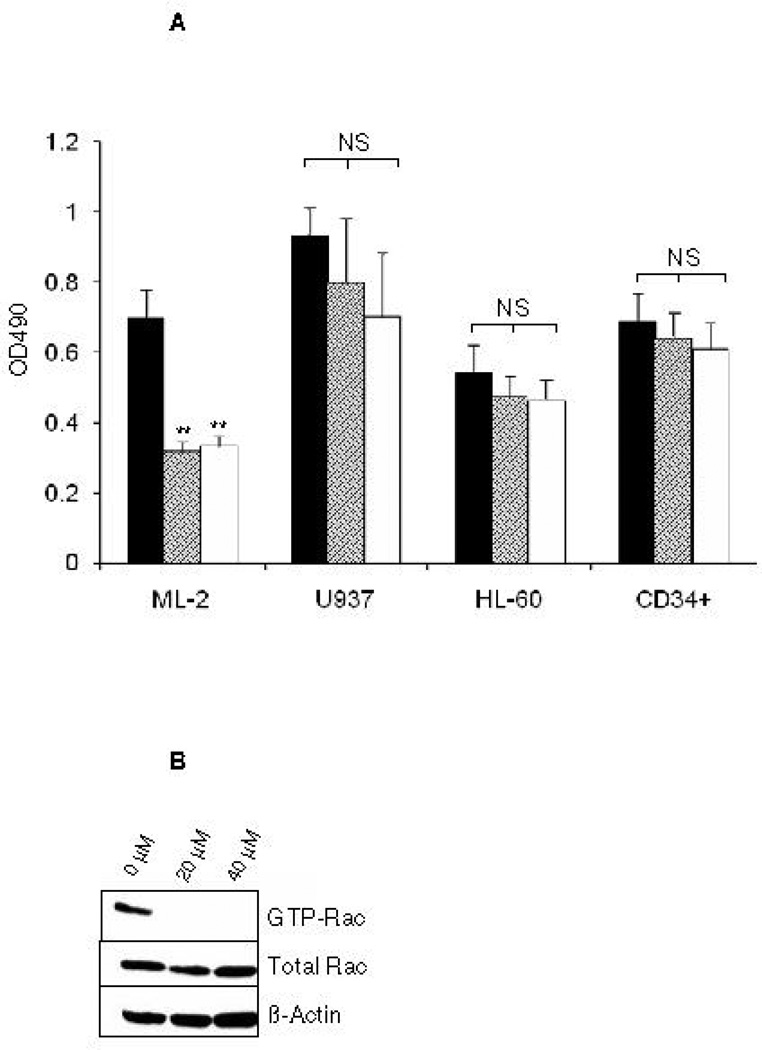
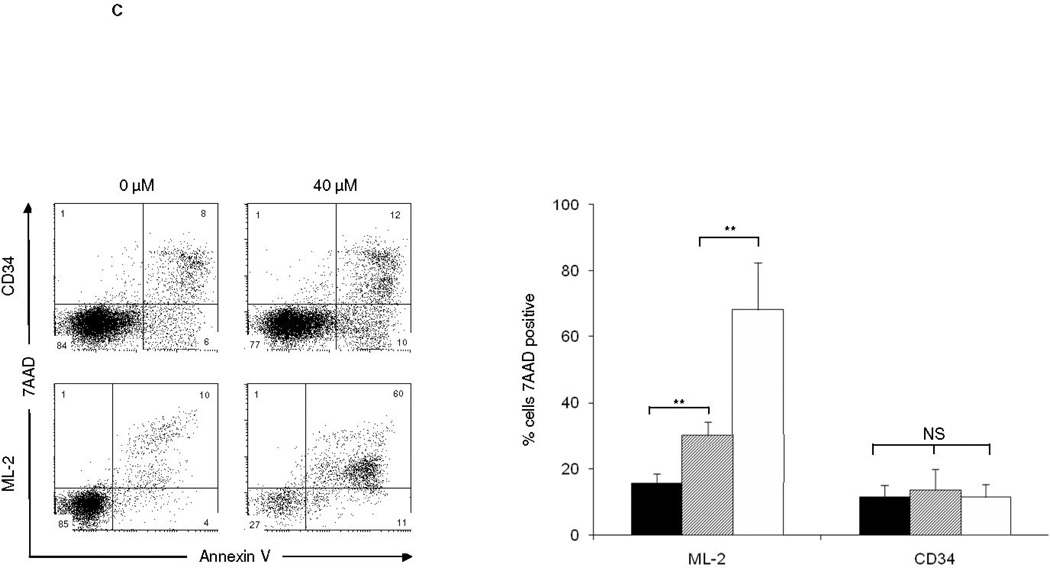
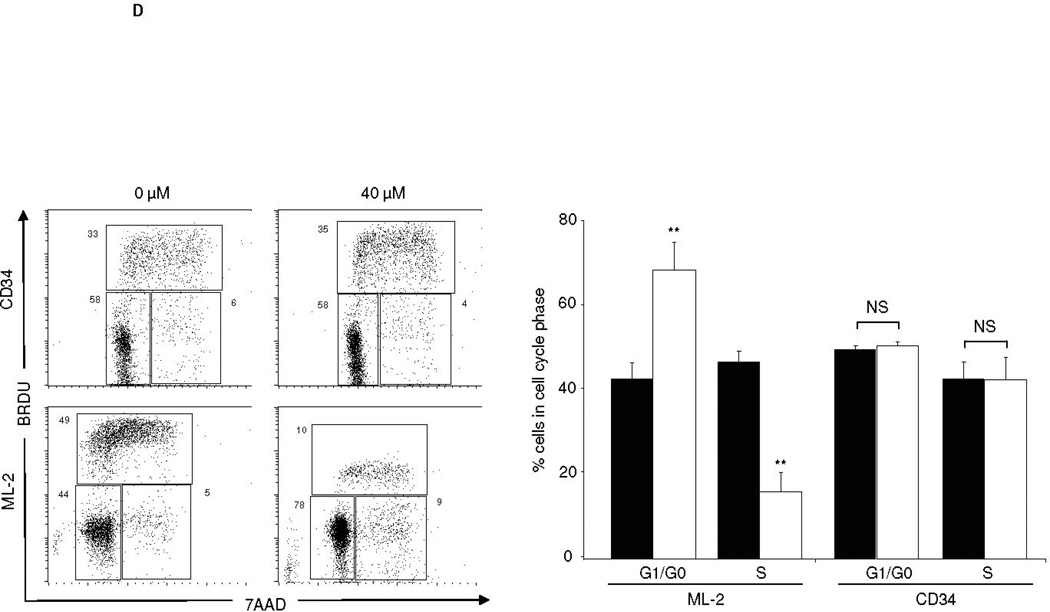
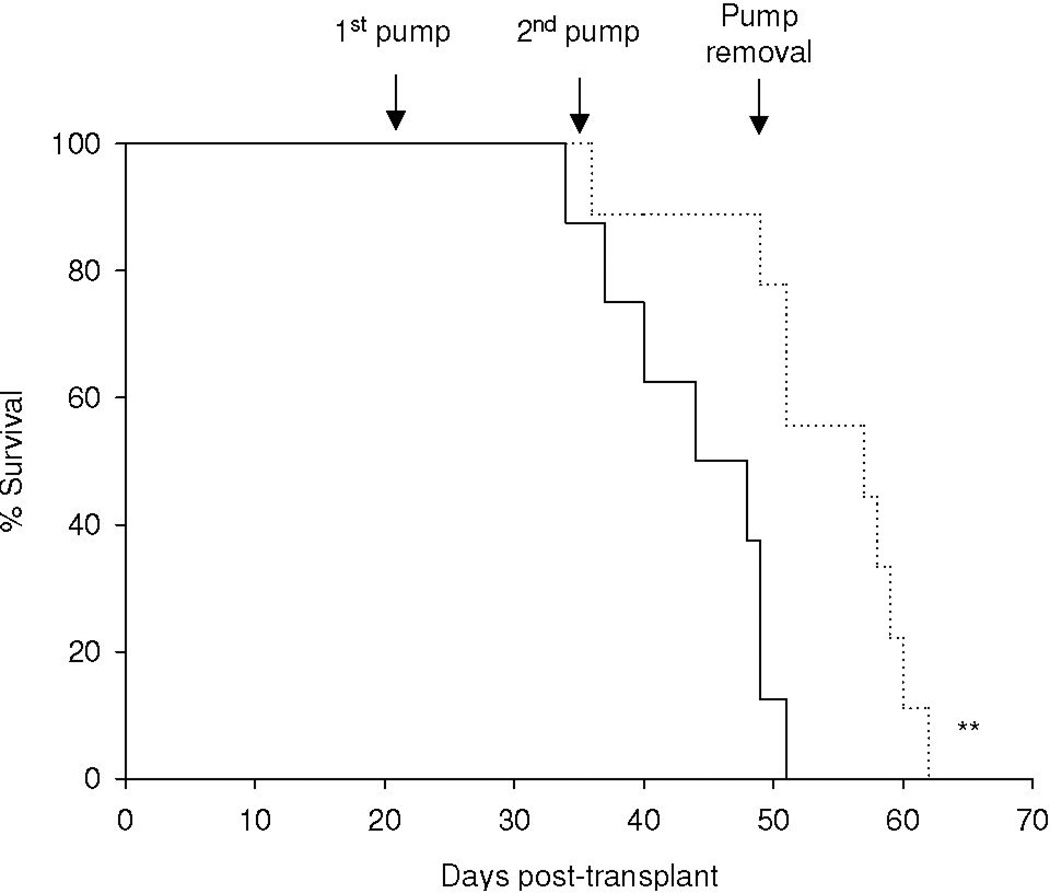
Acute myeloid leukemia (AML) is intrinsically prone to resistance to conventional chemotherapeutic agents. Here we explore the Rac family of small GTPases as novel biologic targets for AML treatment. The Rac subfamily of Rho guanosine triphosphatases (GTPases) plays an essential role in regulating normal hematopoiesis (1). Rac proteins cycle between active GTP-bound and inactive GDP-bound states. Of the three known Rac family members, Rac1 and Rac3 are expressed ubiquitously, while Rac2 is restricted to the hematopoietic system. In hematopoietic cells, Rac proteins integrate signals from growth factor, chemokine, and adhesion receptors to mediate a variety of cellular responses, including cell growth and survival, gene transcription, adhesion, motility, and formation of the actin cytoskeleton (1). We recently identified the Rac GTPases as molecular targets in BCR-ABL-induced myeloproliferative disease (2, 3). However, the role of Rac in AML has thus far not been clearly defined. Elevated levels of GTP-bound Rac have been described in CD34+ cells isolated from patients with AML. In these samples, Rac signaling was identified as a critical mediator of stem/progenitor cell and stroma interaction (4). Recently, Wei et al. observed a critical role of Rac signaling in a disease model of human CD34+ cells transduced with the Mixed Lineage Leukemia (MLL)-AF9 fusion oncogene (5). Interestingly, while MLL-AF9 transduced cells were sensitive to Rac inhibition, cells transduced with the AML-ETO oncogene did not depend on Rac signaling for survival and proliferation. These discrepancies prompted us to further investigate the role of Rac signaling in a panel of human AML cell lines, including the MLL gene-rearranged ML-2 cell line and cell lines not harboring MLL rearrangements such as the histiocytic lymphoma U937 and the acute promyelocytic HL-60 cell line. We demonstrate the presence of GTP-Rac in all cell lines via p21-activated kinase (PAK)-binding domain (PBD) pull-down and immunoblot (data not shown). Compared to purified normal human CD34+ cells, ML-2 cells, which contain a MLL-AF6 translocation (6), showed the most profound inhibition of cell proliferation upon pharmacologic inhibition of Rac using the small molecule Rac inhibitor NSC23766 (7) (Figure 1A). To determine whether a correlation exists between Rac activation and the observed decrease in proliferation of ML-2 cells, we analyzed the effect of NSC23766 on GTP-Rac via PBD pull-down assay and observed abrogation of Rac activation with drug treatment (Figure 1B). We next wanted to determine whether NSC23766 treatment would impact apoptosis (Figure 1C) and/or cell cycle progression of ML-2 cells (Figure 1D). ML-2 cells displayed an increase of early and late apoptosis as measured by Annexin V/7AAD 72 hours after drug exposure (Figure 1C). In addition, Rac inhibition led to increased cell cycle arrest in G0/G1 (Figure 1D). Importantly, these effects were specific to ML-2 cells, as normal CD34+ cells were not significantly affected by NSC23766 exposure. Analogous effects were observed in the MLL-AF9 containing THP-1 cell line (Supplemental Figure 1). In contrast to these MLL gene rearranged cell lines, no significant effect of NSC23677 on cell cycle or apoptosis was observed in U937 cells and only marginal effects were observed in HL- 60 cells (data not shown). To further analyze the potential therapeutic efficacy of NSC23766 in a murine xenograft model, 2 × 107 ML-2 cells were transplanted into irradiated (350 Gy) NOD/SCID mice. Alzet osmotic pumps containing NSC23766 (2 pumps, 75 mM NSC23766 per pump) or PBS were implanted on day 21 post transplant. The pumps were exchanged for new pumps on day 35 and removed on day 49 post transplant. Animals were monitored for survival and bone marrow chimerism of ML-2 cells (human CD45+) was assessed by flow cytometry post-mortem. Animals with less than 15% human CD45+ chimerism were censored from the study (3 animals in the NSC23766- and four animals in the PBS-cohort). We noted a significant difference in the survival of PBS vs. NSC23677-treated animals (Figure 2). Death due to disease progression in the treatment group occurred predominantly following pump removal.
Figure 1.
The Rac-specific inhibitor NSC23766 significantly impacts proliferation, survival, and cell cycle progression of human AML cell lines. (A) Proliferation of a panel of AML cell lines was analyzed by MTS assay 72 hrs after exposure to increasing doses of NSC23766 (n=3, 24 wells per condition). Black bars indicate 0 µM, hatched bars 20 µM, and white bars 40 µM NSC23766. (B) NSC23766 inhibits Rac activation in ML-2 cells. ML-2 cells were cultured in the presence of increasing doses of NSC23766. Lysates were analyzed for active, GTP-Rac. As controls, total lysates were analyzed for Rac and actin expression. (C) Apoptosis of ML-2 and CD34+ cells was analyzed 72 hours after exposure to NSC23766. Left panel depicts a representative dot blot analysis. Numbers indicate percentage of cells per quadrant. Graph depicts percentage of 7AAD-positive cells after exposure to 0 µM (black bars), 20 µM (hatched bars), or 40 µM (white bars) NSC23766 (n=5). (D) Cell cycle analysis of ML-2 and CD34+ cells was performed 48 hours after exposure to NSC23766. Left panel depicts representative dot blot after exposure to 0 or 40 µM NSC23766. Graph depicts percentage of cells in G1/G0 or S phase of cell cycle 48 hours after exposure to 0 µM (black bars) or 40 µM (white bars) NSC23766, respectively (n=5). ** p<0.01, NS = not significant.
Figure 2.
NSC23766 significantly delays the development of leukemia in a murine in vivo model. 2 × 107 ML-2 cells were transplanted into sublethally irradiated NOD/SCID mice. Arrows indicate placement/removal of Alzet osmotic pumps containing PBS (solid line, n=8) or NSC23766 (dotted line, n=9). ** p < 0.01 (log-rank test).
In summary, we demonstrate the impact of Rac inhibition on a panel of human AML cell lines. While the drug target GTP-Rac was present in all cell lines (not shown), the MLL gene-rearranged cell line ML-2 displayed the most profound dependence on Rac signaling. This finding is consistent with recently published findings in human CD34+ cells transduced with the MLL-AF9 oncogene and corroborated by our findings in the MLL-AF9 positive THP-1 cell line (5). The effect of NSC23766 may point to a potential specific vulnerability of MLL rearranged leukemia to Rac inhibition. The mechanism by which the other cell lines bypass this pathway will require further studies. Taken together, our findings highlight the Rac GTPases as potential molecular targets for a subgroup of AML. Development of more potent inhibitors of Rac GTPases is warranted to enable clinical use of this approach.
Supplementary Material
Acknowledgements
Supported by National Institute of Health grant numbers HL69974 and DK62757 (D.A.W.), St. Baldrick’s Foundation Fellowship (L.M.), and CancerFree Kids grant (R.J.S.). The authors would like to thank James Mulloy, Junping Wei, and Michael Milsom for critical review of the manuscript; the Division of Experimental Hematology Translational Trials Development and Support Laboratory for providing normal donor human CD34+ cells.
Footnotes
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu).
References
- 1.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302(5644):445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 2.Thomas EK, Cancelas JA, Zheng Y, Williams DA. Rac GTPases as key regulators of p210-BCR-ABL-dependent leukemogenesis. Leukemia. 2008;22(5):898–904. doi: 10.1038/leu.2008.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas EK, Cancelas JA, Chae HD, Cox AD, Keller PJ, Perrotti D, et al. Rac guanosine triphosphatases represent integrating molecular therapeutic targets for BCR-ABL-induced myeloproliferative disease. Cancer Cell. 2007;12(5):467–478. doi: 10.1016/j.ccr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Rozenveld-Geugien M, Baas IO, van Gosliga D, Vellenga E, Schuringa JJ. Expansion of normal and leukemic human hematopoietic stem/progenitor cells requires rac-mediated interaction with stromal cells. Exp Hematol. 2007;35(5):782–792. doi: 10.1016/j.exphem.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Wei J, Wunderlich M, Fox C, Alvarez S, Cigudosa JC, Wilhelm JS, et al. Microenvironment determines lineage fate in human model of MLL-AF9 leukemia. Cancer Cell. 2008 doi: 10.1016/j.ccr.2008.04.020. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drexler HG, MacLeod RA. Malignant hematopoietic cell lines: in vitro models for the study of anaplastic large-cell lymphoma. Leukemia. 2004;18(10):1569–1571. doi: 10.1038/sj.leu.2403465. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA. 2004;101(20):7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.