Abstract
Japanese encephalitis (JE) is an infectious disease of the central nervous system caused by Japanese encephalitis virus (JEV), a zoonotic mosquito-borne flavivirus. JEV is prevalent in much of Asia and the Western Pacific, with over 4 billion people living at risk of infection. In the absence of antiviral intervention, vaccination is the only strategy to develop long-term sustainable protection against JEV infection. Over the past half-century, a mouse brain-derived inactivated vaccine has been used internationally for active immunization. To date, however, JEV is still a clinically important, emerging, and re-emerging human pathogen of global significance. In recent years, production of the mouse brain-derived vaccine has been discontinued, but 3 new cell culture-derived vaccines are available in various parts of the world. Here we review current aspects of JEV biology, summarize the 4 types of JEV vaccine, and discuss the potential of an infectious JEV cDNA technology for future vaccine development.
Keywords: Japanese encephalitis virus, vaccine, immunization, biodefense, prevention, pathogenesis, virulence, flavivirus
Ecology and Epidemiology
Japanese encephalitis (JE) is an inflammatory disease of the brain caused by Japanese encephalitis virus (JEV).1 JE is the most common cause of viral encephalitis in Asia, from the China-Russia border region in the north to the northern Australia in the south, and from the Western Pacific islands in the east to the India-Pakistan border region in the west (Fig. 1).2-4 Histologically, JE-like outbreaks were recorded in Japan in the late 1800s, but the first confirmed JE case was reported in Japan in 1924,5 followed by Korea (1933), China (1940), the Philippines (1950), India (1955), and a number of other Asian countries thereafter.2,6 Over the past few decades, JE incidence has decreased considerably in some countries (e.g., Japan, South Korea, and Taiwan) but has increased in others (e.g., Bangladesh, Cambodia, India, Indonesia, and Pakistan).6 In the late 1990s, JEV began to emerge in the Torres Strait islands and spread onto the Cape York Peninsula,7-9 posing a serious risk to public health in Australia and raising a significant concern that the virus may continue to spread throughout the world.4,6,10-12
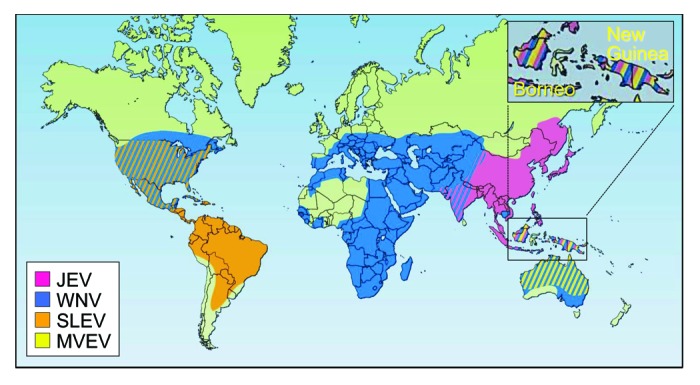
Figure 1. Geographic distribution of four members of the JE serological group: Japanese encephalitis virus (JEV), West Nile virus (WNV), St. Louis encephalitis virus (SLEV), and Murray Valley encephalitis virus (MVEV). Adapted with permission from Macmillan Publishers Ltd: Nature Medicine,4 © 2004.
Today, JE affects ~25 Asian countries, where ~60% of the global population lives at risk of exposure to JEV. In endemic countries, JE occurs primarily among children under the age of 15, and the incidence also increases in the elderly, as protective immunity decreases; however, travel-associated JE can occur at any age because of the absence of protective immunity.13,14 Most JEV infections are subclinical, with a symptomatic-to-asymptomatic ratio of 1:25–1000.15-18 The annual incidence of JE is estimated to be in the range of 50 000–175 000 cases, depending on age group, geographical area, and immunization status.13,19,20 Approximately 20–30% of clinical JE cases are fatal, and ~30–50% of survivors experience serious neurologic, cognitive, or psychiatric complications even years later.13,19,21,22
Genetic and Antigenic Diversity
Based on the nucleotide sequence of the partial or complete viral genome, JEV is divided into five genotypes, GI-GV.23-32 There is a geographic pattern in their distribution, with (1) the Indonesia-Malaysia region having all 5 genotypes isolated; (2) the Australia-New Guinea region having GI and GII; (3) the Taiwan-Philippines region having GII and GIII; (4) the Thailand-Cambodia-Vietnam region having GI, GII, and GIII; (5) the Japan-Korea-China region having GI and GIII; and (6) the India-Sri Lanka-Nepal region having GIII.33 Comprehensive evolutionary studies have suggested that JEV originated from an ancestor in the Indonesia-Malaysia region and diverged there into 5 genotypes, with the 2 older and more divergent genotypes (GIV and GV) still circulating in that region and the 3 more recently evolved genotypes (GI, GII, and GIII) having spread to other geographic locations.29,33 Since 2008, a group of GV JEVs has been isolated in China and South Korea, indicating their recent emergence outside of the Indonesia-Malaysia region.34,35 Although all JEV genotypes form a single serotype,36,37 at least 5 antigenic groups are differentiated by various immunological assays,38-42 demonstrating that some degree of antigenic variation exists among circulating JEVs. Thus, the genetic and antigenic heterogeneity of JEV may have a major impact on JE prevention and control.
Transmission
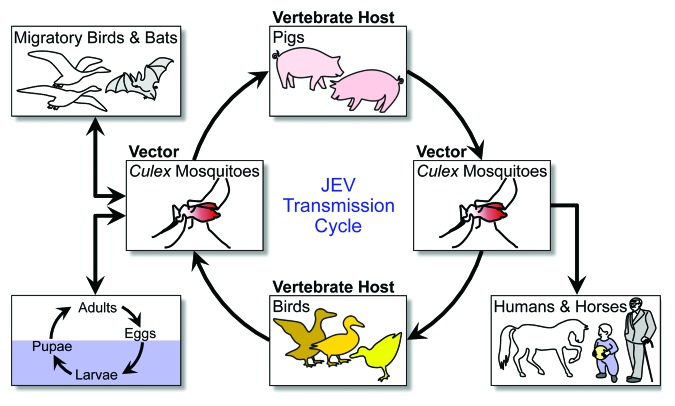
JEV is an arthropod-borne virus (arbovirus) that is transmitted in an enzootic cycle among mosquito vectors and vertebrate hosts, particularly pigs and birds; and humans become infected when bitten by an infected mosquito (Fig. 2).43,44 Although many vertebrate animals can be infected with JEV, domestic pigs are the major virus-amplifying host for virus transmission to humans, not only because they develop high titers and long-lasting viremia after natural infection but also because they live on farms in close proximity to human habitats.45 As another important amplifying host, ardeid birds may contribute to the long-distance dissemination of JEV into new geographic locations, since the virus does not cause any clinical signs in this natural host.4 A variety of mosquito species may act as vectors in the enzootic cycle, but culicine mosquitoes (primarily Culex tritaeniorhynchus) are the principal vector for human infection.45,46 Humans, horses, and other non-avian vertebrates are considered incidental dead-end hosts because they do not produce a level of viremia sufficient to infect new mosquitoes.15,17 In addition to its mosquito-specific horizontal transmission, JEV is also vertically transmitted to the progeny of infected mosquitoes through eggs.47,48
Figure 2. JEV transmission cycle. JEV is amplified in an enzootic cycle that involves mosquito vectors (mainly Culex species) and vertebrate hosts (primarily pigs and birds). Incidentally, JEV is also transmitted to dead-end hosts, such as humans and horses.
There are 2 distinct epidemiological patterns of JE, which mainly reflect climate conditions.2,17,19 In temperate countries (e.g., China, Japan, Nepal, and South Korea), seasonal JE outbreaks occur as the temperature and rainfall increase in the summer months, when JEV is detectable in mosquitoes, pigs, and birds. On the other hand, in tropical and subtropical countries (e.g., Indonesia, Malaysia, the Philippines, Sri Lanka, and Vietnam), sporadic JE cases occur all year round, with a peak during the rainy season. Besides its natural transmission, JEV is also considered to be transmissible through blood transfusion and organ transplantation.49,50
Clinical Presentation
JE has an incubation period of ~5–15 d from the initial exposure to JEV until the appearance of the first symptom.51 Symptomatic JEV infection can cause a spectrum of clinical manifestations15 ranging from undifferentiated febrile illness and aseptic meningitis to acute encephalitis.52-55 The prodromal phase of the disease begins with flu-like non-specific symptoms, including fever, headache, malaise, and vomiting, that may last for several days.56 This mild febrile illness is followed by the acute encephalitic phase, in which a variety of neurological symptoms manifest themselves15 (e.g., mental status changes, focal neurologic deficits, and movement disorders).56-58 JE patients often show a parkinsonian syndrome, which is characterized by tremor, cogwheel rigidity, and hypertonia.15,59,60 Also, a significant proportion of JE patients experience polio-like acute flaccid paralysis.60,61 Convulsions and abnormal behavior are common in children, whereas febrile illness and meningism occur frequently in adults.56,58,62-64 The most common complications associated with a poor prognosis include persistent seizures, motor neuron weakness, cerebellar signs, extrapyramidal disorders, arm flexion deformities, leg hyperextensions, cognitive deficits, language impairments, learning disabilities, and behavioral problems.15,62,63,65
Virology
Genome structure and gene expression
JEV is a member of the genus Flavivirus in the family Flaviviridae.66,67 Within the genus, JEV is the prototype virus of the JE serogroup, which also includes several medically important etiological agents of encephalitis, such as West Nile virus, St. Louis encephalitis virus, and Murray Valley encephalitis virus.68 Taxonomically, JEV is closely related to other clinically important flaviviruses, including yellow fever virus (YFV), dengue virus, and tick-borne encephalitis virus (TBEV).69 Like all flaviviruses, JEV is a small enveloped virus, ~50 nm in diameter, with a single-stranded positive-sense RNA genome that has a 5′ type I cap but lacks a 3′ poly(A) tract.23,70,71 The genome encodes a single long open reading frame (ORF) flanked by 2 short non-coding regions (NCRs) at the 5′ and 3′ ends (Fig. 3A).45,72 In the case of JEV CNU/LP2, a genetically well-characterized virulent strain, the genomic RNA is 10 968 nucleotides in length, consisting of a 95-nucleotide 5′NCR, a 10 299-nucleotide ORF (3432 amino acids plus a stop codon), and a 574-nucleotide 3′NCR.23,73,74
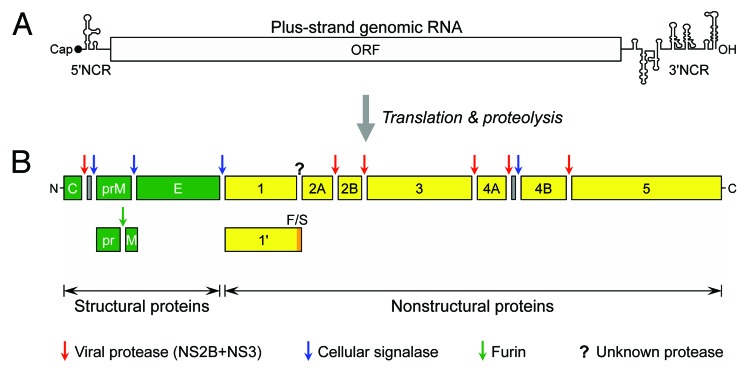
Figure 3. JEV genome structure and gene expression. (A) Genome structure. The plus-strand genomic RNA contains a cap structure, a 5′NCR, a long ORF, and a 3′NCR in a 5′-to-3′ direction. (B) Gene expression. The nascent polyprotein synthesized from the ORF is cleaved by host and viral proteases into at least 3 structural and 7 nonstructural proteins, as indicated. During viral maturation, prM is further processed by furin or a furin-like protease into the pr and M proteins. A derivative of NS1 (NS1') is expressed by a mechanism of frame-shifting which occurs between the codons 8 and 9 of NS2A, resulting in the addition of 52 extra amino acids.
In flaviviruses, the genomic RNA is equivalent to a functional mRNA. Upon infection into a susceptible cell, the ORF encoded in the genome is translated in a cap-dependent manner into a polyprotein precursor, which is co- or post-translationally cleaved by host and viral proteases into a panel of at least 10 functional proteins,45,72 designated C-prM-E-NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5 in an N- to C-terminal direction (Fig. 3B).70 Of these, the 3 N-terminal structural proteins (C; prM, the precursor of M; and E) are required for the formation of infectious virions.75,76 A viral particle has a nucleocapsid, a complex of the genomic RNA with multiple copies of the highly-basic C proteins,77,78 which is enveloped by a host-derived lipid bilayer containing the 2 membrane-anchored surface proteins, prM/M and E.79-81 The 7 C-terminal nonstructural proteins (NS1 to 5) are involved in multiple steps of viral life cycle, i.e., RNA replication,82-86 virus assembly,87-91 and innate immunity evasion.92-98 RNA replication is processed by the 2 largest nonstructural proteins with multiple enzymatic activities: (1) NS3 functions as a serine protease (with its cofactor NS2B),99,100 an RNA-stimulated nucleoside triphosphatase,101 an RNA helicase,102 and an RNA triphosphatase.103 (2) NS5 works as a methyltransferase,104,105 an RNA guanylyltransferase,106 and an RNA-dependent RNA polymerase.107,108 Interestingly, an extended form of NS1 (previously known as NS1') has been reported to be the product of a classical -1 ribosomal frameshifting, which occurs between codons 8 and 9 of NS2A and adds 52 extra amino acids.109,110 Site-directed mutagenesis has suggested that NS1' is involved in viral neuroinvasiveness.109
Replication cycle
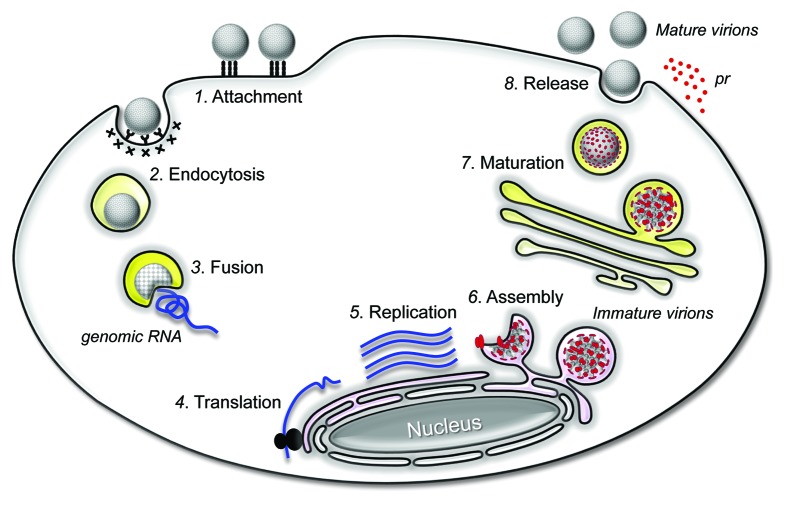
An overview of the flavivirus replication cycle is schematically illustrated in Figure 4. The first step is attachment of the virion to the host cell in a non-specific manner.111-115 Subsequently, the viral E protein is believed to bind with high specificity to an unknown cellular receptor(s) on the cell surface. The particles are then internalized by receptor-mediated, clathrin-dependent endocytosis.116-122 Upon exposure to the acidic conditions in endosomes, the E protein undergoes conformational changes, which trigger fusion of the viral membrane with the cellular endosomal membrane.123-131 Once the viral genomic RNA is uncoated, it is translated into a polyprotein in the endoplasmic reticulum (ER), which is processed by a combination of host and viral proteases to yield the functional structural and nonstructural proteins.45,72 The viral nonstructural proteins, presumably together with a number of host factors, are responsible for the replication of the viral genomic RNA,82,83,85,86,132,133 which takes place in the viral “replication complex” that is associated with the ER-derived membranes.134-136 At the early stage of viral assembly, immature virions are formed by budding of the viral genomic RNA and C proteins into the lumen of the ER, where the prM and E proteins are incorporated into the budding particles.137-140 These immature virions are then transported through the cellular secretory pathway. In the trans-Golgi network (TGN), the immature virions are exposed to the low pH environment and undergo structural changes that render the cleavage site of prM accessible to the cellular protease furin.141 The cleavage of prM to M and the low pH in the TGN induces the maturation of the viral particles,142-145 which is also accompanied by significant structural rearrangements of the prM/M and E proteins.137,146,147 Both completely and partially mature virions are secreted from the infected cells.148
Figure 4. JEV replication cycle. An infectious virion attaches to a target cell by binding to an attachment and entry receptor molecule(s) on the plasma membrane (Step 1). This interaction triggers the cell to internalize the virion by receptor-mediated endocytosis (Step 2). In the endosomes, the low pH induces significant conformational changes in the viral E glycoprotein, triggering the fusion of viral membrane with the host endosomal membrane (Step 3). Upon fusion, the viral genomic RNA is released into the cytoplasm, where it is first translated into the polyprotein precursor in association with the rough ER (Step 4). The polyprotein is processed to yield the mature viral proteins necessary for RNA replication and particle assembly. The genomic RNA is replicated in the replication complex inside virus-induced, ER-derived vesicles (Step 5). Immature progeny virions are formed by budding a complex of the newly synthesized genomic RNA and C proteins into the lumen of the ER, where they acquire the prM and E proteins on their membranes (Step 6). The immature virions are then transported to the Golgi apparatus through the secretory pathway; in the trans-Golgi network, the cleavage of prM to M leads to the maturation of the viral particles (Step 7). Finally, mature virions are released from the cell into the extracellular milieu by exocytosis (Step 8).
Vaccines
There are still no specific drugs available to treat JEV infection.149-152 In the absence of antiviral therapy, JE is managed only with supportive therapies and preventive measures. Based on the mode of transmission, the prevention of JEV is based essentially on 4 strategies, i.e., mosquito control, avoiding mosquito bites, pig immunization, and human immunization.4,12,17,153 Of these, human immunization is the active method of choice to achieve long-term sustainable protection against JE. Currently, there are 4 different types of JE vaccines available for humans in various areas of the world (Table 1): (1) mouse brain-derived killed-inactivated, (2) cell culture-derived live-attenuated, (3) cell culture-derived killed-inactivated, and (4) genetically engineered live-attenuated chimeric vaccines.154-156
Table 1. Summary of 4 different types of JE vaccines.
| Vaccine type | JEV strain | Vaccine name (Manufacturer) |
|---|---|---|
| Mouse brain-derived killed-inactivated | Nakayama/Beijing-1 (P1) | JE-VAX (BIKENa) |
| Cell culture-derived live-attenuated | SA14-14-2 | SA14-14-2 (CDIBPb) |
| Cell culture-derived killed-inactivated | Beijing-1 (P1) | JEBIK V (BIKEN); ENCEVAC, KD-287, or JEIMMUGEN INJ (Kaketsuken) |
| Beijing-3 (P3) | ||
| SA14-14-2 | IC51, IXIARO, JESPECT, or JEEV (Intercell AG) | |
| Cell culture-derived live-attenuated chimeric | SA14-14-2 | ChimeriVax-JE, IMOJEV, JE-CV, or THAIJEV (Sanofi-Aventis) |
aBIKEN, Research Foundation for Microbial Diseases of Osaka University. bCDIBP, Chengdu Institute of Biological Products.
Mouse brain-derived killed-inactivated vaccines
The first licensed JE vaccine was an inactivated mouse brain-derived vaccine based on the prototype JEV strain Nakayama, the first isolate recovered from the brain of a JE patient in 1935 in Japan.2,18 Although its purity has been improved over the course of the past ~60 y, the vaccine was typically produced by inoculating suckling mice intracerebrally with the virus, inactivating the supernatant of the infected mouse brain homogenate with formalin, and purifying inactivated virus suspension by ultracentrifugation.157-159 No mouse myelin basic protein could be detected in the vaccine at a detection limit of 2 ng/ml.51 The mouse brain-derived inactivated Nakayama vaccine was marketed as JE-VAX153,160 and was the only commercially available vaccine worldwide for several decades.18,161,162 Similar mouse brain-derived JE vaccines were produced by multiple manufacturers in India, Japan, Korea, Taiwan, Thailand, and Vietnam. Although highly immunogenic and efficacious,160,163-165 JE-VAX had several drawbacks and limitations, including vaccine-induced adverse events, high production costs, and the need for 2 or 3 primary doses plus boosters.1,51,166-169 In addition to a considerable incidence of local and mild systemic side-effects, there was also a risk of rare but serious allergic and neurologic side-effects.170-179 It could cause post-vaccination cases of acute disseminated encephalomyelitis,51,153,173,180 which have also been associated with a number of other vaccines for rabies, smallpox, measles, mumps, rubella, pertussis, influenza, and hepatitis B.181,182 In light of the availability of the new cell culture-derived JE vaccines, production of JE-VAX was halted in 2006, and all the remaining stock expired in 2011.51,156
From 1989 until the mid-2000s, the mouse brain-derived vaccine had been produced in Japan using two JEV strains, the Nakayama for international distribution and the Beijing-1 (P1) for domestic consumption only; Beijing-1 is a Chinese isolate that originated from the brain of a human patient in 1949.160,183 Initially, the Beijing-1 strain was considered to be able to induce a more potent immunogenicity and elicit broader cross-reacting antibodies against heterologous JEV strains.184 However, several comparative studies showed that both the Nakayama- and Beijing-1-based mouse brain-derived vaccines were equally capable of eliciting high levels of immunogenicity and protective efficacy against a wide range of JEV strains.160,185,186 For decades, the 2 mouse brain-derived vaccines had therefore been used for the effective control of JE in several endemic countries, including South Korea, Japan, Taiwan, and Thailand.18,161,162 In most cases, a 2-dose primary immunization schedule was used, giving the first dose at any age between 1 and 3 years and the second dose 1–4 weeks later; the first booster dose was given at 1 year after primary vaccination, and then repeated boosters were given every 1 to 3 y up to 10–15 y of age.18,21,187
Cell culture-derived live-attenuated vaccine
A live-attenuated cell culture-derived JE vaccine was developed in China based on the SA14-14-2 strain, an attenuated form of the virulent JEV strain SA14 isolated from a pool of Culex pipiens mosquito larvae collected in China in 1954.188,189 The SA14-14-2 strain was generated by serial passage in primary hamster kidney (PHK) cells and in animals (i.e., mice and hamsters), combined with multiple plaque purifications in PHK or primary chick embryo (PCE) cells during the passages.189 The live-attenuated vaccine named SA14-14-2 was first licensed for commercial application in China in 1988 and is currently being produced in PHK cells.189,190 Since its licensure, >300 million doses of SA14-14-2 have been produced for administration to Chinese children, with an excellent record of safety and efficacy.189,190 Over the past decade, SA14-14-2 has been progressively licensed in other Asian countries, including South Korea, Nepal, India, Sri Lanka, Cambodia, Laos, Myanmar, and Thailand. Today, SA14-14-2 is the most widely used JE vaccine in JE-endemic areas.191 In China, SA14-14-2 has been administered to children (9–12 mo) in 2 doses 1 year apart; a booster dose is given at school-entry age.187 To promote progress toward expanding the international licensure of SA14-14-2 outside of Asia, several key issues needed to be addressed that relate to quality control of the adventitious agents in the uncharacterized cells used for vaccine production:13,187,192 (1) maintaining the hamster colonies under specified-pathogen-free conditions; (2) monitoring the vaccine seeds to ensure freedom from adventitious agents; (3) testing batches of the vaccine for attenuated phenotype in suckling mice, weanling mice, and monkeys; and (4) controlling and recording the raw materials (e.g., hamster cells and bovine sera) used to produce the original vaccine seeds.
SA14-14-2 is highly immunogenic, as shown by the high percentage of seroconversion with 1 dose (85%–100%) and near-complete seroconversion with 2 doses given 1 to 3 mo apart.167,188,193-195 Consistent with its high level of immunogenicity, several case-control studies have indicated that SA14-14-2 is highly efficacious in preventing JE, with a high protection efficacy following 1 dose (80%–99%) and almost complete protection after 2 doses (>98%).167,196,197 A recent follow-up study has shown that in a JE-endemic area of Nepal, a high level of neutralizing antibody is maintained in children vaccinated with a single dose of SA14-14-2 after 4 (~90%) and 5 (~64%) years of vaccination.198 In Nepal, 2 case-control studies have also provided evidence of sustained high protection elicited by a single dose of SA14-14-2 at 1 (~98%) and 5 (~96%) years after the initial vaccination.199,200 However, the durability of protective immunity needs further investigation, because in JE-endemic areas, vaccinated individuals are continuously exposed to natural JEV infection. With respect to vaccine safety, no severe vaccine-induced adverse events have been observed.188,201 Thus, SA14-14-2 appears to be effective and safe when administered in a 2-dose regimen. Still, there is a theoretical risk for reversion of the attenuated SA14-14-2 virus to high virulence, which has restricted its extended application to global immunization for the prevention of JEV infection.18,154
Cell culture-derived killed-inactivated vaccines
A PHK cell-derived inactivated JE vaccine was developed using the Beijing-3 (P3) strain, a Chinese isolate recovered in 1949 from the brain of a patient during the Beijing-1 strain epidemic.18,202 Since 1968, this PHK cell-derived inactivated Beijing-3 vaccine has been widely used in China and was adapted to production in African green monkey kidney (Vero) cells.18,203 In China, the Vero cell-derived Beijing-3 vaccine was licensed in 1998 and is now the leading inactivated vaccine for domestic use, but it is being replaced by the live-attenuated SA14-14-2 vaccine.18,202,204 In Japan, another Vero cell-derived inactivated vaccine was produced using the Beijing-1 strain and is currently available under the two trade names: JEBIK V, approved in 2009, and ENCEVAC (also known as KD-287 or JEIMMUGEN INJ.) in 2011.204 In the case of JEBIK V, two clinical trials have shown that a three-dose regimen has superior immunogenicity with a good safety profile, as compared with the mouse brain-derived Beijing-1 vaccine.205,206
Another new Vero cell-derived, inactivated JE vaccine (designated IC51) has been established using the attenuated SA14-14-2 strain.207,208 Since 2009, IC51 has been licensed under one of the three trade names (IXIARO, JESPECT, and JEEV) in many countries, including the US, Europe, Canada, Australia, Hong Kong, Switzerland, and India.51,209-211 In all cases except India, the initial licensure of IC51 is limited to adults aged ≥17 y as a substitute for JE-VAX, which is no longer available.204,212 In India, on the other hand, IC51 is approved for both adults (18 to ≤49 y) and children (1 to <3 y), with the recommendation of 2 primary doses given 28 d apart. In early 2013, the pediatric use of IC51 was also approved for children (2 mo to <17 or 18 y) in the US and Europe. IC51 is formulated with aluminum hydroxide as an adjuvant.209,210 In general, an appropriate adjuvant acts to accelerate, enhance, or prolong the quality of the vaccine-elicited immune responses.213 Aluminum compounds are the most commonly used adjuvants in human vaccination because of their clinical safety, low cost, and strong adjuvanticity with a variety of antigens (albeit with some limitations, such as local reactions, IgE antibody induction, and ineffectiveness to elicit cell-mediated immune responses).214,215 The ideal adjuvant for JE vaccines should enhance the neutralizing antibody response, have the potential for antigen-sparing, and be safe and well-tolerated.216
In adults aged ≥18 y, several clinical trials have indicated that IC51 induces a level of immunogenicity equivalent to or even higher than that of JE-VAX, with a safety and tolerability that have proven to be more favorable.217-221 The good safety profile of IC51 has also been confirmed by post-marketing surveillance, including safety data from 10 phase III clinical trials in >4000 subjects who received at least 1 IC51 vaccination.222 After a 2-dose primary immunization with IC51, the seroprotection rate decreases over time (i.e., 83% at month 6, 58% at month 12, and 48% at month 24), but a booster at 1 or 2 y after the initial vaccination leads to complete seroconversion.223 Recently, an additional clinical trial has confirmed the long-term immunity following a booster dose of IC51 at 15 mo after the primary immunization.224 Similarly, in Indian children aged 1 to 3 y, no apparent difference was observed in the immunogenicity and safety profiles of the IC51 and JE-VAX vaccines.225 Currently, additional clinical trials are ongoing to determine the immunogenicity and safety of the vaccine in children/infants in the US and other countries.204
As part of its ongoing efforts to address the interchangeability of JE vaccines, a single dose of IC51 has been shown to be able to boost immunity in subjects primed with JE-VAX, suggesting that two JEV strains, Nakayama and SA14-14-2, are immunologically similar enough to induce a substantial level of cross-reactive immune responses.226 This finding will have a large impact on heterologous prime-boost vaccination with the Nakayama-based JE-VAX followed by 1 of the 3 SA14-14-2-derived JE vaccines (i.e., SA14-14-2, IC51, or ChimeriVax-JE). Intriguingly, after a single dose of IC51, the JEV-specific neutralizing antibody response is enhanced with pre-existing anti-TBEV immunity, indicating a positive effect of pre-existing immunity against other flaviviruses on the immunogenicity of JE vaccines.227 Furthermore, a phase III clinical study has reported no influence on the immune response to either JEV or hepatitis A virus (HAV) when IC51 and the HAV vaccine HAVRIX1440 are administered concomitantly to healthy subjects.228
Genetically engineered live-attenuated chimeric vaccine
A recombinant, live-attenuated JE vaccine based on a chimeric YF-JE virus (designated ChimeriVax-JE) has also been produced; this vaccine is engineered to express the structural prM and E proteins of JEV SA14-14-2 in the context of YFV 17D, a live-attenuated YF vaccine strain.229,230 The YFV 17D vaccine was chosen as the ideal vector for production of the chimeric virus because of its excellent record of safety and efficacy for the past ~70 y.231 The ChimeriVax-JE virus is generated by using infectious YFV 17D cDNA technology, in which the genes encoding the prM and E proteins of YFV 17D are replaced with the corresponding genes of JEV SA14-14-2.232 This Vero cell-derived ChimeriVax-JE vaccine is also referred as IMOJEV, JE-CV, or THAIJEV and is now commercially available in Australia and Thailand.156,229 In both countries, 1 dose of the vaccine is recommended for subjects 12 mo of age and older, and the need for and timing of a booster dose to extend the duration of protection are being assessed.
A number of earlier studies have shown that the ChimeriVax-JE vaccine is safe, immunogenic, and protective in mouse and nonhuman primate models233-237 as well as in small-scale human studies.37,238,239 Recently, a clinical trial in adults (aged ≥18 y) found that a single dose of IMOJEV generates a level of near-complete seroconversion (~99%) similar to that induced by 3 doses of JE-VAX (~95%), with ~94% of the participants seroconverting within 14 d.240 A 5-y follow-up study has indicated that ~87% of the 1-dose vaccinees who were seroprotected at month 6 were still protected at month 60, and this percentage increased to ~96% with a booster at month 6.241 In Thailand and the Philippines, a clinical trial in children (aged 12 to 18 or 24 mo) has shown that single-dose administration elicits a high protective immune response capable of seroconverting ~95% of naïve toddlers.242,243 There has been no indication of any serious safety concerns related to vaccination.240,241,243 Furthermore, the ChimeriVax-JE virus has been shown to be restricted in its ability to infect and replicate in six different mosquito species following oral feeding of artificial blood meals with high titers of the virus, although all the mosquitoes are susceptible to JEV infection.244,245 ChimeriVax-JE virus is thus less likely to be transmitted by mosquitoes from vaccinated persons to other hosts.
Experimental vaccines
The E protein of JEV has high potential for use as an immunogen capable of eliciting protective immunity, since JEV-neutralizing antibodies alone are sufficient to confer protection against infection.246,247 Ongoing efforts to develop new JE vaccines can be categorized into three different classes: (1) recombinant protein-based vaccines, (2) poxvirus-based vaccines, and (3) plasmid DNA-based vaccines.248
Recombinant protein-based vaccines
In E. coli, antigenic portions of the E protein have been expressed that generate a wide range of neutralizing antibody titers in mice.249-252 In line with this result, it is intriguing to note that a 27-amino acid peptide from the E protein, fused to Johnson grass mosaic virus coat protein to form virus-like particles, has been shown to induce JEV-neutralizing antibodies in mice and protect them against a lethal JEV challenge.253 In a baculovirus-insect cell system, the E protein has also been expressed alone or together with another viral protein, prM or NS1, that elicits neutralizing antibodies and protects mice against a challenge with JEV.254,255 Moreover, in mammalian cells, co-expression of the prM and E proteins has been shown to produce a secreted form of subviral particles capable of inducing JEV-neutralizing antibodies and generating JEV-specific cytotoxic T cells in animals.256-259
Poxvirus-based vaccines
Recombinant poxviruses have been used as viral vectors to deliver JEV antigens (e.g., prM, E, and/or NS1) that can induce protective immunity in mice.260-263 Three major poxviruses that have been demonstrated the potential for JE vaccine development are NYVAC (an attenuated vaccinia), ALVAC (an attenuated canarypox), and MVA (the modified vaccinia Ankara).257,259,264-266 In a clinical trial, NYVAC- and ALVAC-based JE vaccines were found to be well tolerated, but their immunogenicity did not appear to be satisfactory; in particular, the NYVAC-based vaccine elicited JEV-neutralizing antibodies in vaccinia-nonimmune volunteers but not in vaccinia-immune volunteers, suggesting that pre-existing poxvirus immunity may suppress the induction of immune responses to JEV antigens.267 In addition to these poxviruses, adenoviruses have also been explored as a viral vector to express JEV antigens in animals.268
Plasmid DNA-based vaccines
In mice and pigs, immunization of a plasmid encoding the prM and E proteins can induce a range of protective immune responses that include JEV-specific B cells and cytotoxic T cells.256,269-271 In their effort to maximize the immunogenicity and protective efficacy of JEV prM-E DNA immunization, several studies in mice have tested a variety of DNA constructs encoding an intracellular, membrane-anchored, or secreted form of the E protein, with or without the expression of the prM protein.262,272,273 Also, the potential use of cytokine adjuvants has been studied in mice by co-administering a plasmid expressing GM-CSF or IL-12; in both cases, however, the co-inoculation appeared to suppress the cellular and antibody immune responses and protective immunity induced by a JEV DNA vaccine expressing the E, prM-E, or prM-E-NS1 protein.274-276 In addition to the two structural proteins (prM and E), immunization with a plasmid encoding the viral nonstructural protein NS1 can also induce an antibody response with cytolytic activity in a JEV-specific, complement-dependent manner.271,277 The NS1 immunization thus has been shown to be sufficient to protect mice against a lethal infection with JEV, despite having no detectable neutralizing antibodies induced.271
Vaccine efficacy and JEV genotype replacement
Over the past 2 decades, there has been a dramatic shift in the dominant genotype of JEV circulating in JE endemic areas. Historically, GIII was the most widely distributed genotype;33 since the 1990s, however, GI has replaced GIII as the major genotype emerging in many Asian countries,278 including China, Japan, South Korea, Taiwan, Thailand, and Vietnam.279-284 On the other hand, all currently licensed JE vaccines are derived only from GIII JEV strains, i.e., Nakayama, Beijing-1, Beijing-3, and SA14-14-2. This raises a concern that JEV strains of different genotypes exhibit antigenic differences, which may affect vaccine efficacy.186 In mice, a range of variations has been observed in the immunogenicity and protective efficacy of current GIII JE vaccines against heterologous JEV genotypes.37,285,286 In humans, a recent study has found that neutralizing antibodies, elicited by JE-VAX among Taiwanese children, show reduced neutralizing potency against emerging GI JEV strains.157 Similarly, decreased cross-neutralizing responses to heterologous JEV genotypes have also been observed with ChimeriVax-JE.287 In line with these findings, a GI JEV has been isolated from the cerebrospinal fluid (CSF) of a Chinese patient who had been vaccinated with SA14-14-2.288 Moreover, the reduced capacity of neutralizing antibody against different GIII JEV strains has been described in some individuals immunized with JE-VAX, SA14-14-2, and IC51.186,226,289,290 Thus, genotype replacement, in addition to the strain-specific immune response, may have important implications for future JE vaccine development.
Recommendations for the use of JE vaccines among travelers
From 1973 to 2008, a total of 55 JE cases were documented in travelers from non-endemic areas. In a detailed review of 37 patients, 24 (65%) appear to have spent ≥1 mo in JE-endemic areas, and most had factors that could increase the likelihood of acquiring JEV.291 Thus, for most travelers to Asia, the overall risk of JE is generally very low, but it varies depending on risk factors (i.e., location, season, duration, and activity), with an estimated incidence of <1 case per 1 million travelers.51,292-295 According to the United States Advisory Committee on Immunization Practices (ACIP), JE vaccine is recommended for travelers who spend ≥1 mo in JE-endemic areas during a high-risk period of JEV transmission.51 Also, the ACIP recommends that JE vaccine should be considered for individuals (1) traveling to endemic areas for a short period of time (<1 mo) during the JEV transmission season, if they visit outside of an urban area where there is an increased risk of JEV infection; (2) traveling to an area with an ongoing JE outbreak; and (3) traveling to endemic areas, with uncertainty in their destination, duration of stay, or activities.51 JE vaccine is not recommended for short-term travelers whose trips are restricted to urban areas or at times outside of a defined JEV transmission season.51
Immune Responses
Both innate and adaptive immunity are important for the control of JEV infection.296-298 The innate immune response is indicated by a level of interferon-α detected in the plasma and CSF of patients with acute JE,299 but treatment with interferon-α-2a produces no significant improvement in the outcome of JE patients.300 Experimentally, a critical role for interferon-α in resolving JEV infection is suggested by the uncontrolled growth of the virus in mice lacking a functional interferon-α receptor.301 Of the 2 arms of adaptive immunity, the humoral immune response to JEV has been well characterized. In patients with acute JE, JEV-specific IgM and IgG antibodies are typically detectable in serum and CSF within 7 and 30 d after infection, respectively.302 A failure to generate an early, vigorous JEV-specific IgM and IgG antibodies in serum and CSF is associated with a higher risk of severe and fatal outcomes.302-305 In agreement with this finding, a central role of JEV-neutralizing antibodies in the protection against JEV infection has been shown in animals by administering the antibodies before or soon after infection.306-309 Also, a recent study has further highlighted the key role of antibody-mediated immunity in preventing the spread of JEV to the central nervous system (CNS) in mice lacking B cells.310 Thus, protection against JEV depends on virus-specific humoral immunity, and JEV-neutralizing antibodies alone are sufficient for protection.
Unlike humoral immunity, the functional significance of cellular immunity in controlling JEV infection is not well understood. In spider monkeys, which normally produce subclinical symptoms after intracerebral inoculation of JEV, flaccid paralysis develops rapidly when T-cell function is modulated by cyclophosphamide (an immunosuppressive agent).311 In mice, a definite role for CD4+ T cells in protection from lethal JEV infection has been demonstrated by adoptive transfer of JEV-immune cells.312 The same approach has suggested a possible role for CD8+ T cells in JEV clearance, since both CD4+ and CD8+ T cells are reported to be necessary for protecting adult mice against lethal intracerebral challenge with JEV.313 Infection experiments in a group of knockout mice have shown the relative contribution of cell-mediated immunity in protection against JEV, including (1) a critical role for CD4+ T cells in maintaining potent IgM and IgG antibody responses to JEV (using mice that lack major histocompatibility complex class II); and (2) a marginal role for CD8+ T cells in controlling JEV growth in the CNS, with indication that they are dispensable for recovery from JEV infection (using mice that lack key effector molecules [Fas receptor, perforin, or granzymes] of cytolytic CD8+ T cells).310 In agreement with these preclinical data, peripheral blood mononuclear cells from both JE patients and vaccinees have demonstrated JEV-specific CD4+ and CD8+ T-cell proliferation.314 Also, a small number of CD4+ T-cell clones that recognize the JEV E protein in an HLA-restricted manner have been established from JE vaccine recipients.315 Further investigation is needed to better understand the role of cellular immunity in controlling JEV infection.
Reverse Genetics
Reverse genetics is a powerful system for the genetic manipulation of the JEV genomic RNA that allows us to produce recombinant viruses entirely from a molecularly cloned cDNA.45 In 2003, we reported the first description of a reverse genetics system for JEV, developed using the highly virulent strain CNU/LP2.73 This system utilized a bacterial artificial chromosome (BAC) based on a single-copy fertility plasmid (F-plasmid), which houses a genome-length cDNA of JEV (Fig. 5). Run-off transcription of the full-length cDNA in vitro generated synthetic RNAs, which were highly infectious in JEV-susceptible BHK-21 cells and had a specific infectivity of ~1 × 106 PFU/µg; these cells produced a high titer of synthetic viruses (~5 × 106 PFU/ml).73 Importantly, the infectious JEV cDNA was shown to be genetically stable over 180 generations of propagation in E. coli.73 Over the past 10 y, infectious JEV cDNA technology has contributed significantly to our progress in understanding the molecular basis of JEV replication and identifying the viral factors involved in JEV pathogenesis, including (1) the discovery of a complex RNA motif, defined by 3 discontinuous 5-nucleotide-long strands, that is a key element with a crucial regulatory role in the genome replication of JEV and other closely related flaviviruses;316 (2) the construction of a detailed genetic map of the 3′ cis-acting elements in JEV genomic RNA, which regulate the cell type-dependent replication of JEV and perhaps other closely related mosquito-borne flaviviruses;317 and (3) demonstration of the functional importance of the single highly conserved N-glycosylation motif in prM, which is crucial for multiple stages of JEV biology: prM biogenesis, virus release, and pathogenesis.74
Figure 5. JEV reverse genetics. The full-length JEV cDNA is cloned in the bacterial artificial chromosome plasmid pBeloBAC11. The cloned cDNA is modified to have an SP6 or T7 promoter just upstream of the 5′-end of the viral genome and a run-off Xba I site immediately downstream of the viral 3′-end. To prepare a template for in vitro run-off transcription, the full-length cDNA is linearized by Xba I, followed by mung bean nuclease treatment to remove the 5′ overhang left by the Xba I digestion. The linearized cDNA is used as a template for run-off transcription using the SP6 or T7 RNA polymerase, as appropriate, in the presence of the m7G(5′)ppp(5′)A cap structure analog. This transcription reaction generates 5′ capped synthetic RNAs with authentic 5′ and 3′ ends of the viral genome. The synthetic RNAs are then introduced into eukaryotic cells by transfection using various methods such as DEAE-dextran, cationic liposomes, and electroporation. Typically, electroporation of the synthetic RNAs into BHK-21 cells generates a high titer of synthetic virus (~5 × 106 PFU/ml) at 24 h post-transfection.
In recent years, we have also constructed another full-length infectious cDNA for JEV SA14-14-2,318 the most widely used vaccine strain. By employing the same strategy used for JEV CNU/LP2,73 the genomic RNA of SA14-14-2 was first cloned as 4 overlapping cDNA fragments, which were then sequentially joined at natural restriction sites present within the genome into a genome-length cDNA in the BAC plasmid pBeloBAC11 (Yun SI and Lee YM, unpublished). We have observed that molecularly cloned viruses rescued from the infectious SA14-14-2 cDNA have in vitro growth properties and in vivo attenuation phenotypes identical to those of the parental uncloned SA14-14-2 virus (Yun SI and Lee YM, unpublished). This system, together with the virus pair SA14 and SA14-14-2, now offers a unique opportunity to understand the molecular basis of how SA14-14-2 virus is attenuated in virulence. Also, the infectious SA14-14-2 cDNA technology has direct application to the design of a novel and promising class of vaccines to expand and improve the currently available preventive arsenals against infection with JEV and other taxonomically related flaviviruses. Furthermore, this technology will allow us to explore SA14-14-2 as a vaccine vector that can express a heterologous protein and induce protective immune responses against the inserted antigen.
Conclusion
JE is a neurological disease caused by a mosquito-borne JEV. Because of the enzootic nature of its transmission, JEV, unlike smallpox and polio, cannot be completely eliminated. Since its discovery in 1935, JEV has continued to expand its activity into new territories, although several JE vaccines have been made commercially available in different parts of the world. Concern about its spread has been highlighted by the recent emergence and spread of JEV in northern Australia, making it of significant concern for global public health. One of the most important research areas is the development of an ideal JE vaccine: one that is safer, cheaper, and more efficacious and that provides life-long protection with a single dose. The development of such a vaccine will be greatly facilitated by a deepening of our understanding of JEV replication and pathogenesis at the molecular level, which has now become technically feasible with the use of infectious JEV SA14-14-2 cDNA technology. This technology also has huge potential for developing JEV SA14-14-2 as a vaccine vector to deliver a foreign gene(s), as has already been achieved with infectious YFV 17D cDNA technology.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by a National Institutes of Health grant (AI101464), Utah Science Technology and Research funds, and a Korean National Research Foundation grant (2007-0052563). Also, this research was supported by the Utah Agricultural Experiment Station, Utah State University, and approved as journal paper number UAES #8612. Yun SI was supported by a grant from the National Research Foundation (532-2008-1-C00011), funded by the Korean Ministry of Education, Science, and Technology. We thank Dr Deborah McClellan for reading the manuscript.
References
- 1.Solomon T. Control of Japanese encephalitis--within our grasp? N Engl J Med. 2006;355:869–71. doi: 10.1056/NEJMp058263. [DOI] [PubMed] [Google Scholar]
- 2.Endy TP, Nisalak A. Japanese encephalitis virus: ecology and epidemiology. Curr Top Microbiol Immunol. 2002;267:11–48. doi: 10.1007/978-3-642-59403-8_2. [DOI] [PubMed] [Google Scholar]
- 3.Halstead SB, Jacobsen J. Japanese encephalitis vaccines. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. Maryland Heights, MO: Saunders Elsevier, 2008:311-52. [Google Scholar]
- 4.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10(Suppl):S98–109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 5.Rappleye WC. Epidemiology of Japanese B encephalitis. Epidemic encephalitis: Third Report of the Matheson Commission. New York: Columbia University Press, 1939:157. [Google Scholar]
- 6.Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15:1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna JN, Ritchie SA, Phillips DA, Lee JM, Hills SL, van den Hurk AF, Pyke AT, Johansen CA, Mackenzie JS. Japanese encephalitis in north Queensland, Australia, 1998. Med J Aust. 1999;170:533–6. doi: 10.5694/j.1326-5377.1999.tb127878.x. [DOI] [PubMed] [Google Scholar]
- 8.Hanna JN, Ritchie SA, Phillips DA, Shield J, Bailey MC, Mackenzie JS, Poidinger M, McCall BJ, Mills PJ. An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med J Aust. 1996;165:256–60. doi: 10.5694/j.1326-5377.1996.tb124960.x. [DOI] [PubMed] [Google Scholar]
- 9.Mackenzie JS, Johansen CA, Ritchie SA, van den Hurk AF, Hall RA. Japanese encephalitis as an emerging virus: the emergence and spread of Japanese encephalitis virus in Australasia. Curr Top Microbiol Immunol. 2002;267:49–73. doi: 10.1007/978-3-642-59403-8_3. [DOI] [PubMed] [Google Scholar]
- 10.Mackenzie JS, Williams DT, Smith DW. Japanese encephalitis virus: the geographic distribution, incidence, and spread of a virus with a propensity to emerge in new areas. In: Tabor E, ed. Emerging viruses in human populations. Amsterdam: Elsevier, 2007:201-68. [Google Scholar]
- 11.Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85:328–45. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 13.Tsai TF. New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting, Bangkok, Thailand, 13-15 October 1998. Vaccine. 2000;18(Suppl 2):1–25. doi: 10.1016/S0264-410X(00)00037-2. [DOI] [PubMed] [Google Scholar]
- 14.Misra UK, Kalita J. Overview: Japanese encephalitis. Prog Neurobiol. 2010;91:108–20. doi: 10.1016/j.pneurobio.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Solomon T, Vaughn DW. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections. Curr Top Microbiol Immunol. 2002;267:171–94. doi: 10.1007/978-3-642-59403-8_9. [DOI] [PubMed] [Google Scholar]
- 16.Solomon T, Winter PM. Neurovirulence and host factors in flavivirus encephalitis-evidence from clinical epidemiology. Arch Virol. 2004;18(Suppl):161–70. doi: 10.1007/978-3-7091-0572-6_14. [DOI] [PubMed] [Google Scholar]
- 17.Vaughn DW, Hoke CH., Jr. The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol Rev. 1992;14:197–221. doi: 10.1093/oxfordjournals.epirev.a036087. [DOI] [PubMed] [Google Scholar]
- 18.Monath TP. Japanese encephalitis vaccines: current vaccines and future prospects. Curr Top Microbiol Immunol. 2002;267:105–38. doi: 10.1007/978-3-642-59403-8_6. [DOI] [PubMed] [Google Scholar]
- 19.Burke DS, Leake CJ. Japanese encephalitis. In: Monath TP, ed. The arboviruses: epidemiology and ecology. Boca Raton, FL: CRC, 1988:63-92. [Google Scholar]
- 20.Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, Ginsburg AS. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Orga. 2011;89:766-74–774A-774E. doi: 10.2471/BLT.10.085233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO) Japanese encephalitis vaccines. Wkly Epidemiol Rec. 2006;81:331–40. [PubMed] [Google Scholar]
- 22.Ding D, Hong Z, Zhao SJ, Clemens JD, Zhou B, Wang B, Huang MS, Zeng J, Guo QH, Liu W, et al. Long-term disability from acute childhood Japanese encephalitis in Shanghai, China. Am J Trop Med Hyg. 2007;77:528–33. [PubMed] [Google Scholar]
- 23.Yun SI, Kim SY, Choi WY, Nam JH, Ju YR, Park KY, Cho HW, Lee YM. Molecular characterization of the full-length genome of the Japanese encephalitis viral strain K87P39. Virus Res. 2003;96:129–40. doi: 10.1016/S0168-1702(03)00181-3. [DOI] [PubMed] [Google Scholar]
- 24.Pyke AT, Williams DT, Nisbet DJ, van den Hurk AF, Taylor CT, Johansen CA, Macdonald J, Hall RA, Simmons RJ, Mason RJ, et al. The appearance of a second genotype of Japanese encephalitis virus in the Australasian region. Am J Trop Med Hyg. 2001;65:747–53. doi: 10.4269/ajtmh.2001.65.747. [DOI] [PubMed] [Google Scholar]
- 25.Williams DT, Wang LF, Daniels PW, Mackenzie JS. Molecular characterization of the first Australian isolate of Japanese encephalitis virus, the FU strain. J Gen Virol. 2000;81:2471–80. doi: 10.1099/0022-1317-81-10-2471. [DOI] [PubMed] [Google Scholar]
- 26.Paranjpe S, Banerjee K. Phylogenetic analysis of the envelope gene of Japanese encephalitis virus. Virus Res. 1996;42:107–17. doi: 10.1016/0168-1702(96)01306-8. [DOI] [PubMed] [Google Scholar]
- 27.Schuh AJ, Li L, Tesh RB, Innis BL, Barrett AD. Genetic characterization of early isolates of Japanese encephalitis virus: genotype II has been circulating since at least 1951. J Gen Virol. 2010;91:95–102. doi: 10.1099/vir.0.013631-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen WR, Rico-Hesse R, Tesh RB. A new genotype of Japanese encephalitis virus from Indonesia. Am J Trop Med Hyg. 1992;47:61–9. doi: 10.4269/ajtmh.1992.47.61. [DOI] [PubMed] [Google Scholar]
- 29.Mohammed MA, Galbraith SE, Radford AD, Dove W, Takasaki T, Kurane I, Solomon T. Molecular phylogenetic and evolutionary analyses of Muar strain of Japanese encephalitis virus reveal it is the missing fifth genotype. Infect Genet Evol. 2011;11:855–62. doi: 10.1016/j.meegid.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Huong VT, Ha DQ, Deubel V. Genetic study of Japanese encephalitis viruses from Vietnam. Am J Trop Med Hyg. 1993;49:538–44. doi: 10.4269/ajtmh.1993.49.538. [DOI] [PubMed] [Google Scholar]
- 31.Chen WR, Tesh RB, Rico-Hesse R. Genetic variation of Japanese encephalitis virus in nature. J Gen Virol. 1990;71:2915–22. doi: 10.1099/0022-1317-71-12-2915. [DOI] [PubMed] [Google Scholar]
- 32.Uchil PD, Satchidanandam V. Phylogenetic analysis of Japanese encephalitis virus: envelope gene based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent. Am J Trop Med Hyg. 2001;65:242–51. doi: 10.4269/ajtmh.2001.65.242. [DOI] [PubMed] [Google Scholar]
- 33.Solomon T, Ni H, Beasley DW, Ekkelenkamp M, Cardosa MJ, Barrett AD. Origin and evolution of Japanese encephalitis virus in southeast Asia. J Virol. 2003;77:3091–8. doi: 10.1128/JVI.77.5.3091-3098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takhampunya R, Kim HC, Tippayachai B, Kengluecha A, Klein TA, Lee WJ, Grieco J, Evans BP. Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virol J. 2011;8:449. doi: 10.1186/1743-422X-8-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li MH, Fu SH, Chen WX, Wang HY, Guo YH, Liu QY, Li YX, Luo HM, Da W, Duo Ji DZ, et al. Genotype v Japanese encephalitis virus is emerging. PLoS Negl Trop Dis. 2011;5:e1231. doi: 10.1371/journal.pntd.0001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsarev SA, Sanders ML, Vaughn DW, Innis BL. Phylogenetic analysis suggests only one serotype of Japanese encephalitis virus. Vaccine. 2000;18(Suppl 2):36–43. doi: 10.1016/S0264-410X(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 37.Beasley DW, Li L, Suderman MT, Guirakhoo F, Trent DW, Monath TP, Shope RE, Barrett AD. Protection against Japanese encephalitis virus strains representing four genotypes by passive transfer of sera raised against ChimeriVax-JE experimental vaccine. Vaccine. 2004;22:3722–6. doi: 10.1016/j.vaccine.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 38.Ali A, Igarashi A. Antigenic and genetic variations among Japanese encephalitis virus strains belonging to genotype 1. Microbiol Immunol. 1997;41:241–52. doi: 10.1111/j.1348-0421.1997.tb01196.x. [DOI] [PubMed] [Google Scholar]
- 39.Hasegawa H, Yoshida M, Kobayashi Y, Fujita S. Antigenic analysis of Japanese encephalitis viruses in Asia by using monoclonal antibodies. Vaccine. 1995;13:1713–21. doi: 10.1016/0264-410X(95)00099-M. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi Y, Hasegawa H, Oyama T, Tamai T, Kusaba T. Antigenic analysis of Japanese encephalitis virus by using monoclonal antibodies. Infect Immun. 1984;44:117–23. doi: 10.1128/iai.44.1.117-123.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasegawa H, Yoshida M, Fujita S, Kobayashi Y. Comparison of structural proteins among antigenically different Japanese encephalitis virus strains. Vaccine. 1994;12:841–4. doi: 10.1016/0264-410X(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi Y, Hasegawa H, Yamauchi T. Studies on the antigenic structure of Japanese encephalitis virus using monoclonal antibodies. Microbiol Immunol. 1985;29:1069–82. doi: 10.1111/j.1348-0421.1985.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 43.Rosen L. The natural history of Japanese encephalitis virus. Annu Rev Microbiol. 1986;40:395–414. doi: 10.1146/annurev.mi.40.100186.002143. [DOI] [PubMed] [Google Scholar]
- 44.Weaver SC, Barrett AD. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol. 2004;2:789–801. doi: 10.1038/nrmicro1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yun SI, Lee YM. Japanese encephalitis virus: molecular biology and vaccine development. In: Kalitzky M, Borowski P, eds. Molecular biology of the flavivirus. Norwich, United Kingdom: Horizon Scientific Press, 2006:225-71. [Google Scholar]
- 46.Gubler DJ, Kuno G, Markoff L. Flaviviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, eds. Fields virology. Philadelphia, PA: Lippincott Williams & Wilkins Publishers, 2007:1153-252. [Google Scholar]
- 47.Dhanda V, Mourya DT, Mishra AC, Ilkal MA, Pant U, Jacob PG, Bhat HR. Japanese encephalitis virus infection in mosquitoes reared from field-collected immatures and in wild-caught males. Am J Trop Med Hyg. 1989;41:732–6. doi: 10.4269/ajtmh.1989.41.732. [DOI] [PubMed] [Google Scholar]
- 48.Rosen L, Lien JC, Shroyer DA, Baker RH, Lu LC. Experimental vertical transmission of Japanese encephalitis virus by Culex tritaeniorhynchus and other mosquitoes. Am J Trop Med Hyg. 1989;40:548–56. doi: 10.4269/ajtmh.1989.40.548. [DOI] [PubMed] [Google Scholar]
- 49.Iwamoto M, Jernigan DB, Guasch A, Trepka MJ, Blackmore CG, Hellinger WC, Pham SM, Zaki S, Lanciotti RS, Lance-Parker SE, et al. West Nile Virus in Transplant Recipients Investigation Team Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:2196–203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 50.Pealer LN, Marfin AA, Petersen LR, Lanciotti RS, Page PL, Stramer SL, Stobierski MG, Signs K, Newman B, Kapoor H, et al. West Nile Virus Transmission Investigation Team Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236–45. doi: 10.1056/NEJMoa030969. [DOI] [PubMed] [Google Scholar]
- 51.Fischer M, Lindsey N, Staples JE, Hills S, Centers for Disease Control and Prevention (CDC) Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59(RR-1):1–27. [PubMed] [Google Scholar]
- 52.Kuwayama M, Ito M, Takao S, Shimazu Y, Fukuda S, Miyazaki K, Kurane I, Takasaki T. Japanese encephalitis virus in meningitis patients, Japan. Emerg Infect Dis. 2005;11:471–3. doi: 10.3201/eid1103.040285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu Y, Zhaori G, Vene S, Shen K, Zhou Y, Magnius LO, Wahren B, Linde A. Viral etiology of acute childhood encephalitis in Beijing diagnosed by analysis of single samples. Pediatr Infect Dis J. 1996;15:1018–24. doi: 10.1097/00006454-199611000-00017. [DOI] [PubMed] [Google Scholar]
- 54.Lowry PW, Truong DH, Hinh LD, Ladinsky JL, Karabatsos N, Cropp CB, Martin D, Gubler DJ. Japanese encephalitis among hospitalized pediatric and adult patients with acute encephalitis syndrome in Hanoi, Vietnam 1995. Am J Trop Med Hyg. 1998;58:324–9. doi: 10.4269/ajtmh.1998.58.324. [DOI] [PubMed] [Google Scholar]
- 55.Watt G, Jongsakul K. Acute undifferentiated fever caused by infection with Japanese encephalitis virus. Am J Trop Med Hyg. 2003;68:704–6. [PubMed] [Google Scholar]
- 56.Kumar R, Tripathi P, Singh S, Bannerji G. Clinical features in children hospitalized during the 2005 epidemic of Japanese encephalitis in Uttar Pradesh, India. Clin Infect Dis. 2006;43:123–31. doi: 10.1086/505121. [DOI] [PubMed] [Google Scholar]
- 57.Kalita J, Misra UK. Markedly severe dystonia in Japanese encephalitis. Mov Disord. 2000;15:1168–72. doi: 10.1002/1531-8257(200011)15:6<1168::AID-MDS1016>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 58.Rayamajhi A, Singh R, Prasad R, Khanal B, Singhi S. Clinico-laboratory profile and outcome of Japanese encephalitis in Nepali children. Ann Trop Paediatr. 2006;26:293–301. doi: 10.1179/146532806X152818. [DOI] [PubMed] [Google Scholar]
- 59.Diagana M, Preux PM, Dumas M. Japanese encephalitis revisited. J Neurol Sci. 2007;262:165–70. doi: 10.1016/j.jns.2007.06.041. [DOI] [PubMed] [Google Scholar]
- 60.Misra UK, Kalita J. Anterior horn cells are also involved in Japanese encephalitis. Acta Neurol Scand. 1997;96:114–7. doi: 10.1111/j.1600-0404.1997.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 61.Solomon T, Kneen R, Dung NM, Khanh VC, Thuy TT, Ha DQ, Day NP, Nisalak A, Vaughn DW, White NJ. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet. 1998;351:1094–7. doi: 10.1016/S0140-6736(97)07509-0. [DOI] [PubMed] [Google Scholar]
- 62.Kumar R, Mathur A, Kumar A, Sharma S, Chakraborty S, Chaturvedi UC. Clinical features & prognostic indicators of Japanese encephalitis in children in Lucknow (India) Indian J Med Res. 1990;91:321–7. [PubMed] [Google Scholar]
- 63.Solomon T, Dung NM, Kneen R, Thao TT, Gainsborough M, Nisalak A, Day NP, Kirkham FJ, Vaughn DW, Smith S, et al. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain. 2002;125:1084–93. doi: 10.1093/brain/awf116. [DOI] [PubMed] [Google Scholar]
- 64.Misra UK, Kalita J. Seizures in Japanese encephalitis. J Neurol Sci. 2001;190:57–60. doi: 10.1016/S0022-510X(01)00589-5. [DOI] [PubMed] [Google Scholar]
- 65.Schneider RJ, Firestone MH, Edelman R, Chieowanich P, Pornpibul R. Clinical sequelae after japanese encephalitis: a one year follow-up study in Thailand. Southeast Asian J Trop Med Public Health. 1974;5:560–8. [PubMed] [Google Scholar]
- 66.Calisher CH, Gould EA. Taxonomy of the virus family Flaviviridae. Adv Virus Res. 2003;59:1–19. doi: 10.1016/S0065-3527(03)59001-7. [DOI] [PubMed] [Google Scholar]
- 67.Thiel HJ, Collett MS, Gould EA, Heinz FX, Houghton M, Meyers G, Purcell RH, Rice CM. Family Flaviviridae. In: Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA, eds. Virus taxonomy: Eighth Report of the International Committee on Taxonomy of Viruses. San Diego, CA: Elsevier Academic Press, 2005:981-98. [Google Scholar]
- 68.Mackenzie JS, Barrett AD, Deubel V. The Japanese encephalitis serological group of flaviviruses: a brief introduction to the group. Curr Top Microbiol Immunol. 2002;267:1–10. doi: 10.1007/978-3-642-59403-8_1. [DOI] [PubMed] [Google Scholar]
- 69.Gould EA, de Lamballerie X, Zanotto PM, Holmes EC. Origins, evolution, and vector/host coadaptations within the genus Flavivirus. Adv Virus Res. 2003;59:277–314. doi: 10.1016/S0065-3527(03)59008-X. [DOI] [PubMed] [Google Scholar]
- 70.Rice CM, Lenches EM, Eddy SR, Shin SJ, Sheets RL, Strauss JH. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985;229:726–33. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- 71.Sumiyoshi H, Mori C, Fuke I, Morita K, Kuhara S, Kondou J, Kikuchi Y, Nagamatu H, Igarashi A. Complete nucleotide sequence of the Japanese encephalitis virus genome RNA. Virology. 1987;161:497–510. doi: 10.1016/0042-6822(87)90144-9. [DOI] [PubMed] [Google Scholar]
- 72.Lindenbach BD, Thiel HJ, Rice CM. Flaviviridae: the viruses and their replication. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, eds. Fields virology. Philadelphia, PA: Lippincott Williams & Wilkins Publishers, 2007:1101-52. [Google Scholar]
- 73.Yun SI, Kim SY, Rice CM, Lee YM. Development and application of a reverse genetics system for Japanese encephalitis virus. J Virol. 2003;77:6450–65. doi: 10.1128/JVI.77.11.6450-6465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim JM, Yun SI, Song BH, Hahn YS, Lee CH, Oh HW, Lee YM. A single N-linked glycosylation site in the Japanese encephalitis virus prM protein is critical for cell type-specific prM protein biogenesis, virus particle release, and pathogenicity in mice. J Virol. 2008;82:7846–62. doi: 10.1128/JVI.00789-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15:690–8. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mukhopadhyay S, Kuhn RJ, Rossmann MG. A structural perspective of the flavivirus life cycle. Nat Rev Microbiol. 2005;3:13–22. doi: 10.1038/nrmicro1067. [DOI] [PubMed] [Google Scholar]
- 77.Dokland T, Walsh M, Mackenzie JM, Khromykh AA, Ee KH, Wang S. West Nile virus core protein; tetramer structure and ribbon formation. Structure. 2004;12:1157–63. doi: 10.1016/j.str.2004.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma L, Jones CT, Groesch TD, Kuhn RJ, Post CB. Solution structure of dengue virus capsid protein reveals another fold. Proc Natl Acad Sci U S A. 2004;101:3414–9. doi: 10.1073/pnas.0305892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell. 2002;108:717–25. doi: 10.1016/S0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG, Kuhn RJ. Structure of West Nile virus. Science. 2003;302:248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- 81.Zhang W, Chipman PR, Corver J, Johnson PR, Zhang Y, Mukhopadhyay S, Baker TS, Strauss JH, Rossmann MG, Kuhn RJ. Visualization of membrane protein domains by cryo-electron microscopy of dengue virus. Nat Struct Biol. 2003;10:907–12. doi: 10.1038/nsb990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paranjape SM, Harris E. Control of dengue virus translation and replication. Curr Top Microbiol Immunol. 2010;338:15–34. doi: 10.1007/978-3-642-02215-9_2. [DOI] [PubMed] [Google Scholar]
- 83.Markoff L. 5′- and 3′-noncoding regions in flavivirus RNA. Adv Virus Res. 2003;59:177–228. doi: 10.1016/S0065-3527(03)59006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Westaway EG, Mackenzie JM, Khromykh AA. Replication and gene function in Kunjin virus. Curr Top Microbiol Immunol. 2002;267:323–51. doi: 10.1007/978-3-642-59403-8_16. [DOI] [PubMed] [Google Scholar]
- 85.Villordo SM, Gamarnik AV. Genome cyclization as strategy for flavivirus RNA replication. Virus Res. 2009;139:230–9. doi: 10.1016/j.virusres.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brinton MA. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371–402. doi: 10.1146/annurev.micro.56.012302.160654. [DOI] [PubMed] [Google Scholar]
- 87.Patkar CG, Kuhn RJ. Yellow Fever virus NS3 plays an essential role in virus assembly independent of its known enzymatic functions. J Virol. 2008;82:3342–52. doi: 10.1128/JVI.02447-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leung JY, Pijlman GP, Kondratieva N, Hyde J, Mackenzie JM, Khromykh AA. Role of nonstructural protein NS2A in flavivirus assembly. J Virol. 2008;82:4731–41. doi: 10.1128/JVI.00002-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu WJ, Chen HB, Khromykh AA. Molecular and functional analyses of Kunjin virus infectious cDNA clones demonstrate the essential roles for NS2A in virus assembly and for a nonconservative residue in NS3 in RNA replication. J Virol. 2003;77:7804–13. doi: 10.1128/JVI.77.14.7804-7813.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kümmerer BM, Rice CM. Mutations in the yellow fever virus nonstructural protein NS2A selectively block production of infectious particles. J Virol. 2002;76:4773–84. doi: 10.1128/JVI.76.10.4773-4784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pijlman GP, Kondratieva N, Khromykh AA. Translation of the flavivirus kunjin NS3 gene in cis but not its RNA sequence or secondary structure is essential for efficient RNA packaging. J Virol. 2006;80:11255–64. doi: 10.1128/JVI.01559-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morrison J, Aguirre S, Fernandez-Sesma A. Innate immunity evasion by Dengue virus. Viruses. 2012;4:397–413. doi: 10.3390/v4030397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robertson SJ, Mitzel DN, Taylor RT, Best SM, Bloom ME. Tick-borne flaviviruses: dissecting host immune responses and virus countermeasures. Immunol Res. 2009;43:172–86. doi: 10.1007/s12026-008-8065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Diamond MS. Mechanisms of evasion of the type I interferon antiviral response by flaviviruses. J Interferon Cytokine Res. 2009;29:521–30. doi: 10.1089/jir.2009.0069. [DOI] [PubMed] [Google Scholar]
- 95.Liu WJ, Wang XJ, Mokhonov VV, Shi PY, Randall R, Khromykh AA. Inhibition of interferon signaling by the New York 99 strain and Kunjin subtype of West Nile virus involves blockage of STAT1 and STAT2 activation by nonstructural proteins. J Virol. 2005;79:1934–42. doi: 10.1128/JVI.79.3.1934-1942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Muñoz-Jordan JL, Sánchez-Burgos GG, Laurent-Rolle M, García-Sastre A. Inhibition of interferon signaling by dengue virus. Proc Natl Acad Sci U S A. 2003;100:14333–8. doi: 10.1073/pnas.2335168100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Muñoz-Jordán JL, Laurent-Rolle M, Ashour J, Martínez-Sobrido L, Ashok M, Lipkin WI, García-Sastre A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol. 2005;79:8004–13. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo JT, Hayashi J, Seeger C. West Nile virus inhibits the signal transduction pathway of alpha interferon. J Virol. 2005;79:1343–50. doi: 10.1128/JVI.79.3.1343-1350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Falgout B, Miller RH, Lai CJ. Deletion analysis of dengue virus type 4 nonstructural protein NS2B: identification of a domain required for NS2B-NS3 protease activity. J Virol. 1993;67:2034–42. doi: 10.1128/jvi.67.4.2034-2042.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chambers TJ, Grakoui A, Rice CM. Processing of the yellow fever virus nonstructural polyprotein: a catalytically active NS3 proteinase domain and NS2B are required for cleavages at dibasic sites. J Virol. 1991;65:6042–50. doi: 10.1128/jvi.65.11.6042-6050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wengler G, Wengler G. The carboxy-terminal part of the NS 3 protein of the West Nile flavivirus can be isolated as a soluble protein after proteolytic cleavage and represents an RNA-stimulated NTPase. Virology. 1991;184:707–15. doi: 10.1016/0042-6822(91)90440-M. [DOI] [PubMed] [Google Scholar]
- 102.Li H, Clum S, You S, Ebner KE, Padmanabhan R. The serine protease and RNA-stimulated nucleoside triphosphatase and RNA helicase functional domains of dengue virus type 2 NS3 converge within a region of 20 amino acids. J Virol. 1999;73:3108–16. doi: 10.1128/jvi.73.4.3108-3116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wengler G, Wengler G. The NS 3 nonstructural protein of flaviviruses contains an RNA triphosphatase activity. Virology. 1993;197:265–73. doi: 10.1006/viro.1993.1587. [DOI] [PubMed] [Google Scholar]
- 104.Ray D, Shah A, Tilgner M, Guo Y, Zhao Y, Dong H, Deas TS, Zhou Y, Li H, Shi PY. West Nile virus 5′-cap structure is formed by sequential guanine N-7 and ribose 2′-O methylations by nonstructural protein 5. J Virol. 2006;80:8362–70. doi: 10.1128/JVI.00814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Egloff MP, Benarroch D, Selisko B, Romette JL, Canard B. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. EMBO J. 2002;21:2757–68. doi: 10.1093/emboj/21.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Issur M, Geiss BJ, Bougie I, Picard-Jean F, Despins S, Mayette J, Hobdey SE, Bisaillon M. The flavivirus NS5 protein is a true RNA guanylyltransferase that catalyzes a two-step reaction to form the RNA cap structure. RNA. 2009;15:2340–50. doi: 10.1261/rna.1609709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ackermann M, Padmanabhan R. De novo synthesis of RNA by the dengue virus RNA-dependent RNA polymerase exhibits temperature dependence at the initiation but not elongation phase. J Biol Chem. 2001;276:39926–37. doi: 10.1074/jbc.M104248200. [DOI] [PubMed] [Google Scholar]
- 108.Tan BH, Fu J, Sugrue RJ, Yap EH, Chan YC, Tan YH. Recombinant dengue type 1 virus NS5 protein expressed in Escherichia coli exhibits RNA-dependent RNA polymerase activity. Virology. 1996;216:317–25. doi: 10.1006/viro.1996.0067. [DOI] [PubMed] [Google Scholar]
- 109.Melian EB, Hinzman E, Nagasaki T, Firth AE, Wills NM, Nouwens AS, Blitvich BJ, Leung J, Funk A, Atkins JF, et al. NS1′ of flaviviruses in the Japanese encephalitis virus serogroup is a product of ribosomal frameshifting and plays a role in viral neuroinvasiveness. J Virol. 2010;84:1641–7. doi: 10.1128/JVI.01979-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Firth AE, Atkins JF. A conserved predicted pseudoknot in the NS2A-encoding sequence of West Nile and Japanese encephalitis flaviviruses suggests NS1′ may derive from ribosomal frameshifting. Virol J. 2009;6:14. doi: 10.1186/1743-422X-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med. 1997;3:866–71. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]
- 112.Davis CW, Nguyen HY, Hanna SL, Sánchez MD, Doms RW, Pierson TC. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol. 2006;80:1290–301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier JL, Arenzana-Seisdedos F, Desprès P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep. 2003;4:723–8. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pokidysheva E, Zhang Y, Battisti AJ, Bator-Kelly CM, Chipman PR, Xiao C, Gregorio GG, Hendrickson WA, Kuhn RJ, Rossmann MG. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell. 2006;124:485–93. doi: 10.1016/j.cell.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 115.Tassaneetrithep B, Burgess TH, Granelli-Piperno A, Trumpfheller C, Finke J, Sun W, Eller MA, Pattanapanyasat K, Sarasombath S, Birx DL, et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med. 2003;197:823–9. doi: 10.1084/jem.20021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Acosta EG, Castilla V, Damonte EB. Functional entry of dengue virus into Aedes albopictus mosquito cells is dependent on clathrin-mediated endocytosis. J Gen Virol. 2008;89:474–84. doi: 10.1099/vir.0.83357-0. [DOI] [PubMed] [Google Scholar]
- 117.Chen Y, Maguire T, Marks RM. Demonstration of binding of dengue virus envelope protein to target cells. J Virol. 1996;70:8765–72. doi: 10.1128/jvi.70.12.8765-8772.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chu JJ, Leong PW, Ng ML. Analysis of the endocytic pathway mediating the infectious entry of mosquito-borne flavivirus West Nile into Aedes albopictus mosquito (C6/36) cells. Virology. 2006;349:463–75. doi: 10.1016/j.virol.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 119.Chu JJ, Ng ML. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J Virol. 2004;78:10543–55. doi: 10.1128/JVI.78.19.10543-10555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mosso C, Galván-Mendoza IJ, Ludert JE, del Angel RM. Endocytic pathway followed by dengue virus to infect the mosquito cell line C6/36 HT. Virology. 2008;378:193–9. doi: 10.1016/j.virol.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 121.van der Schaar HM, Rust MJ, Waarts BL, van der Ende-Metselaar H, Kuhn RJ, Wilschut J, Zhuang X, Smit JM. Characterization of the early events in dengue virus cell entry by biochemical assays and single-virus tracking. J Virol. 2007;81:12019–28. doi: 10.1128/JVI.00300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van der Schaar HM, Rust MJ, Chen C, van der Ende-Metselaar H, Wilschut J, Zhuang X, Smit JM. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 2008;4:e1000244. doi: 10.1371/journal.ppat.1000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Allison SL, Schalich J, Stiasny K, Mandl CW, Kunz C, Heinz FX. Oligomeric rearrangement of tick-borne encephalitis virus envelope proteins induced by an acidic pH. J Virol. 1995;69:695–700. doi: 10.1128/jvi.69.2.695-700.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bressanelli S, Stiasny K, Allison SL, Stura EA, Duquerroy S, Lescar J, Heinz FX, Rey FA. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 2004;23:728–38. doi: 10.1038/sj.emboj.7600064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Corver J, Ortiz A, Allison SL, Schalich J, Heinz FX, Wilschut J. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Virology. 2000;269:37–46. doi: 10.1006/viro.1999.0172. [DOI] [PubMed] [Google Scholar]
- 126.Liao M, Sánchez-San Martín C, Zheng A, Kielian M. In vitro reconstitution reveals key intermediate states of trimer formation by the dengue virus membrane fusion protein. J Virol. 2010;84:5730–40. doi: 10.1128/JVI.00170-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Modis Y, Ogata S, Clements D, Harrison SC. Structure of the dengue virus envelope protein after membrane fusion. Nature. 2004;427:313–9. doi: 10.1038/nature02165. [DOI] [PubMed] [Google Scholar]
- 128.Nayak V, Dessau M, Kucera K, Anthony K, Ledizet M, Modis Y. Crystal structure of dengue virus type 1 envelope protein in the postfusion conformation and its implications for membrane fusion. J Virol. 2009;83:4338–44. doi: 10.1128/JVI.02574-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stiasny K, Allison SL, Schalich J, Heinz FX. Membrane interactions of the tick-borne encephalitis virus fusion protein E at low pH. J Virol. 2002;76:3784–90. doi: 10.1128/JVI.76.8.3784-3790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Stiasny K, Koessl C, Heinz FX. Involvement of lipids in different steps of the flavivirus fusion mechanism. J Virol. 2003;77:7856–62. doi: 10.1128/JVI.77.14.7856-7862.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stiasny K, Kössl C, Lepault J, Rey FA, Heinz FX. Characterization of a structural intermediate of flavivirus membrane fusion. PLoS Pathog. 2007;3:e20. doi: 10.1371/journal.ppat.0030020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lindenbach BD, Rice CM. Molecular biology of flaviviruses. Adv Virus Res. 2003;59:23–61. doi: 10.1016/S0065-3527(03)59002-9. [DOI] [PubMed] [Google Scholar]
- 133.Westaway EG, Mackenzie JM, Khromykh AA. Kunjin RNA replication and applications of Kunjin replicons. Adv Virus Res. 2003;59:99–140. doi: 10.1016/S0065-3527(03)59004-2. [DOI] [PubMed] [Google Scholar]
- 134.Gillespie LK, Hoenen A, Morgan G, Mackenzie JM. The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. J Virol. 2010;84:10438–47. doi: 10.1128/JVI.00986-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hsu NY, Ilnytska O, Belov G, Santiana M, Chen YH, Takvorian PM, Pau C, van der Schaar H, Kaushik-Basu N, Balla T, et al. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010;141:799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Welsch S, Miller S, Romero-Brey I, Merz A, Bleck CK, Walther P, Fuller SD, Antony C, Krijnse-Locker J, Bartenschlager R. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe. 2009;5:365–75. doi: 10.1016/j.chom.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li L, Lok SM, Yu IM, Zhang Y, Kuhn RJ, Chen J, Rossmann MG. The flavivirus precursor membrane-envelope protein complex: structure and maturation. Science. 2008;319:1830–4. doi: 10.1126/science.1153263. [DOI] [PubMed] [Google Scholar]
- 138.Zhang Y, Corver J, Chipman PR, Zhang W, Pletnev SV, Sedlak D, Baker TS, Strauss JH, Kuhn RJ, Rossmann MG. Structures of immature flavivirus particles. EMBO J. 2003;22:2604–13. doi: 10.1093/emboj/cdg270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Y, Kaufmann B, Chipman PR, Kuhn RJ, Rossmann MG. Structure of immature West Nile virus. J Virol. 2007;81:6141–5. doi: 10.1128/JVI.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lorenz IC, Allison SL, Heinz FX, Helenius A. Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J Virol. 2002;76:5480–91. doi: 10.1128/JVI.76.11.5480-5491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stadler K, Allison SL, Schalich J, Heinz FX. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol. 1997;71:8475–81. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Elshuber S, Allison SL, Heinz FX, Mandl CW. Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J Gen Virol. 2003;84:183–91. doi: 10.1099/vir.0.18723-0. [DOI] [PubMed] [Google Scholar]
- 143.Guirakhoo F, Bolin RA, Roehrig JT. The Murray Valley encephalitis virus prM protein confers acid resistance to virus particles and alters the expression of epitopes within the R2 domain of E glycoprotein. Virology. 1992;191:921–31. doi: 10.1016/0042-6822(92)90267-S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Guirakhoo F, Heinz FX, Mandl CW, Holzmann H, Kunz C. Fusion activity of flaviviruses: comparison of mature and immature (prM-containing) tick-borne encephalitis virions. J Gen Virol. 1991;72:1323–9. doi: 10.1099/0022-1317-72-6-1323. [DOI] [PubMed] [Google Scholar]
- 145.Yu IM, Holdaway HA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. Association of the pr peptides with dengue virus at acidic pH blocks membrane fusion. J Virol. 2009;83:12101–7. doi: 10.1128/JVI.01637-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yu IM, Zhang W, Holdaway HA, Li L, Kostyuchenko VA, Chipman PR, Kuhn RJ, Rossmann MG, Chen J. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science. 2008;319:1834–7. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- 147.Zhang Y, Zhang W, Ogata S, Clements D, Strauss JH, Baker TS, Kuhn RJ, Rossmann MG. Conformational changes of the flavivirus E glycoprotein. Structure. 2004;12:1607–18. doi: 10.1016/j.str.2004.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Junjhon J, Edwards TJ, Utaipat U, Bowman VD, Holdaway HA, Zhang W, Keelapang P, Puttikhunt C, Perera R, Chipman PR, et al. Influence of pr-M cleavage on the heterogeneity of extracellular dengue virus particles. J Virol. 2010;84:8353–8. doi: 10.1128/JVI.00696-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sampath A, Padmanabhan R. Molecular targets for flavivirus drug discovery. Antiviral Res. 2009;81:6–15. doi: 10.1016/j.antiviral.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Botting C, Kuhn RJ. Novel approaches to flavivirus drug discovery. Expert Opin Drug Discov. 2012;7:417–28. doi: 10.1517/17460441.2012.673579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Noble CG, Chen YL, Dong H, Gu F, Lim SP, Schul W, Wang QY, Shi PY. Strategies for development of Dengue virus inhibitors. Antiviral Res. 2010;85:450–62. doi: 10.1016/j.antiviral.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 152.Geiss BJ, Stahla H, Hannah AM, Gari AM, Keenan SM. Focus on flaviviruses: current and future drug targets. Future Med Chem. 2009;1:327–44. doi: 10.4155/fmc.09.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Igarashi A. Control of Japanese encephalitis in Japan: immunization of humans and animals, and vector control. Curr Top Microbiol Immunol. 2002;267:139–52. doi: 10.1007/978-3-642-59403-8_7. [DOI] [PubMed] [Google Scholar]
- 154.Beasley DW, Lewthwaite P, Solomon T. Current use and development of vaccines for Japanese encephalitis. Expert Opin Biol Ther. 2008;8:95–106. doi: 10.1517/14712598.8.1.95. [DOI] [PubMed] [Google Scholar]
- 155.Wilder-Smith A, Halstead SB. Japanese encephalitis: update on vaccines and vaccine recommendations. Curr Opin Infect Dis. 2010;23:426–31. doi: 10.1097/QCO.0b013e32833c1d01. [DOI] [PubMed] [Google Scholar]
- 156.Halstead SB, Thomas SJ. New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev Vaccines. 2011;10:355–64. doi: 10.1586/erv.11.7. [DOI] [PubMed] [Google Scholar]
- 157.Fan YC, Chen JM, Chiu HC, Chen YY, Lin JW, Shih CC, Chen CM, Chang CC, Chang GJ, Chiou SS. Partially neutralizing potency against emerging genotype I virus among children received formalin-inactivated Japanese encephalitis virus vaccine. PLoS Negl Trop Dis. 2012;6:e1834. doi: 10.1371/journal.pntd.0001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sanofi Pasteur Inc. Japanese encephalitis virus vaccine inactivated JE-VAX® (product information). Available from: http://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm123761.pdf (accessed 2013).
- 159.Takaku K, Yamashita T, Osanai T, Yoshida I, Kato M, Goda H, Takagi M, Hirota T, Amano T, Fukai K, et al. Japanese encephalitis purified vaccine. Biken J. 1968;11:25–39. [Google Scholar]
- 160.Hoke CH, Nisalak A, Sangawhipa N, Jatanasen S, Laorakapongse T, Innis BL, Kotchasenee S, Gingrich JB, Latendresse J, Fukai K, et al. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med. 1988;319:608–14. doi: 10.1056/NEJM198809083191004. [DOI] [PubMed] [Google Scholar]
- 161.Chambers TJ, Tsai TF, Pervikov Y, Monath TP. Vaccine development against dengue and Japanese encephalitis: report of a World Health Organization meeting. Vaccine. 1997;15:1494–502. doi: 10.1016/S0264-410X(97)00195-3. [DOI] [PubMed] [Google Scholar]
- 162.Jelinek T. Japanese encephalitis vaccine in travelers. Expert Rev Vaccines. 2008;7:689–93. doi: 10.1586/14760584.7.5.689. [DOI] [PubMed] [Google Scholar]
- 163.Poland JD, Cropp CB, Craven RB, Monath TP. Evaluation of the potency and safety of inactivated Japanese encephalitis vaccine in US inhabitants. J Infect Dis. 1990;161:878–82. doi: 10.1093/infdis/161.5.878. [DOI] [PubMed] [Google Scholar]
- 164.Defraites RF, Gambel JM, Hoke CH, Jr., Sanchez JL, Withers BG, Karabatsos N, Shope RE, Tirrell S, Yoshida I, Takagi M, et al. Japanese encephalitis vaccine (inactivated, BIKEN) in U.S. soldiers: immunogenicity and safety of vaccine administered in two dosing regimens. Am J Trop Med Hyg. 1999;61:288–93. doi: 10.4269/ajtmh.1999.61.288. [DOI] [PubMed] [Google Scholar]
- 165.Sanchez JL, Hoke CH, McCown J, DeFraites RF, Takafuji ET, Diniega BM, Pang LW. Further experience with Japanese encephalitis vaccine. Lancet. 1990;335:972–3. doi: 10.1016/0140-6736(90)91036-A. [DOI] [PubMed] [Google Scholar]
- 166.Plesner AM. Allergic reactions to Japanese encephalitis vaccine. Immunol Allergy Clin North Am. 2003;23:665–97. doi: 10.1016/S0889-8561(03)00102-4. [DOI] [PubMed] [Google Scholar]
- 167.Halstead SB, Tsai TF. Japanese encephalitis vaccines. In: Plotkin SA, Orenstein WA, eds. Vaccines. Philadelphia, PA: WB Saunders, 2004:919-58. [Google Scholar]
- 168.Reisler RB, Danner DK, Gibbs PH. Immunogenicity of an inactivated Japanese encephalitis vaccine (JE-VAX) in humans over 20 years at USAMRIID: using PRNT50 as an endpoint for immunogenicity. Vaccine. 2010;28:2436–41. doi: 10.1016/j.vaccine.2009.12.080. [DOI] [PubMed] [Google Scholar]
- 169.Gambel JM, DeFraites R, Hoke C, Jr., Brown A, Sanchez J, Karabatsos N, Tsai T, Meschievitz C. Japanese encephalitis vaccine: persistence of antibody up to 3 years after a three-dose primary series. J Infect Dis. 1995;171:1074. doi: 10.1093/infdis/171.4.1074. [DOI] [PubMed] [Google Scholar]
- 170.Plesner AM, Arlien-Soborg P, Herning M. Neurological complications to vaccination against Japanese encephalitis. Eur J Neurol. 1998;5:479–85. doi: 10.1046/j.1468-1331.1998.550479.x. [DOI] [PubMed] [Google Scholar]
- 171.Lindsey NP, Staples JE, Jones JF, Sejvar JJ, Griggs A, Iskander J, Miller ER, Fischer M. Adverse event reports following Japanese encephalitis vaccination in the United States, 1999-2009. Vaccine. 2010;29:58–64. doi: 10.1016/j.vaccine.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 172.Plesner AM, Arlien-Søborg P, Herning M. Neurological complications and Japanese encephalitis vaccination. Lancet. 1996;348:202–3. doi: 10.1016/S0140-6736(05)66156-9. [DOI] [PubMed] [Google Scholar]
- 173.Takahashi H, Pool V, Tsai TF, Chen RT, The VAERS Working Group Adverse events after Japanese encephalitis vaccination: review of post-marketing surveillance data from Japan and the United States. Vaccine. 2000;18:2963–9. doi: 10.1016/S0264-410X(00)00111-0. [DOI] [PubMed] [Google Scholar]
- 174.Plesner A, Rønne T, Wachmann H. Case-control study of allergic reactions to Japanese encephalitis vaccine. Vaccine. 2000;18:1830–6. doi: 10.1016/S0264-410X(99)00403-X. [DOI] [PubMed] [Google Scholar]
- 175.Plesner AM, Rønne T. Allergic mucocutaneous reactions to Japanese encephalitis vaccine. Vaccine. 1997;15:1239–43. doi: 10.1016/S0264-410X(97)00020-0. [DOI] [PubMed] [Google Scholar]
- 176.Sakaguchi M, Miyazawa H, Inouye S. Specific IgE and IgG to gelatin in children with systemic cutaneous reactions to Japanese encephalitis vaccines. Allergy. 2001;56:536–9. doi: 10.1034/j.1398-9995.2001.056006536.x. [DOI] [PubMed] [Google Scholar]
- 177.Berg SW, Mitchell BS, Hanson RK, Olafson RP, Williams RP, Tueller JE, Burton RJ, Novak DM, Tsai TF, Wignall FS. Systemic reactions in U.S. Marine Corps personnel who received Japanese encephalitis vaccine. Clin Infect Dis. 1997;24:265–6. doi: 10.1093/clinids/24.2.265. [DOI] [PubMed] [Google Scholar]
- 178.Andersen MM, Rønne T. Side-effects with Japanese encephalitis vaccine. Lancet. 1991;337:1044. doi: 10.1016/0140-6736(91)92707-9. [DOI] [PubMed] [Google Scholar]
- 179.Ruff TA, Eisen D, Fuller A, Kass R. Adverse reactions to Japanese encephalitis vaccine. Lancet. 1991;338:881–2. doi: 10.1016/0140-6736(91)91531-X. [DOI] [PubMed] [Google Scholar]
- 180.Ohtaki E, Matsuishi T, Hirano Y, Maekawa K. Acute disseminated encephalomyelitis after treatment with Japanese B encephalitis vaccine (Nakayama-Yoken and Beijing strains) J Neurol Neurosurg Psychiatry. 1995;59:316–7. doi: 10.1136/jnnp.59.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Davis LE, Booss J. Acute disseminated encephalomyelitis in children: a changing picture. Pediatr Infect Dis J. 2003;22:829–31. doi: 10.1097/01.inf.0000087847.37363.78. [DOI] [PubMed] [Google Scholar]
- 182.Huynh W, Cordato DJ, Kehdi E, Masters LT, Dedousis C. Post-vaccination encephalomyelitis: literature review and illustrative case. J Clin Neurosci. 2008;15:1315–22. doi: 10.1016/j.jocn.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Holbrook MR, Barrett AD. Molecular epidemiology of Japanese encephalitis virus. Curr Top Microbiol Immunol. 2002;267:75–90. doi: 10.1007/978-3-642-59403-8_4. [DOI] [PubMed] [Google Scholar]
- 184.Kitano T, Yabe S, Kobayashi M, Oya A, Ogata T. Immunogenicity of JE Nakayama and Beijing-1 vaccines. JE HFRS Bull. 1986;1:37–41. [Google Scholar]
- 185.Nimmannitya S, Hutamai S, Kalayanarooj S, Rojanasuphot S. A field study on Nakayama and Beijing strains of Japanese encephalitis vaccines. Southeast Asian J Trop Med Public Health. 1995;26:689–93. [PubMed] [Google Scholar]
- 186.Kurane I, Takasaki T. Immunogenicity and protective efficacy of the current inactivated Japanese encephalitis vaccine against different Japanese encephalitis virus strains. Vaccine. 2000;18(Suppl 2):33–5. doi: 10.1016/S0264-410X(00)00041-4. [DOI] [PubMed] [Google Scholar]
- 187.Solomon T. Japanese encephalitis vaccine. In: Jong EC, Zuckerman JN, eds. Travelers' Vaccines. Hamilton, ON: BC Decker, 2004:219-56. [Google Scholar]
- 188.Xin YY, Ming ZG, Peng GY, Jian A, Min LH. Safety of a live-attenuated Japanese encephalitis virus vaccine (SA14-14-2) for children. Am J Trop Med Hyg. 1988;39:214–7. doi: 10.4269/ajtmh.1988.39.214. [DOI] [PubMed] [Google Scholar]
- 189.Yu Y. Development of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and its characteristics. In: Tkachev S, ed. Encephalitis. Open Access: InTech, 2013:181-206. [Google Scholar]
- 190.Yu Y. Phenotypic and genotypic characteristics of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and their stabilities. Vaccine. 2010;28:3635–41. doi: 10.1016/j.vaccine.2010.02.105. [DOI] [PubMed] [Google Scholar]
- 191.World Health Organization (WHO) Global Advisory Committee on Vaccine Safety, 9-10 June 2005. Wkly Epidemiol Rec. 2005;80:242–7. [PubMed] [Google Scholar]
- 192.World Health Organization (WHO). Recommendations to assure the quality, safety and efficacy of Japanese encephalitis vaccine (live, attenuated) for human use: replacement of WHO TRS No. 910 (Annex 3). Available from: http://www.who.int/biologicals/vaccines/WHO_BS_2012_JE_LA_DB_4March2012.pdf (accessed 2013).
- 193.Sohn YM, Park MS, Rho HO, Chandler LJ, Shope RE, Tsai TF. Primary and booster immune responses to SA14-14-2 Japanese encephalitis vaccine in Korean infants. Vaccine. 1999;17:2259–64. doi: 10.1016/S0264-410X(99)00006-7. [DOI] [PubMed] [Google Scholar]
- 194.Tsai TF, Chang GJ, Yu YX. Japanese encephalitis vaccines. In: Plotkin SA, Orenstein WA, eds. Vaccines. Philadelphia, PA: WB Saunders, 1999:672-710. [Google Scholar]
- 195.Tsai TF, Yu YX, Jia LL, Putvatana R, Zhang R, Wang S, Halstead SB. Immunogenicity of live attenuated SA14-14-2 Japanese encephalitis vaccine--a comparison of 1- and 3-month immunization schedules. J Infect Dis. 1998;177:221–3. doi: 10.1086/517358. [DOI] [PubMed] [Google Scholar]
- 196.Hennessy S, Liu Z, Tsai TF, Strom BL, Wan CM, Liu HL, Wu TX, Yu HJ, Liu QM, Karabatsos N, et al. Effectiveness of live-attenuated Japanese encephalitis vaccine (SA14-14-2): a case-control study. Lancet. 1996;347:1583–6. doi: 10.1016/S0140-6736(96)91075-2. [DOI] [PubMed] [Google Scholar]
- 197.Bista MB, Banerjee MK, Shin SH, Tandan JB, Kim MH, Sohn YM, Ohrr HC, Tang JL, Halstead SB. Efficacy of single-dose SA 14-14-2 vaccine against Japanese encephalitis: a case control study. Lancet. 2001;358:791–5. doi: 10.1016/S0140-6736(01)05967-0. [DOI] [PubMed] [Google Scholar]
- 198.Sohn YM, Tandan JB, Yoksan S, Ji M, Ohrr H. A 5-year follow-up of antibody response in children vaccinated with single dose of live attenuated SA14-14-2 Japanese encephalitis vaccine: immunogenicity and anamnestic responses. Vaccine. 2008;26:1638–43. doi: 10.1016/j.vaccine.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 199.Tandan JB, Ohrr H, Sohn YM, Yoksan S, Ji M, Nam CM, Halstead SB. Single dose of SA 14-14-2 vaccine provides long-term protection against Japanese encephalitis: a case-control study in Nepalese children 5 years after immunization. drjbtandan@yahoo.com. Vaccine. 2007;25:5041–5. doi: 10.1016/j.vaccine.2007.04.052. [DOI] [PubMed] [Google Scholar]
- 200.Ohrr H, Tandan JB, Sohn YM, Shin SH, Pradhan DP, Halstead SB. Effect of single dose of SA 14-14-2 vaccine 1 year after immunisation in Nepalese children with Japanese encephalitis: a case-control study. Lancet. 2005;366:1375–8. doi: 10.1016/S0140-6736(05)67567-8. [DOI] [PubMed] [Google Scholar]
- 201.Liu ZL, Hennessy S, Strom BL, Tsai TF, Wan CM, Tang SC, Xiang CF, Bilker WB, Pan XP, Yao YJ, et al. Short-term safety of live attenuated Japanese encephalitis vaccine (SA14-14-2): results of a randomized trial with 26,239 subjects. J Infect Dis. 1997;176:1366–9. doi: 10.1086/517323. [DOI] [PubMed] [Google Scholar]
- 202.Halstead SB, Thomas SJ. Japanese encephalitis: new options for active immunization. Clin Infect Dis. 2010;50:1155–64. doi: 10.1086/651271. [DOI] [PubMed] [Google Scholar]
- 203.Halstead SB, Thomas SJ. New vaccines for Japanese encephalitis. Curr Infect Dis Rep. 2010;12:174–80. doi: 10.1007/s11908-010-0098-z. [DOI] [PubMed] [Google Scholar]
- 204.McArthur MA, Holbrook MR. Japanese encephalitis vaccines. J Bioterror Biodef. 2011;S1:2. doi: 10.4172/2157-2526.S1-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kikukawa A, Gomi Y, Akechi M, Onishi T, Manabe S, Namazue J, Fuke I, Ishikawa T, Okuno Y, Ueda S. Superior immunogenicity of a freeze-dried, cell culture-derived Japanese encephalitis vaccine (inactivated) Vaccine. 2012;30:2329–35. doi: 10.1016/j.vaccine.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 206.Okada K, Iwasa T, Namazue J, Akechi M, Ueda S, JE Vaccine Clinical Study Group Safety and immunogenicity of a freeze-dried, cell culture-derived Japanese encephalitis vaccine (Inactivated) (JEBIK(®)V) in children. Vaccine. 2012;30:5967–72. doi: 10.1016/j.vaccine.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 207.Lyons A, Kanesa-thasan N, Kuschner RA, Eckels KH, Putnak R, Sun W, Burge R, Towle AC, Wilson P, Tauber E, et al. A Phase 2 study of a purified, inactivated virus vaccine to prevent Japanese encephalitis. Vaccine. 2007;25:3445–53. doi: 10.1016/j.vaccine.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 208.Srivastava AK, Putnak JR, Lee SH, Hong SP, Moon SB, Barvir DA, Zhao B, Olson RA, Kim SO, Yoo WD, et al. A purified inactivated Japanese encephalitis virus vaccine made in Vero cells. Vaccine. 2001;19:4557–65. doi: 10.1016/S0264-410X(01)00208-0. [DOI] [PubMed] [Google Scholar]
- 209.Jelinek T. Ixiaro: a new vaccine against Japanese encephalitis. Expert Rev Vaccines. 2009;8:1501–11. doi: 10.1586/erv.09.112. [DOI] [PubMed] [Google Scholar]
- 210.Kollaritsch H, Paulke-Korinek M, Dubischar-Kastner K. IC51 Japanese encephalitis vaccine. Expert Opin Biol Ther. 2009;9:921–31. doi: 10.1517/14712590903042282. [DOI] [PubMed] [Google Scholar]
- 211.Duggan ST, Plosker GL. Japanese encephalitis vaccine (inactivated, adsorbed) [IXIARO] Drugs. 2009;69:115–22. doi: 10.2165/00003495-200969010-00008. [IXIARO] [DOI] [PubMed] [Google Scholar]
- 212.Centers for Disease Control and Prevention (CDC) Recommendations for use of a booster dose of inactivated vero cell culture-derived Japanese encephalitis vaccine: advisory committee on immunization practices, 2011. MMWR Morb Mortal Wkly Rep. 2011;60:661–3. [PubMed] [Google Scholar]
- 213.Vogel FR. Improving vaccine performance with adjuvants. Clin Infect Dis. 2000;30(Suppl 3):S266–70. doi: 10.1086/313883. [DOI] [PubMed] [Google Scholar]
- 214.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82:497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 215.Gupta RK. Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev. 1998;32:155–72. doi: 10.1016/S0169-409X(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 216.Lobigs M, Pavy M, Hall RA, Lobigs P, Cooper P, Komiya T, Toriniwa H, Petrovsky N. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J Gen Virol. 2010;91:1407–17. doi: 10.1099/vir.0.019190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schuller E, Jilma B, Voicu V, Golor G, Kollaritsch H, Kaltenböck A, Klade C, Tauber E. Long-term immunogenicity of the new Vero cell-derived, inactivated Japanese encephalitis virus vaccine IC51 Six and 12 month results of a multicenter follow-up phase 3 study. Vaccine. 2008;26:4382–6. doi: 10.1016/j.vaccine.2008.05.081. [DOI] [PubMed] [Google Scholar]
- 218.Schuller E, Klade CS, Wölfl G, Kaltenböck A, Dewasthaly S, Tauber E. Comparison of a single, high-dose vaccination regimen to the standard regimen for the investigational Japanese encephalitis vaccine, IC51: a randomized, observer-blind, controlled Phase 3 study. Vaccine. 2009;27:2188–93. doi: 10.1016/j.vaccine.2008.12.062. [DOI] [PubMed] [Google Scholar]
- 219.Tauber E, Kollaritsch H, Korinek M, Rendi-Wagner P, Jilma B, Firbas C, Schranz S, Jong E, Klingler A, Dewasthaly S, et al. Safety and immunogenicity of a Vero-cell-derived, inactivated Japanese encephalitis vaccine: a non-inferiority, phase III, randomised controlled trial. Lancet. 2007;370:1847–53. doi: 10.1016/S0140-6736(07)61780-2. [DOI] [PubMed] [Google Scholar]
- 220.Tauber E, Kollaritsch H, von Sonnenburg F, Lademann M, Jilma B, Firbas C, Jelinek T, Beckett C, Knobloch J, McBride WJ, et al. Randomized, double-blind, placebo-controlled phase 3 trial of the safety and tolerability of IC51, an inactivated Japanese encephalitis vaccine. J Infect Dis. 2008;198:493–9. doi: 10.1086/590116. [DOI] [PubMed] [Google Scholar]
- 221.Dubischar-Kastner K, Kaltenboeck A, Klingler A, Jilma B, Schuller E. Safety analysis of a Vero-cell culture derived Japanese encephalitis vaccine, IXIARO (IC51), in 6 months of follow-up. Vaccine. 2010;28:6463–9. doi: 10.1016/j.vaccine.2010.07.040. [DOI] [PubMed] [Google Scholar]
- 222.Schuller E, Klingler A, Dubischar-Kastner K, Dewasthaly S, Müller Z. Safety profile of the Vero cell-derived Japanese encephalitis virus (JEV) vaccine IXIARO(®) Vaccine. 2011;29:8669–76. doi: 10.1016/j.vaccine.2011.08.117. [DOI] [PubMed] [Google Scholar]
- 223.Dubischar-Kastner K, Eder S, Buerger V, Gartner-Woelfl G, Kaltenboeck A, Schuller E, Tauber E, Klade C. Long-term immunity and immune response to a booster dose following vaccination with the inactivated Japanese encephalitis vaccine IXIARO, IC51. Vaccine. 2010;28:5197–202. doi: 10.1016/j.vaccine.2010.05.069. [DOI] [PubMed] [Google Scholar]
- 224.Eder S, Dubischar-Kastner K, Firbas C, Jelinek T, Jilma B, Kaltenboeck A, Knappik M, Kollaritsch H, Kundi M, Paulke-Korinek M, et al. Long term immunity following a booster dose of the inactivated Japanese Encephalitis vaccine IXIARO®, IC51. Vaccine. 2011;29:2607–12. doi: 10.1016/j.vaccine.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 225.Kaltenböck A, Dubischar-Kastner K, Schuller E, Datla M, Klade CS, Kishore TS. Immunogenicity and safety of IXIARO (IC51) in a Phase II study in healthy Indian children between 1 and 3 years of age. Vaccine. 2010;28:834–9. doi: 10.1016/j.vaccine.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 226.Erra EO, Askling HH, Rombo L, Riutta J, Vene S, Yoksan S, Lindquist L, Pakkanen SH, Huhtamo E, Vapalahti O, et al. A single dose of vero cell-derived Japanese encephalitis (JE) vaccine (Ixiaro) effectively boosts immunity in travelers primed with mouse brain-derived JE vaccines. Clin Infect Dis. 2012;55:825–34. doi: 10.1093/cid/cis542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schuller E, Klade CS, Heinz FX, Kollaritsch H, Rendi-Wagner P, Jilma B, Tauber E. Effect of pre-existing anti-tick-borne encephalitis virus immunity on neutralising antibody response to the Vero cell-derived, inactivated Japanese encephalitis virus vaccine candidate IC51. Vaccine. 2008;26:6151–6. doi: 10.1016/j.vaccine.2008.08.056. [DOI] [PubMed] [Google Scholar]
- 228.Kaltenböck A, Dubischar-Kastner K, Eder G, Jilg W, Klade C, Kollaritsch H, Paulke-Korinek M, von Sonnenburg F, Spruth M, Tauber E, et al. Safety and immunogenicity of concomitant vaccination with the cell-culture based Japanese Encephalitis vaccine IC51 and the hepatitis A vaccine HAVRIX1440 in healthy subjects: A single-blind, randomized, controlled Phase 3 study. Vaccine. 2009;27:4483–9. doi: 10.1016/j.vaccine.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 229.Appaiahgari MB, Vrati S. Clinical development of IMOJEV ®--a recombinant Japanese encephalitis chimeric vaccine (JE-CV) Expert Opin Biol Ther. 2012;12:1251–63. doi: 10.1517/14712598.2012.704908. [DOI] [PubMed] [Google Scholar]
- 230.Jones T. A chimeric live attenuated vaccine against Japanese encephalitis. Expert Rev Vaccines. 2004;3:243–8. doi: 10.1586/14760584.3.3.243. [DOI] [PubMed] [Google Scholar]
- 231.Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28:632–49. doi: 10.1016/j.vaccine.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 232.Chambers TJ, Nestorowicz A, Mason PW, Rice CM. Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol. 1999;73:3095–101. doi: 10.1128/jvi.73.4.3095-3101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Monath TP, Myers GA, Beck RA, Knauber M, Scappaticci K, Pullano T, Archambault WT, Catalan J, Miller C, Zhang ZX, et al. Safety testing for neurovirulence of novel live, attenuated flavivirus vaccines: infant mice provide an accurate surrogate for the test in monkeys. Biologicals. 2005;33:131–44. doi: 10.1016/j.biologicals.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 234.Arroyo J, Guirakhoo F, Fenner S, Zhang ZX, Monath TP, Chambers TJ. Molecular basis for attenuation of neurovirulence of a yellow fever Virus/Japanese encephalitis virus chimera vaccine (ChimeriVax-JE) J Virol. 2001;75:934–42. doi: 10.1128/JVI.75.2.934-942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Monath TP, Levenbook I, Soike K, Zhang ZX, Ratterree M, Draper K, Barrett AD, Nichols R, Weltzin R, Arroyo J, et al. Chimeric yellow fever virus 17D-Japanese encephalitis virus vaccine: dose-response effectiveness and extended safety testing in rhesus monkeys. J Virol. 2000;74:1742–51. doi: 10.1128/JVI.74.4.1742-1751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Monath TP, Soike K, Levenbook I, Zhang ZX, Arroyo J, Delagrave S, Myers G, Barrett AD, Shope RE, Ratterree M, et al. Recombinant, chimaeric live, attenuated vaccine (ChimeriVax) incorporating the envelope genes of Japanese encephalitis (SA14-14-2) virus and the capsid and nonstructural genes of yellow fever (17D) virus is safe, immunogenic and protective in non-human primates. Vaccine. 1999;17:1869–82. doi: 10.1016/S0264-410X(98)00487-3. [DOI] [PubMed] [Google Scholar]
- 237.Guirakhoo F, Zhang ZX, Chambers TJ, Delagrave S, Arroyo J, Barrett AD, Monath TP. Immunogenicity, genetic stability, and protective efficacy of a recombinant, chimeric yellow fever-Japanese encephalitis virus (ChimeriVax-JE) as a live, attenuated vaccine candidate against Japanese encephalitis. Virology. 1999;257:363–72. doi: 10.1006/viro.1999.9695. [DOI] [PubMed] [Google Scholar]
- 238.Monath TP, Guirakhoo F, Nichols R, Yoksan S, Schrader R, Murphy C, Blum P, Woodward S, McCarthy K, Mathis D, et al. Chimeric live, attenuated vaccine against Japanese encephalitis (ChimeriVax-JE): phase 2 clinical trials for safety and immunogenicity, effect of vaccine dose and schedule, and memory response to challenge with inactivated Japanese encephalitis antigen. J Infect Dis. 2003;188:1213–30. doi: 10.1086/378356. [DOI] [PubMed] [Google Scholar]
- 239.Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, Marchesani R, Knauber M, Wells KH, Arroyo J, et al. Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine. 2002;20:1004–18. doi: 10.1016/S0264-410X(01)00457-1. [DOI] [PubMed] [Google Scholar]
- 240.Torresi J, McCarthy K, Feroldi E, Méric C. Immunogenicity, safety and tolerability in adults of a new single-dose, live-attenuated vaccine against Japanese encephalitis: Randomised controlled phase 3 trials. Vaccine. 2010;28:7993–8000. doi: 10.1016/j.vaccine.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 241.Nasveld PE, Ebringer A, Elmes N, Bennett S, Yoksan S, Aaskov J, McCarthy K, Kanesa-thasan N, Meric C, Reid M. Long term immunity to live attenuated Japanese encephalitis chimeric virus vaccine: randomized, double-blind, 5-year phase II study in healthy adults. Hum Vaccin. 2010;6:1038–46. doi: 10.4161/hv.6.12.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chokephaibulkit K, Sirivichayakul C, Thisyakorn U, Sabchareon A, Pancharoen C, Bouckenooghe A, Gailhardou S, Boaz M, Feroldi E. Safety and immunogenicity of a single administration of live-attenuated Japanese encephalitis vaccine in previously primed 2- to 5-year-olds and naive 12- to 24-month-olds: multicenter randomized controlled trial. Pediatr Infect Dis J. 2010;29:1111–7. doi: 10.1097/INF.0b013e3181f68e9c. [DOI] [PubMed] [Google Scholar]
- 243.Feroldi E, Pancharoen C, Kosalaraksa P, Watanaveeradej V, Phirangkul K, Capeding MR, Boaz M, Gailhardou S, Bouckenooghe A. Single-dose, live-attenuated Japanese encephalitis vaccine in children aged 12-18 months: randomized, controlled phase 3 immunogenicity and safety trial. Hum Vaccin Immunother. 2012;8:929–37. doi: 10.4161/hv.20071. [DOI] [PubMed] [Google Scholar]
- 244.Bhatt TR, Crabtree MB, Guirakhoo F, Monath TP, Miller BR. Growth characteristics of the chimeric Japanese encephalitis virus vaccine candidate, ChimeriVax-JE (YF/JE SA14--14--2), in Culex tritaeniorhynchus, Aedes albopictus, and Aedes aegypti mosquitoes. Am J Trop Med Hyg. 2000;62:480–4. doi: 10.4269/ajtmh.2000.62.480. [DOI] [PubMed] [Google Scholar]
- 245.Reid M, Mackenzie D, Baron A, Lehmann N, Lowry K, Aaskov J, Guirakhoo F, Monath TP. Experimental infection of Culex annulirostris, Culex gelidus, and Aedes vigilax with a yellow fever/Japanese encephalitis virus vaccine chimera (ChimeriVax-JE) Am J Trop Med Hyg. 2006;75:659–63. [PubMed] [Google Scholar]
- 246.Dowd KA, Pierson TC. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology. 2011;411:306–15. doi: 10.1016/j.virol.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chen HW, Pan CH, Liau MY, Jou R, Tsai CJ, Wu HJ, Lin YL, Tao MH. Screening of protective antigens of Japanese encephalitis virus by DNA immunization: a comparative study with conventional viral vaccines. J Virol. 1999;73:10137–45. doi: 10.1128/jvi.73.12.10137-10145.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kaur R, Vrati S. Development of a recombinant vaccine against Japanese encephalitis. J Neurovirol. 2003;9:421–31. doi: 10.1080/13550280390218454. [DOI] [PubMed] [Google Scholar]
- 249.Verma SK, Kumar S, Gupta N, Vedi S, Bhattacharya SM, Lakshmana Rao PV. Bacterially expressed recombinant envelope protein domain III of Japanese encephalitis virus (rJEV-DIII) elicits Th1 type of immune response in BALB/c mice. Vaccine. 2009;27:6905–9. doi: 10.1016/j.vaccine.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 250.Seif SA, Morita K, Igarashi A. A 27 amino acid coding region of JE virus E protein expressed in E. coli as fusion protein with glutathione-S-transferase elicit neutralizing antibody in mice. Virus Res. 1996;43:91–6. doi: 10.1016/0168-1702(96)01323-8. [DOI] [PubMed] [Google Scholar]
- 251.Mason PW, Dalrymple JM, Gentry MK, McCown JM, Hoke CH, Burke DS, Fournier MJ, Mason TL. Molecular characterization of a neutralizing domain of the Japanese encephalitis virus structural glycoprotein. J Gen Virol. 1989;70:2037–49. doi: 10.1099/0022-1317-70-8-2037. [DOI] [PubMed] [Google Scholar]
- 252.Seif SA, Morita K, Matsuo S, Hasebe F, Igarashi A. Finer mapping of neutralizing epitope(s) on the C-terminal of Japanese encephalitis virus E-protein expressed in recombinant Escherichia coli system. Vaccine. 1995;13:1515–21. doi: 10.1016/0264-410X(95)00097-K. [DOI] [PubMed] [Google Scholar]
- 253.Saini M, Vrati S. A Japanese encephalitis virus peptide present on Johnson grass mosaic virus-like particles induces virus-neutralizing antibodies and protects mice against lethal challenge. J Virol. 2003;77:3487–94. doi: 10.1128/JVI.77.6.3487-3494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.McCown J, Cochran M, Putnak R, Feighny R, Burrous J, Henchal E, Hoke C. Protection of mice against lethal Japanese encephalitis with a recombinant baculovirus vaccine. Am J Trop Med Hyg. 1990;42:491–9. doi: 10.4269/ajtmh.1990.42.491. [DOI] [PubMed] [Google Scholar]
- 255.Matsuura Y, Miyamoto M, Sato T, Morita C, Yasui K. Characterization of Japanese encephalitis virus envelope protein expressed by recombinant baculoviruses. Virology. 1989;173:674–82. doi: 10.1016/0042-6822(89)90580-1. [DOI] [PubMed] [Google Scholar]
- 256.Chang GJ, Hunt AR, Davis B. A single intramuscular injection of recombinant plasmid DNA induces protective immunity and prevents Japanese encephalitis in mice. J Virol. 2000;74:4244–52. doi: 10.1128/JVI.74.9.4244-4252.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Konishi E, Kurane I, Mason PW, Shope RE, Ennis FA. Poxvirus-based Japanese encephalitis vaccine candidates induce JE virus-specific CD8+ cytotoxic T lymphocytes in mice. Virology. 1997;227:353–60. doi: 10.1006/viro.1996.8331. [DOI] [PubMed] [Google Scholar]
- 258.Konishi E, Fujii A, Mason PW. Generation and characterization of a mammalian cell line continuously expressing Japanese encephalitis virus subviral particles. J Virol. 2001;75:2204–12. doi: 10.1128/JVI.75.5.2204-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Konishi E, Pincus S, Paoletti E, Laegreid WW, Shope RE, Mason PW. A highly attenuated host range-restricted vaccinia virus strain, NYVAC, encoding the prM, E, and NS1 genes of Japanese encephalitis virus prevents JEV viremia in swine. Virology. 1992;190:454–8. doi: 10.1016/0042-6822(92)91233-K. [DOI] [PubMed] [Google Scholar]
- 260.Yasuda A, Kimura-Kuroda J, Ogimoto M, Miyamoto M, Sata T, Sato T, Takamura C, Kurata T, Kojima A, Yasui K. Induction of protective immunity in animals vaccinated with recombinant vaccinia viruses that express PreM and E glycoproteins of Japanese encephalitis virus. J Virol. 1990;64:2788–95. doi: 10.1128/jvi.64.6.2788-2795.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Konishi E, Pincus S, Fonseca BA, Shope RE, Paoletti E, Mason PW. Comparison of protective immunity elicited by recombinant vaccinia viruses that synthesize E or NS1 of Japanese encephalitis virus. Virology. 1991;185:401–10. doi: 10.1016/0042-6822(91)90788-D. [DOI] [PubMed] [Google Scholar]
- 262.Jan LR, Yang CS, Henchal LS, Sumiyoshi H, Summers PL, Dubois DR, Lai CJ. Increased immunogenicity and protective efficacy in outbred and inbred mice by strategic carboxyl-terminal truncation of Japanese encephalitis virus envelope glycoprotein. Am J Trop Med Hyg. 1993;48:412–23. doi: 10.4269/ajtmh.1993.48.412. [DOI] [PubMed] [Google Scholar]
- 263.Mason PW, Pincus S, Fournier MJ, Mason TL, Shope RE, Paoletti E. Japanese encephalitis virus-vaccinia recombinants produce particulate forms of the structural membrane proteins and induce high levels of protection against lethal JEV infection. Virology. 1991;180:294–305. doi: 10.1016/0042-6822(91)90034-9. [DOI] [PubMed] [Google Scholar]
- 264.Nam JH, Wyatt LS, Chae SL, Cho HW, Park YK, Moss B. Protection against lethal Japanese encephalitis virus infection of mice by immunization with the highly attenuated MVA strain of vaccinia virus expressing JEV prM and E genes. Vaccine. 1999;17:261–8. doi: 10.1016/S0264-410X(98)00156-X. [DOI] [PubMed] [Google Scholar]
- 265.Konishi E, Pincus S, Paoletti E, Shope RE, Wason PW. Avipox virus-vectored Japanese encephalitis virus vaccines: use as vaccine candidates in combination with purified subunit immunogens. Vaccine. 1994;12:633–8. doi: 10.1016/0264-410X(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 266.Wang F, Feng X, Zheng Q, Hou H, Cao R, Zhou B, Liu Q, Liu X, Pang R, Zhao J, et al. Multiple linear epitopes (B-cell, CTL and Th) of JEV expressed in recombinant MVA as multiple epitope vaccine induces a protective immune response. Virol J. 2012;9:204. doi: 10.1186/1743-422X-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kanesa-thasan N, Smucny JJ, Hoke CH, Marks DH, Konishi E, Kurane I, Tang DB, Vaughn DW, Mason PW, Shope RE. Safety and immunogenicity of NYVAC-JEV and ALVAC-JEV attenuated recombinant Japanese encephalitis virus--poxvirus vaccines in vaccinia-nonimmune and vaccinia-immune humans. Vaccine. 2000;19:483–91. doi: 10.1016/S0264-410X(00)00191-2. [DOI] [PubMed] [Google Scholar]
- 268.Li P, Zheng QS, Wang Q, Li Y, Wang EX, Liu JJ, Cao RB, Chen PY. Immune responses of recombinant adenoviruses expressing immunodominant epitopes against Japanese encephalitis virus. Vaccine. 2008;26:5802–7. doi: 10.1016/j.vaccine.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 269.Konishi E, Yamaoka M, Khin-Sane-Win, Kurane I, Mason PW. Induction of protective immunity against Japanese encephalitis in mice by immunization with a plasmid encoding Japanese encephalitis virus premembrane and envelope genes. J Virol. 1998;72:4925–30. doi: 10.1128/jvi.72.6.4925-4930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Konishi E, Yamaoka M, Kurane I, Mason PW. Japanese encephalitis DNA vaccine candidates expressing premembrane and envelope genes induce virus-specific memory B cells and long-lasting antibodies in swine. Virology. 2000;268:49–55. doi: 10.1006/viro.1999.0142. [DOI] [PubMed] [Google Scholar]
- 271.Lin YL, Chen LK, Liao CL, Yeh CT, Ma SH, Chen JL, Huang YL, Chen SS, Chiang HY. DNA immunization with Japanese encephalitis virus nonstructural protein NS1 elicits protective immunity in mice. J Virol. 1998;72:191–200. doi: 10.1128/jvi.72.1.191-200.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ashok MS, Rangarajan PN. Immunization with plasmid DNA encoding the envelope glycoprotein of Japanese Encephalitis virus confers significant protection against intracerebral viral challenge without inducing detectable antiviral antibodies. Vaccine. 1999;18:68–75. doi: 10.1016/S0264-410X(99)00180-2. [DOI] [PubMed] [Google Scholar]
- 273.Kaur R, Sachdeva G, Vrati S. Plasmid DNA immunization against Japanese encephalitis virus: immunogenicity of membrane-anchored and secretory envelope protein. J Infect Dis. 2002;185:1–12. doi: 10.1086/338015. [DOI] [PubMed] [Google Scholar]
- 274.Gao N, Chen W, Zheng Q, Fan DY, Zhang JL, Chen H, Gao GF, Zhou DS, An J. Co-expression of Japanese encephalitis virus prM-E-NS1 antigen with granulocyte-macrophage colony-stimulating factor enhances humoral and anti-virus immunity after DNA vaccination. Immunol Lett. 2010;129:23–31. doi: 10.1016/j.imlet.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 275.Chen H, Gao N, Fan D, Wu J, Zhu J, Li J, Wang J, Chen Y, An J. Suppressive effects on the immune response and protective immunity to a JEV DNA vaccine by co-administration of a GM-CSF-expressing plasmid in mice. PLoS One. 2012;7:e34602. doi: 10.1371/journal.pone.0034602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chen HW, Pan CH, Huan HW, Liau MY, Chiang JR, Tao MH. Suppression of immune response and protective immunity to a Japanese encephalitis virus DNA vaccine by coadministration of an IL-12-expressing plasmid. J Immunol. 2001;166:7419–26. doi: 10.4049/jimmunol.166.12.7419. [DOI] [PubMed] [Google Scholar]
- 277.Krishna VD, Rangappa M, Satchidanandam V. Virus-specific cytolytic antibodies to nonstructural protein 1 of Japanese encephalitis virus effect reduction of virus output from infected cells. J Virol. 2009;83:4766–77. doi: 10.1128/JVI.01850-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Pan XL, Liu H, Wang HY, Fu SH, Liu HZ, Zhang HL, Li MH, Gao XY, Wang JL, Sun XH, et al. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J Virol. 2011;85:9847–53. doi: 10.1128/JVI.00825-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Huang JH, Lin TH, Teng HJ, Su CL, Tsai KH, Lu LC, Lin C, Yang CF, Chang SF, Liao TL, et al. Molecular epidemiology of Japanese encephalitis virus, Taiwan. Emerg Infect Dis. 2010;16:876–8. doi: 10.3201/eid1605.091055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Yun SM, Cho JE, Ju YR, Kim SY, Ryou J, Han MG, Choi WY, Jeong YE. Molecular epidemiology of Japanese encephalitis virus circulating in South Korea, 1983-2005. Virol J. 2010;7:127. doi: 10.1186/1743-422X-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Nga PT, del Carmen Parquet M, Cuong VD, Ma SP, Hasebe F, Inoue S, Makino Y, Takagi M, Nam VS, Morita K. Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: implications for frequent introductions of JEV from Southeast Asia to East Asia. J Gen Virol. 2004;85:1625–31. doi: 10.1099/vir.0.79797-0. [DOI] [PubMed] [Google Scholar]
- 282.Chen YY, Fan YC, Tu WC, Chang RY, Shih CC, Lu IH, Chien MS, Lee WC, Chen TH, Chang GJ, et al. Japanese encephalitis virus genotype replacement, Taiwan, 2009-2010. Emerg Infect Dis. 2011;17:2354–6. doi: 10.3201/eid1712.110914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Nitatpattana N, Dubot-Pérès A, Gouilh MA, Souris M, Barbazan P, Yoksan S, de Lamballerie X, Gonzalez JP. Change in Japanese encephalitis virus distribution, Thailand. Emerg Infect Dis. 2008;14:1762–5. doi: 10.3201/eid1411.080542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ma SP, Yoshida Y, Makino Y, Tadano M, Ono T, Ogawa M. Short report: a major genotype of Japanese encephalitis virus currently circulating in Japan. Am J Trop Med Hyg. 2003;69:151–4. [PubMed] [Google Scholar]
- 285.Liu X, Yu Y, Li M, Liang G, Wang H, Jia L, Dong G. Study on the protective efficacy of SA14-14-2 attenuated Japanese encephalitis against different JE virus isolates circulating in China. Vaccine. 2011;29:2127–30. doi: 10.1016/j.vaccine.2010.12.108. [DOI] [PubMed] [Google Scholar]
- 286.Van Gessel Y, Klade CS, Putnak R, Formica A, Krasaesub S, Spruth M, Cena B, Tungtaeng A, Gettayacamin M, Dewasthaly S. Correlation of protection against Japanese encephalitis virus and JE vaccine (IXIARO(®)) induced neutralizing antibody titers. Vaccine. 2011;29:5925–31. doi: 10.1016/j.vaccine.2011.06.062. [DOI] [PubMed] [Google Scholar]
- 287.Nasveld PE, Marjason J, Bennett S, Aaskov J, Elliott S, McCarthy K, Kanesa-Thasan N, Feroldi E, Reid M. Concomitant or sequential administration of live attenuated Japanese encephalitis chimeric virus vaccine and yellow fever 17D vaccine: randomized double-blind phase II evaluation of safety and immunogenicity. Hum Vaccin. 2010;6:906–14. doi: 10.4161/hv.6.11.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhang JS, Zhao QM, Guo XF, Zuo SQ, Cheng JX, Jia N, Wu C, Dai PF, Zhao JY. Isolation and genetic characteristics of human genotype 1 Japanese encephalitis virus, China, 2009. PLoS One. 2011;6:e16418. doi: 10.1371/journal.pone.0016418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ferguson M, Johnes S, Li L, Heath A, Barrett A. Effect of genomic variation in the challenge virus on the neutralization titres of recipients of inactivated JE vaccines--report of a collaborative study on PRNT50 assays for Japanese encephalitis virus (JE) antibodies. Biologicals. 2008;36:111–6. doi: 10.1016/j.biologicals.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 290.Shyu WR, Wang YC, Chin C, Chen WJ. Assessment of neutralizing antibodies elicited by a vaccine (Nakayama) strain of Japanese encephalitis virus in Taiwan. Epidemiol Infect. 1997;119:79–83. doi: 10.1017/S095026889700753X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Hills SL, Griggs AC, Fischer M. Japanese encephalitis in travelers from non-endemic countries, 1973-2008. Am J Trop Med Hyg. 2010;82:930–6. doi: 10.4269/ajtmh.2010.09-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Shlim DR, Solomon T. Japanese encephalitis vaccine for travelers: exploring the limits of risk. Clin Infect Dis. 2002;35:183–8. doi: 10.1086/341247. [DOI] [PubMed] [Google Scholar]
- 293.Fischer M, Griggs A, Staples JE. Japanese encephalitis. In: Brunette GW, ed. CDC Health information for international travel 2010. Atlanta, GA: US Department of Health and Human Services, Public Health Service, 2009:74-81. [Google Scholar]
- 294.Hatz C, Werlein J, Mutsch M, Hufnagel M, Behrens RH. Japanese encephalitis: defining risk incidence for travelers to endemic countries and vaccine prescribing from the UK and Switzerland. J Travel Med. 2009;16:200–3. doi: 10.1111/j.1708-8305.2009.00334.x. [DOI] [PubMed] [Google Scholar]
- 295.Buhl MR, Lindquist L. Japanese encephalitis in travelers: review of cases and seasonal risk. J Travel Med. 2009;16:217–9. doi: 10.1111/j.1708-8305.2009.00333.x. [DOI] [PubMed] [Google Scholar]
- 296.Unni SK, Růžek D, Chhatbar C, Mishra R, Johri MK, Singh SK. Japanese encephalitis virus: from genome to infectome. Microbes Infect. 2011;13:312–21. doi: 10.1016/j.micinf.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 297.Ye J, Zhu B, Fu ZF, Chen H, Cao S. Immune evasion strategies of flaviviruses. Vaccine. 2013;31:461–71. doi: 10.1016/j.vaccine.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 298.Müllbacher A, Lobigs M, Lee E. Immunobiology of mosquito-borne encephalitic flaviviruses. Adv Virus Res. 2003;60:87–120. doi: 10.1016/S0065-3527(03)60003-5. [DOI] [PubMed] [Google Scholar]
- 299.Burke DS, Morill JC. Levels of interferon in the plasma and cerebrospinal fluid of patients with acute Japanese encephalitis. J Infect Dis. 1987;155:797–9. doi: 10.1093/infdis/155.4.797. [DOI] [PubMed] [Google Scholar]
- 300.Solomon T, Dung NM, Wills B, Kneen R, Gainsborough M, Diet TV, Thuy TT, Loan HT, Khanh VC, Vaughn DW, et al. Interferon alfa-2a in Japanese encephalitis: a randomised double-blind placebo-controlled trial. Lancet. 2003;361:821–6. doi: 10.1016/S0140-6736(03)12709-2. [DOI] [PubMed] [Google Scholar]
- 301.Lee E, Lobigs M. Mechanism of virulence attenuation of glycosaminoglycan-binding variants of Japanese encephalitis virus and Murray Valley encephalitis virus. J Virol. 2002;76:4901–11. doi: 10.1128/JVI.76.10.4901-4911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Burke DS, Nisalak A, Ussery MA, Laorakpongse T, Chantavibul S. Kinetics of IgM and IgG responses to Japanese encephalitis virus in human serum and cerebrospinal fluid. J Infect Dis. 1985;151:1093–9. doi: 10.1093/infdis/151.6.1093. [DOI] [PubMed] [Google Scholar]
- 303.Libraty DH, Nisalak A, Endy TP, Suntayakorn S, Vaughn DW, Innis BL. Clinical and immunological risk factors for severe disease in Japanese encephalitis. Trans R Soc Trop Med Hyg. 2002;96:173–8. doi: 10.1016/S0035-9203(02)90294-4. [DOI] [PubMed] [Google Scholar]
- 304.Leake CJ, Burke DS, Nisalak A, Hoke CH. Isolation of Japanese encephalitis virus from clinical specimens using a continuous mosquito cell line. Am J Trop Med Hyg. 1986;35:1045–50. doi: 10.4269/ajtmh.1986.35.1045. [DOI] [PubMed] [Google Scholar]
- 305.Burke DS, Lorsomrudee W, Leake CJ, Hoke CH, Nisalak A, Chongswasdi V, Laorakpongse T. Fatal outcome in Japanese encephalitis. Am J Trop Med Hyg. 1985;34:1203–10. doi: 10.4269/ajtmh.1985.34.1203. [DOI] [PubMed] [Google Scholar]
- 306.Zhang MJ, Wang MJ, Jiang SZ, Ma WY. Passive protection of mice, goats, and monkeys against Japanese encephalitis with monoclonal antibodies. J Med Virol. 1989;29:133–8. doi: 10.1002/jmv.1890290211. [DOI] [PubMed] [Google Scholar]
- 307.Gupta AK, Lad VJ, Koshy AA. Protection of mice against experimental Japanese encephalitis virus infections by neutralizing anti-glycoprotein E monoclonal antibodies. Acta Virol. 2003;47:141–5. [PubMed] [Google Scholar]
- 308.Goncalvez AP, Chien CH, Tubthong K, Gorshkova I, Roll C, Donau O, Schuck P, Yoksan S, Wang SD, Purcell RH, et al. Humanized monoclonal antibodies derived from chimpanzee Fabs protect against Japanese encephalitis virus in vitro and in vivo. J Virol. 2008;82:7009–21. doi: 10.1128/JVI.00291-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kimura-Kuroda J, Yasui K. Protection of mice against Japanese encephalitis virus by passive administration with monoclonal antibodies. J Immunol. 1988;141:3606–10. [PubMed] [Google Scholar]
- 310.Larena M, Regner M, Lee E, Lobigs M. Pivotal role of antibody and subsidiary contribution of CD8+ T cells to recovery from infection in a murine model of Japanese encephalitis. J Virol. 2011;85:5446–55. doi: 10.1128/JVI.02611-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Nathanson N, Cole GA. Fatal Japanese encephalitis virus infection in immunosuppresed spider monkeys. Clin Exp Immunol. 1970;6:161–6. [PMC free article] [PubMed] [Google Scholar]
- 312.Biswas SM, Ayachit VM, Sapkal GN, Mahamuni SA, Gore MM. Japanese encephalitis virus produces a CD4+ Th2 response and associated immunoprotection in an adoptive-transfer murine model. J Gen Virol. 2009;90:818–26. doi: 10.1099/vir.0.008045-0. [DOI] [PubMed] [Google Scholar]
- 313.Murali-Krishna K, Ravi V, Manjunath R. Protection of adult but not newborn mice against lethal intracerebral challenge with Japanese encephalitis virus by adoptively transferred virus-specific cytotoxic T lymphocytes: requirement for L3T4+ T cells. J Gen Virol. 1996;77:705–14. doi: 10.1099/0022-1317-77-4-705. [DOI] [PubMed] [Google Scholar]
- 314.Konishi E, Kurane I, Mason PW, Innis BL, Ennis FA. Japanese encephalitis virus-specific proliferative responses of human peripheral blood T lymphocytes. Am J Trop Med Hyg. 1995;53:278–83. doi: 10.4269/ajtmh.1995.53.278. [DOI] [PubMed] [Google Scholar]
- 315.Aihara H, Takasaki T, Matsutani T, Suzuki R, Kurane I. Establishment and characterization of Japanese encephalitis virus-specific, human CD4(+) T-cell clones: flavivirus cross-reactivity, protein recognition, and cytotoxic activity. J Virol. 1998;72:8032–6. doi: 10.1128/jvi.72.10.8032-8036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Song BH, Yun SI, Choi YJ, Kim JM, Lee CH, Lee YM. A complex RNA motif defined by three discontinuous 5-nucleotide-long strands is essential for Flavivirus RNA replication. RNA. 2008;14:1791–813. doi: 10.1261/rna.993608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Yun SI, Choi YJ, Song BH, Lee YM. 3′ cis-acting elements that contribute to the competence and efficiency of Japanese encephalitis virus genome replication: functional importance of sequence duplications, deletions, and substitutions. J Virol. 2009;83:7909–30. doi: 10.1128/JVI.02541-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Song BH, Yun GN, Kim JK, Yun SI, Lee YM. Biological and genetic properties of SA₁₄-14-2, a live-attenuated Japanese encephalitis vaccine that is currently available for humans. J Microbiol. 2012;50:698–706. doi: 10.1007/s12275-012-2336-6. [DOI] [PubMed] [Google Scholar]