Abstract
Programmed Death Ligand 1 (PD-L1, also known as B7 homolog 1 (B7-H1) or CD274) is a major obstacle to anti-tumor immunity because it (i) tolerizes/anergizes tumor-reactive T cells by binding to its receptor PD-1 (CD279); (ii) renders tumor cells resistant to CD8+ T cell and FasL-mediated lysis; and (iii) tolerizes T cells by reverse signalling through T cell-expressed CD80. PD-L1 is abundantly present in the tumor microenvironment where it is expressed by many malignant cells as well as by immune cells and vascular endothelial cells. The critical role of PD-L1 in obstructing anti-tumor immunity has been demonstrated in multiple animal models and in recent clinical trials. This article reviews the mechanisms by which PD-L1 impairs anti-tumor immunity and discusses established and experimental strategies for maintaining T cell activation in the presence of PD-L1-expressing cells in the tumor microenvironment.
Keywords: Tumor Immunity, Tolerance/Suppression/Anergy, T cells
The Programmed Death-1 (PD-1)2 pathway is essential for maintaining peripheral T cell tolerance, and is critical for attenuating autoimmunity and maintaining T cell homeostasis. However, this pathway is also a deterrent to anti-tumor immunity. Advanced cancer patients who have failed all other therapies have impressive responses when treated with mAbs that block this pathway either as monotherapy or in combination with mAbs that block signaling through CTLA-4 (1-4). The PD-1 pathway includes the receptor, PD-1 (CD279) and two ligands, PD-L1 (programmed death ligand-1; also named B7 homolog 1 (B7-H1) or CD274) and PD-L2 (B7-DC or CD273). The receptor and its ligands are type 1 transmembrane proteins and are members of the B7/CD28 family of ligands and receptors that includes both costimulatory (CD28) and coinhibitory (PD-1, CTLA-4) receptors. The ligands PD-L1 and PD-L2 are coinhibitory, whereas CD80 is costimulatory when bound to CD28, but coinhibitory when bound to CTLA-4 (figure 1A). PD-1 consists of a single extracellular IgV domain, a transmembrane region, and a cytoplasmic domain that includes an ITIM and immunoreceptor tyrosine-based switch motif (ITSM) (5, 6). PD-L1 consists of extracellular IgV and IgC domains, a transmembrane region, and an intracellular domain (7) (figure 1B). Because PD-L1 is an established impediment to antitumor immunity and is either constitutively expressed or induced on most carcinoma cells and can also be expressed by immune cells relevant in tumor immunity (e.g. dendritic cells, myeloid cells, and T cells), this review focuses on the role of the PD-1 pathway in antitumor immunity.
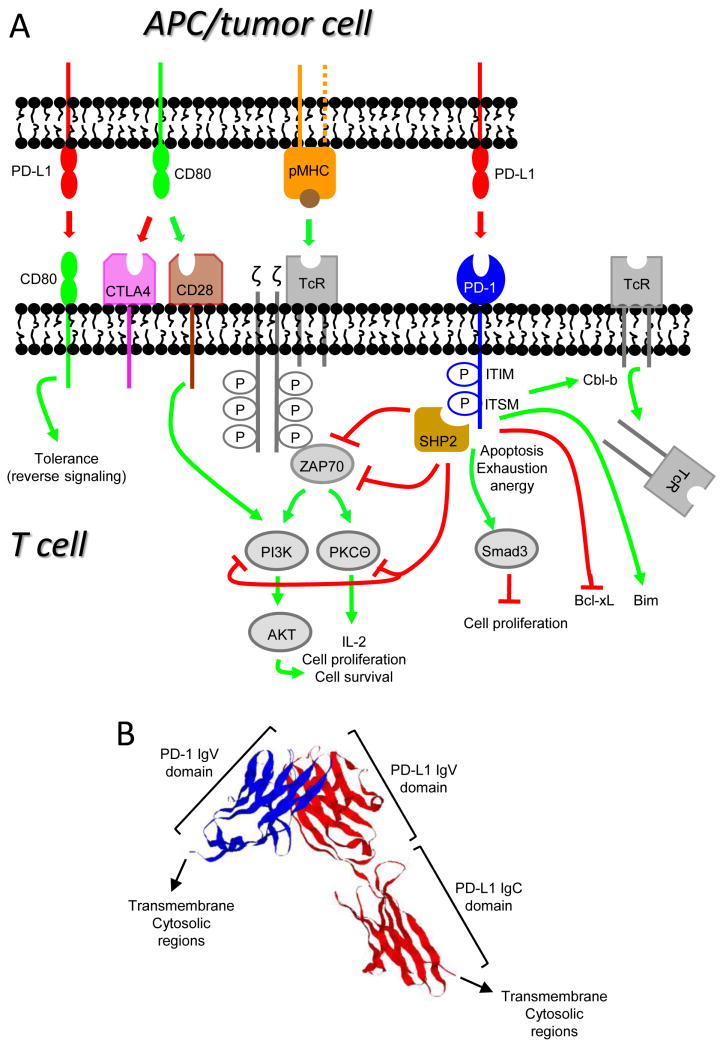
Figure 1. B7 and CD28 family members deliver costimulatory and coinhibitory signals to T cells.
(A) T cells are activated when their TcR and CD28 receive antigen-specific signals delivered by peptide/MHC complexes (pMHC) and costimulatory signals such as CD80 (B7.1), respectively. Activated T cells up-regulate PD-1 and can then be suppressed by interaction with PD-L1+ cells. Signaling through PD-1 results in T cell apoptosis, exhaustion, and/or anergy, and involves phosphorylation of SHP2 which blocks the activation of ZAP70, AKT, PI3K, and PKCΘ which mediate the down-stream events that culminate in activation through the TcR. PD-1 signaling also activates Cbl-b and Smad3 which down-regulate cell surface expression of the TcR and cell proliferation, respectively, inhibits the anti-apoptotic gene Bcl-xL, and activates the pro-apoptotic gene Bim. PD-L1 also tolerizes peripheral T cells by reverse signaling through T cell-expressed CD80. Green and red lines indicate pathways that are activated and suppressed, respectively. (B) Crystal structure of the extracellular PD-L1:PD-1 complex (human PD-L1 and murine PD-1). PD-L1 (shown in red) consists of extracellular IgV and IgC domains. PD-1 (shown in blue) consists of a single extracellular IgV-like domain. (Structure is from (79)).
The PD-1 pathway is a negative regulator of activated T cells
The role of PD-1 in programmed cell death (apoptosis) was first recognized in the early 1990's (5). It was subsequently shown that PD-1 expression on activated T cells results in T cell death, and it was proposed that the autoimmunity observed in PD-1 knockout mice was due to a breakdown of tolerance to self antigens (8). PD-1 and the receptors CD28 and CTLA-4 share structural and functional characteristics, suggesting that the ligand for PD-1 might be similar to the ligands for CD28 and CTLA-4, CD80 (B7.1) and CD86 (B7.2). By screening human and mouse databases for genes with sequence homology to CD80, both the human and mouse ligands for PD-1 were identified (9, 10). Soon after its discovery, PD-L1 was recognized as a cancer immunotherapy target due to its wide-spread expression on many cancer cells, and because blockade of the PD-1 pathway reduced tumor progression, while over-expression of PD-L1 promoted tumor progression in mice (11-14).
Because the PD-1 pathway plays a central role in down-regulating activated T cells in the periphery, it is important during infection and autoimmunity, as well as in tumor immunity. Multiple studies with PD-1-deficient mice demonstrate its critical role in dampening down T cell responses after the clearance of pathogens and in preventing autoimmunity. In contrast to CTLA-4, which predominantly regulates the early stages of T cell activation, PD-1 acts on activated T cells (reviewed in (15)). PD-1 itself is a marker of activated T cells since its expression is induced only after T cell activation. The pathway appears to effect both the ability of activated T cells to kill tumor cells (16), as well as the survival of activated T cells (17).
Both tumor and immune cells express PD-L1 which is regulated at the transcriptional and translational levels
Many human tumor cells either constitutively express or are induced to express PD-L1. These include cervical, pancreatic, urothelial, gastric, esophageal, renal cell, hepatocellular, head and neck squamous cell, ovarian, breast, non-small cell lung, and bladder carcinomas, as well as cutaneous and uveal melanoma, various leukemias, multiple myeloma, and glioma. PD-L1 is present in the cytoplasm and plasma membrane of both mouse and human tumors; however, not all tumors or all cells within a tumor express PD-L1 (14, 18). Activated immune cells including dendritic cells (DC), NK cells, macrophages, monocytes, B cells, and T cells, as well as non-hematopoietic cells may also express PD-L1 (9, 10, 13, 19). PD-L1 expression is induced by multiple pro-inflammatory molecules including Type 1 and Type 2 IFNs, TNFα, LPS, GM-CSF, and VEGF, as well as the cytokines IL-10 and IL-4, with IFNγ being the most potent inducer (20, 21). Since IFNγ and TNFα are produced by activated type 1 T cells, and GM-CSF and VEGF are produced by a variety of tumor stromal cells, the tumor microenvironment up-regulates PD-L1 expression and thereby promotes immune suppression. This latter effect is called “adaptive immune resistance” since the tumor protects itself by inducing PD-L1 in response to IFNγ-produced by activated T cells (18).
PD-L1 is regulated at both the transcriptional and translational levels. Phosphatidylinositol-3 kinase (PI3K) activation of Akt is essential for transcription of PD-L1 mRNA (22), and inhibition of the PI3K pathway reduces the immune resistance of PD-L1+ tumor cells (23) (figure 1A). PD-L1 is also regulated by anaplastic lymphoma kinase (ALK) via the STAT3 transcription factor (24). In addition, the PD-L1 promoter region contains an NF-κB responsive element, and pharmacological inhibition of NF-κB activation inhibits PD-L1 expression (21). At the translational level, PD-L1 expression is suppressed by the phosphatase and tensin homolog (PTEN) gene. Cancer cells frequently contain mutated PTEN, which activates the S6K1 gene. The S6K1 gene, in turn, shifts PD-L1 mRNA to polysomes and hence increases the translation of PD-L1 mRNA and increases plasma membrane expression of PD-L1 (22). Micro RNAs also translationally regulate PD-L1 expression. miRNA513 is complementary to the 3′ untranslated region of PD-L1 and prevents PD-L1 mRNA translation. Treatment with IFNγ down-regulates miRNA513 and thereby facilitates PD-L1 mRNA translation (25).
PD-L1 and PD-1 mediate immune suppression by multiple mechanisms
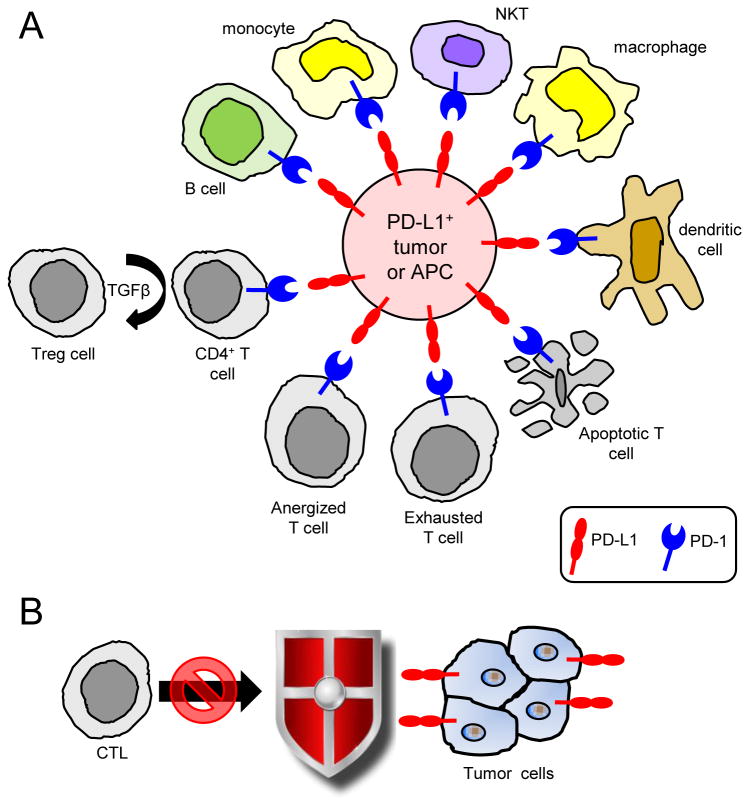
PD-L1 and PD-1 suppress anti-tumor immunity and promote tumor progression by inactivating T cells, protecting tumor cells, and activating tumor-suppressive cell populations (figure 2). Activated NKT (26), B cells (27), and DC (28) can also express PD-1 and be suppressed through the PD-1 pathway. Early studies demonstrated that PD-L1+ murine and human tumor cells induce apoptosis of activated T cells and that antibody blocking of PD-L1 facilitates anti-tumor immunity (13, 14). PD-L1+ APC anergize autoreactive T cells (29) and the anergy is reversed by mAbs to PD-L1 (30). It is likely that the persistence of PD-L1+ tumor cells similarly anergizes, functionally inactivates, and/or tolerizes, PD-1+ tumor-reactive and tumor infiltrating T cells (TIL). Blockade of PD-1 and Tim-3 (T cell immunoglobulin mucin), a marker of T cell exhaustion, reverses exhaustion and reduces tumor growth (31).
Figure 2. Multiple lymphoid and myeloid cell populations express PD-1 and are inhibited by PD-L1+ tumor cells or antigen presenting cells.
(A) Binding of PD-L1+ cells to PD-1+ activated T cells can result in T cell dysfunction by causing T cell anergy, T cell exhaustion, T cell apoptosis, and by inducing the differentiation of T regulatory cells. PD-1 is also expressed by activated B cells, monocytes, NKT cells, macrophages, and dendritic cells and suppresses these cells. (B) PD-L1 also impairs anti-tumor immunity by protecting PD-L1+ tumor cells from CTL, presumably by inhibiting Fas or granzyme B-mediated cytolysis.
Ligand binding to PD-1 blocks the downstream signaling events triggered by antigen/MHC engagement of the TcR and costimulation through CD28, resulting in impaired T cell activation and IL-2 production (Figure 1A). Signaling through the TcR requires phosphorylation of the tyrosine kinase ZAP70. Once docked on phosphorylated tyrosine residues of the TcR-associated CD3ζ chain, phosphorylated ZAP70 activates downstream adapter molecules and enzymes. PD-1 engagement reduces the phosphorylation of ZAP70 and hence inhibits downstream signaling events. PD-1 ligation also prevents phosphorylation of PKCθ which is essential for IL-2 production (32), and arrests T cells in the G1 phase blocking proliferation. PD-1 mediates this affect by activating Smad3, a factor that arrests cycling (33).
PD-1 also reduces T cell survival by impacting apoptotic genes. During T cell activation, CD28 ligation sustains T cell survival by driving expression of the anti-apoptotic gene Bcl-xL. PD-1 prevents Bcl-xL expression by inhibiting PI3K activation which is essential for up-regulation of Bcl-xL. Since CD28-mediated up-regulation of Bcl-xL is relatively unaffected by CTLA-4 ligation, these experiments demonstrated that PD-1 and CTLA-4 suppress via distinct mechanisms (34). Signaling through PD-1 also prevents the conversion of functional CD8+ T effector memory cells into CD8+ central memory cells (35) and thus reduces long-term immune memory which might protect against future metastatic disease. This effect occurs because PD-1 ligation decreases T cells survival by upregulating the pro-apoptotic factor Bim (36).
PD-1 ligation also facilitates down-modulation of the TcR. The TcR is down-regulated as a part of a normal T cell response, and is controlled in part by the E3 ubiquitin ligase Cbl-b (37). PD-L1 binding to PD-1 increases Cbl-b expression and therefore increases TcR down-regulation (38), which may serve as another method tumor cells use to escape T cell immunity. mAb blockade of PD-1 and PD-L1 leads to a decrease in Cbl-b expression, which abrogates TcR internalization.
Microscopy studies revealed some of the morphological events that occur during PD-1-mediated T cell suppression. Successful T cell activation involves the accumulation of naïve T cells around APC. Stable engagement of PD-1 by PD-L1 restricts this homing by limiting T cell mobility, and thus prevents efficient antigen presentation (39). During T cell activation, involved receptors redistribute in the T cell plasma membrane and form peripheral and central supramolecular activation clusters (p-SMAC, c-SMAC). T cell activation involves binding of receptors within the microclusters with ligands on the APC. When bound by ligand, PD-1 translocates to microclusters within the c-SMAC. The phosphatase SHP2 is then transiently bound to the ITSM motif of PD-1 and blocks activation of down-stream mediators (40), including the kinases Akt and PI3K which are activated through CD28 (32).
PD-L1 also promotes tumor progression by protecting PD-L1+ tumor cells from CTL-mediated and Fas-mediated lysis (“molecular shield”)(41), as well as by reverse signaling through CD80 into T cells. Reverse signaling results in tolerizing CD80+ T cells in the periphery and is due to the binding of PD-L1 to T cell-expressed CD80 (42). CD80:PD-L1 interactions restrain self-reactive T cells in an autoimmune setting (43) and therefore their inhibition may facilitate antitumor immunity.
The PD-1 pathway is also involved in generating immune suppressive T regulatory cells (Tregs). APC-expressed PD-L1 induces natural Tregs in the thymus and converts peripheral naïve CD4+ T cells to inducible Tregs (iTregs). The latter conversion involves TGFβ, and is the result of blocking the Akt/mTOR pathway. PD-L1 simultaneously sustains the survival and increases the suppressive activity of iTregs by maintaining and increasing their expression of Foxp3 (44). Engagement of PD-1 also converts mature Th1+ CD4 T cells to Foxp3+ Tregs (45, 46). PD-1 induced expansion of natural T regs is amplified by IFNγ driven up-regulation of PD-L1 and reverse signaling through nTreg CD80 (47).
Expression of PD-L1 may be a biomarker for prognosis
Many immunohistochemical studies have assessed if tumor cell expression of PD-L1 is associated with tumor progression and/or survival (48-54). The majority of these studies concluded that higher expression of PD-L1 in the tumor microenvironment correlates with poor prognosis and reduced survival time and/or tumor grade. These studies used a variety of anti-PD-L1 antibodies, some of which are not optimal for histology. Most of these studies considered cells PD-L1+ if they expressed either cytoplasmic or plasma membrane staining. Since cell surface, but not cytoplasmic, PD-L1 is physiologically relevant, the validity of these reports is controversial. In studies of melanoma (18) and Merkel cell carcinoma (55) patients using the well-validated 5H1 anti-PD-L1 antibody and plasma membrane PD-L1 expression as the criterion, tumor cell expression of PD-L1 positively correlated with improved survival. Using the same antibody and assessment criteria, melanoma and non-small cell lung cancer PD-L1 expression positively correlated with improved survival following PD-1 antibody therapy (2, 56). These latter findings were interpreted as confirming the concept that increased PD-L1 was due to the presence of IFNγ-secreting tumor-reactive TIL. However, studies using the same assessment criteria and anti-PD-L1 antibody, revealed an inverse correlation between renal cell carcinoma expression of PD-L1 and survival (57), and identified PD-L1 as a prognostic indicator of poor survival for urothelial carcinoma patients receiving BCG therapy (58). The differences between these reports could be due to differences between different types of cancer. Additional studies are needed to resolve this issue.
PD-L1 is also present in some cancer patients in a soluble form. Soluble PD-L1 in the serum of some renal cell carcinoma patients induced apoptosis of PD-1+ T cells and correlated with tumors of advanced stage and grade and increased risk of dying (59). Similarly, soluble PD-L1 in the plasma of patients with large B cell lymphoma was identified as a biomarker of poor survival (60).
PD-1 or PD-L1 blockade enhances anti-tumor immunity in mouse models, but combination therapies are more effective
The first report demonstrating that blockade of the PD-1 pathway facilitated tumor rejection used antibodies to PD-L1 and PD-1-deficient mice PD-1 (11). Several studies immediately thereafter confirmed these initial findings (13, 61), and many subsequent studies expanded the efficacy of PD-1 pathway blockade to multiple types of tumors. For example, adoptive transfer of ex vivo activated tumor-specific T cells followed by in vivo treatment with mAbs to PD-L1 delayed tumor progression and increased survival of mice with squamous cell carcinoma (62) and acute myelogenous leukemia (46). In mice with B16 melanoma, simultaneous inhibition of CTLA-4 and PD-1 combined with administration of a Flt3 ligand-transduced tumor cell vaccine (FVAX) dramatically increased survival rates and viable TIL that produced higher levels of IFNγ and TNFα, and increased the ratio of T effector cells to both Tregs and myeloid-derived suppressor cells (MDSC)(63). Similar findings were observed for a combination therapy of mAbs to PD-L1 or PD-1 with a GM-CSF vaccine (GVAX) in mice with CT26 colon carcinoma or ID8 ovarian carcinoma (64). In a murine rhabdomyosarcoma system, monotherapy with anti-PD-1 mAbs had limited therapeutic effect; however, inclusion of mAbs against CXCR2, a receptor expressed by MDSC, synergized with antibody therapy (65).
PD-1 pathway mAb treatment has also been combined with mAb therapies aimed at facilitating T cell activation by triggering costimulatory molecules. Treatment with mAbs to the costimulatory molecule 4-1BB (CD137) has therapeutic efficacy. However, in mice with PD-L1+ tumor cells this therapy is not effective and concomitant treatment with mAbs to PD-1 or PD-L1 is needed (66). CD137 therapy also synergizes with mAbs to PD-1 or PD-L1 and GVAX or FVAX (67). The latter studies are particularly noteworthy because late stage tumors were responsive.
Exhausted/dysfunctional TIL of cancer patients express PD-1 (68), and also express other receptors that inhibit T cell activation. Lymphocyte activation gene (LAG-3, CD223) and Tim-3 are two such additional coinhibitory T cell receptors. Combination therapy with mAbs to PD-1 plus LAG-3 reduced tumor progression of MC38 colon carcinoma and Sa1N fibrosarcoma, and was significantly more effective than either mAb alone. Treatment of melanomas containing few TIL with immunostimulatory RNA polyIC increased type I IFN, which in turn up-regulated PD-L1. Although polyIC by itself temporarily restrained tumor progression, addition of PD-1 blockade extended survival time (69). These studies should be viewed with caution since therapy was usually initiated early during tumor growth when stromal cells and vasculature were likely to be minimal; however, they do indicate that PD-1 pathway inhibition is most effective when combined with treatments that activate the immune system.
PD-1 pathway blockade has also been combined with chemotherapy or radiotherapy. Early treatment of mice with Panc02 adenocarcinoma with gemcitabine, the standard of care for pancreatic cancer, combined with PD-1 blockade increased CD8+ TIL and intratumoral immune activation, and improved survival (70). Combination of irradiation with mAbs to PD-L1 is much more effective than either therapy alone and rendered mice with MC38 colon carcinoma or TUBO breast carcinoma resistant to rechallenge with tumor. The combination therapy is most likely effective since irradiation up-regulates PD-L1 expression in the tumor microenvironment and concomitant administration of mAbs to PD-L1 minimizes this suppression (71).
mAbs to PD-L1 and PD-1 have therapeutic efficacy in some cancer patients
The critical role of the PD-1 pathway in human cancer was firmly established in recent clinical trials in which 17-28% of patients with advanced cancers had partial or complete remissions following treatment with mAbs to PD-L1 or PD-1 (1, 2). Follow-up studies with a subset of patients demonstrated that post-treatment responses lasted as long as three years, and that a partial responder who had tumor recurrence responded to reinduction therapy (72). PD-1 mAb therapy was also effective in 28% of advanced melanoma patients who were non-responsive to Ipilimumab therapy (anti-CTLA-4 mAbs)(73), and Ipilimumab plus anti-PD-1 mAb therapy yielded an objective response rate of 53% (3). These responses are impressive because they occurred in advanced cancer patients who were non-responsive to conventional therapies. However, the response rates also indicate that inhibition of the PD-1 pathway by itself is insufficient for the treatment of all patients.
Novel strategies for inhibiting PD-L1/PD-1-mediated immune suppression: beyond mAbs
Immunotherapy strategies that inhibit the PD-1 pathway are based on the hypothesis that eliminating PD-1-mediated suppression permits the natural development of tumoricidal T cells. These strategies will be effective provided the tumor is sufficiently immunogenic and activated APC can present antigen. However, it is likely that not all cancer patients will have spontaneously activated tumor-reactive T cells and that strategies aimed at directly activating T cells will be needed for optimal antitumor immunity. This concept is supported by murine studies which demonstrated that concurrently blocking the PD-1 pathway and using a vaccine or other means to activate tumor-reactive T cells is more efficacious than PD-1 monotherapy (63, 64, 67). These findings have led to the development of strategies that combine PD-1 pathway blockade and enhancement of antigen presentation in a single reagent.
Soluble CD80 simultaneously blocks the PD-1 pathway and costimulates T cells through CD28
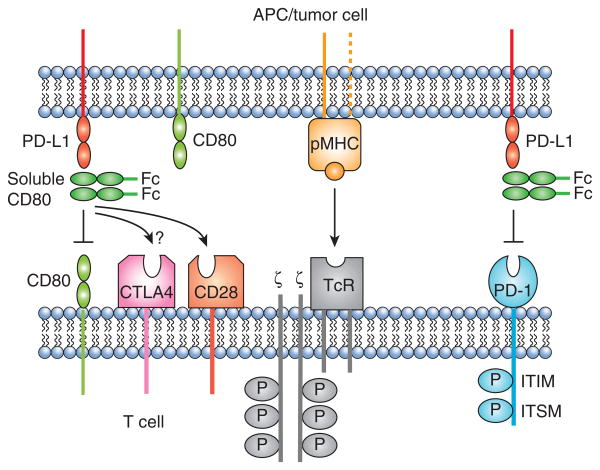
The finding that CD80 binds to PD-L1 with a binding affinity approximately equal to its affinity for CD28 (74) suggested that CD80 could bind to PD-L1 and sterically block PD-1:PD-L1 interactions. Studies with human and mouse tumor cells demonstrated that soluble CD80 (CD80-Fc consisting of the extracellular CD80 IgV and IgC regions fused to an IgG Fc domain) and membrane-bound CD80 prevented PD-1 from binding to PD-L1+ tumor cells and maintained IFNγ production by activated PD-1+ CD4+ and CD8+ T cells (75, 76).
Surprisingly, soluble CD80 was more effective than mAbs to PD-L1 or PD-1 in maintaining T cell activation suggesting that CD80-Fc not only prevented PD-1:PD-L1 binding, but also mediated other effects (76). Studies with CD28-deficient mice and with antibodies that prevented CD80:CD28 binding demonstrated that CD80 also costimulated T cells via CD28 (77). Although not shown experimentally, soluble CD80 may also prevent T cell apoptosis by reverse signaling of PD-L1 into CD80+ T cells, since the binding of soluble CD80 to PD-L1 could block PD-L1 from binding to T cell-expressed CD80. PD-1 mAb cannot prevent reverse signaling; however, PD-L1 mAbs could, provided they block the PD-L1 binding site for CD80.
There are potential drawbacks to soluble CD80 as an immunotherapeutic. Although soluble CD80 has the potential to suppress via CTLA-4, sustained production of IFNγ and blocking studies with mAbs to CTLA-4 in the presence of CD80-Fc suggested that this suppression does not occur (Horn and Ostrand-Rosenberg, unpublished data). The absence of CTLA-4-mediated suppression may be due to the inability of CD80-Fc to cross-link CTLA-4, or because PD-L1 blocks the CTLA-4 binding site on CD80. If the latter is occurring, then it is interesting that the CTLA-4, but not the CD28, binding site on CD80 is affected since the two sites were considered to overlap (78). Figure 3 summarizes the known ligand-receptor interactions that soluble CD80 impacts, as well as additional interactions that soluble CD80 has the potential to impact.
Figure 3. Soluble CD80 may facilitate anti-tumor immunity through three distinct mechanisms.
Soluble CD80 may (i) prevent PD-L1+ cells (tumor cells, lymphoid cells, or other PD-L1+ cells) from anergizing PD-1+ activated T cells; (ii) prevent PD-L1+ cells from anergizing CD80+ T cells by reverse signaling through T cell-expressed CD80; and (iii) enhance T cell activation by costimulating tumor antigen-specific T cells through CD28.
Conclusions
Clinical trials and animal studies have established PD-1 and PD-L1 as major deterrents to T cell-mediated anti-tumor immunity. Other coinhibitory receptor-ligand systems may also contribute to T cell dysfunction in individuals with cancer. However, the PD-1 pathway may play a key role because PD-L1 is expressed by most tumor cells as well as by immune cells and other host cells in the tumor microenvironment. Pre-clinical and on-going clinical trials are demonstrating that treatments combining PD-1 pathway blockade with inhibition of other coinhibitory pathways as well as combination treatments that enhance antigen presentation may yield positive responses in even more patients. The clinical findings are very encouraging and have raised intense interest in cancer immunotherapy. Although much is known about the PD-1 pathway in tumor immunity, many issues remain unexplored. For example, does therapy with mAbs to PD-1 or PD-L1, or soluble CD80 inhibit tolerance induction by reverse signaling by PD-L1 into CD80+ T cells? Does mAb or soluble CD80 therapy generate memory T cells capable of protecting against recurrent disease? Will combining immunotherapies be additive, synergistic, or redundant? Which immune therapies when combined with inhibition of the PD-1 pathway will be most efficacious? Does blockade of the PD-1 pathway result in activation of only tumor-reactive T cells or also T cells that are autoreactive to non-tumor antigens and that could cause undesirable autoimmunity? Will immunotherapies such as soluble CD80 that combine blockade of the PD-1 pathway with costimulation of tumor-reactive T cells in a single reagent be more effective than combinations of monotherapies? Would a soluble CD80 engineered to lack the CTLA-4 binding site provide enhanced T cell activation? Addressing these questions will provide mechanistic information on how blockade of the PD-1 pathway facilitates anti-tumor immunity, and is likely to provide new insights for designing better immunotherapeutics for the treatment of cancer. Thus, additional basic immunology studies are an essential complement to on-going clinical trials.
Footnotes
These studies were supported by NIH RO1CA84232. STH was partially supported by NIH T32 GM066706. LAH was partially supported by US Dept. Education grant P200A090094-11.
Abbreviations used in this paper: ALK: anaplastic lymphoma kinase; B7-H1: B7 homolog 1 (CD274, PD-L1); DC: dendritic cells; FVAX: Flt3 ligand cancer vaccine; GVAX: GM-CSF cancer vaccine; iTregs: inducible T regulatory cells; ITSM: immunoreceptor tyrosine switch motif; LAG-3: lymphocyte activating gene 3; MDSC: myeloid-derived suppressor cells; nTreg: natural T regulatory cells; PD-1: programmed death-1; PD-L1: programmed death ligand-1; PTEN: phosphatase and tensin homolog gene; SMAC: supramolecular activation cluster; TIL: tumor-infiltrating T cells; Tim-3: T cell immunoglobulin mucin
References
- 1.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, Burke MM, Caldwell A, Kronenberg SA, Agunwamba BU, Zhang X, Lowy I, Inzunza HD, Feely W, Horak CE, Hong Q, Korman AJ, Wigginton JM, Gupta A, Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, Leming PD, Lipson EJ, Puzanov I, Smith DC, Taube JM, Wigginton JM, Kollia GD, Gupta A, Pardoll DM, Sosman JA, Hodi FS. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol. 2014;32:1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinohara T, Taniwaki M, Ishida Y, Kawaichi M, Honjo T. Structure and chromosomal localization of the human PD-1 gene (PDCD1) Genomics. 1994;23:704–706. doi: 10.1006/geno.1994.1562. [DOI] [PubMed] [Google Scholar]
- 7.Freeman GJ. Structures of PD-1 with its ligands: sideways and dancing cheek to cheek. Proc Natl Acad Sci U S A. 2008;105:10275–10276. doi: 10.1073/pnas.0805459105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 9.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion 10.1038/70932. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 10.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, Greenfield EA, Freeman GJ. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 13.Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, Krzysiek R, Knutson KL, Daniel B, Zimmermann MC, David O, Burow M, Gordon A, Dhurandhar N, Myers L, Berggren R, Hemminki A, Alvarez RD, Emilie D, Curiel DT, Chen L, Zou W. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med. 2003;9:562–567. doi: 10.1038/nm863. [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 15.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 17.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, Chen L. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saudemont A, Jouy N, Hetuin D, Quesnel B. NK cells that are activated by CXCL10 can kill dormant tumor cells that resist CTL-mediated lysis and can express B7-H1 that stimulates T cells. Blood. 2005;105:2428–2435. doi: 10.1182/blood-2004-09-3458. [DOI] [PubMed] [Google Scholar]
- 20.Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res. 2013;19:1021–1034. doi: 10.1158/1078-0432.CCR-12-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo A, Yamashita T, Tamura H, Zhao W, Tsuji T, Shimizu M, Shinya E, Takahashi H, Tamada K, Chen L, Dan K, Ogata K. Interferon-gamma and tumor necrosis factor-alpha induce an immunoinhibitory molecule, B7-H1, via nuclear factor-kappaB activation in blasts in myelodysplastic syndromes. Blood. 2010;116:1124–1131. doi: 10.1182/blood-2009-12-255125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC, Mischel PS, Stokoe D, Pieper RO. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 23.Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, Simko JP, Waldman FM, Pieper RO, Parsa AT. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28:306–312. doi: 10.1038/onc.2008.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, Wang HY, Wysocka M, Cheng M, Ruggeri BA, Wasik MA. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci U S A. 2008;105:20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gong AY, Zhou R, Hu G, Li X, Splinter PL, O'Hara SP, LaRusso NF, Soukup GA, Dong H, Chen XM. MicroRNA-513 regulates B7-H1 translation and is involved in IFN-gamma-induced B7-H1 expression in cholangiocytes. J Immunol. 2009;182:1325–1333. doi: 10.4049/jimmunol.182.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Cheng L, Wondimu Z, Swain M, Santamaria P, Yang Y. Cutting edge: CD28 engagement releases antigen-activated invariant NKT cells from the inhibitory effects of PD-1. J Immunol. 2009;182:6644–6647. doi: 10.4049/jimmunol.0804050. [DOI] [PubMed] [Google Scholar]
- 27.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao S, Wang S, Zhu Y, Luo L, Zhu G, Flies S, Xu H, Ruff W, Broadwater M, Choi IH, Tamada K, Chen L. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113:5811–5818. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selenko-Gebauer N, Majdic O, Szekeres A, Hofler G, Guthann E, Korthauer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, Knapp W, Stockl J. B7-H1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol. 2003;170:3637–3644. doi: 10.4049/jimmunol.170.7.3637. [DOI] [PubMed] [Google Scholar]
- 30.Tsushima F, Yao S, Shin T, Flies A, Flies S, Xu H, Tamada K, Pardoll DM, Chen L. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, Chaudhary D. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 33.Patsoukis N, Sari D, Boussiotis VA. PD-1 inhibits T cell proliferation by upregulating p27 and p15 and suppressing Cdc25A. Cell Cycle. 2012;11:4305–4309. doi: 10.4161/cc.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charlton JJ, Chatzidakis I, Tsoukatou D, Boumpas T, Garinis GA, Mamalaki C. Programmed death-1 shapes memory phenotype CD8 T cell subsets in a cell-intrinsic manner. J Immunol. 2013;190:6104–6114. doi: 10.4049/jimmunol.1201617. [DOI] [PubMed] [Google Scholar]
- 36.Gibbons RM, Liu X, Pulko V, Harrington SM, Krco CJ, Kwon ED, Dong H. B7-H1 limits the entry of effector CD8 (+) T cells to the memory pool by upregulating Bim. Oncoimmunology. 2012;1:1061–1073. doi: 10.4161/onci.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shamim M, Nanjappa SG, Singh A, Plisch EH, LeBlanc SE, Walent J, Svaren J, Seroogy C, Suresh M. Cbl-b regulates antigen-induced TCR down-regulation and IFN-gamma production by effector CD8 T cells without affecting functional avidity. J Immunol. 2007;179:7233–7243. doi: 10.4049/jimmunol.179.11.7233. [DOI] [PubMed] [Google Scholar]
- 38.Karwacz K, Bricogne C, MacDonald D, Arce F, Bennett CL, Collins M, Escors D. PD-L1 co-stimulation contributes to ligand-induced T cell receptor down-modulation on CD8+ T cells. EMBO Mol Med. 2011;3:581–592. doi: 10.1002/emmm.201100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S, Liu Y, Strome SE, Chen L, Tamada K. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paterson AM, Brown KE, Keir ME, Vanguri VK, Riella LV, Chandraker A, Sayegh MH, Blazar BR, Freeman GJ, Sharpe AH. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol. 2011;187:1097–1105. doi: 10.4049/jimmunol.1003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, Levine BL, June CH, Medin JA, Fowler DH. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3:111ra120. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, Sharpe AH, Vallera DA, Azuma M, Levine BL, June CH, Murphy WJ, Munn DH, Blazar BR. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116:2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yi T, Li X, Yao S, Wang L, Chen Y, Zhao D, Johnston HF, Young JS, Liu H, Todorov I, Forman SJ, Chen L, Zeng D. Host APCs augment in vivo expansion of donor natural regulatory T cells via B7H1/B7.1 in allogeneic recipients. J Immunol. 2011;186:2739–2749. doi: 10.4049/jimmunol.1002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 49.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, Ajarim D, Al-Tweigeri T, Dermime S. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 52.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56:1173–1182. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 54.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Lipson EJ, Vincent JG, Loyo M, Kagohara LT, Luber BS, Wang H, Xu H, Nayar SK, Wang TS, Sidransky D, Anders RA, Topalian SL, Taube JM. PD-L1 expression in the Merkel cell carcinoma microenvironment: association with inflammation, Merkel cell polyomavirus and overall survival. Cancer Immunol Res. 2013;1:54–63. doi: 10.1158/2326-6066.CIR-13-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, Sengupta S, Frank I, Parker AS, Zincke H, Blute ML, Sebo TJ, Cheville JC, Kwon ED. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 58.Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, Fradet Y, Lacombe L, Kwon ED. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109:1499–1505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 59.Frigola X, Inman BA, Lohse CM, Krco CJ, Cheville JC, Thompson RH, Leibovich B, Blute ML, Dong H, Kwon ED. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–1923. doi: 10.1158/1078-0432.CCR-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossille D, Gressier M, Damotte D, Maucort-Boulch D, Pangault C, Semana G, Le Gouill S, Haioun C, Tarte K, Lamy T, Milpied N, Fest T. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014 doi: 10.1038/leu.2014.137. epub ahead of print 10.1038/leu.2014.137. [DOI] [PubMed] [Google Scholar]
- 61.Iwai Y, Terawaki S, Honjo T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int Immunol. 2005;17:133–144. doi: 10.1093/intimm/dxh194. [DOI] [PubMed] [Google Scholar]
- 62.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, Salomao D, Cheville J, Hirano F, Lin W, Kasperbauer JL, Ballman KV, Chen L. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 63.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duraiswamy J, Kaluza KM, Freeman GJ, Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73:3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, Kaplan RN, Mackall CL. Disruption of CXCR2-Mediated MDSC Tumor Trafficking Enhances Anti-PD1 Efficacy. Sci Transl Med. 2014;6:237ra267. doi: 10.1126/scitranslmed.3007974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 67.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–6912. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, Rosenberg SA. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bald T, Landsberg J, Lopez-Ramos D, Renn M, Glodde N, Jansen P, Gaffal E, Steitz J, Tolba R, Kalinke U, Limmer A, Jonsson G, Holzel M, Tuting T. Immune Cell-Poor Melanomas Benefit from PD-1 Blockade after Targeted Type I IFN Activation. Cancer Discov. 2014;4:674–687. doi: 10.1158/2159-8290.CD-13-0458. [DOI] [PubMed] [Google Scholar]
- 70.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 71.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lipson EJ, Sharfman WH, Drake CG, Wollner I, Taube JM, Anders RA, Xu H, Yao S, Pons A, Chen L, Pardoll DM, Brahmer JR, Topalian SL. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, Dronca R, Gangadhar TC, Patnaik A, Zarour H, Joshua AM, Gergich K, Elassaiss-Schaap J, Algazi A, Mateus C, Boasberg P, Tumeh PC, Chmielowski B, Ebbinghaus SW, Li XN, Kang SP, Ribas A. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haile S, Bosch JJ, Agu N, Zeender A, Somasundaram P, Srivastava MK, Rodel S, Wolf J, Ksander BR, Ostrand-Rosenberg S. Tumor cell programmed death ligand-1-mediated T cell suppression is overcome by co-expression of CD80. J Immunol. 2011;186:6822–6829. doi: 10.4049/jimmunol.1003682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haile ST, Dalal SP, Clements V, Tamada K, Ostrand-Rosenberg S. Soluble CD80 restores T cell activation and overcomes tumor cell programmed death ligand 1-mediated immune suppression. J Immunol. 2013;191:2829–2836. doi: 10.4049/jimmunol.1202777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haile S, Horn LH, Ostrand-Rosenberg S. A Soluble Form of CD80 Enhances Anti-tumor Immunity by Neutralizing Programmed Death Ligand-1 and Simultaneously Providing Costimulation. Cancer Immunol Res. 2014;2:610–615. doi: 10.1158/2326-6066.CIR-13-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peach RJ, Bajorath J, Naemura J, Leytze G, Greene J, Aruffo A, Linsley PS. Both extracellular immunoglobin-like domains of CD80 contain residues critical for binding T cell surface receptors CTLA-4 and CD28. J Biol Chem. 1995;270:21181–21187. doi: 10.1074/jbc.270.36.21181. [DOI] [PubMed] [Google Scholar]
- 79.Lin DY, Tanaka Y, Iwasaki M, Gittis AG, Su HP, Mikami B, Okazaki T, Honjo T, Minato N, Garboczi DN. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci U S A. 2008;105:3011–3016. doi: 10.1073/pnas.0712278105. [DOI] [PMC free article] [PubMed] [Google Scholar]