Abstract
Tetanus is caused by the tetanus neurotoxin (TeNT), a 150 kDa single polypeptide molecule which is cleaved into an active two-chain molecule composed of a 50 kDa N-terminal light (L) and a 100 kDa C-terminal heavy (H) chains. Recently, extensive effort has focused on characterization of TeNT binding receptors and toxin neutralization by monoclonal antibodies (mAbs). Toxin binding inhibition and neutralization is routinely assessed either in vitro by the ganglioside GT1b binding inhibition assay or in vivo using an animal model. These two assay systems have never been compared. In the present study, we report characterization of eleven mAbs against different parts of TeNT. The toxin inhibitory and neutralization activity of the mAbs was assessed in vitro and in vivo respectively. Our data demonstrated that seven mAbs bind to fragment C of the heavy chain, two mAbs react with the light chain, one mAb recognizes both chains and one mAb reacts with neither light chain nor fragment C. Six fragment C specific mAbs were able to inhibit TeNT binding to GT1b ganglioside in vitro but three failed to neutralize the toxin in vivo. One in vitro inhibitory mAb (1F3E3) was found to synergize with the in vivo neutralizing mAbs to reduce toxin lethal activity in vivo. Sequencing of the immunoglobulin heavy and light chain variable region genes revealed that the three in vivo neutralizing mAbs were derived from a common origin. Altogether, our data suggests that fragment C specific mAbs contribute to toxin neutralization in both systems, though some of the GT1b binding inhibitory mAbs may not be able to neutralize TeNT in vivo.
Keywords: tetanus neurotoxin, GT1b binding assay, in vivo neutralization assay, neutralizing mAbs
Introduction
Tetanus is a potentially fatal disease associated with high mortality caused by the Clostridium tetani neurotoxin. Tetanus neurotoxin (TeNT) is released from bacteria as a single-chain polypeptide of 150 kDa and is subsequently cleaved to generate an active di-chain toxin, in which the light (LC) and heavy chains (HC) are linked by a single disulphide bond. The 50 kDa LC contains the HExxH zinc protease consensus motif and acts as a zinc-dependent endopeptidase.1 The 100 kDa HC is composed of two distinct functional domains, the N-terminal half (HN: 50 kDa) which is important for LC translocation and the carboxy terminal half (HC or fragment C: 50 kDa) which has a key role in binding to the neuron gangliosides.1,2 Fragment C is further subdivided into two subdomains: the proximal HCN subdomain and the extreme carboxy subdomain, HCC. This latter subdomain (HCC) holds the key amino acid residues responsible for the binding activity of the CNTs.3,4
Although TeNT mechanism of action is well characterized, the sites through which TeNT and its receptor bind are not fully explored. Several studies indicated that TeNT binds to its receptor via selected regions (lipid rafts) that are enriched in gangliosides (notably gangliosides of the 1b series), cholesterol, and GPI-anchored glycoproteins.5-7 This gave direct support for the dual receptor model in which gangliosides and glycosylated proteins such as synaptic vesicle protein SV2A and SV2B are involved in TeNT binding.8 Considering the gangliosides as the main part of tetanus toxin binding receptor, GT1b binding assay was exploited to evaluate in vitro inhibition of TeNT binding activity.9-11
We have recently assessed the inhibitory potential of four TeNT specific mAbs (1F3E3, 1F2C2, 1F3B3, and 1F4E11) using the in vitro GT1b binding assay.12 However, there is no detailed information about the validity and relevance of this method to the results obtained from in vivo experiments. Here, we report characterization of four previously reported12 and seven new anti-TeNT-specific murine mAbs recognizing different epitopes of the tetanus toxin. The TeNT inhibitory activity of these mAbs was determined in vitro by the GT1b binding assay and neutralization activity of these mAbs was assessed in vivo using toxin neutralization assay in mice.
Results
Production and specificity assessment of anti-TeNT mAbs
The fusion of splenocytes from hyperimmunized mice with SP2/0 myeloma cells resulted in establishment of hybridoma cells. All actively proliferating hybridomas were initially screened based on reactivity to tetanus toxoid and toxin by an indirect ELISA. Eleven stable antibody producing hybridomas reacting with both tetanus toxoid and toxin were selected. All selected hybridomas were subcloned and stable clones producing TeNT-specific mAbs were finally selected. The isotype of the mAbs was found to be either IgG2a (8 clones) or IgG2b (3 clone) (Table 1). For further characterization of anti-TeNTmAbs, ELISA was performed using tetanus toxin subfragments. Seven mAbs (1F3E3, 1F2C2, 1F1E12, 1F2C8, 2C9B6, 3B3D9, and 1E4D5) showed positive reactivity to fragment C, two mAbs (1F3C3 and 1F3B3) reacted only with light chain, one mAb (1F4E11) bound to both fragment C and the light chain and one mAb (5E6B10) reacted neither with fragment C nor light chain (Table 1). The affinity constant (Kaff) of mAbs varied between 6.6 ×107 to 3.79 × 109 as determined by ELISA (Table1).
Table 1. Determination of isotype and specificity of tetanus toxin specific monoclonal antibodies.
| mAbs | Isotype | Toxoid | Toxin | Fragment C | Light chain | Affinity Constant |
|---|---|---|---|---|---|---|
| 1F3E3 | IgG2a | + | + | + | − | 1.78 × 109 |
| 1F2C2 | IgG2a | + | + | + | − | 7.1 × 108 |
| 1F1E12 | IgG2a | + | + | + | − | 8.1 × 108 |
| 1F2C8 | IgG2a | + | + | + | − | 1.9 × 109 |
| 1F3C3 | IgG2a | + | + | − | + | 6.6 × 107 |
| 1F3B3 | IgG2a | + | + | − | + | 7.0 × 107 |
| 1F4E11 | IgG2b | + | + | + | + | 2.1 × 108 |
| 2C9B6 | IgG2a | + | + | + | − | 1.98 × 108 |
| 3B3D9 | IgG2b | + | + | + | − | 9.06 × 108 |
| 5E6B10 | IgG2a | + | + | − | − | 1.8 × 109 |
| 1E4D5 | IgG2b | + | + | + | − | 3.79 × 109 |
Cross-inhibition of binding of monoclonal antibodies to tetanus toxin
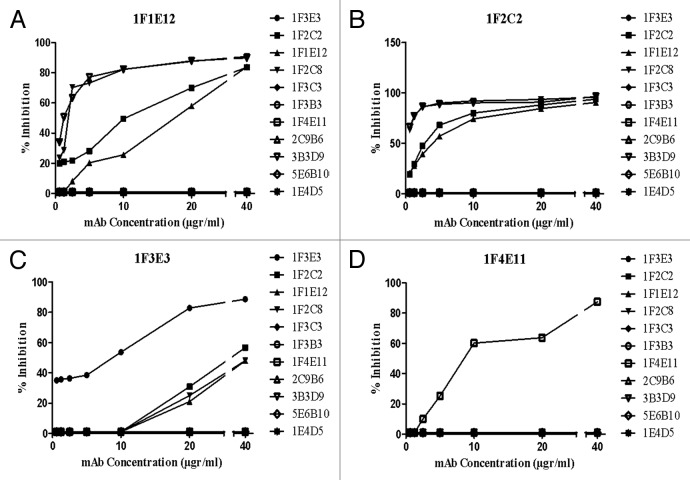
Epitope specificity of mAbs was determined by antibody binding competition ELISA assay. 1F4E11, 1F1E12, 1F2C2, and 1F3E3 were used as detectors (enzyme conjugated) and other mAbs as competitors (unconjugated) in a multistage ELISA assay. Increasing concentrations of the competitor mAb (from 0.612 to 40 µg/ml) were used to inhibit binding of the detector mAb to tetanus toxin. Our results demonstrated that four anti fragment C antibodies (1F2C2, 1F1E12, 1F2C8, and 3B3D9) completely inhibited binding of each other to tetanus toxin even at the low concentration (1.25 µgr/ml) (Fig. 1A and B). Moreover 1F2C2, 1F1E12, and 1F2C8, but not 3B3D9 partially inhibited binding of IF3E3 to tetanus toxin at the highest concentration (40 µgr/ml) (Fig. 1C). No cross-inhibition was observed for the other mAbs (Fig. 1D).
Figure 1. Representative cross-inhibition profile of TeNT-specific monoclonal antibody binding to tetanus toxin. Increasing concentrations of the competitor mAbs were used to inhibit binding of the enzyme conjugated detector mAbs (1F1E2, 1F2C2, 1F3E3, and 1F4E11) to tetanus toxin. The results show percent inhibition of the binding of detector mAb to tetanus toxin.
Inhibition of TeNT binding to Ganglioside GT1b
The ability of each mAb to block TeNT binding to immobilized ganglioside GT1b was assessed by an indirect ELISA. As shown in Figure 2, 1F3E3, 1F2C2, 1F1E12, and 1F2C8 mAbs completely inhibited binding of TeNT to GT1b even at a very low concentration (0.312 µg/ml), whereas 2C9B6 and 3B3D9 induced a similar binding inhibitory activity only at higher concentrations (5–10 µg/ml). Three of the mAbs (1F3C3, 1F3B3, and 1F4E11) did not block TeNT binding to its receptor even at the highest concentration (10 µg/ml) employed in this study.
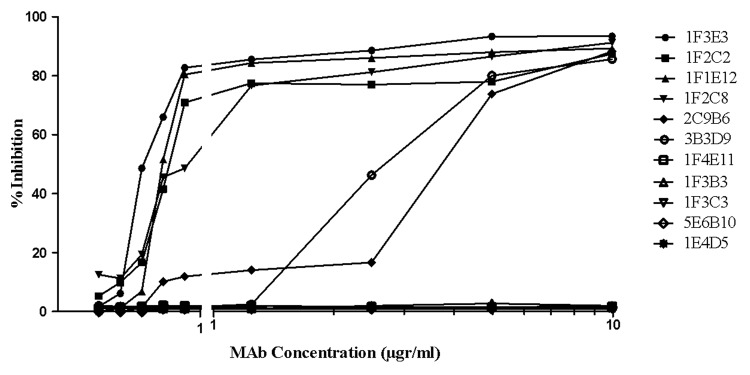
Figure 2. Inhibition of binding of tetanus toxin to ganglioside GT1b by the monoclonal antibodies. An appropriate concentration (20 µg /ml) of tetanus toxin in the presence or absence of increasing concentrations of mAbs was allowed to bind to ganglioside GT1b. Percent inhibition of TeNT binding was calculated by dividing of average OD of each concentration of neutralizing mAbs by the average OD of toxin alone.
In vivo TeNT neutralization assay in mice
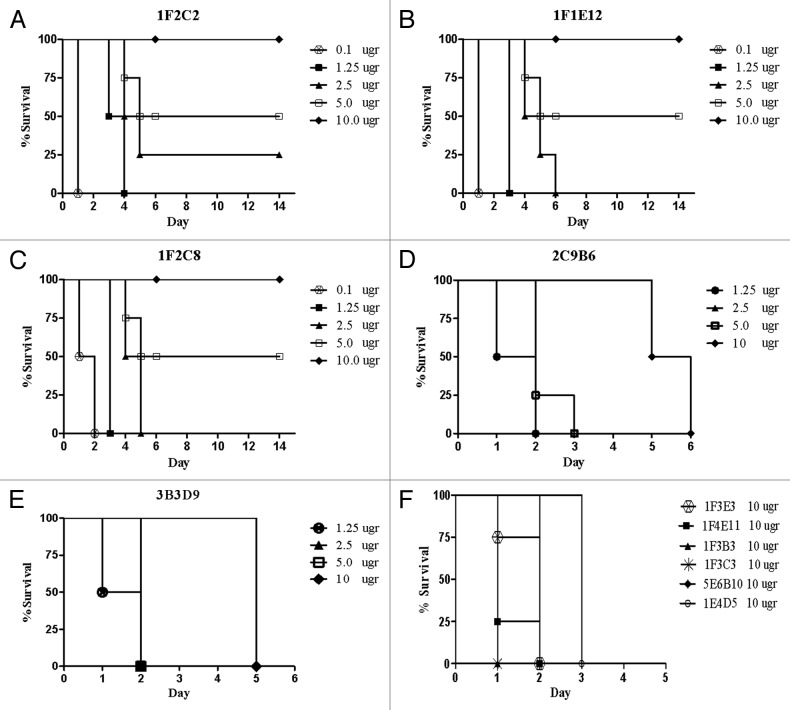
A combination of 10 MLD (mean lethal dose) of TeNT and different concentrations of mAbs was injected intraperitoneally in BALB/c mice. Mice injected with toxin alone or in combination with commercial polyclonal anti-tetanus immunoglobulin (TIG) were included as controls. Three out of seven fragment C specific mAbs, including 1F2C2, 1F1E12, and 1F2C8 neutralized the toxin in mice (Fig. 3A-C), whereas 2C9B6 and 3B3D9 mAbs that showed partial neutralization activity of TeNT in vitro were not substantially effective in vivo and caused only a delay in the death of mice up to 6 d (Fig. 3D and E). Surprisingly, 1F3E3 mAb that had shown the highest in vitro inhibitory activity did not neutralize the toxin in vivo even at the highest concentration (10 µgr/ml) employed in this study (Fig. 3F). As expected, none of the five GT1b negative mAbs, neutralized TeNT in vivo (Fig. 3F). Next, we investigated the synergistic effects of the mAbs. As shown in Figure 4A, the results revealed that 1F2C2 and 1F3E3, which were able to neutralize the toxin in vitro, have synergistic effect in vivo. A minimum of 1.25 µg of 1F2C2 mAb in combination with 1F3E3 neutralized the toxin completely and displayed neutralization efficiency similar to that obtained with 10 µg of 1F2C2 mAb alone (Fig. 4A). No synergistic effects were observed using a combination of other mAbs (Fig. 4B).
Figure 3. Tetanus toxin inhibitory effect of monoclonal antibodies in vivo. BALB/c mice were injected with a constant amount of toxin (10 MLD) mixed with different concentrations of six mAbs (A–F).
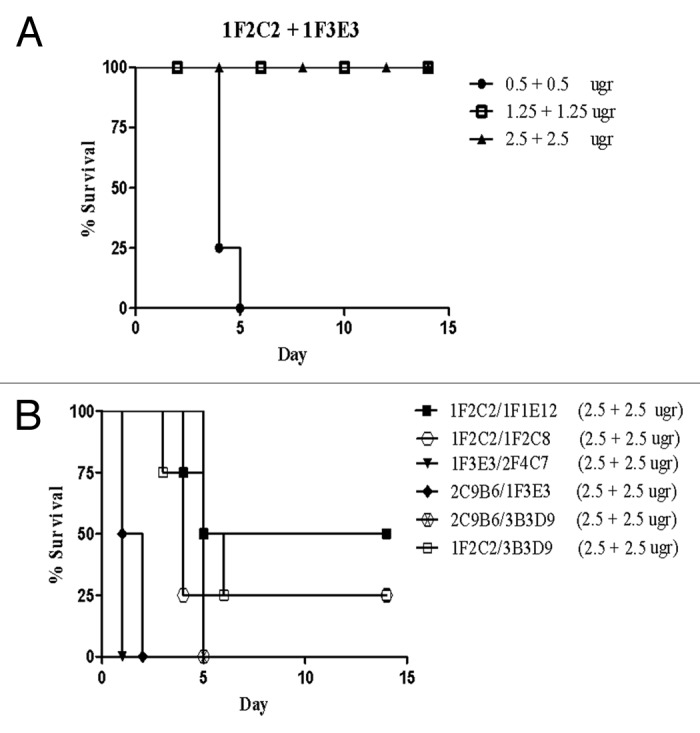
Figure 4. Neutralizing activity of two combinations of monoclonal antibodies (A and B). Equal concentrations of two mAbs were mixed and incubated with 10 MLD of tetanus toxin before injection into mice.
Different concentrations of the commercial human polyclonal anti-TeNT immunoglobulin with known IU were tested (5, 10, 50, 125, and 1000 µgr) (Fig. 5). The minimum concentration required to completely neutralize TeNT and protect mice was found to be 125 μg.
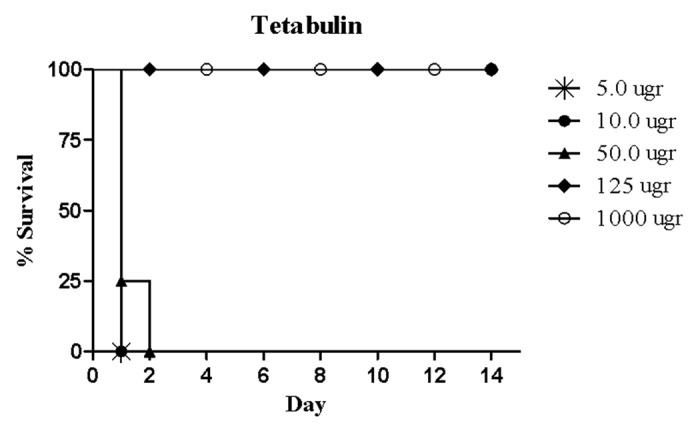
Figure 5. Protective capacity of standard human polyclonal anti tetanus immunoglobulin (TIG) in BALB/c mice. Different concentrations of TIG were mixed with 10 MLD of TeNT and injected intraperitoneally.
Immunoglobulin heavy and light chain variable region genes expressed by the hybridoma clones
To determine whether these clones are distinct or have been derived from the same origin, the IGHV and IGLV genes expressed in nine clones were amplified and sequenced using VH and VL specific primers. Analysis of the rearrangement patterns and sequences of the V (D) J genes indicates establishment of 3 hybridomas (1F2C2, 1F1E12, and 1F2C8) from a single clone. These clones displayed similar characteristics and utilized the same VH gene from the IGHV3–6*02 heavy chain family recombined with IGHJ3*01 and IGHD5–5*01. All hybridomas in this group expressed the same IGKV6–17*01 and IGKJ1*01 of the kappa light chain gene family (Table 2).
Table 2. Immunoglobulin heavy and light chain variable region genes usage by the TeNT-specific hybridoma clones.
| VH | DH | JH | Vκ | Jκ | VH CDR3 | VL CDR3 | |
|---|---|---|---|---|---|---|---|
| 1F3E3 | IGHV1–9*01 | IGHD3–2*01 | IGHJ3*01 | IGKV19–93*01 | IGKJ2*01 | CARKTVRATYPDW | CLQYDNLLAF |
| 1F2C2 | IGHV3–6*02 | IGHD5–5*01 | IGHJ3*01 | IGKV6–17*01 | IGKJ1*01 | CAREGVLPESW | CLQHSSTPRTF |
| 1F1E12 | IGHV3–6*02 | IGHD5–5*01 | IGHJ3*01 | IGKV6–17*01 | IGKJ1*01 | CAREGVLPESW | CLQHSSTPRTF |
| 1F2C8 | IGHV3–6*02 | IGHD5–5*01 | IGHJ3*01 | IGKV6–17*01 | IGKJ1*01 | CAREGVLPESW | CLQHSSTPRTF |
| 1F3C3 | IGHV1–35*01 | IGHD2–10*01 | IGHJ1–01 | IGKV5–48*01 | IGKJ4*01 | CARRAYYGNSFSWPFDVW | CQQSNSWPFTF |
| 1F3B3 | IGHV1–39*01 | IGHD2–10*01 | IGHJ1*01 | IGKV4–59*01 | IGKJ2*01 | CARRAYYGNSYWYFDVW | CQQWSSNPPTF |
| 1F4E11 | IGHV1–4*01 | IGHD5–7*01 | IGHJ3*01 | IGKV12–41*01 | IGKJ1*01 | CARSASPLTWFAYW | CQHFWTTPWTF |
| 2C9B6 | IGHV5–2*01 F | IGHD2–1*01 F | IGHJ3*01 F | IGKV3–12*01 F | IGKJ2*01 F | CARGGYGNPFAYW | CQHIRELDRF |
| 3B3D9 | IGHV3–6*02 F | IGHD1–1*02 F | IGHJ3*02 P | IGKV3–12*01 F | IGKJ2*01 F | CAREGVIFGYW | CQHIRELTTF |
| 5E6B10 | Not determined | ||||||
| 1E4D5 | Not determined | ||||||
Discussion
In the present study, we report characterization of seven new and four previously described anti-TeNT specific mAbs12 all of which react with the tetanus toxin and toxoid. Further characterization of the mAbs with subfragments of the light and heavy chain of TeNT revealed that seven mAbs (1F3E3, 1F2C2, 1F1E12, 1F2C8, 2C9B6, 3B3D9, and 1E4D5) bind to fragment C, while the other four mAbs recognized either LC alone (1F3B3 and 1F3C3), both LC and fragment C (1F4E11), or none of them (5E6B10). 1F4E11 mAb may recognize a conformational epitope requiring both chains for expression or a homologous epitope expressed in both chains of TeNT.
Tetanus toxin fragment C contains “universal epitopes” for human CD4+ T cells which are easily processed and bind to different human class II molecules. This property enables “universal epitopes” to sensitize CD4+ T cells of most or all individuals.13,14 Taken together, fragment C could be considered an immunodominant part of the toxin to which the humoral immune response is predominantly elicited. This proposition is consistent with our findings showing that the majority of mAbs react with this part of the toxin.
The results of cross-inhibition studies showed that four out of seven anti fragment C mAbs (1F2C2, 1F1E12, 1F2C8, and 3B3D9) entirely inhibit binding of each other to tetanus toxin (Fig. 1A and B). Surprisingly 1F2C2, 1F1E12, and 1F2C8 but not 3B3D9 were able to partially inhibit binding of 1F3E3 to tetanus toxin and this inhibitory effect only was achieved at the highest concentration of the inhibitor mAbs (40 µg/ml) (Fig. 1C). Our VH and VL sequencing data showed that these three mAbs were derived from a single hybridoma clone. These results suggest that this group of mAbs (1F2C2, 1F1E12, and 1F2C8) and 3B3D9 and 1F3E3 recognize three distinct, but spatially or linearly very close or overlapping epitopes located within fragment C of TeNT.
In the next step we assessed the ability of mAbs to inhibit binding activity or neutralize the toxin using both the in vitro GT1b binding assay and the in vivo using toxin neutralization assay in mouse. As previously mentioned, gangliosides of the 1b series are considered to be the main part of clostridial neurotoxin receptor. Therefore, the GT1b binding assay has been widely utilized9-11,15 as a suitable method to study the inhibitory activity of anti- TeNT antibodies. As shown in Figure 1, six out of seven anti-fragment C antibodies (1F3E3, 1F2C2, 1F1E12, 1F2C8, 2C9B6, and 3B3D9) inhibited TeNT binding to its receptor dose dependently, whereas the anti-LC antibody (1F3B3 and 1F3C3) or anti-light and anti-fragment C antibody (1F4E11) failed to block the binding of the TeNT to GT1b ganglioside. Given that the receptor binding domain and critical residues for TeNT binding are located in fragment C, antibodies against this region are expected to be neutralizing, a phenomenon also observed elsewhere.10 In addition, monoclonal antibody neutralizing activity was investigated using the mouse passive protection assay. Intriguingly, 1F3E3, which displayed the highest inhibitory activity in the GT1b binding assay, was not able to neutralize the toxin in mice. However, the other three fragment C specific mAbs were able to neutralize the toxin to the same extent in both assay systems (Fig. 3A– C), whereas 2C9B6 and 3B3D9 mAbs were not so much effective and induced only a delay in death of mice up to 6 d (Fig. 3D and E).
As previously described, fragment C is composed of two almost identical sub-domains: HCN and HCC. HCN has the carbohydrate-binding moiety structure conserved between BoNTs and TeNT. In contrast, HCC holds the highest sequence divergence throughout the CNT family which probably contributes to the distinct binding properties found between TeNT and BoNTs.16-18Shiavo and coworkers showed that a 15-kDa putative glycoprotein has been involved in membrane activity of fragment C and HCC subdomain binding activity. The same group also suggested that GPI anchored protein Thy-1 can interact with TeNT and mimic ganglioside binding properties.5,19 Moreover, the results of atomic-force microscopy/total internal-reflection fluorescence microscopy (TMAFM/TIRFM) suggest that fragment C binding to neuronal cells is dependent on both the ganglioside concentration and the pH of the medium.20 Taken together, it has been suggested that different factors including ganglioside concentration, GPI-anchored proteins and pH of the microenvironment play major roles in TeNT binding, retrograde transportation and TeNT intoxication of neurons. These different binding properties of fragment C may explain the differences observed between the in vitro and in vivo TeNT neutralizing results of the mAbs in our study. Moreover several studies showed that antibodies against not only fragment C but also fragment B (light chain + HN) and light chain are able to neutralize TeNT21-25 showing that GT1b assay lonely cannot forecast TeNT neutralization in vivo.
Another interesting finding of this study was the synergistic neutralizing effect of the GT1b binding inhibitory mAb 1F3E3 (inhibitory in vitro but non-inhibitory in vivo) with the in vivo neutralizing 1F2C2 mAb (Fig. 4A). Low concentration of these two mAbs provided complete protection in mice. These two antibodies displayed an in vivo potency several times higher than the commercial TIG antibody preparation (Fig. 5). Cooperative binding effects of antibodies directed against different epitopes have been previously reported.26,27 Cooperative binding is caused by conformational changes of a protein in which binding of one antibody thermodynamically or entropically facilitate binding of the next one. Such cooperative or synergistic effect has been reported for antibodies or scFvs binding to non-overlapping or independent epitopes of the tetanus toxin.28In this study, 1F3E3/1F2C2 exhibits marked synergistic effects in neutralization of TeNT. Furthermore as shown in Figure 1C, 1F2C2 and 1F3E3 recognize two distinct but very close or overlapping epitopes within fragment C suggesting the importance of these epitopes in TeNT neutralization.
Finally, comparable in vitro and in vivo characteristics of 1F2C2, 1F1E12, and 1F2C8 mAbs led us to determine whether these mAbs are distinct or have been derived from the same clone. VH and VL genes sequencing analysis of nine hybridoma clones revealed expression of seven different VH-VL combinations. 1F2C2, 1F1E12, and 1F2C8 mAbs which had the same characteristics expressed the same VH and VL genes and thus are derived from a single clone (Table 2). These mAbs were selected from separate plates seeded with fused splenocytes from an immunized mouse which suggests extensive stimulation and expansion of the original B cell clone in response to the corresponding immunodominant epitope of TeNT.
Materials and Methods
Mouse immunization and anti-TeNTmAbs production
Female BALB/c mice (6–8 weeks age) were given an intraperitoneal injection of tetanus toxoid (40 μgr) and three additional booster injections (10 μgr) at three week intervals. Splenocytes of hyperimmunized mice were harvested and fused with the mouse myeloma cell line SP2/0 (National Cell Bank of Iran). Polyethylene glycol (PEG) 1500 (Sigma) was used for fusion of splenocytes from hyperimmunized mice with SP2/0 cells at a 4:1 ratio. Fused cells were grown in hypoxanthine/ aminopterin/ thymidine (HAT) selective medium (Sigma) and cloned by the limiting dilution assay. Supernatants of growing cells were tested for presence of antibody with tetanus toxoid and toxin specificity by indirect ELISA. Immunized mouse serum was used as positive control. All positive hybridomas were further subcloned to generate stable clones.
mAb isotype determination and purification
The isotype of the heavy and light chains of selected clones was determined using IsoStrip mouse mAb isotyping kit (Roche Diagnostics) according to the manufacturer’s recommendations. Isotype of positive clones was also confirmed by ELISA using goat anti-mouse IgG1 and IgG2a isotypes (Sigma). All clones were injected intraperitoneally in BALB/c mice one week after injection of 0.5 ml pristane (Sigma) to obtain ascitic fluid. Ascitic fluids were harvested and mAbs purified using HiTrap™ SPG column (GE Healthcare).
mAb specificity determination by ELISA
To investigate mAbs specificity, ELISA was performed using tetanus toxin, toxoid, and tetanus toxin subfragments. Briefly, appropriate concentration of fragment C (1 µg/ml) (Sigma), light chain (1 µg/ml) (List Biological Labs), tetanus toxin (10 µgr/ml), and tetanus toxoid (10 µg/ml) (Razi Vaccine and Serum Research Institute) in phosphate buffer saline (PBS, 0.15 M, pH = 7.2) were coated onto the wells of a microtiter ELISA plate (Maxisorp) overnight at 4 °C. After washing, the plate was blocked using blocking buffer (PBS-Tween 20 containing 3% non-fat skim milk) at 37 °C for 1.5 h. After blocking and washing, 100 µl of 1 µg/ml purified antibody was added and incubated 1.5 h at 37 °C. Appropriate dilution of HRP-conjugated rabbit anti-mouse (prepared in our lab) was subsequently added and the reaction revealed with 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate. Finally, the reaction was stopped with 20% H2SO4 and the optical density (OD) measured by a multiscan ELISA reader (OrganonTeknika) at 450 nm.
Affinity constant determination
The affinity constant (Kaff) of our mAbs was determined by ELISA method.12 Briefly, 2000, 1000, 500, 250, 125, and 60 ng/ml tetanus toxoid was coated on wells of a micotiter ELISA and serial concentrations of mAbs (from 30 to 2000 ng/ml) in blocking buffer were added into each coated well and incubated at 37 °C for 1.5 h. After washing step, all wells were incubated with HRP-conjugated sheep anti-mouse Ig for 1.5 h at 37 °C. Washing was repeated and ODs were measured following addition of TMB substrate solution. Sigmoidal curves of ODs vs. the logarithm of antibody concentrations were drawn. The antibody concentration resulting in 50% of the maximum absorbance value ([Ab]t) at a particular antigen coating concentration was selected for the affinity calculation using the formula Kaff = 1/2(2 [Ab´]t − [Ab]t). [Ab´]t and [Ab]t represent the antibody concentrations resulting in 50% of the maximum absorbance value at two consecutive concentrations of coated antigen where [Ag] = 2 [Ag´]. Final Kaff value was the mean of such calculations for at least three antigen concentrations.
Assessment of epitope specificity of monoclonal antibodies by competition ELISA
Epitope specificity of mAbs was determined by competition ELISA as described previously.12 Briefly 10 µg/ml of TeNT was coated on wells of a micotiter ELISA plate and blocked with 3% skin milk-PBS/T 0.05%. Unconjugated mAbs in final concentrations of 40, 20, 10, 5, 2.5, 1.25, and 0.612 µg/ml were added together with 1 µg/ml of HRP-conjugated mAbs (prepared in our lab) and incubated at 37 °C for 1.5 h. Washing steps were repeated and ODs were measured after addition of TMB substrate solution and then stopping solution. Percent of inhibition for each mAb was calculated based on the following formula: Percent of inhibition = ([ODNI − ODWI]/ODNI) × 100, where ODNI represents OD obtained in absence of a competitor mAb and ODWI represents OD obtained in presence of a competitor mAb.
Assessment of inhibitory activity of mAbs on tetanus toxin binding to ganglioside GT1b
The ability of mAbs to inhibit binding of TeNT to GT1b ganglioside was assessed by a modification of a previously described procedure.10 Briefly, a concentration of TeNT that resulted in saturation of ganglioside GT1b binding was chosen for use in the assay (20 µg /ml) and incubated with an equal volume of serial concentrations of anti-TeNTmAb (starting from 10 µg /ml) for 2 h at room temperature. Microtiter ELISA plates were coated with GT1b (Sigma) (10 µg /ml in methanol, 100 µl/well) and plates were left at room temperature overnight to allow evaporation of methanol. The plates were then blocked with 1% BSA/PBS for 2 h at room temperature. After washing four times with PBS-Tween 20, 100 µl of each TeNT/antibody mixture (preincubated for 2–3 h) was added to wells and incubated for 2 h. Plates were washed 4 times and then incubated 2 h with HRP-conjugated human anti TeNTpolyclonalantibodies (produced in our lab). Following addition of TMB substrate solution and then adding the stop solution, the ODs were measured at 450 nm by a multiscan ELISA reader.
Toxin-neutralizing assay in mice
The biological activity of all mAbs, either individually or in combination, was determined using a tetanus toxin neutralization assay in mice. The mean lethal dose (MLD) of the standard toxin was determined from a dose–response curve. Various concentrations of purified mAbs (from 1.0 μg to 10 μg) were diluted in PBS and incubated with 10 MLD of the toxin for 2 h at 37 °C. In parallel, human polyclonal anti- tetanus immunoglobulin (TIG) (Baxter AG) was also mixed with the toxin under the same condition. Then 0.2 ml of the mixture (TeNT/ mAbs and TeNT alone) was injected intraperitoneally into BALB/c mice (6–8 weeks age). After injection, mice were followed for the next 14 d to check for any signs of paralysis or death. Mice surviving 2 weeks after challenge were considered protected.
VH/VL cloning and sequencing
Total RNA from TeNT specific clones was extracted and cDNA was synthesized from 1µgr of total RNA. Two microliters of each cDNA was used to amplify the mouse VH and VL gene family using specific degenerated primers:
5′-CAGGTSMARCTGCAGSAGTCWGG-3′as sense primer for VH and
5′-AGGGGCCAGTGGATAGACAG ATGG-3′ as antisense primer for VH and
5′-GAHRTTSWGN TSACYCAGWC TCCA-‘3 as sense primer for Vκ and
5′-TGGTGGGAAG ATGGATACAG-‘3 as antisense primer for Vκ.
PCR was performed in 40 cycles, initially 3 min at 94 °C followed by 90 °C for 20 s, 60 °C for 30 s, 72 °C for 30 s, and 10 min at 72 °C for the final extension. PCR products were finally visualized by electrophoresis on 1% agarose gel containing ethidium bromide. PCR products were extracted using the GF-1 Nucleic Acid Extraction Kit (Vivantis). Gel purified PCR products were cloned into pGEM-T easy vector system Ι (Promega) according to the manufacturer's instructions. Sequencing of selected clones was performed for both directions using the BigDye Terminator Cycle Sequencing Reaction Kit (Applied Biosystems), T7, and SP6 primers.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Jalal Khoshnoodi and Ahmad Ali Bayat for their technical assistance.
References
- 1.Lacy DB, Tepp W, Cohen AC, DasGupta BR, Stevens RC. Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat StructBiol. 1998;5:898–902. doi: 10.1038/2338. [DOI] [PubMed] [Google Scholar]
- 2.Swaminathan S, Eswaramoorthy S. Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat StructBiol. 2000;7:693–9. doi: 10.1038/78005. [DOI] [PubMed] [Google Scholar]
- 3.Schiavo G, Matteoli M, Montecucco C. Neurotoxins affecting neuroexocytosis. Physiol Rev. 2000;80:717–66. doi: 10.1152/physrev.2000.80.2.717. [DOI] [PubMed] [Google Scholar]
- 4.Montecucco C, Schiavo G. Structure and function of tetanus and botulinum neurotoxins. Q Rev Biophys. 1995;28:423–72. doi: 10.1017/S0033583500003292. [DOI] [PubMed] [Google Scholar]
- 5.Herreros J, Ng T, Schiavo G. Lipid rafts act as specialized domains for tetanus toxin binding and internalization into neurons. MolBiol Cell. 2001;12:2947–60. doi: 10.1091/mbc.12.10.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herreros J, Lalli G, Schiavo G. C-terminal half of tetanus toxin fragment C is sufficient for neuronal binding and interaction with a putative protein receptor. Biochem J. 2000;347:199–204. doi: 10.1042/0264-6021:3470199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montecucco C, Rossetto O, Schiavo G. Presynaptic receptor arrays for clostridial neurotoxins. Trends Microbiol. 2004;12:442–6. doi: 10.1016/j.tim.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Swaminathan S. Molecular structures and functional relationships in clostridial neurotoxins. FEBS J. 2011;278:4467–85. doi: 10.1111/j.1742-4658.2011.08183.x. [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson B, Whitmore E, Tiru M. Neutralization of tetanus toxin by human monoclonal antibodies directed against tetanus toxin fragment C. Hybridoma. 1993;12:699–708. doi: 10.1089/hyb.1993.12.699. [DOI] [PubMed] [Google Scholar]
- 10.Fitzsimmons SP, Clark KC, Wilkerson R, Shapiro MA. Inhibition of tetanus toxin fragment C binding to gangliosideG(T1b) by monoclonal antibodies recognizing different epitopes. Vaccine. 2000;19:114–21. doi: 10.1016/S0264-410X(00)00115-8. [DOI] [PubMed] [Google Scholar]
- 11.Schengrund CL, DasGupta BR, Ringler NJ. Binding of botulinum and tetanus neurotoxins to ganglioside GT1b and derivatives thereof. J Neurochem. 1991;57:1024–32. doi: 10.1111/j.1471-4159.1991.tb08253.x. [DOI] [PubMed] [Google Scholar]
- 12.Yousefi M, Tahmasebi F, Younesi V, Razavi A, Khoshnoodi J, Bayat AA, Abbasi E, Rabbani H, Jeddi-Tehrani M, Shokri F. Characterization of neutralizing monoclonal antibodies directed against tetanus toxin fragment C. J Immunotoxicol. 2013 doi: 10.3109/1547691X.2013.763872. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 13.Diethelm-Okita BM, Okita DK, Banaszak L, Conti-Fine BM. Universal epitopes for human CD4+ cells on tetanus and diphtheria toxins. J Infect Dis. 2000;181:1001–9. doi: 10.1086/315324. [DOI] [PubMed] [Google Scholar]
- 14.Tymciu S, Durieux-Alexandrenne C, Wijkhuisen A, Créminon C, Frobert Y, Grassi J, Couraud JY, Boquet D. Enhancement of antibody responses in DNA vaccination using a vector encoding a universal T-helper cell epitope. DNA Cell Biol. 2004;23:395–402. doi: 10.1089/104454904323145281. [DOI] [PubMed] [Google Scholar]
- 15.Holmgren J, Elwing H, Fredman P, Svennerholm L. Polystyrene-adsorbed gangliosides for investigation of the structure of the tetanus-toxin receptor. Eur J Biochem. 1980;106:371–9. doi: 10.1111/j.1432-1033.1980.tb04583.x. [DOI] [PubMed] [Google Scholar]
- 16.Emsley P, Fotinou C, Black I, Fairweather NF, Charles IG, Watts C, Hewitt E, Isaacs NW. The structures of the H(C) fragment of tetanus toxin with carbohydrate subunit complexes provide insight into ganglioside binding. J BiolChem. 2000;275:8889–94. doi: 10.1074/jbc.275.12.8889. [DOI] [PubMed] [Google Scholar]
- 17.Fotinou C, Emsley P, Black I, Ando H, Ishida H, Kiso M, Sinha KA, Fairweather NF, Isaacs NW. The crystal structure of tetanus toxin Hc fragment complexed with a synthetic GT1b analogue suggests cross-linking between ganglioside receptors and the toxin. J BiolChem. 2001;276:32274–81. doi: 10.1074/jbc.M103285200. [DOI] [PubMed] [Google Scholar]
- 18.Rummel A, Bade S, Alves J, Bigalke H, Binz T. Two carbohydrate binding sites in the H(CC)-domain of tetanus neurotoxin are required for toxicity. J MolBiol. 2003;326:835–47. doi: 10.1016/S0022-2836(02)01403-1. [DOI] [PubMed] [Google Scholar]
- 19.Herreros J, Lalli G, Montecucco C, Schiavo G. Tetanus toxin fragment C binds to a protein present in neuronal cell lines and motoneurons. J Neurochem. 2000;74:1941–50. doi: 10.1046/j.1471-4159.2000.0741941.x. [DOI] [PubMed] [Google Scholar]
- 20.Slade AL, Schoeniger JS, Sasaki DY, Yip CM. In situ scanning probe microscopy studies of tetanus toxin-membrane interactions. Biophys J. 2006;91:4565–74. doi: 10.1529/biophysj.105.080457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gigliotti F, Insel RA. Protective human hybridoma antibody to tetanus toxin. J Clin Invest. 1982;70:1306–9. doi: 10.1172/JCI110730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trabaud MA, Lery L, Desgranges C. Human monoclonal antibodies with a protective activity against tetanus toxin. APMIS. 1989;97:671–6. doi: 10.1111/j.1699-0463.1989.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 23.Kamei M, Hashizume S, Sugimoto N, Ozutsumi K, Matsuda M. Establishment of stable mouse/human-human hybrid cell lines producing large amounts of anti-tetanus human monoclonal antibodies with high neutralizing activity. Eur J Epidemiol. 1990;6:386–97. doi: 10.1007/BF00151713. [DOI] [PubMed] [Google Scholar]
- 24.Arunachalam B, Ghosh S, Talwar GP, Raghupathy R. A single human monoclonal antibody that confers total protection from tetanus. Hybridoma. 1992;11:165–79. doi: 10.1089/hyb.1992.11.165. [DOI] [PubMed] [Google Scholar]
- 25.Ahnert-Hilger G, Bizzini B, Goretzki K, Müller H, Völckers C, Habermann E. Monoclonal antibodies against tetanus toxin and toxoid. Med MicrobiolImmunol. 1983;172:123–35. doi: 10.1007/BF02124513. [DOI] [PubMed] [Google Scholar]
- 26.GreenspanNS, CooperLJ. Cooperative binding by mouse IgG3 antibodies: implications for functional affinity, effector function, and isotype restriction. Springer seminars in immunopathology: Springer, 1993:275-91. [DOI] [PubMed] [Google Scholar]
- 27.Greenspan NS. Affinity, complementarity, cooperativity, and specificity in antibody recognition. Curr Top MicrobiolImmunol. 2001;260:65–85. doi: 10.1007/978-3-662-05783-4_5. [DOI] [PubMed] [Google Scholar]
- 28.Scott N, Qazi O, Wright MJ, Fairweather NF, Deonarain MP. Characterisation of a panel of anti-tetanus toxin single-chain Fvs reveals cooperative binding. MolImmunol. 2010;47:1931–41. doi: 10.1016/j.molimm.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]