Abstract
Disease caused by human papillomavirus (HPV) remains common, despite preventive vaccines and screening strategies. Globally, HPVs cause one-third of infection-associated cancers. The indolent clinical course of the precursor intraepithelial lesions provide an opportunity to understand immunologic obstacles posed by the microenvironment of incipient disease, and how they might be overcome. Results from recent therapeutic HPV vaccine clinical trials suggest that relevant immune responses may be sequestered at the lesion site, and are difficult to detect in the circulation. In this Cancer Immunology at the Crossroads article, we outline current understanding of the risk, diagnosis, and treatment of HPV infection-associated cancers, and suggest that quantitative tissue-based endpoints should be included whenever possible in the evaluation of immune-based therapies.
Introduction
On a global scale, approximately 1 in 6 new cancer diagnoses are attributable to an infectious pathogen [1, 2]. Malignancies caused by human papillomaviruses (HPV) comprise one-third of infection-associated cancers, including cancers of the cervix, vagina, vulva, anus, and oropharynx. Today, despite the existence of strategies to either detect or prevent cervical HPV disease, cervical cancers cause 7.5% of cancer deaths in women [3]. Because these cervical neoplastic lesions are relatively accessible, and non-self antigens are required for disease, these lesions present an opportunity to increase our understanding of the mechanisms governing immune responses in the cervical mucosa. Developing immune therapies for disease caused by HPV is important because existing strategies for early detection and prevention of disease are both cumbersome and expensive, and so, HPV disease remains common. In the U.S., the annual cost of HPV disease per birth cohort of girls is estimated to be over $6.5 billion (Table 1) [3-7].
Table 1.
Annual cost of HPV-associated disease for one birth cohort of girls
| Cervical cancer screening* | $5,740,000,000 |
| Cervical cancer | $350,000,000 |
| Other anogenital cancers | $127,000,000 |
| Oropharyngeal cancer | $38,000,000 |
| Anogenital warts | $220,000,000 |
| Respiratory papillomatosis | $151,000,000 |
| TOTAL | >$6.5 BILLION |
Routine screening accounts for ~63% of the cost; follow-up accounts for the other 37%
Cost of vaccinating one birth cohort of girls: ~$1 billion ($500/girl × 2 million girls)
In part, the expense of preventing HPV disease reflects our incomplete understanding of the risk of disease. Most people are exposed to HPVs shortly after the onset of sexual activity, and most of these people clear their infection without intervention nor being aware of being infected [8, 9]. In unvaccinated persons, the lifetime risk of infection at least once with an oncogenic HPV type is estimated to be 80% [10]. Our relatively recent ability to test routine clinical specimens obtained at the time of cervical screening for the presence of oncogenic strains of HPV has presented the clinical conundrum of how to identify early HPV lesions that present a true risk for cancer. Fewer than one-third of women referred for colposcopic evaluation of an abnormal pap smear or a positive HPV test have confirmed disease that requires treatment [11]. No screening algorithms have been validated for HPV disease of the oropharynx, penis, anus, vulva, or vagina. The incidence of HPV-associated malignancies in these anatomic sites for which screening strategies have not yet been validated continues to increase, particularly in the oropharynx [12]. Although the currently available prophylactic vaccines provide protection against HPV16, the genotype most commonly associated with HPV malignancies, to date, uptake has been uneven. Because HPV exposure occurs with the onset of sexual activity, and because the currently available prophylactic vaccines have no therapeutic effect, it is likely that HPV infections will continue to contribute to the global burden of disease for the foreseeable future.
Immune therapies for HPV disease
Both squamous cervical cancers (SCCx) and their immediate precursor lesion, cervical intraepithelial neoplasia 2/3 (CIN2/3), are associated with integration of the viral genome into the host genome, and subsequent functionally obligate, constitutive expression of two viral gene products, E6 and E7 [13, 14]. Expression of both E6 and E7 is required, but not sufficient, for initiation and persistence of disease.
Because CIN lesions are both accessible and clinically indolent, they present an opportunity to explicate mechanisms of immune-mediated clearance of disease.
The need for objective, quantitative methods for tissue studies can be illustrated by the history of immune therapies for HPV disease. To date, although much has been learned about the immunobiology of HPV disease, and preclinical models demonstrate therapeutic efficacy of E6- and E7-specific T cells against solid tumors expressing these antigens, in humans, therapeutic vaccinations targeting the E6 and E7 antigens have yielded limited success. Although E6 and E7 are not “self” antigens, they have shown limited immunogenicity in peripheral blood lymphocytes in the vectors tested to date. Several recent publications, however, suggest that peripheral vaccination can indeed induce immune responses in target lesions. Two trials of therapeutic vaccination in subjects with vulvar intraepithelial neoplasia (VIN) have reported rates of complete regression (CR) that were approximately ten-fold higher than what would be expected without intervention [15, 17]. Untreated, the rate of spontaneous regression of VIN lesions is less than 5% [16]. One trial tested vaccination with long peptides spanning the length of E6 and E7, administered intramuscularly with incomplete Freund’s adjuvant, in subjects with HPV16-positive high grade VIN [17]. This trial reported a 47% CR rate. Another trial tested vaccination with fusion protein (L2E6E7) in sequence with imiquimod, a topical Toll-like receptor (TLR) 7/8 agonist, applied to the lesion [15]. These investigators reported a CR rate of 63%. In both trials an immune response provoked by the immunologic intervention had had effect on the lesions, including peripheral blood T-cell responses to vaccine antigens. However, although the responses were detectable, they required several days of ex vivo expansion for detection. The design of these trials differed from canonical phase I trials in that the 52-week time period between the initial trial intervention and the time of the histologic endpoint was far longer than most other trials. These trials suggest that it takes time for an immune response to be effective, even in a host with only intraepithelial disease, and that relevant immune responses may be recruited to the site of antigen, not lingering in the peripheral blood.
Indeed, recently, we reported evidence of striking changes in target tissues after therapeutic vaccination, in subjects with HPV16+ CIN2/3 [18]. Seven weeks after vaccination with a heterologous DNA prime-recombinant vaccinia virus vector-based boost regimen, although immune responses to vaccine antigens in the blood were increased only modestly, we found lymphoid aggregates composed of proliferating, clonally expanded CD8 T cells that localized specifically in residual dysplastic mucosa, in subjects who had residual disease. Intralesional CD8+ infiltrates were increased in residual lesions, which also showed evidence of squamous cell apoptosis. These findings were congruent with the clinical regressions obtained in the VIN clinical trials, suggesting that in the case of immune therapies for intraepithelial HPV disease, that the indolent clinical behavior of these lesions permits a longer period of observation post-treatment that would allow an immune response to eliminate the disease. They also demonstrate the value of including tissue endpoints before and after immunotherapeutic interventions, if at all possible.
Tissue-resident immune cells
Until relatively recently, much of our understanding of the kinetics, functional polarization, lineage commitment, and homeostasis of immune cells has been derived from studies performed on either peripheral blood or splenic lymphocytes. In an adult human, most antigen-experienced T cells reside in peripheral tissues, most of which are non-sterile barrier epithelia [19, 20]. A growing body of literature attests to the concept of tissue tropism of immune memory cells. Most infectious pathogens have a pronounced and specific tropism for a specific tissue site; memory T cells are tropic for the tissue in which they encountered their cognate antigen. Tissue-resident T cells have been described now in many peripheral tissues, including the skin, lung, gastrointestinal tract, central nervous system, liver, and the lower female genital tract [20]. The mechanisms governing the recruitment, functional polarization, potential for functional plasticity, homeostasis, and retention of tissue-resident T cells in humans, are only now beginning to be understood.
Tissue analyses of clinical specimens are limited by the small absolute size of tissue, which becomes available only after pathologic diagnosis. The development of sophisticated, high throughput analytic technologies now provides opportunities to derive a great deal of information directly ex vivo from limited specimens of unmanipulated cells. These studies hold great promise for enhancing our understanding of mechanisms of immune-cell recruitment, repertoire, functional polarization, and cell retention, which must be informed and guided meticulously by tissue image analyses to minimize the odds of deriving heterogeneous data that could obscure or mask a dynamic clinical picture. Although isolation of segregated cell subsets in the epithelial or stromal compartments is time-consuming, these kinds of studies will provide insights regarding interactions between cell types involved in specific histologic contexts. Studies of unmanipulated cells obtained directly from tissue specimens are likely to provide very different insights about mechanisms of immune activation, retention, and homeostasis in the periphery than those that are known from experiments using circulating lymphocytes.
Quantitative digital image analysis of lesions
In HPV-associated disease, all squamous cancers are thought to arise from untreated high-grade squamous intraepithelial lesions. However, in the cervix, not all CIN2/3 lesions progress to invasive cancer. We and others have reported that in a timeframe of 4-6 months, ~35% of CIN2/3 lesions undergo spontaneous regression [22, 23]. CIN2/3 lesions caused by monoinfection with HPV16 are less likely to regress (20-30%). Although regression is mediated presumably by an adaptive immune response, peripheral blood T-cell responses to E6 and E7 are modest, require ex vivo manipulation to be detected, and do not distinguish persons whose lesions regress. This observation raises the question of whether it is possible to identify immune responses in the target lesion that could predict either disease outcome or the likelihood of a clinical response to immune manipulation.
In fact, the intensity of CD8 + T-cell infiltrates in dysplastic cervical epithelium does predict clinical outcome. A prospective cohort study of low-grade squamous intraepithelial lesions (CIN1) reported an association between the intensity of intraepithelial granzyme B+/CD8+ cells and subsequent regression [24]. CIN2/3 lesions in which CD8+ T-cell infiltrates gain access to dysplastic epithelium are more likely to undergo regression than lesions in which the T-cell infiltrates are restricted to the stroma subjacent to the dysplastic epithelium [25]. In contrast, in CIN2/3 lesions that do not regress, CD8 T-cell infiltrates are restricted to the stroma, failing to infiltrate the dysplastic epithelium. This observation is common to many human solid tumors.
The presence of tertiary lymphoid structures in the stroma abutting primary cancers is associated with better prognosis with conventional anticancer treatment modalities in many tumor settings [26-30]. Whether these structures comprise tumor-specific effector cells, or if they arise from tissue-resident immune cells, or are composed of cells recruited from the periphery is not yet well understood. Tertiary lymphoid structures are also observed in autoimmune diseases, such as rheumatoid arthritis, and in many chronic inflammatory conditions, such as inflammatory bowel disease. The question of whether primary immune responses can be generated and maintained in peripheral tissues is of central importance to the fields of autoimmunity and tumor immunology.
One approach to tissue analyses involves using quantitative digital image analysis to inform high throughput molecular studies on single-cell populations. The intensity and co-localization of immune-cell infiltrates of interest can be quantified objectively on a tissue section, using immunohistochemical or immunofluorescence staining and digital image analysis. For example, one could identify regions of interest precisely and distinctly, including the neoplastic epithelium and the immediate subjacent stroma, and the normal epithelium and the stroma immediately subjacent. While immunohistochemical staining can be performed with two colors on a given tissue section, it is now possible to perform multiplex immunofluorescence staining on a single tissue section for as many as 6 or 7 molecular analytes of interest. The intensity and distribution of the different fluors can be deconvoluted to determine spatial relationships, for example, between intra-tumoral (epithelial) CD8+ infiltrates and tissue macrophages in the adjacent stroma. This kind of information can be layered with antibodies that detect changes in methylation, or apoptotic markers, or markers that reflect immune activation, such as PD-L1. This approach is very similar to the idea of flow-cytometry phenotyping of individual cells, except that tissue-based studies provide a histologic context. These quantitative measures can be performed on longitudinally obtained tissue specimens from individual subjects, or in cross-sectional studies to identify histologic configurations that are associated with prognosis, or that could be used to monitor effects of an intervention.
Single-cell laser capture microdissection
Unlike circulating lymphocytes, tissue-resident lymphocytes have undergone fewer replicative cycles, and are thought to have greater potential for functional plasticity [31]. The contributions of stromal cell subsets to lesion persistence or progression are also incompletely understood. The use of high throughput technologies applied to human tissue specimens, to date, has relied on a “grind-and-find” approach. Now, the availability of laser-capture microdissection microscopes that are capable of isolating single cells has opened the door to performing in-depth studies of unmanipulated tissue-resident cell populations. Rapid immunohistochemical staining can be performed on cryosections to identify specific cell populations of interest. Using pattern-recognition software, the stained cells can be identified readily, and isolated directly into polymerase-chain reaction (PCR) tubes for molecular analyses. Although labor-intensive and painstaking, these types of studies will provide data derived from specifically chosen cell subsets in specific histologic contexts. (Table 2)
Table 2.
Image analysis-guided molecular profiling of cell subsets in the tumor microenvironment:
| SPECIMEN TYPE |
METHOD | ANALYSES |
|---|---|---|
| Formalin-fixed paraffin embedded tissue (FFPE) |
Digital image analysis (brightfield or multiplex immunofluorescence) |
quantify intensity, distribution, and colocalization of molecular analytes of interest |
| FFPE or flash- frozen tissue |
Immuno-laser capture microdissection (tissue compartments, or individual cells, or individual structures) |
TCR deep sequencing, clonal analysis, epigenetic profiling, mRNA transcriptomes |
| primary tissue explants |
isolate viable T cells | phenotyping, single cell
TCR sequencing, functional polarization |
| isolate passage one fibroblasts | ||
| endocervical cytobrush specimens |
isolate viable T cells from longitudinally obtained specimens: Single cell toolbox: microengraving, imaging |
phenotyping, cytokine kinetics, RNAseq, FISH |
| peripheral blood (PBMC and plasma) |
ELISPOT, intracellular
cytokine staining, flow cytometry |
quantify antigen-specific response |
| exosome signature |
Clinical trials: intelligent design
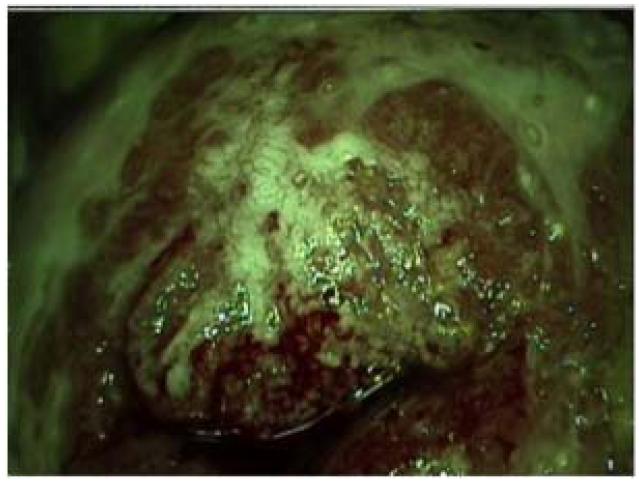
One mechanism by which invasive solid tumors evade effector immune cells is by developing neovasculature that does not express adhesion molecules that mediate egress from the circulation into the tissue. In the clinical setting of cervical HPV disease, high-grade cervical intraepithelial neoplasia can be identified visually using relatively simple technology. A dilute acetic acid wash is applied to the cervix (3% vinegar). Dysplastic cells have a higher nuclear to cytoplasmic ratio than do normal cells, and so dehydrate more readily when exposed to vinegar. When viewed through a low-magnification lens, through a green filter, these dehydrated cells appear white (‘aceto-white’). In addition to aceto-white changes, high-grade lesions are also identified by a pathognomonic pattern of neovasculature, termed “mosaicism”. (FIGURE 1) Cervical T cells express the α4β7 homing integrin. It is now clear that, similar to invasive tumors, one mechanism by which CIN2/3 can prevent recruited effector cells from accessing dysplastic epithelium is because the neovasculature in persistent CIN2/3 has downregulated the expression of MAdCAM, the ligand for α4β7 [25]. This finding underscores the need to activate lesional vascular endothelium, as a therapeutic goal, in combination with other immunotherapeutic modalities.
Figure 1.
Colposcopic appearance of cervical intraepithelial neoplasia 2/3 CIN2/3. Sharply demarcated, dense acetowhite lesion, with pathognomonic pattern of neovasculature, termed “mosaicism”. Adapted from Trimble et al. (25). (Originally published in The Journal of Immunology Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, et al. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol. 2010;185:7107-14. Copyright © 2010 The American Association of Immunologists, Inc.)
Modulating the lesion microenvironment directly
Finally, intraepithelial lesions present an opportunity to learn what is happening with the immune response in either persistent infection, or in the early stages of development of intraepithelial lesions. It is also possible to manipulate the lesion microenvironment directly, for example, with topical TLR agonists. Studies have suggested several mechanisms by which this intervention, applied concurrently, might potentiate an effector cell response. Clark and colleagues demonstrated that in cutaneous squamous cancers, imiquimod appeared to potentiate recruitment of effector cells from the periphery into the epithelial compartment of the lesions, and that this was associated with tumor apoptosis and regression [32]. Cutaneous squamous cancers evade recruited effector cells by downregulating the expression of the adhesion molecule, e-selectin, a ligand that supports the entry of CLA+ T cells into both normal and inflamed skin [33]. Topical imiquimod upregulated the expression of e-selectin, and was associated with an influx of CD8+ T cells expressing the skin-homing integrin, CLA. TNFα also induced the expression of e-selectin on skin microvessels. TLR agonists have also been shown, in some settings, to mediate tumor apoptosis directly [34]. Indeed, in a murine model, investigators have demonstrated that peripheral vaccination with HPV16 E7 long peptides, followed by intravaginal imiquimod, increased by 5-fold the infiltration of vaccine-induced CD8+ T cells in the vaginal mucosa [35]. Currently, our group is conducting a clinical trial in which patients with HPV16+ CIN2/3 undergo peripheral therapeutic vaccination with a heterologous DNA prime-recombinant vaccinia (TA-HPV) boost regimen, and they also receive imiquimod, applied directly to the cervix (NCT00788164).
The beginning of the end of CIN2/3?
In the end, because preinvasive cervical HPV disease is accessible, tissue-based analyses can provide insights into immunologic obstacles posed by the microenvironment of incipient disease, and how they might be overcome. Tissue studies in human HPV disease have already identified some barriers in CIN2/3 that are now a therapeutic target in concert with vaccination. Studies of either regressing or persistent lesions can provide unique insights into how the microenvironment of a developing tumor impacts the host’s ability to successfully or unsuccessfully address the nascent tumor.
Intraepithelial lesions associated with HPVs are clinically indolent, and are also directly accessible – two features that could allow for making clinical management decisions based on tissue monitoring. As colposcopically-directed biopsies are much less invasive or destructive than a cone resection, patients whose lesional biopsies showed evidence of an effector immune response could potentially be monitored and saved from more invasive surgery, whereas patients whose lesions did not manifest an effector response could proceed to therapeutic resection. These kinds of treatment management decisions will be predicated on a better understanding of what constitutes a clinically meaningful tissue immune response.
Acknowledgments
Funding support: R01 CA142691 (Trimble, PI); 1R21CA123876 (Trimble, PI); 2 P50 CA098252 (Trimble, Project 3 PI); 5P30-CA6973-48 (Clinical Staff Investigator).
Footnotes
Conflict of Interest declaration: I have no conflicts of interest to declare.
References
- 1.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol. 2012;13:607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 2.Forman D, de Martel C, Lacey CJ, Soerjomataram I, LOrtet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J, Sgin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg. 1995;121:1386–91. doi: 10.1001/archotol.1995.01890120044008. [DOI] [PubMed] [Google Scholar]
- 5.Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol. 2008;198:500 e1–7. doi: 10.1016/j.ajog.2008.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Insinga RP, Ye X, Singhal PK, Carides GW. Healthcare resource use and costs associated with cervical, vaginal and vulvar cancers in a large U.S. health plan. Gynecol Oncol. 2008;111:188–96. doi: 10.1016/j.ygyno.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 7.Hoy T, Singhal PK, Willey VJ, Insinga RP. Assessing incidence and economic burden of genital warts with data from a US commercially insured population. Curr Med Res Opin. 2009;25:2343–51. doi: 10.1185/03007990903136378. [DOI] [PubMed] [Google Scholar]
- 8.Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998;338:423–8. doi: 10.1056/NEJM199802123380703. [DOI] [PubMed] [Google Scholar]
- 9.Holowaty P, MIller AB, Rohan T, To T. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst. 1999;91:252–8. doi: 10.1093/jnci/91.3.252. [DOI] [PubMed] [Google Scholar]
- 10.Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32(Suppl 1):S16–24. doi: 10.1016/j.jcv.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Whiteside MA, Siegel EM, Unger ER. Human papillomavirus and molecular considerations for cancer risk. Cancer. 2008;113(10 Suppl):2981–94. doi: 10.1002/cncr.23750. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46(4 Suppl):S20–6. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Hudson JB, Bedell MA, McCance DJ, Laiminis LA. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J Virol. 1990;64:519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werness B, Levine A, Howley P. Association of human papillomavirus types 16 and 18 proteins with p53. Science. 1990;248:76–9. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 15.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, Kitchener HC. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer. 2010;102:1129–36. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Seters M, van Beurden M, de Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol. 2005;97:645–51. doi: 10.1016/j.ygyno.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–47. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 18.Maldonado L, Teague JE, Morrow MP, Jotoval I, Wu TC, Wang C, et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med. 2014;6(221):221ra13. doi: 10.1126/scitranslmed.3007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner DL, Gordon CL, Farber DL. Tissue-resident T cells, in situ immunity and transplantation. Immunol Rev. 2014;258:150–66. doi: 10.1111/imr.12149. [DOI] [PubMed] [Google Scholar]
- 20.Farber DL, Yudanin NA, Restifo NP. Human memory T cells: generation, compartmentalization and homeostasis. Nat Rev Immunol. 2014;14:24–35. doi: 10.1038/nri3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14:1294–301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 22.Melnikow J, Nuoco J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol. 1998;92(4 Pt 2):727–35. doi: 10.1016/s0029-7844(98)00245-2. [DOI] [PubMed] [Google Scholar]
- 23.Trimble CL, Piantadosi S, Gravitt P, Ronnett B, Pizer E, Elko A, et al. Spontaneous regression of high-grade cervical dysplasia: effects of human papillomavirus type and HLA phenotype. Clin Cancer Res. 2005;11:4717–23. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo YL, Sterling J, Damay I, Coleman N, Crawford R, van der Burg SH, Stanley M. Characterising the local immune responses in cervical intraepithelial neoplasia: a crosssectional and longitudinal analysis. BJOG. 2008;115:1616–21. doi: 10.1111/j.1471-0528.2008.01936.x. discussion 1621-2. [DOI] [PubMed] [Google Scholar]
- 25.Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, et al. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J Immunol. 2010;185:7107–14. doi: 10.4049/jimmunol.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silina K, Rulle U, Kalnina Z, Kine A. Manipulation of tumour-infiltrating B cells and tertiary lymphoid structures: a novel anti-cancer treatment avenue? Cancer Immunol Immunother. 2014;63:643–62. doi: 10.1007/s00262-014-1544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pimenta EM, Barnes BJ. Role of Tertiary Lymphoid Structures (TLS) in Anti-Tumor Immunity: Potential Tumor-Induced Cytokines/Chemokines that Regulate TLS Formation in Epithelial-Derived Cancers. Cancers (Basel) 2014;6:969–97. doi: 10.3390/cancers6020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goc J, Fridman WH, Sautes-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology. 2013;2:e26836. doi: 10.4161/onci.26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goc J, Fridman WH, Hammond SA, Sautes-Fridman C, Dieu-Nosjean MC. Tertiary lymphoid structures in human lung cancers, a new driver of antitumor immune responses. Oncoimmunology. 2014;3:e28976. doi: 10.4161/onci.28976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pages F, Galon J, Dieu-Nosjean MC, Tartour E, Sautes-Fridman, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 31.Murphy KM. Stockinger B. Effector T cell plasticity: flexibility in the face of changing circumstances. Nat Immunol. 2010;11:674–80. doi: 10.1038/ni.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang SJ, Hijnen D, Murphy GF, Kupper TS, Calarese AW, Mollet IG, et al. Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin. J Invest Dermatol. 2009;129:2676–85. doi: 10.1038/jid.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, et al. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205:2221–34. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salaun B, Coste I, Rissoan MC, Lebecque SJ, Renno T. TLR3 can directly trigger apoptosis in human cancer cells. J Immunol. 2006;176:4894–901. doi: 10.4049/jimmunol.176.8.4894. [DOI] [PubMed] [Google Scholar]
- 35.Domingos-Pereira S, Decrausaz L, Derre L, Bobst M, Romero P, Schiller JT, et al. Intravaginal TLR agonists increase local vaccine-specific CD8 T cells and human papillomavirus-associated genital-tumor regression in mice. Mucosal Immunol. 2013;6:393–404. doi: 10.1038/mi.2012.83. [DOI] [PMC free article] [PubMed] [Google Scholar]