Abstract
Hilar cholangiocarcinoma (CCA) is a difficult malignancy to treat surgically given its anatomical location and its frequent association with primary sclerosing cholangitis (PSC). Neoadjuvant chemoradiotherapy followed by liver transplantation in lymph node negative patients has been advanced by select liver transplant centers for treatment of patients with unresectable disease. This approach has most commonly used external beam radiotherapy combined with biliary brachytherapy and 5-FU based chemotherapy. Our center has recently embarked on a protocol utilizing stereotactic body radiation therapy (SBRT) followed by capecitabine in lymph node negative patients until liver transplantation. We therefore retrospectively determined tolerability and pathologic response in this pilot study. Over a three year period 17 patients with unresectable hilar CCA were evaluated for treatment under this protocol. In all, 12 patients qualified for neoadjuvant therapy and were treated with SBRT (50–60 Gy, 3–5 fractions over two weeks). Following one week of rest, capecitabine was initiated at 1330 mg/m2/day and continued until liver transplantation. During neoadjuvant therapy, there were a total of 35 adverse events with cholangitis and palmar/plantar erythrodysesthesia being the most common. Capecitabine dose reductions were required on 5 occasions. Ultimately, 9 patients were listed for transplant and 6 patients received a liver transplant. Explant pathology of hilar tumors showed at least a partial treatment response in five patients with extensive tumor necrosis and fibrosis noted. Additionally, high apoptotic and low proliferative indices were measured on histological examination. Eleven transplant-related complications occurred, and one-year survival after transplant was 83%. In this pilot study, neoadjuvant therapy with SBRT, capecitabine, and liver transplantation for unresectable CCA demonstrated acceptable tolerability. Further studies will determine the overall future efficacy of this therapy.
Keywords: Malignancy/liver transplantation, sclerosing cholangitis, bile duct cancer, biliary neoplasms, external beam radiotherapy
Introduction
Treatment of hilar cholangiocarcinoma (CCA) is most effective when hepatic resection is performed and negative surgical margins can be achieved. Unfortunately, only 35–40% of presenting patients are able to undergo resection secondary to bilateral vascular/biliary involvement, metastatic disease, or underlying hepatic disease including primary sclerosing cholangitis (PSC) (1). Pioneering work by the Mayo Rochester group demonstrated the feasibility and efficacy of using neoadjuvant chemoradiotherapy, staging for negative hilar lymph nodes, and followed by orthotopic liver transplantation (OLT) (2, 3). This therapeutic approach to hilar CCA has resulted in survival rates that are similar to patients receiving OLT for other forms of liver disease (4). Therefore in 2008, the United Network for Organ Sharing (UNOS) began to accept listing of unresectable hilar CCA patients provided that centers had an established protocol utilizing neoadjuvant chemoradiotherapy along with demonstration of negative lymph node status for metastatic disease.
Recent neoadjuvant chemoradiotherapy protocols have primarily used a combination of external beam radiotherapy (given over several weeks) along with brachytherapy via biliary catheterization to a total dose of 45–55 Gy (4). Chemotherapy has traditionally been 5-FU infusion based followed by capecitibine following radiation therapy. Our center has developed the novel use of stereotactic body radiation therapy (SBRT) in the treatment of hepatic malignancies (5, 6). Safety and efficacy has been demonstrated for these cancers often in many patients with underlying liver disease or cirrhosis (5, 7). SBRT has the advantage to deliver high doses of radiotherapy to confined areas while sparing toxicity to surrounding structures or parenchyma. SBRT has the added advantage of therefore allowing a shorter treatment course, often in 3–5 fractions in a two week period.
We therefore decided to utilize our center’s expertise in SBRT, a recently developed therapy in treatment of hepatic malignancies, in our neoadjuvant chemoradiotherapy protocol followed by OLT for patients with unresectable CCA and lymph node negative disease. The aims of this pilot study were to determine the 1) overall tolerability of this regimen with respect to side effects and adverse events and 2) the pathologic response by histologic evaluation of transplant explanted specimens. We also sought to examine tolerability as it was related to reaching successful transplantation and any influence on transplant related complications. While significant adverse events (SAEs) occurred, these were often well tolerated as nine patients were listed and six of these were transplanted to date. Significant tumor responses were noted on histology suggesting that this regimen utilizing SBRT results in acceptable tumor control with tolerable side effects until definitive therapy with transplantation.
Methods
Patient Selection
This retrospective study was approved by the University of Michigan Institutional Review Board (IRB). Patients with hilar CCA were identified by review in our Multidisciplinary Liver Tumor Clinic and Board. Patients’ diagnosis and treatment followed an existing, Transplant Committee treatment protocol at the University of Michigan. Diagnosis and inclusion criteria were similar to that described by the Mayo Rochester group previously (2, 3). Patients were required to have a malignant appearing hilar biliary stricture above the cystic duct and a CA19-9 level greater than 100 ng/mL, transcatheter biopsy/brush cytology positive for adenocarcinoma, or an associated mass on cross sectional imaging 3.0 cm or less in maximal radial diameter. Patients who were determined to be unresectable based upon bilateral vascular or biliary involvement or due to underlying liver disease (PSC) were further evaluated by our Multdisciplinary Liver Transplant Committee. Staging included CT or MRI of the abdomen and CT of the chest. PET was used selectively for indeterminate lesions noted on initial cross-sectional imaging. Patients were excluded if they had any overt evidence of distant disease or regional lymph node metastasis. Patients were likewise excluded if there was any prior attempted surgical resection, open or percutaneous biopsy, or prior treatment. Finally, endoscopic ultrasound (EUS) with fine needle aspiration of hilar lymph nodes was routinely performed and, if positive, patients were excluded from entry into the protocol (8).
Neoadjuvant Protocol and Transplantation
Patients found to be acceptable transplant candidates and meeting inclusion criteria for the neoadjuvant protocol were treated with SBRT at 50–60 Gy total dose divided over 3–5 fractions (10–20 Gy/fraction) (5, 6) as our routine institutional practice. Briefly, patients underwent CT simulation with a customized vacuum body mold. Active breathing control was utilized to eliminate breathing-related tumor motion. Tumors were delineated on MRI, and these images were fused with the CT for planning. To account for setup variation, 5mm radial and 8 mm superior and inferior margins were added to generate the planning target volume (PTV). SBRT was delivered using 8–16 non-opposed, non-coplanar, static 6 and 16 MV photon beams. Radiation dose was prescribed to the isodose surface covering 99.5% of the PTV (typically 75–85% of maximum dose). Daily cone beam CT was utilized prior to each treatment for image-guidance. SBRT was completed in two weeks and following a one week rest, patients were initiated on capecitabine 1330 mg/m2/day in two divided oral doses rounded to the nearest 500 mg. Staging operation was performed 4–6 weeks after initiation of SBRT following a three day hold of capecitabine. The staging operation was performed in a completely laparoscopic or laparoscopic assisted manner. Examination for extrahepatic disease was performed along with hepatic ultrasound. Excisional biopsy of hilar lymph nodes was performed and submitted to permanent pathology. Patients were removed from the protocol for any evidence of peritoneal metastasis or lymph node metastasis. Capecitabine was reinitiated at discharge and continued until transplantation. Capecitabine was held for a one week rest every six weeks while listed.
Once final pathology was completed following the staging operation, patients were listed for liver transplantation. The regional review board was asked to grant a MELD exception of 22 points (9) with an additional 3 point increment if wait time went beyond three months. Staging CT scans were repeated every three months to evaluate for stability of disease while on the wait list. The transplant procedure was performed as previously described with care taken to divide hilar structures as distally as possible as well as remove any additional hilar lymph nodes (2, 3). Frozen sections were performed on the distal bile duct to verify whether pancreaticoduodenectomy would be necessary. Vena cava sparing technique was used along with use of donor iliac artery conduit for donor hepatic arterial reconstruction to the recipient supraceliac aorta to avoid arterial complications (10). Biliary reconstruction was performed using a hepaticojejunostomy. Capecitabine was discontinued and immunosuppression was initiated according to our center’s standard protocol using a prednisone taper, mycophenolate mofetil, and tacrolimus. Hepatic duplex was performed on postoperative days 1, 7, and 21 with subsequent contrast enhanced CT or MRI performed every three months postoperative for the first year then every six months thereafter.
Patient Monitoring
One week following SBRT, capecitabine was initiated and patients were monitored for signs of toxicity weekly by laboratory and symptom assessment. In patients that experienced a clear treatment related toxicity of grade 2 or greater, capecitabine was held until grade 1 or less was achieved. Capecitabine was then resumed with a daily dose reduction 500 mg. All adverse events were defined as those occurring after initiation of neoadjuvant therapy and were determined upon retrospective review of the medical history with serious adverse events (SAEs) defined as those that resulted in hospitalization, escalated level of care, or significant clinical intervention. An SAE related to cholangitis was treated with urgent antibiotics and biliary tube interrogation.
Tumor Histological Analysis
Tumor explants following transplantation were paraffin-embedded and sectioned for routine hematoxylin and eosin staining and reviewed by a gastrointestinal pathologist. Additional serial sections were deparaffinized in xylene, rehydrated in descending alcohol concentrations, and followed by heat-induced antigen retrieval by boiling in citrate buffer. Antibodies for cytokeratin-7 (CK-7) (clone OV-TL 12/30, Dako, USA) and Ki-67 (clone MIB-1, Dako) were used for immunohistochemical (IHC) staining following blockade of endogenous peroxidase and protein according to the manufacturer’s instructions (11, 12). Biotinylated Link and Streptavidin-HRP (Dako) were subsequently incubated followed by DAB chromogen incubation (Dako) for development. TUNEL staining was performed according to the manufacturer’s procedure (ApopTag® Plus Peroxidase In Situ Apoptosis Kit, # S7101, Millipore, USA). Slides were examined with 40× magnification from 2–3 representative areas in each tumor specimen whereupon Ki-67 and TUNEL positive cells were determined for at least 200 CK-7+ tumor cells per patient. Proliferation index (PI) and apoptosis index (AI) were calculated as percentage of Ki-67 positive CK-7 cells and percentage of TUNEL positive CK-7 cells, respectively (13), and were compared to tumors surgically resected that had not undergone neoadjuvant therapy as a control.
Results
Patient Presentation
Seventeen patients with unresectable hilar CCA were evaluated for the neoadjuvant chemoradiotherapy protocol and transplantation following review in our multidisciplinary liver tumor and transplant programs. Baseline demographics (Table 1) showed that the majority of patients were male and 24% of patients had underlying PSC as an etiology with 71% being “de novo” or no predisposing etiology. One patient had an underlying type IV choledochal cyst. Ten patients (59%) had a measurable mass on cross sectional imaging with an overall mean tumor diameter of 1.42 cm. Five patients (29%) had brushings positive for adenocarcinoma on cholangiography along with a malignant appearing stricture as their diagnostic criteria. Two patients had a malignant appearing stricture along with an elevated CA19-9 as their only presenting diagnostic criteria.
Table 1.
Presentation of Patients with Unresectable Hilar Cholangiocarcinoma
| Patients Evaluated | 17 |
| Age (mean) | 58 |
| Male/Female | 12/5 |
| PSC (%) | 4 (24) |
| De novo (%) | 12 (71) |
| Other Etiology (%) | 1 (5) |
| Mean Mass Size (range) | 1.42 (0–3.2) |
| CA19-9 (mean) | 429 |
| Mass (%) | 10 (59) |
| Brushing positive only (%) | 5 (29) |
| PTC (%) | 13 (76) |
Ultimately, 12 patients (71%) were able to initiate neoadjuvant therapy (Table 2 and Supp. Fig. 1) with 5 patients excluded from entering neoadjuvant therapy_due to positive findings on EUS lymph node (LN) aspirate (1 patient), peritoneal metastasis on early diagnostic laparoscopy (3 patients), and newly symptomatic coronary artery disease (1 patient). Diagnostic laparoscopy was performed in these patients due to clinical concern of higher disease stage despite initial eligibility on cross-sectional imaging and EUS screening.
Table 2.
Neoadjuvant Chemoradiotherapy and Transplant Protocol Completion
| Patients Evaluated | 17 |
| Patients Ruled Out: | 5 |
| EUS positive (%) | 1 (6) |
| Early Progression (peritoneal disease) (%) | 3 (18) |
| New Co-morbidity (%) | 1 (6) |
| Initiated Neoadjuvant Therapy | 12 |
| Stage Laparoscopy Positive (% of treated) | 3 (25) |
| Lymph Node | 2 |
| Peritoneal Metastasis | 1 |
| Listed for Transplant (% of treated) | 9 (75) |
| Removed From List | 2 |
| Transplanted | 6 |
| Waiting | 1 |
Protocol Completion and Tolerability
Radiation therapy for a total dose of 50–60 Gy was achieved in all 12 patients, although one patient was treated with a non-SBRT protocol due to concern of longitudinal extension; ultimately, this patient had peritoneal metastases on later staging laparoscopy. The other 11 patients received SBRT in 3–5 fractions and went on to initiate capecitabine as scheduled. During neoadjuvant treatment, there were 35 adverse events which occurred after the initiation of therapy_with 14 of these being significant enough to be classified as an SAE (Table 3). SAEs occurred in 6 patients (50% of treated patients), with cholangitis related to PTC tube dysfunction being the most significant and common etiology. Other SAEs were related to dehydration (3 episodes) and occurred in the same patients with cholangitis. The most common non-SAE adverse events were palmar-plantar erythrodysesthesia (6 patients), diarrhea (2 patients), and wound infection following the staging operation (2 patients). No other gastrointestinal toxicity such as gastritis or ulcer disease was noted. There were a total of 5 dose reductions of capecitabine among 4 patients. All 12 patients underwent staging laparoscopy or laparoscopic assisted exploration and portal lymph node sampling 4–6 weeks from completion of SBRT. One patient was found to have peritoneal disease and 2 patients were found to have positive lymph nodes on final pathology (Supp. Fig. 1). Therefore 9 patients were formally listed for transplantation with MELD exception scores of 22 points. The need for elective and urgent biliary interventions was common, as a mean of 7 such interventions were required per patient during the treatment and transplant wait time period. One patient was ultimately removed from the list due to concerns of decreasing performance status along with weight lossand another was removed at the time of exploration with transplant intent, when a peritoneal metastasis was identified. Therefore the dropout rate following neoadjuvant therapy due to a positive staging operation (3 patients), due to disease progression (one patient) or lack of tolerability (one patient) combined for 42%.
Table 3.
Tolerability of Neoadjuvant Therapy and Transplant Outcomes
| Adverse Events (total) | 35 |
| SAEs (total) | 14 |
| SAE per Patient (range) | 1 (0–6) |
| Other AEs (total) | 21 |
| AE per Patient (range) | 2 (0–8) |
| Most Frequent AEs (% of treated patients) | |
| Cholangitis | 6 (50) |
| Plantar/Palmar Erythrodysesthesia | 6 (50) |
| Diarrhea | 2 (17) |
| Wound Infection (post staging procedure) | 2 (17) |
| Dose Reductions | 5 |
| Biliary Interventions (mean) | 7 |
| Total Transplants | 6 |
| Mean Wait Time (days) | 92 |
| Cold Ischemia Time (mean min.) | 344 |
| Warm Ischemia Time (mean min.) | 27 |
| Length of Stay (median) | 12.5 |
| Post-transplant Complications | 11 |
| One Year Survival (%) | 83 |
| Tumor Explant Pathology | |
| Tumor Size (mean cm) | 1.95 |
| Neurovascular Invasion (no. patients) | 3 |
| Positive Lymph Node (no. patients) | 1 |
| Complete Response (%) | 1 (17) |
| Partial Response (%) | 4 (67) |
| No Response (%) | 1 (17) |
Transplantation and Tumor Responses
Ultimately 6 patients have undergone deceased donor OLT with one patient currently actively listed (Table 3). The mean and median wait timeswere 92 and 88 days, respectively (range 29–201 days). Cold and warm ischemic times were a mean of 344 minutes and 27 minutes, respectively. One patient required a concomitant pancreaticoduodenectomy secondary to an initially positive distal bile duct margin. All patients underwent supraceliac aorta interposition graft to the hepatic artery using donor iliac conduit. There was a total of 11 transplant related complications (Table 3). One patient was free of any complication. One year survival was 83% with one patient dying after discharge of presumed cardiovascular collapse 21 days following transplantation. The median follow up time is greater than 14 months to date. There were two re-explorations for bleeding secondary to pancreatic leak, however there were no vascular, biliary, or hepatic insufficiency complications to date. Median length of stay following OLT was 12.5 days.
Routine clinical pathologic review of tumor explants showed at least a partial response for five of six patientswith one patient demonstrating a complete response histologically. One patient was a non-responder._Responding patients had evidence of significant necrosis and fibrosis on routine histologic examination (Fig. 1). Patients with a partial response had only minute foci (< 0.2 cm) of viable tumor cells remaining. We therefore performed further examination using IHC staining and showed that in contrast to patients that had undergone surgical resection without neoadjuvant therapy (control), patients that had been treated neoadjuvantly followed by transplantation had_a low proliferative index (Ki-67+) among CK-7+ tumor cells (Fig. 2, 3, and Supp. Fig. 2) in all patients (range 0–17%). Additionally, a high apoptotic index (TUNEL+/CK-7+) was noted among remaining tumor cells (range 2–51%) in comparison to non-treated (control) patients. The one patient with a complete response based upon clinical histologic examination, had an undetectable proliferative index, but the highest apoptotic index among CK-7+ tumor cells at 51% (Supp. Fig. 2). Mean gross tumor size at explant was 1.95 cm. Three patients had evidence of neurovascular invasion with one of these patients having a positive lymph node and another patient having a subsequent peritoneal implant noted on final pathology. Both of these patients ultimately recurred and succumbed to disease after systemic therapy at 16 and 30 months following transplantation. Four of the six explants (67%) were noted to have peri-hilar cirrhosis or fibrosis in the liver parenchyma not involved by tumor. Two of these patients were without a diagnosis of PSC and had de novo hilar CCA.
Figure 1. Histology of hilar CCAs following neoadjuvant therapy.
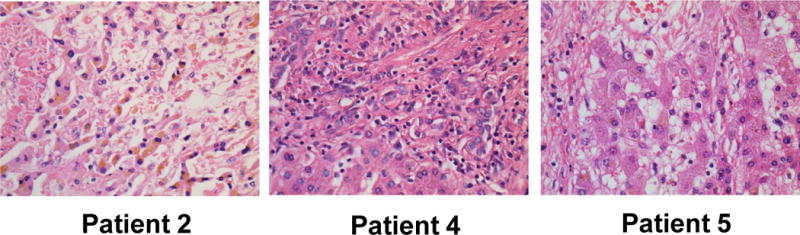
Representative patient hilar tumors with partial clinical pathologic responses (n=3) following transplantation were stained with hematoxylin and eosin (H&E) under 40× magnification are shown.
Figure 2. Immunohistochemistry for proliferating and apoptotic CCA cells.
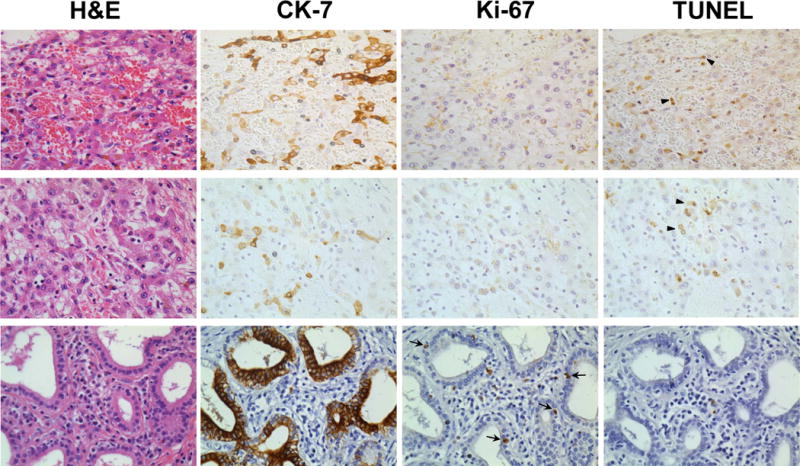
Two representative patients with partial pathologic responses (Patient 2, top row and Patient 5, middle row) are shown. A non-treated, surgically resected CCA is shown for comparison (bottom row)._Serial sections for H&E stain, CK-7 stain for CCA cells, Ki-67 stain for proliferation, and TUNEL staining for apoptosis are indicated and performed as described in Methods. Arrows indicate dual positive TUNEL+/CK-7+ cells. 40× magnification shown.
Figure 3. Proliferative and apoptotic index of hilar CCAs following neoadjuvant therapy.
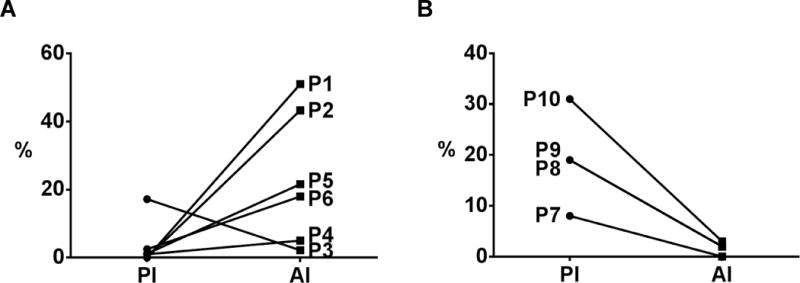
Patients hilar CCAs (n=6) following neoadjuvant therapy and transplantation (A) underwent IHC for CK-7, Ki-67, and TUNEL staining as shown in Fig. 2 and as described in Methods. Proliferative index (PI) and apoptotic indexes (AI) were calculated as the percentage of Ki-67 positive CK-7 cells and percentage of TUNEL positive CK-7 cells, respectively. Patients who underwent resection only (no neoadjuvant therapy, n=4) are shown for comparison (B).
Discussion
Hilar CCA continues to be a challenging treatment problem given the often distant as well as local extent of disease. It is estimated that 30% of patients present with advanced disease and of those with disease confined to the liver hilum, as low as 50% will undergo an R0 resection due to bilateral biliary and vascular invasion (1). Additionally, many patients will have underlying PSC, which further limits safe resection. While early experiences began to explore whether liver transplantation might be a therapeutic option for patients with unresectable disease (14), the Mayo Rochester group pioneered the use of neoadjuvant chemoradiotherapy in addition to rigorous surgical staging before transplantation with overall results that are similar to transplantation for other disease etiologies (2, 3). The ability to achieve a R0 resection and the presence of negative lymph nodes are the most significant positive prognostic factors for the surgical therapy of hilar cholangiocarcinoma (1, 15, 16). Consequently, evaluation of lymph nodes using a combination of EUS-guided biopsy and surgical staging is critically important to selecting patients for this transplant therapy (8).
Experience with neoadjuvant chemoradiotherapy utilizing external beam radiation therapy, brachytherapy, and 5-FU based chemotherapy has shown significant tumor responses to date but requires a significant number of therapeutic sessions in which to deliver this therapy. SBRT has the ability to concentrate radiation dose to a confined anatomical area over a shorter number of fractions, while minimizing adjacent toxicities. Our center has been a forerunner in the use of SBRT for a variety of hepatic malignancies which has been shown to be safe and effective, even in the presence of underlying liver disease (5, 6). Therefore we sought to build on this experience by using a combination of SBRT in five fractions or less followed by oral capecitabine for the neoadjuvant treatment of unresectable hilar CCA patients before transplantation. Our total dose of 50–60 Gy in 3–5 twice-weekly treatments is therefore similar to, if not more biologically intense, than the originally described protocol of external beam given over 30 fractions along with supplemental brachytherapy (3, 10).
The present analysis represents a pilot study that seeks to examine our initial experience with this algorithm and determine overall tolerability. We also sought to examine tumor responses histologically in explants following transplantation. Overall our protocol was well tolerated other than the expected adverse events related to cholangitis and the need for continuous biliary decompression. Over the course of our experience, episodes of cholangitis requiring admission and urgent biliary intervention appeared to dissipate with the majority of episodes occurring in our earlier patients and 50% of patients being completely free of such episodes. It is possible that increasing coordination of our multidisciplinary care during the course of our protocol accounted for a gradual reduction in cholangitis related events though it is difficult to generalize in this cohort size. Palmar-plantar erythrodysesthesia was the next most common adverse event, associated with capecitabine therapy, however only 5 dose reductions were necessary. One patient required removal from the list due to declining performance status after a significant waiting time. No patients experienced decompensation of liver disease following neoadjuvant therapy. Therefore the overall dropout rate due to disease progression or lack of tolerability was somewhat comparable with previous experiences in that we experienced a rate of 42% whereas other groups experienced a 31% dropout rate (17). Care must be taken when comparisons are made between this pilot study and other experiences such as those at the Mayo, Rochester. Indeed, our cohort included only 24% of patients with PSC as a predisposing etiology when compared to the approximately 70% experienced elsewhere. These differences are difficult to explain but may be due to referral biases, differential use of FISH analysis for diagnosis, or due to our small size cohort.
Transplant related complications did not appear to be particularly increased, although one early death was noted following an initially uncomplicated course due to presumed cardiovascular collapse of unknown cause. Length of stay following transplant was otherwise acceptable at a median of 12.5 days and no patients experienced biliary, vascular, or hepatic insufficiency complications. This compares favorably with other experiences which have documented arterial complications in as high as 21% and portal venous complications in as high as 22% of patients (10), and as a result, use iliac artery donor conduits to the recipient aorta (10) which was also performed in our protocol.
Tumor responses to neoadjuvant therapy were demonstrated based upon explant analysis showing extensive necrosis of hilar tumors with 4 of 6 patients demonstrating less than a 0.2 cm foci of residual carcinoma and one patient having no viable tumor remaining. However, more detailed analysis using IHC showed that this patient had minute evidence of residual tumor cells based upon CK-7 IHC analysis. While small foci of tumor cells remained, low proliferative and high apoptotic indexes were noted among remaining CK-7+ cells and could reflect even greater tumor control than indicated on routine histologic analysis (12). Other experiences have indicated perhaps a greater tumor eradication rate (17), however direct comparisons are difficult due to perhaps varying disease burdens at entry and the non-prospective nature of pathologic evaluation in the current study as well as previous reported experiences. Nonetheless, it is hoped that further analysis of tumor explants will allow us to continue to improve neoadjuvant therapy while minimizing toxicity based upon this pilot experience. Unfortunately, our protocol failed to identify two patients with more advanced disease, one with a positive lymph node and one with a peritoneal metastasis at the time of transplant. The isolated peritoneal implant is difficult to explain, but may be related to unrecognized perforation due to numerous complicated biliary interventions required in this patient. Percutaneous biliary decompression by itself has not been previously shown to be a risk factor for tumor recurrence following transplantation (3). Patients with previous known percutaneous, transduodenal, or operative biopsies of the primary mass had otherwise been excluded from this protocol as well as other reported protocols due to risk of tumor seeding.
It must be emphasized that the present study is a pilot to primarily examine the overall tolerability and feasibility of utilizing SBRT, capecitabine, and transplantation for unresectable hilar CCA. While this regimen appears to have acceptable tolerability, more patients and longer follow up will be necessary to measure the overall long term oncologic benefits with this treatment regimen and allow additional comparisons to other currently utilized regimens. Explant analysis demonstrated evidence of pathologic response in that a partial to complete response was achieved in almost all patients. This treatment in this pilot study, utilizing SBRT, allowed for a short radiation treatment interval and allowed for an acceptable rate of protocol completion and listing for transplantation. This regimen, therefore, appears to be a reasonable multi-modality therapy worthy of additional study as a treatment of unresectable hilar CCA.
Supplementary Material
Supplemental Figure 1. Flowchart depicting patients evaluated, initiated on therapy, and transplant status. The patients that were ruled out before entering the protocol or were removed following initiation of therapy at the time of staging laparoscopy are also indicated. Additional patient details are provided in Table 2 and in the text.
Supplemental Figure 2. Proliferative and apoptotic rates for individual patients after neoadjuvant therapy. Proliferative index (PI) and apoptotic index (AI) was determined as described in Fig. 3 on neoadjuvant therapy patients (top) and on non-treated surgically resected patients (bottom).
Acknowledgments
The authors would like to thank all the patients and families who have undergone treatment for cholangiocarcinoma at the University of Michigan. The authors would also like to thank Drs. Charles Rosen and Julie Heimbach from the Mayo Clinic, Rochester for many helpful discussions.
This work was partially supported by the Tissue Histology Core and Tumor Procurement Program (Comprehensive Cancer Center) and also by NIH grantCA151414 (THW).
Footnotes
There are no conflicts to disclose.
The following abbreviations are used in this manuscript: 5-FU (5-fluorouracil), AE (adverse event), AI (apoptotic index), CCA (cholangiocarcinoma), EUS (endoscopic ultrasound), Gy (Gray), OLT (orthotopic liver transplant), PI (proliferative index), m (meter), MELD (Model for End-stage Liver Disease), mg (milligrams), PSC (primary sclerosing cholangitis), SAE (significant adverse event), SBRT (stereotactic body radiation therapy), UNOS (United Network for Organ Sharing).
References
- 1.Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, Youssef BM, Klimstra D, Blumgart LH. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. discussion 517–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Vreede I, Steers JL, Burch PA, Rosen CB, Gunderson LL, Haddock MG, Burgart L, Gores GJ. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000;6:309–316. doi: 10.1053/lv.2000.6143. [DOI] [PubMed] [Google Scholar]
- 3.Heimbach JK, Gores GJ, Haddock MG, Alberts SR, Pedersen R, Kremers W, Nyberg SL, Ishitani MB, Rosen CB. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703–1707. doi: 10.1097/01.tp.0000253551.43583.d1. [DOI] [PubMed] [Google Scholar]
- 4.Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88–98 e83. doi: 10.1053/j.gastro.2012.04.008. quiz e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Normolle D, Pan C, Ben-Josef E, Lawrence T. Adaptive trial of personalized radiotherapy for intrahepatic cancer. Per Med. 2010;7:197–204. doi: 10.2217/pme.10.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu E, Stenmark MH, Schipper MJ, Caoili EM, Lee OE, Ben-Josef E, Lawrence TS, Feng M. Stereotactic body radiation therapy for primary and metastatic liver tumors. Transl Onc. 2013 doi: 10.1593/tlo.12448. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor JK, Trotter J, Davis GL, Dempster J, Klintmalm GB, Goldstein RM. Long-term outcomes of stereotactic body radiation therapy in the treatment of hepatocellular cancer as a bridge to transplantation. Liver Transpl. 2012;18:949–954. doi: 10.1002/lt.23439. [DOI] [PubMed] [Google Scholar]
- 8.Gleeson FC, Rajan E, Levy MJ, Clain JE, Topazian MD, Harewood GC, Papachristou GI, Takahashi N, Rosen CB, Gores GJ. EUS-guided FNA of regional lymph nodes in patients with unresectable hilar cholangiocarcinoma. Gastrointest Endosc. 2008;67:438–443. doi: 10.1016/j.gie.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Gores GJ, Gish RG, Sudan D, Rosen CB. Model for end-stage liver disease (MELD) exception for cholangiocarcinoma or biliary dysplasia. Liver Transpl. 2006;12:S95–97. doi: 10.1002/lt.20965. [DOI] [PubMed] [Google Scholar]
- 10.Mantel HT, Rosen CB, Heimbach JK, Nyberg SL, Ishitani MB, Andrews JC, McKusick MA, Haddock MG, Alberts SR, Gores GJ. Vascular complications after orthotopic liver transplantation after neoadjuvant therapy for hilar cholangiocarcinoma. Liver Transpl. 2007;13:1372–1381. doi: 10.1002/lt.21107. [DOI] [PubMed] [Google Scholar]
- 11.Lau SK, Prakash S, Geller SA, Alsabeh R. Comparative immunohistochemical profile of hepatocellular carcinoma, cholangiocarcinoma, and metastatic adenocarcinoma. Hum Pathol. 2002;33:1175–1181. doi: 10.1053/hupa.2002.130104. [DOI] [PubMed] [Google Scholar]
- 12.Rijken AM, Umezawa A, van Gulik TM, Bosma A, Polak MM, Offerhaus GJ, Obertop H, Gouma DJ. Prognostic value of cell proliferation (Ki-67 antigen) and nuclear DNA content in clinically resectable, distal bile duct carcinoma. Ann Surg Oncol. 1998;5:699–705. doi: 10.1007/BF02303480. [DOI] [PubMed] [Google Scholar]
- 13.Navarrete MA, Maier CM, Falzoni R, Quadros LG, Lima GR, Baracat EC, Nazario AC. Assessment of the proliferative, apoptotic and cellular renovation indices of the human mammary epithelium during the follicular and luteal phases of the menstrual cycle. Breast Cancer Res. 2005;7:R306–313. doi: 10.1186/bcr994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singal A, Welling TH, Marrero JA. Role of liver transplantation in the treatment of cholangiocarcinoma. Expert Rev Anticancer Ther. 2009;9:491–502. doi: 10.1586/era.09.5. [DOI] [PubMed] [Google Scholar]
- 15.Hemming AW, Reed AI, Fujita S, Foley DP, Howard RJ. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693–699. doi: 10.1097/01.sla.0000160701.38945.82. discussion 699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocha FG, Matsuo K, Blumgart LH, Jarnagin WR. Hilar cholangiocarcinoma: the Memorial Sloan-Kettering Cancer Center experience. J Hepatobiliary Pancreat Surg. 2009 doi: 10.1007/s00534-009-0205-4. [DOI] [PubMed] [Google Scholar]
- 17.Murad SD, Kim WR, Therneau T, Gores GJ, Rosen CB, Martenson JA, Alberts SR, Heimbach JK. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology. 2012;56:972–981. doi: 10.1002/hep.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Flowchart depicting patients evaluated, initiated on therapy, and transplant status. The patients that were ruled out before entering the protocol or were removed following initiation of therapy at the time of staging laparoscopy are also indicated. Additional patient details are provided in Table 2 and in the text.
Supplemental Figure 2. Proliferative and apoptotic rates for individual patients after neoadjuvant therapy. Proliferative index (PI) and apoptotic index (AI) was determined as described in Fig. 3 on neoadjuvant therapy patients (top) and on non-treated surgically resected patients (bottom).


