Abstract
Background: Infants are at the highest risk for meningococcal disease and a broadly protective and safe vaccine is an unmet need in this youngest population. We evaluated the immunogenicity and safety of a 4-dose infant/toddler regimen of MenACWY-CRM given at 2, 4, 6, and 12 months of age concomitantly with pentavalent diphtheria-tetanus-acellular pertussis-Hemophilus influenzae type b-inactivated poliovirus-combination vaccine (DTaP-IPV/Hib), hepatitis B vaccine (HBV), 7- or 13-valent conjugate pneumococcal vaccine (PCV), and measles, mumps, and rubella vaccine (MMR).
Results: Four doses of MenACWY-CRM induced hSBA titers ≥8 in 89%, 95%, 97%, and 96% of participants against serogroups A, C, W-135, and Y, respectively. hSBA titers ≥8 were present in 76–98% of participants after the first 3 doses. A categorical linear analysis incorporating vaccine group and study center showed responses to routine vaccines administered with MenACWY-CRM were non-inferior to routine vaccines alone, except for seroresponse to the pertussis antigen fimbriae. The reactogenicity profile was not affected when MenACWY-CRM was administered concomitantly with routine vaccines.
Conclusion: MenACWY-CRM administered with routine concomitant vaccinations in young infants was well tolerated and induced highly immunogenic responses against each of the serogroups without significant interference with the immune responses to routine infant vaccinations.
Methods: Healthy 2 month old infants were randomized to receive MenACWY-CRM with routine vaccines (n = 258) or routine vaccines alone (n = 271). Immunogenicity was assessed by serum bactericidal assay using human complement (hSBA). Medically attended adverse events (AEs), serious AEs (SAEs) and AEs leading to study withdrawal were collected throughout the study period.
Keywords: meningococcal disease, conjugate vaccine, immunogenicity, safety, infants
Introduction
Invasive meningococcal disease remains one of the most devastating bacterial infections worldwide and can cause serious disability or death in an otherwise healthy person in a matter of hours.1,2 Annually, an estimated 500 000 cases of invasive meningococcal disease occur worldwide, causing 50 000 deaths.3 The vast majority of invasive meningococcal disease (>90%) can be attributed to 1 of 5 immunologically distinct serogroups: A, B, C, W-135, and Y.4,5 A quadrivalent meningococcal vaccine against serogroups A, C, W-135, and Y offers a broad protection against Neisseria meningitidis infection.
The distribution of serogroups varies geographically, with serogroups B and C predominating in Europe, Australia, and New Zealand, serogroups A, C, and W-135 most common in Asia and Africa and serogroups B, C, and Y predominating in the Americas.6 Infants in their first year of life are at the highest risk for meningococcal disease, with peak incidence occurring in the first 6 mo.7,8 While serogroup C meningococcal conjugate vaccines have had a profound effect where widely employed,9,10 a safe and effective vaccine that broadly protects infants from 2 mo of age against meningococcal disease is an unmet need.11
MenACWY-CRM (Menveo®, Novartis Vaccines and Diagnostics) is a quadrivalent (A, C, W-135, and Y) meningococcal vaccine conjugated to CRM197, a nontoxic mutant of diphtheria toxin, as the carrier protein.12 Previous phase 2 and 3 studies demonstrated that MenACWY-CRM elicited highly immunogenic responses and was well-tolerated in adults,13-15 adolescents,13,16,17 children,18,19 and importantly, young infants.20-23
Adding novel vaccines to infant vaccination schedules generally necessitates concomitant administration and requires evidence that vaccines can be given simultaneously without negatively impacting the safety and immunogenicity of either of the vaccines. It was recently demonstrated that MenACWY-CRM induced safe, robust immune responses in infants and toddlers without concern for clinically relevant interference with routine vaccines (pentavalent diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus combination vaccine [DTaP-HBV-IPV; Pediarix®, GlaxoSmithKline], Hemophilus influenzae type b-tetanus conjugate vaccine [PRP-T; ActHib®, Sanofi Pasteur], 7-valent pneumococcal conjugate vaccine [PCV7; Prevnar®, Pfizer],20 and measles, mumps, rubella, and varicella vaccine [MMRV; ProQuad®, Merck and Co]).21
Here we present the results of a phase 3 study conducted in healthy infants to assess the immunogenicity and safety of MenACWY-CRM when co-administered with routine vaccines including pentavalent diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Hemophilus influenzae type b-combination vaccine (DTaP-IPV/Hib; Pentacel®, Sanofi Pasteur), hepatitis B vaccine (HBV; Engerix-B®, GlaxoSmithKline), and either PCV7 or 13-valent pneumococcal conjugate vaccine (PCV13; Prevnar13®, Wyeth) at 2, 4, and 6 mo of age, and measles, mumps, and rubella vaccine (MMR; M-M-R®II, Merck and Co) and either PCV7 or PCV13 at 12 mo of age. The immunogenicity and safety of DTaP-IPV/Hib, PCV, and HBV, and the safety of MMR when co-administered with MenACWY-CRM are presented.
Results
Enrolment, study flow and demographics
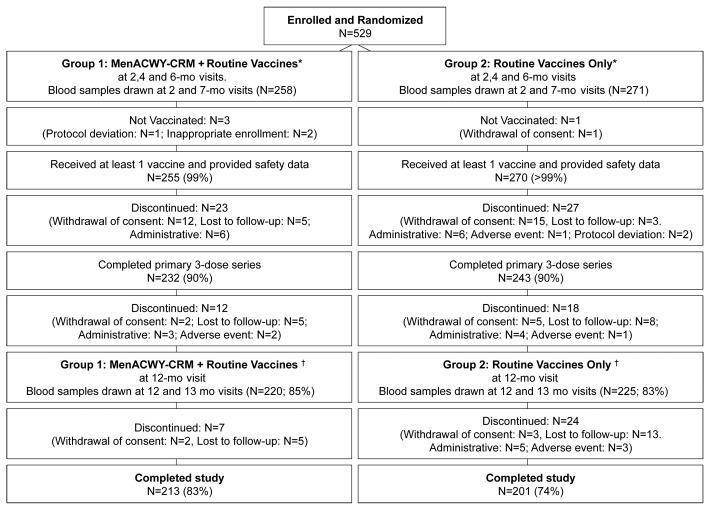
A total of 529 infants were enrolled and randomized, 258 to MenACWY-CRM + Routine and 271 to Routine Only. Of these, 213 in group MenACWY-CRM + Routine and 201 in group Routine Only completed the study (Fig. 1). With 200 evaluable subjects per group however, the power of the study for the primary immunogenicity objectives combining the 4 serogroups was still >96%.
Figure 1. Subject disposition flowchart. *Routine vaccines at 2, 4, and 6 mo visits included DTaP-IPV/Hib, HBV, and either PCV-7 or PCV-13. †Routine vaccines at 12 mo visit included MMR and either PCV-7 or PCV-13.
Both groups were similar in age, gender, and weight and the majority of participants were Caucasian (Table 1).
Table 1. Study population demographics.
| MenACWY-CRM + Routine (N = 258) |
Routine Only (N = 271) |
|
|---|---|---|
| Age, days (Mean ± SD) | 64.7 ± 6.5 | 65.4 ± 7.4 |
| Male, n (%) | 133 (52) | 141 (52) |
| Female, n (%) | 125 (48) | 130 (48) |
| Weight, kg (Mean ± SD) | 5.4 ± 0.7* | 5.4 ± 0.7 |
| Ethnicity | ||
|---|---|---|
| Asian, n (%) | 3 (1) | 1 (<1) |
| Black, n (%) | 20 (8) | 29 (11) |
| Caucasian, n (%) | 168 (65) | 177 (65) |
| Hispanic, n (%) | 44 (17) | 45 (17) |
| Other, n (%) | 23 (9) | 19 (7) |
| Met entry criteria, n (%) | 254 (98) | 266 (98) |
SD, standard deviation; *N = 257
Immunogenicity
Immunogenicity analyses were performed on the per protocol (PP) set: 411 (78%) participants after the 3-dose infant series (MenACWY-CRM + Routine, n = 202; Routine Only, n = 209), and 352 (67%) after the full 4-dose infant/toddler series (MenACWY-CRM + Routine, n = 172: Routine Only, n = 180). The primary immunogenicity analysis was also conducted on the modified intent to treat (MITT) set: 428 (81%) participants after the infant series and 439 (83%) participants after the toddler dose.
Responses to meningococcal ACWY antigens
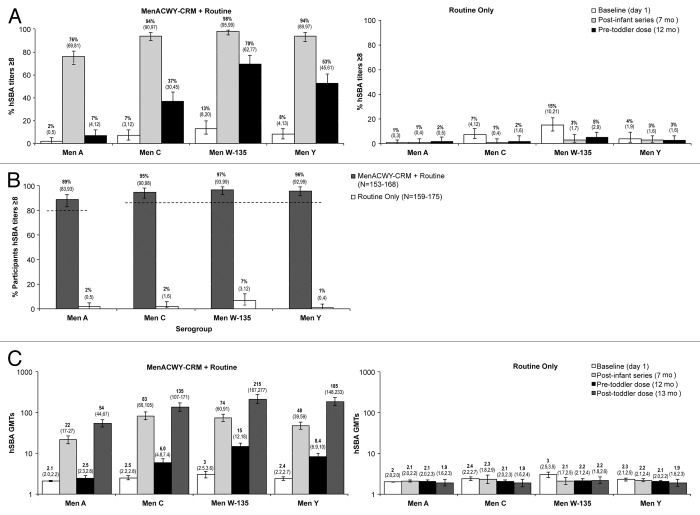
The primary immunogenicity objective was sufficiency of the immune response following 4 doses of MenACWY-CRM in terms of the percentage of participants with hSBA titers ≥8. A post-vaccination hSBA titer ≥8 is used as an accepted and conservative correlate of protection against invasive meningococcal disease.24 In the MenACWY-CRM + Routine group, 1 mo following the primary course, 76%, 94%, 98%, and 94% of infants demonstrated hSBA titers ≥8 against serogroups A, C, W-135, and Y, respectively (Fig. 2A [post-infant series at 7 mo of age]). A ≥4-fold increase in hSBA titers against serogroups A, C, W-135, and Y was achieved in 78%, 94%, 93%, and 93% of participants, respectively. At 12 mo of age (before the toddler dose), 7%, 37%, 70%, and 53% of infants demonstrated hSBA titers ≥8 against serogroups A, C, W-135, and Y, respectively (Fig. 2A).
Figure 2. (A) Percentage of participants (95% CI) in MenACWY-CRM + Routine (left) and Routine Only (right) groups with hSBA titers ≥8 at baseline, 1-mo following the 3-dose infant series (7 mo) and prior to the toddler dose, at 12 mo, per serogroup. (B) Immunogenicity results after 4 doses of MenACWY-CRM. Percentage of participants (95% CI) with hSBA titers ≥8 at 1-mo after the 4th vaccination (13 mo) in MenACWY-CRM + Routine and Routine Only groups, per serogroup.* Dashed lines illustrate criterion for response sufficiency, i.e., LL95%CI ≥85% for serogroups C, W-135, and Y and ≥80% for serogroup A after 4 doses of MenACWY-CRM. (C) GMTs (95% CI) in MenACWY-CRM + Routine (left) and Routine only (right) groups before vaccination, 1-mo after the 3-dose infant series (7 mo), prior to toddler dose (12 mo), and 1-mo following toddler dose (13 mo), per serogroup.
At 13 mo of age (1 mo post-toddler dose) 89%, 95%, 97%, and 96% of toddlers who received MenACWY-CRM + Routine vaccines achieved hSBA titers ≥8 against serogroups A, C, W-135, and Y, respectively (Fig. 2B). The lower limit (LL) of the 2-sided 95% confidence intervals (LL95%CIs) were 83%, 90%, 93%, and 92% for serogroups A, C, W-135, and Y, respectively, which exceeded the prespecified criteria, demonstrating that the primary endpoint was achieved. As expected, these percentages were very low (1–7%) in participants who received routine vaccines only. The conclusions did not change when these analyses were performed on the MITT population.
Following the 3-dose infant series (at 7 mo), robust increases in geometric mean titers (GMTs) (Fig. 2C) were observed in the MenACWY-CRM + Routine group, with 10-, 30-, 27-, and 20-fold increases for serogroups A, C, W-135, and Y, respectively. Immediately prior to the 4th dose at 12 mo, 7%, 37%, 70%, and 53% of toddlers who received MenACWY-CRM + Routine vaccines still demonstrated hSBA titers ≥8 against serogroups A, C, W-135, and Y, respectively (Fig. 2A), and GMTs were 2.5, 6.0, 15.0, and 8.4 for the respective serogroups.
One month following the toddler dose, 89%, 92%, 95%, and 96% of participants showed a ≥4-fold increase in pre-toddler dose titers against serogroups A, C, W-135, and Y, respectively. Robust increases in GMTs were also observed in this group with 21-, 23-, 14-, and 22-fold increases against serogroups A, C, W-135, and Y, respectively (Fig. 2C).
Responses to co-administered antigens
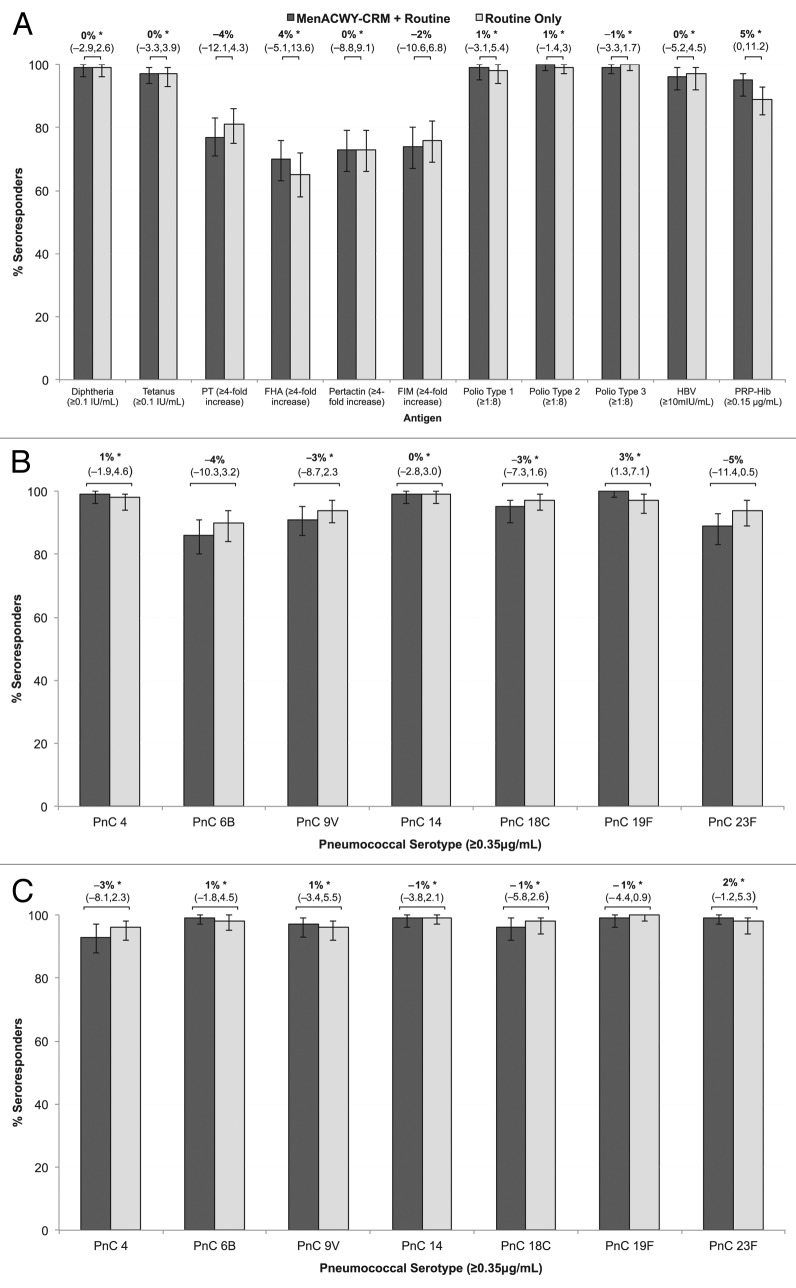
Following the 3-dose infant series (Fig. 3A), the LL95%CIs for geometric mean concentration (GMC) ratios for pertussis antigens were all >0.67 and the LL95%CIs for GMC ratios for all other antigens were >0.5 (non-inferiority criterion met). Non-inferiority criteria for the difference in seroresponse rates were met for all of the antigens except pertussis antigens pertussis toxin (PT) (LL95%CI: –12.1%) and fimbriae (FIM) (LL95%CI: –10.6%) (Fig. 3A), and pneumococcal serotypes 6B (LL95%CI: –10.3%) and 23F (LL95%CI: –11.4%), only marginally exceeding the non-inferiority threshold (Fig. 3B). Post-hoc analyses incorporating group and study center were performed for these 4 antibody responses. After adjustment for center differences, responses to PT, 6B, and 23F met non-inferiority criteria (LL95%CI: –3.9, –1.8, and –2.3, respectively). However, the CI for the response to FIM remained just outside the non-inferiority boundary (group difference – 2%, [95%CI: –10.2, 5.9]).
Figure 3. Seroresponse in participants with and without MenACWY-CRM after routine vaccinations at 2, 4, 6, and 12 mo. The difference in percentage of seroresponders (95% CI) is shown. (A) Percentage of participants with seroresponse to concomitant vaccinations DTaP-IPV/Hib 1-mo after the 3-dose infant series, at 7 mo. (B) Percentage of participants with seroresponse to pneumococcal conjugate 1-mo after the 3-dose infant series, at 7 mo. (C) Percentage of participants with seroresponse to pneumococcal conjugate 1-mo after the 4th vaccine dose, at 13 mo. *Non-inferiority criteria met for the difference in percentage of seroresponders, i.e., LL95%CI ≥–5% for poliovirus and ≥–10% for all other antigens.
Following the 4th dose of PCV at 12 mo of age, the LL95%CIs for group differences in seroresponse rates were > –10% for all pneumococcal antigens, and the LL95%CIs for GMC ratios for all antigens were >0.5 (non–inferiority criteria met) (Fig. 3C).
Although all participants received a 4-dose series of concomitant PCV, individual participants received 0–4 doses of PCV13 due to the transition from PCV7 to PCV13 that occurred during the study. Table 2 shows the ratio of pneumococcal conjugate polysaccharide (PnC) concentrations (MenACWY-CRM + Routine/Routine Only) for each serotype common to both vaccines, per number of PCV13 doses received. The ratios of PnC concentrations were consistently >1 in participants who received 4 doses of PCV13, but no conclusions can be drawn due to the fact that very few participants received 4 doses of PCV13.
Table 2. Ratio of concentrations of antibodies against PnC antigens (MenACWY-CRM + Routine/Routine Only) by PnC serotype and number of PCV13 doses.
| Number of doses per PCV vaccine | 0 PCV13, 4 PCV7 | 1 PCV13, 3 PCV7 | 2 PCV13, 2 PCV7 | 3 PCV13, 1 PCV7 | 4 PCV13, 0 PCV7 |
|---|---|---|---|---|---|
| Number of participants1 | 66 (34/32) | 63 (31/32) | 90 (48/42) | 102 (41/61) | 10 (7/3) |
| Serotype2 | |||||
| 4 | 0.75 (0.51–1.11) | 1.19 (0.71–2.0) | 1.11 (0.78–1.58) | 0.85 (0.58–1.26) | 4.12 (0.8–21) |
| 6b | 0.60 (0.36–1) | 0.71 (0.42–1.18) | 0.78 (0.51–1.18) | 0.82 (0.56–1.18) | 2.41 (0.58–10) |
| 9v | 0.64 (0.43–0.97) | 0.94 (0.6–1.46) | 0.98 (0.7–1.38) | 0.84 (0.57–1.24) | 3.19 (1.43–7.09) |
| 14 | 0.75 (0.46–1.23) | 1.26 (0.7–2.27) | 1.25 (0.88–1.77) | 0.9 (0.61–1.32) | 3.29 (0.37–29) |
| 18C | 0.67 (0.47–0.96) | 1.06 (0.68–1.68) | 1.19 (0.82–1.71) | 1.03 (0.72–1.48) | 2.08 (1.04–4.17) |
| 19F | 0.63 (0.43–0.9) | 0.94 (0.54–1.63) | 0.94 (0.67–1.3) | 1.02 (0.72–1.46) | 1.42 (0.23–8.73) |
| 23F | 0.79 (0.53–1.19) | 0.95 (0.54–1.67) | 0.93 (0.63–1.37) | 0.73 (0.48–1.12) | 1.33 (0.2–8.67) |
Note: 1Numbers of participants are reported as total N (NMenACWY-CRM+Routine / NRoutine Only). 2GMC ratios were computed as GMCMenACWY-CRM+Routine / GMCRoutine Only and are reported as GMC ratio (CI).
Reactogenicity and Safety
Of 529 enrolled participants, 525 (99%) were exposed to ≥1 study vaccination and contributed to the safety analyses. Adverse event (AE) rates were similar between groups, with low rates of “possibly vaccine-related” events (Table 3). Noting that there was no placebo control group, the most commonly reported AE by preferred term was upper respiratory infection (56% MenACWY-CRM + Routine, 57% Routine Only), followed by otitis media (39% both groups), and conjunctivitis (23% MenACWY-CRM + Routine, 19% Routine Only). Overall, serious AEs (SAEs) were reported in 21 participants (8%) receiving MenACWY-CRM + Routine vaccines and in 20 participants (7%) receiving Routine Only. No SAEs were considered vaccine-related. There were few study withdrawals due to AEs (1% MenACWY-CRM + Routine, 2% Routine Only group) and none were vaccine-related. There were no deaths.
Table 3. Adverse events reported in ≥5% of participants in the MenACWY-CRM + Routine and Routine Only groups.
| N (%) | MenACWY-CRM + Routine | Routine Only |
|---|---|---|
| Between 2 and 4 months | ||
| Any AE | 99 (39%) | 105 (39%) |
| AEs reported in ≥5% of participants | ||
| Otitis Media | 12 (5%) | 16 (6%) |
| Upper respiratory tract infection | 46 (18%) | 37 (14%) |
| Between 4 and 6 months | ||
|---|---|---|
| Any AE | 118 (49%) | 114 (45%) |
| AEs reported in ≥5% of participants | ||
| Otitis Media | 21 (17%) | 17 (7%) |
| Upper respiratory tract infection | 31 (13%) | 39 (15%) |
| Between 6 and 12 months | ||
|---|---|---|
| Any AE | 187 (78%) | 197 (79%) |
| AEs reported in ≥5% of participants | ||
| Conjunctivitis | 25 (10%) | 17 (7%) |
| Diarrhea | 18 (8%) | 15 (6%) |
| Teething | 11 (5%) | - |
| Vomiting | - | 14 (6%) |
| Pyrexia | 24 (10%) | 25 (10%) |
| Bronchiolitis | - | 12 (5%) |
| Candidiasis | 11 (5%) | - |
| Gastroenteritis | 22 (9%) | 15 (6%) |
| Otitis Media | 68 (28%) | 63 (25%) |
| Otitis Media Acute | 14 (6%) | 19 (8%) |
| Pharyngitis | 14 (6%) | 14 (6%) |
| Upper respiratory tract infection | 86 (36%) | 94 (38%) |
| Viral infection | 20 (8%) | 25 (10%) |
| Cough | 16 (7%) | 13 (5%) |
| Within 28 Days After 12-Month | ||
|---|---|---|
| Any AE | 80 (36%) | 78 (35%) |
| AEs reported in ≥5% of participants | ||
| Otitis Media | 21 (10%) | 21 (9%) |
| Upper respiratory tract infection | 28 (13%) | 19 (8%) |
| Between 13 and 18 months (End of study) | ||
|---|---|---|
| Any AE | 137 (64%) | 137 (67%) |
| AEs reported in ≥5% of participants | ||
| Conjunctivitis | 21 (10%) | 15 (7%) |
| Pyrexia | 14 (7%) | - |
| Otitis Media | 54 (25%) | 53 (26%) |
| Otitis Media Acute | 17 (8%) | - |
| Rhinitis | 10 (5%) | - |
| Upper respiratory tract infection | 53 (25%) | 52 (25%) |
| Viral infection | 15 (7%) | 21 (10%) |
| Dermatitis Diaper | - | 10 (5%) |
Discussion
Young children are at highest risk for meningococcal disease, especially in the first year of life.7,25,26 The development of a sufficiently immunogenic vaccine to broadly protect infants from meningococcal disease has proven challenging. Recent studies in this vulnerable population have demonstrated promising results for MenACWY-CRM as indicated by robust immune responses against serogroups A, C, W-135, and Y and a well-tolerated safety profile.20-23 To incorporate new vaccines into routine childhood immunization schedules, evidence is required that vaccines can be given concomitantly without compromising their immune responses and reactogenicity and safety profiles.
Results from the present study demonstrate that 4 doses of MenACWY-CRM given at 2, 4, 6, and 12 mo of age with concomitant pentavalent DTaP-IPV/Hib, hepatitis B, 7- or 13-valent PCV and MMR vaccines induced robust immune responses against all 4 serogroups, as evidenced by 89%, 95%, 97%, and 96% of participants with hSBA titers ≥8 against serogroups A, C, W-135, and Y, respectively. GMTs increased 14–23-fold from pre- to post-toddler dose and 89–96% of participants showed a ≥4-fold increase. Importantly, robust immune responses were also observed after the 3-dose infant series of MenACWY-CRM + Routine with 76%, 94%, 98%, and 94% of infants achieving hSBA titers ≥8 against serogroups A, C, W-135, and Y, respectively.
These findings are in line with recently published pivotal phase 3 infant data,20 in which another pentavalent DTaP-HBV-IPV was co-administered. In that study, hSBA titers ≥8 were present in 94–100% of participants after the full 4-dose series and in 67–97% after the 3-dose infant series. Antibody persistence was demonstrated before the 4th dose in 12–69% of participants, and GMTs increased 4.5–38-fold from before to after dose 4.
The study by Klein et al. also demonstrated significantly higher GMTs for all serogroups after a 4th dose of MenACWY-CRM at 12 mo, compared with a first dose given at 12 mo (range of ratios 4.5–38). These findings support previous results demonstrating that meningococcal conjugate vaccines induce immunological memory, bringing about an elevated immune response to subsequent vaccination.22,27 By contrast, only modest immunogenicity after a 3-dose infant series was observed with a quadrivalent meningococcal vaccine conjugated to diphtheria toxoid.28 In that study, immunogenicity was tested by SBA using rabbit complement (rSBA), which results in higher titers compared with when human complement is used. One month after the 3-dose series, rSBA titers ≥8 were present in 54–92% of children, depending on the serogroup and the dose of polysaccharide contained in the vaccine.28 The role of carrier proteins may be of relevance in this context, as previous studies have demonstrated differential immunogenicity in Hib vaccines using diphtheria toxoid or CRM197 carrier proteins.29,30
The percentages of subjects with hSBA titers ≥8 were lower against serogroup A than the other serogroups, an observation that has been reported in other studies.20,23,31,32 Measures of immunogenicity by rSBA after MenACWY-CRM have yielded much higher titers with prolonged persistence of antibodies.33 However, the clinical relevance of these differences between serogroup responses are yet to be determined.
As has been observed with other glycoconjugate vaccines,34-37 as well as previous MenACWY-CRM studies,20,23,31 there was evidence of waning antibodies after infant vaccination by 12 mo of age. In the appropriate epidemiologic setting, these data support the importance of the dose in the second year of life, which this study and others have demonstrated to elicit an anamnestic response.20,23,31
Immune responses to routine infant vaccinations (pneumococcal, IPV, HBV, Hib, DTaP) were generally not affected when administered concomitantly with MenACWY-CRM. Non-inferiority of the immune response to all routine vaccine antigens was demonstrated after the 3-dose infant series and after the toddler dose at 12 mo, except for slight differences in the post-infant seroresponse rates for pertussis antigens pertactin (PRN) and FIM, and pneumococcal serotypes 6B and 23F, although the non-inferiority criterion for the GMC ratio was achieved. Klein et al. recently demonstrated that responses to routine vaccines with MenACWY-CRM were non-inferior to routine vaccines alone, except for the seroresponse rate for pneumococcal serotype 6B (after the 3-dose infant series but not after dose 4) and PRN after dose 3. After adjustment for center differences in our study, only FIM continued to exceed the non-inferiority CI boundary for seroresponse rate. Non-inferiority was achieved for this antigen in terms of GMC ratio, and the difference in percentage of seroresponders between MenACWY-CRM + Routine and Routine Only groups was only 2% (Fig. 3A); however, the clinical significance of this is unclear.
This study has some limitations, including a high withdrawal rate. In total 115 subjects (22% of enrolled population) withdrew from the study early, mainly due to withdrawal of informed consent not related to AEs (8%), lost to follow-up (7%), or administrative reasons (5%). The higher than expected drop-out rate decreases the power to conclude on non-inferiority for routine childhood vaccines (especially for pneumococcal antigens at 1 mo after the third vaccination). Furthermore, the study was conducted during the transition from PCV7 to PCV13, and therefore the subjects may have received a mix of Prevnar 7® and Prevnar 13®. As a result, only data for the 7 serogroups that are common in both vaccines (4, 6B, 9V, 14, 18C, 19F, and 23F) were evaluated.
Overall, all vaccines were well tolerated and the safety profile was not affected when MenACWY-CRM was administered concomitantly with routine vaccines. Altogether, the current findings support recently published data that MenACWY-CRM is highly immunogenic and well tolerated in healthy infants without major concern for clinical immunological interference with routine infant vaccination.20,21
Conclusions
The full 4-dose series of MenACWY-CRM administered with routine concomitant pentavalent DTaP-IPV/Hib, HBV, and PCV vaccines at 2, 4, and 6 mo and with PCV and MMR vaccines at 12 mo of age was well tolerated and induced highly immunogenic responses against all 4 serogroups without clinically relevant interference with immune responses to routine vaccinations.
Methods
Study design
This phase 3, randomized, open-label, controlled, multi-center study was conducted at 42 sites in the United States, 3 sites in Australia, and 1 site in Canada from November 2009–November 2011 (Clinicaltrials.gov identifier: NCT01000311) and was designed according to Good Clinical Practice and the 1975 Declaration of Helsinki. Ethics review committees of participating centers approved the protocol and amendments, and written informed consent was obtained from parents/legal guardians prior to enrolment.
Participants
A total of 529 healthy 2 mo old infants were randomized 1:1 to receive 4 doses of MenACWY-CRM co-administered with routine vaccines (MenACWY-CRM + Routine; n = 258) at 2, 4, 6, and 12 mo of age or routine vaccines alone (Routine Only; n = 271) (Fig. 1). Randomization was performed using site-specific envelopes, whereby each site was assigned a set of randomization blocks containing the 1:1 ratio with a block size of 4.
Eligible participants were healthy infants aged 2 mo (55–89 d, inclusive) at the time of enrolment. Exclusion criteria included exposure to or vaccination against: N. meningitidis; C. diphtheriae; C. tetani; B. pertussis; poliovirus; Hib; pneumococcus; or receipt of the second dose of HBV. Other exclusion criteria were history or likelihood of anaphylaxis or adverse reactions to vaccine components; history or ongoing (chronic) illness likely to interfere with results; impairment/alteration of the immune system; significant acute or chronic infection within 7 d, or fever ≥38 °C within 3 d of enrolment; receipt of blood, blood products and/or plasma derivatives or any parenteral immunoglobulin preparation; and previous receipt or intent to receive any investigational agents or vaccines prior to study completion. Also, family or household members of study staff and participants whose parents/legal representatives were planning to leave the area during the study were excluded.
Vaccines
MenACWY-CRM (lot numbers X79P45I1 and 091601) was prepared by extemporaneous mixing of the lyophilized MenA component with the liquid MenCWY component before intramuscular injection. Each vaccine contained 10 μg of meningococcal serogroup A and 5 μg each of capsular polysaccharide of serogroups C, W-135, and Y conjugated to CRM197 in a 0.5 mL dose.17 The 4-dose infant/toddler MenACWY-CRM regimen consisted of a 3-dose infant series (at 2, 4, and 6 mo) followed by a toddler dose at 12 mo.
Routine vaccines included DTaP-IPV/Hib, HBV, and PCV at 2, 4, and 6 mo of age and MMR and PCV at 12 mo (Fig. 1). During the study, there was a transition from PCV7 to PCV13, and infants received the latter in accordance with national recommendations. Vaccines were administered into the anterolateral region of either thigh, except the MMR vaccine, which was administered subcutaneously to the deltoid region of the arm.
Immunogenicity
Blood samples (2–5 mL) were obtained for immunogenicity analyses before and 1-mo after the 3-dose infant vaccinations (at 2 and 7 mo), and before and 1-mo after the 12-mo toddler dose (at 12 and 13 mo). For hSBA and HBV, sera were analyzed using validated methods at the Novartis laboratory (Clinical Serology, Novartis Vaccines). For all other antigens, sera were analyzed at two external contract labs; one lab analyzed antigens against DTaP-IPV/Hib and the other analyzed antigens against PCV. The laboratory staff who performed the serology assays were blinded to study group allocation and visit associated with the samples.
Immunogenicity was assessed by serum bactericidal assay using human complement (hSBA), expressed as hSBA GMTs, percentage of participants with hSBA titers ≥8, and with ≥4-fold increase in prevaccination titers. Immune responses to routine vaccines were assessed by standard ELISA or neutralization test methods (poliovirus). Seroprotection was defined as antibody concentration ≥0.1 IU/mL for diphtheria and tetanus, 1:8 dilution for poliovirus types 1, 2, and 3, 0.15 μg/mL for PRP/Hib, 10 mIU/mL for hepatitis B, and 0.35 μg/mL for pneumococcal serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. For pertussis antigens PT, filamentous hemagglutinin, PRN and FIM types 2 and 3, response was defined as antibody concentrations ≥4 times the LL of quantification for initially seronegative participants, or ≥4-fold increase in pre-vaccination antibody concentration for initially seropositive participants.
Safety
Participants were observed for ≥15 min after receipt of each vaccination to monitor for immediate reactions and parents/guardians were provided with a worksheet to record AEs until the next study visit. Reports of medically attended AEs, SAEs, and AEs leading to study withdrawal were collected throughout the study (up to 18 mo of age). AEs were classified by the investigator as not vaccine-related, possibly related, or probably related.
Statistical analyses
Analyses were performed using SAS software version 8.2 or higher (SAS Institute, Cary).
A sample of 260 participants per group was estimated to provide adequate power to assess the primary and key secondary endpoints. The primary immunogenicity objective was sufficiency of the immune response following 4 doses of MenACWY-CRM in terms of the percentage of participants with hSBA titers ≥8 at 1-mo post-4th dose, for each serogroup. The criterion for response sufficiency was LL95%CI ≥85% for serogroups C, W-135, and Y and ≥80% for serogroup A, as previously agreed with the Center for Biologics Evaluation and Research (CBER). With 200 evaluable subjects per study group, the power of the study to demonstrate the sufficiency of immune response for each serogroup was >99% and >96% for the 4 serogroups combined (the primary immunogenicity objective). The key secondary objective was non-inferiority of routine vaccinations (PCV, IPV, HBV, Hib, and DTaP) when administered concomitantly compared with administration without MenACWY-CRM. Non-inferiority criteria were the LL95%CI of the GMC ratio of MenACWY-CRM + Routine/Routine Only >0.67 for pertussis antigens and >0.50 for all other antigens. For non-inferiority, the LL95%CI for the difference in seroresponse rates ([MenACWY-CRM + Routine] − [Routine Only]) would be > –5% for poliovirus and > –10% for all other antigens. A pooled analysis of responses to the 7 shared serotypes of any combination of PCV7 and PCV13 was performed.
Log10-transformed antibody titers were analyzed using an analysis of variance model that included group and center effects for each serogroup or antigen, and GMTs/GMCs, their ratios, and corresponding LL95%CI were calculated. Titers below the limit of detection were set at half that limit. To further explore non-inferiority of seroresponses, a categorical linear model analysis that incorporated group and center in the model was performed.
Safety data were summarized as the number and percentage of participants reporting an event, per group. Immunogenicity analyses were run on the PP set: participants who received all relevant vaccine doses correctly, provided serum samples at appropriate time points, and had no major protocol violations. Primary immunogenicity analyses were also conducted on the MITT set: participants who received a study vaccination and provided ≥1 evaluable serum sample after baseline. Safety was analyzed for all participants exposed to study vaccines who provided safety data.
Disclosure of Potential Conflicts of Interest
T.M.N. received travel support from GSK, CSL, Novartis, Sanfi-Pasteur, and Wyeth (Pfizer) for participation in investigator meetings or for the presentation of scientific data at research meetings, and honoraria for participation in a GSK independent data monitoring and safety board for an unrelated vaccine. His institution has received project-specific research funding from the same organizations to conduct clinical trials. He is a member of the WHO SAGE (Scientific Advisory Group of Experts on immunization), and chairs the Australian Government’s Australian Technical Advisory Group on Immunisation (ATAGI). M.D.N. has received travel grants from Pfizer (previously Wyeth Australia) to present independent research at international meetings, and currently and previously has been the principal investigator for clinical trials sponsored by Abbott, Baxter, CSL, GSK, MedImmune, Merck, Novartis, Sanofi-Pasteur, Wyeth, and Pfizer. L.B., M.H., T.O., and P.M.D. are employees of Novartis Vaccines and Diagnostics.
Acknowledgments
Medical writing assistance was provided by Patricia de Groot, PhD, CHC-Europe. Kathleen Jenks, Raju Gautam and Amanda Prowse assisted with manuscript preparation. The authors thank the following persons for their contributions to the conduct of the study: Dr Jodie McVernon, Dr Peter Howard, Marita Kefford, Emily Bailey, and Sharon Trevorrow (VIRGo, Melbourne); Dr Raymond Chuk, Dr Uyen Thi Duong, Aaron Buckner, Rai Halstead (Qpid, Brisbane).
References
- 1.Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344:1378–88. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 2.Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, Harnden A, Mant D, Levin M. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006;367:397–403. doi: 10.1016/S0140-6736(06)67932-4. [DOI] [PubMed] [Google Scholar]
- 3.WHO Meningococcal position paper. Wkly Epidemiol Rec. 2002;44:329–40. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. The Pink Book: Course Textbook - 12th Edition (April 2011) http://www.cdc.gov/vaccines/pubs/pinkbook/mening.html Accessed 6 June 2012.
- 5.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 6.Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Sáfadi MA. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(Suppl 2):B26–36. doi: 10.1016/j.vaccine.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, Pondo T, Arnold KE, Baumbach J, Bennett N, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50:184–91. doi: 10.1086/649209. [DOI] [PubMed] [Google Scholar]
- 8.Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med. 2010;362:1511–20. doi: 10.1056/NEJMra0906357. [DOI] [PubMed] [Google Scholar]
- 9.Bettinger JA, Scheifele DW, Le Saux N, Halperin SA, Vaudry W, Tsang R, Canadian Immunization Monitoring Program, Active (IMPACT) The impact of childhood meningococcal serogroup C conjugate vaccine programs in Canada. Pediatr Infect Dis J. 2009;28:220–4. doi: 10.1097/INF.0b013e31819040e7. [DOI] [PubMed] [Google Scholar]
- 10.Gray SJ, Trotter CL, Ramsay ME, Guiver M, Fox AJ, Borrow R, Mallard RH, Kaczmarski EB, Meningococcal Reference Unit Epidemiology of meningococcal disease in England and Wales 1993/94 to 2003/04: contribution and experiences of the Meningococcal Reference Unit. J Med Microbiol. 2006;55:887–96. doi: 10.1099/jmm.0.46288-0. [DOI] [PubMed] [Google Scholar]
- 11.Stoddard J, Dougherty N. Universal immunization of infants against Neisseria meningitidis: addressing the remaining unmet medical need in the prevention of meningitis and septicemia. Hum Vaccin. 2010;6:219–33. doi: 10.4161/hv.6.2.10330. [DOI] [PubMed] [Google Scholar]
- 12.Bröker M, Cooper B, Detora LM, Stoddard JJ. Critical appraisal of a quadrivalent CRM(197) conjugate vaccine against meningococcal serogroups A, C W-135 and Y (Menveo) in the context of treatment and prevention of invasive disease. Infect Drug Resist. 2011;4:137–47. doi: 10.2147/IDR.S12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gasparini R, Conversano M, Bona G, Gabutti G, Anemona A, Dull PM, Ceddia F. Randomized trial on the safety, tolerability, and immunogenicity of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, administered concomitantly with a combined tetanus, reduced diphtheria, and acellular pertussis vaccine in adolescents and young adults. Clin Vaccine Immunol. 2010;17:537–44. doi: 10.1128/CVI.00436-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reisinger KS, Baxter R, Block SL, Shah J, Bedell L, Dull PM. Quadrivalent meningococcal vaccination of adults: phase III comparison of an investigational conjugate vaccine, MenACWY-CRM, with the licensed vaccine, Menactra. Clin Vaccine Immunol. 2009;16:1810–5. doi: 10.1128/CVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stamboulian D, Lopardo G, Lopez P, Cortes-Barbosa C, Valencia A, Bedell L, Karsten A, Dull PM. Safety and immunogenicity of an investigational quadrivalent meningococcal CRM(197) conjugate vaccine, MenACWY-CRM, compared with licensed vaccines in adults in Latin America. Int J Infect Dis. 2010;14:e868–75. doi: 10.1016/j.ijid.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Arguedas A, Soley C, Loaiza C, Rincon G, Guevara S, Perez A, Porras W, Alvarado O, Aguilar L, Abdelnour A, et al. Safety and immunogenicity of one dose of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, when administered to adolescents concomitantly or sequentially with Tdap and HPV vaccines. Vaccine. 2010;28:3171–9. doi: 10.1016/j.vaccine.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 17.Jackson LA, Baxter R, Reisinger K, Karsten A, Shah J, Bedell L, Dull PM, V59P13 Study Group Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin Infect Dis. 2009;49:e1–10. doi: 10.1086/599117. [DOI] [PubMed] [Google Scholar]
- 18.Black S, Klein NP, Shah J, Bedell L, Karsten A, Dull PM. Immunogenicity and tolerability of a quadrivalent meningococcal glycoconjugate vaccine in children 2-10 years of age. Vaccine. 2010;28:657–63. doi: 10.1016/j.vaccine.2009.10.104. [DOI] [PubMed] [Google Scholar]
- 19.Halperin SA, Gupta A, Jeanfreau R, Klein NP, Reisinger K, Walter E, Bedell L, Gill C, Dull PM. Comparison of the safety and immunogenicity of an investigational and a licensed quadrivalent meningococcal conjugate vaccine in children 2-10 years of age. Vaccine. 2010;28:7865–72. doi: 10.1016/j.vaccine.2010.09.092. [DOI] [PubMed] [Google Scholar]
- 20.Klein NP, Reisinger KS, Johnston W, Odrljin T, Gill CJ, Bedell L, Dull P. Safety and immunogenicity of a novel quadrivalent meningococcal CRM-conjugate vaccine given concomitantly with routine vaccinations in infants. Pediatr Infect Dis J. 2012;31:64–71. doi: 10.1097/INF.0b013e31823dce5c. [DOI] [PubMed] [Google Scholar]
- 21.Klein NP, Shepard J, Bedell L, Odrljin T, Dull P. Immunogenicity and safety of a quadrivalent meningococcal conjugate vaccine administered concomitantly with measles, mumps, rubella, varicella vaccine in healthy toddlers. Vaccine. 2012;30:3929–36. doi: 10.1016/j.vaccine.2012.03.080. [DOI] [PubMed] [Google Scholar]
- 22.Perrett KP, Snape MD, Ford KJ, John TM, Yu LM, Langley JM, McNeil S, Dull PM, Ceddia F, Anemona A, et al. Immunogenicity and immune memory of a nonadjuvanted quadrivalent meningococcal glycoconjugate vaccine in infants. Pediatr Infect Dis J. 2009;28:186–93. doi: 10.1097/INF.0b013e31818e037d. [DOI] [PubMed] [Google Scholar]
- 23.Snape MD, Perrett KP, Ford KJ, John TM, Pace D, Yu LM, Langley JM, McNeil S, Dull PM, Ceddia F, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA. 2008;299:173–84. doi: 10.1001/jama.2007.29-c. [DOI] [PubMed] [Google Scholar]
- 24.Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine. 2009;27(Suppl 2):B112–6. doi: 10.1016/j.vaccine.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 25.Tapsall J, Australian Meningococcal Surveillance Programme Annual report of the Australian Meningococcal Surveillance Programme, 2007--Amended. Commun Dis Intell Q Rep. 2009;33:1–9. [PubMed] [Google Scholar]
- 26.Trotter CL, Chandra M, Cano R, Larrauri A, Ramsay ME, Brehony C, Jolley KA, Maiden MC, Heuberger S, Frosch M. A surveillance network for meningococcal disease in Europe. FEMS Microbiol Rev. 2007;31:27–36. doi: 10.1111/j.1574-6976.2006.00060.x. [DOI] [PubMed] [Google Scholar]
- 27.Kelly DF, Snape MD, Clutterbuck EA, Green S, Snowden C, Diggle L, Yu LM, Borkowski A, Moxon ER, Pollard AJ. CRM197-conjugated serogroup C meningococcal capsular polysaccharide, but not the native polysaccharide, induces persistent antigen-specific memory B cells. Blood. 2006;108:2642–7. doi: 10.1182/blood-2006-01-009282. [DOI] [PubMed] [Google Scholar]
- 28.Rennels M, King J, Jr., Ryall R, Papa T, Froeschle J. Dosage escalation, safety and immunogenicity study of four dosages of a tetravalent meninogococcal polysaccharide diphtheria toxoid conjugate vaccine in infants. Pediatr Infect Dis J. 2004;23:429–35. doi: 10.1097/01.inf.0000126297.28952.f8. [DOI] [PubMed] [Google Scholar]
- 29.Heath PT. Haemophilus influenzae type b conjugate vaccines: a review of efficacy data. Pediatr Infect Dis J. 1998;17(Suppl):S117–22. doi: 10.1097/00006454-199809001-00005. [DOI] [PubMed] [Google Scholar]
- 30.Knuf M, Kowalzik F, Kieninger D. Comparative effects of carrier proteins on vaccine-induced immune response. Vaccine. 2011;29:4881–90. doi: 10.1016/j.vaccine.2011.04.053. [DOI] [PubMed] [Google Scholar]
- 31.Blanchard-Rohner G, Snape MD, Kelly DF, O’Connor D, John T, Clutterbuck EA, Ohene-Kena B, Klinger CL, Odrljin T, Pollard AJ. The B-cell response to a primary and booster course of MenACWY-CRM₁₉₇ vaccine administered at 2, 4 and 12 months of age. Vaccine. 2013;31:2441–8. doi: 10.1016/j.vaccine.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 32.Findlow H, Plikaytis BD, Aase A, Bash MC, Chadha H, Elie C, Laher G, Martinez J, Herstad T, Newton E, et al. Investigation of different group A immunoassays following one dose of meningococcal group A conjugate vaccine or A/C polysaccharide vaccine in adults. Clin Vaccine Immunol. 2009;16:969–77. doi: 10.1128/CVI.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gill CJ, Baxter R, Anemona A, Ciavarro G, Dull P. Persistence of immune responses after a single dose of Novartis meningococcal serogroup A, C, W-135 and Y CRM-197 conjugate vaccine (Menveo®) or Menactra® among healthy adolescents. Hum Vaccin. 2010;6:881–7. doi: 10.4161/hv.6.11.12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–7. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 35.MacLennan JM, Shackley F, Heath PT, Deeks JJ, Flamank C, Herbert M, Griffiths H, Hatzmann E, Goilav C, Moxon ER. Safety, immunogenicity, and induction of immunologic memory by a serogroup C meningococcal conjugate vaccine in infants: A randomized controlled trial. JAMA. 2000;283:2795–801. doi: 10.1001/jama.283.21.2795. [DOI] [PubMed] [Google Scholar]
- 36.Snape MD, Kelly DF, Green B, Moxon ER, Borrow R, Pollard AJ. Lack of serum bactericidal activity in preschool children two years after a single dose of serogroup C meningococcal polysaccharide-protein conjugate vaccine. Pediatr Infect Dis J. 2005;24:128–31. doi: 10.1097/01.inf.0000151029.58752.27. [DOI] [PubMed] [Google Scholar]
- 37.Blanchard Rohner G, Snape MD, Kelly DF, John T, Morant A, Yu LM, Borkowski A, Ceddia F, Borrow R, Siegrist CA, et al. The magnitude of the antibody and memory B cell responses during priming with a protein-polysaccharide conjugate vaccine in human infants is associated with the persistence of antibody and the intensity of booster response. J Immunol. 2008;180:2165–73. doi: 10.4049/jimmunol.180.4.2165. [DOI] [PubMed] [Google Scholar]