistical significance was determined by comparing groups of ΔCt values using a 2-tailed Student t test, and ΔΔCt values were then determined by comparing the averages of the 2 groups.
Quantification of Lipids
Lipids were directly assayed using reagents for cholesterol (No. 3313018), triglycerides (No. 3034658), and high-density lipoproteins (No. 3034569), all from Roche Diagnostics.
Aortic Sinus Immunostaining
Primary antibodies were to citrullinated histone H3 (Abcam), Ly-6G (BD Pharmingen), F4/80 (Abcam), and MPO (Dako).
Western Blotting
Protein was prepared from dissected aortas using TriPure Isolation Reagent. Primary antibodies were specific to citrullinated histone H3 (Abcam) and α-tubulin (Sigma).
Detection of Neutrophil-Platelet Aggregates
This was similar to what has been previously described,18 with neutrophil-platelet aggregates (Ly-6G+CD61+) quantified in fresh heparinized blood.
Neutrophil Depletion
Neutrophils were depleted as described.4 In brief, depletion was with intraperitoneal injection of monoclonal antibody 1A8 (BioXCell, West Lebanon, NH). Mice were specifically treated with 100 μg of the antibody every other day from weeks 8 to 18. The control antibody 2A3 was also from BioXCell.
Statistical Analysis and Oversight
Unless otherwise indicated, results are presented as the mean and standard error of the mean (SEM), and statistical analysis was performed using Student t test in GraphPad Prism software version 5. All protocols were approved by the Committee on Use and Care of Animals of the University of Michigan.
Results
PAD Inhibition With Cl-amidine Reduces Atherosclerosis and Arterial Thrombosis in Apoe−/− Mice
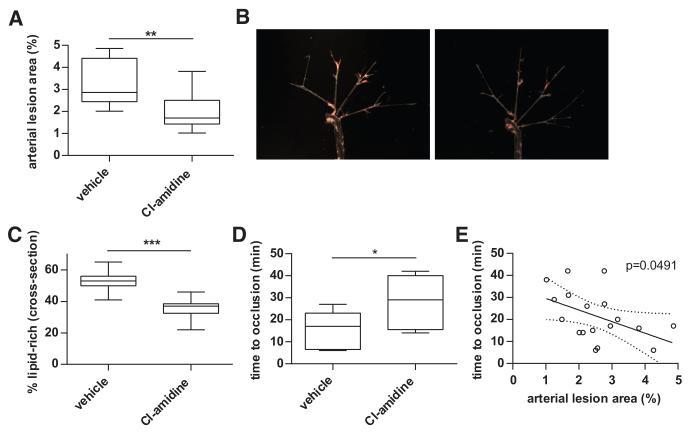
Apoe−/− mice were treated with either Cl-amidine (10 mg/kg per day) or an equal volume of PBS (vehicle) by daily subcutaneous injection, beginning at 7 weeks and through euthanasia at 18 weeks (n=10/group). Mice were fed high-fat chow beginning at 8 weeks and until euthanasia. After 11 weeks of exposure to Cl-amidine, atherosclerotic lesion area was significantly reduced when compared with vehicle-treated mice by analysis of en face oil red O lesion area (Figure 1A and 1B), as well as by quantification of cross-sectional atherosclerotic lesion area (Figure 1C). There was a statistically significant correlation between the 2 methods of analysis (for en face versus cross-sectional, linear regression P=0.0296). Furthermore, mice treated with Cl-amidine displayed prolongation of time to thrombosis in a photochemical injury model (Figure 1D). There was a statistically significant inverse correlation between lesion area and time to thrombosis (Figure 1E).
Figure 1. Peptidylarginine deiminase (PAD) inhibition with Cl-amidine reduces atherosclerosis and arterial thrombosis in Apoe−/− mice.
Apoe−/− mice exposed to high-fat chow were treated with vehicle or Cl-amidine from 7 to 18 weeks of age (n=10/group). A, Atherosclerotic lesions were quantified in arterial trees after en face Oil Red O staining. B, Representative arterial trees from vehicle-treated (left) and Cl-amidine-treated (right) mice. C, Atherosclerosis was also scored by quantifying the lipid-rich region of the intima (containing foam cells and cholesterol clefts) in cross-section as a percentage of total intimal area. D, Carotid artery thrombosis was induced by photochemical injury, and time to occlusion was determined. E, Correlation between arterial lesion area and time to carotid occlusion (n=20); the best fit line and 95% confidence intervals are plotted. For A, C, and D, boxes represent the median, 25th percentile, and 75th percentile; whiskers delineate the minimum and maximum values. *P<0.05; **P<0.01; ***P<001.
In a separate experiment, we fed Apoe−/− mice high-fat chow from 8 to 22 weeks, and started Cl-amidine treatment at 18 weeks. Under these conditions, there was no statistical difference in atherosclerotic lesion area at 22 weeks (P=0.89 with n=10 mice per group). Taken together, these results indicate that pharmacological inhibition of PADs can significantly reduce both atherosclerosis and arterial thrombosis in Apoe−/− mice when initiated as a preventive strategy.
Apoe−/− Mice Demonstrate Enhanced NET Formation and Develop AutoAbs to NETs
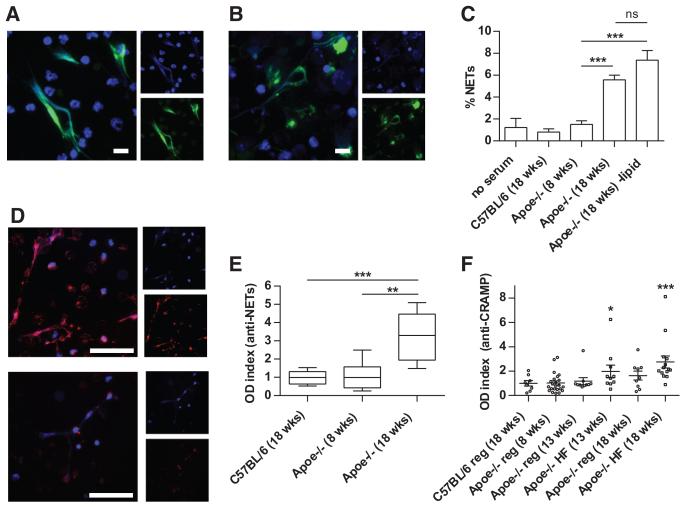
To address the mechanisms by which Cl-amidine mitigates vascular damage, we explored whether NET formation was accelerated in Apoe−/− mice. Indeed, Apoe−/− NETs displayed typical morphology and contained established protein markers of NETs including citrullinated histone H3 (H3-Cit) and MPO (Figure 2A and 2B). We then tested whether Apoe−/− serum could stimulate NET formation (Figure 2C). Neutrophils isolated from young Apoe−/− mice have a low rate of NET formation (<1%) in the absence of serum. Although serum from control C57BL/6 mice and young Apoe−/− mice did not stimulate NET formation, serum from aged, high-fat chow-fed Apoe−/− mice was significantly stimulatory (Figure 2C). This stimulation was not abrogated by lipid depletion (Figure 2C). These findings suggest that circulating factors in mice with atherosclerotic lesions are stimulatory to NET formation, independent of lipid content.
Figure 2. Apoe−/− mice demonstrate enhanced neutrophil extracellular trap (NET) formation and develop autoantibodies to NETs.
A and B, Representative immunofluorescence staining of nonpermeabilized Apoe−/− neutrophils for citrullinated histone H3 (H3-Cit; A) and MPO (B). DNA is stained blue, and the indicated protein green. Scale bars=10 μm. C, Bone marrow neutrophils were isolated from 8-week-old Apoe−/− mice. Neutrophils were incubated in the presence of 10% serum from the indicated mice for 4 hours (n=5 mice per group). ***P<0.001. D, Apoe−/− NETs were fixed and incubated with 1% serum from 18-week-old Apoe−/− mice (top) or 8-week-old Apoe−/− mice (bottom). Detection of bound antibodies was with Texas-Red-conjugated anti-immunoglobulin G; DNA is stained blue. Scale bars=50 μm. E, NET proteins were prepared as described in Methods and used to coat plates for the enzyme-linked immunosorbent assay (ELISA). Optical density (OD) index normalizes data to the average value for C57BL/6 mice. Box-and-whisker plots show data for 8 mice per group, with boxes representing the median, 25th percentile, and 75th percentile; whiskers delineate the minimum and maximum values. **P<0.01; ***P<0.001. F, An ELISA for anti-cathelicidin-related antimicrobial peptide (CRAMP) was performed as described in Methods. Some Apoe−/− mice were placed on high-fat chow (HF) beginning at 8 weeks of age; others remained on regular chow (reg). OD index normalizes data to the average value for control mice. Mean and SEM are plotted, with n≥8 per group. *P<0.05 and ***P<0.001 when compared with C57BL/6 control mice.
Increased titers of autoantibodies (autoAbs) have previously been demonstrated in Apoe−/− mice, as well as in humans with idiopathic atherosclerosis.5,37 To investigate this observation in more detail, and to better understand its relevance to NETs, we tested whether immunoglobulin G isolated from Apoe−/− mice could recognize NETs, as determined by immunofluorescence microscopy and enzyme-linked immunosorbent assay. Serum immunoglobulin G from aged mice bound NETs at a dilution that gave undetectable binding with serum from younger Apoe−/− mice (Figure 2D and 2E). CRAMP, the murine ortholog of human LL37, is known to be externalized in NETs. LL37/CRAMP has been implicated in triggering autoimmune response in SLE,12 and to play a prominent role in atherosclerosis development in murine systems.6 When Apoe−/− mice were fed a high-fat chow diet, they developed increased titers of anti-CRAMP autoAbs (Figure 2F). Overall, these results suggest that Apoe−/− mice develop an immune response directed at autoantigens externalized in the NETs, reminiscent of what has been previously described in SLE models.26
IFN Expression Is Upregulated in Atherosclerotic Lesions
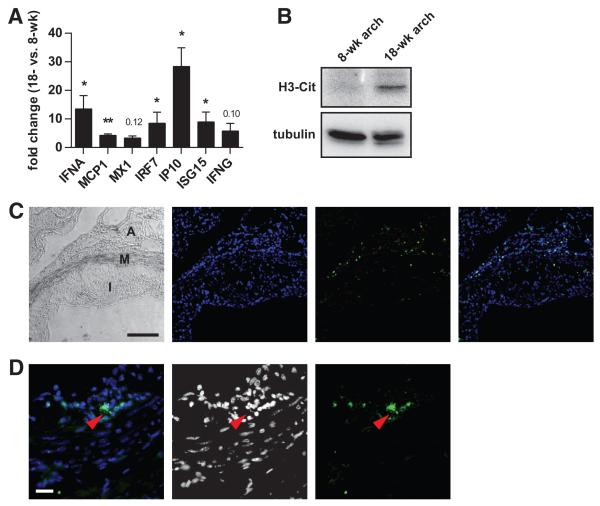
Several groups have shown that NETs signal through toll-like receptors to stimulate IFN-α production by pDCs in SLE,11-13 a concept also recently suggested for atherosclerosis.5,16 By quantitative PCR, we determined the expression of type I and II IFNs (IFN-α and IFN-γ, respectively), as well as a panel of IFN-responsive genes, in aortic tissue. We confirmed enhanced expression of the IFN-α gene, as well as several of the IFN-responsive genes, in aortic arches from older mice fed high-fat chow, as compared with younger mice (Figure 3A). In contrast, there were no significant differences in these genes when comparing spleens isolated from the same mice (data not shown).
Figure 3. Interferon expression and histone citrullination are upregulated in atherosclerotic lesions.
A, Apoe−/− mice were placed on high-fat chow beginning at 8 weeks of age. RNA was isolated from aortic arches of 8- and 18-week-old Apoe−/− mice. Fold change in gene expression was calculated for 18-week-old mice, relative to 8-week-old mice (n=5). Mean and SEM are plotted. *P<0.05, **P<0.01, and ***P<0.001; P values that did not reach significance are indicated. B, Protein was prepared from aortic arches of the indicated Apoe−/− mice. Protein from 5 mice per group was pooled and 20 μg of total protein was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis before Western blotting with the indicated antibodies. C, Low magnification view of an atherosclerotic lesion with a phase-contrast image showing intima (I), media (M), and adventitia (A). DNA is stained blue, and MPO is stained green, with an overlay to the far right. Scale bar=100 μm. D, A higher magnification view of the media/adventitia interface shows an MPO-positive cell in more detail. Extracellular MPO (green) juxtaposed with decondensed DNA (blue) is seen (red arrowhead); in the middle, the DNA channel is shown in grayscale to improve contrast. Scale bar=20 μm.
We also detected markers of NETs in the aortic arches of older mice fed high-fat chow, as compared with younger mice. By Western blotting, H3-Cit protein was significantly upregulated (Figure 3B). Furthermore, by immunofluorescence of aortic sinus plaques, MPO-positive cells could be detected infiltrating the media and adventitia (Figure 3C). In many cases, the MPO-positive cells demonstrated nuclear decondensation and extracellular MPO, where the MPO staining overlapped with DNA (Figure 3D), a staining pattern consistent with NETs. Overall, these observations indicate that diseased arteries are associated with enhanced neutrophil netting and local IFN responses, with a time course and localization that parallels the development of atherosclerosis.
Cl-Amidine Abrogates NET Formation
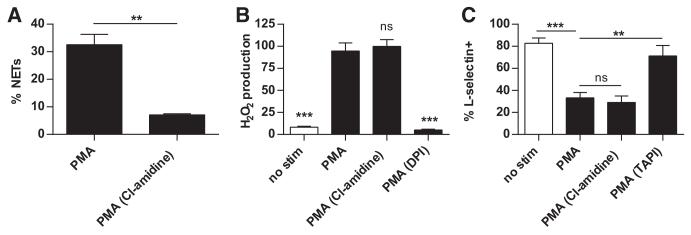
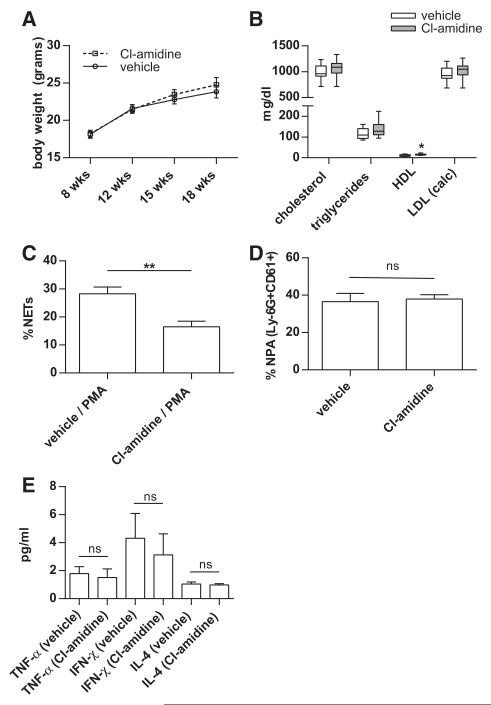
Because Apoe−/− mice displayed evidence of enhanced NET formation and IFN responses, we tested the impact of PAD inhibition on these parameters. First, we demonstrated in vitro that Cl-amidine was relatively specific for NET inhibition. Although Cl-amidine robustly blocked NET formation by neutrophils (Figure 4A), neither H2O2 production (Figure 4B) nor L-selectin shedding (Figure 4C) was affected. When administered in vivo, Cl-amidine treatment did not alter weight (Figure 5A), and lipid profile was also largely unchanged, except for a slight (3.8 mg/dL) increase in HDL levels with Cl-amidine, when compared with vehicle treatment (Figure 5B). Although NET formation was significantly reduced in mice treated with Cl-amidine (Figure 5C), the regulation of neutrophil adherence molecules was not affected based on quantification of neutrophil-platelet aggregates (Figure 5D). Furthermore, with Cl-amidine treatment, no significant changes were detected in circulating levels of TNF-α, IFN-γ, IL-4, anti-CRAMP, or total immunoglobulin G (Figure 5E, and data not shown). In summary, Cl-amidine had a relatively specific effect on NET formation, without targeting other neutrophil functions, or autoantibody titers. Combined with previous work by us and others with this agent,26,27 we propose that Cl-amidine primarily impacts the immune system through abrogation of NET formation.
Figure 4. Cl-amidine abrogates neutrophil extracellular trap (NET) formation, but not H2O2 production or L-selectin shedding.
A, Bone marrow neutrophils were isolated from 8-week-old Apoe−/− mice. Neutrophils were stimulated with phorbol-12-myristate-13-acetate (PMA) in the presence or absence of 200 μmol/L Cl-amidine, and NET formation was quantified by immunofluorescence microscopy. B, Cl-amidine treatment does not alter H2O2 production. Apoe−/− bone marrow neutrophils were stimulated with PMA in the presence of inhibitors as indicated. The PMA-stimulated sample was arbitrarily set at 100% H2O2 production; statistical comparisons are to this group. DPI=NADPH oxidase inhibitor. C, Cl-amidine treatment does not alter L-selectin shedding. Neutrophils were stimulated with PMA in the presence of inhibitors as indicated. Surface staining was then with anti-Ly-6G (to confirm the identity of neutrophils) and anti-L-selectin, before analysis by flow cytometry. Data are presented as the percentage of Ly-6G+ that are also L-selectin+. All experiments were repeated at least 3 times. **P<0.01; ***P<0.001.
Figure 5. Peptidylarginine deiminase (PAD) inhibition abrogates neutrophil extracellular trap (NET) formation and alters anti-NET autoantibody profiles in Apoe−/− mice.
High-fat chow-fed Apoe−/− mice were treated with vehicle or Cl-amidine from 7 to 18 weeks of age. A, Body weight was recorded at the indicated time points. B, Serum was collected at 18 weeks of age and total cholesterol, triglycerides, and HDL were determined by direct measurement; LDL was calculated. *P<0.05; no other comparison of vehicle versus Cl-amidine was significant C, Bone marrow neutrophils were isolated at 18 weeks of age and stimulated with phorbol-12-myristate-13-acetate (PMA). **P<0.01. D, Neutrophil-platelet aggregates (NPA) were determined in blood at 18 weeks of age. NPA were defined as events positive for both Ly-6G and CD61. The percentage is calculated relative to total Ly-6G-positive cells. E, Serum cytokine levels were measured by multiplex assay; ns=not significant. A-E, n=10 per group. For A, C, D, and E, the mean and SEM are plotted. For B, boxes represent the median, 25th percentile, and 75th percentile; whiskers delineate the minimum and maximum values.
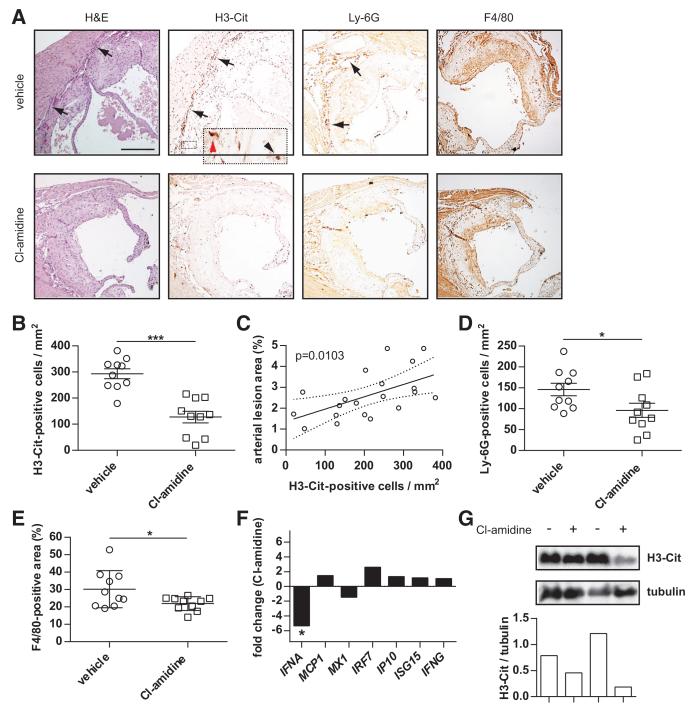
PAD Inhibition Reduces the Recruitment of Netting Neutrophils to the Media and Adventitia of Apoe−/− Aortic Sinus Lesions
To further understand the mechanism by which PAD inhibition reduces atherosclerosis, we analyzed the inflammatory infiltrates of aortic sinus arterial lesions. Although F4/80 (macrophage) staining highlighted areas of the intima bordering on the vessel lumen, a distinctly different pattern was observed for Ly-6G (neutrophil) and H3-Cit (netting neutrophils) staining, which highlighted cells clustering in the media and at the media-adventitia interface (see Figure 6A for representative staining). Quantification of these areas revealed a significant decrease in both H3-Cit- and Ly-6G-positive cells when mice were treated with Cl-amidine (Figure 6B-D). Furthermore, there was a significant reduction in F4/80-positive area within the intima of Cl-amidine-treated mice (Figure 6E), although H3-Cit staining did no colocalize with this region. Importantly, the number of H3-Cit-positive cells correlated directly with lesion area (Figure 6C). In contrast, neither Ly-6G-positive cells nor F4/80-positive staining showed a statistically significant correlation (data not shown). These data demonstrate that Cl-amidine can reduce the infiltration of macrophages into the intima, and of netting neutrophils into the media and adventitia.
Figure 6. Peptidylarginine deiminase (PAD) inhibition reduces the recruitment of netting neutrophils to the media and adventitia of Apoe−/− aortic sinus lesions.
A, Aortic sinuses from the 18-week-old mice presented in Figure 1 were sectioned and stained by immunohistochemistry for neutrophil extracellular traps (citrullinated histone H3 [H3-Cit]), neutrophils (Ly-6G), and macrophages (F4/80). Representative staining is shown, with a neutrophil and H3-Cit-rich infiltrate at the interface between the intima and the media/adventitia (arrows). The inset shows a relatively intact H3-Cit-positive cell with polymorphonuclear morphology (black arrowhead) as well as a cell with decondensed morphology (red arrowhead). Scale bar=250 μm. B, Quantification of H3-Cit-positive cells in the media/adventitia. C, Correlation between the number of H3-Cit-positive cells and arterial lesion area; the best fit line and 95% confidence intervals are plotted. D and E, Quantification of Ly-6G-positive cells in the media/adventitia (D), and F4/80-positive area in the intima (E). B, D, and E, n=10 per group, and mean and SEM are plotted. *P<0.05; ***P<0.001. F, PAD inhibition reduces IFN-α expression in the aortic arch of Apoe−/− mice. RNA was prepared from aortic arches at 18 weeks of age. The data are expressed as fold change (positive value indicates activation; and negative value, repression) for Cl-amidine-treated mice relative to vehicle-treated mice (n=10 per group). *P<0.05; no other Cl-amidine versus vehicle comparison reached statistical significance. G, Protein was prepared from aortic arches at 18 weeks of age. Each lane represents protein pooled from 5 similarly treated mice, with H3-Cit and α-tubulin detected by Western blotting. Data are plotted as the ratio of H3-Cit density to α-tubulin density for each sample. The Western blot portion of the experiment was performed twice with similar results.
Given that NETs are recognized promoters of IFN-α production in SLE,11-13 as well as atherosclerosis,5,16 we predicted that reduced NET formation in arterial lesions would also lead to downregulation of IFN-α expression. Indeed, by quantitative PCR, we found a 5-fold repression of the IFNA gene with Cl-amidine treatment (Figure 6F). This repression was not seen in the spleens of the same animals (data not shown) nor was it seen for the IFNG gene. The only IFN-responsive gene to show a slight trend toward repression was MX1 (the tested gene most selective for IFN-α as compared with IFN-γ),38 although this did not reach statistical significance (Figure 6F). Cl-amidine also downregulated H3-Cit protein by Western blot in the same samples for which quantitative PCR was performed (Figure 6G). To summarize, PAD inhibition represses IFN-α synthesis, probably by blocking NET formation.
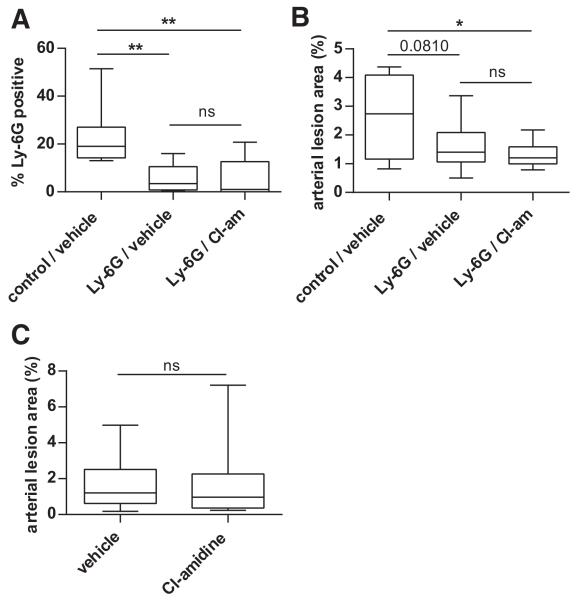
Cl-Amidine Does Not Protect Against Atherosclerosis in Neutropenic or in Type I IFN Receptor-Deficient Mice
It has previously been shown that neutrophil depletion with an anti-Ly-6G antibody protects against atherosclerosis in Apoe−/− mice.4 Here, we hypothesized that if Cl-amidine were primarily providing its protective effects by inhibiting neutrophil function, then Cl-amidine would mitigate atherosclerosis after neutrophil depletion. Using a published treatment regimen,4 Apoe−/− mice were administered either a control or anti-Ly-6G antibody, from 8 to 18 weeks. Mice were at the same time treated with Cl-amidine or vehicle, as above, from 7 to 18 weeks. With this regimen, Ly-6G-positive neutrophils remained effectively depleted at 18 weeks of age (Figure 7A). Furthermore, there was a strong trend toward reduction in atherosclerosis with anti-Ly-6G treatment (Figure 7B, compare the first and second conditions). Importantly, in the background of neutrophil depletion, Cl-amidine did not provide any further protection (Figure 7B, compare the second and third conditions).
Figure 7. Cl-amidine does not protect against atherosclerosis in neutropenic mice.
A, Apoe−/− mice exposed to high-fat chow were treated with vehicle or Cl-amidine from 7 to 18 weeks of age as indicated. Mice were also treated with either anti-Ly-6G or a control antibody from 8 to 18 weeks of age as indicated. At 18 weeks of age, peripheral blood was collected and anti-Ly-6G-positive cells were determined by flow cytometry as a percentage of total circulating leukocytes. B, Mice were treated as in A, and atherosclerotic lesions were quantified in arterial trees by en face Oil Red O staining. C, Apoe−/− Ifnαβr−/− mice lack the type I IFN receptor. These mice were exposed to high-fat chow, and were treated with vehicle or Cl-amidine from 7 to 18 weeks of age as indicated. Atherosclerotic lesions were quantified in arterial trees by en face Oil Red O staining. For all experiments, box- and-whisker plots show data for 10 mice per group, with boxes representing the median, 25th percentile, and 75th percentile; whiskers delineate the minimum and maximum values. *P<0.05; **P<0.01; ns indicates not significant. One P value that approaches significance is denoted.
Our group has previously shown that atherosclerosis is reduced in Apoe−/− mice that also carry a mutation in the type I IFN receptor gene.18 Similar to neutropenic mice, these Apoe−/− Ifnαβr−/− mice were not protected by treatment with Cl-amidine (Figure 7C). In summary, Cl-amidine does not protect against atherosclerosis in the background of neutrophil depletion or type I IFN receptor deletion, suggesting that Cl-amidine likely acts through a neutrophil-based pathway, such as NET formation, and the induction of type I IFN responses in the artery.
Discussion
Recent studies have observed the infiltration of netting neutrophils into the atheromatous lesions of mice.4-6 Indeed, in murine systems, depletion of either whole neutrophils or the NET component CRAMP can protect against atherosclerosis,4,6 whereas treatment with exogenously prepared CRAMP-DNA complexes can accelerate disease.5 Netting neutrophils can also be detected in the blood of patients with severe coronary atherosclerosis,39 as well as in the atherosclerotic plaques themselves.40 Furthermore, in human plaques, PAD4 has been observed deiminating fibrinogen to generate a novel rheumatoid arthritis autoantigen.41 Although the cellular sources of this PAD4 have not been explored,41 neutrophils are a prime candidate. Our group recently showed that PAD inhibition reduces NET formation, alters markers of autoimmunity, and potently mitigates vascular damage in a murine model of SLE,26 a disease process that is highly dependent on type I IFNs like IFN-α.42 Although disruption of PAD activity has been considered in a model of venous thrombosis,43 it has not been evaluated in a pure model of arterial damage or atherosclerosis.
We now report that Apoe−/− mice are protected from atherosclerosis when treated with the PAD inhibitor Cl-amidine. We also show that PAD inhibition abrogates NET release, mitigates arterial type I IFN responses, and reduces the number of netting neutrophils that infiltrate the media and adventitia of atheromatous lesions. The fact that we did not see further protection with Cl-amidine in neutropenic mice, or in mice lacking the type I IFN receptor, suggests that the protective effects of Cl-amidine are primarily through downregulation of neutrophil and IFN pathways. And because Cl-amidine specifically targets NET formation, but not other neutrophil functions,26,27 we have now demonstrated in vivo a causative role for neutrophil netting in the development of murine atherosclerosis. Importantly, previous studies by our group and others have not identified a role for PAD inhibition in modulation of the phenotype and function of other immune cells, including lymphocytes and natural killer cells.26,27
Cl-amidine prolonged the time to carotid thrombosis in these mice, which could be related to mitigation of vascular disease burden since an inverse correlation between lesion area and time to thrombosis was observed. Furthermore, previous work indicates that NETs infiltrate and stimulate venous and arterial thrombosis through effects on platelets and coagulation factors19,26; it is therefore plausible that the decrease in prothrombotic phenotype was attributable, at least in part, to impaired neutrophil function. Indeed, in venous injury models, neutrophil PAD4 has been found to be critical for clots to form in vivo.41 Our results newly support an important role for neutrophil posttranslational modifications (deimination) and chromatin decondensation (NET formation) in thrombus formation in animal models of atherosclerosis. This has potentially important clinical implications, given the well-recognized role of arterial thrombosis in atherosclerosis-mediated tissue ischemia.
Our group and others have shown that type I IFNs are proatherogenic and have pleiotropic deleterious effects in the vasculature.14-18 Furthermore, depletion of IFN-producing pDCs reduces plaque area and macrophage recruitment,16 and protein-DNA complexes derived from NETs activate pDCs to accelerate atherosclerosis in Apoe−/− mice.5 Indeed, both NETs and CRAMP have been detected at the luminal surface of early atherosclerotic lesions, whereas depletion of either pDCs or CRAMP protects against atherosclerosis.5,6 A notable difference in our study is that, as has also been reported by others,2,44 neutrophils were detected in the media and the media-adventitia interface, and were only rarely found along the luminal surface of lesions. It should be noted that we quantified cells at a later time point than some other studies,5,6 and did not use live image capture; as such, cells transiently associated with the endothelial surface could have been lost during processing for immunohistochemistry. It is certainly possible that neutrophils and NETs play important roles both in the initiating events of atherogenesis such as endothelial damage,5,6 and in later events like the IFN-mediated recruitment of macrophages to developing lesions; indeed, a reduction in arterial macrophage infiltration was evident after Cl-amidine treatment.
Although Cl-amidine was effective as a preventive strategy for plaque formation, significant changes were not seen if started once atheroma formation was well underway. This is in line with previous work demonstrating a particular role for neutrophils in the early stages of murine atherosclerosis.4 Future studies should continue to address, in various murine and human systems, whether more targeted PAD inhibition might have therapeutic effects in reversing atherosclerosis rather than preventing it. Furthermore, given the well-recognized association between inflammatory disorders and atherosclerosis,45-48 this preventive data could still have significant clinical implications for the subset of patients known to be prone to accelerated atherosclerosis.
Whether the modest increase in HDL induced by Cl-amidine could have played a role in plaque inhibition is unclear and will require further study. We previously reported that Apoe−/− mice that lack type I IFN receptor signaling display increases in HDL.18 As such, it is possible that the effect of Cl-amidine on HDL quantity is through abrogation of proinflammatory cellular responses mediated by type I IFNs.
In conclusion, a possible therapeutic intervention for humans, pharmacological PAD inhibition, significantly decreases atherosclerosis burden and mitigates arterial thrombosis in the Apoe−/− model. Future studies should focus on more specific PAD4 inhibition by chemical and genetic strategies, as well as different dosing strategies for already described PAD inhibitors like Cl-amidine. Other areas needing investigation include the potential implications of atheroma-derived PAD activity beyond NET formation,41 and the different circulating factors—such as autoAbs, inflammatory cytokines, and activated platelets—that may predispose neutrophils toward netting. Overall, our observations further support the notion that innate immune responses, specifically NETs and type I IFNs, play prominent roles in the pathogenesis of atherosclerosis. These pathways should be further studies as therapeutic targets to mitigate atherosclerosis and thrombotic risk in the general population.
Novelty and Significance.
What Is Known?
Neutrophil depletion protects against atherosclerosis in mice.
Neutrophil extracellular traps (NETs), chromatin-based structures released by neutrophils to capture and kill pathogens, are highly stimulatory to the immune system, sometimes with deleterious consequences.
NETs have been implicated in vascular damage in other diseases, such as systemic lupus erythematosus (SLE).
Peptidylarginine deiminase (PAD) inhibition is an effective, and relatively specific, means for blocking NET formation by neutrophils.
What New Information Does This Article Contribute?
Atherosclerosis-prone Apoe−/− mice have evidence of accelerated NET formation, as well as an autoimmune response to NETs.
PAD inhibition not only blocks NET formation but also significantly protects against both atherosclerosis and arterial thrombosis, strongly suggesting a causative role for NET formation in atherosclerosis.
NETs stimulate type I interferon production in murine atherosclerosis, a process that is effectively blocked by PAD inhibition.
Neutrophils infiltrate atherosclerotic plaques and, in mice, neutrophil depletion has been shown to protect against atherosclerosis. Neutrophils, and in particular NET formation, have been implicated in the vascular damage of other inflammatory diseases, such as SLE, possibly through the stimulation of type I IFN production and endothelial cell cytotoxicity. Here, we tested whether pharmacological blockade of NET formation by inhibition of PAD enzymes could protect against atherosclerosis. Indeed, Apoe−/− mice were protected from both atherosclerosis and arterial thrombosis when NET formation was prevented with a PAD inhibitor. Blocking NETs also led to downregulation of type I interferons in diseased arteries. Importantly, PAD inhibition was not effective in the setting of neutrophil depletion or interferon receptor mutation, thereby implicating both pathways in NET-mediated arterial damage. This article adds to the existing literature by strongly arguing for a causative role of NETs in atherosclerosis. Furthermore, and in contrast to neutrophil depletion or genetic mutation, NETs were inhibited here by a pharmacological approach that might someday be applicable to humans. Future studies should further assess these pathways as therapeutic targets to mitigate atherosclerosis and thrombotic risk in the general population.
Acknowledgments
Microscopy was performed in the Center for Live Cell Imaging at the University of Michigan. This work used the Chemistry Core of the Michigan Diabetes Research and Training Center funded by DK020572 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Sources of Funding
This work was supported by the National Institutes of Health (NIH) through Public Health Service grant HL088419 (to M.J. Kaplan). J.S. Knight was supported by a Rheumatology Research Foundation Rheumatology Scientist Development Award. P.R. Thompson is supported by NIH grants GM079357 and CA151304. This article was prepared while W. Zhao and M.J. Kaplan were employed at the University of Michigan. The opinions expressed in this article are the authors’ own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the US government.
Nonstandard Abbreviations and Acronyms
- autoAb
autoantibody
- CRAMP
cathelicidin-related antimicrobial peptide
- H3-Cit
citrullinated histone H3
- IFN
interferon
- MPO
myeloperoxidase
- NET
neutrophil extracellular trap
- PAD
peptidylarginine deiminase
- pDC
plasmacytoid dendritic cell
- SLE
systemic lupus erythematosus
Footnotes
Disclosures
None.
References
- 1.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110:875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 2.van Leeuwen M, Gijbels MJ, Duijvestijn A, Smook M, van de Gaar MJ, Heeringa P, de Winther MP, Tervaert JW. Accumulation of myeloperoxidase-positive neutrophils in atherosclerotic lesions in LDLR−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:84–89. doi: 10.1161/ATVBAHA.107.154807. [DOI] [PubMed] [Google Scholar]
- 3.Rotzius P, Thams S, Soehnlein O, Kenne E, Tseng CN, Björkström NK, Malmberg KJ, Lindbom L, Eriksson EE. Distinct infiltration of neutrophils in lesion shoulders in ApoE−/− mice. Am J Pathol. 2010;177:493–500. doi: 10.2353/ajpath.2010.090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drechsler M, Megens RT, van Zandvoort M, Weber C, Soehnlein O. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 5.Döring Y, Manthey HD, Drechsler M, et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation. 2012;125:1673–1683. doi: 10.1161/CIRCULATIONAHA.111.046755. [DOI] [PubMed] [Google Scholar]
- 6.Döring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res. 2012;110:1052–1056. doi: 10.1161/CIRCRESAHA.112.265868. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol. 2012;198:773–783. doi: 10.1083/jcb.201203170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7:e32366. doi: 10.1371/journal.pone.0032366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z, Punaro M, Baisch J, Guiducci C, Coffman RL, Barrat FJ, Banchereau J, Pascual V. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra20. doi: 10.1126/scitranslmed.3001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lande R, Ganguly D, Facchinetti V, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med. 2011;3:73ra19. doi: 10.1126/scitranslmed.3001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villanueva E, Yalavarthi S, Berthier CC, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol. 2011;187:538–552. doi: 10.4049/jimmunol.1100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goossens P, Gijbels MJ, Zernecke A, Eijgelaar W, Vergouwe MN, van der Made I, Vanderlocht J, Beckers L, Buurman WA, Daemen MJ, Kalinke U, Weber C, Lutgens E, de Winther MP. Myeloid type I interferon signaling promotes atherosclerosis by stimulating macrophage recruitment to lesions. Cell Metab. 2010;12:142–153. doi: 10.1016/j.cmet.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Fu Q, Cui H, Qu B, Pan W, Shen N, Bao C. Interferon-alpha priming promotes lipid uptake and macrophage-derived foam cell formation: A novel link between interferon-alpha and atherosclerosis in lupus. Arthritis Rheum. 2011;63:492–502. doi: 10.1002/art.30165. [DOI] [PubMed] [Google Scholar]
- 16.Macritchie N, Grassia G, Sabir SR, Maddaluno M, Welsh P, Sattar N, Ialenti A, Kurowska-Stolarska M, McInnes IB, Brewer JM, Garside P, Maffia P. Plasmacytoid dendritic cells play a key role in promoting atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2012;32:2569–2579. doi: 10.1161/ATVBAHA.112.251314. [DOI] [PubMed] [Google Scholar]
- 17.Niessner A, Shin MS, Pryshchep O, Goronzy JJ, Chaikof EL, Weyand CM. Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation. 2007;116:2043–2052. doi: 10.1161/CIRCULATIONAHA.107.697789. [DOI] [PubMed] [Google Scholar]
- 18.Thacker SG, Zhao W, Smith CK, Luo W, Wang H, Vivekanandan-Giri A, Rabquer BJ, Koch AE, Pennathur S, Davidson A, Eitzman DT, Kaplan MJ. Type I interferons modulate vascular function, repair, thrombosis, and plaque progression in murine models of lupus and atherosclerosis. Arthritis Rheum. 2012;64:2975–2985. doi: 10.1002/art.34504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs TA, Brill A, Wagner DD. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Brühl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rohrbach AS, Slade DJ, Thompson PR, Mowen KA. Activation of PAD4 in NET formation. Front Immunol. 2012;3:360. doi: 10.3389/fimmu.2012.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leshner M, Wang S, Lewis C, Zheng H, Chen XA, Santy L, Wang Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front Immunol. 2012;3:307. doi: 10.3389/fimmu.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Causey CP, Jones JE, Slack JL, Kamei D, Jones LE, Subramanian V, Knuckley B, Ebrahimi P, Chumanevich AA, Luo Y, Hashimoto H, Sato M, Hofseth LJ, Thompson PR. The development of N-α-(2-carboxyl) benzoyl-N(5)-(2-fluoro-1-iminoethyl)-l-ornithine amide (o-F-amidine) and N-α-(2-carboxyl)benzoyl-N(5)-(2-chloro-1-iminoethyl)-l-ornithine amide (o-Cl-amidine) as second generation protein arginine deiminase (PAD) inhibitors. J Med Chem. 2011;54:6919–6935. doi: 10.1021/jm2008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight JS, Zhao W, Luo W, Subramanian V, O’Dell AA, Yalavarthi S, Hodgin JB, Eitzman DT, Thompson PR, Kaplan MJ. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013;123:2981–2993. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, Luo Y, Levitt B, Glogowska M, Chandra P, Kulik L, Robinson WH, Arend WP, Thompson PR, Holers VM. N-α-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186:4396–4404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo Y, Arita K, Bhatia M, Knuckley B, Lee YH, Stallcup MR, Sato M, Thompson PR. Inhibitors and inactivators of protein arginine deiminase 4: functional and structural characterization. Biochemistry. 2006;45:11727–11736. doi: 10.1021/bi061180d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Causey CP, Thompson PR. An improved synthesis of haloaceteamidine-based inactivators of protein arginine deiminase 4 (PAD4) Tetrahedron Lett. 2008;49:4383–4385. doi: 10.1016/j.tetlet.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo W, Wang H, Ohman MK, Guo C, Shi K, Wang J, Eitzman DT. P-selectin glycoprotein ligand-1 deficiency leads to cytokine resistance and protection against atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 2012;220:110–117. doi: 10.1016/j.atherosclerosis.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohman MK, Shen Y, Obimba CI, Wright AP, Warnock M, Lawrence DA, Eitzman DT. Visceral adipose tissue inflammation accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2008;117:798–805. doi: 10.1161/CIRCULATIONAHA.107.717595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boxio R, Bossenmeyer-Pourié C, Steinckwich N, Dournon C, Nüsse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 33.Serezani CH, Aronoff DM, Jancar S, Mancuso P, Peters-Golden M. Leukotrienes enhance the bactericidal activity of alveolar macrophages against Klebsiella pneumoniae through the activation of NADPH oxidase. Blood. 2005;106:1067–1075. doi: 10.1182/blood-2004-08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hafezi-Moghadam A, Thomas KL, Prorock AJ, Huo Y, Ley K. L-selectin shedding regulates leukocyte recruitment. J Exp Med. 2001;193:863–872. doi: 10.1084/jem.193.7.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Denny MF, Yalavarthi S, Zhao W, Thacker SG, Anderson M, Sandy AR, McCune WJ, Kaplan MJ. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 2010;184:3284–3297. doi: 10.4049/jimmunol.0902199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thacker SG, Duquaine D, Park J, Kaplan MJ. Lupus-prone New Zealand Black/New Zealand White F1 mice display endothelial dysfunction and abnormal phenotype and function of endothelial progenitor cells. Lupus. 2010;19:288–299. doi: 10.1177/0961203309353773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Huang Z, Lu H, Lin H, Wang Z, Chen X, Ouyang Q, Tang M, Hao P, Ni J, Xu D, Zhang M, Zhang Q, Lin L, Zhang Y. Apolipoprotein E-knockout mice show increased titers of serum anti-nuclear and anti-dsDNA antibodies. Biochem Biophys Res Commun. 2012;423:805–812. doi: 10.1016/j.bbrc.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 38.Aebi M, Fäh J, Hurt N, Samuel CE, Thomis D, Bazzigher L, Pavlovic J, Haller O, Staeheli P. cDNA structures and regulation of two interferon-induced human Mx proteins. Mol Cell Biol. 1989;9:5062–5072. doi: 10.1128/mcb.9.11.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, Gallant M, Martinod K, Ten Cate H, Hofstra L, Crijns HJ, Wagner DD, Kietselaer BL. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol. 2013;33:2032–2040. doi: 10.1161/ATVBAHA.113.301627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Megens RT, Vijayan S, Lievens D, Döring Y, van Zandvoort MA, Grommes J, Weber C, Soehnlein O. Presence of luminal neutrophil extra-cellular traps in atherosclerosis. Thromb Haemost. 2012;107:597–598. doi: 10.1160/TH11-09-0650. [DOI] [PubMed] [Google Scholar]
- 41.Sokolove J, Sharpe O, Brennan M, Lahey LJ, Kao AH, Krishnan E, Edmundowicz D, Lepus CM, Wasko MC, Robinson WH. Citrullination within the atherosclerotic plaque: A potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum. 2013 doi: 10.1002/art.37961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elkon KB, Wiedeman A. Type I IFN system in the development and mani-festations of SLE. Curr Opin Rheumatol. 2012;24:499, 505. doi: 10.1097/BOR.0b013e3283562c3e. [DOI] [PubMed] [Google Scholar]
- 43.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A. 2013;110:8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Ye D, Wang J, Calpe-Berdiel L, Azzis SB, Van Berkel TJ, Van Eck M. Stage-specific remodeling of atherosclerotic lesions upon cholesterol lowering in LDL receptor knockout mice. Am J Pathol. 2011;179:1522–1532. doi: 10.1016/j.ajpath.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cohen Tervaert JW. Cardiovascular disease due to accelerated atherosclerosis in systemic vasculitides. Best Pract Res Clin Rheumatol. 2013;27:33–44. doi: 10.1016/j.berh.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 46.Knight JS, Kaplan MJ. Cardiovascular disease in lupus: insights and updates. Curr Opin Rheumatol. 2013;25:597–605. doi: 10.1097/BOR.0b013e328363eba3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papagoras C, Voulgari PV, Drosos AA. Atherosclerosis and cardiovascular disease in the spondyloarthritides, particularly ankylosing spondylitis and psoriatic arthritis. Clin Exp Rheumatol. 2013;31:612–620. [PubMed] [Google Scholar]
- 48.Gkaliagkousi E, Gavriilaki E, Doumas M, Petidis K, Aslanidis S, Stella D. Cardiovascular risk in rheumatoid arthritis: pathogenesis, diagnosis, and management. J Clin Rheumatol. 2012;18:422–430. doi: 10.1097/RHU.0b013e31827846b1. [DOI] [PubMed] [Google Scholar]