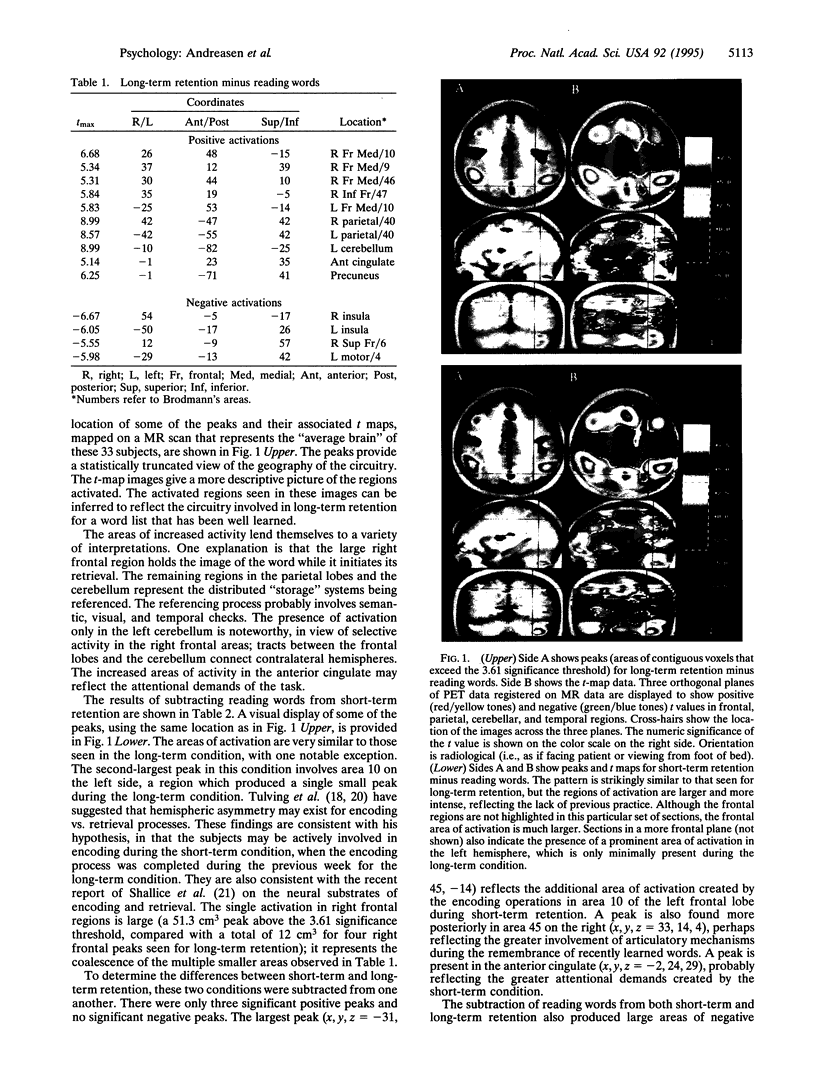
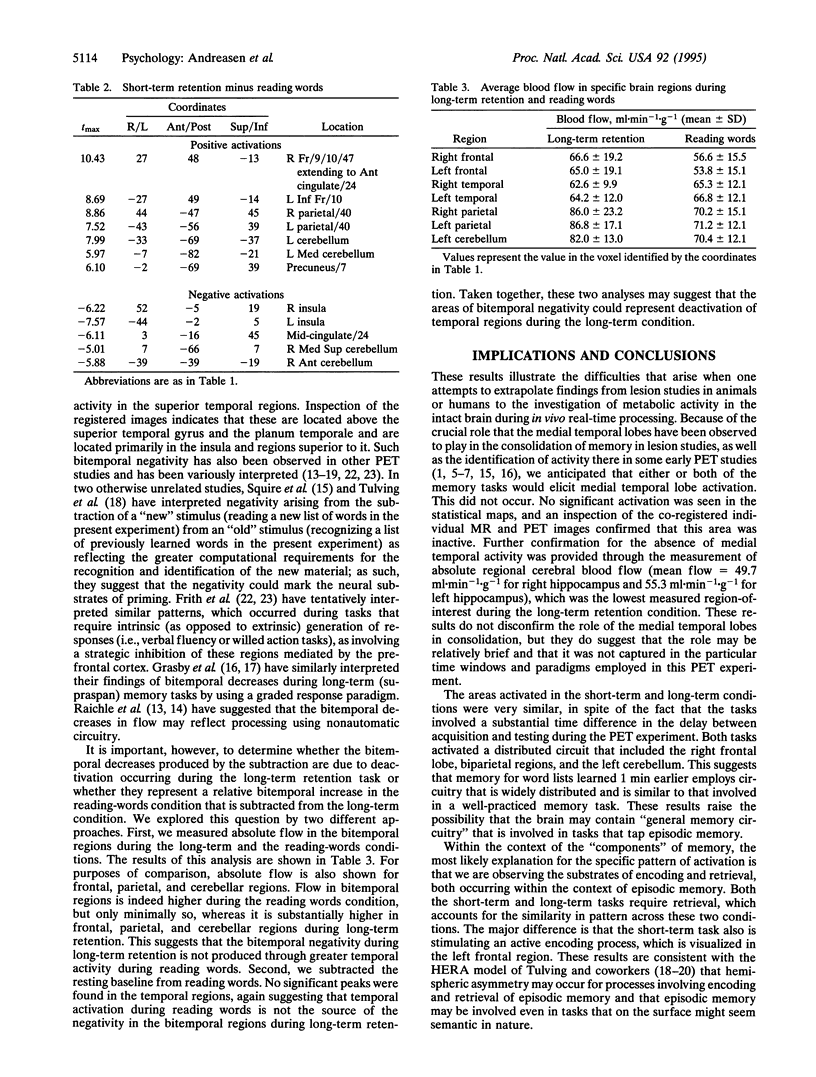
Abstract
Short-term and long-term retention of experimentally presented words were compared in a sample of 33 healthy normal volunteers by the [15O]H2O method with positron emission tomography (PET). The design included three conditions. For the long-term condition, subjects thoroughly studied 18 words 1 week before the PET study. For the short-term condition, subjects were shown another set of 18 words 60 sec before imaging, with instructions to remember them. For the baseline condition, subtracted from the two memory conditions, subjects read a third set of words that they had not previously seen in the experiment. Similar regions were activated in both short-term and long-term conditions: large right frontal areas, biparietal areas, and the left cerebellum. In addition, the short-term condition also activated a relatively large region in the left prefrontal region. These complex distributed circuits appear to represent the neural substrates for aspects of memory such as encoding, retrieval, and storage. They indicate that circuitry involved in episodic memory has much larger cortical and cerebellar components than has been emphasized in earlier lesion studies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreasen N. C., Arndt S., Swayze V., 2nd, Cizadlo T., Flaum M., O'Leary D., Ehrhardt J. C., Yuh W. T. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994 Oct 14;266(5183):294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- Andreasen N. C., Cizadlo T., Harris G., Swayze V., 2nd, O'Leary D. S., Cohen G., Ehrhardt J., Yuh W. T. Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci. 1993 Spring;5(2):121–130. doi: 10.1176/jnp.5.2.121. [DOI] [PubMed] [Google Scholar]
- Andreasen N. C., Cohen G., Harris G., Cizadlo T., Parkkinen J., Rezai K., Swayze V. W., 2nd Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci. 1992 Spring;4(2):125–133. doi: 10.1176/jnp.4.2.125. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The concept of working memory: a view of its current state and probable future development. Cognition. 1981 Aug-Dec;10(1-3):17–23. doi: 10.1016/0010-0277(81)90020-2. [DOI] [PubMed] [Google Scholar]
- Friston K. J., Frith C. D., Liddle P. F., Frackowiak R. S. Investigating a network model of word generation with positron emission tomography. Proc Biol Sci. 1991 May 22;244(1310):101–106. doi: 10.1098/rspb.1991.0057. [DOI] [PubMed] [Google Scholar]
- Frith C. D., Friston K. J., Liddle P. F., Frackowiak R. S. A PET study of word finding. Neuropsychologia. 1991;29(12):1137–1148. doi: 10.1016/0028-3932(91)90029-8. [DOI] [PubMed] [Google Scholar]
- Frith C. D., Friston K., Liddle P. F., Frackowiak R. S. Willed action and the prefrontal cortex in man: a study with PET. Proc Biol Sci. 1991 Jun 22;244(1311):241–246. doi: 10.1098/rspb.1991.0077. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. S. Development of cortical circuitry and cognitive function. Child Dev. 1987 Jun;58(3):601–622. [PubMed] [Google Scholar]
- Grasby P. M., Frith C. D., Friston K. J., Bench C., Frackowiak R. S., Dolan R. J. Functional mapping of brain areas implicated in auditory--verbal memory function. Brain. 1993 Feb;116(Pt 1):1–20. doi: 10.1093/brain/116.1.1. [DOI] [PubMed] [Google Scholar]
- Grasby P. M., Frith C. D., Friston K., Frackowiak R. S., Dolan R. J. Activation of the human hippocampal formation during auditory-verbal long-term memory function. Neurosci Lett. 1993 Dec 12;163(2):185–188. doi: 10.1016/0304-3940(93)90378-x. [DOI] [PubMed] [Google Scholar]
- Hurtig R. R., Hichwa R. D., O'Leary D. S., Boles Ponto L. L., Narayana S., Watkins G. L., Andreasen N. C. Effects of timing and duration of cognitive activation in [15O]water PET studies. J Cereb Blood Flow Metab. 1994 May;14(3):423–430. doi: 10.1038/jcbfm.1994.53. [DOI] [PubMed] [Google Scholar]
- Kapur S., Craik F. I., Tulving E., Wilson A. A., Houle S., Brown G. M. Neuroanatomical correlates of encoding in episodic memory: levels of processing effect. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2008–2011. doi: 10.1073/pnas.91.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D. N., Pelizzari C. A., Chen G. T., Chen C. T., Cooper M. D. Retrospective geometric correlation of MR, CT, and PET images. Radiology. 1988 Dec;169(3):817–823. doi: 10.1148/radiology.169.3.3263666. [DOI] [PubMed] [Google Scholar]
- Miller E. K., Li L., Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science. 1991 Nov 29;254(5036):1377–1379. doi: 10.1126/science.1962197. [DOI] [PubMed] [Google Scholar]
- Mishkin M. Memory in monkeys severely impaired by combined but not by separate removal of amygdala and hippocampus. Nature. 1978 May 25;273(5660):297–298. doi: 10.1038/273297a0. [DOI] [PubMed] [Google Scholar]
- Ojemann G. A., Creutzfeldt O., Lettich E., Haglund M. M. Neuronal activity in human lateral temporal cortex related to short-term verbal memory, naming and reading. Brain. 1988 Dec;111(Pt 6):1383–1403. doi: 10.1093/brain/111.6.1383. [DOI] [PubMed] [Google Scholar]
- Petrides M., Alivisatos B., Evans A. C., Meyer E. Dissociation of human mid-dorsolateral from posterior dorsolateral frontal cortex in memory processing. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M., Alivisatos B., Meyer E., Evans A. C. Functional activation of the human frontal cortex during the performance of verbal working memory tasks. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):878–882. doi: 10.1073/pnas.90.3.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M. E., Fiez J. A., Videen T. O., MacLeod A. M., Pardo J. V., Fox P. T., Petersen S. E. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex. 1994 Jan-Feb;4(1):8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Raichle M. E. Images of the mind: studies with modern imaging techniques. Annu Rev Psychol. 1994;45:333–356. doi: 10.1146/annurev.ps.45.020194.002001. [DOI] [PubMed] [Google Scholar]
- Raichle M. E., Martin W. R., Herscovitch P., Mintun M. A., Markham J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med. 1983 Sep;24(9):790–798. [PubMed] [Google Scholar]
- SCOVILLE W. B., MILNER B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957 Feb;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T., Fletcher P., Frith C. D., Grasby P., Frackowiak R. S., Dolan R. J. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994 Apr 14;368(6472):633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Squire L. R., Ojemann J. G., Miezin F. M., Petersen S. E., Videen T. O., Raichle M. E. Activation of the hippocampus in normal humans: a functional anatomical study of memory. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1837–1841. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L. R., Zola-Morgan S. The medial temporal lobe memory system. Science. 1991 Sep 20;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Tulving E., Kapur S., Craik F. I., Moscovitch M., Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E., Kapur S., Markowitsch H. J., Craik F. I., Habib R., Houle S. Neuroanatomical correlates of retrieval in episodic memory: auditory sentence recognition. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2012–2015. doi: 10.1073/pnas.91.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K. J., Evans A. C., Marrett S., Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992 Nov;12(6):900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S. M., Squire L. R. The primate hippocampal formation: evidence for a time-limited role in memory storage. Science. 1990 Oct 12;250(4978):288–290. doi: 10.1126/science.2218534. [DOI] [PubMed] [Google Scholar]