Abstract
Background: The present study aimed to evaluate immunogenicity and safety of the 2012/2013 seasonal influenza vaccine (Optaflu®) after the World Health Organization recommended two new strains for the composition.
Results: Twenty-one days post-vaccination geometric mean titers (GMTs) against A(H1N1), A(H3N2) and the B strain were 528, 935, and 201 for adults and 272, 681, and 101 for elderly subjects, respectively. The proportion of subjects with a HI titer of ≥ 40 against the three strains A(H1N1), A(H3N2) and B was 98%, 100%, and 98% in adults and 100%, 100%, and 85% in elderly subjects, respectively. Optaflu® met the CHMP criteria of the Committee for Medicinal Products for Human Use (CPMP/BWP/214/96). Pre-vaccination titers indicated seroprotection against the A(H1N1), the A(H3N2) and the B strain in 56%, 86%, and 54% of the adults and in 61%, 85%, and 40% of the elderly with highest titers against the A(H3N2) strain. In the safety analysis injection site pain (37%) and myalgia (31%) were the most common local and systemic reactions. No serious adverse events were recorded.
Conclusion: The 2012/2013 seasonal influenza vaccine Optaflu® showed good immunogenicity and an acceptable safety profile in both adults and elderly.
Methods: In this trial, 126 subjects (63 adults ≥18 to ≤60 y, 63 elderly ≥61 y) were vaccinated with a single dose Optaflu® containing each of the three virus strains recommended for the 2012/2013 season (A/California/7/2009(H1N1)-like strain, A/Victoria/361/2011(H3N2)-like strain, and B/Wisconsin/1/2010-like strain). Immunogenicity was assessed by hemagglutinin inhibition (HI) and single radial hemolysis (SRH) assays on day 22, the safety profile was investigated throughout the whole study period.
Keywords: influenza, H1N1, pandemic, trivalent vaccine, Optaflu, H3N2
Introduction
Influenza is still a major health concern worldwide and one of the main causes of respiratory illness resulting in 3 to 5 million severe cases each year.1-3 During the 2011/2012 season influenza was responsible for cumulative hospitalization rates of up to 30.4 per 100 000 population aged ≥65 y in the United States.4
During the influenza season, severity of the illness is closely related to the circulating seasonal virus strains and the population immunity.5 Because of antigenic drift, these strains differ from one influenza season to another necessitating extensive worldwide surveillance on the predominating virus strains.6 While for the northern hemisphere's 2012/2013 seasonal influenza vaccine two new influenza A(H3N1) and B strains were recommended by the World Health Organization (WHO), the A(H1N1) virus has been part of seasonal influenza vaccines since the new influenza (“swine flu“) pandemic in 2009. Since there are frequent reports that vaccine-induced immunity might be waning over time, it is consequently relevant to (1) assess this vaccines immunity and safety for the 2012/2013 influenza season and (2) gather sero-epidemiologic data about protection levels against current strains in the population.7,8
To date vaccinations remain the most important method of influenza disease prevention and one of the core strategies in pandemic influenza preparedness.9,10 Until 1995, when the WHO recommended mammalian cell lines as an alternative culture technique for vaccine production, vaccines were mainly produced in the allantoic cavity of embryonated hen eggs, which harbors certain disadvantages concerning production flexibility and restrictions of administration to patients with egg allergy.11-14
Cell culture-derived influenza vaccines (CCIV), in contrast, can be made available within shorter periods of time, which enables incorporation of late emerging strains.15 Immunogenicity and safety of CCIV have been frequently shown to be comparable to egg-based vaccines.16,17 The Optaflu® vaccine is produced in a specifically developed cell line cloned from Madin Darby Canine Kidney (MDCK) tissue and was approved by the European Medicines Agency in 2007.11,18 Previous clinical trials of Optaflu® indicated good safety and immunogenicity in terms of helping to enhance immunity to influenza.19,20
The aim of the present study was to evaluate the immunogenicity, safety and tolerability of Optaflu®, northern hemisphere formulation 2012/2013 in compliance with current EU guidelines in adult and elderly subjects.
Results
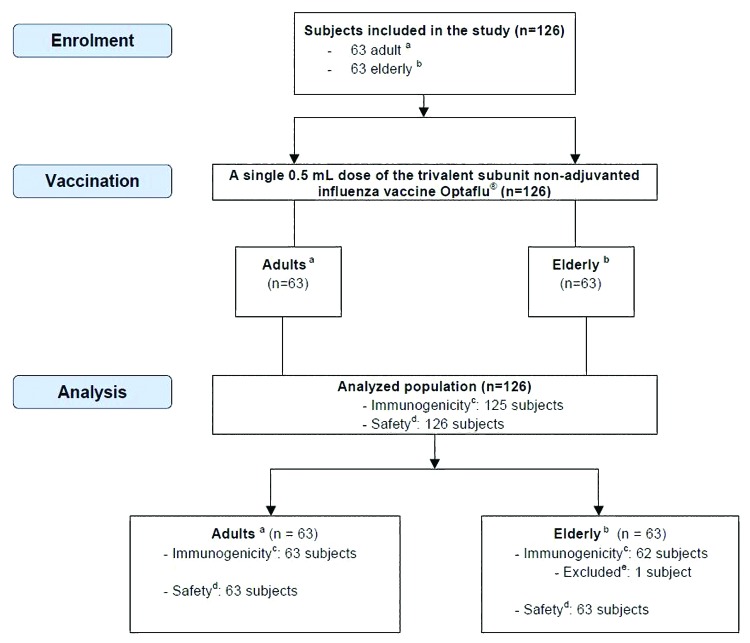
A total of 126 subjects were enrolled in the study, 63 each were included in the adult and the elderly group. Baseline and demographic data are shown in Table 1. All of the 126 subjects received the study vaccination and 125 subjects provided appropriate serum samples before and after vaccination for immunogenicity assessments. One elderly subject ≥61 y did not attend the study site on day 22 for blood sampling and was consequently excluded from HI and SRH per protocol set (Fig. 1).
Table 1. Demographic and baseline characteristics.
| Enrolled Subjects a | |||
|---|---|---|---|
| Age 18–60 y | Age ≥61 y | Total | |
| n = 63 | n = 63 | n = 126 | |
| Age (years, SD) | 37.4 ± 11.4 | 68.0 ± 4.7 | 52.7 ± 17.7 |
| Sex | |||
| Male n (%) | 30 (48) | 32 (51) | 62 (49) |
| Female n (%) | 33 (52) | 31 (49) | 64 (51) |
| Weight (kg, SD) | 78.44 ± 14.97 | 76.35 ± 13.63 | 77.40 ± 14.30 |
| Height (cm, SD) | 173.3 ± 10.6 | 173.3 ± 9.2 | 173.3 ± 9.9 |
| BMI (kg/m2, SD) | 26.0 ± 4.0 | 25.3 ± 3.1 | 25.7 ± 3.6 |
| Previous seasonal influenza vaccination | |||
| No n (%) | 43 (68) | 30 (48) | 73 (58) |
| Yes n (%) | 20 (32) | 33 (52) | 53 (42) |
| Ethnicity | |||
| White n (%) | 62 (98) | 63 (100) | 125 (99) |
| Native Hawaiian or other Pacific Islander n (%) |
1 (2) | 0 | 1 (<1) |
aAll subjects who were enrolled in the study (i.e., attended the first clinical visit).
Figure 1. Inclusion and exclusion of subjects. aAdults (≥18–≤60 y); belderly (≥61 y); cimmunogenicity was analyzed as per protocol using HI and SRH assays according to CHMP criteria; dsafety assessment was conducted by collection of any solicited local and systemic reactions and collection of adverse events (adverse events were defined as solicited reactions persisting after day 4 or other than solicited local and systemic reactions reported during the study period); eone subject was not available for visit 3 and was therefore excluded from immunogenicity analysis
Immunogenicity
Hemagglutinin (HI) titers at baseline (day 1) and 21 d after vaccination are summarized in Table 2.
Table 2. Vaccine Immunogenicity assessment by HI assay.
| Subjectsa ≥18 to ≤60 y of age | Subjectsa ≥61 y of age | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | A(H1N1) | A(H3N2) | B | A(H1N1) | A(H3N2) | B | ||||||||
| Pre vaccination (day1) | ||||||||||||||
| GMTb (95%-CI)c | 40 (26–62) |
147 (101–212) |
36 (26–48) |
47 (34–66) |
162 (111–236) |
28 (22–37) |
||||||||
| HI titer ≥40 d (n/Ne, %) | 35/63 | 56% | 54/63 | 86% | 34/63 | 54% | 38/62 | 61% | 53/62 | 85% | 25/62 | 40% | ||
| (95%-CI) | (42–68%) | (41–67%) | (41–67%) | (48–73%) | (74–93%) | (28–54%) | ||||||||
| Post vaccination (day22) | ||||||||||||||
| CHMPf | CHMPf | |||||||||||||
| Seroconversiong (n/N,%) | 19/20 | 95% | 2/2 | 100% | 5/5 | 100% | 6/6 | 100% | 2/2 | 100% | 4/5 | 80% | ||
| Significant increase in antibody titersh (n/N, %) | 27/43 | 63% | 40/61 | 66% | 35/58 | 60% | 30/56 | 54% | 26/60 | 43% | 22/57 | 39% | ||
| Seroconversion or significant increase | >40% | 46/63 | 73% | 42/63 | 67% | 40/63 | 63% | >30% | 36/62 | 58% | 28/62 | 45% | 26/62 | 42% |
| (95%-CI) | (60–83%) | (54–78%) | (50–75%) | (45–70%) | (32–58%) | (30–55%) | ||||||||
| GMT | 528 | 935 | 201 | 272 | 681 | 101 | ||||||||
| (95%-CI) | (392–710) | (743–1178) | (155–259) | (211–352) | (533–869) | (78–130) | ||||||||
| GM increasei | >2.5 | 13 | 6.38 | 5.63 | >2.0 | 5.79 | 4.21 | 3.54 | ||||||
| (95%-CI) | (8.75–20) | (4.5–9.06) | (4.11–7.7) | (4.12–8.12) | (2.96–5.97) | (2.65–4.72) | ||||||||
| HI titer ≥40 (n/N, %) | >70% | 62/63 | 98% | 63/63 | 100% | 62/63 | 98% | >60% | 62/62 | 100% | 62/62 | 100% | 53/62 | 85% |
| (95%-CI) | 91–100% | 94–100% | 91–100% | 94–100% | 94–100% | 74–93% | ||||||||
a Subjects in per protocol set; bGMT, geometric mean titer; c95%-CI, 95%- confidence interval; dproportion of subjects with a HI titer ≥40; en/N, subject with a respective immune response (n) as part of the number of subjects of the (sub-) population (N); fCHMP, Criteria according to the Committee for Medicinal Products for Human Use; gSeroconversion, proportion of subjects with antibody increase from <10 pre vaccination to ≥40 post vaccination; hsignificant increase, proportion of subjects with an antibody titer of ≥10 pre vaccination and at least 4-fold antibody increase post vaccination; iGM increase, geometric mean increase.
Pre-vaccination geometric mean titers (GMTs) against A(H1N1), A(H3N2), and the B strain were measured with 40, 147, and 36 for adults and 47, 162, and 28 for elderly subjects, respectively. Twenty-one days post-vaccination GMTs against A(H1N1), A(H3N2), and the B strain were 528, 935, and 201 for adults and 272, 681, and 101 for elderly subjects, respectively.
Seroprotection, which has been associated with a 50% reduction in illness relative to no detectable antibody in healthy younger adults, has been determined by a HI titer ≥40. The proportion of subjects with a HI titer of ≥40 after vaccination against the three strains A(H1N1), A(H3N2), and B was 98%, 100%, and 98% for adults and 100%, 100%, and 85% for elderly subjects, respectively.
Geometric mean fold rises (GMFR) were overall higher in adult subjects (5.63–13) than in elderly subjects (3.54–5.79). For subjects who had antibody titers of <10 prior to the vaccination seroconversion was observed in 95% to 100% of subjects in the adult group and in 80% to 100% of subjects in the elderly group. In subjects with already pre-existing antibody titers of ≥10 a significant (at least 4-fold) increase of the GMT had to be achieved to meet CHMP criteria. Rates for significant increases in antibody titers against the three strains A(H1N1), A(H3N2) and B were measured with 63%, 66%, and 60% for the adults and 54%, 43%, and 39% for the elderly, respectively.
In the HI assay, all three CHMP licensure criteria were met for all strains contained in the vaccine in both age groups.
The highest GMFR on day 22 was measured against the A(H1N1) strain in both groups with 13 in the adult group and 5.79 in the elderly group. Across both groups, the weakest immunogenicity as measured by post-vaccination GMTs and GMFR was observed against the B strain. Serologic analyses from serum samples obtained on day 1 showed moderate pre-vaccination immunity.
Pre-vaccination HI titers ≥40 against the A(H1N1), the A(H3N2), and the B strain were detected in 56%, 86%, and 54% of the adults and in 61%, 85%, and 40% of the elderly. The highest pre-vaccination GMTs were measured against the A(H3N2) strain in both age groups.
Safety and tolerability
In total, 60% of subjects (71% of adults and 49% of elderly) reported any solicited local or systemic reaction. As shown in Table 3, systemic reactions were more frequent than local reactions (47% vs. 41%). Across the age groups, the most frequent solicited local injection site reaction was pain (37%), followed by ecchymosis (4%), swelling (2%), and induration (2%). Among systemic reactions, myalgia (31%), fatigue (17%), and headache (17%) were most common. The majority of solicited local and systemic reactions in both age groups was mild (84.2% of local and 84.0% of systemic reactions) or moderate (15.8% of local and 16.0% of systemic reactions). No subject reported severe local or systemic reactions. Most solicited local (82.7%) and systemic reactions (98.3%) resolved until day 4 and all resolved before the subject’s study termination. Local and systemic reactions, which persisted beyond day 4 were treated as adverse events (AEs). These treatment emergent AEs were reported by 9 (7.1%) subjects in relation to local injection site reactions and by 1 (0.8%) subject who reported headache as systemic reaction.
Table 3. Local injection site and systemic reactions.
| Number (%) of subjects a | ||||
|---|---|---|---|---|
| 18–60 y | ≥61 y | Total | ||
| (n = 63) | (n = 63) | (n = 126) | ||
| Any | 45 (71%) | 31 (49%) | 76 (60%) | |
| Injection site reactions (Day 1 – Day 4 post vaccination) | ||||
| Any | 30 (48%) | 22 (35%) | 52 (41%) | |
| Ecchymosis (mm) | Any | 1 (2%) | 4 (6%) | 5 (4%) |
| >50 mm | 0 | 0 | 0 | |
| Erythema (mm) | Any | 1 (2%) | 0 | 1 (1%) |
| >50 mm | 0 | 0 | 0 | |
| Induration (mm) | Any | 0 | 2 (3%) | 2 (2%) |
| >50 mm | 0 | 0 | 0 | |
| Swelling (mm) | Any | 1 (2%) | 2 (3%) | 3 (2%) |
| >50 mm | 0 | 0 | 0 | |
| Pain | Any | 29 (46%) | 17 (27%) | 46 (37%) |
| Severe | 0 | 0 | 0 | |
| Systemic reactions (Day 1–Day 4 post vaccination) | ||||
| Any | 39 (62%) | 20 (32%) | 59 (47%) | |
| Chills/ shivering | Any | 1 (2%) | 1 (2%) | 2 (2%) |
| Severe | 0 | 0 | 0 | |
| Malaise | Any | 5 (8%) | 2 (3%) | 7 (6%) |
| Severe | 0 | 0 | 0 | |
| Myalgia | Any | 27 (43%) | 12 (19%) | 39 (31%) |
| Severe | 0 | 0 | 0 | |
| Arthralgia | Any | 2 (3%) | 0 | 2 (2% |
| Severe | 0 | 0 | 0 | |
| Headache | Any | 13 (21%) | 4 (6%) | 17 (13%) |
| Severe | 0 | 0 | 0 | |
| Sweating | Any | 10 (16%) | 7 (11%) | 17 (13%) |
| Severe | 0 | 0 | 0 | |
| Fatigue | Any | 15 (24%) | 7 (11%) | 22 (17%) |
| Severe | 0 | 0 | 0 | |
| Fever | 0 | 0 | 0 | |
| Other reactions | ||||
| Temperature | <36 °C | 3 (5%) | 11 (17%) | 14 (11%) |
| ≥40 | 0 | 0 | 0 | |
a Local injection site and systemic reactions were recorded for all subjects who provided post vaccination safety data (safety set)
In total, 17% of subjects (14% of adults and 19% of elderly) reported AEs (treatment emergent and non-treatment emergent). Most AEs were mild, only two AEs (abdominal abscess, erysipelas) were moderate and no AE was severe. Both, abdominal abscess and erysipelas were not related to the vaccination.
In 11% of subjects the investigators judged the AEs as possibly or probably related to the study vaccine (Table 4). The majority (83%) of the possibly or probably related AEs were solicited local and systemic reactions continuing beyond day 4 (treatment emergent AEs). The most common ones were injection site erythema (3%) and injection site hemorrhage (3%), whereby the latter has to be interpreted as local hematoma.
Table 4. Overview of subjects with other adverse events.
| Number (%) of subjects a | |||
|---|---|---|---|
| 18–60 y | ≥61 y | Total | |
| (n = 63) | (n = 63) | (n = 126) | |
| Any AEs | 9 (14%) | 12 (19%) | 21 (17%) |
| At least possibly related AEs | 5 (8%) | 7 (11%) | 12 (10%) |
| Serious AEs | 0 | 0 | 0 |
| At least possibly related SAEs | 0 | 0 | 0 |
| AEs leading to withdrawal | 0 | 0 | 0 |
aOther adverse events were recorded for all subjects who provided post vaccination safety data (safety set)
Most of the non-treatment emergent AEs were classified as mild, only one AE (carpal tunnel syndrome) was classified as moderate. Two AEs (eye inflammation, arthropod bite) did not resolve within the study period. One subject suffered from flu-like symptoms, thus a swap sample was taken and influenza was ruled out by PCR. No serious adverse events (SAEs) were reported in this study.
Discussion
This study was conducted to evaluate the safety, tolerability and immunogenicity of a trivalent inactivated surface antigen influenza vaccine produced in mammalian cell culture. The primary endpoint was to assess antibody levels against the three strains of influenza recommended by the WHO for the 2012/2013 northern hemisphere influenza season after vaccination. The cell-culture derived 2012/2013 Optaflu® influenza vaccine met all three CHMP immunogenicity criteria both in adults and elderly.
The recommendations for the 2012/2013 seasonal influenza composition included two new strains (A/Victoria/361/2011 (H3N2)-like virus and a B/Wisconsin/1/2010-like virus) since influenza activity surveillance until February 2012 indicated increasing infection rates with these strains in the northern hemisphere.21 The pandemic strain A(H1N1) that evolved in March 2009 in Mexico had been part of seasonal influenza vaccines since the 2010/2011 season.
Compared with two other clinical trials from Germany, we found comparatively high pre-vaccination titers for the A(H1N1) strain. However, these two trials have been conducted earlier after the rise of the pandemic A(H1N1) strain in 2009 and the several opportunities for natural infection and previous vaccination since then have to be taken into account.22,23
Nevertheless, almost 60% of our overall population had already achieved HI titers ≥40 against the A(H1N1) strain prior to vaccination and thus fulfilled one of the CHMP criteria even on day 1.
Post-vaccination GMTs against the A(H1N1) strain were higher for adults than for elderly and showed the highest increase of all strains contained in the vaccine after 21 d. These findings are in line with earlier immunogenicity studies.24,25
Post-vaccination titers after seasonal influenza vaccination were frequently described to wane relatively quickly, which contrasts our finding of high pre-vaccination titers.7,26,27 Since only 42% of our overall population was previously vaccinated with seasonal influenza vaccines, naturally acquired infection might have largely contributed to the higher number of seropositive individuals. In particular between September 2011 and February 2012 relevant circulation of the A(H1N1) strain was observed.21 A significant number of A(H1N1)-infected individuals remain unaware of relevant clinical signs and symptoms, thus they do not necessarily recall previous influenza-like illness later on but may still be protected against subsequent infection.22
The highest post-vaccination GMT was measured against the A(H3N2) strain, which already revealed the highest HI titers in the baseline analysis on day 1. Percentages of subjects with HI titers ≥40 were high and almost equal between the two age groups. This particular strain had never been part of a previous vaccine composition.
Previous studies however indicated that there might be sufficient antigenic similarity between different earlier A(H3N2) strains, which could explain a certain degree of cross reactivity and consequently higher baseline titers against the newly introduced strain.28 This aspect was also discussed for the pandemic A(H1N1) strain, for which a distinct level of cross-protection was achieved by earlier seasonal influenza vaccinations.29,30 Whether vaccine-induced cross-reactivity of earlier A(H3N2) strains or previous natural infection with the current or a similar A(H3N2) strain was responsible for the high baseline titers is however not possible to distinguish by using HI assays.31 Latest data of the 2012/2013 influenza season indicated A(H3N2) as the most frequent co-circulating strain after A(H1N1).32
The post-vaccination titers for the B/Wisconsin/1/2010-strain increased moderately, but represented the lowest post-vaccination HI titers across the three strains on day 22. Compared with earlier trials, GMTs for the B strain were overall similar.22,33-35
Typical for vaccine trials, there is a potential for selection bias, as participants may tend to favor the idea of preventive vaccinations.22,24,36 Latest vaccination rates from Germany date from 2009/10 when 47.5% of individuals ≥60 y of age had been vaccinated.37 Phone polls by the Robert Koch Institute (RKI) in the following years indicated declining rates.38 In our trial 52% of the elderly subjects reported earlier seasonal influenza vaccinations.
The vaccine showed a robust safety profile. Local and systemic reactions were similar to other studies on seasonal influenza vaccines.22,39 Most AEs possibly or probably related to the study vaccine were solicited local or systemic reactions lasting longer than 4 d after vaccination. None of them has been severe and all AEs resolved until study termination.
Cell culture-derived influenza vaccines (CCIV) have been proven to be adequate alternatives for conventional egg-based vaccines showing equivalent immune responses.40 Because CCIV provide a series of advantages they are likely to gain in importance in the future.41
Concluding the results of the present study we are able to confirm that the MDCK-derived season 2012/2013 influenza vaccine Optaflu® generates good antibody levels, and has an acceptable safety profile.
Materials and Methods
Study design and objectives
All subjects were enrolled at the Bernhard Nocht Center for Clinical Trials (www.bncct.de), University Medical Center Hamburg-Eppendorf, Germany, between July and August 2012. The trial was designed as a single treatment arm, open-label study to evaluate the immunogenicity and safety for the 2012/2013 northern hemisphere’s seasonal influenza vaccine. The primary immunogenicity objective was to evaluate the antibody response to each influenza vaccine antigen at approximately 21 d after a single intramuscular injection of Optaflu® in adult and elderly subjects. Secondary objective was to evaluate the safety and tolerability in compliance with the requirements of the current EU recommendations for clinical trials related to yearly licensing of influenza vaccines (CPMP/BWP214/96). The trial is registered at Eudra-CT (2011-006277-25) and on clinicaltrials.gov (NCT01640314).42,43
Participants
For a minimum of 100 subjects contributing to the final analysis, the aim was to enrol a total of 126 participants accounting for incomplete data sets and lost to follow-up. It was intended to recruit half of the subjects into the age groups ≥18 to ≤60 y (adults) and ≥61 y (elderly), respectively. The main exclusion criteria were: Psychiatric illness or cognitive impairment that could have interfered with the subject’s ability to participate in the study, a serious chronic or acute disease, significant immunodeficiency, laboratory confirmed seasonal or pandemic influenza disease or seasonal or pandemic influenza vaccination 6 months prior to the study enrolment, receipt of any other vaccine within 4 weeks prior to enrolment and females who were pregnant, breastfeeding or refusing to use an acceptable method of birth control for the whole duration of the study. Pregnancy in women of childbearing potential was ruled out by using a urine pregnancy test before administration of the study vaccine. The sponsor carries out separate vaccine trials for pregnant women to ensure uniformity of the study population.
After written informed consent was given, participants were vaccinated (day 1). A follow-up visit was scheduled three weeks later (day 22 −1/+4). On day 5 (+3 d) a phone interview assessing safety and tolerability was conducted. Blood samples were taken on day 1 prior to study vaccine administration and on day 22 (−1/+4).
Investigational product
Each subject received a single intramuscular injection of a 0.5 mL dose of the trivalent subunit non-adjuvanted influenza vaccine Optaflu® containing purified viral envelope-glycoproteins neuraminidase (NA) and hemagglutinin (HA) derived from MDCK cell culture-based viral amplification. Each pre-filled vaccine syringe was adjusted to contain 15 μg of HA of each of the three virus strains recommended for the 2012/2013 season. For the northern hemisphere the following strains were recommended by the WHO: A/California/7/2009 pdm09 (H1N1)-like strain, A/Victoria/361/2011 (H3N2)-like strain, and B/Wisconsin/1/2010-like strain. The actual strains contained in the vaccine were: A/California/7/2009 pdm09 (H1N1)-like strain used A/Brisbane/10/2010 wild type, A/Victoria/361/2011 (H3N2)-like strain used A/Victoria/361/2011, IVR-165 and B/Wisconsin/1/2010-like strain used B/Wisconsin/1/2010 wild type.
Immunogenicity assessment
Blood samples of approximately 10 ml were obtained prior to and 21 d (study day 22, window: −1 to +5 d) after vaccination. Serum samples were prepared within the hour, stored at temperatures below −20° C and shipped to Novartis Vaccines Clinical Serology Laboratory in Marburg, Germany. Serum antibody titers were measured by hemagglutinin inhibition (HI) assays using WHO reference antigens.44 For confirmatory purposes antibodies were additionally quantified using single radial hemolysis (SRH) assays. A titer as determined by HI assay test was defined as the reciprocal value of the dilution, e.g., a dilution of 1:40 represented a titer of 40. The detection limit of the HI assay was 10. All sera were tested in duplicate, if an individual result was below the detection limit it was set to 5. Seroprotection was defined as a titer ≥40.45,46 Seroconversion or significant increase were defined as an antibody titer increase from <10 pre vaccination to ≥40 post-vaccination or an antibody titer of ≥10 pre-vaccination and at least 4-fold increase post-vaccination. For SRH assays, seroconversion and significant increase were defined as an increase from SRH area ≤4mm2 pre-vaccination to SRH area ≥25mm2 post-vaccination or an at least 50% increase in area from positive pre-vaccination serum, respectively. All immunogenicity assessments were related to the CHMP criteria of the Committee for Medicinal Products for Human Use (CPMP/BWP/214/96) for the respective age group.47 Briefly, in subjects ≥18 to ≤60 y one of the following criteria had to be met for each strain: A proportion of >70% achieving an HI titer ≥40 or an SRH area ≥25 mm2; a proportion >40% with seroconversion or with significant increase in HI titer or SRH area and/or an increase of >2.5 in the geometric mean titer (GMT). In subjects ≥61 y, these criteria were: >60% achieving an HI titer ≥40 or an SRH area ≥25 mm2; >30% with seroconversion or with significant increase in HI titer or SRH area and/or an increase in GMT of >2.0.
Safety assessment
After vaccination on day 1, all study participants were monitored at the study site for at least 30 min. A diary card was dispensed to each subject to record all solicited local and systemic reactions from day 1 up to and including day 4 in order to indicate reactogenicity. The following solicited local and systemic reactions were investigated: ecchymosis (local hematoma), erythema, swelling, induration and (injection site) pain as well as fever (axillary temperature ≥38 °C), chills/shivering, malaise, headache, myalgia, arthralgia, sweating and fatigue, respectively. Adverse events (AEs) were defined as solicited local or systemic reactions persisting beyond day 4 or unsolicited local and systemic reactions / events reported during the entire study period.
Solicited local and systemic reactions were classified as mild (transient with no limitation in normal daily activity), moderate (some limitation in normal daily activity) or severe (unable to perform normal daily activity). For local injection site reactions a diameter of 10–≤25mm determined a mild reaction, a diameter of 26–≤50mm a moderate reaction and a diameter >50mm a severe reaction, respectively.
AEs and serious adverse events (SAE) as well as concomitant medications were recorded for the whole study period up to and including day 22. AEs were monitored until their resolution and their relationship to the investigational product was determined by the investigator.48
Statistical analysis
This study was conducted in compliance with the sample size requirements of the CHMP guideline on harmonization of requirements for influenza vaccines (CPMP/BWP/214/96). There was no statistical null hypothesis associated with the immunogenicity objective.
Disclosure of Potential Conflicts of Interest
The vaccine trial has been conducted at the Bernhard Nocht Center for Clinical Trials with financial compensation by Novartis Vaccines and Diagnostics. S.M. and K.L. are employees of the sponsor.
Acknowledgments
The authors would like to thank the entire staff at the Bernhard Nocht Center for Clinical Trials (BNCCT), in particular Mrs. Sabine Eberhardt and Mrs Anja Wentzin, for their enduring and valuable work.
Glossary
Abbreviations:
- AE
adverse event
- CCIV
cell culture-derived influenza vaccines
- CHMP
Committee for Medicinal Products for Human Use
- GM
geometric mean
- GMFR
geometric mean fold rises
- GMT
geometric mean titer
- HA
hemagglutinin
- HI
hemagglutinin inhibition
- MDCK
Madin Darby Canine Kidney
- NA
neuraminidase
- PCR
polymerase chain reaction
- SAE
serious adverse event
- SRH
single radial hemolysis
- WHO
World Health Organization
References
- 1.World Health Organization. (2009) Influenza (seasonal) Available: http://www.who.int/mediacentre/factsheets/fs211/en/index.html Accessed: 06.12.2012
- 2.Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, Bridges CB, Grijalva CG, Zhu Y, Bernstein DI, et al. New Vaccine Surveillance Network The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 3.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NS, Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57(RR-7):1–60. [PubMed] [Google Scholar]
- 4.Center for Disease Control and Prevention (CDC). (2012) Update: Influenza Activity — United States, 2011–12 Season and Composition of the 2012–13 Influenza Vaccine Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a4.htm Accessed: 06.12.2012.
- 5.Center for Disease Control and Prevention (CDC). (2012) Flu Symptoms and Severity Available: http://www.cdc.gov/flu/about/disease/symptoms.htm Accessed: 06.12.2012
- 6.Chen J, Deng YM. Influenza virus antigenic variation, host antibody production and new approach to control epidemics. Virol J. 2009;6:30. doi: 10.1186/1743-422X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cramer J, Mac T, Hogan B, Stauga S, Eberhardt S, Wichmann O, Mertens T, Burchard G. Influenza A(H1N1)pdm09 antibodies after pandemic and trivalent seasonal influenza vaccination as well as natural infection in November 2010 in Hamburg, Germany. Euro Surveill. 2012;17:17. [PubMed] [Google Scholar]
- 8.Pebody R, Hardelid P, Fleming D, McMenamin J, Andrews N, Robertson C, Thomas D, Sebastianpillai P, Ellis J, Carman W, et al. Effectiveness of seasonal 2010/11 and pandemic influenza A(H1N1)2009 vaccines in preventing influenza infection in the United Kingdom: mid-season analysis 2010/11. Euro Surveill. 2011;16:16. [PubMed] [Google Scholar]
- 9.Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, Lilac HA, Hall H, Klimov A, Fukuda K. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA. 2000;284:1655–63. doi: 10.1001/jama.284.13.1655. [DOI] [PubMed] [Google Scholar]
- 10.Frey S, Vesikari T, Szymczakiewicz-Multanowska A, Lattanzi M, Izu A, Groth N, Holmes S. Clinical efficacy of cell culture–derived and egg‐derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis. 2010;51:997–1004. doi: 10.1086/656578. [DOI] [PubMed] [Google Scholar]
- 11.Doroshenko A, Halperin SA. Trivalent MDCK cell culture-derived influenza vaccine Optaflu (Novartis Vaccines) Expert Rev Vaccines. 2009;8:679–88. doi: 10.1586/erv.09.31. [DOI] [PubMed] [Google Scholar]
- 12.Stöhr K. Vaccinate before the next pandemic? Nature. 2010;465:161. doi: 10.1038/465161a. [DOI] [PubMed] [Google Scholar]
- 13.Ulmer JB, Valley U, Rappuoli R. Vaccine manufacturing: challenges and solutions. Nat Biotechnol. 2006;24:1377–83. doi: 10.1038/nbt1261. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization Cell culture as a substrate for the production of influenza vaccines: memorandum from a WHO meeting. Bull World Health Organ. 1995;73:431–5. [PMC free article] [PubMed] [Google Scholar]
- 15.Wright PF. Vaccine preparedness--are we ready for the next influenza pandemic? N Engl J Med. 2008;358:2540–3. doi: 10.1056/NEJMp0803650. [DOI] [PubMed] [Google Scholar]
- 16.Ambrozaitis A, Groth N, Bugarini R, Sparacio V, Podda A, Lattanzi M. A novel mammalian cell-culture technique for consistent production of a well-tolerated and immunogenic trivalent subunit influenza vaccine. Vaccine. 2009;27:6022–9. doi: 10.1016/j.vaccine.2009.07.083. [DOI] [PubMed] [Google Scholar]
- 17.Szymczakiewicz-Multanowska A, Groth N, Bugarini R, Lattanzi M, Casula D, Hilbert A, Tsai T, Podda A. Safety and immunogenicity of a novel influenza subunit vaccine produced in mammalian cell culture. J Infect Dis. 2009;200:841–8. doi: 10.1086/605505. [DOI] [PubMed] [Google Scholar]
- 18.European Medicines Agency. (2008) Optaflu Available: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000758/human_med_000952.jsp&mid=WC0b01ac058001d124 Accessed: 06.12.2012
- 19.Vesikari T, Block SL, Guerra F, Lattanzi M, Holmes S, Izu A, Gaitatzis N, Hilbert AK, Groth N. Immunogenicity, safety and reactogenicity of a mammalian cell-culture-derived influenza vaccine in healthy children and adolescents three to seventeen years of age. Pediatr Infect Dis J. 2012;31:494–500. doi: 10.1097/INF.0b013e31824bb179. [DOI] [PubMed] [Google Scholar]
- 20.Groth N, Montomoli E, Gentile C, Manini I, Bugarini R, Podda A. Safety, tolerability and immunogenicity of a mammalian cell-culture-derived influenza vaccine: a sequential Phase I and Phase II clinical trial. Vaccine. 2009;27:786–91. doi: 10.1016/j.vaccine.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. (2012) Recommended composition of influenza virus vaccines for use in the 2012-2013 northern hemisphere influenza season Available: http://www.who.int/influenza/vaccines/virus/recommendations/201202_recommendation.pdf Accessed: 08.12.2012.
- 22.Loebermann M, Anders G, Brestrich G, Fritzsche C, Klammt S, Boršo D, Frimmel S, Riebold D, Reisinger EC. Safety and immunogenicity of a trivalent single dose seasonal influenza vaccine containing pandemic A(H1N1) antigen in younger and elderly subjects: a phase III open-label single-arm study. Vaccine. 2011;29:1228–34. doi: 10.1016/j.vaccine.2010.11.092. [DOI] [PubMed] [Google Scholar]
- 23.Loebermann M, Voss U, Meyer S, Bosse D, Fritzsche C, Klammt S, Frimmel S, Riebold D, Reisinger EC. Clinical trial to evaluate the safety and immunogenicity of a trivalent surface antigen seasonal influenza vaccine produced in mammalian cell culture and administered to young and elderly adults with and without A(H1N1) pre-vaccination. PLoS One. 2013;8:e70866. doi: 10.1371/journal.pone.0070866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatz C, Cramer JP, Vertruyen A, Schwarz TF, von Sonnenburg F, Borkowski A, Lattanzi M, Hilbert AK, Cioppa GD, Leroux-Roels G. A randomised, single-blind, dose-range study to assess the immunogenicity and safety of a cell-culture-derived A/H1N1 influenza vaccine in adult and elderly populations. Vaccine. 2012;30:4820–7. doi: 10.1016/j.vaccine.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–35. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 26.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–69. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 27.Bateman AC, Kieke BA, Irving SA, Meece JK, Shay DK, Belongia EA. Effectiveness of Monovalent A(H1N1)pdm09 and 2010-11 Trivalent Inactivated Influenza Vaccines in Wisconsin During the 2010-11 Season. J Infect Dis. 2013 doi: 10.1093/infdis/jit020. [DOI] [PubMed] [Google Scholar]
- 28.Lessler J, Riley S, Read JM, Wang S, Zhu H, Smith GJ, Guan Y, Jiang CQ, Cummings DA. Evidence for antigenic seniority in influenza A (H3N2) antibody responses in southern China. PLoS Pathog. 2012;8:e1002802. doi: 10.1371/journal.ppat.1002802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iorio AM, Bistoni O, Galdiero M, Lepri E, Camilloni B, Russano AM, Neri M, Basileo M, Spinozzi F. Influenza viruses and cross-reactivity in healthy adults: humoral and cellular immunity induced by seasonal 2007/2008 influenza vaccination against vaccine antigens and 2009 A(H1N1) pandemic influenza virus. Vaccine. 2012;30:1617–23. doi: 10.1016/j.vaccine.2011.12.107. [DOI] [PubMed] [Google Scholar]
- 30.Luytjes W, Enouf V, Schipper M, Gijzen K, Liu WM, van der Lubben M, Meijer A, van der Werf S, Soethout EC. HI responses induced by seasonal influenza vaccination are associated with clinical protection and with seroprotection against non-homologous strains. Vaccine. 2012;30:5262–9. doi: 10.1016/j.vaccine.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 31.Hayden F, Palese P. (2009) Influenza virus. In: Richman D, Whitley R, Hayden F, editors, editors. Clinical Virology: ASM press. pp. 943-976. [Google Scholar]
- 32.International Society for Infectious Diseases. (2013) PROMED - Influenza (18): European region update 08 Feb 2013 Available: http://www.promedmail.org/ Accessed: 08.02.2013.
- 33.Campbell JD, Chambers CV, Brady RC, Caldwell MC, Bennett NL, Fourneau MA, Jain VK, Innis BL. Immunologic non-inferiority of a newly licensed inactivated trivalent influenza vaccine versus an established vaccine: a randomized study in US adults. Hum Vaccin. 2011;7:81–8. doi: 10.4161/hv.7.1.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song JY, Cheong HJ, Woo HJ, Wie SH, Lee JS, Chung MH, Kim YR, Jung SI, Park KH, Kim TH, et al. Immunogenicity and safety of trivalent inactivated influenza vaccine: a randomized, double-blind, multi-center, phase 3 clinical trial in a vaccine-limited country. J Korean Med Sci. 2011;26:191–5. doi: 10.3346/jkms.2011.26.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allwinn R, Bickel M, Lassmann C, Wicker S, Friedrichs I. “Trivalent influenza vaccination of healthy adults 3 years after the onset of swine-origin H1N1 pandemic: restricted immunogenicity of the new A/H1N1v constituent?”. Med Microbiol Immunol. 2013;202:125–30. doi: 10.1007/s00430-012-0259-9. [DOI] [PubMed] [Google Scholar]
- 36.Kulkarni PS, Manjunath K, Agarkhedkar S, Group of SII IIV Studies Safety and immunogenicity of an adjuvanted whole virion, inactivated A (H1N1) 2009 influenza vaccine in young and elderly adults, and children. Vaccine. 2012;31:20–2. doi: 10.1016/j.vaccine.2012.10.081. [DOI] [PubMed] [Google Scholar]
- 37.Böhmer MM, Walter D, Falkenhorst G, Müters S, Krause G, Wichmann O. Barriers to pandemic influenza vaccination and uptake of seasonal influenza vaccine in the post-pandemic season in Germany. BMC Public Health. 2012;12:938. doi: 10.1186/1471-2458-12-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robert Koch Institut (RKI). (2013) Infektionsschutz - Wie viele Menschen lassen sich gegen die saisonale Influenza impfen? Available: http://www.rki.de/SharedDocs/FAQ/Impfen/Influenza/FAQ06.html Accessed: 19.10.2013.
- 39.Brydak LB, Tadeusz S, Magdalena M. Antibody response to influenza vaccination in healthy adults. Viral Immunol. 2004;17:609–15. doi: 10.1089/vim.2004.17.609. [DOI] [PubMed] [Google Scholar]
- 40.Reisinger KS, Block SL, Izu A, Groth N, Holmes SJ. Subunit influenza vaccines produced from cell culture or in embryonated chicken eggs: comparison of safety, reactogenicity, and immunogenicity. J Infect Dis. 2009;200:849–57. doi: 10.1086/605506. [DOI] [PubMed] [Google Scholar]
- 41.Ampofo WK, Baylor N, Cobey S, Cox NJ, Daves S, Edwards S, Ferguson N, Grohmann G, Hay A, Katz J, et al. Improving influenza vaccine virus selection: report of a WHO informal consultation held at WHO headquarters, Geneva, Switzerland, 14-16 June 2010. Influenza Other Respi Viruses 2012 6: 142-152, e141-145. [Google Scholar]
- 42.EU Clinical Trials Register. (2012) A Phase III Open Label, Uncontrolled, Multi Center Study to Evaluate Safety and Immunogenicity of a Surface, Antigen, Inactivated, Influenza Vaccine Produced in Mammalian Cell Culture (Optaflu®), Formulation 2012/2013, when Administered to adult and elderly subjects. Available: https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-006277-25/DE Accessed: 02.02.2013.
- 43.ClinicalTrials.gov. (2012) Safety and Immunogenicity of a Cell Derived Subunit Trivalent Nonadjuvated Influenza Study Vaccine in Adults Aged 18 Years and Above Available: http://clinicaltrials.gov/ct2/show/NCT01640314?term=NCT01640314&rank=1 Accessed: 21.12. 2012.
- 44.Keitel WA, Couch RB, Cate TR, Hess KR, Baxter B, Quarles JM, Atmar RL, Six HR. High doses of purified influenza A virus hemagglutinin significantly augment serum and nasal secretion antibody responses in healthy young adults. J Clin Microbiol. 1994;32:2468–73. doi: 10.1128/jcm.32.10.2468-2473.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003;115:63–73. [PubMed] [Google Scholar]
- 46.Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979;35:69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- 47.The Committee for Proprietary Medicinal Products (CHMP). (2009) Note for guidance on harmonisation of requirements for influenza vaccines. CHMP/BWP/214/96. Available: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf Accessed: 29.11.2012.
- 48.Brighton Collaboration. (2012) Vaccine Safety Research Standards Available: https://brightoncollaboration.org/public Accessed: 07.12.2012.