Abstract
The self-adjuvanting lipid core peptide (LCP) system offers a safe alternative vaccine delivery strategy, eliminating the need for additional adjuvants such as CpG Alum. In this study, we adopted the LCP as a scaffold for an epitope located on the surface of the cathepsin D hemoglobinase (Sm-CatD) of the human blood fluke Schistosoma mansoni. Sm-CatD plays a pivotal role in digestion of the fluke’s bloodmeal and has been shown to be efficacious as a subunit vaccine in a murine model of human schistosomiasis. Using molecular modeling we showed that S. mansoni cathepsin D possesses a predicted surface exposed α-helix (A263K) that corresponds to an immunodominant helix and target of enzyme–neutralizing antibodies against Necator americanus APR-1 (Na-APR-1), the orthologous protease and vaccine antigen from blood-feeding hookworms. The A263K epitope was engineered as two peptide variants, one of which was flanked at both termini with a coil maintaining sequence, thereby promoting the helical characteristics of the native A263K epitope. Some of the peptides were fused to a self-adjuvanting lipid core scaffold to generate LCPs. Mice were vaccinated with unadjuvanted peptides, peptides formulated with Freund’s adjuvants, or LCPs. Antibodies generated to LCPs recognized native Sm-CatD within a soluble adult schistosome extract, and almost completely abolished its enzymatic activity in vitro. Using immunohistochemistry we showed that anti-LCP antibodies bound to the native Sm-CatD protein in the esophagus and anterior regions of the gastrodermis of adult flukes. Vaccines offer an alternative control strategy in the fight against schistosomiasis, and further development of LCPs containing multiple epitopes from this and other vaccine antigens should become a research priority.
Keywords: cathepsin D, peptide antigen, vaccine delivery, lipopeptide, schistosomiasis, self-adjuvanting, conformational epitope
Introduction
An effective and safe vaccine against schistosomiasis is a public health priority. The disease, caused by numerous species of trematodes from the genus Schistosoma, affects some of the world’s poorest populations. It is estimated that more than 200 million people are affected worldwide, most of which are infections with S. mansoni and S. hematobium in sub-Saharan Africa.1 Despite the availability of inexpensive and effective chemotherapy with praziquantel, human schistosomiasis continues to plague large regions of the world’s tropics and sub-tropics. Praziquantel is effective at eliminating current infection but does not protect against reinfection. Moreover, there are concerns about the emergence of resistance to praziquantel due to reports on reduced efficacy of the drug in Egypt and Senegal,2 and generation of praziquantel resistant schistosomes under laboratory conditions.3 The development of a vaccine to reduce the disease burden caused by schistosomiasis worldwide is vital to control schistosomiasis, and should be an essential component of a toolbox aimed at eliminating this disease.4
The aspartic proteases (APR) have been extensively characterized as vaccine candidates against blood-feeding helminth parasites.5 In Schistosoma spp.. the protease is referred to as cathepsin D (CatD). Schistosomes acquire most of their nutrients via ingestion of blood and subsequent proteolytic digestion of major blood proteins such as hemoglobin.6 In S. mansoni and S. japonicum, CatD plays an integral role in the initiation of the digestion cascade within the gastrodermis of the flukes.7-9 Transcripts for CatD are present in all stages of the parasite and the enzyme is also capable of digesting immunoglobulin and serum components such as albumin.10-12 Vaccination with recombinant forms of S. japonicum CatD in the mouse model of schistosomiasis achieved significant reductions in total worm burden across numerous trials.13 RNA interference targeting the gene encoding S. mansoni CatD resulted in a lethal phenotype (growth retardation) and the reduction in the ability of the parasite to effectively digest Hb in vitro.14
While it is possible to generate recombinant Sm-CatD for small-scale vaccine testing, high-yield expression of eukaryotic proteases is problematic.15,16 Moreover, the most efficacious vaccines for helminth infections are likely to require multiple antigens or fragments thereof, and must induce high titer antibody responses that are long-lasting. Currently, most adjuvants approved for use in subunit vaccines for human use are those based on aluminum salts. Although these adjuvants are safe, they are considered weak stimulators of the immune system.17,18 The self-adjuvanting lipid core peptides (LCPs) offer an alternative to the use of aluminum and are an easier platform for large-scale production. They consist of a synthetic, non-microbial, lipo-peptide adjuvant based on lipo-amino acids attached to numerous polylysine branches. These branches provide a scaffold for conjugation of multiple peptide epitopes.19 The synthetic LCP system has been shown to be immunostimulatory by targeting dendritic cells and inducing their activation.20 The system has undergone rigorous pre-clinical assessment in murine vaccine trials for Group A Streptococcus,21 cancer22 and malaria.23 LCPs constructed with the conserved epitopes from the M protein on the surface of Streptococcus pyogenes can stimulate long lasting, high titer antibody responses that induce protective immunity in a mouse model of disease.20
Hookworms and schistosomes, while members of distinct phyla, share some aspects of their parasitic life cycles in humans, including percutaneous entry into the host, an intravascular sojourn through the lungs, and an absolute nutritional requirement for blood.24 The hookworm ortholog of Sm-CatD, Ac-APR-1, protected dogs against challenge infection with A. caninum infective larvae when administered as a recombinant protein adjuvanted with AS03.25 Purified IgG from vaccinated animals decreased the catalytic activity of the recombinant enzyme in vitro, and the antibody bound in situ to the intestines of worms recovered from vaccinated dogs, implying that vaccination induced antibodies which were ingested by feeding worms, and that these antibodies interfered with the ability of the parasite to digest blood.25 Enzyme neutralizing antibodies were targeted to A291Y, a helical loop located adjacent to the APR-1 active site cleft.16,26 We recently generated an LCP based on A291Y, the first parasitic helminth LCP of any kind, and showed that vaccination of mice with this LCP induced antibodies that reduced the enzymatic activity of Na-APR-1 in vitro.15
Herein we apply LCP technology for the first time to a human platyhelminth pathogen. LCPs corresponding to the Sm-A263K epitope derived from Sm-CatD were synthesized with or without coil promoting sequences and induced antibodies that bound to and neutralized the enzymatic activity of the native protein in schistosome extracts and fixed tissue sections. This study forms the basis for ongoing studies using LCPs containing multiple antigenic peptides to develop multi-epitope vaccines to control schistosomiasis.
Results
Sm-CatD molecular model
The three-dimensional structure of Sm-CatD was predicted based on molecular modeling using the crystal structure of human cathepsin E as a template (Fig. 1). An overlay of Sm-CatD with the template highlights the similarity between the 2-folds. Residues A263 and G264 form part of a β-strand and residues T266 to K275 are in a α-helical conformation in the predicted structure. One face of the helix is surface exposed with residues 266, 267, 270, 271, 274, and 275 having a high degree of solvent accessible surfaces, and therefore likely to be accessible to antibody binding.
Figure 1. Molecular model of Sm-CatD. (A) Superimposition of the Sm-CatD model (red ribbon with cyan catalytic Asp residues) on the template structure of human cathepsin E (blue ribbon with cyan catalytic Asp residues, PDB code 1TZS). The RMSD over the Cα-atoms of the model and the template is 0.17 Å. Residues Ala263-Lys275 are highlighted in yellow. (B) Surface representation of the cathepsin D model highlighting residues Ala263-Lys275 in yellow. Residues 266, 267, 270, 271, 274, and 275 are on the external face of the helix and have solvent accessible surfaces greater than 25%. The diagram was generated using MOLMOL and PyMOL.
Synthesis and characterization of peptides and LCPs
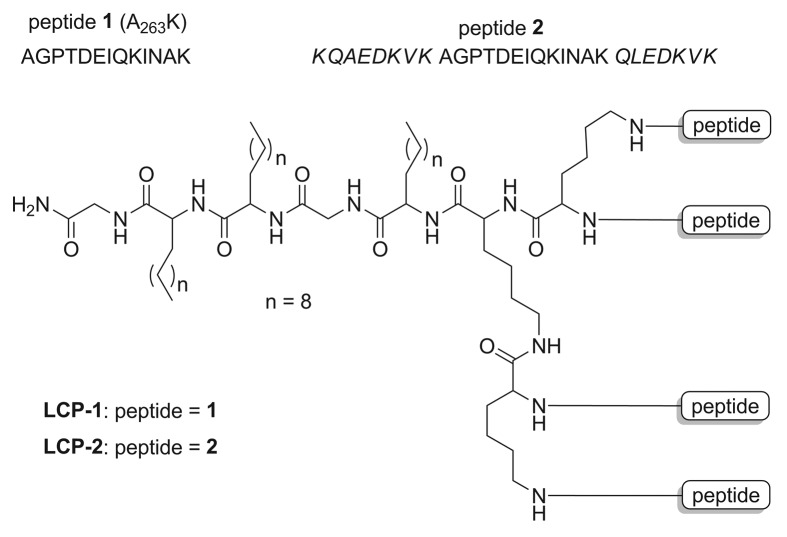
Pure peptides and tetra-branched LCP constructs (Fig. 2) were readily synthesized using the step-wise Boc-SPSS as in previous studies.15,19 The CD spectra analysis suggested that the peptides adopted a somewhat random coil rather than a helical conformation, with negative bands observed at 201 nm and 200 nm for peptides 1 and 2, respectively (Fig. 3). Spectra for LCP-1 and LCP-2 indicated that the lipophilic LCPs adopted α-helical conformations, with double negative bands at 205 nm and shallow negative at 223 nm for LCP-1, and double negative bands at 203 nm and shallow negative at 222 nm for LCP-2. Both LCPs had positive bands near 195 nm, whereas the less ordered peptides displayed strong negative bands near 195 nm.
Figure 2. Structure of A263K peptides -1 and -2 and LCP constructs -1 and -2. Peptide 1 (A263K) numbering starts at the beginning (Val-1) of the pro-domain of Sm-CatD after removal of the signal peptide. Peptide 1 is flanked by the helicity inducing sequence from the yeast protein GCN4 (italicized) to form peptide 2.
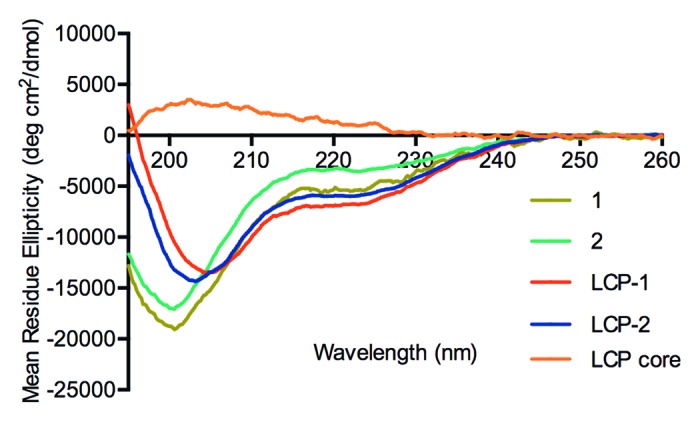
Figure 3. CD spectra of peptides and LCPs. Spectra were acquired at 1 nm intervals from 260 nm to 190 nm, were the average of 3 individual scan and are reported in units of mean residue ellipticity [θ].
Toxicological evaluation
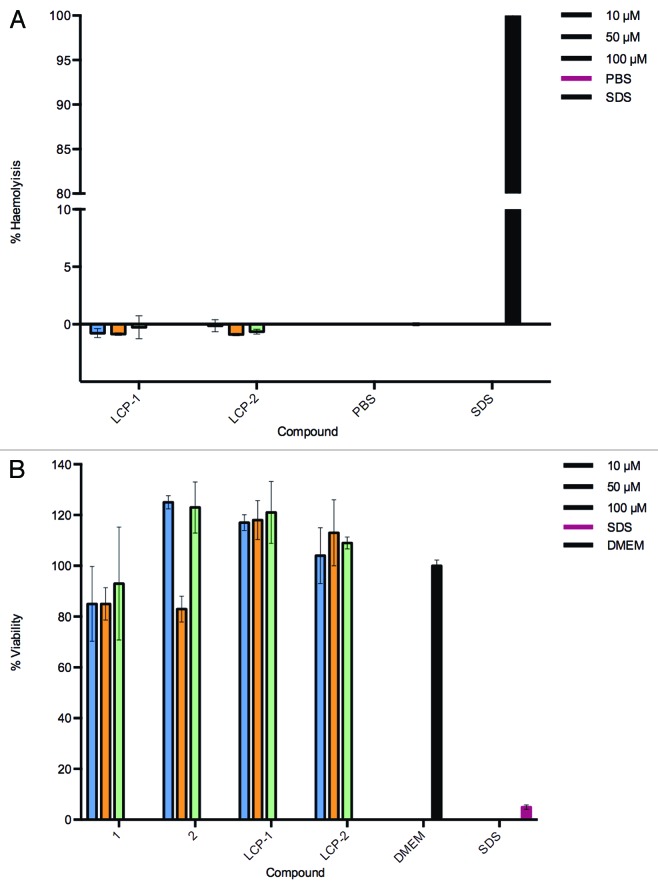
A basic toxicological evaluation of all compounds was performed using hemolysis and MTT assays. LCP-1 and LCP-2 were assessed for toxicity to red blood cells and were shown to be non-hemolytic, even at high concentrations of 100 µM (Fig. 4A). In addition, toxicity of the synthesized compounds was measured to Caco-2 cells (Fig. 4B). LCP-1 and LCP-2 did not alter the cell viability for all LCP concentrations ≤100 µM.
Figure 4. Absence of toxicity for LCP-1 and LCP-2. (A) Hemolytic potential of lipid core peptides (LCP) was measured by comparing the absorbance (540 nm) of blood samples incubated with the LCP vaccine candidates with that of samples incubated with a positive control (SDS, 100% hemolysis) and a negative control (PBS, 0%). Mean and SD of triplicate samples is shown. (B) 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide cell viability assays in triplicate were performed on the Caco-2 cell line with varying concentrations of compounds (10–100 µM).
Antibody response to immunization
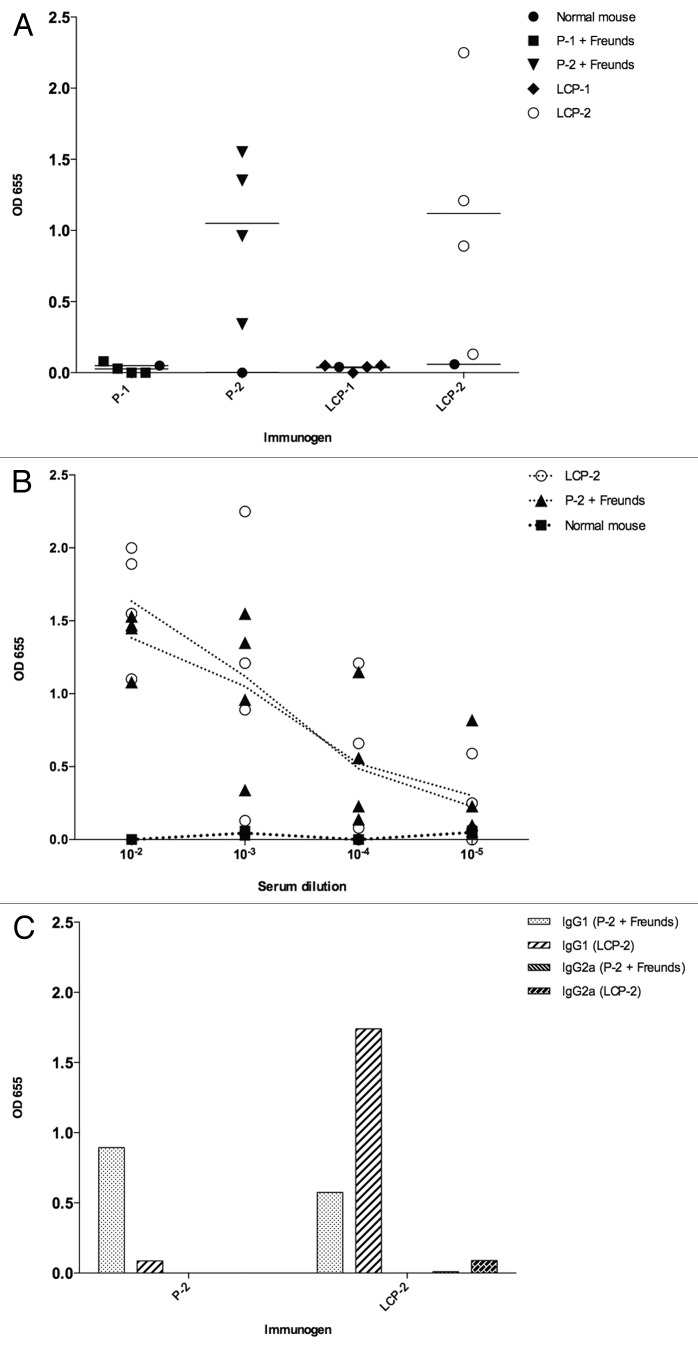
Neither Freund’s adjuvanted peptide 1 nor LCP-1 induced an IgG response in immunized mice, irrespective of the peptide/LCP coated on the microtiter plate (not shown). Both Freund’s adjuvanted peptide 2 and LCP-2 induced IgG responses in all immunized mice, although we observed substantial variation between individual mice within each group (Fig. 5A). Mice immunized with peptide 2 alone (without adjuvant) did not produce an antibody response (not shown). IgG endpoint titers of 10−5 were obtained for the lowest responding mice vaccinated with both Freund’s adjuvanted peptide 2 and LCP-2. The endpoint titer for the high responding mice was not determined (Fig. 5B). Insufficient sera were available to determine IgG subclass responses from individual mice, so we pooled the remaining sera within each group for subclass characterization. Mice immunized with Freund’s adjuvanted peptide 2 mounted a strong IgG1 response that was detected when plates were coated with peptide 2 or LCP-2 (Fig. 5C). Mice immunized with LCP-2 mounted a strong IgG1 response to LCP-2 but a much weaker response was detected when the plate was coated with peptide 2. We did not detect strong IgG2a responses to any of the immunogens.
Figure 5. Mouse antibody responses to their respective immunogens. (A) Vaccination of mice with both peptide-2 (P-2) formulated with Freund’s adjuvants and lipid core peptide 2 (LCP-2) induced IgG responses in mice. (B) Mice immunized with peptide-2+ Freund’s and LCP-2 are still binding at an antibody titer of 10−5. (C) IgG antibodies from pooled serum raised to peptide-2 recognize both peptide 2 and LCP-2. In contrast, pooled IgG1 antibodies generated to LCP-2 strongly bind to LCP-2 but only weakly recognize peptide 2. Only weak IgG2a responses were detected to LCP-2 in pooled serum raised to LCP-2. Antibody binding is presented as blank-corrected OD655 at a serum dilution of 1:1000. Panels A and B depict single groups of four mice and are representative of duplicate experiments.
Anti-LCP antibodies neutralize native cathepsin D activity in schistosome extracts
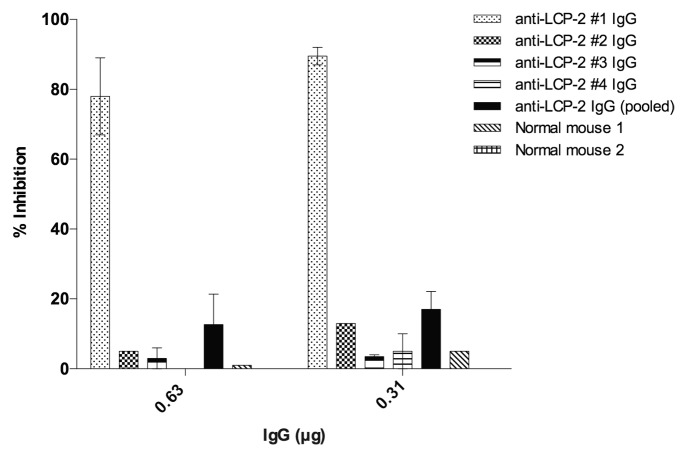
Catalytic cleavage of MoCAc-GKPILFFRLK by native Sm-CatD in schistosome extracts was inhibited by up to 90% in the presence of 0.3 μg purified anti-LCP-2 IgG from mouse #1 (Fig. 6). Reproducible knockdown of activity was also achieved with 1.25 μg of anti-LCP-2 IgG from mouse #3 (mean 25% reduction) but limited availability of purified IgG from most of the mice except for mouse #1 precluded conducting this assay with >1.25 μg of IgG (Fig. S1). Importantly, purified normal mouse serum used in this assay did not inhibit the activity of Sm-CatD at any of the concentrations tested. Due to limited availability of purified antibody, we could not conduct this assay in a reliable fashion with IgG from mice immunized with peptide 2 + CFA.
Figure 6. Anti-LCP-2 IgG neutralizes the catalytic activity of native Sm-CatD in soluble adult schistosome extracts. IgG from mouse #1 (immunized with LCP-2) strongly inhibits the enzymatic digestion of MoCAc-GKPILFFRLK by native Sm-CatD in schistosome extracts. Relative fluorescence units (RFU) are corrected to enzyme-free wells that contained substrate alone. Percent inhibition of enzymatic activity with each IgG was determined by establishing the baseline fluorescence using control IgG.
Adult worm localization
IgG derived from pooled anti-LCP-2 sera bound within the esophagus and gastrodermis of adult S. mansoni flukes (Fig. 7A). When serial sections of adult flukes were observed and photographed under identical exposure settings, intense fluorescence was observed in the gastrointestinal tract, the site of expression of Sm-CatD.7 Substantially less intense fluorescence was observed in serial sections cut from the same tissue block and treated and visualized under identical conditions but probed with negative control mouse IgG (Fig. 7B). Background fluorescence of the schistosome gastrodermis when probed with anti-mouse IgG has been reported27 and is due to the presence of residual mouse IgG derived from the parasite’s bloodmeal. Pooled serum from schistosome infected mice was used as a positive control and showed intense fluorescence of many tissues including the tegument, esophagus and gastrodermis (Fig. 7C).
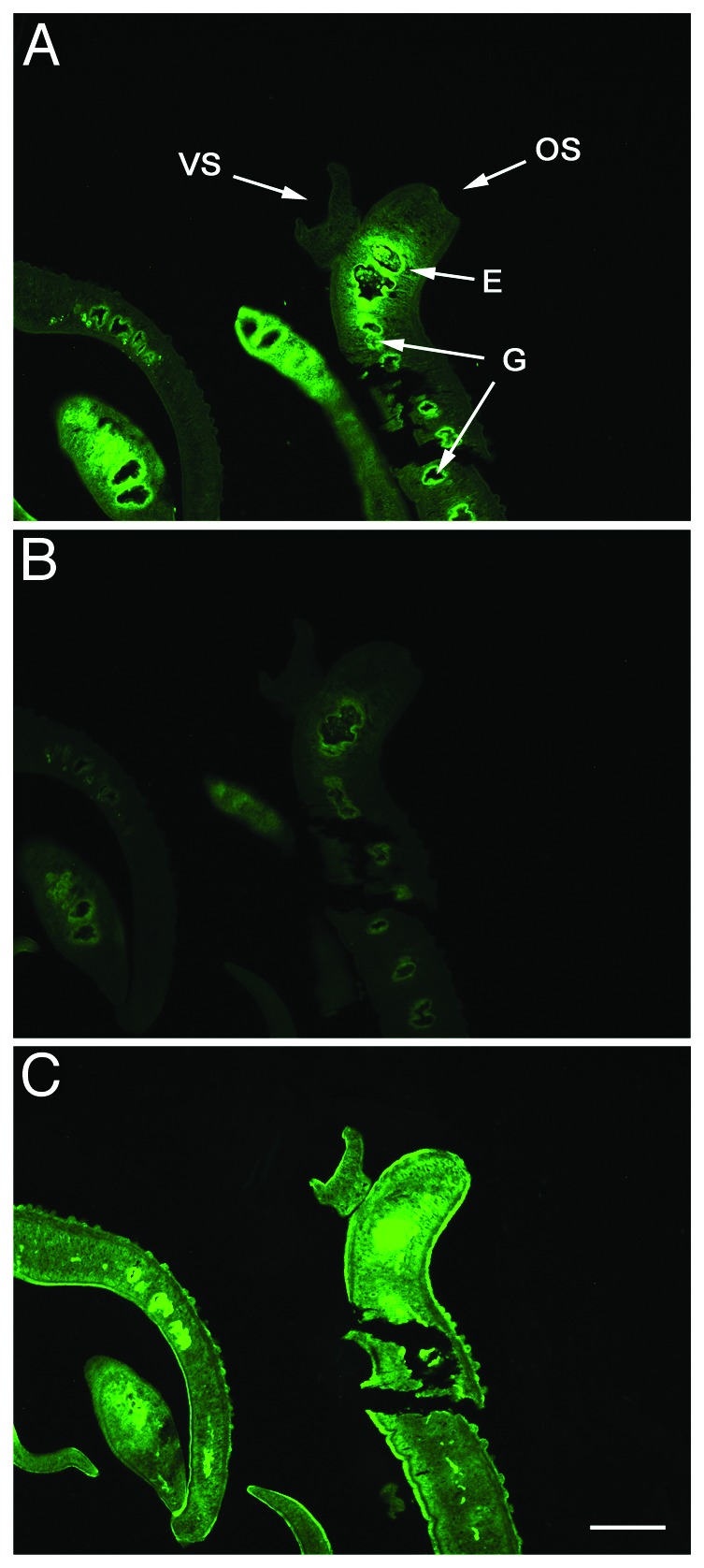
Figure 7. Anti-LCP-2 sera binds to Sm-CatD in the gastrointestinal tract of adult schistosomes. Histological sections of an S. mansoni adult worm pair probed with (A) pooled anti-LCP2 sera, (B) normal mouse serum, and (C) pooled sera from three schistosome infected mice. Arrows pointing to male, VS, ventral sucker; OS, oral sucker; E, esophagus; G, gastrodermis, 100x magnification.
Discussion
Blood-feeding helminths of diverse taxonomic origins have convergently evolved various processes to facilitate their parasitic existences. One such example is the molecular digestion of host blood proteins.5 In hookworms, APR-1 initiates hemoglobin digestion28,29 and this process can be inhibited by targeting the A291Y surface exposed helical epitope.15,26 A291Y is highly antigenic and the target of antibodies that neutralize the activity of APR-1 in vitro.26 When this epitope was fused to coil promoting flanking residues and synthesized as a LCP, it generated protease neutralizing antibodies when used to vaccinate mice.15 Herein we have shown that the orthologous epitope from S. mansoni cathepsin D, Sm-A263K, has remarkably similar characteristics and can be produced using the same LCP platform, and moreover, this construct induces enzyme-neutralizing antibodies which bind to the native protein in the schistosome alimentary canal.
Four different forms of the A263K peptide were synthesized for characterization. The peptide alone (peptide 1), the peptide conjugated at both N- and C-termini to the yeast GCN4 coil promoting sequence30 (peptide 2), and each of these two peptides linked to a lipid core to generate LCP-1 and LCP-2 respectively. CD analysis confirmed the helical nature of the two LCPs but not the corresponding unadjuvanted peptides, irrespective of the presence of GCN4 in LCP-2. We have observed a similar phenomenon with helical peptides derived from other proteins of a range of pathogens due to their conjugation to a dendritic LCP core.31-33 Such helicity induction was also observed in other dendritic systems, presumably due to dense packing of peptides in these structures.34
To assess the antigenicity of the LCPs and Freund’s adjuvanted peptides, mice were immunized and IgG responses were measured to each immunogen. The constructs without the lipid core were formulated with Fruend’s adjuvant to render them immunogenic. Indeed, even when formulated with Freund’s complete and incomplete adjuvants, only peptide 2 (containing the GCN4 sequence) induced an antibody response. Neither Freund’s adjuvanted peptide 1 nor LCP-1 induced an antibody response, indicating that the GCN4 sequence incorporated into peptide 2/LCP-2 was essential for immunogenicity, despite its inability to induce helicity (in water) in the absence of a lipid core. This result was likely due to the larger size of peptide 2 (13 residues for peptide 1 vs. 28 residues for peptide 2), and is in agreement with our findings for the corresponding A291Y helical peptide derived from hookworm APR-1.26 Thus, the GCN4 peptide might be important for inducing helicity of a peptide in a biological environment (during presentation by dendritic cells), or alternatively, GCN4 peptide serve as a linker and allows proper processing of the epitopes attached to the LCP core upon uptake by dendritic cells. Antibodies were generated by most of the mice that were immunized with LCP-2, however the antibody response was inconsistent between mice in two separate experiments (data not shown). Such variation in antibody production has been reported for Streptococcus LCPs,35,36 and was overcome by inclusion of the universal T helper cell epitope, P25.37 Future Sm-CatD constructs will incorporate T helper epitopes in an effort to boost IgG titers as well as consistency between individual animals.
As expected, antibodies produced to both peptide 2 and LCP-2 bound most strongly to their corresponding immunogens. However, pooled anti-LCP-2 IgG1 bound very weakly to peptide 2 coated on the microtiter plate. Despite the fact that both peptide 2 and LCP-2 had the same peptide sequence, CD spectra showed that LCP-2 adopted an α-helical structure whereas peptide-2 did not, accounting for the differences in IgG1 binding. IgG1 dominated the humoral response to both peptide 2 and LCP-2, indicating a T helper type 2 (Th2) response to the immunogen. A similar IgG1 response was detected when mice were immunized with an LCP-based helical peptide vaccine for Group A Streptococcus.38 Precisely what constitutes a protective immune response to human schistosomiasis is a contentious issue.2 Th2 oriented responses characterized by IgE and IL-5 are thought to drive protection in children and individuals who are treated multiple times with the drug praziquantel. On the other hand, Th1 responses characterized primarily by IFN-γ responses to larval antigens are thought to drive protection in naturally resistant individuals who have not had praziquantel therapy.39 The apparent discrepancy in resistance phenotypes likely reflects the chronic exposure of drug-treated individuals to schistosome egg antigens and subsequent Th2 biased response of these individuals compared with the Th1 oriented response of naturally resistant individuals who never seem to develop patent infections and therefore avoid the development of Th2 skewed responses. Tailoring a vaccine toward one phenotype or the other therefore depends on the target population for such a vaccine—chronically infected older individuals or helminth naïve children.
To further explore the vaccine potential of LCP-2, we assessed whether antibodies generated to LCP-2 were able to bind to the native Sm-CatD protein and neutralize its catalytic properties. By silencing the expression of the Sm-catD gene, Morales et al.14 showed that Sm-CatD is the only enzyme in the schistosome that is capable of cleaving MoCAc-GKPILFFRLK at low pH. We therefore concluded herein that anti-LCP-2 IgG mediated inhibition of proteolytic cleavage of this peptide substrate by schistosome somatic extracts provided strong evidence that these antibodies were indeed enzyme-neutralizing, and would therefore potentially interrupt digestion of the schistosome’s bloodmeal.7,9 This finding also provides further evidence that the A262K peptide adopted a native α-helical fold within the LCP-2 construct. Enzyme-neutralizing capacity was not however detected in all of the immunized mice that generated antibodies, suggesting that different mice recognized different epitopes within the 28 amino acid peptide. Future work in this area will focus on fine epitope mapping to confirm the minimal epitopes recognized by each individual mouse and link this information to the neutralizing capacity of the antibodies.
Sm-CatD is pivotal to the digestion of hemoglobin, but also cleaves IgG, complement C3 and serum albumin.11 In S. japonicum CatD has previously been localized to the cecal lumen, gastrodermis and the tegument.7,40,41 In S. mansoni however, the catD mRNA has been identified in gastrodermal tissue42 but has not been immunolocalized until now. Using anti-LCP-2 IgG we localized Sm-CatD to the esophagus and gastrodermis of adult male and female S. mansoni, the site where digestion of blood proteins is initiated. Reproducible, albeit relatively weak, fluorescence was detected in the gastrointestinal tract of flukes probed with normal mouse serum under identical microscope/camera settings, and likely represents mouse immunoglobulins bound to the enteric tube during feeding as previously described.27 Probing of the parasites with anti-LCP-2 sera resulted in a reproducibly strong fluorescence signal in the gastrodermis that was brighter than the background fluorescence observed with control sera.
We did not test the efficacy of Sm-CatD LCPs in a murine schistosomiasis challenge experiment for a number of reasons. A schistosomiasis vaccine is unlikely to be a stand-alone intervention, but rather one component of an integrated control strategy that links repeated chemotherapy with vaccination in endemic areas, a scenario that is difficult to reproduce in mouse models of infection. Moreover, we believe that a schistosomiasis subunit vaccine based on recombinant proteins or peptides is likely to require at least two antigens to achieve sufficient efficacy.2 Therefore, an efficacious LCP-based vaccine would likely require epitopes from multiple proteins.21 We suspect that protection in a mouse model with Sm-CatD LCP-2 alone would be modest at best, and propose that protective epitopes from multiple antigens incorporating the P25 T helper epitope be identified for incorporation into a multi-epitope LCP. One of the leading vaccine candidate antigens for human schistosomiasis is the tegument membrane protein Sm-TSP-2.4,43 We recently showed that vaccination of mice with a chimera of Sm-TSP-2 fused to a fragment of the hookworm cathepsin D protease (Na-APR-1) that contains the Na-A291Y peptide (corresponding peptide to Sm-A263K described herein) resulted in greater protection against S. mansoni challenge than that obtained with Sm-TSP-2 alone.44 Further studies should therefore incorporate Sm-A263K into a P25 bearing multi-epitope LCP-based vaccine with protective epitopes from other lead vaccine antigens including Sm-TSP-2, Smp80-calpain45 and Sh-GST28.46
Materials and Methods
Molecular model of Sm-CatD
The three-dimensional structure of Sm-CatD was predicted using the ModWeb interface, which uses Modeler for model building.47 Sixty models were calculated and three models selected for analysis. The model with the highest sequence identity (51%) used a crystal structure of an activation intermediate of human cathepsin E (PDB code 1TZS) as a template.
Materials
Protected L-amino acids were from Novabiochem and Mimotopes. 4-Methylbenzhydrylamine hydrochloride salt (pMBHA) resin was from Peptides International Inc. 1-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) was from Mimotopes. HPLC grade acetonitrile and N,N-dimethylformamide (DMF) were from Ajax Finechem. Trifluoroacetic acid (TFA) was from Merck. All other reagents were purchased from Sigma-Aldrich. Microwave assisted fluorenylmethyloxycarbonyl-solid phase peptide synthesis (FmocSPPS) was performed using SPS mode on a Discovery reactor (CME Corporation). An AKel-F HF apparatus (Peptide Institute) was used for HF cleavage. Electrospray Ionization Mass Spectrometry was performed on a Perkin-Elmer-Sciex API3000 instrument with Analyst 1.4 software (Applied Biosystems/ MDS Sciex). Analytical RP-HPLC was performed on an Agilent instrument with a 1.0 mL/min flow rate and detection at 214 nm. Separation was achieved by running a gradient mode of 0–100% solvent B over 40 min with solvent A (0.1% TFA/H2O); 2 different solvents were used as solvent B: B1—90% MeCN/0.1% TFA/H2O; B2—90% MeOH/0.1% TFA/H2O. Purification was performed by preparative RP-HPLC using a Waters Delta 600 system with a 10.0 mL/min flow rate. Compounds were detected at 230 nm. Separations were performed with solvent A and solvent B1 on either a Vydac preparative C4 column (214TP1022; 10 mm, 22 × 250 mm) or a Vydac preparative C18 column (218TP1022; 10 mm, 22 × 250 mm). CD spectra were measured on a JASCO J-710 spectropolarimeter using a quartz cuvette of 1 mm path length at 23 °C. The circular dichroism spectra were measured in water with 10% v/v trifluoroethanol (TFE).
Synthesis of peptide epitopes and LCPs
Peptides 1 (AGPTDEIQKINAK) and 2 (KQAEDKVK-AGPTDEIQKINAK- QLEDKVK) (Fig. 2) were synthesized using manual Boc-SPPS at 0.1 mM scale with pMBHA (0.45 mM NH2/g) resin. The synthesis was performed in a similar manner to that previously reported.15,19,48 Briefly, Boc-deprotection was performed with neat TFA (2 × 1 min), followed by a 1 min DMF flow wash. Boc-protected amino acids (4.2 equiv.) were pre-activated with 0.5 M HBTU/DMF solution (4.0 equiv.), and N,N-Diisopropylethylamine (DIPEA) (6.2 equiv.). Then, coupling with pre-activated amino acids was performed for 30 min, except for lipoamino acids (2-[R/S]-[(tert-butoxycarbonyl)amino]-dodecanoic acid) which were allowed to react for one hour.32 Double coupling was performed for all amino acids. The peptides and LCP constructs were cleaved from the resin using HF.49
Peptide 1
(AGPTDEIQKINAK). Yield: 56%. Molecular Weight: 1425.5.
[M+2H+]2+ m/z 713.6 (calc. 713.8), [M+3H+]3+ m/z 476.0 (calc. 476.2). tR = 15.00 (0–100% Solvent B1, 30 min; C18).
Peptide 2
(KQAEDKVK AGPTDEIQKINAK QLEDKVK). Yield: 33%. Molecular Weight: 3151.6. [M+3H+]3+ m/z 1051.7 (calc. 1051.5), [M+4H+]4+ m/z 788.8 (calc. 788.9). tR = 13.96 (0–100% Solvent B1, 30 min; C18), tR = 21.77 (0–100% Solvent B2, 30 min; C18).
LCP-1
Yield: 16%. Molecular Weight: 6741.8.
[M+5H+]5+ m/z 1349.7 (calc. 1349.4), [M+6H+]6+ m/z 1124.8 (calc. 1124.6). tR = 20.87, 21.17, 21.31 (0–100% Solvent B1, 30 min; C4), tR = 30.48 (0–100% Solvent B2, 30 min; C4).
LCP-2
Yield: 24%. Molecular Weight: 13813.9.
[M+11H+]11+ m/z 1257.0 (calc. 1256.81), [M+12H+]12+ m/z 1151.9 (calc. 1152.16). tR = 18.58 (0–100% Solvent B1, 30 min; C4), tR = 28.23 (0–100% Solvent B2, 30 min; C4).
Hemolysis Assay
Using a standard hemolysis assay, the capacity of the LCP compounds to induce hemolysis was examined. Whole Blood was collected from a healthy human volunteer with written informed consent (protocol approved by the University of Queensland Ethics Committee, approval number 2009000661). The toxicity of the LCPs on cells was tested at 10, 50, and 100 µM at 37 °C for one hour. SDS was used as the positive control for hemolyisis and PBS as negative control. After one hour, the plate was centrifuged at 750 g for 15 min and 75 µL of supernatant per well was transferred to a new 96 well plate. The absorbance at 540 nm was recorded by UV spectrometer. The data was calculated according to a standard formula:
% Hemolysis = ([A540-minA540]/[maxA540-minA540]) × 100%
where: A540 is the average absorption of the compound at 540 nm; minA540 is the average absorption of PBS; maxA540 is the average absorption of SDS
MTT Assay
The toxicity of the compounds was examined by methylthiazol tetrazolium (MTT) assay (Fig. 2). Caco-2 cells were cultured in a flask with Dulbecco's Modified Eagle Medium (DMEM) at 37 °C to reach 80% confluence. The cells were split into a 96 well plate (100 µL of cells per well) prior to the MTT test to let the cells adhere to the surface. The compounds were prepared in three concentrations of 10, 50, and 100 µM in PBS. The culture medium was removed and 100 µL of compound in solution was added to each well. After 24 h of incubation at 37° C, the solutions were removed and 20 µL of MTT followed by 80 µL of DMEM medium was added to each well. The plates were incubated for 4 h, centrifuged at 750 g for 5 min, and the supernatant discarded. DMSO (50 μl) was added to each well to dissolve the MTT crystals. UV absorbance readings were taken at 570 nm. PBS was used as a blank and SDS as a negative control. The toxicity was calculated according to a standard formula:
% Viability = ([A540]/[maxA540]) × 100%
where: A540 is the average absorption of compound at 540 nm; maxA540 is the average absorption of DMEM (positive control)
The experiment was performed in triplicate.
Intraperitoneal immunization of mice with constructs
Two groups of four female BALB/c mice (4–6 weeks old) were intraperitoneally injected with 30 µg of either peptide 1 + Freund’s adjuvants (Sigma-Aldrich), peptide 2 alone, peptide 2 + Freund’s adjuvants, LCP-1 or LCP-2 in 200 µL of PBS. For peptides 1 and 2 formulated with the Freund’s adjuvants, 100 µL (30 µg) of peptide was mixed with an equal volume of complete Freund’s adjuvant (CFA) for the first immunization and incomplete Freund’s adjuvant (IFA) for the subsequent immunizations. Mice were injected 4 times on days 0, 21, 33, and 43. Mice were euthanized on day 70 and the blood was collected via cardiac puncture. Sera were separated from clotted blood by centrifugation at 1500 g for 10 min. All animal protocols used were approved by the James Cook University Ethics Committee (A1484) in accordance with guidelines established by the National Health and Medical Research Council of Australia.
ELISA
Antibodies generated to the LCP constructs or peptides were measured using indirect ELISA as previously described.15 IgG subclasses were detected using goat anti-mouse IgG1 or goat anti-mouse IgG2a horsereadish peroxidase (Novex). Absorbance was measured at 655 nm. All samples were measured in duplicate for which the average was taken subtracting the background. The background was calculated as the average of all wells receiving sera (1:100) in the absence of the peptide or LCP bound to the plate.
Cathepsin D neutralization assay
IgG from mice immunised with LCP-2 was purified using either protein G sepharose (Millipore) or Dynabeads® Protein G (Novex) and eluted as previously described.16,26 After purification, IgG was concentrated and buffer exchanged into PBS using Nanosep centrifugal devices (Pall) as per the manufacturer’s protocol. Soluble worm antigen preparation (SWAP) was prepared by homogenizing frozen S. mansoni in ice-cold phosphate buffered saline using a QIAGEN TissueLyser at 25 Hz for 10 min. The homogenate was then centrifuged for 15 min at 1500 g. The supernatant was removed and further centrifuged at 14 000 g for 10 min. The supernatant was then collected and filtered through a 0.45 μm syringe filter. Protein concentration was determined using the Pierce BCA protein assay kit (Thermo Scientific). SWAP (1.75 μg) was incubated with 1.25, 0.63, 0.31, and 0.16 μg of purified IgG in 50 mM sodium acetate. Reactions were performed in black 384 well plates (Greiner Bio One) in 50 µl; the substrate 7-methoxycoumarin-4-acetyl-GKPILFFRLK(DNP)-d-Arg-amide (MoCAc-GKPILFFRLK) (Sigma-Aldrich) was added to a final concentration of 1.8 mM, and the fluorescence generated by substrate hydrolysis was measured as described16 using a POLARstar Omega microplate reader (BMG Labtech). The baseline for raw fluorescence was set to blank against a negative control sample containing SWAP, MoCAc-GKPILFFRLK and 0.4 mM pepstatin A (Sigma-Aldrich). Inhibition of enzymatic activity was analyzed as a percentage reduction of the fluorescence generated from an equivalent reaction containing equal amounts of control IgG (Millipore) at 1.25, 0.63, 0.31, and 0.16 μg.
Immunohistochemistry
Adult S. mansoni worm pairs recovered from infected C57BL/6 mice were washed in perfusion buffer (25 mM C6H9Na3O9, 150 mM NaCl) and fixed in 10% formalin. Fixed worm pairs were embedded in paraffin wax and cut on a rotary microtome at 4 μm thickness (Micron). Slides were dewaxed by rinsing three times in a xylene bath and rehydrated through an ethanol series, twice in 100%, 90%, 80%, 70% ethanol for 3 min and finally 10 mM sodium citrate buffer three times for 5 min. Immunofluorescence was performed by blocking the slides in 5% goat serum in 0.1 M phosphate buffer for 1 h at room temperature. Primary antibody (pooled LCP-2 sera) was applied at dilution of 1:10 overnight at 4 °C. Slides were then rinsed twice with 0.1 M phosphate buffer for 5 min followed by incubation with a 1:50 dilution of fluorescein-conjugated goat anti-mouse IgG (H+L) (Jackson ImmunoResearch Laboratories) for 1 h at room temperature. Slides were washed again and counterstained with DAPI NucBlue™ FIXED cell stain (Molecular Probes). Slides were prepared in Gel Mount™ Aqueous Mounting Medium (Sigma-Aldrich). Fluorescence images were taken using the Axio Imager.M1 and associated Zen 2012 imaging software (Zeiss).
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- LCP
lipid core peptide
- Sm-CatD
Schistosoma mansoni cathepsin D
- APR1
Aspartic protease I
- Na-APR-1
Necator americanus APR-1
- Hb
hemoglobin
- A263K
AGPTDEIQKINAK
- A291Y
AGPKAQVEAIQKY
- SWAP
soluble worm antigen preparation
References
- 1.Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412. doi: 10.1371/journal.pntd.0000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21:225–42. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fallon PG, Doenhoff MJ. Drug-resistant schistosomiasis: resistance to praziquantel and oxamniquine induced in Schistosoma mansoni in mice is drug specific. Am J Trop Med Hyg. 1994;51:83–8. doi: 10.4269/ajtmh.1994.51.83. [DOI] [PubMed] [Google Scholar]
- 4.Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol. 2010;8:814–26. doi: 10.1038/nrmicro2438. [DOI] [PubMed] [Google Scholar]
- 5.Pearson MS, Ranjit N, Loukas A. Blunting the knife: development of vaccines targeting digestive proteases of blood-feeding helminth parasites. Biol Chem. 2010;391:901–11. doi: 10.1515/bc.2010.074. [DOI] [PubMed] [Google Scholar]
- 6.Brindley PJ, Kalinna BH, Dalton JP, Day SR, Wong JYM, Smythe ML, McManus DP. Proteolytic degradation of host hemoglobin by schistosomes. Mol Biochem Parasitol. 1997;89:1–9. doi: 10.1016/S0166-6851(97)00098-4. [DOI] [PubMed] [Google Scholar]
- 7.Brindley PJ, Kalinna BH, Wong JYM, Bogitsh BJ, King LT, Smyth DJ, Verity CK, Abbenante G, Brinkworth RI, Fairlie DP, et al. Proteolysis of human hemoglobin by schistosome cathepsin D. Mol Biochem Parasitol. 2001;112:103–12. doi: 10.1016/S0166-6851(00)00351-0. [DOI] [PubMed] [Google Scholar]
- 8.Becker MM, Harrop SA, Dalton JP, Kalinna BH, McManus DP, Brindley PJ. Cloning and characterization of the Schistosoma japonicum aspartic proteinase involved in hemoglobin degradation. J Biol Chem. 1995;270:24496–501. doi: 10.1074/jbc.270.41.24496. [DOI] [PubMed] [Google Scholar]
- 9.Delcroix M, Sajid M, Caffrey CR, Lim KC, Dvorák J, Hsieh I, Bahgat M, Dissous C, McKerrow JH. A multienzyme network functions in intestinal protein digestion by a platyhelminth parasite. J Biol Chem. 2006;281:39316–29. doi: 10.1074/jbc.M607128200. [DOI] [PubMed] [Google Scholar]
- 10.Morales ME, Kalinna BH, Heyers O, Mann VH, Schulmeister A, Copeland CS, Loukas A, Brindley PJ. Genomic organization of the Schistosoma mansoni aspartic protease gene, a platyhelminth orthologue of mammalian lysosomal cathepsin D. Gene. 2004;338:99–109. doi: 10.1016/j.gene.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Verity CK, Loukas A, McManus DP, Brindley PJ. Schistosoma japonicum cathepsin D aspartic protease cleaves human IgG and other serum components. Parasitology. 2001;122:415–21. doi: 10.1017/S0031182001007521. [DOI] [PubMed] [Google Scholar]
- 12.Verity CK, McManus DP, Brindley PJ. Developmental expression of cathepsin D aspartic protease in Schistosoma japonicum. Int J Parasitol. 1999;29:1819–24. doi: 10.1016/S0020-7519(99)00126-5. [DOI] [PubMed] [Google Scholar]
- 13.Verity CK, McManus DP, Brindley PJ. Vaccine efficacy of recombinant cathepsin D aspartic protease from Schistosoma japonicum. Parasite Immunol. 2001;23:153–62. doi: 10.1046/j.1365-3024.2001.00369.x. [DOI] [PubMed] [Google Scholar]
- 14.Morales ME, Rinaldi G, Gobert GN, Kines KJ, Tort JF, Brindley PJ. RNA interference of Schistosoma mansoni cathepsin D, the apical enzyme of the hemoglobin proteolysis cascade. Mol Biochem Parasitol. 2008;157:160–8. doi: 10.1016/j.molbiopara.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skwarczynski M, Dougall AM, Khoshnejad M, Chandrudu S, Pearson MS, Loukas A, Toth I. Peptide-based subunit vaccine against hookworm infection. PLoS One. 2012;7:e46870. doi: 10.1371/journal.pone.0046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson MS, Bethony JM, Pickering DA, de Oliveira LM, Jariwala A, Santiago H, Miles AP, Zhan B, Jiang D, Ranjit N, et al. An enzymatically inactivated hemoglobinase from Necator americanus induces neutralizing antibodies against multiple hookworm species and protects dogs against heterologous hookworm infection. FASEB J. 2009;23:3007–19. doi: 10.1096/fj.09-131433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schijns VEJC, Lavelle EC. Trends in vaccine adjuvants. Expert Rev Vaccines. 2011;10:539–50. doi: 10.1586/erv.11.21. [DOI] [PubMed] [Google Scholar]
- 18.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–96. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 19.Skwarczynski M, Toth I. Lipid-Core-Peptide System for Self-Adjuvanting Synthetic Vaccine Delivery. In: Mark SS, ed. Bioconjugation Protocols: Humana Press, 2011:297-308. [DOI] [PubMed] [Google Scholar]
- 20.Olive C, Moyle PM, Toth I. Towards the development of a broadly protective group a streptococcal vaccine based on the Lipid-Core Peptide system. Curr Med Chem. 2007;14:2976–88. doi: 10.2174/092986707782794069. [DOI] [PubMed] [Google Scholar]
- 21.Zhong W, Skwarczynski M, Toth I. Lipid Core Peptide System for Gene, Drug, and Vaccine Delivery. Aust J Chem. 2009;62:956–67. doi: 10.1071/CH09149. [DOI] [Google Scholar]
- 22.Moyle PM, Olive C, Ho M-F, Pandey M, Dyer J, Suhrbier A, Fujita Y, Toth I. Toward the development of prophylactic and therapeutic human papillomavirus type-16 lipopeptide vaccines. J Med Chem. 2007;50:4721–7. doi: 10.1021/jm070287b. [DOI] [PubMed] [Google Scholar]
- 23.Apte SH, Groves PL, Skwarczynski M, Fujita Y, Chang C, Toth I, Doolan DL. Vaccination with lipid core peptides fails to induce epitope-specific T cell responses but confers non-specific protective immunity in a malaria model. PLoS One. 2012;7:e40928. doi: 10.1371/journal.pone.0040928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotez PJ, Bethony JM, Oliveira SC, Brindley PJ, Loukas A. Multivalent anthelminthic vaccine to prevent hookworm and schistosomiasis. Expert Rev Vaccines. 2008;7:745–52. doi: 10.1586/14760584.7.6.745. [DOI] [PubMed] [Google Scholar]
- 25.Loukas A, Bethony JM, Mendez S, Fujiwara RT, Goud GN, Ranjit N, Zhan B, Jones K, Bottazzi ME, Hotez PJ. Vaccination with recombinant aspartic hemoglobinase reduces parasite load and blood loss after hookworm infection in dogs. PLoS Med. 2005;2:e295. doi: 10.1371/journal.pmed.0020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson MS, Pickering DA, Tribolet L, Cooper L, Mulvenna J, Oliveira LM, Bethony JM, Hotez PJ, Loukas A. Neutralizing antibodies to the hookworm hemoglobinase Na-APR-1: implications for a multivalent vaccine against hookworm infection and schistosomiasis. J Infect Dis. 2010;201:1561–9. doi: 10.1086/651953. [DOI] [PubMed] [Google Scholar]
- 27.Thors C, Jokiranta TS, Meri T, Kairemo K, Meri S, Linder E. Immunoglobulin uptake and processing by Schistosoma mansoni. Parasite Immunol. 2006;28:421–8. doi: 10.1111/j.1365-3024.2006.00839.x. [DOI] [PubMed] [Google Scholar]
- 28.Ranjit N, Zhan B, Hamilton B, Stenzel D, Lowther J, Pearson M, Gorman J, Hotez P, Loukas A. Proteolytic degradation of hemoglobin in the intestine of the human hookworm Necator americanus. J Infect Dis. 2009;199:904–12. doi: 10.1086/597048. [DOI] [PubMed] [Google Scholar]
- 29.Williamson AL, Brindley PJ, Abbenante G, Prociv P, Berry C, Girdwood K, Pritchard DI, Fairlie DP, Hotez PJ, Dalton JP, et al. Cleavage of hemoglobin by hookworm cathepsin D aspartic proteases and its potential contribution to host specificity. FASEB J. 2002;16:1458–60. doi: 10.1096/fj.02-0181fje. [DOI] [PubMed] [Google Scholar]
- 30.Hollenbeck JJ, McClain DL, Oakley MG. The role of helix stabilizing residues in GCN4 basic region folding and DNA binding. Protein Sci. 2002;11:2740–7. doi: 10.1110/ps.0211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skwarczynski M, Kamaruzaman KA, Srinivasan S, Zaman M, Lin IC, Batzloff MR, Good MF, Toth I. M-protein-derived conformational peptide epitope vaccine candidate against Group A Streptococcus. Curr Drug Deliv. 2013;10:39–45. doi: 10.2174/1567201811310010007. [DOI] [PubMed] [Google Scholar]
- 32.Skwarczynski M, Dougall AM, Khoshnejad M, Chandrudu S, Pearson MS, Loukas A, Toth I. Peptide-based subunit vaccine against hookworm infection. PLoS One. 2012;7:e46870. doi: 10.1371/journal.pone.0046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skwarczynski M, Ahmad Fuaad AAH, Rustanti L, Ziora ZM, Aqil M, Batzloff MR, Good MF, Toth I, Group A. Streptococcal Vaccine Candidates based on the Conserved Conformational Epitope from M Protein. Drug Deliv Lett. 2011;1:2–8. [Google Scholar]
- 34.Skwarczynski M, Zaman M, Urbani CN, Lin IC, Jia Z, Batzloff MR, Good MF, Monteiro MJ, Toth I. Polyacrylate dendrimer nanoparticles: a self-adjuvanting vaccine delivery system. Angew Chem Int Ed Engl. 2010;49:5742–5. doi: 10.1002/anie.201002221. [DOI] [PubMed] [Google Scholar]
- 35.Hayman WA, Toth I, Flinn N, Scanlon M, Good MF. Enhancing the immunogenicity and modulating the fine epitope recognition of antisera to a helical group A streptococcal peptide vaccine candidate from the M protein using lipid-core peptide technology. Immunol Cell Biol. 2002;80:178–87. doi: 10.1046/j.1440-1711.2002.01067.x. [DOI] [PubMed] [Google Scholar]
- 36.Olive C, Batzloff M, Horváth A, Clair T, Yarwood P, Toth I, Good MF. Potential of lipid core peptide technology as a novel self-adjuvanting vaccine delivery system for multiple different synthetic peptide immunogens. Infect Immun. 2003;71:2373–83. doi: 10.1128/IAI.71.5.2373-2383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdel-Aal AB, Batzloff MR, Fujita Y, Barozzi N, Faria A, Simerska P, Moyle PM, Good MF, Toth I. Structure-activity relationship of a series of synthetic lipopeptide self-adjuvanting group a streptococcal vaccine candidates. J Med Chem. 2008;51:167–72. doi: 10.1021/jm701091d. [DOI] [PubMed] [Google Scholar]
- 38.Skwarczynski M, Zaman M, Urbani CN, Lin IC, Jia Z, Batzloff MR, Good MF, Monteiro MJ, Toth I. Polyacrylate dendrimer nanoparticles: a self-adjuvanting vaccine delivery system. Angew Chem Int Ed Engl. 2010;49:5742–5. doi: 10.1002/anie.201002221. [DOI] [PubMed] [Google Scholar]
- 39.Corrêa-Oliveira R, Caldas IR, Gazzinelli G. Natural versus drug-induced resistance in Schistosoma mansoni infection. Parasitol Today. 2000;16:397–9. doi: 10.1016/S0169-4758(00)01740-3. [DOI] [PubMed] [Google Scholar]
- 40.Bogitsh BJ, Kirschner KF. Schistosoma japonicum: immunocytochemistry of adults using heterologous antiserum to bovine cathepsin D. Exp Parasitol. 1987;64:213–8. doi: 10.1016/0014-4894(87)90145-7. [DOI] [PubMed] [Google Scholar]
- 41.Bogitsh BJ, Kirschner KF. Schistosoma japonicum: ultrastructural localization of a hemoglobinase using mercury labeled pepstatin. Exp Parasitol. 1986;62:211–5. doi: 10.1016/0014-4894(86)90025-1. [DOI] [PubMed] [Google Scholar]
- 42.Nawaratna SSK, McManus DP, Moertel L, Gobert GN, Jones MK. Gene Atlasing of digestive and reproductive tissues in Schistosoma mansoni. PLoS Negl Trop Dis. 2011;5:e1043. doi: 10.1371/journal.pntd.0001043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, Don TA, McManus DP, Correa-Oliveira R, Loukas A. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med. 2006;12:835–40. doi: 10.1038/nm1430. [DOI] [PubMed] [Google Scholar]
- 44.Pearson MS, Pickering DA, McSorley HJ, Bethony JM, Tribolet L, Dougall AM, Hotez PJ, Loukas A. Enhanced protective efficacy of a chimeric form of the schistosomiasis vaccine antigen Sm-TSP-2. PLoS Negl Trop Dis. 2012;6:e1564. doi: 10.1371/journal.pntd.0001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmad G, Zhang W, Torben W, Ahrorov A, Damian RT, Wolf RF, White GL, Carey DW, Mwinzi PN, Ganley-Leal L, et al. Preclinical prophylactic efficacy testing of Sm-p80-based vaccine in a nonhuman primate model of Schistosoma mansoni infection and immunoglobulin G and E responses to Sm-p80 in human serum samples from an area where schistosomiasis is endemic. J Infect Dis. 2011;204:1437–49. doi: 10.1093/infdis/jir545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riveau G, Deplanque D, Remoué F, Schacht A-M, Vodougnon H, Capron M, Thiry M, Martial J, Libersa C, Capron A. Safety and immunogenicity of rSh28GST antigen in humans: phase 1 randomized clinical study of a vaccine candidate against urinary schistosomiasis. PLoS Negl Trop Dis. 2012;6:e1704. doi: 10.1371/journal.pntd.0001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pieper U, Webb BM, Barkan DT, Schneidman-Duhovny D, Schlessinger A, Braberg H, Yang Z, Meng EC, Pettersen EF, Huang CC, et al. ModBase, a database of annotated comparative protein structure models, and associated resources. Nucleic Acids Res. 2011;39:D465–74. doi: 10.1093/nar/gkq1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Skwarczynski M, Parhiz BH, Soltani F, Srinivasan S, Kamaruzaman KA, Lin I-C, Toth I. Lipid Peptide Core Nanoparticles as Multivalent Vaccine Candidates against Streptococcus pyogenes. Aust J Chem. 2012;65:35–9. doi: 10.1071/CH11292. [DOI] [Google Scholar]
- 49.Skwarczynski M, Toth I. Lipid-Core-Peptide System for Self-Adjuvanting Synthetic Vaccine Delivery. In: Mark SS, ed. Bioconjugation Protocols: Strategies and Methods, Second Edition, 2011:297-308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.