Abstract
This chapter focuses on the biology of the major facilitative membrane folate transporters, the reduced folate carrier (RFC), and the proton-coupled folate transporter (PCFT). Folates are essential vitamins, and folate deficiency contributes to a variety of heath disorders. RFC is ubiquitously expressed and is the major folate transporter in mammalian cells and tissues. PCFT mediates intestinal absorption of dietary folates. Clinically relevant antifolates such as methotrexate (MTX) are transported by RFC, and the loss of RFC transport is an important mechanism of MTX resistance. PCFT is abundantly expressed in human tumors and is active under pH conditions associated with the tumor microenvironment. Pemetrexed (PMX) is an excellent substrate for PCFT as well as for RFC. Novel tumor-targeted antifolates related to PMX with selective membrane transport by PCFT over RFC are being developed. The molecular picture of RFC and PCFT continues to evolve relating to membrane topology, N-glycosylation, energetics, and identification of structurally and functionally important domains and amino acids. The molecular bases for MTX resistance associated with loss of RFC function, and for the rare autosomal recessive condition, hereditary folate malabsorption (HFM), attributable to mutant PCFT, have been established. From structural homologies to the bacterial transporters GlpT and LacY, homology models were developed for RFC and PCFT, enabling new mechanistic insights and experimentally testable hypotheses. RFC and PCFT exist as homo-oligomers, and evidence suggests that homo-oligomerization of RFC and PCFT monomeric proteins may be important for intracellular trafficking and/or transport function. Better understanding of the structure and function of RFC and PCFT should facilitate the rational development of new therapeutic strategies for cancer as well as for HFM.
1. INTRODUCTION
The folates are members of the B family of vitamins that require active membrane transport systems for cellular uptake (Matherly & Goldman, 2003; Zhao, Diop-Bove, Visentin, & Goldman, 2011). Studies of the membrane transport of folate cofactors over the past five decades have established its nutritional importance in providing cofactors for essential metabolic reactions required for cell proliferation and tissue repair. Transport is also required for uptake of antifolate drugs such as methotrexate (MTX) into tumor cells and is a major determinant of chemotherapeutic efficacy for these agents (Matherly, Hou, & Deng, 2007; Zhao & Goldman, 2003).
The reduced folate carrier (RFC; SLC19A1) belongs to the solute carrier (SLC) group of transporters and is the principal mechanism by which folates and clinically used antifolates are delivered to mammalian cells and tissues from the systemic circulation at neutral pH (Matherly et al., 2007). Although a transporter with unique properties from RFC was recognized for over 30 years to mediate intestinal absorption of folates, and more recently to drive uptake of the new generation antifolate pemetrexed (PMX) into tumor cells, it was not until 2006 that this system was cloned and characterized (Qiu et al., 2006). Thus, the proton-coupled folate transporter (PCFT; SLC46A1) was recognized as distinct from RFC or other known transporters capable of transporting folates such as the family of organic anion transporters (Matherly et al., 2007; Rizwan & Burckhardt, 2007). Identification of loss-of-function mutations in PCFT from human subjects with hereditary folate malabsorption (HFM) provided the molecular basis for this rare condition and unequivocally established PCFT as the principal mechanism for intestinal absorption of dietary folates (Qiu et al., 2006).
This review focuses on the biology of the major facilitative folate transporters, RFC and PCFT, including their physiology and structural and functional properties.
2. THE BIOLOGICAL ROLES OF FOLATES AND THERAPY WITH ANTIFOLATES
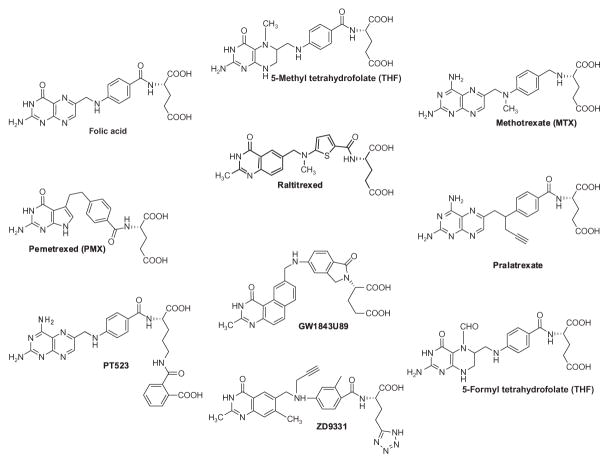
Folate is the generic term for water-soluble members of the B class of vitamins that are required for normal tissue growth and development in mammalian cells. The biological importance of reduced folates relates to their essential roles in one-carbon transfer reactions leading to thymidylate, purine nucleotides, serine, and methionine (Stokstad, 1990). Folates are also required for vitamin-B12-dependent synthesis of methionine, a precursor of S-adenosyl methionine, which is needed for methylation of DNA, histones, lipids, and neurotransmitters (Lu, 2000). Folic acid (Fig. 4.1) is the synthetic form of the metabolically important folates found in cells that differ in the level of oxidation of the pteridine ring, the nature of the one-carbon substituent at the N5 and N10 positions, and the extent of γ-glutamate conjugation (Stokstad, 1990). The major circulating folate is 5-methyl tetrahydrofolate (THF) (Fig. 4.1). Following cellular uptake, 5-methyl THF is converted to various THF polyglutamates required for folate-dependent metabolic reactions (Stokstad, 1990).
Figure 4.1.
Structures of folates and clinically relevant antifolates. RFC substrates including MTX, PMX, ratitrexed, pralatrexate, 5-methyl THF, and 5-formyl THF are all excellent PCFT substrates. However, the antifolates PT523 and GW1843U89 do not appear to be transported by PCFT. RFC has a low affinity but PCFT has a high affinity for folic acid. GW1843U89 is an excellent substrate for human RFC, but a poor substrate for murine RFC.
Mammalian cells, unlike bacteria, cannot synthesize folates de novo. Hence, folate requirements must be met entirely from dietary sources. Traditionally, folates are derived from foods such as liver and dark green leafy vegetables. In the United States and Canada, the fortification of cereals, grains, and bread with folic acid now represents an important source of dietary folate and has resulted in a rise in folate levels in tissues and blood (Jacques, Selhub, Bostom, Wilson, & Rosenberg, 1999).
Folates are hydrophilic molecules that are anions at physiological pH and thus cross biological membranes poorly by diffusion. Reflecting this, mammalian cells have evolved sophisticated uptake systems for facilitating cellular uptake of folate cofactors (Kugel Desmoulin, Hou, Gangjee, & Matherly, 2012; Matherly & Goldman, 2003; Zhao et al., 2011). Major facilitative folate transporters include: (i) the RFC, an anionic exchanger and the major route of delivery of folates to systemic tissues at physiological pH (Matherly & Goldman, 2003; Matherly et al., 2007); and (ii) PCFT, like RFC, a member of the superfamily of solute carriers, but with an acidic pH optimum and distinct transport specificities (Kugel Desmoulin et al., 2012; Zhao & Goldman, 2007). In certain epithelial tissues such as renal cells and macrophages, folate receptors (FRs) α and β are embedded in the plasma membrane by a glycosylphosphatidylinositol anchor and mediate uptake of folates into cells via receptor-mediated endocytosis at neutral to mildly acidic pHs (Elnakat & Ratnam, 2004; Kamen & Smith, 2004). Whereas RFC is ubiquitously expressed in human tissues (Whetstine, Flatley, & Matherly, 2002), PCFT is more narrowly expressed, although substantial levels of PCFT were detected in the duodenum and jejunum, kidney, and liver, as well as choroid plexus (Kugel Desmoulin et al., 2012). FRα is expressed in the proximal renal tubules, choroid plexus, uterus, and placenta, whereas FRβ is expressed in placenta and hematopoietic cells, and in activated macrophages (Elnakat & Ratnam, 2004).
Proliferating tumor cells have unique metabolic requirements characterized by enhanced nutrient uptake and metabolic pathways to support the biosynthesis of macromolecules needed for cell growth and division. These pathways include the folate-dependent de novo synthesis of purine nucleotides and thymidylate. Structural analogs of folate compounds (i.e., antifolates) have been developed and continue to be important drugs for treating a variety of cancers (Gonen & Assaraf, 2012a; Kugel Desmoulin et al., 2012; Visentin, Zhao, & Goldman, 2012a) and nonmalignant diseases such as psoriasis and rheumatoid arthritis (Chladek et al., 1998; Wessels, Huizinga, & Guchelaar, 2008). Clinically relevant antifolates include MTX, PMX, raltitrexed, and pralatrexate (Fig. 4.1). These classic antifolates use the same transporters as physiological folates to enter tumor cells, particularly RFC (Kugel Desmoulin et al., 2012; Matherly et al., 2007). Membrane transport of antifolates is an important determinant of clinical efficacy for cancer, and impaired transport is a common mechanism of drug resistance (Zhao & Goldman, 2003). In recent years, there has been emphasis on developing tumor-targeted folate-based therapies reflecting selective cellular uptake of folate-conjugated cytotoxins or cytotoxic antifolates by FRs or the PCFT by tumor cells (Deng, Wang, et al., 2008; Deng et al., 2009; Gibbs et al., 2005; Kugel Desmoulin et al., 2012, 2011a, 2010; Wang et al., 2012, 2010, 2011; Xia & Low, 2010; Yang, Vlashi, & Low, 2012). This has fostered a new paradigm, namely the rational development of tumor-targeted therapies based on tumor-specific expression and/or function of the major folate transporters.
In the following sections, we focus on biological properties of RFC and PCFT, including structure, function, and mechanisms of facilitative transport.
3. REDUCED FOLATE CARRIER (SLC19A1)
3.1. Properties of RFC
As noted above, RFC is expressed ubiquitously and is widely considered to be the major transport system for folates in mammalian cells and tissues (Matherly et al., 2007). Very high levels of RFC transcripts are detected in liver and placenta, with appreciable levels in other tissues, including kidney, lung, bone marrow, intestine, brain, and portions of the central nervous system (Whetstine et al., 2002). By immunohistochemistry in mouse tissues, RFC was detected at the basolateral membrane of the renal tubule epithelium, the apical brush border membrane of the small intestine and colon, hepatocyte membranes, the apical surface of the choroid plexus, and the apical membrane of the cells lining the spinal canal (Wang, Zhao, Russell, & Goldman, 2001). Reflecting its physiological importance, low levels of RFC could result in a number of pathophysiological states associated with folate deficiency, ranging from cardiovascular disease, fetal abnormalities, neurological disorders, to possibly cancer (Matherly, 2004; Matherly et al., 2007). RFC is important for development since inactivating both RFC alleles by targeted homologous recombination in mice is embryonic lethal (Zhao, Russell, et al., 2001).
The properties of RFC-mediated transport in both human and rodent models have been comprehensively studied in numerous laboratories (Matherly et al., 2007). Investigators have used commercially available radioactive MTX as a surrogate substrate in functional studies on RFC because MTX is not metabolized over short intervals and because of the ease and accuracy of influx determinations and of distinguishing between free and protein-bound drug within cells (Goldman, Lichtenstein, & Oliverio, 1968). With minor exceptions, transport properties are remarkably conserved among species, in large part reflecting significant conservation of primary sequence (see below). The murine RFC was cloned in 1994 (Dixon, Lanpher, Chiu, Kelley, & Cowan, 1994), followed shortly thereafter by the human RFC by a number of laboratories (Moscow et al., 1995; Prasad, Ramamoorthy, Leibach, & Ganapathy, 1995; Williams & Flintoff, 1995; Wong, Proefke, Bhushan, & Matherly, 1995), thus paving the way to further detailed studies of RFC structure and function. The human RFC gene is localized to chromosome 21q22.3 (Moscow et al., 1995) and is subjected to complex regulation involving multiple promoters and noncoding exons that likely ensures its ubiquitous tissue expression (Matherly et al., 2007).
For most reduced folates and many antifolate substrates including clinically relevant drugs such as MTX and pralatrexate, transport by RFC is saturable at low micromolar concentrations (Kt ~ 1–5 μM) (Matherly et al., 2007). The novel δ-hemiphthaloylorinithine antifolate PT523 (Fig. 4.1) is one of the best substrates for human RFC with a Kt value less than 1 μM (Rosowsky, Bader, Wright, Keyomarsi, & Matherly, 1994). Likewise, the benzoquinazoline antifolate GW1843U89 (Fig. 4.1) has a reported Kt of 0.33 μM and is an excellent substrate for human RFC (Vmax/Kt = 20.3), although it is a surprisingly poor substrate for the murine RFC (Vmax/Kt =0.25) (Duch et al., 1993). The affinity of RFC for folic acid is orders of magnitude less than for reduced folates (Goldman et al., 1968; Westerhof et al., 1995). While RFC transport is not stereospecific for 5-methyl THF (Fig. 4.1) (Sirotnak & Donsbach, 1974; White, Bailey, & Goldman, 1978), the (6R) isomer of 5-formyl THF (Fig. 4.1) has a far lower affinity than the natural (6S) stereoisomer (Sirotnak et al., 1979). Thus, the (6R) stereoisomer of 5-formyl THF in leucovorin [(6R,S)5-formyl THF] should have no impact on cellular uptake of the (6S) stereoisomer of 5-formyl THF.
RFC is an anion transporter, and its activity is competitively inhibited by inorganic anions such as chloride, bicarbonate, and phosphate, or by structurally diverse organic anions such as adenine nucleotides and thiamine phosphate (Goldman, 1971a). Furthermore, replacing anionic buffers with nonanionic HEPES–sucrose buffers results in a dramatic stimulation of concentrative uptake of MTX (Henderson & Zevely, 1983b). When cells are incubated in anion-free buffers (without glucose) and preloaded with radio-labeled MTX, the rate of MTX efflux is inhibited. Efflux can be induced upon addition of both inorganic and organic anions (e.g., chloride, sulfate, phosphate, thiamine pyrophosphate, AMP, ADP, and 5-formyl THF) (Henderson & Zevely, 1980, 1981).
Folates include a glutamate moiety for which both α- and γ-carboxyl groups are ionized at physiological pH. Replacing the glutamate with valine or 2-aminosuberate in the novel antifolate substrate ICI-198,583 preserved binding to RFC (Westerhof et al., 1995). Modifications of the glutamate γ-carboxyl group appear to be well tolerated; replacing either the glutamate with a δ-hemiphthaloylornithine in PT523 (Fig. 4.1) or the γ-carboxyl with a tetrazole in ZD9331 (Fig. 4.1) preserved substrate activity (Jansen, 1999; Rosowsky et al., 1994). A series of diaminofuro[2,3-d]pyrimidine antifolates with hydrogen or methyl substitutions at the α- or γ-carboxyl groups of the terminal glutamate were used to systematically assess the importance of these groups for substrate binding to RFC (Deng, Hou, et al., 2008). Analogs with only a single α-carboxyl but no γ-carboxyl were potent inhibitors of MTX transport by RFC, whereas analogs with only a γ-carboxyl group but no α-carboxyl group were poor inhibitors. Thus, the α-carboxyl group is important for binding of (anti)folate substrates to RFC.
Transport by RFC is temperature-dependent and sodium-independent (Goldman, 1971a; Goldman et al., 1968) and shows a distinctly neutral pH optimum. Transport decreases dramatically below pH 7 (Sierra, Brigle, Spinella, & Goldman, 1997). Transport by RFC is concentrative, although the energy source for this uphill process is not directly linked to ATP hydrolysis (Goldman, 1971a; Henderson & Zevely, 1983a). Concentrative uptake by RFC is driven by gradients of organic anions that bind and exit cells via RFC and block folate export via this same mechanism (Goldman, 1971a). Consistent with this model, influx of radiolabeled MTX by RFC is enhanced (trans-stimulated) in cells “preloaded” with high concentrations of 5-formyl or 5-methyl THF (Goldman, 1971b). Similarly, in studies with membrane vesicles loaded with sulfate or phosphate anions, uptake of MTX in the “trans” compartment was dramatically stimulated via a counter-transport mechanism (Yang, Sirotnak, & Dembo, 1984). Finally, thiamine pyrophosphate and ZMP (5-aminoimidazole-4-carboxamide ribonucleo-tide) are bona fide RFC substrates, which, when present intracellularly, can trans-stimulate folate influx by RFC while inhibiting (anti)folate export (Visentin, Zhao, & Goldman, 2012b; Zhao, Gao, et al., 2001). Collectively, these studies support a model whereby concentrative folate uptake by RFC is driven by a counter-transport mechanism involving an anionic cellular metabolite, perhaps an organic phosphate. However, neither the identity of the actual physiologic counter-anion(s) nor the detailed mechanism by which bidirectional anion fluxes are coupled is firmly established.
3.2. Structure and function of RFC
RFC belongs to the major facilitator superfamily (MFS) of transporters. MFS proteins transport an assortment of substrates including amino acids, neurotransmitters, sugars, vitamins, nucleosides, and organic phosphate in a uniport, symport, or antiport fashion (Chang, Lin, Keith Studley, Tran, & Saier, 2004). While no structure of RFC is yet available, three-dimensional crystal structures are available for RFC structural homologs including the bacterial lactose/proton symporter (LacY) (Abramson et al., 2003) and the inorganic phosphate/glycerol-3-phosphate antiporter (GlpT) (Huang, Lemieux, Song, Auer, & Wang, 2003) from which to draw mechanistic inferences and to generate predictive homology models (Hou, Ye, Haska, & Matherly, 2006) that can be experimentally tested. By analogy with these bacterial transporters, membrane transport of folates by RFC would be expected to involve a physical movement of the carrier within the plasma membrane accompanied by alternate accessibilities of the aqueous substrate-binding cavity between the intra- and extracellular compartments. For RFC, as noted above, transport is driven by extrusion of anions down a large (intra- to extracellular) concentration gradient.
While the functional properties of RFC in cultured murine leukemia cells were first documented over 40 years ago (Goldman et al., 1968), it was only following cloning of RFCs from multiple species (Dixon et al., 1994; Moscow et al., 1995; Prasad et al., 1995; Williams & Flintoff, 1995; Williams, Murray, Underhill, & Flintoff, 1994; Wong et al., 1995) that it became possible to explore structural determinants of RFC transport that impact its physiology and pharmacology. These include its membrane topology, N-glycosylation, functionally and structurally important domains and amino acids, and, more recently, tertiary and quaternary structures.
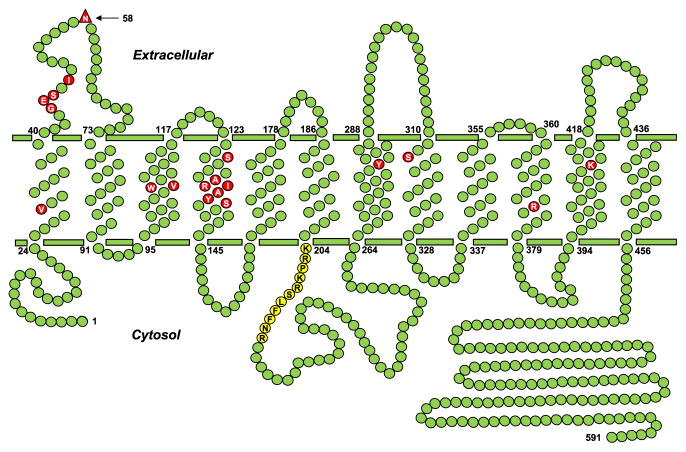
RFC is characterized by 12 transmembrane domains (TMDs) and cytoplasmically oriented N- and C-termini (Fig. 4.2) (Cao & Matherly, 2004; Ferguson & Flintoff, 1999; Liu & Matherly, 2002). Human RFC includes 591 amino acids and is N-glycosylated at Asn58 in the loop domain connecting TMD1 and TMD2 (Matherly, Czajkowski, & Angeles, 1991; Wong, Zhang, Proefke, & Matherly, 1998). RFCs from various species are conserved (64–66% between humans and rodents) with substantial conservation within the TMDs and the lowest homologies in the loop domain connecting TMD6 and TMD7, and in the N- and C-termini (Matherly & Hou, 2008). In structure–function studies of RFC, investigators have sought to identify domains and/or residues that contribute to binding and/or translocation of (anti)folate substrates, as well as TMD helix packing associations that facilitate folate substrate binding and translocation.
Figure 4.2.
Human RFC topology model. A topology model is shown for human RFC, with 12 TMDs, internal N- and C-termini, and a loop domain connecting TMD6 and 7. The structurally and functionally important amino acids, as described in the text, are shown as red circles. A conserved stretch of amino acids (Lys204–Arg214) in the TMD6/TMD7 loop domain, which is important for transport activity, is shown as yellow circles. N-glycosylation occurs at Asn58, which is labeled as a red triangle.
Deletions of the N- and C-termini exclusive of the TMDs from ectopically expressed human RFC did not impact surface targeting or transport activity in HEK-293 and HuTu-80 cells (Marchant, Subramanian, Parker, & Said, 2002). While the same findings were obtained with rodent RFCs for the N-terminus, the impact of deleting the C-terminal domain ranged from modest to a complete loss of expression (Sadlish, Williams, & Flintoff, 2002; Sharina, Zhao, Wang, Babani, & Goldman, 2002). Larger deletions of human RFC that included TMD segments abolished surface targeting (Marchant et al., 2002). The linker domain connecting TMD6 and TMD7 in RFC (Fig. 4.2) is poorly conserved with the exception of a Lys204–Arg214 segment. Deleting segments (49 or 60 amino acids; positions 215–263 and 204–263, respectively) of the TMD6/TMD7 linker from human RFC abolished transport activity (Liu, Witt, & Matherly, 2003). Interestingly, replacing the deleted segments with 73 or 84 amino acid segments of nonhomologous sequence from SLC19A2 restored transport, although there was an absolute requirement for the human RFC 204–214 peptide sequence. Finally, when human RFC was expressed as individual TMD1–6 and TMD7–12 half molecules, transport activity was restored (Witt, Stapels, & Matherly, 2004). These studies establish that neither the N- or C-termini nor the TMD6/TMD7 linker is critical for folate substrate binding and membrane translocation. The primary role of the TMD6-TMD7 loop domain is to provide appropriate spacing between the TMD1–6 and TMD7–12 segments for optimal carrier function.
Studies with RFC mutants have implicated conserved amino acids in TMD1, TMD2, TMD4, TMD8, TMD10, and TMD11 as important for transport (Fig. 4.2) (Matherly et al., 2007). Structurally or functionally important amino acids identified from mutant studies include (numbers based on human RFC sequence) Val29, Gly44, Glu45, Ser46, Ile48, Val106, Trp107, Ser127, Ala132, Arg133, Tyr281, Ser313, and Arg373 (Brigle, Spinella, Sierra, & Goldman, 1995; Drori, Jansen, Mauritz, Peters, & Assaraf, 2000; Jansen et al., 1998; Liu & Matherly, 2001; Roy, Tolner, Chiao, & Sirotnak, 1998; Sadlish et al., 2002; Sharina et al., 2002; Wong et al., 1999; Zhao, Assaraf, & Goldman, 1998; Zhao, Gao, Babani, & Goldman, 2000; Zhao, Gao, & Goldman, 1999). Mutation of Lys404 in TMD11 of murine RFC (Lys411 in human RFC) to leucine resulted in 11- and 4-fold increases in Ki values for 5-methyl THF and 5-formyl THF, respectively (Sharina, Zhao, Wang, Babani, & Goldman, 2001). Binding of the γ-carboxyl group of MTX was localized to Lys411 of human RFC based on N-hydroxysuccinimide [3H]MTX radioaffinity-labeling results (Deng, Hou, et al., 2008).
To identify domains that form the putative substrate-binding pocket/translocation pathway, Hou and coworkers (Hou, Stapels, Haska, & Matherly, 2005; Hou et al., 2006) used scanning cysteine accessibility methods (SCAM) with a functional “Cys-less” human RFC in which the 11 cysteine residues were replaced with serines (Cao & Matherly, 2003). In these studies, 282 cysteine mutants (in the Cys-less background) were individually expressed in RFC-null HeLa cells and treated with 2-sulfonatoethyl methanethiosulfonate (MTSES) so as to identify positions that are aqueous accessible (in spite of their TMD localization) and that may participate in folate substrate binding and/or translocation (Hou et al., 2005, 2006). From the patterns of transport inhibition by MTSES and protection from MTSES inhibition by leucovorin, residues localized in TMD4, TMD5, TMD7, TMD8, TMD10, and TMD11 were implicated in forming the putative substrate-binding pocket (Hou et al., 2005, 2006). Of the 282 RFC cysteine mutants tested, only 10 were completely inactive. Interestingly, these included a stretch of amino acids in TMD4 (Arg133, Ile134, Ala135, Tyr136, Ser138), Tyr281 in TMD7, Ser313 in TMD8, and Arg373 in TMD10. As described above, Arg133, Ser313, and Arg373 were previously implicated as structurally or functionally important (Liu & Matherly, 2001; Sadlish et al., 2002; Sharina et al., 2002; Zhao et al., 1999).
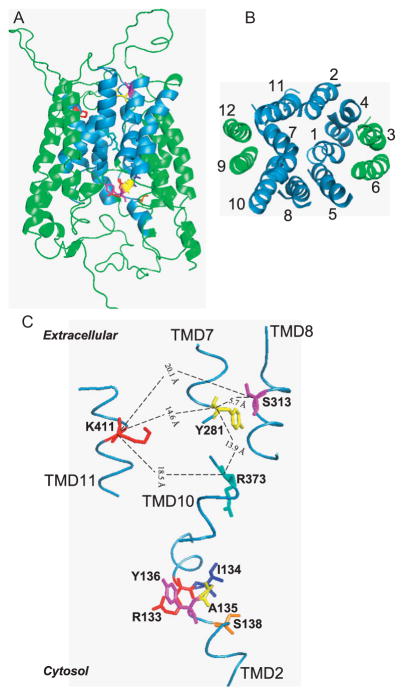
From the solved structures of the LacY and GlpT proteins, a three-dimensional homology model for human RFC was generated that included a membrane translocation pathway formed from TMD1, TMD2, TMD4, TMD5, TMD7, TMD8, TMD10, and TMD11, and mechanistically important roles for Tyr281, Ser313, Arg373, and Lys411 (Fig. 4.3) (Hou et al., 2006). Based on this homology model, site-directed mutagen-esis of Ser313 (localized to TMD8) and homo-bifunctional cross-linking between TMD8 and juxtaposed TMD5 were used to further examine the functional significance of Ser313 and TMD8 in RFC membrane transport (Hou, Wu, Ye, Cherian, & Matherly, 2010). The results showed that there are substantial differences between structurally diverse RFC (anti)folate substrates in their binding to RFCs with mutated Ser313, and in inducing conformational changes involving the proximal end of TMD8, determined by protein cross-linking. Thus, there is direct support for an essential role of TMD8 and Ser313 in binding and/or membrane translocation of (anti)folate substrates (Hou, Wu, et al., 2010).
Figure 4.3.
Three-dimensional (3D) homology models of human RFC. A 3D model for human RFC is presented, based on structure alignments between RFC and LacY/GlpT and experimental data. (A) A side view of the RFC for which the extended C-terminal segment is truncated at Lys479. TMD1, TMD2, TMD4, and TMD5 of the N-terminal region and TMD7, TMD8, TMD10, and TMD11 of the C-terminal region are involved in formation of the hydrophilic binding site for anionic folates (colored sky blue). TMD3, TMD6, TMD9, and TMD12 are buried in the lipid bilayer and do not directly participate in substrate binding (colored green). Panel (A) also depicts key amino acids (shown in assorted colors) that may contribute to the binding pocket for anionic folate substrates, as described in the text. (B) A cytosolic view of only the TMD segments of the human RFC molecule so that the order of helix packing can be seen easily. TMD coloring is the same as described in (A). (C) Enhanced view of the hypothetical substrate-binding site comprising the same key amino acids depicted in (A), including Lys411, Ser313, Tyr281, and Arg373, as described in the text. Other residues that may contribute to the substrate-binding pocket are also shown and include Arg133, Ile134, Ala135, Tyr136, and Ser138. The physical distances between the αcarboxyl groups of Lys411, Ser313, Tyr281, and Arg373 are given in angstroms. This figure was originally published in The Journal of Biological Chemistry, by Hou et al. (2006). © The American Society for Biochemistry and Molecular Biology.
Human RFC, like many MFS proteins, exists as a homo-oligomer (Hou & Matherly, 2009). RFC monomers appear to function independently (i.e., oligomerization is not a prerequisite for transport) (Hou, Cherian, Drews, Wu, & Matherly, 2010). Nonetheless, co-folding of RFC monomers to form oligomeric RFC seems to be functionally important for trafficking from the ER to the cell surface (Hou & Matherly, 2009). Co-expressing wild-type and inactive mutant S138C RFCs produces a dominant-negative phenotype due to decreased surface expression of both mutant and wild-type RFCs. Thus, it appears that RFC oligomerization may serve an important regulatory function. Clearly, further characterization of oligomeric RFC, including structural and regulatory determinants of oligomer formation, is essential, given its potential importance to understanding the broader physiologic and pharmacologic role of this transporter.
4. PROTON-COUPLED FOLATE TRANSPORTER (SLC46A1)
4.1. Properties of PCFT
In 2006, a new folate transporter was identified—PCFT (Qiu et al., 2006). While PCFT also belongs to the superfamily of solute carriers, it is functionally distinct from the RFC in that PCFT functions optimally at acidic rather than neutral pH, and it shows several differences in its specificities for particular(anti) folate substrates (Kugel Desmoulin et al., 2012; Zhao & Goldman, 2007).
The cloning of PCFT provided a molecular basis for the long-recognized but uncharacterized low pH folate transport activity in many normal and malignant cells, most notably in the small intestine (Assaraf, Babani, & Goldman, 1998; Henderson & Strauss, 1990; Kuhnel, Chiao, & Sirotnak, 2000; Sierra & Goldman, 1998; Zhao, Gao, Hanscom, & Goldman, 2004; Zhao & Goldman, 2007). The critical role of PCFT in intestinal absorption of dietary folates was confirmed by the demonstration of loss-of-function mutations in the human PCFT gene in patients with HFM (Atabay et al., 2010; Borzutzky et al., 2009; Diop-Bove, Jain, Scaglia, & Goldman, 2013; Lasry et al., 2008; Mahadeo et al., 2011, 2010; Meyer et al., 2010; Min et al., 2008; Qiu et al., 2006; Shin et al., 2011, 2010; Zhao et al., 2007). PCFT knockout mice were developed that largely recapitulate the HFM syndrome seen in humans (Salojin et al., 2011). These mice fail to absorb dietary folates, resulting in undetectable levels of serum folate and elevated plasma homocysteine.
Within the intestine, the highest PCFT levels are found at the apical brush border membrane of the proximal jejunum and duodenum, while levels decrease markedly in other segments of the intestine and colon (Inoue et al., 2008; Qiu et al., 2006, 2007; Urquhart et al., 2010). Other major sites of PCFT expression include the kidney, placenta, spleen, the sinusoidal membrane of the liver, the basolateral membrane of the choroid plexus, and the retinal pigment epithelium (Inoue et al., 2008; Kugel Desmoulin et al., 2012; Qiu et al., 2006; Umapathy et al., 2007; Wollack et al., 2008; Zhao, Min, et al., 2009). PCFT is abundantly expressed in human tumor cell lines (e.g., breast, lung, ovarian, and lung) and at very low levels in human leukemia cell lines (Gonen, Bram, & Assaraf, 2008; Kugel Desmoulin et al., 2012). While PCFT is likely to be active in the acidic microenvironment characterizing the upper GI and in solid tumors, it seems unlikely that PCFT represents an important mechanism of folate uptake in most normal tissues in which it is expressed. From results in HFM patients, PCFT is important for folate transport across the choroid plexus (Wollack et al., 2008; Zhao, Min, et al., 2009). Clearly, the physiologic role of PCFT in tissues not normally associated with a low pH microclimate needs to be clarified. It is conceivable that PCFT transports 5-methyl THF and related folates in normal tissues in areas of localized acidification or when expressed at high levels (Zhao, Matherly, & Goldman, 2009). However, at comparatively neutral pHs of most tissues, RFC is more efficient at delivering reduced folates than PCFT (Zhao et al., 2008).
Investigators have extensively characterized the transport properties of PCFT expressed in transfected cell lines and in Xenopus oocytes microinjected with PCFT cRNAs (Kugel Desmoulin et al., 2011b, 2010; Nakai et al., 2007; Qiu et al., 2006; Wang et al., 2011; Zhao & Goldman, 2007). A distinguishing characteristic of PCFT is its acidic optimum (pH 5–5.5) (Nakai et al., 2007; Qiu et al., 2006; Zhao & Goldman, 2007). As the pH increases from pH 5.5, transport decreases dramatically. Above pH 7, transport is almost undetectable, although the extent of residual transport varies for different substrates (Kugel Desmoulin et al., 2011a, 2010; Wang et al., 2010, 2011; Zhao & Goldman, 2007; Zhao et al., 2008). RFC substrates including MTX, PMX, raltitrexed, aminopterin, pralatrexate, 5-methyl THF, and 5-formyl THF are all PCFT substrates (Deng et al., 2009; Kugel Desmoulin et al., 2012; Menter et al., 2012; Zhao et al., 2008). However, the antifolates PT523 and GW1843U89 do not appear to be transported by PCFT (Deng et al., 2009; Zhao & Goldman, 2007). In contrast to RFC, PCFT has similar Kts for transport of reduced folates (5-methyl THF, 5-formyl THF) and folic acid (Zhao & Goldman, 2007). PCFT is stereospecific for (6S)5-formyl THF, and for L- over D-aminopterin (Menter et al., 2012; Zhao & Goldman, 2007). Whereas the 5-substituted pyrrolo[2,3-d]pyrimidine antifolate PMX is among the best substrates for transport by PCFT (Zhao & Goldman, 2007), a novel series of 6-subsitituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate analogs were recently reported with affinities and transport activities for PCFT comparable to those for PMX (Kugel Desmoulin et al., 2011a; Kugel Desmoulin, Wang, et al., 2012; Wang et al., 2010, 2011). For the lead compound of this series, PCFT transport by malignant mesothelioma xenografts in severe combined immunodeficient mice resulted in potent antitumor effects in early- and late-stage disease (Cherian et al., 2013). Notably, the binding affinities for both 5- and 6-substituted pyrrolo[2,3-d]pyrimidine analogs appear to be less affected by pH than for other PCFT substrates.
PCFT activity is not affected by removing extracellular Na+, K+, Ca2+, Mg2+, or Cl− (Qiu et al., 2006) or by changing membrane potential (Inoue et al., 2008), data consistent with an electroneutral mode of PCFT transport. However, there is a requirement for an inwardly directed proton gradient for PCFT-mediated folate transport based on the following observations. (i) Dissipation of the transmembrane proton gradient by a proton ionophore in Xenopus oocytes (Qiu et al., 2006) and a K+/H+-exchanging ionophore in HEK293 cells (Inoue et al., 2008) reduced transport by PCFT. (ii) A transvesicular pH gradient was reported to result in increased unidirectional folate transport and substantial transmembrane folate concentration gradients in rabbit jejunum, from the low pH to the high pH compartment, consistent with a proton-coupled process (Schron, Washington, & Blitzer, 1985). (iii) Treatment of HeLa cells with nitrate or bisulfite inhibited PCFT transport by abolishing the pH gradient (Zhao, Visentin, Suadicani, & Goldman,2013). Finally,(iv) cellular acidification accompanied folate transport into Xenopus oocytes, confirming proton coupling (Unal, Zhao, et al., 2009).
Since folates are bivalent anions, this means that more than two protons must be co-transported with each folate molecule to account for the net positive charge of the PCFT–folate–proton complex (Qiu et al., 2006, 2007). Thus, PCFT functions as a folate–proton symporter such that the downhill flow of protons is coupled to the uphill flow of folates into cells. Interestingly, PCFT can also function in the absence of a transmembrane pH gradient. Under these conditions, folate transport is driven by membrane potentials (Qiu et al., 2006; Umapathy et al., 2007), similar to the divalent metal-ion transporter DMT1 (Mackenzie, Ujwal, Chang, Romero, & Hediger, 2006). At low pH, PCFT exhibited channel-like activities (i.e., protons can flow independent of folates) (Mahadeo et al., 2010; Unal, Zhao, et al., 2009).
4.2. Structure and function of PCFT
Human PCFT includes 459 amino acids and has a predicted molecular mass of 49.8 kDa. PCFT is predicted to contain 12 TMDs comprised of hydrophobic stretches of amino acids with cytosolic-oriented N- and C-termini (Fig. 4.4). This structure has been experimentally confirmed by immunofluorescence staining of hemagglutinin (HA)-tagged PCFT constructs and by SCAM with MTSEA (2-aminoethyl methanethiosulfonate)-biotin (Unal, Zhao, Qiu, & Goldman, 2008; Zhao, Unal, Shin, & Goldman, 2010). PCFT has two N-glycosylation sites (Asn58 and Asn68) in the loop domain between TMD1 and TMD2 (Unal et al., 2008). Mutating either of the asparagine residues to glutamine had no effect on PCFT expression and function (Unal et al., 2008). However, mutating both Asn58 and Asn68 to glutamine decreased transport activity to 40% of that for wild-type PCFT. In contrast to RFC which is expressed at the basolateral membrane (see above), PCFT (with a C-terminal, yellow fluorescent protein tag) was localized to the apical membrane of Madin-Darby canine kidney and Caco-2 cells (Subramanian, Marchant, & Said, 2008). Truncating the C-terminus of human PCFT at position 449 did not impact apical membrane targeting or transporter activity (Subramanian et al., 2008).
Figure 4.4.
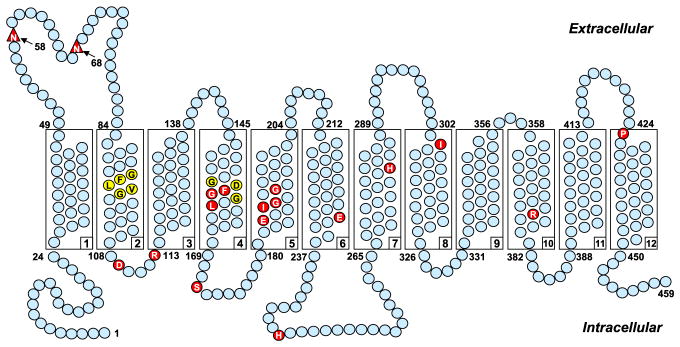
Schematic structure of human PCFT membrane topology. A topology model is shown for human PCFT, with 12 TMDs and internal N- and C-termini. Structurally or functionally important amino acids, as determined from published mutagenesis studies and in patients with hereditary folate malabsorption (HFM), are shown as red circles. GXXXG putative oligomerization motifs are shown as yellow circles (Phe157 and Gly158 in the G155XXXG159 motif are shown as red circles because they are also structurally and functionally important). N-glycosylation occurs at Asn58 and Asn68 (shown as red triangles).
A number of structurally and functionally important amino acids in human PCFT have been identified. This includes molecular characterization of human PCFT mutants that result in loss of function in HFM cases, and systematic mutagenesis of these HFM mutants. From considerations of species homologies and amino acid charge characteristics in relation to their TMD localization, other residues have been identified as important to PCFT transport. Thus, residues have been implicated as critical to proton coupling (i.e., Glu185 (TMD5); Unal, Zhao, & Goldman, 2009) or substrate binding (i.e., His281 (TMD7); Unal, Zhao, et al., 2009), or both (i.e., Arg376 (TMD10); Mahadeo et al., 2010) (Fig. 4.4).
His247, which is localized to the loop region separating TMD6 and TMD7, is predicted to lie in the cytoplasmic opening of the water-filled translocation pathway where it interacts with Ser172 to limit substrate access to the folate-binding pocket (thus determining substrate selectivity) (Unal, Zhao, et al., 2009). His281 faces the extracellular region in TMD7 (Fig. 4.4) and is believed to play an important role in PCFT protonation, which augments substrate binding to the carrier (Unal, Zhao, et al., 2009).
The conserved segment (DXXGRR) linking TMD2 and TMD3 (residues 109–114), including a β-turn, is of particular interest (Lasry et al., 2008; Shin et al., 2010; Subramanian et al., 2008; Zhao et al., 2007). Key residues in this stretch include Asp109 and Arg113 that are likely to play important roles in substrate binding and/or translocation since both conservative and nonconservative replacements completely abolish transport (Lasry, Berman, Glaser, Jansen, & Assaraf, 2009; Shin et al., 2010). According to a PCFT homology model based on the GlpT template, Arg113 protrudes into a hydrophobic cavity formed by TMD1, TMD3, TMD4, and TMD6 (Lasry et al., 2008). However, this has not been experimentally confirmed.
Other residues that may be functionally important in the human PCFT include Leu161 (TMD4), Glu232 (TMD6), and Ile304 (TMD8) (Fig. 4.4) (Zhao, Shin, Diop-Bove, Ovits, & Goldman, 2011). Loss of transport was associated with a reduced rate of carrier translocation (Glu232Gly mutant) or lower substrate affinities (Ile304Phe and Leu161Arg mutants). Mutating Pro425 to Arg in the loop junction flanking TMD12 (Fig. 4.4) eliminated binding of MTX and other substrates to PCFT, but preserved binding of PMX (Shin, Zhao, Yap, Fiser, & Goldman, 2012). Given its location, Pro425 is unlikely to directly participate in substrate binding. Rather, mutation at this position likely produces a conformational change that selectively inhibits binding of particular (anti)folate substrates. Finally, from mutant studies, Gly189 and Gly192 (TMD5) were implicated as functionally important (Zhao, Shin, Fiser, & Goldman, 2012).
In SCAM studies on human PCFT, membrane-impermeable methane-thiosulfonate reagents reacted with Cys replacements at positions 157, 158, and 161 in TMD4 and at position 188 in TMD5 (Shin, Zhao, Fiser, & Goldman, 2013; Zhao et al., 2012). Reactions could be protected by PMX, placing these residues within or near the substrate-binding site(s) in human PCFT.
Based on the results with several complementary techniques, the human PCFT forms homo-oligomers both in detergent solution and in situ (Hou et al., 2012). Oligomerization of PCFT was demonstrated by protein cross-linking of the ectopically expressed carrier with 1,1-methanediyl bismethanethiosulfonate (MTS-1-MTS), and by fluorescence resonance energy transfer between co-expressed YPet- and ECFP*-tagged PCFT monomers (Hou et al., 2012). Physical associations between HA- and His10-tagged PCFT monomers were established by co-expression in PCFT-null HeLa cells and co-binding to nickel affinity columns. Furthermore, the PCFT dimer was shown to be the dominant species by blue native gel electrophoresis (Hou et al., 2012). The functional significance of oligomerization was established by co-expression of wild-type and mutant Pro425Arg PCFT forms in PCFT-null HeLa cells. In these experiments, there was a “dominant-positive” functional phenotype, establishing positive cooperativity between PCFT monomers and a functional “rescue” of inactive mutant PCFT by wild-type protein (Hou et al., 2012). Based on these results, an “alternate access” model for PCFT transport, analogous to that suggested for LacY (Abramson et al., 2003) and adapted from that for monomeric PCFT (Unal et al., 2009), was proposed (Hou et al., 2012) that incorporates a role for PCFT oligomerization.
Duddempudi, Nakashe, Blanton, and Jansen (2013) suggested that PCFT may not oligomerize in plasma membranes prepared by polymerization with colloidal silica and polyacrylic acid from Xenopus oocytes or in Chinese hamster ovary cells. However, this conclusion was based on a limited number of methods. Furthermore, since different methods and metrics were employed in this report and the more extensive study of Hou et al. (2012), it is impossible to reconcile the conclusions of these reports.
The human PCFT primary sequence includes GXXXG motifs in TMD2 (amino acids 93–97) and TMD4 (amino acids 155–159) (Fig. 4.4) that are analogous to “dimerization motifs” in other amphipathic proteins (Duan, Wu, & You, 2011; Polgar et al., 2010). Mutating Gly93 and Gly97 to Ala did not inhibit transport activity or oligomer formation, based on thiol-reactive (MTS-1-MTS) protein cross-linking (Zhao et al., 2012). Unfortunately, parallel studies on the GXXXG motif in TMD4 were not performed. Interestingly, a TMD6 interface between PCFT monomers was suggested by the finding that MTS-1-MTS treatment generated cross-links between Cys229 (in TMD6) in individual PCFT monomers, but not when Cys229 was mutated to Ser (Zhao et al., 2012).
5. SUMMARY
This chapter focuses on the biology of the major facilitative membrane transport systems for folate cofactors, RFC and PCFT. Folates are essential vitamins, and folate deficiency has been implicated in a variety of heath disorders ranging from cardiovascular disease to neurodegenerative disease and cancer (Lucock, 2000; Matherly, 2004). Further, folate-based therapeutics (e.g., antifolates) continue to occupy an important niche for treating cancer as well as other pathological conditions (Gonen & Assaraf, 2012a; Kugel Desmoulin et al., 2012; Salojin et al., 2011; Visentin et al., 2012a). These include recent FDA-approved drugs such as PMX and pralatrexate, with many more folate-based therapies in the pipeline. Losses of RFC or PCFT transport can have potential physiological and developmental consequences, particularly in the context of folate deficiency (Matherly, 2004; Matherly et al., 2007; Zhao et al., 2009), or may result in drug resistance with antifolates used for cancer therapy (Gonen & Assaraf, 2012b; Matherly et al., 2007; Zhao & Goldman, 2003).
RFC was functionally characterized beginning in the late 1960s as a neutral pH membrane transport mechanism for clinically relevant antifolates such as MTX that when lost resulted in MTX resistance (Goldman et al., 1968; Sirotnak, Kurita, & Hutchison, 1968). RFC was cloned and its molecular features were exhaustively documented beginning in the mid-1990s (Matherly & Hou, 2008; Matherly et al., 2007). It was soon recognized that RFC was the major folate transport system in mammalian cells, consistent with its ubiquitous tissue expression (Whetstine et al., 2002) and its absolute requirement for fetal development (Zhao, Russell, et al., 2001). By contrast, although an acidic pH transporter had long been recognized, the molecular characteristics of PCFT were not demonstrated until 2005 when it was originally classified as a heme transporter (Shayeghi et al., 2005). By 2006, PCFT was redefined as the molecular entity responsible for the acidic folate transport activity involved in intestinal uptake of dietary folates (Qiu et al., 2006). Studies soon followed that established that a low pH transport activity detected in many human solid tumor cells was in fact PCFT, and that PCFT was abundantly expressed in a wide range of human tumors and was highly active at pH conditions approximating those found in the tumor microen-vironment (Kugel Desmoulin et al., 2011a). This soon led to the development of novel tumor-targeted therapies typified by 6-substituted pyrrolo [2,3-d]pyrimidine antifolates with selective membrane transport by PCFT over RFC (Kugel Desmoulin et al., 2012).
An important prerequisite for effective clinical applications of RFC and PCFT transport associated with folate deficiency or in targeted therapy of cancer is understanding of the structure and function of these critical transport systems. Indeed, since their cloning, structure–function research has led to remarkably detailed molecular information, including the membrane topology, N-glycosylation, energetics, and roles of functionally and structurally important domains and amino acids for both RFC and PCFT. These insights have shed important new light on the causal basis for chemotherapy resistance to MTX associated with the loss of RFC function, and for the rare autosomal recessive condition, HFM, involving mutations in the PCFT gene and protein. Based on structural homologies to the bacterial MFS transporters GlpT and LacY, homology models for human RFC and human PCFT have been developed. The models provide new mechanistic insights and experimentally testable hypotheses that could further facilitate efforts to design a new generation of targeted therapeutics with selective membrane transport by PCFT over RFC. Unfortunately, detailed X-ray diffraction studies on membrane proteins such as RFC and PCFT remain a formidable challenge.
Structure and function considerations for human RFC and human PCFT must now include higher-order structures such as homo-oligomers that may impact transport mechanisms and regulate transporter expression and function. Indeed, evidence suggests that formation of homo-oligomers between monomeric RFC and PCFT proteins may be essential for intracellular trafficking and/or for carrier function. Formation of PCFT or RFC oligomers, including both wild-type and mutant forms, may also be important to clinical phenotypes involving mutant transporters in patients with HFM or in cancer patients being treated with cytotoxic antifolates. Clearly, better understanding of the broader significance and structural determinants of these higher-order oligomeric structures will be an important area for future study as these may lead to approaches for modulating the formation of oligomers for therapeutic impact.
Acknowledgments
This study was supported by Grant R01 CA53535 from the National Cancer Institute, National Institutes of Health.
References
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301(5633):610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- Assaraf YG, Babani S, Goldman ID. Increased activity of a novel low pH folate transporter associated with lipophilic antifolate resistance in Chinese hamster ovary cells. The Journal of Biological Chemistry. 1998;273(14):8106–8111. doi: 10.1074/jbc.273.14.8106. [DOI] [PubMed] [Google Scholar]
- Atabay B, Turker M, Ozer EA, Mahadeo K, Diop-Bove N, Goldman ID. Mutation of the proton-coupled folate transporter gene (PCFT-SLC46A1) in Turkish siblings with hereditary folate malabsorption. Pediatric Hematology and Oncology. 2010;27(8):614–619. doi: 10.3109/08880018.2010.481705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borzutzky A, Crompton B, Bergmann AK, Giliani S, Baxi S, Martin M, et al. Reversible severe combined immunodeficiency phenotype secondary to a mutation of the proton-coupled folate transporter. Clinical Immunology. 2009;133(3):287–294. doi: 10.1016/j.clim.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigle KE, Spinella MJ, Sierra EE, Goldman ID. Characterization of a mutation in the reduced folate carrier in a transport defective L1210 murine leukemia cell line. The Journal of Biological Chemistry. 1995;270(39):22974–22979. doi: 10.1074/jbc.270.39.22974. [DOI] [PubMed] [Google Scholar]
- Cao W, Matherly LH. Characterization of a cysteine-less human reduced folate carrier: Localization of a substrate-binding domain by cysteine-scanning mutagenesis and cysteine accessibility methods. The Biochemical Journal. 2003;374(Pt 1):27–36. doi: 10.1042/BJ20030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Matherly LH. Analysis of the membrane topology for transmembrane domains 7–12 of the human reduced folate carrier by scanning cysteine accessibility methods. The Biochemical Journal. 2004;378(Pt 1):201–206. doi: 10.1042/BJ20031288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang AB, Lin R, Keith Studley W, Tran CV, Saier MH., Jr Phylogeny as a guide to structure and function of membrane transport proteins. Molecular Membrane Biology. 2004;21(3):171–181. doi: 10.1080/09687680410001720830. [DOI] [PubMed] [Google Scholar]
- Cherian C, Kugel Desmoulin S, Wang L, Polin L, White K, Kushner J, et al. Therapeutic targeting malignant mesothelioma with a novel 6-substituted pyrrolo[2,3-d] pyrimidine thienoyl antifolate via its selective uptake by the proton-coupled folate transporter. Cancer Chemotherapy and Pharmacology. 2013;71(4):999–1011. doi: 10.1007/s00280-013-2094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chladek J, Martinkova J, Simkova M, Vaneckova J, Koudelkova V, Nozickova M. Pharmacokinetics of low doses of methotrexate in patients with psoriasis over the early period of treatment. European Journal of Clinical Pharmacology. 1998;53(6):437–444. doi: 10.1007/s002280050404. [DOI] [PubMed] [Google Scholar]
- Deng Y, Hou Z, Wang L, Cherian C, Wu J, Gangjee A, et al. Role of lysine 411 in substrate carboxyl group binding to the human reduced folate carrier, as determined by site-directed mutagenesis and affinity inhibition. Molecular Pharmacology. 2008;73(4):1274–1281. doi: 10.1124/mol.107.043190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Wang Y, Cherian C, Hou Z, Buck SA, Matherly LH, et al. Synthesis and discovery of high affinity folate receptor-specific glycinamide ribonucleotide formyltransferase inhibitors with antitumor activity. Journal of Medicinal Chemistry. 2008;51(16):5052–5063. doi: 10.1021/jm8003366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Zhou X, Kugel Desmoulin S, Wu J, Cherian C, Hou Z, et al. Synthesis and biological activity of a novel series of 6-substituted thieno[2,3-d]pyrimidine antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors over the reduced folate carrier and proton-coupled folate transporter for cellular entry. Journal of Medicinal Chemistry. 2009;52(9):2940–2951. doi: 10.1021/jm8011323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diop-Bove N, Jain M, Scaglia F, Goldman ID. A novel deletion mutation in the proton-coupled folate transporter (PCFT; SLC46A1) in a Nicaraguan child with hereditary folate malabsorption. Gene. 2013;527(2):673–674. doi: 10.1016/j.gene.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon KH, Lanpher BC, Chiu J, Kelley K, Cowan KH. A novel cDNA restores reduced folate carrier activity and methotrexate sensitivity to transport deficient cells. The Journal of Biological Chemistry. 1994;269(1):17–20. [PubMed] [Google Scholar]
- Drori S, Jansen G, Mauritz R, Peters GJ, Assaraf YG. Clustering of mutations in the first transmembrane domain of the human reduced folate carrier in GW1843U89-resistant leukemia cells with impaired antifolate transport and augmented folate uptake. The Journal of Biological Chemistry. 2000;275(40):30855–30863. doi: 10.1074/jbc.M003988200. [DOI] [PubMed] [Google Scholar]
- Duan P, Wu J, You G. Mutational analysis of the role of GXXXG motif in the function of human organic anion transporter 1 (hOAT1) International Journal of Biochemistry and Molecular Biology. 2011;2(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Duch DS, Banks S, Dev IK, Dickerson SH, Ferone R, Heath LS, et al. Biochemical and cellular pharmacology of 1843U89, a novel benzoquinazoline inhibitor of thymidylate synthase. Cancer Research. 1993;53(4):810–818. [PubMed] [Google Scholar]
- Duddempudi PK, Nakashe P, Blanton MP, Jansen M. The monomeric state of the proton-coupled folate transporter represents the functional unit in the plasma membrane. The FEBS Journal. 2013;280(12):2900–2915. doi: 10.1111/febs.12293. [DOI] [PubMed] [Google Scholar]
- Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Advanced Drug Delivery Reviews. 2004;56(8):1067–1084. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Ferguson PL, Flintoff WF. Topological and functional analysis of the human reduced folate carrier by hemagglutinin epitope insertion. The Journal of Biological Chemistry. 1999;274(23):16269–16278. doi: 10.1074/jbc.274.23.16269. [DOI] [PubMed] [Google Scholar]
- Gibbs DD, Theti DS, Wood N, Green M, Raynaud F, Valenti M, et al. BGC 945, a novel tumor-selective thymidylate synthase inhibitor targeted to alpha-folate receptor-overexpressing tumors. Cancer Research. 2005;65(24):11721–11728. doi: 10.1158/0008-5472.CAN-05-2034. [DOI] [PubMed] [Google Scholar]
- Goldman ID. The characteristics of the membrane transport of amethopterin and the naturally occurring folates. Annals of the New York Academy of Sciences. 1971a;186:400–422. doi: 10.1111/j.1749-6632.1971.tb46996.x. [DOI] [PubMed] [Google Scholar]
- Goldman ID. A model system for the study of heteroexchange diffusion: Methotrexate-folate interactions in L1210 leukemia and Ehrlich ascites tumor cells. Biochimica et Biophysica Acta. 1971b;233(3):624–634. doi: 10.1016/0005-2736(71)90162-3. [DOI] [PubMed] [Google Scholar]
- Goldman ID, Lichtenstein NS, Oliverio VT. Carrier-mediated transport of the folic acid analogue, methotrexate, in the L1210 leukemia cell. The Journal of Biological Chemistry. 1968;243(19):5007–5017. [PubMed] [Google Scholar]
- Gonen N, Assaraf YG. Antifolates in cancer therapy: Structure, activity and mechanisms of drug resistance. Drug Resistance Updates. 2012a;15(4):183–210. doi: 10.1016/j.drup.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Gonen N, Assaraf YG. Antifolates in cancer therapy: Structure, activity and mechanisms of drug resistance. Drug Resistance Updates. 2012b;15(4):183–210. doi: 10.1016/j.drup.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Gonen N, Bram EE, Assaraf YG. PCFT/SLC46A1 promoter methylation and restoration of gene expression in human leukemia cells. Biochemical and Biophysical Research Communications. 2008;376(4):787–792. doi: 10.1016/j.bbrc.2008.09.074. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Strauss BP. Characteristics of a novel transport system for folate compounds in wild-type and methotrexate-resistant L1210 cells. Cancer Research. 1990;50(6):1709–1714. [PubMed] [Google Scholar]
- Henderson GB, Zevely EM. Transport of methotrexate in L1210 cells: Effect of ions on the rate and extent of uptake. Archives of Biochemistry and Biophysics. 1980;200(1):149–155. doi: 10.1016/0003-9861(80)90341-0. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Zevely EM. Anion exchange mechanism for transport of methotrexate in L1210 cells. Biochemical and Biophysical Research Communications. 1981;99(1):163–169. doi: 10.1016/0006-291x(81)91727-7. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Zevely EM. Structural requirements for anion substrates of the methotrexate transport system in L1210 cells. Archives of Biochemistry and Biophysics. 1983a;221(2):438–446. doi: 10.1016/0003-9861(83)90162-5. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Zevely EM. Use of non-physiological buffer systems in the analysis of methotrexate transport in L1210 cells. Biochemistry International. 1983b;6(4):507–515. [PubMed] [Google Scholar]
- Hou Z, Cherian C, Drews J, Wu J, Matherly LH. Identification of the minimal functional unit of the homo-oligomeric human reduced folate carrier. The Journal of Biological Chemistry. 2010;285(7):4732–4740. doi: 10.1074/jbc.M109.086033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Kugel Desmoulin S, Etnyre E, Olive M, Hsiung B, Cherian C, et al. Identification and functional impact of homo-oligomers of the human proton-coupled folate transporter. The Journal of Biological Chemistry. 2012;287(7):4982–4995. doi: 10.1074/jbc.M111.306860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Matherly LH. Oligomeric structure of the human reduced folate carrier: Identification of homo-oligomers and dominant-negative effects on carrier expression and function. The Journal of Biological Chemistry. 2009;284(5):3285–3293. doi: 10.1074/jbc.M807206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Stapels SE, Haska CL, Matherly LH. Localization of a substrate binding domain of the human reduced folate carrier to transmembrane domain 11 by radioaffinity labeling and cysteine-substituted accessibility methods. The Journal of Biological Chemistry. 2005;280(43):36206–36213. doi: 10.1074/jbc.M507295200. [DOI] [PubMed] [Google Scholar]
- Hou Z, Wu J, Ye J, Cherian C, Matherly LH. Substrate-specific binding and conformational changes involving Ser313 and transmembrane domain 8 of the human reduced folate carrier, as determined by site-directed mutagenesis and protein cross-linking. The Biochemical Journal. 2010;430(2):265–274. doi: 10.1042/BJ20100181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Ye J, Haska CL, Matherly LH. Transmembrane domains 4, 5, 7, 8, and 10 of the human reduced folate carrier are important structural or functional components of the transmembrane channel for folate substrates. The Journal of Biological Chemistry. 2006;281(44):33588–33596. doi: 10.1074/jbc.M607049200. [DOI] [PubMed] [Google Scholar]
- Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301(5633):616–620. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- Inoue K, Nakai Y, Ueda S, Kamigaso S, Ohta KY, Hatakeyama M, et al. Functional characterization of PCFT/HCP1 as the molecular entity of the carrier-mediated intestinal folate transport system in the rat model. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2008;294(3):G660–G668. doi: 10.1152/ajpgi.00309.2007. [DOI] [PubMed] [Google Scholar]
- Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. The New England Journal of Medicine. 1999;340(19):1449–1454. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- Jansen G. Receptor-and carrier-mediated transport systems for folates and antifolates. In: Jackman AL, editor. Antifolate drugs in cancer therapy. Totowa, NJ: Humana Press Inc; 1999. pp. 293–321. [Google Scholar]
- Jansen G, Mauritz R, Drori S, Sprecher H, Kathmann I, Bunni M, et al. A structurally altered human reduced folate carrier with increased folic acid transport mediates a novel mechanism of antifolate resistance. The Journal of Biological Chemistry. 1998;273(46):30189–30198. doi: 10.1074/jbc.273.46.30189. [DOI] [PubMed] [Google Scholar]
- Kamen BA, Smith AK. A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Advanced Drug Delivery Reviews. 2004;56(8):1085–1097. doi: 10.1016/j.addr.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Kugel Desmoulin S, Hou Z, Gangjee A, Matherly LH. The human proton-coupled folate transporter: Biology and therapeutic applications to cancer. Cancer Biology & Therapy. 2012;13(14):1355–1373. doi: 10.4161/cbt.22020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugel Desmoulin S, Wang L, Hales E, Polin L, White K, Kushner J, et al. Therapeutic targeting of a novel 6-substituted pyrrolo [2,3-d]pyrimidine thienoyl antifolate to human solid tumors based on selective uptake by the proton-coupled folate transporter. Molecular Pharmacology. 2011a;80(6):1096–1107. doi: 10.1124/mol.111.073833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugel Desmoulin S, Wang L, Hales E, Polin L, White K, Kushner J, et al. Therapeutic targeting of a novel 6-substituted pyrrolo [2,3-d]pyrimidine thienoyl antifolate to human solid tumors based on selective uptake by the proton-coupled folate transporter. Molecular Pharmacology. 2011b;80(6):1096–1107. doi: 10.1124/mol.111.073833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugel Desmoulin S, Wang L, Polin L, White K, Kushner J, Stout M, et al. Functional loss of the reduced folate carrier enhances the antitumor activities of novel antifolates with selective uptake by the proton-coupled folate transporter. Molecular Pharmacology. 2012;82(4):591–600. doi: 10.1124/mol.112.079004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugel Desmoulin S, Wang Y, Wu J, Stout M, Hou Z, Fulterer A, et al. Targeting the proton-coupled folate transporter for selective delivery of 6-substituted pyrrolo[2,3-d]pyrimidine antifolate inhibitors of de novo purine biosynthesis in the chemotherapy of solid tumors. Molecular Pharmacology. 2010;78(4):577–587. doi: 10.1124/mol.110.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnel JM, Chiao JH, Sirotnak FM. Contrasting effects of oncogene expression on two carrier-mediated systems internalizing folate compounds in Fisher rat 3T3 cells. Journal of Cellular Physiology. 2000;184(3):364–372. doi: 10.1002/1097-4652(200009)184:3<364::AID-JCP11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Lasry I, Berman B, Glaser F, Jansen G, Assaraf YG. Hereditary folate mal-absorption: A positively charged amino acid at position 113 of the proton-coupled folate transporter (PCFT/SLC46A1) is required for folic acid binding. Biochemical and Biophysical Research Communications. 2009;386(3):426–431. doi: 10.1016/j.bbrc.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Lasry I, Berman B, Straussberg R, Sofer Y, Bessler H, Sharkia M, et al. A novel loss-of-function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function. Blood. 2008;112(5):2055–2061. doi: 10.1182/blood-2008-04-150276. [DOI] [PubMed] [Google Scholar]
- Liu XY, Matherly LH. Functional interactions between arginine-133 and aspartate-88 in the human reduced folate carrier: Evidence for a charge-pair association. The Biochemical Journal. 2001;358(Pt 2):511–516. doi: 10.1042/0264-6021:3580511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XY, Matherly LH. Analysis of membrane topology of the human reduced folate carrier protein by hemagglutinin epitope insertion and scanning glycosylation insertion mutagenesis. Biochimica et Biophysica Acta. 2002;1564(2):333–342. doi: 10.1016/s0005-2736(02)00467-4. [DOI] [PubMed] [Google Scholar]
- Liu XY, Witt TL, Matherly LH. Restoration of high-level transport activity by human reduced folate carrier/ThTr1 thiamine transporter chimaeras: Role of the transmembrane domain 6/7 linker region in reduced folate carrier function. The Biochemical Journal. 2003;369(Pt 1):31–37. doi: 10.1042/BJ20020419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC. S-Adenosylmethionine. The International Journal of Biochemistry & Cell Biology. 2000;32(4):391–395. doi: 10.1016/s1357-2725(99)00139-9. [DOI] [PubMed] [Google Scholar]
- Lucock M. Folic acid: Nutritional biochemistry, molecular biology, and role in disease processes. Molecular Genetics and Metabolism. 2000;71(1–2):121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- Mackenzie B, Ujwal ML, Chang MH, Romero MF, Hediger MA. Divalent metal-ion transporter DMT1 mediates both H+-coupled Fe2+ transport and uncoupled fluxes. Pflügers Archiv. 2006;451(4):544–558. doi: 10.1007/s00424-005-1494-3. [DOI] [PubMed] [Google Scholar]
- Mahadeo KM, Diop-Bove N, Ramirez SI, Cadilla CL, Rivera E, Martin M, et al. Prevalence of a loss-of-function mutation in the proton-coupled folate transporter gene (PCFT-SLC46A1) causing hereditary folate malabsorption in Puerto Rico. The Journal of Pediatrics. 2011;159(4):623–627. doi: 10.1016/j.jpeds.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadeo K, Diop-Bove N, Shin D, Unal ES, Teo J, Zhao R, et al. Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption. American Journal of Physiology. Cell Physiology. 2010;299(5):C1153–C1161. doi: 10.1152/ajpcell.00113.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant JS, Subramanian VS, Parker I, Said HM. Intracellular trafficking and membrane targeting mechanisms of the human reduced folate carrier in mammalian epithelial cells. The Journal of Biological Chemistry. 2002;277(36):33325–33333. doi: 10.1074/jbc.M205955200. [DOI] [PubMed] [Google Scholar]
- Matherly LH. Human reduced folate carrier gene and transcript variants: Functional, physiologic, and pharmacologic consequences. Current Pharmacogenetics. 2004;2:287–298. [Google Scholar]
- Matherly LH, Czajkowski CA, Angeles SM. Identification of a highly gly-cosylated methotrexate membrane carrier in K562 human erythroleukemia cells up-regulated for tetrahydrofolate cofactor and methotrexate transport. Cancer Research. 1991;51(13):3420–3426. [PubMed] [Google Scholar]
- Matherly LH, Goldman DI. Membrane transport of folates. Vitamins and Hormones. 2003;66:403–456. doi: 10.1016/s0083-6729(03)01012-4. [DOI] [PubMed] [Google Scholar]
- Matherly LH, Hou Z. Structure and function of the reduced folate carrier a paradigm of a major facilitator superfamily mammalian nutrient transporter. Vitamins and Hormones. 2008;79:145–184. doi: 10.1016/S0083-6729(08)00405-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matherly LH, Hou Z, Deng Y. Human reduced folate carrier: Translation of basic biology to cancer etiology and therapy. Cancer Metastasis Reviews. 2007;26(1):111–128. doi: 10.1007/s10555-007-9046-2. [DOI] [PubMed] [Google Scholar]
- Menter A, Thrash B, Cherian C, Matherly LH, Wang L, Gangjee A, et al. Intestinal transport of aminopterin enantiomers in dogs and humans with psoriasis is stereoselective: Evidence for a mechanism involving the proton-coupled folate transporter. The Journal of Pharmacology and Experimental Therapeutics. 2012;342(3):696–708. doi: 10.1124/jpet.112.195479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E, Kurian MA, Pasha S, Trembath RC, Cole T, Maher ER. A novel PCFT gene mutation (p.Cys66LeufsX99) causing hereditary folate malabsorption. Molecular Genetics and Metabolism. 2010;99(3):325–328. doi: 10.1016/j.ymgme.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SH, Oh SY, Karp GI, Poncz M, Zhao R, Goldman ID. The clinical course and genetic defect in the PCFT gene in a 27-year-old woman with hereditary folate malabsorption. The Journal of Pediatrics. 2008;153(3):435–437. doi: 10.1016/j.jpeds.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscow JA, Gong M, He R, Sgagias MK, Dixon KH, Anzick SL, et al. Isolation of a gene encoding a human reduced folate carrier (RFC1) and analysis of its expression in transport-deficient, methotrexate-resistant human breast cancer cells. Cancer Research. 1995;55(17):3790–3794. [PubMed] [Google Scholar]
- Nakai Y, Inoue K, Abe N, Hatakeyama M, Ohta KY, Otagiri M, et al. Functional characterization of human proton-coupled folate transporter/heme carrier protein 1 heterologously expressed in mammalian cells as a folate transporter. The Journal of Pharmacology and Experimental Therapeutics. 2007;322(2):469–476. doi: 10.1124/jpet.107.122606. [DOI] [PubMed] [Google Scholar]
- Polgar O, Ierano C, Tamaki A, Stanley B, Ward Y, Xia D, et al. Mutational analysis of threonine 402 adjacent to the GXXXG dimerization motif in transmembrane segment 1 of ABCG2. Biochemistry (Mosc) 2010;49(10):2235–2245. doi: 10.1021/bi902085q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad PD, Ramamoorthy S, Leibach FH, Ganapathy V. Molecular cloning of the human placental folate transporter. Biochemical and Biophysical Research Communications. 1995;206(2):681–687. doi: 10.1006/bbrc.1995.1096. [DOI] [PubMed] [Google Scholar]
- Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127(5):917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- Qiu A, Min SH, Jansen M, Malhotra U, Tsai E, Cabelof DC, et al. Rodent intestinal folate transporters (SLC46A1): Secondary structure, functional properties, and response to dietary folate restriction. American Journal of Physiology. Cell Physiology. 2007;293(5):C1669–C1678. doi: 10.1152/ajpcell.00202.2007. [DOI] [PubMed] [Google Scholar]
- Rizwan AN, Burckhardt G. Organic anion transporters of the SLC22 family: Biopharmaceutical, physiological, and pathological roles. Pharmaceutical Research. 2007;24(3):450–470. doi: 10.1007/s11095-006-9181-4. [DOI] [PubMed] [Google Scholar]
- Rosowsky A, Bader H, Wright JE, Keyomarsi K, Matherly LH. Synthesis and biological activity of N omega-hemiphthaloyl-alpha, omega-diaminoalkanoic acid analogues of aminopterin and 3′,5-dichloroaminopterin. Journal of Medicinal Chemistry. 1994;37(14):2167–2174. doi: 10.1021/jm00040a008. [DOI] [PubMed] [Google Scholar]
- Roy K, Tolner B, Chiao JH, Sirotnak FM. A single amino acid difference within the folate transporter encoded by the murine RFC-1 gene selectively alters its interaction with folate analogues. Implications for intrinsic antifolate resistance and directional orientation of the transporter within the plasma membrane of tumor cells. The Journal of Biological Chemistry. 1998;273(5):2526–2531. doi: 10.1074/jbc.273.5.2526. [DOI] [PubMed] [Google Scholar]
- Sadlish H, Williams FM, Flintoff WF. Cytoplasmic domains of the reduced folate carrier are essential for trafficking, but not function. The Biochemical Journal. 2002;364(Pt 3):777–786. doi: 10.1042/BJ20011361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salojin KV, Cabrera RM, Sun W, Chang WC, Lin C, Duncan L, et al. A mouse model of hereditary folate malabsorption: Deletion of the PCFT gene leads to systemic folate deficiency. Blood. 2011;117(18):4895–4904. doi: 10.1182/blood-2010-04-279653. [DOI] [PubMed] [Google Scholar]
- Schron CM, Washington C, Jr, Blitzer BL. The transmembrane pH gradient drives uphill folate transport in rabbit jejunum. Direct evidence for folate/hydroxyl exchange in brush border membrane vesicles. The Journal of Clinical Investigation. 1985;76(5):2030–2033. doi: 10.1172/JCI112205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharina IG, Zhao R, Wang Y, Babani S, Goldman ID. Mutational analysis of the functional role of conserved arginine and lysine residues in transmembrane domains of the murine reduced folate carrier. Molecular Pharmacology. 2001;59(5):1022–1028. doi: 10.1124/mol.59.5.1022. [DOI] [PubMed] [Google Scholar]
- Sharina IG, Zhao R, Wang Y, Babani S, Goldman ID. Role of the C-terminus and the long cytoplasmic loop in reduced folate carrier expression and function. Biochemical Pharmacology. 2002;63(9):1717–1724. doi: 10.1016/s0006-2952(02)00955-3. [DOI] [PubMed] [Google Scholar]
- Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, et al. Identification of an intestinal heme transporter. Cell. 2005;122(5):789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Shin DS, Mahadeo K, Min SH, Diop-Bove N, Clayton P, Zhao R, et al. Identification of novel mutations in the proton-coupled folate transporter (PCFT-SLC46A1) associated with hereditary folate malabsorption. Molecular Genetics and Metabolism. 2011;103(1):33–37. doi: 10.1016/j.ymgme.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DS, Min SH, Russell L, Zhao R, Fiser A, Goldman ID. Functional roles of aspartate residues of the proton-coupled folate transporter (PCFT-SLC46A1); a D156Y mutation causing hereditary folate malabsorption. Blood. 2010;116(24):5162–5169. doi: 10.1182/blood-2010-06-291237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DS, Zhao R, Fiser A, Goldman ID. Role of the fourth transmembrane domain in proton-coupled folate transporter function as assessed by the substituted cysteine accessibility method. American Journal of Physiology. Cell Physiology. 2013;304(12):C1159–C1167. doi: 10.1152/ajpcell.00353.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin DS, Zhao R, Yap EH, Fiser A, Goldman ID. A P425R mutation of the proton-coupled folate transporter causing hereditary folate malabsorption produces a highly selective alteration in folate binding. American Journal of Physiology. Cell Physiology. 2012;302(9):C1405–C1412. doi: 10.1152/ajpcell.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra EE, Brigle KE, Spinella MJ, Goldman ID. pH dependence of methotrexate transport by the reduced folate carrier and the folate receptor in L1210 leukemia cells. Further evidence for a third route mediated at low pH. Biochemical Pharmacology. 1997;53(2):223–231. doi: 10.1016/s0006-2952(96)00730-7. [DOI] [PubMed] [Google Scholar]
- Sierra EE, Goldman ID. Characterization of folate transport mediated by a low pH route in mouse L1210 leukemia cells with defective reduced folate carrier function. Biochemical Pharmacology. 1998;55(9):1505–1512. doi: 10.1016/s0006-2952(97)00673-4. [DOI] [PubMed] [Google Scholar]
- Sirotnak FM, Chello PL, Moccio DM, Kisliuk RL, Combepine G, Gaumont Y, et al. Stereospecificity at carbon 6 of fomyltetrahydrofolate as a competitive inhibitor of transport and cytotoxicity of methotrexate in vitro. Biochemical Pharmacology. 1979;28(19):2993–2997. doi: 10.1016/0006-2952(79)90599-9. [DOI] [PubMed] [Google Scholar]
- Sirotnak FM, Donsbach RC. Stereochemical characteristics of the folate-antifolate transport mechanism in L1210 Leukemia cells. Cancer Research. 1974;34(2):371–377. [PubMed] [Google Scholar]
- Sirotnak FM, Kurita S, Hutchison DJ. On the nature of a transport alteration determining resistance to amethopterin in the L1210 leukemia. Cancer Research. 1968;28(1):75–80. [PubMed] [Google Scholar]
- Stokstad ELR. Historical perspective on key advances in the biochemistry and physiology of folates. In: Picciano MF, Stokstad ELR, Greogory JF, editors. Contemporary issues in clinical nutrition: Folic acid metabolism in health and disease. New York: Wiley-Liss; 1990. pp. 1–21. [Google Scholar]
- Subramanian VS, Marchant JS, Said HM. Apical membrane targeting and trafficking of the human proton-coupled transporter in polarized epithelia. American Journal of Physiology. Cell Physiology. 2008;294(1):C233–C240. doi: 10.1152/ajpcell.00468.2007. [DOI] [PubMed] [Google Scholar]
- Umapathy NS, Gnana-Prakasam JP, Martin PM, Mysona B, Dun Y, Smith SB, et al. Cloning and functional characterization of the proton-coupled electrogenic folate transporter and analysis of its expression in retinal cell types. Investigative Ophthalmology & Visual Science. 2007;48(11):5299–5305. doi: 10.1167/iovs.07-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. The Journal of Biological Chemistry. 2009;284(26):17846–17857. doi: 10.1074/jbc.M109.008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal ES, Zhao R, Goldman ID. Role of the glutamate 185 residue in proton translocation mediated by the proton-coupled folate transporter SLC46A1. American Journal of Physiology. Cell Physiology. 2009;297(1):C66–C74. doi: 10.1152/ajpcell.00096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT) Biochimica et Biophysica Acta. 2008;1778(6):1407–1414. doi: 10.1016/j.bbamem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart BL, Gregor JC, Chande N, Knauer MJ, Tirona RG, Kim RB. The human proton-coupled folate transporter (hPCFT): Modulation of intestinal expression and function by drugs. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2010;298(2):G248–G254. doi: 10.1152/ajpgi.00224.2009. [DOI] [PubMed] [Google Scholar]
- Visentin M, Zhao R, Goldman ID. The antifolates. Hematology/Oncology Clinics of North America. 2012a;26(3):629–648. doi: 10.1016/j.hoc.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visentin M, Zhao R, Goldman ID. Augmentation of reduced folate carrier-mediated folate/antifolate transport through an antiport mechanism with 5-aminoimidazole-4-carboxamide riboside monophosphate. Molecular Pharmacology. 2012b;82(2):209–216. doi: 10.1124/mol.112.078642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cherian C, Kugel Desmoulin S, Mitchell-Ryan S, Hou Z, Matherly LH, et al. Synthesis and biological activity of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl regioisomers as inhibitors of de novo purine biosynthesis with selectivity for cellular uptake by high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier. Journal of Medicinal Chemistry. 2012;55(4):1758–1770. doi: 10.1021/jm201688n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cherian C, Kugel Desmoulin S, Polin L, Deng Y, Wu J, et al. Synthesis and antitumor activity of a novel series of 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitors of purine biosynthesis with selectivity for high affinity folate receptors and the proton-coupled folate transporter over the reduced folate carrier for cellular entry. Journal of Medicinal Chemistry. 2010;53(3):1306–1318. doi: 10.1021/jm9015729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Kugel Desmoulin S, Cherian C, Polin L, White K, Kushner J, et al. Synthesis, biological, and antitumor activity of a highly potent 6-substituted pyrrolo[2,3-d]pyrimidine thienoyl antifolate inhibitor with proton-coupled folate transporter and folate receptor selectivity over the reduced folate carrier that inhibits beta-glycinamide ribonucleotide formyltransferase. Journal of Medicinal Chemistry. 2011;54(20):7150–7164. doi: 10.1021/jm200739e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao R, Russell RG, Goldman ID. Localization of the murine reduced folate carrier as assessed by immunohistochemical analysis. Biochimica et Biophysica Acta. 2001;1513(1):49–54. doi: 10.1016/s0005-2736(01)00340-6. [DOI] [PubMed] [Google Scholar]
- Wessels JA, Huizinga TW, Guchelaar HJ. Recent insights in the pharmacological actions of methotrexate in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2008;47(3):249–255. doi: 10.1093/rheumatology/kem279. [DOI] [PubMed] [Google Scholar]
- Westerhof GR, Schornagel JH, Kathmann I, Jackman AL, Rosowsky A, Forsch RA, et al. Carrier- and receptor-mediated transport of folate antagonists targeting folate-dependent enzymes: Correlates of molecular-structure and biological activity. Molecular Pharmacology. 1995;48(3):459–471. [PubMed] [Google Scholar]
- Whetstine JR, Flatley RM, Matherly LH. The human reduced folate carrier gene is ubiquitously and differentially expressed in normal human tissues: Identification of seven non-coding exons and characterization of a novel promoter. The Biochemical Journal. 2002;367(Pt 3):629–640. doi: 10.1042/BJ20020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JC, Bailey BD, Goldman ID. Lack of stereospecificity at carbon 6 of methyltetrahydrofolate transport in Ehrlich ascites tumor cells. Carrier-mediated transport of both stereoisomers. The Journal of Biological Chemistry. 1978;253(1):242–245. [PubMed] [Google Scholar]
- Williams FM, Flintoff WF. Isolation of a human cDNA that complements a mutant hamster cell defective in methotrexate uptake. The Journal of Biological Chemistry. 1995;270(7):2987–2992. doi: 10.1074/jbc.270.7.2987. [DOI] [PubMed] [Google Scholar]
- Williams FM, Murray RC, Underhill TM, Flintoff WF. Isolation of a hamster cDNA clone coding for a function involved in methotrexate uptake. The Journal of Biological Chemistry. 1994;269(8):5810–5816. [PubMed] [Google Scholar]
- Witt TL, Stapels SE, Matherly LH. Restoration of transport activity by co-expression of human reduced folate carrier half-molecules in transport-impaired K562 cells: Localization of a substrate binding domain to transmembrane domains 7–12. The Journal of Biological Chemistry. 2004;279(45):46755–46763. doi: 10.1074/jbc.M408696200. [DOI] [PubMed] [Google Scholar]
- Wollack JB, Makori B, Ahlawat S, Koneru R, Picinich SC, Smith A, et al. Characterization of folate uptake by choroid plexus epithelial cells in a rat primary culture model. Journal of Neurochemistry. 2008;104(6):1494–1503. doi: 10.1111/j.1471-4159.2007.05095.x. [DOI] [PubMed] [Google Scholar]
- Wong SC, Proefke SA, Bhushan A, Matherly LH. Isolation of human cDNAs that restore methotrexate sensitivity and reduced folate carrier activity in methotrexate transport-defective Chinese hamster ovary cells. The Journal of Biological Chemistry. 1995;270(29):17468–17475. doi: 10.1074/jbc.270.29.17468. [DOI] [PubMed] [Google Scholar]
- Wong SC, Zhang L, Proefke SA, Matherly LH. Effects of the loss of capacity for N-glycosylation on the transport activity and cellular localization of the human reduced folate carrier. Biochimica et Biophysica Acta. 1998;1375(1–2):6–12. doi: 10.1016/s0005-2736(98)00118-7. [DOI] [PubMed] [Google Scholar]
- Wong SC, Zhang L, Witt TL, Proefke SA, Bhushan A, Matherly LH. Impaired membrane transport in methotrexate-resistant CCRF-CEM cells involves early translation termination and increased turnover of a mutant reduced folate carrier. The Journal of Biological Chemistry. 1999;274(15):10388–10394. doi: 10.1074/jbc.274.15.10388. [DOI] [PubMed] [Google Scholar]
- Xia W, Low PS. Folate-targeted therapies for cancer. Journal of Medicinal Chemistry. 2010;53(19):6811–6824. doi: 10.1021/jm100509v. [DOI] [PubMed] [Google Scholar]
- Yang CH, Sirotnak FM, Dembo M. Interaction between anions and the reduced folate/methotrexate transport system in L1210 cell plasma membrane vesicles: Directional symmetry and anion specificity for differential mobility of loaded and unloaded carrier. The Journal of Membrane Biology. 1984;79(3):285–292. doi: 10.1007/BF01871067. [DOI] [PubMed] [Google Scholar]
- Yang J, Vlashi E, Low P. Folate-linked drugs for the treatment of cancer and inflammatory diseases. Sub-Cellular Biochemistry. 2012;56:163–179. doi: 10.1007/978-94-007-2199-9_9. [DOI] [PubMed] [Google Scholar]
- Zhao R, Assaraf YG, Goldman ID. A mutated murine reduced folate carrier (RFC1) with increased affinity for folic acid, decreased affinity for methotrexate, and an obligatory anion requirement for transport function. The Journal of Biological Chemistry. 1998;273(30):19065–19071. doi: 10.1074/jbc.273.30.19065. [DOI] [PubMed] [Google Scholar]
- Zhao R, Diop-Bove N, Visentin M, Goldman ID. Mechanisms of membrane transport of folates into cells and across epithelia. Annual Review of Nutrition. 2011;31:177–201. doi: 10.1146/annurev-nutr-072610-145133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Gao F, Babani S, Goldman ID. Sensitivity to 5,10-dideazatetrahydrofolate is fully conserved in a murine leukemia cell line highly resistant to methotrexate due to impaired transport mediated by the reduced folate carrier. Clinical Cancer Research. 2000;6(8):3304–3311. [PubMed] [Google Scholar]
- Zhao R, Gao F, Goldman ID. Discrimination among reduced folates and methotrexate as transport substrates by a phenylalanine substitution for serine within the predicted eighth transmembrane domain of the reduced folate carrier. Biochemical Pharmacology. 1999;58(10):1615–1624. doi: 10.1016/s0006-2952(99)00257-9. [DOI] [PubMed] [Google Scholar]
- Zhao R, Gao F, Hanscom M, Goldman ID. A prominent low-pH methotrexate transport activity in human solid tumors: Contribution to the preservation of methotrexate pharmacologic activity in HeLa cells lacking the reduced folate carrier. Clinical Cancer Research. 2004;10(2):718–727. doi: 10.1158/1078-0432.ccr-1066-03. [DOI] [PubMed] [Google Scholar]
- Zhao R, Gao F, Wang Y, Diaz GA, Gelb BD, Goldman ID. Impact of the reduced folate carrier on the accumulation of active thiamin metabolites in murine leukemia cells. The Journal of Biological Chemistry. 2001;276(2):1114–1118. doi: 10.1074/jbc.M007919200. [DOI] [PubMed] [Google Scholar]
- Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;22(47):7431–7457. doi: 10.1038/sj.onc.1206946. [DOI] [PubMed] [Google Scholar]
- Zhao R, Goldman ID. The molecular identity and characterization of a proton-coupled folate transporter—PCFT; biological ramifications and impact on the activity of pemetrexed. Cancer Metastasis Reviews. 2007;26(1):129–139. doi: 10.1007/s10555-007-9047-1. [DOI] [PubMed] [Google Scholar]
- Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: Intestinal absorption and transport into systemic compartments and tissues. Expert Reviews in Molecular Medicine. 2009;11:e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Min SH, Qiu A, Sakaris A, Goldberg GL, Sandoval C, et al. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood. 2007;110(4):1147–1152. doi: 10.1182/blood-2007-02-077099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis. The Journal of Biological Chemistry. 2009;284(7):4267–4274. doi: 10.1074/jbc.M807665200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Qiu A, Tsai E, Jansen M, Akabas MH, Goldman ID. The proton-coupled folate transporter: Impact on pemetrexed transport and on antifolates activities compared with the reduced folate carrier. Molecular Pharmacology. 2008;74(3):854–862. doi: 10.1124/mol.108.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Russell RG, Wang Y, Liu L, Gao F, Kneitz B, et al. Rescue of embryonic lethality in reduced folate carrier-deficient mice by maternal folic acid supplementation reveals early neonatal failure of hematopoietic organs. The Journal of Biological Chemistry. 2001;276(13):10224–10228. doi: 10.1074/jbc.c000905200. [DOI] [PubMed] [Google Scholar]
- Zhao R, Shin DS, Diop-Bove N, Ovits CG, Goldman ID. Random mutagenesis of the proton-coupled folate transporter (SLC46A1), clustering of mutations, and the bases for associated losses of function. The Journal of Biological Chemistry. 2011;286(27):24150–24158. doi: 10.1074/jbc.M111.236539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Shin DS, Fiser A, Goldman ID. Identification of a functionally critical GXXG motif and its relationship to the folate binding site of the proton-coupled folate transporter (PCFT-SLC46A1) American Journal of Physiology Cell Physiology. 2012;303(6):C673–C681. doi: 10.1152/ajpcell.00123.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Unal ES, Shin DS, Goldman ID. Membrane topological analysis of the proton-coupled folate transporter (PCFT-SLC46A1) by the substituted cysteine accessibility method. Biochemistry (Moscow) 2010;49(13):2925–2931. doi: 10.1021/bi9021439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Visentin M, Suadicani SO, Goldman ID. Inhibition of the proton-coupled folate transporter (PCFT-SLC46A1) by bicarbonate and other anions. Molecular Pharmacology. 2013;84(1):95–103. doi: 10.1124/mol.113.085605. [DOI] [PMC free article] [PubMed] [Google Scholar]