Abstract
Objective
To provide insight into mitochondrial function in vivo, we evaluated the 3D spatial relationship between capillaries, mitochondria, and muscle fibers in live mice.
Methods
3D volumes of in vivo murine Tibialis anterior muscles were imaged by multi-photon microscopy (MPM). Muscle fiber type, mitochondrial distribution, number of capillaries, and capillary-to-fiber contact were assessed. The role of myoglobin-facilitated diffusion was examined in myoglobin knockout mice. Distribution of GLUT4 was also evaluated in the context of the capillary and mitochondrial network.
Results
MPM revealed that 43.6 ± 3.3% of oxidative fiber capillaries had ≥ 50% of their circumference embedded in a groove in the sarcolemma, in vivo. Embedded capillaries were tightly associated with dense mitochondrial populations lateral to capillary grooves and nearly absent below the groove. Mitochondrial distribution, number of embedded capillaries, and capillary-to-fiber contact were proportional to fiber oxidative capacity and unaffected by myoglobin knockout. GLUT4 did not preferentially localize to embedded capillaries.
Conclusions
Embedding capillaries in the sarcolemma may provide a regulatory mechanism to optimize delivery of oxygen to heterogeneous groups of muscle fibers. We hypothesize that mitochondria locate to paravascular regions due to myofibril voids created by embedded capillaries, not to enhance the delivery of oxygen to the mitochondria.
Keywords: Two-photon microscopy, 3D quantitative imaging, muscle mitochondria, myoglobin, vasculature, microcirculation, GLUT4
Introduction
Aerobic exercise training can increase skeletal muscle mitochondrial content two-fold (13, 27). However, maximal whole body oxygen uptake (VO2max) concomitantly increases by only ~15% (13, 27). Thus, VO2max is not primarily limited by mitochondrial capacity to consume oxygen, but rather, by the ability to deliver and transfer oxygen to mitochondria within skeletal muscle (17, 83). As such, the spatial relationship between capillaries, mitochondria, and the muscle fiber becomes crucial to the study of skeletal muscle mitochondrial function in vivo.
Beginning with the pioneering work of Krogh (47), the capillary supply of oxygen to skeletal muscle has been examined extensively over the past 90 years (14, 16, 33, 36, 68). Early studies focused largely on capillary density (number of capillaries per unit area) and found a strong correlation with muscle mitochondrial content for a wide range of species and muscles (36). Although both capillary density and the capillary-to-fiber ratio have been found to be good markers of oxidative capacity in skeletal muscle under a variety of conditions (61), this does not always hold true, e.g. in the rat plantaris muscle (68). Better indices of maximal oxygen exchange capacity seem to be those which account for capillary geometry, such as the size of the capillary-to-fiber interface, the length of capillaries per fiber volume, or capillary volume density, each of which is found to be proportional to muscle mitochondrial content (33, 68, 84).
Most skeletal muscle morphology knowledge comes from analysis of non-sequential 2D images of muscle fibers that have been prepared with a fixative or from previously frozen biopsies (11, 14, 16, 33, 35, 36, 53, 54, 68). While each method has its own advantages and disadvantages, fiber and/or capillary morphology may be significantly altered due to the shrinking associated with fixation and dehydration or the muscle contraction that occurs in biopsies (33). Further, determining capillary geometry from 2D images can be difficult, often requiring the estimation and modeling of capillary anisotropy (54). To overcome some of these limitations, confocal microscopy has recently been implemented in a series of studies to evaluate the 3D relationship between capillaries and muscle fibers (22, 37–39, 48, 59, 60). Although these studies used fixed or frozen tissue sections and were limited by the depth of penetration of confocal microscopy (12), they began to demonstrate the power of the 3D approach to evaluate capillary networks in skeletal muscle (37). We recently developed a method of collecting large field-of-view 3D volumes with submicron resolution using multi-photon microscopy (78). This method was used to image the 3D capillary network of skeletal muscle in vivo (78), however, muscle fibers were not imaged and, therefore, no quantitative data on relationship between the capillary network, muscle fibers, and mitochondria was provided.
Due to the critical nature of the spatial relationship between capillaries and mitochondria in skeletal muscle and our continuing work evaluating skeletal muscle mitochondrial function in vivo with multi-photon microscopy (6, 74, 79), we set out to determine the 3D spatial relationship between capillaries, mitochondria, and three fiber types of varying oxidative capacity within the murine Tibialis anterior (TA) muscle in vivo. Preliminary results showed that a significant fraction of the capillaries associated with muscle fibers were embedded in grooves in the sarcolemma, particularly in the most oxidative fibers. As a result, the role of these capillary grooves was investigated further by comparing the in vivo 3D capillary network in skeletal muscle of mice with and without myoglobin (Mb). Due to the role of Mb in oxygen diffusion in muscle (86) and a previous report of an increased capillary density in the soleus of Mb knockout (KO) mice (28), we hypothesized that the loss of Mb would be compensated for by an increase in capillary to fiber contact through more frequent or extensive capillary embedding. Delivery of molecules other than oxygen could also be the impetus for embedding capillaries in the sarcolemma of muscle fibers. To explore this possibility, mice expressing green fluorescent protein (GFP) labeled glucose transporter 4 (GLUT4) were examined to determine the relationship between embedded capillaries and glucose uptake into the muscle with the hypothesis that GLUT4 would localize to capillaries embedded in sarcolemmal grooves.
Materials and Methods
Mice
C57BL/6 mice, 2 – 4 months old, were purchased from Taconic Farms (Germantown, NY). Mb knockout (KO) mice were obtained from Jürgen Schrader (Heinrich Heine University, Düsseldorf) after being generated as described in (77). Transgenic GLUT4-GFP mice, 3 – 5 months old, were derived as described previously (52). All mice were fed ad libitum and kept on a 12 hour light, 12 hour dark cycle at 20–26°C.
Animal Preparation for Imaging
All procedures were approved by the National Heart, Lung, and Blood Institute Animal Care and Use Committee and performed in accordance with the guidelines described in the Animal Care and Welfare Act (7 USC 2142 § 13). Mice were anaesthetized and the Tibialis anterior (TA) muscle prepared for imaging as described previously (6). Vasculature (di-8-ANEPPS) and interstitial space (carboxyfluorescein succinimidyl ester, CFSE) dyes were injected into the heparinized jugular vein at a rate of 10 μl/min via syringe pump until the fluorescent signal was sufficient to begin imaging (40).
GLUT4-GFP mice were fasted for 4 hours prior to preparation of the TA. Images were acquired before and after 15 minutes of electrical stimulation of the muscle. Electrical signals (2 V, 0.05 ms pulse width, 5 Hz) were sent from the stimulator (Grass S88, Grass Instruments, Quincy, MA) to needle electrodes inserted into the muscle on both sides of the TA to induce isometric, twitch contractions of the TA.
Image Acquisition
Images were collected using a custom LabVIEW program for rapid overlapping volume acquisition and reconstruction (ROVAR) applied to a Leica TCS SP5 II upright, resonant scanning, multi-photon microscope as described previously (78). Either a Leica 20X, 1.0 NA or Nikon 25X, 1.1 NA water immersion objective was used with an objective heater maintained at 37°C (Warner Instruments, Harnden, CT). The exposed muscle was imaged with a pulsed Ti:sapphire laser tuned to 720 nm (Spectra Physics, Irvine, CA, U.S.A.), with three emission filters tuned to 435–495 nm, 510–550 nm, and 570–640 nm, separating the signals generated from mitochondrial NAD(P)H, CFSE, and di-8-ANEPPS, respectively. Laser power output was controlled by the ROVAR software and increased as a function of image depth in order to preserve image intensity with depth as well as increase the maximal imaging depth.
Image Analysis
Image volumes were stitched together using a freely available plug-in (69) for ImageJ software (National Institutes of Health, Bethesda, MD) as described previously (78). Image analysis was then divided into two portions to quantify the degree of capillary to fiber contact: 1) automatic image segmentation to locate and classify capillary structures from the di-8-ANEPPS signal, and 2) manual image segmentation to identify fiber boundaries and fiber type from the NAD(P)H and CFSE signals.
Automated vessel segmentation began with a flat-field correction to improve the non-uniformity of the detected fluorescence signal across different depths of the tissue. This correction was performed by estimating spatial signal intensity variation from the maximum intensity projections of 3 orthogonal image planes. Polynomial intensity-surface fitting was employed to approximate a global signal intensity profile along each plane. A volumetric signal intensity profile was then reconstructed from these planar image intensity profiles and used for global intensity normalization. Homomorphic filtering was then used to simultaneously normalize image brightness and sharpen image features followed by anisotropic filtering to enhance vessels along their principal directions, thus suppressing noise efficiently while preserving vessels boundaries. White top-hat morphological processing was implemented using a 3D sphere-shape with a diameter equal to 11 μm to further enhance the capillary signal and to separate capillaries from larger vessels. Finally, a minimum cross-entropy thresholding algorithm (51) was applied to determine the optimal threshold for minimizing the cross-entropy between the original image and its thresholded version and was used to produce binary images of the capillary structures.
The segmented, binary vessel images and raw NAD(P)H and interstitial dye channels were then individually loaded into a custom contour drawing and analysis program written in IDL (Exelis Visual Information Solutions, Boulder, CO). The NAD(P)H fluorescence signal was taken as primarily from the mitochondrial pool based on our prior topology and functional responses of the signal (74) and enhancement by Complex I binding of NADH (9). Thus, NAD(P)H intensity was considered indicative of mitochondrial content within the muscle fibers and used for fiber typing similar to commonly used histochemical stains of mitochondrial enzymes (64). Fiber boundaries were manually traced for 10 – 30 fibers on sequential images within each 3D volume using the interstitial dye and NAD(P)H signals. Using the fiber boundaries and the segmented vessels, fiber and vessel surface areas, volumes, and contact was assessed for each fiber and associated vessel (see Figure S1 for description). Paravascular (PV) mitochondrial density relative to intermyofibrillar (IMF) mitochondrial density was determined by dividing the mean NAD(P)H intensity within 5 μm of the fiber boundary by the mean NAD(P)H intensity of the rest of the fiber. No differences in intra- or inter-observer variability were found between the two users of the fiber tracing program.
Transmission Electron Microscopy
TEM images were obtained to confirm multi-photon microscopy observations using more conventional techniques. Mouse hindlimbs were stabilized and immersion fixed in 5% glutaraldehyde in 0.12 M sodium cacodylate, pH 7.35 for 15 minutes in vivo. TA muscles were excised, placed into fresh, cold fixative, and cut into small strips. Fixed muscles were postfixed with 1% osmium tetroxide, stained with 1% uranyl acetate, dehydrated with an ethanol series and propylene oxide, and embedded in Epon. Samples were sectioned with an ultramicrotome, placed on grids, and stained again with uranyl acetate and lead citrate prior to imaging on a JEM 1400 electron microscope (JEOL USA, Peabody MA) with an AMT XR-111 digital camera (Advanced Microscopy Techniques Corporation, Woburn MA). Fiber type in TEM images was primarily determined by volume of mitochondria present (35), however, in cross-sectional images, fiber size and myofibril shape (26) were also used to determine fiber type.
Scanning Electron Microscopy
SEM images were obtained to confirm multi-photon microscopy observations using more conventional techniques. Within 1–1.5 minutes following death by cervical dislocation, the soleus muscle was excised and one half was immediately fixed in a 1% glutaraldehyde and 0.5% paraformaldehyde solution in a 60 mM cacodylate buffer, pH 7.4. Fixed samples were rinsed twice in 67 mM cacodylate buffer, immersed in DMSO for 5–10 min, and snap frozen in liquid nitrogen-cooled isopentane for 5–10 seconds. Frozen samples were immediately fractured by applying lateral pressure within a liquid nitrogen-cooled cracking instrument. The resulting fractured specimens, approximately 0.5 – 3mm in size, were rinsed three times in 67 mM cacodylate buffer and postfixed in 1% osmium tetroxide for one hour. Samples were subsequently transferred to 0.1% osmium tetroxide for 72 – 96 hours (63), before being dehydrated in sequential steps of ethanol (25%, 50%, 75%, and 100% twice) and dried with CO2 in a Bal-Tec Critical Point Dryer. Specimens were mounted on stubs covered with carbon disks, gold coated (15 nm) in a Polaron SEM Coating Unit, and examined on a Stereoscan 240 Scanning Electron Microscope. Digital micrographs were captured at different magnifications with the Orion 6.60.6 software.
Differential Gel Electrophoresis
DIGE was used to evaluate the proteomic profile of the Mb KO and WT mice and to demonstrate that Mb had indeed been knocked out. To prepare heart and skeletal muscle homogenates from Mb KO and WT mice, 0.1 g of tissue was placed in 900 μL of 280 mM sucrose, 10 mM HEPES, 1 mM EDTA, 1 mM EGTA, pH 7.1 and homogenized at 40% power for 15 sec on ice using a Virtishear homogenizer (Virtis, Gardiner, NY). One mg protein was then pelleted and resuspended in 250 μl lysis buffer (15 mM Tris-HCl, 7 M urea, 2 M thiourea, and 4% CHAPS (w/v)) and kept on ice with frequent vortexing for 5 minutes. Samples were then spun at 16,000 g for 10 minutes at 4°C and the supernatant transferred to a fresh microcentrifuge tube. This was repeated two more times. To compare proteins with isoelectric points (pIs) from pH 3 – 11, we used 2-D electrophoresis (2-DE) as described previously (1, 2, 24, 65). Samples from Mb KO (25 μg), Mb WT (25 μg), and a combination of Mb KO and Mb WT (12.5 μg each) were mixed with 0.3 nmol Cy5, Cy3, or Cy2 dyes, respectively, (GE Healthcare, Piscataway, NJ) in 1 μl dimethylformamide and incubated in the dark for 30 minutes on ice. To quench protein binding to the CyDyes, 1 μl of 10 mM lysine was then added to each sample and incubated on ice for at least 15 minutes. Samples were combined and added to rehydration solution (7 M urea, 2 M thiourea, 4% CHAPS (w/v), 13 mM DTT, 1% pH 3–11 NL Pharmalyte (v/v), and 2 μl of Destreak reagent) to a final volume of 210 μl and placed on ice for 5 min before being loaded onto 11 cm Immobiline DryStrip gels (pH 3–11 NL, Sigma-Aldrich, St. Louis, MO). Isoelectric focusing (IEF) was achieved by active rehydration for 12 h at 30 V followed by stepwise application of 500V (1 h), 1,000 V (1 h), a gradient to 6,000 V (2 h), and a final step at 6,000 V (1.2 h) for a total of ~15,000 Vh (Ettan IPG Phor2). After IEF, gel strips were incubated in SDS equilibration solution (50 mM Tris-HCl (pH 8.8), 6M urea, 30% glycerol, and 2% SDS) plus 0.05 g DTT for 10 minutes. The gel strip was then placed atop an 8 – 16% Tris-HCl gel, sealed with 0.5% agarose plus bromophenol blue, and run in TGS buffer (25 mM Tris, 192 mM glycine, 0.1% w/v SDS, pH 8.3) at room temperature for 10 minutes at 50 V and 70 minutes at 180 V. Upon completion of SDS-PAGE, the Cy2, Cy3, and Cy5 images were scanned sequentially on a Typhoon variable mode imager at a resolution of 100 μm using excitation at 488, 532, and 633 nm, respectively, and emission bandpass filters of 520 ± 20, 580 ± 15, and 670 ± 15 nm, respectively.
Tissue Mitochondrial Content
Mitochondrial content in Mb KO and WT tissues was evaluated as the amount of cytochrome a, a3 (cyt a) present. To prepare tissue homogenates, 0.1 g of trimmed and minced tissue was added to 0.9 ml isolation buffer and homogenized at 40% power for 15 sec on ice using a Virtishear (Virtis, Gardiner, NY). Cyt a content was determined as described previously (7) for 3 paired Mb KO and WT hearts and 4 paired Mb KO and WT TA muscles.
Statistical Analyses
Differences among the three fiber types and between Mb KO and WT mice were tested using a two-way ANOVA (± myoglobin x fiber type) with a Tukey’s honest significance post hoc test. Significant differences were determined using a p-value of 0.05.
Results
In Vivo Skeletal Muscle Capillary Network
3D datasets of in vivo mouse skeletal muscle fibers, mitochondria, and blood vessels with 850 nm x-, y-, and z-pixel sizes were collected to examine the spatial relationships between them. Figure 1 shows representative 2D images of the murine TA muscle longitudinally and in cross-section. Movies of a complete 3D dataset showing flowing blood can be found in Supplemental Videos 1 and 2. The green signal in these datasets represents endogenous mitochondrial NAD(P)H thus giving information on the spatial location and density of mitochondria in each fiber. Interstitial space between muscle fibers is shown in blue and is captured through the use of an intravenous fluorescent dye (CFSE) that diffuses out of the vessels, providing clear boundaries between muscle fibers. Blood vessels are imaged by the fluorescent di-8-ANEPPS dye, which is also injected intravenously but does not leave the vessels (40).
Figure 1.
2D images of a large field-of-view, 3D image stack collected from C57BL/6 mouse Tibialis anterior muscle in vivo. A) Representative XY image plane provides longitudinal view of in vivo skeletal muscle fibers. B) Representative YZ image plane shows cross-sectional view of in vivo skeletal muscle fibers. Green color represents endogenous mitochondrial NAD(P)H fluorescence. Red color is from di-8-ANEPPS fluorescent dye injected into the vasculature. Blue color is from fluorescent CFSE dye in the interstitial space. Because CFSE was also injected into the vasculature, the signal from the residual CFSE remaining in the blood vessels was subtracted out using the di-8-ANEPPS signal to yield just the CFSE signal from the interstitial space. X-, Y-, and Z-pixel sizes are 0.85 μm. Images sizes are 825 μm in X and Y and 280 μm in Z. Movies of this 3D image stack showing flowing blood can be found in Supplemental Videos 1 and 2. Images and movies are representative of five 3D datasets collected from three C57BL/6 mice.
The 3D, in vivo skeletal muscle capillary network is visualized in Figure 2. The di-8-ANEPPS signal from the dataset shown in Figure 1 was rendered using ImageJ software and shows the high connectivity in all three dimensions of the skeletal muscle vasculature imaged in vivo with multi-photon microscopy. A movie showing 360° rotations of these 3D renderings can be found in Supplemental Video 3. 3D vasculature networks were then segmented to capture the volume and surface area measurements for each vessel.
Figure 2.
3D renderings of in vivo skeletal muscle vasculature network from Figure 1. Top left: View of the di-8-ANEPPS signal from above the XY plane. Top right: View from below the XY plane. Bottom left: View from the YZ plane. Bottom right: View from the XZ plane. X-, Y-, and Z-pixel sizes are 0.85 μm. Images sizes are 825 μm in X and Y and 280 μm in Z. Movie showing 360° rotation of these 3D renderings can be found in Supplemental Video 3.
To create 3D renderings of muscle fibers complete with surface area and volume measurements, a custom fiber tracing program was used to draw fiber boundaries on sequential 2D cross-sectional images. Figure 3 shows an example of a 2D image used to trace fibers and the resultant 3D renderings of representative oxidative and glycolytic fibers. Unexpectedly, we noticed that many fibers contained grooves at the surface where capillaries were embedded (see Figure 3B). Thus, these capillary grooves became the focus of further study to confirm and quantify their presence.
Figure 3.
3D fiber tracing. A) Single cross-section image of in vivo muscle fibers with fiber boundaries manually traced. Blue is endogenous mitochondrial NAD(P)H, green is dye in the interstitial space, and orange is segmented vasculature. Colors used to represent each signal were altered from Figure 1 to enhance visualization of fiber boundaries for tracing. B) Representative 3D renderings of oxidative (left) and glycolytic (right) muscle fibers.
Confirmation of Capillaries Embedded in the Sarcolemma
Higher resolution (200 nm x-, y-, and z-pixel size) 3D images of in vivo skeletal muscle were collected to provide a better view on how capillaries interact with muscle fibers. Figure 4 shows a series of 2D images from a representative oxidative muscle fiber surrounded by capillaries (movie showing flowing blood is found in Supplemental Video 4). Just as with the lower resolution images shown in Figures 1 and 3, the capillaries appear to be wrapped around the fiber in grooves. Note that mitochondria, represented by the green NAD(P)H signal in Figure 4, seem highly concentrated in the areas directly lateral, but not deep to the embedded capillaries.
Figure 4.
Series of higher resolution 2D skeletal muscle images in vivo. A) XY image from just above oxidative muscle fiber. B) XY image at the surface of oxidative muscle fiber. C) XY image just below surface of oxidative muscle fiber. D) XY image in the center of oxidative muscle fiber. X-, Y-, and Z-pixel sizes are 0.2 μm. Field of view is 102.4 × 102.4 μm. Quantification of image depth relative to fiber surface was confounded by slow tissue drift in the z-direction caused by the intensity of light necessary to collect high resolution images. Movie of this 3D image stack showing flowing blood can be found in Supplemental Video 4.
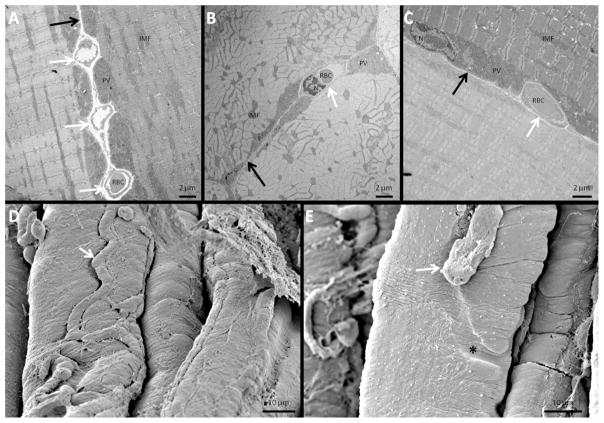
The representative TEM images of immersion fixed muscle shown in Figures 5A–C confirmed that the embedding of capillaries in the sarcolemma of oxidative muscle fibers were not simply an artifact of multi-photon microscopy imaging. Figure 5A shows a longitudinal image of two adjacent oxidative fibers with three capillaries embedded equally in each fiber. A cross-sectional image of two adjacent oxidative fibers is shown in Figure 5B with equal sharing of a single capillary. Figure 5C shows a longitudinal section of an oxidative fiber next to a glycolytic fiber; however, the shared capillary embeds only into the oxidative fiber, suggesting capillary embedding may occur preferentially in these more aerobic fibers. Consistent with the in vivo images in Figure 1 and more clearly in Figure 4, mitochondria in the TEM images were concentrated in the areas lateral, but not deep to the embedded capillaries. Additionally, the thickness of the paravascular (PV) pool of mitochondria always appeared to be matched to the depth of the adjacent embedded capillary.
Figure 5.
Electron microscopy images of fixed C57BL/6 mouse skeletal muscle fibers. A) TEM image of two longitudinally-sectioned adjacent oxidative muscle fibers with three capillaries shared between them. B) TEM image of two cross-sectioned adjacent oxidative fibers with one capillary between them. C) TEM image of longitudinally-sectioned adjacent glycolytic and oxidative fibers with one capillary between them. D) SEM image showing a capillary running along a groove on the top of a muscle fiber. E) SEM image showing a fractured capillary revealing a groove (black asterisk) on the surface of a muscle fiber. White arrows point to embedded capillaries. Black arrows point to fiber boundaries. PV: paravascular mitochondria. IMF: intermyofibrillar mitochondria. RBC: red blood cell. N: nucleus.
The SEM image of freeze-fractured muscle in Figure 5D clearly shows a capillary in a groove running along the surface of a muscle fiber. Figure 5E depicts a capillary which was broken off by the freeze-fracture procedure leaving an empty groove behind on the surface of the muscle fiber.
Myoglobin Knockout Mice
The protein profiles of heart and skeletal muscle in Mb KO (red spots) and WT (green spots) mice are very similar as evident by the predominance of yellow spots shown in Figure 6. The primary difference was that Mb was not detected in either the heart or skeletal muscle samples from the Mb KO mice. Additionally, mitochondrial Complex IV content was no different between either the hearts (43.7 ± 2.2 and 38.5 ± 3.1 nmol cyt a/g wet) or TA muscles (8.2 ± 0.6 and 8.1 ± 0.5 nmol cyt a/g wet) of Mb WT and KO mice, respectively. The TA muscles of Mb WT and KO mice also were no different with respect to fiber typing by in vivo NAD(P)H intensity (Figure 7A) and consisted of a majority of glycolytic muscle fibers (68.2 ± 1.6% and 75.1 ± 3.2%, respectively). There were no differences in fiber cross-sectional area or fiber perimeter between Mb KO and WT mice (Figures 7B–C) though intermediate and oxidative fibers in the KO mice had smaller cross-sectional areas than glycolytic fibers (Figure 7B). Fiber surface area-to-volume ratios increased from glycolytic to intermediate to oxidative fibers but were no different between Mb KO and WT mice (Figure 7D). PV mitochondrial density relative to IMF mitochondrial density was higher in oxidative than glycolytic fibers in both Mb WT and KO mice (Figure 8A).
Figure 6.
2D-DIGE protein profiles of heart and skeletal muscle of Mb WT and KO mice. A) 2D heart protein profile. B) 2D TA muscle protein profile. Red spots represent higher abundance of proteins in Mb KO tissues, green spots represent higher abundance of proteins in Mb WT tissues, and yellow spots represent proteins with equal abundance in Mb WT and KO tissues. Images shown are representative of 4 paired experiments from each tissue.
Figure 7.
In vivo muscle fiber type and morphology in Mb KO and WT mice. A) Fiber types as a percentage of total fibers. B) Fiber cross-sectional area for each fiber type. C) Fiber perimeter for each fiber type. D) Fiber surface area-to-volume ratios for each fiber type. N = 61, 13, and 8 for Mb WT glycolytic, intermediate, and oxidative fibers, respectively with 457 ± 13 sequential images analyzed per fiber. N = 58, 15, and 7 for Mb KO glycolytic, intermediate, and oxidative fibers, respectively with 559 ± 39 sequential images analyzed per fiber. Data are from 4 pairs of WT and KO mice. * denotes significant difference from glycolytic fibers. # denotes significant difference from intermediate fibers. + denotes significant difference from Mb WT mice.
Figure 8.
In vivo mitochondrial and capillary distribution in the skeletal muscle of Mb KO and WT mice. A) PV mitochondrial density relative to IMF mitochondrial density for each fiber type. B) Number of capillaries per fiber for each fiber type. C) Number of capillaries at least 50% embedded for each fiber type. D) Percentage of capillaries embedded for each fiber type. For mitochondrial density measurements, N = 4 fibers of each fiber type for both Mb KO and WT mice. For capillary measures, N = 61, 13, and 8 for Mb WT glycolytic, intermediate, and oxidative fibers, respectively with 457 ± 13 sequential images analyzed per fiber. N = 58, 15, and 7 for Mb KO glycolytic, intermediate, and oxidative fibers, respectively with 559 ± 39 sequential images analyzed per fiber. Data are from 4 pairs of WT and KO mice. * denotes significant difference from glycolytic fibers. # denotes significant difference from intermediate fibers. + denotes significant difference from Mb WT mice.
The number of capillaries per fiber increased with fiber oxidative capacity, and there were more capillaries per fiber in Mb WT than KO in both glycolytic and oxidative fibers (Figure 8B). Glycolytic fibers had fewer embedded capillaries than intermediate or oxidative fibers (Figure 8C), and Mb KO glycolytic fibers had a greater percentage of embedded capillaries compared to Mb WT glycolytic fibers (Figure 8D).
Fiber-to-vessel contact area relative to total fiber surface area, fiber-to-vessel contact area per capillary, total vessel volume supporting a fiber, and fiber volume embedded with capillaries all increased as a function of fiber oxidative capacity (Figure 9). Glycolytic fibers from Mb KO mice had less fiber-to-vessel contact relative to fiber surface area and lower total vessel volume than Mb WT mice (Figures 9A and C).
Figure 9.
In vivo vessel-to-muscle fiber morphology in Mb KO and WT mice. A) Fiber-to-vessel contact area relative to total fiber surface area for each fiber type. B) Fiber-to-vessel contact area relative to total vessel surface area for each fiber type. C) Total volume of vessels supporting each fiber type. D) Volume of fibers embedded with capillaries for each fiber type. N = 61, 13, and 8 for Mb WT glycolytic, intermediate, and oxidative fibers, respectively with 457 ± 13 sequential images analyzed per fiber. N = 58, 15, and 7 for Mb KO glycolytic, intermediate, and oxidative fibers, respectively with 559 ± 39 sequential images analyzed per fiber. Data are from 4 pairs of WT and KO mice. * denotes significant difference from glycolytic fibers. # denotes significant difference from intermediate fibers. + denotes significant difference from Mb WT mice.
GLUT4-GFP Mice
The potential relationship between capillary embedding and glucose uptake into muscle was investigated by in vivo imaging of mice expressing GLUT4 labeled with GFP. Figure 10 shows longitudinal and cross-sectional views of the GLUT4-GFP signal in skeletal muscle in vivo before and after stimulating GLUT4 translocation through muscle contractions. Prior to muscle contraction, GLUT4 was largely present in vesicles located throughout the muscle fibers (Figures 10A and C). After contraction, there was less GLUT4 in vesicles and an increase in signal at the sarcolemma and t-tubules (Figures 10B and D). The white arrows in Figure 10 point to the negative signal caused by blood vessels and show no evidence of an increased localization of GLUT4 near embedded capillaries either before or after stimulating GLUT4 translocation. We were unable to find any evidence of increased localization of GLUT4 to embedded capillaries after searching throughout the multi-photon microcopy accessible portions of TA muscles of six mice. Movies of the image stacks shown in Figure 10 can be found in Supplemental Videos 5–8.
Figure 10.
GLUT4-GFP localization to embedded capillaries. A) Representative XY image plane provides longitudinal view of basal skeletal muscle GLUT4-GFP signal in vivo. B) Representative XY image plane provides longitudinal view of post-contraction skeletal muscle GLUT4-GFP signal in vivo. C) Representative YZ image plane shows cross-sectional view of basal skeletal muscle GLUT4-GFP signal in vivo. D) Representative YZ image plane shows cross-sectional view of post-contraction skeletal muscle GLUT4-GFP signal in vivo. Arrows point to the negative signal left by blood vessels. Movies for A-D can be found in Supplemental Videos 5–8, respectively. Images are representative of experiments on 6 mice.
Discussion
In vivo, 3D microscopy revealed that a significant portion of capillaries associated with skeletal muscle fibers are embedded in grooves in the sarcolemma. Although neuromuscular junctions are well-known to involve grooves, pits, and/or depressions in the sarcolemma and have been described in many species and models for over 50 years (15, 18, 58, 71, 81), we were unable to find any previous quantitative description of capillaries embedded in the sarcolemma of muscle fibers. Thirty years ago, several skeletal muscle SEM studies showed images of capillary grooves along the surface of muscle fibers (15, 34, 55); however, these were just noted observations, and there was no quantitative analysis or discussion of the physiological relevance of these grooves.
Implications for Oxygen Diffusion
Vessel to fiber contact area is perhaps the most reliable measure of the structural capacity for oxygen flux from capillary to mitochondria in skeletal muscle (53) and has been shown to correlate well with mitochondrial content even when the capillary to fiber ratio does not (68). In the current analysis, the capillary contact area was enhanced in oxidative fibers compared to glycolytic fibers by three major factors: 1) the ~85% more capillaries associated with each fiber (Figure 8B), 2) the greater number of embedded capillaries (Figure 8C) resulting in a 70–100% higher capillary contact area per capillary (Figure 9B) and 3) the 18–32% smaller size of the fiber leading to smaller diffusion distances (Figure 7B). The net result is a more than three-fold higher capacity for oxygen delivery to the oxidative fibers based on the increased surface area contact in these fibers (Figure 9A). Noteworthy is that the greater capillary contact area per capillary and the greater number of capillaries each contribute about equally to the increased capacity for oxygen delivery to oxidative fibers.
Based on these observations, we propose that embedding capillaries in the sarcolemma of skeletal muscle fibers provides an additional level of control over oxygen delivery beyond simply increasing the number of capillaries. This mechanism may be particularly important for the optimal delivery of oxygen to heterogeneous groups of muscle fibers. Skeletal muscle in both humans (42) and mice (11) rarely consist of a single fiber type and, instead, is comprised of a mix of several muscle fiber types with varying aerobic capacities. As depicted in Figures 1B, 3A, and 5C, oxidative and glycolytic fibers are often located directly adjacent to one another. Thus, modulating oxygen delivery capacity simply by matching the number of capillaries surrounding a fiber to its aerobic capacity becomes a near impossible task as adjacent oxidative and glycolytic fibers commonly share ~25% of their respective fiber boundaries. Increasing the number of capillaries in this shared boundary may provide the necessary oxygen to the oxidative fiber but may result in oversupply of oxygen to the glycolytic fiber and the associated toxic effects (57). Conversely, leaving capillaries out of this shared boundary may be optimal for the glycolytic fiber but result in a deficient oxygen supply to the oxidative fiber. By controlling the depth of embedding into the sarcolemma of each of the two adjacent fibers, capillaries can be placed in this shared boundary while maintaining an appropriate balance between oxygen supply and demand. Examples of modulating the depth of embedding to match the respective oxygen demands of two adjacent fibers are shown in Figure 5. In Figures 5A and 5B, two oxidative fibers are adjacent to one another, and the shared capillaries are equally embedded into each fiber. In Figure 5C, an oxidative and a glycolytic fiber share a boundary, and the shared capillary is preferentially embedded into the oxidative fiber. It is important to note that capillaries do embed into glycolytic and intermediate fibers, albeit to a lesser degree than in oxidative fibers (Figure 8C). However, out of the 13 in vivo, 3D datasets and 107 TEM images collected, we did not find a single example of a capillary preferentially embedding into the more glycolytic of two adjacent fibers.
Relationship between Capillary Embedding and Myoglobin
Mb is a cytosolic hemoprotein which is nearly homogeneously distributed throughout the muscle, including near embedded capillaries (46), and is believed to enhance oxygen transport from the sarcolemma to the mitochondria (85) via simple mass action effects. Although initial reports that Mb KO mice exhibited no phenotype with regard to cardiac function or exercise capacity challenged the role of Mb in oxygen transport (23), subsequent investigations revealed that Mb KO mice undergo a number of compensatory adaptations (25, 28, 56), including a 21% increase in capillary density in the soleus (28). For these reasons, Mb KO mice were considered a good model to further investigate the role of capillary embedding in skeletal muscle fibers. We hypothesized that there would be a greater degree of embedding in the Mb KO mice along with increased capillary density to compensate for decreased oxygen diffusion capacity. However, we found no difference in the number of embedded capillaries between Mb KO and WT mice (Figure 8C). In fact, there were slightly fewer capillaries associated with Mb KO muscle fibers (Figure 8B), and overall, we found very little difference between Mb KO and WT muscle fibers and their capillary networks (Figures 7–9). Furthermore, we surprisingly found no molecular adaptation, at least within the sensitivity limits of 2D DIGE, in muscle protein composition in the KO muscle with the exception of Mb itself, confirming that the knockout occurred (Figure 6). No changes in myofilament or mitochondrial protein content were detected suggesting that, even though the assumed ability to deliver oxygen to the mitochondria was compromised, the ratio of myofilaments to mitochondria remained constant. It would be reasonable to assume that if the mitochondria were comprised by a lack of oxygen delivery, that it would take more mitochondria to support a given amount myofilaments, however, this was not observed.
With regard to the PV mitochondria themselves, it is unlikely that Mb does facilitate the diffusion over the short distances from the capillary to these mitochondria, with the possible exception of those in the taper far away from the capillary if intra-mitochondrial coupling does not occur. However, the fact that the number of these embedded capillary regions was not altered to compensate for the removal of the Mb contribution to oxygen diffusion is surprising. The simplest explanation of these results is that the capillary density and embedding are not influenced by Mb facilitated oxygen diffusion on the cellular level. Coupled to the minor changes in exercise capacity in these animals (23), these results suggest that Mb facilitated diffusion may only play an important role under maximum work conditions, which laboratory mice rarely experience, and, thus, would have little or no impact on the overall oxygen delivery under normal conditions. Thus, no molecular or morphological modification of the tissue was observed. It may be interesting to explore the effects of maximal exercise training on the morphology and proteomic composition in the Mb KO mice, however, this was outside the scope of the current study.
Another role of Mb in muscle may be as a nitrite reductase (31). The largest source of nitric oxide (NO) is the vascular endothelial cells (80), therefore, the proximity of embedded capillaries to paravascular mitochondria leaves myoglobin less opportunity to reduce NO as compared to mitochondria deep in the muscle. Indeed, the mitochondria themselves are very powerful at metabolizing NO (21), thus, the PV mitochondria may indeed be playing a role in consuming vascular NO production (21). In any event, no morphological or biochemical adaptations were detected with the elimination of myoglobin nitrite reductase activity.
Mitochondrial Pool Associated with Capillary Embedding
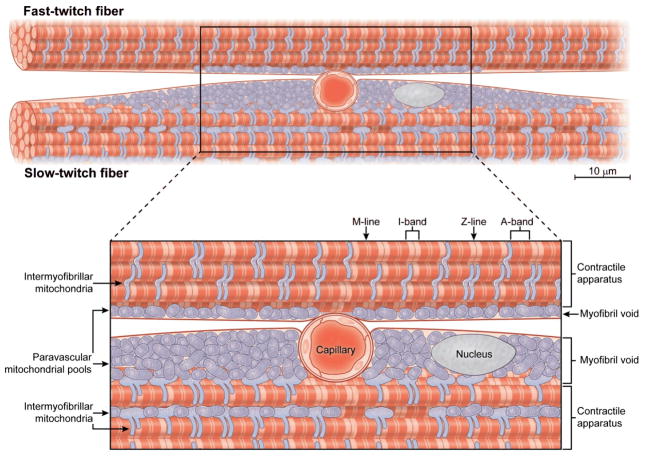
A very reproducible mitochondrial distribution around embedded capillaries was observed in this study both in fixed histology and in vivo microscopy experiments, as well as anecdotally in previous reports (36, 73, 82). Nuclei are also commonly co-localized in this paravascular region (70, 72). Examination of the basement membranes by electron microscopy does not reveal any remarkable differences between the shared basement membrane of the sarcolemma capillary groove and the free sarcolemma, although this does not preclude differences in molecular composition (29). Based on our experience in measuring mitochondrial distribution within skeletal muscles in vivo in this study of mice, as well as previously in rabbits and rats (40, 74, 75), we have concluded that most of the so called “subsarcolemmal” mitochondria are actually associated with a pool of mitochondria surrounding capillaries in oxidative muscle fibers and can perhaps be more aptly described as “paravascular” (PV) mitochondria.
It is unclear what guides the PV mitochondrial topology. One hypothesis is that the mitochondria are located near the capillaries for oxygen availability or vascular signaling, including NO as discussed above. Another possibility is that the PV mitochondrial topology is driven by the simple fact that the capillary groove creates a myofibril void in the cytosol that is filled with mitochondria and/or nuclei not interfering with muscle action. We believe the following observations are more consistent with the hypothesis that the PV mitochondria are the result of the mechanical requirements of the fiber and not the proximity to the oxygen source: 1) the co-localization of the PV mitochondrial pool with nuclei, which have no specific requirement for oxygen, 2) the horizontal extension of the PV mitochondrial pool for many microns away from the capillary limiting any diffusion advantage, 3) mitochondria do not encircle the capillary but only run laterally in the mechanical “eddy” of the embedded capillary, and 4) Mb removal, and the implied decrease in oxygen delivery capacity, had no effect on the prevalence of the PV mitochondrial pool. This mechanical hypothesis suggests that a muscle fiber needs a specific volume of mitochondria to support contractile activity and that the mitochondria, along with the nuclei, are simply placed where they will not interfere with the continuous end to end run of the contractile fibers. The mechanical “eddy” created by the capillary groove in the sarcolemma to enhance overall oxygen “capture” and improve oxygen delivery to the overall fiber is an appropriate location to place the mitochondria and nuclei and is shown schematically in Figure 11. Critical for a better analysis is the actual structure of the mitochondria in these regions. Are the PV mitochondria coupled (66, 67), permitting the transmission of energy via the mitochondrial membrane potential throughout the PV region from the capillary oxygen source? Obtaining 3D images with nanometer resolution electron microscopy (61) may offer further insight into the structural and functional relationships between capillaries and both PV and IMF pools of mitochondria.
Figure 11.
Schematic representation of capillary preferentially embedding in the sarcolemma of a slow-twitch skeletal muscle fiber. The embedded capillary creates a myofibril void in the cytosol that is consequently filled with paravascular mitochondria and nuclei lateral but not deep to the capillary. Intermyofibrillar mitochondria are often located in pairs on each side of the z-lines as well as in between and parallel to the myofibrils.
Role of the Capillary-to-Fiber Relationship in Glucose Transport
The embedding of the capillaries within muscle fibers may provide enhanced delivery of other vascular molecules to the muscle rather than just oxygen. One possibility is glucose, which is a major substrate to supply energy for muscle contraction. Similar to oxygen consumption, glucose uptake into skeletal muscle increases incrementally to match the rate of muscle contractions (43). Glucose uptake during exercise is facilitated by the translocation of a glucose transporter protein, GLUT4, to the cell surface (50), and, just as with mitochondrial content, GLUT4 content is highest in aerobic muscles (32, 41). We hypothesized that GLUT4 would localize to the embedded capillaries, and in particular, to the area of the capillary closer to the center of the fiber where mitochondria are not present. As shown in Figure 10, we did not find an increase in density of GLUT4 translocated to the sarcolemma/capillary grooves compared to the rest of the cell surface either before or after a bout of electrically stimulated muscle contractions. These data suggest that the embedding of the capillary does not enhance glucose uptake in these muscles since the transporters are not concentrated in this region to take advantage of the increased capillary contact area. This conclusion is not surprising since glycolytic fibers run almost exclusively from glucose driving a glycolytic ATP production and have very low capillary contact areas implying that the interstitial supply of glucose to the sarcolemma is adequate for glucose uptake in both oxidative and glycolytic fibers.
Limitations
The use of multi-photon microscopy to evaluate skeletal muscle morphology in vivo offers several advantages over traditional methods including the ability to collect large, 3D datasets and to avoid the artifacts caused by fixation or tissue biopsies (33). However, to achieve adequate resolution deep into the muscle, removal of the skin and layers of fascia on top of the muscle is required. This invasive process may alter the morphology of the muscle possibly due to inflammation, tissue damage, exposure of the muscle fibers to the open environment, or other factors. Inflammation has been shown to both increase and decrease vascular perfusion depending on the circumstances (3, 49). However, as we restricted our analysis to capillaries and capillaries neither dilate nor constrict (44), any inflammation caused by exposing the muscle likely did not alter the results presented here. We are also confident that tissue damage did not play a significant role in this study for at least three reasons. First, major damage to the muscle during surgical exposure causes the fibers to lose their parallel alignment, and as shown in Figures 1–4, the muscle fibers imaged here remain aligned to one another. Second, minor damage which may only puncture a fiber but not alter its overall structure would result in the CFSE dye entering the fiber, and although we have observed this in poorly prepared muscles, we did not observe this in the datasets used here. Finally, because muscle exposure occurred prior to injection of the fluorescent vasculature dye, any damage to the vasculature caused by surgery would be predicted to result in a disconnected or partially unlabeled vascular network. As shown in Figure 2, the vascular network was highly connected. Moreover, because the vascular network leaves negative images in the mitochondrial NAD(P)H signal due to capillary embedding, if some capillaries were not labeled with dye, we would note a mismatch between the negative images in the NAD(P)H signal and the signal from the vascular dye, and this did not occur here. Thus, for this primarily anatomical study, we do not believe that the surgical exposure significantly compromised the conclusions reached.
Another concern with exposing the muscle for in vivo imaging is that the extracellular environment surrounding the fibers may be altered. High resolution imaging of skeletal muscle in vivo typically involves either immersion of hindlimb muscles in saline solution (20, 74), superfusion of saline solution over an exteriorized muscle (5, 45), or the use of an iso-osmotic optical gel (75, 76). For these studies, we chose to use a custom made carbomer gel with a refractive index similar to water (75) as it allowed the muscle to remain intact within the leg, and using a small volume of viscous gel (~ 0.5 mL) lowers the potential dilution of the extracellular components compared to immersion in a larger volume of salt solution. It should be noted that a spherical aberration collar correction for the water immersion lens improves the point spread function with this carbomer gel suggesting that the gel does not have a refractive index identical to water. Significant alterations in the osmolarity of the extracellular milieu would be predicted to cause shrinking or swelling of the muscle over time (19). Although we have observed and quantified rapid tissue swelling/shrinking after inducing death or during continuous illumination of a muscle volume (6), there was little tissue drift observed in these studies where the focal plane was constantly changing.
Quantitation of the vessel to fiber relationships relies on the collection of images which accurately portray the true geometry within the muscle. In a multi-photon microscope image, the object is distorted by the point spread function (PSF) of the microscope (30) and tissue inner filter effects (74). Under ideal conditions where diffraction limited resolution is achieved, the resolution is three times worse in the axial direction than in the transverse direction for the objective lens used here (87). Further, this optimal resolution may only be achieved at the sample surface with an objective collar properly set to correct for the spherical aberrations induced by the optical coupling gel. Image resolution is further degraded by depth dependent spherical aberrations caused by the refractive index mismatch between the tissue and immersion medium. As a result, axial and transverse resolutions decrease approximately linearly with depth, with differing rates, beyond a modest imaging depth (> ~50 μm) (10). In our system, these combined effects result in a stretching of images in the z-direction (e.g. several capillaries in Figures 1B and 3A) which may alter the spatial relationships between fibers, vessels, and mitochondria. This elongation in the axial direction may cause an overestimation of how much of the muscle volume is accounted for by embedded capillaries (Figure 9D), but should not change the determination of whether a capillary is 50% embedded or not as the relationship between the center of a capillary and the fiber boundary (Supplemental Figure 1) should remain unaffected. In addition, the distortions caused by the PSF should be similar among the different fiber types and between Mb KO and WT mice. Myoglobin, hemoglobin and the mitochondrial cytochromes are known secondary inner filter agents in muscle. However, secondary inner filter effects should primarily affect the intensity of the images with depth and not the spatial resolution, and thereby have little effect on the morphology outcomes reported here. The PSF may also vary in the transverse direction due to tissue refractive index inhomogeneities and may be further distorted by two-photon excitation saturation (8). For these reasons, compensation of the distortions mentioned above would require spatially variant 3D image deconvolution (62). This method requires knowledge of the local PSF across the 3D volume, information that remains a challenge to acquire in live tissue. As a result, image deconvolution was not applied on these images.
Another integral part of quantifying the vessel to fiber relationship was the segmentation of the vascular network. This process applies filtering and intensity thresholds on the vessel images to create binary images and thereby allow for determination of vessel sizes and volumes in 3D. Determining the appropriate threshold is critical to the accuracy of the data as too low a threshold results in vessels that are too large and too high a threshold results in vessel exclusion. Because vessels come in various sizes within a muscle and image resolution and intensity decrease with imaging depth, the optimal threshold for different regions within a volume is likely different. Unfortunately, we are unable to optimize the threshold for different regions within a volume at this time. Therefore, we chose to err on the side of including all vessels which likely resulted in the overestimation of the size of some of the vessels. The data in Figures 8B-D counting capillaries and embedded capillaries should be unaffected by this decision. Any overestimation of vessel sizes would be reflected in the data in Figure 9 describing the fiber-to-vessel contact, vessel volume, and fiber volume embedded. However, vessel size inaccuracies should be uniform across all fiber types and should also be similar between Mb KO and WT muscles. Thus, while some of the absolute values reported here may be slightly overestimated, the fiber type and Mb KO and WT comparisons remain valid, as well as the number of embedded capillaries. Development of data collection and post-processing tools to normalize the intensity and PSF in all regions of a complex volume of living tissue would be very beneficial to overcoming these limitations in future in vivo morphological investigations.
While multi-photon microscopy allows for much deeper 3D imaging than confocal microscopy (12), we were still unable to image through the depth of the entire TA muscle in vivo. As a result, our in vivo analyses were limited to the superficial regions of the muscle where micron resolution could be maintained. Imaging depths ranged from 250 – 650 μm in the Mb WT and KO muscles with an average depth of 414 ± 40 μm compared to the full depth of a mouse TA of about 2000 μm (11). Deeper portions of skeletal muscle are reported to have greater capillary densities than those near the surface (4, 16, 73), and, therefore, it is possible that the relationships reported here only pertain to the superficial fibers we could image. However, the differences in capillary density reported for different regions within the same muscle also corresponded to changes in fiber oxidative capacity and/or fiber type (4, 16, 73). Thus, as Romanul (73) noted when assessing different regions within a muscle, “…every muscle fiber is surrounded by a number of capillaries which is directly proportionate to the oxidative metabolic activity of the fiber.” As the in vivo analyses reported here in Figures 7 – 9 were done on individual fibers based on their oxidative capacity and not on groups of fibers within a region or field of view, we believe the relationships reported here, particularly the degree of capillary embedding, hold true throughout the entire mouse TA. Though we were unable to evaluate fibers at different depths, we did compare the fiber-to-vessel contact values (as in Figure 9A) among five 3D datasets from three different C57BL/6 TA muscles (data not shown). There were no differences in either oxidative or glycolytic fiber-to-vessel contact among any of the datasets suggesting again that there are no regional differences in capillarity when fiber oxidative capacity is accounted for, at least in the superficial portion of the TA.
Summary
Examination of the 3D skeletal muscle microvasculature network in vivo revealed that a significant fraction of capillaries are embedded in specialized grooves in the sarcolemma of muscle fibers. These capillary grooves may provide an additional level of control over oxygen supply to adjacent muscle fibers by modulating the degree of fiber-to-vessel contact through alterations in the depth of embedding. Capillary embedding also results in myofibril voids lateral to the vessels that are filled with mitochondria and nuclei. The presence of mitochondria lateral but not deep to embedded capillaries suggests that mitochondrial distribution near the sarcolemma of skeletal muscle cells is not optimized for oxygen diffusion, but rather, mitochondria are located where they avoid the contractile apparatus. Thus, capillary embedding increases the capacity for oxygen diffusion into a muscle cell and, by creating myofibril voids, increases the cellular volume available for mitochondria, thereby allowing for the required volume of mitochondria to support contractile activity without interfering with the contractile process.
Supplementary Material
Figure S1: Morphology variables measured. 2D fiber and vessel circumferences become surface areas in 3D. 2D fiber and vessel areas become volumes in 3D. Variables are measured for each traced fiber and every vessel in contact with the respective fiber.
Perspectives.
The spatial relationship between capillaries, mitochondria, and muscle fibers is critical to the oxygen delivery, and, thus, aerobic work capacity of the muscle, yet the 3D associations of these structures in live tissue remain unclear. Evaluation of the 3D spatial relationship between capillaries, mitochondria, and muscle fibers in vivo revealed a significant fraction of capillaries embedded in grooves in the sarcolemma of muscle fibers. These specialized sarcolemma/capillary grooves may offer an additional level of control over oxygen delivery to heterogeneous populations of muscle fibers and thereby provide a novel tool to explore how oxygen delivery may be compromised with aging, disease, or other pathologies.
Acknowledgments
The authors wish to thank Kathryn White (EM Research Services, Newcastle University) for expert technical assistance in preparing and visualizing the SEM samples and Dr. Bertrand Lucotte (NHLBI) for discussions and advice regarding the technical limitations of two-photon microscopy. Additionally, we are grateful to Jürgen Schrader (Heinrich-Heine-Universität) for kindly providing us with the myoglobin knockout mice.
Grants
This study was supported by Intramural Funding of the Division of Intramural Research, National Heart, Lung, and Blood Institute.
List of Abbreviations
- VO2max
maximal whole body oxygen uptake
- Mb
myoglobin
- GFP
green fluorescent protein
- GLUT4
glucose transporter type 4
- TA
Tibialis anterior
- KO
knockout
- WT
wild type
- di-8-ANEPPS
di-8-[butyl] amino-naphthyl-ethylenepyridinium-propyl-sulfonate
- CFSE
carboxyfluorescein succinimidyl ester
- NA
numerical aperture
- ROVAR
rapid overlapping volume acquisition and reconstruction
- NAD(P)H
nicotinamide adenine dinucleotide (phosphate)
- DMSO
dimethylsulfoxide
- TEM
transmission electron microscopy
- SEM
scanning electron microscopy
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- EGTA
ethylene glycol tetraacetic acid
- EDTA
ethylenediaminetetraacetic acid
- DIGE
differential in gel electrophoresis
- IEF
isoelectric focusing
- DTT
dithiothreitol
- CHAPS
3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- ANOVA
analysis of variance
- PV
paravascular
- IMF
intermyofibrillar
- PSF
point spread function
References
- 1.Aponte AM, Phillips D, Harris RA, Blinova K, French S, Johnson DT, Balaban RS. 32P labeling of protein phosphorylation and metabolite association in the mitochondria matrix. Methods Enzymol. 2009;457:63–80. doi: 10.1016/S0076-6879(09)05004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aponte AM, Phillips D, Hopper RK, Johnson DT, Harris RA, Blinova K, Boja ES, French S, Balaban RS. Use of (32)P to study dynamics of the mitochondrial phosphoproteome. Journal of proteome research. 2009;8:2679–2695. doi: 10.1021/pr800913j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armour J, Tyml K, Lidington D, Wilson JX. Ascorbate prevents microvascular dysfunction in the skeletal muscle of the septic rat. J Appl Physiol. 2001;90:795–803. doi: 10.1152/jappl.2001.90.3.795. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong RB, Delp MD, Goljan EF, Laughlin MH. Distribution of blood flow in muscles of miniature swine during exercise. J Appl Physiol. 1987;62:1285–1298. doi: 10.1152/jappl.1987.62.3.1285. [DOI] [PubMed] [Google Scholar]
- 5.Bagher P, Segal SS. The mouse cremaster muscle preparation for intravital imaging of the microcirculation. J Vis Exp. 2011 doi: 10.3791/2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakalar M, Schroeder JL, Pursley R, Pohida TJ, Glancy B, Taylor J, Chess D, Kellman P, Xue H, Balaban RS. Three-dimensional motion tracking for high-resolution optical microscopy, in vivo. J Microsc. 2012;246:237–247. doi: 10.1111/j.1365-2818.2012.03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balaban RS, Mootha VK, Arai A. Spectroscopic determination of cytochrome c oxidase content in tissues containing myoglobin or hemoglobin. Anal Biochem. 1996;237:274–278. doi: 10.1006/abio.1996.0239. [DOI] [PubMed] [Google Scholar]
- 8.Berland K, Shen G. Excitation saturation in two-photon fluorescence correlation spectroscopy. Appl Opt. 2003;42:5566–5576. doi: 10.1364/ao.42.005566. [DOI] [PubMed] [Google Scholar]
- 9.Blinova K, Levine RL, Boja ES, Griffiths GL, Shi ZD, Ruddy B, Balaban RS. Mitochondrial NADH fluorescence is enhanced by complex I binding. Biochemistry. 2008;47:9636–9645. doi: 10.1021/bi800307y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth MJ, Wilson T. Refractive-index-mismatch induced aberrations in single-photon and two-photon microscopy and the use of aberration correction. J Biomed Opt. 2001;6:266–272. doi: 10.1117/1.1382808. [DOI] [PubMed] [Google Scholar]
- 11.Burkholder TJ, Fingado B, Baron S, Lieber RL. Relationship between muscle fiber types and sizes and muscle architectural properties in the mouse hindlimb. Journal of morphology. 1994;221:177–190. doi: 10.1002/jmor.1052210207. [DOI] [PubMed] [Google Scholar]
- 12.Centonze VE, White JG. Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophys J. 1998;75:2015–2024. doi: 10.1016/S0006-3495(98)77643-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies KJ, Packer L, Brooks GA. Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch Biochem Biophys. 1981;209:539–554. doi: 10.1016/0003-9861(81)90312-x. [DOI] [PubMed] [Google Scholar]
- 14.Dawson JM, Hudlicka O. Changes in the microcirculation in slow and fast skeletal muscles with long term limitations of blood supply. Cardiovascular research. 1990;24:390–395. doi: 10.1093/cvr/24.5.390. [DOI] [PubMed] [Google Scholar]
- 15.Desaki J, Uehara Y. The overall morphology of neuromuscular junctions as revealed by scanning electron microscopy. J Neurocytol. 1981;10:101–110. doi: 10.1007/BF01181747. [DOI] [PubMed] [Google Scholar]
- 16.Deveci D, Marshall JM, Egginton S. Relationship between capillary angiogenesis, fiber type, and fiber size in chronic systemic hypoxia. Am J Physiol Heart Circ Physiol. 2001;281:H241–252. doi: 10.1152/ajpheart.2001.281.1.H241. [DOI] [PubMed] [Google Scholar]
- 17.di Prampero PE. Metabolic and circulatory limitations to VO2 max at the whole animal level. J Exp Biol. 1985;115:319–331. doi: 10.1242/jeb.115.1.319. [DOI] [PubMed] [Google Scholar]
- 18.Edwards GA, Ruska H, De Harven E. Electron microscopy of peripheral nerves and neuromuscular junctions in the wasp leg. J Biophys Biochem Cytol. 1958;4:107–114. doi: 10.1083/jcb.4.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eichelberger L. The Distribution of Water and Electrolytes between Blood and Skeletal Muscle in Experimental Hypertension. J Exp Med. 1943;77:205–213. doi: 10.1084/jem.77.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang H, Chen M, Ding Y, Shang W, Xu J, Zhang X, Zhang W, Li K, Xiao Y, Gao F, Shang S, Li JC, Tian XL, Wang SQ, Zhou J, Weisleder N, Ma J, Ouyang K, Chen J, Wang X, Zheng M, Wang W, Cheng H. Imaging superoxide flash and metabolism-coupled mitochondrial permeability transition in living animals. Cell Res. 2011;21:1295–1304. doi: 10.1038/cr.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.French S, Giulivi C, Balaban RS. Nitric oxide synthase in porcine heart mitochondria: evidence for low physiological activity. Am J Physiol Heart Circ Physiol. 2001;280:H2863–2867. doi: 10.1152/ajpheart.2001.280.6.H2863. [DOI] [PubMed] [Google Scholar]
- 22.Fujino H, Kondo H, Murakami S, Nagatomo F, Fujita N, Takeda I, Ishihara A, Roy RR. Differences in capillary architecture, hemodynamics, and angiogenic factors in rat slow and fast plantarflexor muscles. Muscle Nerve. 2012;45:242–249. doi: 10.1002/mus.22267. [DOI] [PubMed] [Google Scholar]
- 23.Garry DJ, Ordway GA, Lorenz JN, Radford NB, Chin ER, Grange RW, Bassel-Duby R, Williams RS. Mice without myoglobin. Nature. 1998;395:905–908. doi: 10.1038/27681. [DOI] [PubMed] [Google Scholar]
- 24.Glancy B, Balaban RS. Protein composition and function of red and white skeletal muscle mitochondria. Am J Physiol Cell Physiol. 2011;300:C1280–1290. doi: 10.1152/ajpcell.00496.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Godecke A, Flogel U, Zanger K, Ding Z, Hirchenhain J, Decking UK, Schrader J. Disruption of myoglobin in mice induces multiple compensatory mechanisms. Proc Natl Acad Sci U S A. 1999;96:10495–10500. doi: 10.1073/pnas.96.18.10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldspink G. Alterations in Myofibril Size and Structure During Growth, Exercise, and Changes in Environmental Temperature. Comprehensive Physiology. 2011:539–554. [Google Scholar]
- 27.Gollnick PD, Armstrong RB, Saltin B, Saubert CWt, Sembrowich WL, Shepherd RE. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973;34:107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- 28.Grange RW, Meeson A, Chin E, Lau KS, Stull JT, Shelton JM, Williams RS, Garry DJ. Functional and molecular adaptations in skeletal muscle of myoglobin-mutant mice. Am J Physiol Cell Physiol. 2001;281:C1487–1494. doi: 10.1152/ajpcell.2001.281.5.C1487. [DOI] [PubMed] [Google Scholar]
- 29.Gulati AK, Reddi AH, Zalewski AA. Changes in the basement membrane zone components during skeletal muscle fiber degeneration and regeneration. J Cell Biol. 1983;97:957–962. doi: 10.1083/jcb.97.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hell SW, Soukka J, Hänninen PE. Two- and multiphoton detection as an imaging mode and means of increasing the resolution in far-field light microscopy: A study based on photon-optics. Bioimaging. 1995;3:64–69. [Google Scholar]
- 31.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, Steinhoff HJ, Goedecke A, Schrader J, Gladwin MT, Kelm M, Rassaf T. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henriksen EJ, Bourey RE, Rodnick KJ, Koranyi L, Permutt MA, Holloszy JO. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol. 1990;259:E593–598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- 33.Hepple RT, Mathieu-Costello O. Estimating the size of the capillary-to-fiber interface in skeletal muscle: a comparison of methods. J Appl Physiol. 2001;91:2150–2156. doi: 10.1152/jappl.2001.91.5.2150. [DOI] [PubMed] [Google Scholar]
- 34.Holley JA, Fahim MA. Scanning electron microscopy of mouse muscle microvasculature. Anat Rec. 1983;205:109–117. doi: 10.1002/ar.1092050202. [DOI] [PubMed] [Google Scholar]
- 35.Hoppeler H, Hudlicka O, Uhlmann E. Relationship between mitochondria and oxygen consumption in isolated cat muscles. J Physiol. 1987;385:661–675. doi: 10.1113/jphysiol.1987.sp016513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoppeler H, Mathieu O, Weibel ER, Krauer R, Lindstedt SL, Taylor CR. Design of the mammalian respiratory system. VIII Capillaries in skeletal muscles. Respir Physiol. 1981;44:129–150. doi: 10.1016/0034-5687(81)90080-3. [DOI] [PubMed] [Google Scholar]
- 37.Janacek J, Cebasek V, Kubinova L, Ribaric S, Erzen I. 3D visualization and measurement of capillaries supplying metabolically different fiber types in the rat extensor digitorum longus muscle during denervation and reinnervation. J Histochem Cytochem. 2009;57:437–447. doi: 10.1369/jhc.2008.953018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janacek J, Cvetko E, Kubinova L, Travnik L, Erzen I. A novel method for evaluation of capillarity in human skeletal muscles from confocal 3D images. Microvasc Res. 2011;81:231–238. doi: 10.1016/j.mvr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 39.Janacek J, Kreft M, Cebasek V, Erzen I. Correcting the axial shrinkage of skeletal muscle thick sections visualized by confocal microscopy. J Microsc. 2012;246:107–112. doi: 10.1111/j.1365-2818.2011.03594.x. [DOI] [PubMed] [Google Scholar]
- 40.Jobsis PD, Rothstein EC, Balaban RS. Limited utility of acetoxymethyl (AM)-based intracellular delivery systems, in vivo: interference by extracellular esterases. J Microsc. 2007;226:74–81. doi: 10.1111/j.1365-2818.2007.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johannsson E, Waerhaug O, Bonen A. Effect of cross-reinnervation on the expression of GLUT-4 and GLUT-1 in slow and fast rat muscles. Am J Physiol. 1996;270:R1355–1360. doi: 10.1152/ajpregu.1996.270.6.R1355. [DOI] [PubMed] [Google Scholar]
- 42.Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- 43.Katz A, Broberg S, Sahlin K, Wahren J. Leg glucose uptake during maximal dynamic exercise in humans. Am J Physiol. 1986;251:E65–70. doi: 10.1152/ajpendo.1986.251.1.E65. [DOI] [PubMed] [Google Scholar]
- 44.Kaul S. The role of capillaries in determining coronary blood flow reserve: Implications for stress-induced reversible perfusion defects. J Nucl Cardiol. 2001;8:694–700. doi: 10.1067/mnc.2001.119690. [DOI] [PubMed] [Google Scholar]
- 45.Kindig CA, Richardson TE, Poole DC. Skeletal muscle capillary hemodynamics from rest to contractions: implications for oxygen transfer. J Appl Physiol. 2002;92:2513–2520. doi: 10.1152/japplphysiol.01222.2001. [DOI] [PubMed] [Google Scholar]
- 46.Krenacs T, Molnar E, Dobo E, Dux L. Fibre typing using sarcoplasmic reticulum Ca2+-ATPase and myoglobin immunohistochemistry in rat gastrocnemius muscle. Histochem J. 1989;21:145–155. doi: 10.1007/BF01007489. [DOI] [PubMed] [Google Scholar]
- 47.Krogh A. The anatomy and physiology of capillaries. New Haven: Yale university press; etc; 1922. [Google Scholar]
- 48.Kubinova L, Janacek J, Ribaric S, Cebasek V, Erzen I. Three-dimensional study of the capillary supply of skeletal muscle fibres using confocal microscopy. J Muscle Res Cell Motil. 2001;22:217–227. doi: 10.1023/a:1012201314440. [DOI] [PubMed] [Google Scholar]
- 49.Lam FY, Ferrell WR. Acute inflammation in the rat knee joint attenuates sympathetic vasoconstriction but enhances neuropeptide-mediated vasodilatation assessed by laser Doppler perfusion imaging. Neuroscience. 1993;52:443–449. doi: 10.1016/0306-4522(93)90170-k. [DOI] [PubMed] [Google Scholar]
- 50.Lauritzen HP. Insulin- and contraction-induced glucose transporter 4 traffic in muscle: insights from a novel imaging approach. Exerc Sport Sci Rev. 2013;41:77–86. doi: 10.1097/JES.0b013e318275574c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li CH, Lee CK. Minimum Cross Entropy Thresholding. Pattern Recogn. 1993;26:617–625. [Google Scholar]
- 52.Lizunov VA, Stenkula KG, Lisinski I, Gavrilova O, Yver DR, Chadt A, Al-Hasani H, Zimmerberg J, Cushman SW. Insulin stimulates fusion, but not tethering, of GLUT4 vesicles in skeletal muscle of HA-GLUT4-GFP transgenic mice. Am J Physiol Endocrinol Metab. 2012;302:E950–960. doi: 10.1152/ajpendo.00466.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathieu-Costello O, Hepple RT. Muscle structural capacity for oxygen flux from capillary to fiber mitochondria. Exerc Sport Sci Rev. 2002;30:80–84. doi: 10.1097/00003677-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Mathieu O, Cruz-Orive LM, Hoppeler H, Weibel ER. Estimating length density and quantifying anisotropy in skeletal muscle capillaries. J Microsc. 1983;131:131–146. doi: 10.1111/j.1365-2818.1983.tb04240.x. [DOI] [PubMed] [Google Scholar]
- 55.Mazanet R, Franzini-Armstrong C. Scanning electron microscopy of pericytes in rat red muscle. Microvasc Res. 1982;23:361–369. doi: 10.1016/s0026-2862(82)80008-3. [DOI] [PubMed] [Google Scholar]
- 56.Meeson AP, Radford N, Shelton JM, Mammen PP, DiMaio JM, Hutcheson K, Kong Y, Elterman J, Williams RS, Garry DJ. Adaptive mechanisms that preserve cardiac function in mice without myoglobin. Circ Res. 2001;88:713–720. doi: 10.1161/hh0701.089753. [DOI] [PubMed] [Google Scholar]
- 57.Menger MD, Steiner D, Messmer K. Microvascular ischemia-reperfusion injury in striated muscle: significance of “no reflow”. Am J Physiol. 1992;263:H1892–1900. doi: 10.1152/ajpheart.1992.263.6.H1892. [DOI] [PubMed] [Google Scholar]
- 58.Miledi R, Slater CR. Electrophysiology and electron-microscopy of rat neuromuscular junctions after nerve degeneration. Proc R Soc Lond B Biol Sci. 1968;169:289–306. doi: 10.1098/rspb.1968.0012. [DOI] [PubMed] [Google Scholar]
- 59.Murakami S, Fujino H, Takeda I, Momota R, Kumagishi K, Ohtsuka A. Comparison of capillary architecture between slow and fast muscles in rats using a confocal laser scanning microscope. Acta Med Okayama. 2010;64:11–18. doi: 10.18926/AMO/32859. [DOI] [PubMed] [Google Scholar]
- 60.Murakami S, Fujita N, Kondo H, Takeda I, Momota R, Ohtsuka A, Fujino H. Abnormalities in the fiber composition and capillary architecture in the soleus muscle of type 2 diabetic Goto-Kakizaki rats. TheScientificWorldJournal. 2012;2012:680189. doi: 10.1100/2012/680189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murphy GE, Lowekamp BC, Zerfas PM, Chandler RJ, Narasimha R, Venditti CP, Subramaniam S. Ion-abrasion scanning electron microscopy reveals distorted liver mitochondrial morphology in murine methylmalonic acidemia. J Struct Biol. 2010;171:125–132. doi: 10.1016/j.jsb.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niesner RA, Andresen V, Gunzer M. Intravital two-photon microscopy: focus on speed and time resolved imaging modalities. Immunol Rev. 2008;221:7–25. doi: 10.1111/j.1600-065X.2008.00582.x. [DOI] [PubMed] [Google Scholar]
- 63.Ogata T, Yamasaki Y. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat Rec. 1997;248:214–223. doi: 10.1002/(SICI)1097-0185(199706)248:2<214::AID-AR8>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 64.Peter JB, Barnard RJ, Edgerton VR, Gillespie CA, Stempel KE. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972;11:2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- 65.Phillips D, Aponte AM, French SA, Chess DJ, Balaban RS. Succinyl-CoA synthetase is a phosphate target for the activation of mitochondrial metabolism. Biochemistry. 2009;48:7140–7149. doi: 10.1021/bi900725c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Picard M, Gentil BJ, McManus MJ, White K, St Louis K, Gartside SE, Wallace DC, Turnbull DM. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J Appl Physiol. 2013 doi: 10.1152/japplphysiol.00819.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Picard M, White K, Turnbull DM. Mitochondrial morphology, topology, and membrane interactions in skeletal muscle: a quantitative three-dimensional electron microscopy study. J Appl Physiol. 2013;114:161–171. doi: 10.1152/japplphysiol.01096.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poole DC, Mathieu-Costello O. Relationship between fiber capillarization and mitochondrial volume density in control and trained rat soleus and plantaris muscles. Microcirculation. 1996;3:175–186. doi: 10.3109/10739689609148286. [DOI] [PubMed] [Google Scholar]
- 69.Preibisch S, Saalfeld S, Tomancak P. Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics. 2009;25:1463–1465. doi: 10.1093/bioinformatics/btp184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ralston E, Lu Z, Biscocho N, Soumaka E, Mavroidis M, Prats C, Lomo T, Capetanaki Y, Ploug T. Blood vessels and desmin control the positioning of nuclei in skeletal muscle fibers. J Cell Physiol. 2006;209:874–882. doi: 10.1002/jcp.20780. [DOI] [PubMed] [Google Scholar]
- 71.Reger JF. Studies on the fine structure of normal and denervated neuromuscular junctions from mouse gastrocnemius. J Ultrastruct Res. 1959;2:269–282. doi: 10.1016/s0022-5320(59)80001-0. [DOI] [PubMed] [Google Scholar]
- 72.Roberts WM. Sodium channels near end-plates and nuclei of snake skeletal muscle. J Physiol. 1987;388:213–232. doi: 10.1113/jphysiol.1987.sp016611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romanul FC. Capillary Supply and Metabolism of Muscle Fibers. Arch Neurol. 1965;12:497–509. doi: 10.1001/archneur.1965.00460290053007. [DOI] [PubMed] [Google Scholar]
- 74.Rothstein EC, Carroll S, Combs CA, Jobsis PD, Balaban RS. Skeletal muscle NAD(P)H two-photon fluorescence microscopy in vivo: topology and optical inner filters. Biophys J. 2005;88:2165–2176. doi: 10.1529/biophysj.104.053165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rothstein EC, Nauman M, Chesnick S, Balaban RS. Multi-photon excitation microscopy in intact animals. J Microsc. 2006;222:58–64. doi: 10.1111/j.1365-2818.2006.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rudolf R, Mongillo M, Magalhaes PJ, Pozzan T. In vivo monitoring of Ca(2+) uptake into mitochondria of mouse skeletal muscle during contraction. J Cell Biol. 2004;166:527–536. doi: 10.1083/jcb.200403102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schlieper G, Kim JH, Molojavyi A, Jacoby C, Laussmann T, Flogel U, Godecke A, Schrader J. Adaptation of the myoglobin knockout mouse to hypoxic stress. Am J Physiol Regul Integr Comp Physiol. 2004;286:R786–792. doi: 10.1152/ajpregu.00043.2003. [DOI] [PubMed] [Google Scholar]
- 78.Schroeder JL, Bakalar M, Pohida TJ, Balaban RS. Rapid overlapping-volume acquisition and reconstruction (ROVAR): automated 3D tiling for high-resolution, large field-of-view optical microscopy. J Microsc. 2011;243:103–110. doi: 10.1111/j.1365-2818.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schroeder JL, Luger-Hamer M, Pursley R, Pohida T, Chefd’hotel C, Kellman P, Balaban RS. Short communication: Subcellular motion compensation for minimally invasive microscopy, in vivo: evidence for oxygen gradients in resting muscle. Circ Res. 2010;106:1129–1133. doi: 10.1161/CIRCRESAHA.109.211946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shen W, Hintze TH, Wolin MS. Nitric oxide. An important signaling mechanism between vascular endothelium and parenchymal cells in the regulation of oxygen consumption. Circulation. 1995;92:3505–3512. doi: 10.1161/01.cir.92.12.3505. [DOI] [PubMed] [Google Scholar]
- 81.Shotton DM, Heuser JE, Reese BF, Reese TS. Postsynaptic membrane folds of the frog neuromuscular junction visualized by scanning electron microscopy. Neuroscience. 1979;4:427–435. doi: 10.1016/0306-4522(79)90106-4. [DOI] [PubMed] [Google Scholar]
- 82.Suarez RK, Lighton JR, Brown GS, Mathieu-Costello O. Mitochondrial respiration in hummingbird flight muscles. Proc Natl Acad Sci U S A. 1991;88:4870–4873. doi: 10.1073/pnas.88.11.4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wagner PD. New ideas on limitations to VO2max. Exerc Sport Sci Rev. 2000;28:10–14. [PubMed] [Google Scholar]
- 84.Weibel ER, Bacigalupe LD, Schmitt B, Hoppeler H. Allometric scaling of maximal metabolic rate in mammals: muscle aerobic capacity as determinant factor. Respir Physiol Neurobiol. 2004;140:115–132. doi: 10.1016/j.resp.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Wittenberg BA, Wittenberg JB. Transport of oxygen in muscle. Annu Rev Physiol. 1989;51:857–878. doi: 10.1146/annurev.ph.51.030189.004233. [DOI] [PubMed] [Google Scholar]
- 86.Wittenberg JB, Wittenberg BA. Myoglobin function reassessed. J Exp Biol. 2003;206:2011–2020. doi: 10.1242/jeb.00243. [DOI] [PubMed] [Google Scholar]
- 87.Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Morphology variables measured. 2D fiber and vessel circumferences become surface areas in 3D. 2D fiber and vessel areas become volumes in 3D. Variables are measured for each traced fiber and every vessel in contact with the respective fiber.