Abstract
In the United States, mortality as a result of lung infections consistently ranks in the top ten leading causes of death, accounting for over 50,000 deaths annually. Moreover, there are more than 140,000 deaths annually as a result of chronic lung diseases, some of which may be complicated by an infectious process. The lung is constantly exposed to the environment and consequently, susceptible to infectious complications caused by bacterial, viral, fungal and parasitic pathogens. Indeed, we are continually faced with the threat of morbidity and mortality associated with annual influenza virus infections, new respiratory viruses (such as SARS-CoV) as well as lung infections caused by antibiotic-resistant “ESKAPE pathogens” (three of which target the lung). This review will highlight innate immune receptors and cell types that function to protect against infectious challenges to the respiratory system yet may also be associated with exacerbations in chronic lung diseases.
Introduction
The major function of the respiratory system is to procure O2 and to eliminate CO2 from the body, thus breathing is a physiologic function required to sustain life. However, in an aberrant view, breathing may paradoxically be considered as contributing to mortality. This is because with every breath, toxins, noxious gases, pollutants, particulates and allergens may be introduced into the lungs. Moreover, indoor and outdoor air quality and environmental sampling studies detect enumerable microorganism concentrations per cubic meter in public buildings, homes and even healthcare facilities (1) (2). Altogether, these environmental exposures may ultimately lead to inflammatory and pathological changes that increase the risk of infection. Indeed, although community-acquired pneumonia and influenza results in more than 50,000 deaths in the U.S., chronic lower respiratory diseases are the third leading cause of death (> 140,000) in the U.S (http://www.cdc.gov/nchs/fastats/deaths.htm). These chronic lower respiratory diseases largely include such diseases as asthma and chronic obstructive pulmonary disease (COPD), both of which have known associations with microorganisms (3) (4). This association can be viewed in the proverbial “chicken or the egg” sense: exposure to microorganisms may cause inflammatory and pathological changes that result in the development of asthma or COPD, or conversely, asthma or COPD may result in a lung microenvironment that is conducive to the acquisition of microorganisms and subsequent infectious exacerbations. This article will focus primarily on innate recognition and cellular host defense mechanisms that drive the elimination of pathogens from the lung that may also contribute to lung diseases such as asthma and COPD.
Non-TLR innate immune receptors functioning in the lung
NOD-like receptors (NLRs)
Nucleotide-binding oligomerization domain (NOD)-containing receptors (NLRs) are a family of more than 20 intracellularly-localized receptors that recognize numerous pathogen associated molecular patterns (PAMPs; microbial associated factors recognized by the innate immune system) and damage associated molecular patterns (DAMPs; non-microbial products generated during inflammation and tissue injury) including bacterial flagellin, lipoproteins, toxins and muramyl-dipeptide (reviewed in (5)). NLRs came to prominence over 12 years ago when mutations in the NOD2 receptor were found to be associated with susceptibility to Crohn's disease (6). Coming on the heels of the initial discovery and subsequent intensive study of TLRs in innate immune responses (extensively reviewed in (7)]), these findings launched an explosion of research into non-TLRs that were equally important in innate immune responses to pathogens. The NLRs may be subdivided into signaling (NOD1, NOD2), inflammasome generating (NLRP3, NLRC4) and immunoregulatory (NLRX1, NLRP6, NLRP12) (8) (5). NLRs have been studied in lung immune responses to bacterial infections including K. pneumoniae (9), Pseudomonas aeruginosa (10) Streptococcus pneumoniae (11), S. aureus (12) and Mycobacterium tuberculosis (13) and viral infections such as influenza (14) and RSV (15).
RIG-I-like receptors (RLRs)
Retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs) include three DExD/H box RNA helicases, RIG-I, melanoma differentiation factor 5 (MDA5) and laboratory of genetics and physiology 2 (LGP-2). While RIG-I and MDA5 recognize RNA in the cytosol (reviewed in (16)), LGP-2 does not and is rather thought to be a negative regulator of RIG-1 and MDA5 (17). Intriguingly however, LGP-2 overexpression results in improved survival despite similar viral titers as wild-type mice, yet in the presence of reduced antiviral and inflammatory responses (lower IFN-α, β and λ as well as RANTES and TNF-α levels), after influenza exposure (18). Ligation of RIG-I and MDA5 leads to activation of the adaptor protein MAVS (19) and subsequent induction of type I antiviral and associated inflammatory responses via IRF3 and IRF7 (20) (19). RIG-I initiates immune responses to influenza (21), RSV (22) and human metapneumovirus (23). Although there is some overlap (22) (24), MDA5 may show specificity over RIG-I for some viruses, such as parainfluenza (25). In fact, recent evidence suggests that MDA5 is not only required for lung innate immune responses to parainfluenza (26), but is also required for regulating chronic inflammation after infection (27). Recently, it was demonstrated that mice deficient in the guanine nucleotide exchange factor GEF-H1 were shown to lack RIG-I and MDA5-dependent phosphorylation of IRF3 and were more susceptible to lung infection with influenza A (28). Studies have also shown that some viruses have become adept at evading RIG-1 and MDA5-mediated events. For example, the NS1 protein of influenza A virus may bind to the RIG-I–IPS1 complex and blocks downstream signaling (29). Similarly, the V proteins of many paramyxoviruses interact with MDA5 and may inhibit its function (30). More recently, the 4a protein of the Middle East respiratory syndrome coronavirus (MERS-CoV) inhibits PACT, a cellular dsRNA-binding protein which binds to RIG-I and MDA5 to activate IFN production (31). Although more prominently studied in antiviral responses, studies have shown that RLRs (primarily RIG-I) may also participate in innate responses to lung bacterial pathogens, such as Legionella pneumophila (32).
C-type lectin receptors (CLRs)
CLRs are a large, conserved family of pattern recognition receptors that primarily bind carbohydrate ligands via a carbohydrate recognition domain (CRD) or C-type lectin-like domain (CTLD) (33). Currently, there are 17 known CLR subgroups (34). The most well-described CLRs include group II (calcium-dependent with single CRDs), group V (calcium-independent with single CTLDs) and group VI (calcium-dependent with multiple CRDs). Prominent members of group II CLRs are DC-SIGN, Mincle, SIGNR and Dectin-2 and primarily recognize mannose containing ligands (35). With respect to lung infections, group II CLRs are associated with the recognition of and subsequent binding/entry of or innate responsiveness to Mycobacterium spp. (36), K. pneumoniae (37), S. pneumonia (38), Histoplasma capsulatum (39), Cryptococcus neoformans (40), influenza (41) and SARS (42).
The most prominent member of group V CLRs is the beta-glucan receptor Dectin-1. Dectin-1 is reported to mediate multiple innate immune responses upon myeloid cell recognition of various lung fungal pathogens, including Aspergillus fumigatus (43), Coccidioides immitis (44) and Pneumocystis carinii (45). Although Mycobacterium spp. do not have beta-glucans in their cell wall, Dectin-1 may promote innate cellular responses to this pathogen via recognition of an unknown ligand (46). However, data argues both for (47) and against (48) a role for Dectin-1 in host defense against Mycobacterium spp. Recent studies have focused on a role for Dectin-1 in A. fumigatus-associated asthma. In a chronic live A. fumigatus conidia exposure model, BALB/c mice displayed significantly more TNF-α-producing DCs and macrophages in the lung compared to BL/6 mice, which was dependent on Dectin-1 (49). In our own work, we proceeded this study by showing that Dectin-1 dependent IL-22 signaling contributed to the development of AHR, pro-allergic and pro-inflammatory cytokine and chemokine production, neutrophil recruitment and IL-17A and IL-22 production (50). In another study employing a different fungal asthma model, lower asthma severity in mice deficient in TLR9 correlated with significantly lower Dectin-1 mRNA expression (51), yet this group reported in a subsequent study that TLR6 deficient mice had more severe fungal asthma despite lower Dectin-1 expression and Th17 development (52). Another study investigating A. versicolor-associated asthma demonstrated no effect on AHR in the absence of Dectin-1, although Dectin-1 drove Th17 responses (53). In contrast, Cladosporium cladosporioides-associated asthma resulted in elevated Th2 responses and AHR, which was not dependent on Dectin-1 (53). However, beta-glucans in the C. cladosporioides cell wall may be exposed after heat-killing the organism, which then results in Dectin-1 dependent responses (54). Finally, although Dectin-1 is most recognized as an essential initiator of the innate immune response against various fungal pathogens, Dectin-1 has also been shown to bind an unidentified ligand on T cells and can regulate T cell activation and responses (55).
The most prominent members of group VI CLRs is the macrophage mannose receptor (MR) and DEC-205. Similar to group II CLRs, the ligand specificity of group VI is also mannan/mannose moieties, although MR may also bind sialyl LewisX antigen as well as n-acetylglucosamine (GlcNAc) (35). In turn, the pathogens recognized by the MR are similar to that in group II CLRs and include Mycobacterium spp. (56), K. pneumoniae (57), S. pneumonia (57) and C. neoformans (58). DEC-205 binds ligands on lung associated pathogens such as Yersinia pestis plasminogen activator and Escherichia coli K12 strains (59) and has been targeted in vaccine studies for inducing lung immunity to Y. pestis (60) and M. tuberculosis (61).
Scavenger receptors
Scavenger receptors are a diverse range of receptors consisting of eight different classes with a myriad of ligand specificity ranging from host proteins to microbial components (62). The best-studied scavenger receptors are those found in class A, which include SR-A1 and MARCO. Early studies with SR-A1 identified it as a potential PRR for the bacterial components (63) with subsequent studies identifying a prominent role for SR-A1 in immunity against S. pneumonia (64). However, in a surprising recent finding, SR-A1 deficient mice were observed to be more resistant to polymicrobial sepsis, as lung NF-κB activity was attenuated in the absence of SR-A1, indicating that SR-A1 plays a role in pathophysiology of sepsis/shock (65). Similarly, studies with the lung fungus C. neoformans has shown that SR-A1 deficient mice are more resistant to infection as a result of lower Th2 responses, suggesting that C. neoformans may employ SR-A1 to interfere with the development of anti-cryptococcal Th1 responses (66). In contrast, mice double deficient in SR-A1 and CD36 (see below) demonstrate resistance to peritoneal S. aureus infection, but increase susceptibility to S. aureus lung infection (67), suggesting tissue-specific roles for some scavenger receptors in host defense. Like SR-A1, MARCO also plays a critical role in immune against S. pneumonia (68) and, based on binding studies, may also play a role in innate lung responses E. coli and S. aureus (69). Both MARCO and SR-A1 also appear to play a role in regulating allergic responses in the lung at the level of DC migration (70). MARCO may also contribute to detrimental inflammatory responses during influenza infection (71). CD36 is the prototype class B scavenger receptor and is best known for binding to Plasmodium spp. in addition to the induction of anti-malarial proinflammatory responses (72). However, malaria infection is often accompanied by acute lung injury with recent data suggesting that CD36 functions to sequester Plasmodium spp. which results in the complicating inflammatory response (73). CD36 binds the LprA liporprotein of M. tuberculosis to drive macrophage and DC responsiveness (74), although CD36 deficient mice do not appear to be susceptible to acute or chronic M. tuberculosis infection, unless this is combined with SR-AI/II deficiency (75).
LOX-1 is a member of class E scavenger receptors and shares homology with CLRs as it is one of only two scavenger receptors to possess a CTLD (62). Although well studied in atherosclerosis, binding studies support a putative role for LOX-1 in immune responsiveness to E. coli and S. aureus (76). Another study has shown that blocking LOX-1 improves morbidity during acute lung injury (77), suggesting that LOX-1 signaling contributes to lung pathophysiology, similar to that proposed for SR-A1. Airway epithelial-expressed LOX-1 has recently been implicated in the recognition of double-stranded RNA viruses in the lung (78). The lone member of class G scavenger receptors is SR-PSOX (79), which is identical to the chemokine CXCL16, and thus structurally unique among scavenger receptors (80). Other studies show that expression of CXCR6 (CXCL16 receptor) on lung T cells is a correlate of local protective immunity against M. tuberculosis (81). CXCL16 may also play a role in lung NKT cell homeostasis, as these cells are significantly reduced in mice deficient in the CXCR6 (82). Moreover, NKT cells are elevated in the lungs of germ-free mice leading to increased morbidity in an asthma model, which correlated with increased lung expression of CXCL16 (83).
Cellular effectors of lung innate immunity
Epithelial cells
Epithelial cells serve not only as a physical barrier to the outside environment but also represent one of the first lines of innate host defense against respiratory pathogens (84). The respiratory system is divided into the upper airway tract, composed of the nasal sinuses and pharynx, and the lower tract, composed of the trachea, which successively branches in bronchi, bronchioles, and the alveoli where exchange of O2 and CO2 occurs. The respiratory tract is lined with several types of pseudo-stratified epithelial cells connect by tight junctions that perform a variety of innate host defense functions in the airways including particulate-sweeping by ciliated columnar cells, mucus production by goblet cells and surfactant production by Clara cells (85). The alveoli are composed of type I alveolar epithelial cells, which are primarily responsible for gas exchange and type II alveolar epithelial cells, which serve primarily as immune responders (86). Mucociliary clearance is a key component of innate lung epithelium host defense. Mucins produced by goblet cells are rapidly hydrated into mucus, which traps pathogens and allows for their continual removal from the distal airways via by movement by ciliated epithelial cells into the pharynx where it is swallowed (87). In addition to barrier protection and mucus production, epithelial cells directly contribute to microbial killing via dual oxidase (Duox) expression on the apical surface of epithelial cells, which converts hydrogen peroxide to lactoperoxidase and subsequently antimicrobial hypothiocyanite ions (88). Airway epithelial cells also secrete antiviral type I IFN, lactoferrin, β-defensins and NO in response to many respiratory infections (89). Studies in both humans and animals show that airway epithelial cells express many pattern recognition receptors (PRRs) and produce numerous cytokines and chemokines involved in the recruitment of both innate and adaptive cell type (90) (91) (92,93).
Alveolar macrophages
Along with epithelial cells, alveolar macrophages in the lung are an additional first-line defense mechanism against invading pathogens (94). Alveolar macrophages are responsible for clearing all foreign particles or pathogens that enter the alveoli. These cells are highly phagocytic, express numerous PRRs and produce an extensive array of pro- and anti-inflammatory cytokines, chemokines, and leukotrienes and this are crucial for providing the initial innate immune recognition and response signals (95). Alveolar macrophage host defense capabilities are often determined by their plasticity between classically activated M1 macrophages and alternatively activated M2 macrophages (reviewed extensively in (96)). Conventionally, it was thought that alveolar macrophages were the terminal differentiation state of blood monocytes in the lung after they progress through an interstitial macrophage state (97) or a parenchymal lung macrophage state (98) (which could be the same cell population). Other studies in mice suggest that fetal monocytes are responsible for alveolar macrophages from the lung within the first week of life (99). However, other murine studies suggest that alveolar macrophages may be established before birth and differentiation through monocytes is not required (100). Interestingly, in Th2 associated lung inflammation, studies have shown that development of M2 macrophages occurs not through precursors from the blood, but by local proliferation of macrophages in response to IL-4 (101). Collectively, these studies support both an embryonic and fetal origin of lung macrophages. Although the host defense aspects of these observations are not completely clear, we can speculate that the need for immediate surveillance of inhaled particles, antigens and pathogens has evolutionarily necessitated the presence of alveolar macrophages in the lung at or shortly after birth. Indeed, alveolar macrophages from neonatal mice express PRRs such as TLR4 and TLR2 and are responsive to LPS and zymosan (102).
Neutrophils
Responding to the various chemokines produced by macrophages and epithelial cells, including IL-8/KC, MIP-1α and β and MIP-2, neutrophils are recruited into the lung as part of an ongoing inflammatory response where their predominant function is the intracellular and extracellular killing of microbes. Neutrophil killing is an essential aspect of host defense in a variety of bacterial and fungal pulmonary infections including A. fumigatus (103), Bordetella pertussis (104), P. aeruginosa (105), S. pneumoniae (106) and K. pneumoniae (107). Like alveolar macrophages, neutrophils express numerous PRRs, mediate microbial killing through production of ROS, secretion of azurophilic granule contents (myeloperoxidase, elastase, defensins, specific granule contents (lactoferrin, cathelicidins), gelatinase granule contents (lysozyme) (108) and via the formation of neutrophil extracellular traps (NETs) (109).
Dendritic cells
In order to preserve the delicate architecture of the lung that facilitates gas exchange, alveolar macrophages are designed to dispose of invading organisms before they have a chance to initiate a more robust inflammatory response. However if the alveolar macrophages are overwhelmed, microbes are more likely to encounter pulmonary dendritic cells. In mice, there are three types of dendritic cells in the naïve lung: CD11b+CD103− conventional dendritic cells (cDC) that reside in the lamina propria, CD11b−CD103+ cDC that express tight junctions and intercalates between airway epithelia cells in order to sample airway environment and plasmacytoid dendritic cells (pDCs) found in the conducting airways (110). During inflammatory responses, a fourth type of dendritic cell, monocyte-derived FcεRI+ inflammatory dendritic cells (mDCs), may be found in the lung (110). DCs robustly express TLRs, NLRs, CLRs and RLRs which allow them to sense a wide variety of innate stimuli. Upon activation, cDCs, and pDCs mature and migrate to lung draining lymph nodes where they present antigen in order to direct a T cell response (111). In addition, cDCs, mDCs and pDCs also contribute to innate antiviral response (influenza, RSV) (112) (113) and M. tuberculosis (114) lung infection through the production of type 1 IFNs.
γδ T cells
Since the discovery of γδ T cells, they have remained a fascinating heterogeneous subset of cells, involved in both innate and adaptive immune responses. They are evolutionarily conserved as homologs can be found in jawless vertebrates and although γδ T cells originate from the same thymic precursor as αβ T cells; they appear to be involved in several non-redundant functions (115). Unlike traditional αβ T cells, γδ T cells express a contrasting TCR, which is not MHC restricted (116). γδ T cells were first described in the lung more than 25 years ago and were identified to be as high as 8–20% of CD3+ cells in the lung (117). We now know that γδ T cells play an early protective role in the lung during infection with pathogens such as K. pneumoniae (118), M. tuberculosis (119), S. aureus (120) and S. pneumonia (121). γδ T cells are important sources of “innate IL-17A” in the lung during infection with A. fumigatus (122) and C. neoformans (123).
Innate lymphoid cells
Innate helper cells/innate lymphoid cells (ILC) are thought to be the innate counterparts to T helper subsets based on their respective cytokine production: IFN-γ (Th1) from ILC1 subset, IL-5 and IL-13 (Th2) from the ILC2 subset and IL-17/IL-22 (Th17/Th22) from ILC3 subset (124). Innate helper type-2 cells (ILC2), also called nuocytes (125) or natural helper cells (126), are part of the innate lymphoid cell (ILC) family that are developmentally related along with NK cells (ILC1) and lymphoid tissue inducer (LTi) cells (ILC3). Early studies putatively suggested an ILC2 population existed in the lung after the production of IL-5 and IL-13 was observed in mice lacking conventional T and B cells (127). ILC2s exert a powerful anti-parasitic defense against Nippostrongylus brasiliensis and are sufficient for worm expulsion mediated through production of IL-13 (128), ILC2s also promote tissue repair during influenza infection (129). However ILC2 in the lungs can also play a role in the exacerbation of airway hyper-responsiveness seen in asthma, as IL-25 and IL-33 promote the expansion of IL-13-producing ILC2s that then stimulate mature DCs to migrate to the draining lymph node where they then promote allergic Th2 cell responses (130). ILC3s are found predominantly in mucosal tissues like the gut, yet ILC3s have been identified as sources of innate IL-17 and IL-22 early after exposure to bacterial pathogens such as S. pneumoniae (131) or in models of experimental asthma (132) (133).
Antimicrobial immune responses complicating chronic lung diseases in humans
As referred to earlier, recent mortality data (CDC, 2011) indicated that approximately 2.5 million people die in the US each year with nearly 200,000 of these associated with a lung infection (~50,000 deaths from influenza, pneumonia) or a lung disease (~140,000+ from asthma, COPD etc.). With respect to the latter, disease coding data indicates that these lung diseases may be associated with infectious complications. To this end, it is easy to speculate that in asthma or COPD, immune responses during microbial exposure may serve to exacerbate disease (3) (4). For example, studies have shown that lung infection with H. influenzae induces NLRP3 expression (134). This is hypothesized to be a potentially immunopathogenic mechanism in COPD, as H. influenzae is strongly associated with COPD and individuals with COPD have elevated levels of uric acid (135), which activate NLRP3 (136). Thus, a consequence of H. influenzae exposure in COPD is the upregulation and activation of NLRP3 inflammatory signals that could lead to more severe lung disease. Genetic data has shown that SNPs in Nod1 and Nod2 are associated with increased risk of asthma (137) (138) while recent studies have implicated genetic mutations in SR-A1 in development of or exacerbations in COPD (139) (140). It is tempting to speculate that mutations in PRRs such as NOD1, NOD2 and SR-A1 may result in increased colonization/exposure or sub-clinical infection with microorganisms that could lead to enhanced inflammatory responses and subsequent increased asthma or COPD severity. In contrast to lower PRR expression, differential expression of cellular receptors or numbers of cellular effectors may also contribute to immunopathogenesis in lung diseases. For example, although the function of the scavenger receptor/chemokine CXCL16 in lung host defense is not completely clear, its expression on CD8+ T cells in the lung correlates with disease severity in COPD (141). Furthermore, a recent study investigating the distribution of γδ T cells in the lungs of human subjects with COPD made the surprising finding of significantly lower numbers of γδ T cells in sputum and lung lavage fluid from those with COPD, which correlated with lung function decline (142). Collectively, these observations lay the foundation for examining CXCL16/CXCR6 expression and function as well as γδ T cells in lung infection models of organisms that are commonly associated with COPD (4). Finally, defects in lung epithelium barrier and mucus production, which often leads to hyper-neutrophilic inflammation in the lungs, coupled with recurrent infections and exacerbations are the hallmarks of many human chronic pulmonary diseases such as asthma, CF and COPD (143) (144) (145).
Conclusions
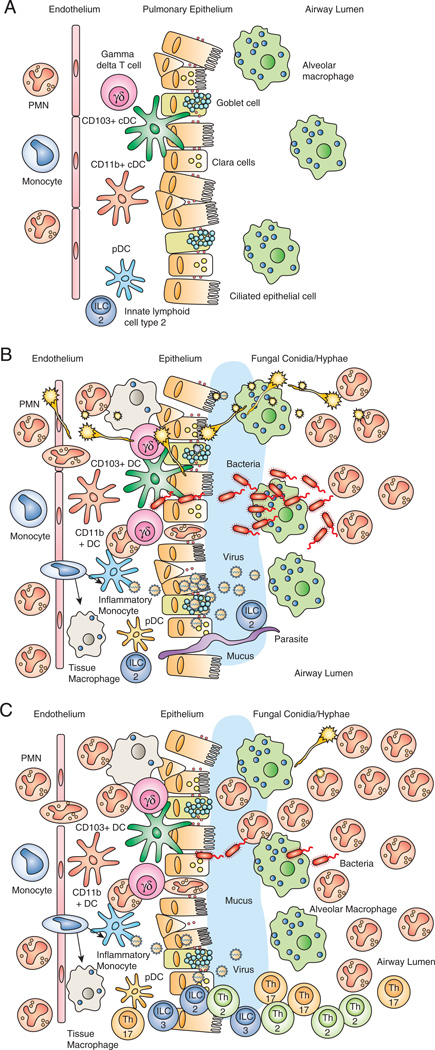
As the lung is continually exposed to the environment, innate immune mechanisms must but equipped to handle the recognition of a diverse array of foreign ligands (Table 1) and respond in a rapid and robust manner in order to clear invading pathogens before they functionally compromise the lung. The importance of innate immunity is reinforced by the identification of numerous genetic polymorphisms that result in lung infections (146). However, innate host defense against lung pathogens may consequently come at the price of developing or exacerbating a lung-specific condition such as asthma or COPD. This complex system is illustrated in Figure 1 whereby the homeostatic lung is poised to react to microbial exposure via epithelial cells, alveolar macrophages, DCs, ILCs and γδ T cells (Figure 1A). Exposure to a bacterial, viral or fungal pathogen results in the activation of these cell types, initiating an inflammatory cascade resulting in the recruitment of neutrophils (Figure 1B). However, in some instances, exposure to or prolonged colonization with an organism results in persistent recruitment or presence of T helper (Th2; Th17) or inflammatory ILCs (ILC2; ILC3) that may result in a hypersensitivity reaction and the development of asthma (Figure 1C).
Table 1.
Non-TLR PRRs in innate lung defense
| PRR Family | Ligand | Lung-associated pathogen | |
|---|---|---|---|
| NLR | NOD-like receptors | ||
| NOD1, NOD2 | DAP, MDP | Lung response to K. pneumoniae, P. aeruginosa, S. pneumoniae, S. aureus, M. tuberculosis, influenza, and RSV | |
| RLR | RIG-1-like receptors | ||
| RIG-1, MDA5 | Cytosolic RNA | induction of type 1 antiviral response | |
| LGP-2 | None | potential negative regulator of RIG-1 and MDA5 | |
| CLR | C-type lectin receptors: | ||
| Group II (calcium-dependent, single CRDs) | |||
| DC-SIGN, Mincle, SIGNR, Dectin-2 | Mannose-containing ligands | Recognition of Mycobacterium spp., K. pneumoniae, S. pneumoniae, H. capsulatum, C. neoformans, influenza, and SARS | |
| Group V (calcium-independent, single CTLDs) | |||
| Dectin-1 | Beta-glucan | Mediates innate immune responses against several fungal pathogens: A. fumigatus, C. immitis, and P. carinii as well as host defense against Mycobacterium spp. | |
| Group VI (calcium dependent, multiple CRDs) | |||
| Mannose Receptor (MR) | Mannan/mannose moieties, sialyl LewisX antigen, GlcNAc | Bacterial Recognition: Mycobacterium spp., K. pneumoniae, S. pneumoniae, and C. neoformans; potential role in induction of allergic response | |
| DEC-205 | Mannan/mannose moieties | Bacterial recognition: Y. pestis, M. tuberculosis, E. coli strain K12 | |
| SR | Scavenger Receptors | ||
| Class A | |||
| SR-A1 | Bacterial components, LPS, LTA, CpG | Recognition of S. pneumoniae, pathophysiology of sepsis/shock, DC migration | |
| MARCO | LPS, LTA, CpG | Defense against S. pneumoniae, E. coli, and S. aureus, DC migration | |
| Class B | |||
| CD36 | Plasmodium spp., C. neoformans, Beta-glucan, Gram (−) bacteria | Pro-inflammatory responses | |
| Class E | |||
| LOX-1 | Gram (+/−) bacteria | Immune response against E. coli and S. aureus, recognition of double-stranded viruses in the lung | |
| Class G | |||
| SR-PSOX | CXCR6, Gram (+/−) bacteria | Identical to CXCL16, protective immunity to M. tuberculosis, potential role in NKT homeostasis | |
Figure 1.
(A) The lung at baseline is constantly exposed to fungal spores, bacteria and viral particles through alveolar macrophages phagocytosis, IFN production by epithelial cells and the mucocilliary escalator. (B) If these defenses become overwhelmed during an active infection, a robust inflammatory process involving alveolar macrophages, DCs, γδ T cells, ILCs, neutophils and epithelial cells commences and involves a variety of antimicrobial mediators. (C) However, during persistent exposure, the inflammatory response remains, contributing to the exacerbation of chronic lung diseases like COPD and asthma through an abundance of neutrophils, Th2/Th17, ILC2 and ILC3 cells.
Acknowledgments
This work was supported by PHS grants T32 HL007517 (J.W.), R21 HL117090, R01 HL096702 and R01 HL119770 (all to C.S.).
References
- 1.Sessa R, Di PM, Schiavoni G, Santino I, Altieri A, Pinelli S, Del PM. Microbiological indoor air quality in healthy buildings. New Microbiol. 2002;25:51–56. [PubMed] [Google Scholar]
- 2.Menegueti MG, Ferreira LR, Silva MF, Silva AS, Bellissimo-Rodrigues F. Assessment of microbiological air quality in hemato-oncology units and its relationship with the occurrence of invasive fungal infections: an integrative review. Rev. Soc. Bras. Med. Trop. 2013;46:391–396. doi: 10.1590/0037-8682-0022-2013. [DOI] [PubMed] [Google Scholar]
- 3.Edwards MR, Bartlett NW, Hussell T, Openshaw P, Johnston SL. The microbiology of asthma. Nat. Rev. Microbiol. 2012;10:459–471. doi: 10.1038/nrmicro2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rangelov K, Sethi S. Role of infections. Clin Chest Med. 2014;35:87–100. doi: 10.1016/j.ccm.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaput C, Sander LE, Suttorp N, Opitz B. NOD-Like Receptors in Lung Diseases. Front Immunol. 2013;4:393. doi: 10.3389/fimmu.2013.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 7.O'Neill LA, Golenbock D, Bowie AG. The history of Toll-like receptors - redefining innate immunity. Nat. Rev. Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 8.Liu D, Rhebergen AM, Eisenbarth SC. Licensing Adaptive Immunity by NOD-Like Receptors. Front Immunol. 2013;4:486. doi: 10.3389/fimmu.2013.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regueiro V, Moranta D, Frank CG, Larrarte E, Margareto J, March C, Garmendia J, Bengoechea JA. Klebsiella pneumoniae subverts the activation of inflammatory responses in a NOD1-dependent manner. Cell Microbiol. 2011;13:135–153. doi: 10.1111/j.1462-5822.2010.01526.x. [DOI] [PubMed] [Google Scholar]
- 10.Travassos LH, Carneiro LA, Girardin SE, Boneca IG, Lemos R, Bozza MT, Domingues RC, Coyle AJ, Bertin J, Philpott DJ, Plotkowski MC. Nod1 participates in the innate immune response to Pseudomonas aeruginosa. J Biol. Chem. 2005;280:36714–36718. doi: 10.1074/jbc.M501649200. [DOI] [PubMed] [Google Scholar]
- 11.Davis KM, Nakamura S, Weiser JN. Nod2 sensing of lysozyme-digested peptidoglycan promotes macrophage recruitment and clearance of S. pneumoniae colonization in mice. J Clin Invest. 2011;121:3666–3676. doi: 10.1172/JCI57761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kapetanovic R, Jouvion G, Fitting C, Parlato M, Blanchet C, Huerre M, Cavaillon JM, Adib-Conquy M. Contribution of NOD2 to lung inflammation during Staphylococcus aureus-induced pneumonia. Microbes. Infect. 2010;12:759–767. doi: 10.1016/j.micinf.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L, Veyrier F, Kobayashi KS, Flavell RA, Gros P, Behr MA. NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J Immunol. 2008;181:7157–7165. doi: 10.4049/jimmunol.181.10.7157. [DOI] [PubMed] [Google Scholar]
- 14.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-Tekippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, Morris IR, Allen IC, Ting JP, Bose S. TLR2/MyD88/NF-kappaB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One. 2012;7:e29695. doi: 10.1371/journal.pone.0029695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vabret N, Blander JM. Sensing Microbial RNA in the Cytosol. Front Immunol. 2013;4:468. doi: 10.3389/fimmu.2013.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saito T, Hirai R, Loo YM, Owen D, Johnson CL, Sinha SC, Akira S, Fujita T, Gale M., Jr Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc. Natl. Acad. Sci. U. S. A. 2007;104:582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Si-Tahar M, Blanc F, Furio L, Chopy D, Balloy V, Lafon M, Chignard M, Fiette L, Langa F, Charneau P, Pothlichet J. Protective Role of LGP2 in Influenza Virus Pathogenesis. J Infect. Dis. 2014;210:214–223. doi: 10.1093/infdis/jiu076. [DOI] [PubMed] [Google Scholar]
- 19.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–682. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 21.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 22.Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, Garcia-Sastre A, Katze MG, Gale M., Jr Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82:335–345. doi: 10.1128/JVI.01080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao S, Bao X, Liu T, Lai S, Li K, Garofalo RP, Casola A. Role of retinoic acid inducible gene-I in human metapneumovirus-induced cellular signalling. J Gen. Virol. 2008;89:1978–1986. doi: 10.1099/vir.0.2008/000778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banos-Lara MR, Ghosh A, Guerrero-Plata A. Critical role of MDA5 in the interferon response induced by human metapneumovirus infection in dendritic cells and in vivo. J Virol. 2013;87:1242–1251. doi: 10.1128/JVI.01213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Childs K, Stock N, Ross C, Andrejeva J, Hilton L, Skinner M, Randall R, Goodbourn S. mda-5, but not RIG-I, is a common target for paramyxovirus V proteins. Virology. 2007;359:190–200. doi: 10.1016/j.virol.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 26.Yount JS, Gitlin L, Moran TM, Lopez CB. MDA5 participates in the detection of paramyxovirus infection and is essential for the early activation of dendritic cells in response to Sendai Virus defective interfering particles. J Immunol. 2008;180:4910–4918. doi: 10.4049/jimmunol.180.7.4910. [DOI] [PubMed] [Google Scholar]
- 27.Kim WK, Jain D, Sanchez MD, Koziol-White CJ, Matthews K, Ge MQ, Haczku A, Panettieri RA, Jr, Frieman MB, Lopez CB. Deficiency of melanoma differentiation-associated protein 5 results in exacerbated chronic postviral lung inflammation. Am. J Respir. Crit Care Med. 2014;189:437–448. doi: 10.1164/rccm.201307-1338OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang HS, Zhao Y, Song JH, Liu S, Wang N, Terhorst C, Sharpe AH, Basavappa M, Jeffrey KL, Reinecker HC. GEF-H1 controls microtubule-dependent sensing of nucleic acids for antiviral host defenses. Nat. Immunol. 2014;15:63–71. doi: 10.1038/ni.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mibayashi M, Martinez-Sobrido L, Loo YM, Cardenas WB, Gale M, Jr, Garcia-Sastre A. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J Virol. 2007;81:514–524. doi: 10.1128/JVI.01265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-beta promoter. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siu KL, Yeung ML, Kok KH, Yuen KS, Kew C, Lui PY, Chan CP, Tse H, Woo PC, Yuen KY, Jin DY. Middle east respiratory syndrome coronavirus 4a protein is a double-stranded RNA-binding protein that suppresses PACT-induced activation of RIG-I and MDA5 in the innate antiviral response. J Virol. 2014;88:4866–4876. doi: 10.1128/JVI.03649-13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Monroe KM, McWhirter SM, Vance RE. Identification of host cytosolic sensors and bacterial factors regulating the type I interferon response to Legionella pneumophila. PLoS Pathog. 2009;5:e1000665. doi: 10.1371/journal.ppat.1000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hardison SE, Brown GD. C-type lectin receptors orchestrate antifungal immunity. Nat. Immunol. 2012;13:817–822. doi: 10.1038/ni.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van den Berg LM, Gringhuis SI, Geijtenbeek TB. An evolutionary perspective on C-type lectins in infection and immunity. Ann. N. Y. Acad. Sci. 2012;1253:149–158. doi: 10.1111/j.1749-6632.2011.06392.x. [DOI] [PubMed] [Google Scholar]
- 35.Sancho D, Reis e Sousa Signaling by myeloid C-type lectin receptors in immunity and homeostasis. Annu. Rev. Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, Akira S, Yoshikai Y, Yamasaki S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp. Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steichen AL, Binstock BJ, Mishra BB, Sharma J. C-type lectin receptor Clec4d plays a protective role in resolution of Gram-negative pneumonia. J Leukoc. Biol. 2013;94:393–398. doi: 10.1189/jlb.1212622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geijtenbeek TB, Groot PC, Nolte MA, van Vliet SJ, Gangaram-Panday ST, van Duijnhoven GC, Kraal G, van Oosterhout AJ, van KY. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood. 2002;100:2908–2916. doi: 10.1182/blood-2002-04-1044. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, LeBert V, Hung CY, Galles K, Saijo S, Lin X, Cole GT, Klein BS, Wuthrich M. C-type lectin receptors differentially induce th17 cells and vaccine immunity to the endemic mycosis of North America. J Immunol. 2014;192:1107–1119. doi: 10.4049/jimmunol.1302314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mansour MK, Latz E, Levitz SM. Cryptococcus neoformans glycoantigens are captured by multiple lectin receptors and presented by dendritic cells. J Immunol. 2006;176:3053–3061. doi: 10.4049/jimmunol.176.5.3053. [DOI] [PubMed] [Google Scholar]
- 41.Hillaire ML, Nieuwkoop NJ, Boon AC, de MG, Vogelzang-van Trierum SE, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. Binding of DC-SIGN to the hemagglutinin of influenza A viruses supports virus replication in DC-SIGN expressing cells. PLoS One. 2013;8:e56164. doi: 10.1371/journal.pone.0056164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang ZY, Huang Y, Ganesh L, Leung K, Kong WP, Schwartz O, Subbarao K, Nabel GJ. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werner J, Metz AE, Horn D, Faro-Trindade I, Schoeb TR, Hewitt MM, Schwiebert LM, Brown GD, Steele C. Requisite role for the Dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viriyakosol S, Jimenez MP, Gurney MA, Ashbaugh ME, Fierer J. Dectin-1 is required for resistance to coccidioidomycosis in mice. MBio. 2013;4 doi: 10.1128/mBio.00597-12. e00597-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steele C, Marrero L, Swain S, Harmsen AG, Zheng M, Brown GD, Gordon S, Shellito JE, Kolls JK. Alveolar Macrophage-mediated Killing of Pneumocystis carinii f. sp. muris Involves Molecular Recognition by the Dectin-1 {beta}-Glucan Receptor. J. Exp. Med. 2003;198:1677–1688. doi: 10.1084/jem.20030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yadav M, Schorey JS. The beta-glucan receptor dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood. 2006;108:3168–3175. doi: 10.1182/blood-2006-05-024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Das R, Koo MS, Kim BH, Jacob ST, Subbian S, Yao J, Leng L, Levy R, Murchison C, Burman WJ, Moore CC, Scheld WM, David JR, Kaplan G, MacMicking JD, Bucala R. Macrophage migration inhibitory factor (MIF) is a critical mediator of the innate immune response to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E2997–E3006. doi: 10.1073/pnas.1301128110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marakalala MJ, Guler R, Matika L, Murray G, Jacobs M, Brombacher F, Rothfuchs AG, Sher A, Brown GD. The Syk/CARD9-coupled receptor Dectin-1 is not required for host resistance to Mycobacterium tuberculosis in mice. Microbes. Infect. 2011;13:198–201. doi: 10.1016/j.micinf.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fei M, Bhatia S, Oriss TB, Yarlagadda M, Khare A, Akira S, Saijo S, Iwakura Y, Fallert Junecko BA, Reinhart TA, Foreman O, Ray P, Kolls J, Ray A. TNF-alpha from inflammatory dendritic cells (DCs) regulates lung IL-17A/IL-5 levels and neutrophilia versus eosinophilia during persistent fungal infection. Proc Natl Acad Sci U S A. 2011;108:5360–5365. doi: 10.1073/pnas.1015476108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lilly LM, Gessner MA, Dunaway CW, Metz AE, Schwiebert L, Weaver CT, Brown GD, Steele C. The beta-Glucan Receptor Dectin-1 Promotes Lung Immunopathology during Fungal Allergy via IL-22. J Immunol. 2012;189:3653–3660. doi: 10.4049/jimmunol.1201797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramaprakash H, Ito T, Standiford TJ, Kunkel SL, Hogaboam CM. TLR9 modulates immune responses to Aspergillus fumigatus conidia in immunodeficient and allergic mice. Infect Immun. 2009;77:108–119. doi: 10.1128/IAI.00998-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moreira AP, Cavassani KA, Ismailoglu UB, Hullinger R, Dunleavy MP, Knight DA, Kunkel SL, Uematsu S, Akira S, Hogaboam CM. The protective role of TLR6 in a mouse model of asthma is mediated by IL-23 and IL-17A. J Clin Invest. 2011;121:4420–4432. doi: 10.1172/JCI44999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mintz-Cole RA, Gibson AM, Bass SA, Budelsky AL, Reponen T, Hershey GK. Dectin-1 and IL-17A suppress murine asthma induced by Aspergillus versicolor but not Cladosporium cladosporioides due to differences in beta-glucan surface exposure. J Immunol. 2012;189:3609–3617. doi: 10.4049/jimmunol.1200589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mintz-Cole RA, Brandt EB, Bass SA, Gibson AM, Reponen T, Khurana Hershey GK. Surface availability of beta-glucans is critical determinant of host immune response to Cladosporium cladosporioides. J Allergy Clin Immunol. 2013;132:159–169. doi: 10.1016/j.jaci.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willment JA, Gordon S, Brown GD. Characterization of the human beta-glucan receptor and its alternatively spliced isoforms. J Biol Chem. 2001;276:43818–43823. doi: 10.1074/jbc.M107715200. [DOI] [PubMed] [Google Scholar]
- 56.Schlesinger LS, Kaufman TM, Iyer S, Hull SR, Marchiando LK. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol. 1996;157:4568–4575. [PubMed] [Google Scholar]
- 57.Zamze S, Martinez-Pomares L, Jones H, Taylor PR, Stillion RJ, Gordon S, Wong SY. Recognition of bacterial capsular polysaccharides and lipopolysaccharides by the macrophage mannose receptor. J Biol. Chem. 2002;277:41613–41623. doi: 10.1074/jbc.M207057200. [DOI] [PubMed] [Google Scholar]
- 58.Dan JM, Kelly RM, Lee CK, Levitz SM. Role of the mannose receptor in a murine model of Cryptococcus neoformans infection. Infect. Immun. 2008;76:2362–2367. doi: 10.1128/IAI.00095-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang SS, Park CG, Zhang P, Bartra SS, Plano GV, Klena JD, Skurnik M, Hinnebusch BJ, Chen T. Plasminogen activator Pla of Yersinia pestis utilizes murine DEC-205 (CD205) as a receptor to promote dissemination. J Biol. Chem. 2008;283:31511–31521. doi: 10.1074/jbc.M804646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Do Y, Didierlaurent AM, Ryu S, Koh H, Park CG, Park S, Perlin DS, Powell BS, Steinman RM. Induction of pulmonary mucosal immune responses with a protein vaccine targeted to the DEC-205/CD205 receptor. Vaccine. 2012;30:6359–6367. doi: 10.1016/j.vaccine.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dong H, Stanek O, Salvador FR, Langer U, Morillon E, Ung C, Sebo P, Leclerc C, Majlessi L. Induction of protective immunity against Mycobacterium tuberculosis by delivery of ESX antigens into airway dendritic cells. Mucosal. Immunol. 2013;6:522–534. doi: 10.1038/mi.2012.92. [DOI] [PubMed] [Google Scholar]
- 62.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 63.Dunne DW, Resnick D, Greenberg J, Krieger M, Joiner KA. The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proc. Natl. Acad. Sci. U. S. A. 1994;91:1863–1867. doi: 10.1073/pnas.91.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arredouani MS, Yang Z, Imrich A, Ning Y, Qin G, Kobzik L. The macrophage scavenger receptor SR-AI/II and lung defense against pneumococci and particles. Am. J Respir. Cell Mol. Biol. 2006;35:474–478. doi: 10.1165/rcmb.2006-0128OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ozment TR, Ha T, Breuel KF, Ford TR, Ferguson DA, Kalbfleisch J, Schweitzer JB, Kelley JL, Li C, Williams DL. Scavenger receptor class a plays a central role in mediating mortality and the development of the pro-inflammatory phenotype in polymicrobial sepsis. PLoS Pathog. 2012;8:e1002967. doi: 10.1371/journal.ppat.1002967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu Y, Dayrit JK, Davis MJ, Carolan JF, Osterholzer JJ, Curtis JL, Olszewski MA. Scavenger receptor A modulates the immune response to pulmonary Cryptococcus neoformans infection. J Immunol. 2013;191:238–248. doi: 10.4049/jimmunol.1203435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blanchet C, Jouvion G, Fitting C, Cavaillon JM, Adib-Conquy M. Protective or deleterious role of scavenger receptors SR-A and CD36 on host resistance to Staphylococcus aureus depends on the site of infection. PLoS One. 2014;9:e87927. doi: 10.1371/journal.pone.0087927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arredouani M, Yang Z, Ning Y, Qin G, Soininen R, Tryggvason K, Kobzik L. The scavenger receptor MARCO is required for lung defense against pneumococcal pneumonia and inhaled particles. J Exp. Med. 2004;200:267–272. doi: 10.1084/jem.20040731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Palecanda A, Paulauskis J, Al-Mutairi E, Imrich A, Qin G, Suzuki H, Kodama T, Tryggvason K, Koziel H, Kobzik L. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J Exp. Med. 1999;189:1497–1506. doi: 10.1084/jem.189.9.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arredouani MS, Franco F, Imrich A, Fedulov A, Lu X, Perkins D, Soininen R, Tryggvason K, Shapiro SD, Kobzik L. Scavenger Receptors SR-AI/II and MARCO limit pulmonary dendritic cell migration and allergic airway inflammation. J Immunol. 2007;178:5912–5920. doi: 10.4049/jimmunol.178.9.5912. [DOI] [PubMed] [Google Scholar]
- 71.Ghosh S, Gregory D, Smith A, Kobzik L. MARCO regulates early inflammatory responses against influenza: a useful macrophage function with adverse outcome. Am J Respi Cell Mol Biol. 2011;45:1036–1044. doi: 10.1165/rcmb.2010-0349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gowda NM, Wu X, Kumar S, Febbraio M, Gowda DC. CD36 contributes to malaria parasite-induced pro-inflammatory cytokine production and NK and T cell activation by dendritic cells. PLoS One. 2013;8:e77604. doi: 10.1371/journal.pone.0077604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lovegrove FE, Gharib SA, Pena-Castillo L, Patel SN, Ruzinski JT, Hughes TR, Liles WC, Kain KC. Parasite burden and CD36-mediated sequestration are determinants of acute lung injury in an experimental malaria model. PLoS Pathog. 2008;4:e1000068. doi: 10.1371/journal.ppat.1000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drage MG, Pecora ND, Hise AG, Febbraio M, Silverstein RL, Golenbock DT, Boom WH, Harding CV. TLR2 and its co-receptors determine responses of macrophages and dendritic cells to lipoproteins of Mycobacterium tuberculosis. Cell Immunol. 2009;258:29–37. doi: 10.1016/j.cellimm.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Court N, Vasseur V, Vacher R, Fremond C, Shebzukhov Y, Yeremeev VV, Maillet I, Nedospasov SA, Gordon S, Fallon PG, Suzuki H, Ryffel B, Quesniaux VF. Partial redundancy of the pattern recognition receptors, scavenger receptors, and C-type lectins for the long-term control of Mycobacterium tuberculosis infection. J Immunol. 2010;184:7057–7070. doi: 10.4049/jimmunol.1000164. [DOI] [PubMed] [Google Scholar]
- 76.Shimaoka T, Kume N, Minami M, Hayashida K, Sawamura T, Kita T, Yonehara S. LOX-1 supports adhesion of Gram-positive and Gram-negative bacteria. J Immunol. 2001;166:5108–5114. doi: 10.4049/jimmunol.166.8.5108. [DOI] [PubMed] [Google Scholar]
- 77.Zhang P, Liu MC, Cheng L, Liang M, Ji HL, Fu J. Blockade of LOX-1 prevents endotoxin-induced acute lung inflammation and injury in mice. J Innate Immun. 2009;1:358–365. doi: 10.1159/000161070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dieudonne A, Torres D, Blanchard S, Taront S, Jeannin P, Delneste Y, Pichavant M, Trottein F, Gosset P. Scavenger receptors in human airway epithelial cells: role in response to double-stranded RNA. PLoS One. 2012;7:e41952. doi: 10.1371/journal.pone.0041952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shimaoka T, Kume N, Minami M, Hayashida K, Kataoka H, Kita T, Yonehara S. Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR-PSOX, on macrophages. J Biol Chem. 2000;275:40663–40666. doi: 10.1074/jbc.C000761200. [DOI] [PubMed] [Google Scholar]
- 80.Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat. Immunol. 2000;1:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- 81.Lee LN, Ronan EO, de LC, Franken KL, Ottenhoff TH, Tchilian EZ, Beverley PC. CXCR6 is a marker for protective antigen-specific cells in the lungs after intranasal immunization against Mycobacterium tuberculosis. Infect. Immun. 2011;79:3328–3337. doi: 10.1128/IAI.01133-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Germanov E, Veinotte L, Cullen R, Chamberlain E, Butcher EC, Johnston B. Critical role for the chemokine receptor CXCR6 in homeostasis and activation of CD1d-restricted NKT cells. J Immunol. 2008;181:81–91. doi: 10.4049/jimmunol.181.1.81. [DOI] [PubMed] [Google Scholar]
- 83.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bartlett JA, Fischer AJ, McCray PB., Jr Innate immune functions of the airway epithelium. Contrib. Microbiol. 2008;15:147–163. doi: 10.1159/000136349. [DOI] [PubMed] [Google Scholar]
- 85.Sanders CJ, Doherty PC, Thomas PG. Respiratory epithelial cells in innate immunity to influenza virus infection. Cell Tissue Res. 2011;343:13–21. doi: 10.1007/s00441-010-1043-z. [DOI] [PubMed] [Google Scholar]
- 86.Chuquimia OD, Petursdottir DH, Periolo N, Fernandez C. Alveolar epithelial cells are critical in protection of the respiratory tract by secretion of factors able to modulate the activity of pulmonary macrophages and directly control bacterial growth. Infect. Immun. 2013;81:381–389. doi: 10.1128/IAI.00950-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adler KB, Tuvim MJ, Dickey BF. Regulated Mucin Secretion from Airway Epithelial Cells. Front Endocrinol. 2013;4:1–8. doi: 10.3389/fendo.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib. Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vareille M, Kieninger E, Edwards MR, Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin Microbiol. Rev. 2011;24:210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sajjan US. Susceptibility to viral infections in chronic obstructive pulmonary disease: role of epithelial cells. Curr. Opin. Pulm. Med. 2013;19:125–132. doi: 10.1097/MCP.0b013e32835cef10. [DOI] [PubMed] [Google Scholar]
- 91.John G, Yildirim AO, Rubin BK, Gruenert DC, Henke MO. TLR-4-mediated innate immunity is reduced in cystic fibrosis airway cells. Am J Respir. Cell Mol Biol. 2010;42:424–431. doi: 10.1165/rcmb.2008-0408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parker D, Prince A. Epithelial uptake of flagella initiates proinflammatory signaling. PLoS One. 2013;8:e59932-e. doi: 10.1371/journal.pone.0059932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xie XH, Law HK, Wang LJ, Li X, Yang XQ, Liu EM. Lipopolysaccharide induces IL-6 production in respiratory syncytial virus-infected airway epithelial cells through the toll-like receptor 4 signaling pathway. Pediatr. Res. 2009;65:156–162. doi: 10.1203/PDR.0b013e318191f5c6. [DOI] [PubMed] [Google Scholar]
- 94.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 95.Hiraiwa K, van Eeden SF. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediators. Inflamm. 2013;2013:619523. doi: 10.1155/2013/619523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Boorsma CE, Draijer C, Melgert BN. Macrophage heterogeneity in respiratory diseases. Mediators. Inflamm. 2013;2013:769214. doi: 10.1155/2013/769214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179:3488–3494. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 99.Guilliams M, De K, Henri IS, Post S, Vanhoutte L, De PS, Deswarte K, Malissen B, Hammad H, Lambrecht BN. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J Exp. Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van RN, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Empey KM, Hollifield M, Garvy BA. Exogenous heat-killed Escherichia coli improves alveolar macrophage activity and reduces Pneumocystis carinii lung burden in infant mice. Infect. Immun. 2007;75:3382–3393. doi: 10.1128/IAI.00174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mircescu MM, Lipuma L, van RN, Pamer EG, Hohl TM. Essential role for neutrophils but not alveolar macrophages at early time points following Aspergillus fumigatus infection. J Infect. Dis. 2009;200:647–656. doi: 10.1086/600380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moreno G, Errea A, Van ML, Roberts R, Leger H, Sirard JC, Benecke A, Rumbo M, Hozbor D. Toll-like receptor 4 orchestrates neutrophil recruitment into airways during the first hours of Bordetella pertussis infection. Microbes. Infect. 2013;15:708–718. doi: 10.1016/j.micinf.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 105.Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, Taylor-Cousar JL, Saavedra MT, Randell SH, Vasil ML, Burns JL, Moskowitz SM, Nick JA. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One. 2011;6:e23637. doi: 10.1371/journal.pone.0023637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hahn I, Klaus A, Janze AK, Steinwede K, Ding N, Bohling J, Brumshagen C, Serrano H, Gauthier F, Paton JC, Welte T, Maus UA. Cathepsin G and neutrophil elastase play critical and nonredundant roles in lung-protective immunity against Streptococcus pneumoniae in mice. Infect. Immun. 2011;79:4893–4901. doi: 10.1128/IAI.05593-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cai S, Batra S, Lira SA, Kolls JK, Jeyaseelan S. CXCL1 regulates pulmonary host defense to Klebsiella Infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. J Immunol. 2010;185:6214–6225. doi: 10.4049/jimmunol.0903843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cowburn AS, Condliffe AM, Farahi N, Summers C, Chilvers ER. Advances in neutrophil biology: clinical implications. Chest. 2008;134:606–612. doi: 10.1378/chest.08-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hahn S, Giaglis S, Chowdhury CS, Hosli I, Hasler P. Modulation of neutrophil NETosis: interplay between infectious agents and underlying host physiology. Semin. Immunopathol. 2013;35:439–453. doi: 10.1007/s00281-013-0380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guilliams M, Lambrecht BN, Hammad H. Division of labor between lung dendritic cells and macrophages in the defense against pulmonary infections. Mucosal. Immunol. 2013;6:464–473. doi: 10.1038/mi.2013.14. [DOI] [PubMed] [Google Scholar]
- 111.Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol. 2012;30:243–270. doi: 10.1146/annurev-immunol-020711-075021. [DOI] [PubMed] [Google Scholar]
- 112.Helft J, Manicassamy B, Guermonprez P, Hashimoto D, Silvin A, Agudo J, Brown BD, Schmolke M, Miller JC, Leboeuf M, Murphy KM, Garcia-Sastre A, Merad M. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J Clin Invest. 2012;122:4037–4047. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Davidson S, Kaiko G, Loh Z, Lalwani A, Zhang V, Spann K, Foo SY, Hansbro N, Uematsu S, Akira S, Matthaei KI, Rosenberg HF, Foster PS, Phipps S. Plasmacytoid dendritic cells promote host defense against acute pneumovirus infection via the TLR7-MyD88-dependent signaling pathway. J Immunol. 2011;186:5938–5948. doi: 10.4049/jimmunol.1002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schreiber HA, Harding JS, Hunt O, Altamirano CJ, Hulseberg PD, Stewart D, Fabry Z, Sandor M. Inflammatory dendritic cells migrate in and out of transplanted chronic mycobacterial granulomas in mice. J Clin Invest. 2011;121:3902–3913. doi: 10.1172/JCI45113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Prinz I, Silva-Santos B, Pennington DJ. Functional development of gammadelta T cells. Eur. J Immunol. 2013;43:1988–1994. doi: 10.1002/eji.201343759. [DOI] [PubMed] [Google Scholar]
- 117.Augustin A, Kubo RT, Sim GK. Resident pulmonary lymphocytes expressing the gamma/delta T-cell receptor. Nature. 1989;340:239–241. doi: 10.1038/340239a0. [DOI] [PubMed] [Google Scholar]
- 118.Moore TA, Moore BB, Newstead MW, Standiford TJ. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol. 2000;165:2643–2650. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- 119.Saunders BM, Frank AA, Cooper AM, Orme IM. Role of gamma delta T cells in immunopathology of pulmonary Mycobacterium avium infection in mice. Infect. Immun. 1998;66:5508–5514. doi: 10.1128/iai.66.11.5508-5514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheng P, Liu T, Zhou WY, Zhuang Y, Peng LS, Zhang JY, Yin ZN, Mao XH, Guo G, Shi Y, Zou QM. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC. Immunol. 2012;13:38. doi: 10.1186/1471-2172-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nakasone C, Yamamoto N, Nakamatsu M, Kinjo T, Miyagi K, Uezu K, Nakamura K, Higa F, Ishikawa H, O'Brien RL, Ikuta K, Kaku M, Fujita J, Kawakami K. Accumulation of gamma/delta T cells in the lungs and their roles in neutrophil-mediated host defense against pneumococcal infection. Microbes. Infect. 2007;9:251–258. doi: 10.1016/j.micinf.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 122.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, Segal BH, Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 123.Wozniak KL, Kolls JK, Wormley FL., Jr Depletion of neutrophils in a protective model of pulmonary cryptococcosis results in increased IL-17A production by gammadelta T cells. BMC. Immunol. 2012;13:65. doi: 10.1186/1471-2172-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. Innate lymphoid cells--a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 125.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 127.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 128.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. U. S. A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, Kubota M, Turner D, Diamond JM, Goldrath AW, Farber DL, Collman RG, Wherry EJ, Artis D. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, Flynn RJ, Sayers I, Hall IP, McKenzie AN. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 131.Van ML, Carnoy C, Cayet D, Ivanov S, Porte R, Deruy E, Chabalgoity JA, Renauld JC, Eberl G, Benecke AG, Trottein F, Faveeuw C, Sirard JC. Activation of Type 3 Innate Lymphoid Cells and Interleukin 22 Secretion in the Lungs During Streptococcus pneumoniae Infection. J Infect. Dis. 2014 doi: 10.1093/infdis/jiu106. [DOI] [PubMed] [Google Scholar]
- 132.Taube C, Tertilt C, Gyulveszi G, Dehzad N, Kreymborg K, Schneeweiss K, Michel E, Reuter S, Renauld JC, Arnold-Schild D, Schild H, Buhl R, Becher B. IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease. PLoS One. 2011;6:e21799. doi: 10.1371/journal.pone.0021799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim HY, Lee HJ, Chang YJ, Pichavant M, Shore SA, Fitzgerald KA, Iwakura Y, Israel E, Bolger K, Faul J, DeKruyff RH, Umetsu DT. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rotta Detto LJ, Rohmann K, Droemann D, Kujath P, Rupp J, Goldmann T, Dalhoff K. Haemophilus Influenzae Infection Upregulates the NLRP3 Inflammasome and Leads to Caspase-1-Dependent Secretion of Interleukin-1beta - A Possible Pathway of Exacerbations in COPD. PLoS One. 2013;8:e66818. doi: 10.1371/journal.pone.0066818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bartziokas K, Papaioannou AI, Loukides S, Papadopoulos A, Haniotou A, Papiris S, Kostikas K. Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur. Respir. J. 2014;43:43–53. doi: 10.1183/09031936.00209212. [DOI] [PubMed] [Google Scholar]
- 136.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 137.Weidinger S, Klopp N, Rummler L, Wagenpfeil S, Novak N, Baurecht HJ, Groer W, Darsow U, Heinrich J, Gauger A, Schafer T, Jakob T, Behrendt H, Wichmann HE, Ring J, Illig T. Association of NOD1 polymorphisms with atopic eczema and related phenotypes. J Allergy Clin Immunol. 2005;116:177–184. doi: 10.1016/j.jaci.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 138.Kabesch M, Peters W, Carr D, Leupold W, Weiland SK, von ME. Association between polymorphisms in caspase recruitment domain containing protein 15 and allergy in two German populations. J Allergy Clin Immunol. 2003;111:813–817. doi: 10.1067/mai.2003.1336. [DOI] [PubMed] [Google Scholar]
- 139.Ohar JA, Hamilton RF, Jr, Zheng S, Sadeghnejad A, Sterling DA, Xu J, Meyers DA, Bleecker ER, Holian A. COPD is associated with a macrophage scavenger receptor-1 gene sequence variation. Chest. 2010;137:1098–1107. doi: 10.1378/chest.09-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thomsen M, Nordestgaard BG, Tybjaerg-Hansen A, Dahl M. Scavenger receptor AI/II truncation, lung function and COPD: a large population-based study. J Intern Med. 2011;269:340–348. doi: 10.1111/j.1365-2796.2010.02308.x. [DOI] [PubMed] [Google Scholar]
- 141.Freeman CM, Curtis JL, Chensue SW. CC chemokine receptor 5 and CXC chemokine receptor 6 expression by lung CD8+ cells correlates with chronic obstructive pulmonary disease severity. Am J Pathol. 2007;171:767–776. doi: 10.2353/ajpath.2007.061177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Urboniene D, Babusyte A, Lotvall J, Sakalauskas R, Sitkauskiene B. Distribution of gammadelta and other T-lymphocyte subsets in patients with chronic obstructive pulmonary disease and asthma. Respir. Med. 2013;107:413–423. doi: 10.1016/j.rmed.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 143.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hayes E, Pohl K, McElvaney NG, Reeves EP. The cystic fibrosis neutrophil: a specialized yet potentially defective cell. Arch. Immunol Ther. Exp. (Warsz.) 2011;59:97–112. doi: 10.1007/s00005-011-0113-6. [DOI] [PubMed] [Google Scholar]
- 145.Hoenderdos K, Condliffe A. The neutrophil in chronic obstructive pulmonary disease. Am J Respir. Cell Mol Biol. 2013;48:531–539. doi: 10.1165/rcmb.2012-0492TR. [DOI] [PubMed] [Google Scholar]
- 146.Mizgerd JP. Acute lower respiratory tract infection. N Engl J Med. 2008;358:716–727. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]