Abstract
Though primarily enteric pathogens, Salmonellae are responsible for a considerable yet under-appreciated global burden of invasive disease. In South and South-East Asia, this manifests as enteric fever caused by serovars Typhi and Paratyphi A. In sub-Saharan Africa, a similar disease burden results from invasive nontyphoidal Salmonellae, principally serovars Typhimurium and Enteritidis. The existing Ty21a live-attenuated and Vi capsular polysaccharide vaccines target S. Typhi and are not effective in young children where the burden of invasive Salmonella disease is highest. After years of lack of investment in new Salmonella vaccines, recent times have seen increased interest in the area led by emerging-market manufacturers, global health vaccine institutes and academic partners. New glycoconjugate vaccines against S. Typhi are becoming available with similar vaccines against other invasive serovars in development. With other new vaccines under investigation, including live-attenuated, protein-based and GMMA vaccines, now is an exciting time for the Salmonella vaccine field.
Keywords: vaccines, Salmonella, nontyphoidal, typhoid, enteric, global health, glycoconjugate, GMMA
Protection Against more than Diarrhea
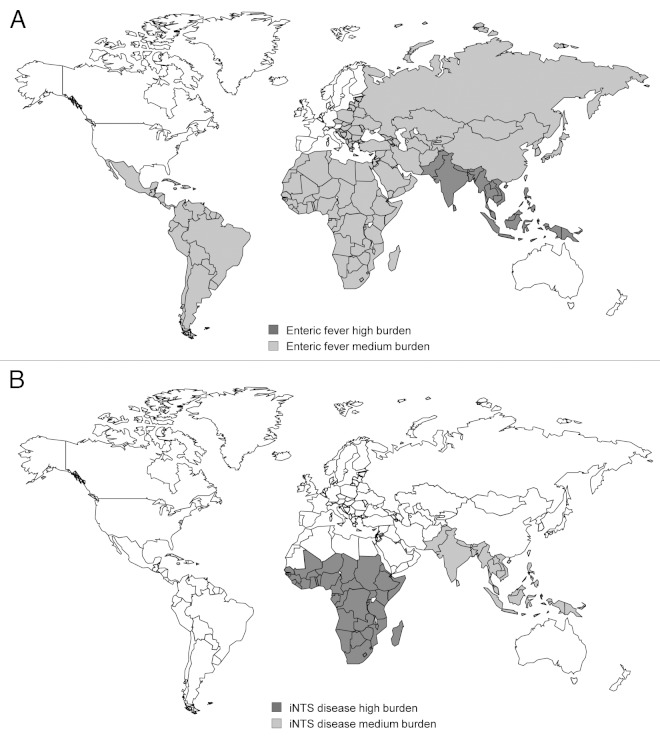
Serovars of the Gram-negative bacterium Salmonella enterica are usually associated with food-borne diarrheal illness in high-income countries. Such gastrointestinal disease is normally self-limiting and rarely life-threatening. Perhaps surprisingly, Salmonella has not been identified as a principal etiological agent of diarrhea in developing countries.1 Nevertheless, Salmonellae are responsible for a huge global disease burden through two forms of invasive illness: enteric fever and invasive nontyphoidal Salmonella (iNTS) disease. Enteric fever is principally caused by Salmonella enterica serovar Typhi (S. Typhi), for which the disease is also called typhoid fever, and S. Paratyphi A. Disease due to both serovars is a major problem in South and South-East Asia (Fig. 1A). S. Typhi is the leading pathogen isolated from blood cultures in South Asia,2 though in some areas enteric fever caused by S. Paratyphi A is more common.5 The annual global burden of disease due to typhoid fever was estimated at 21.7 million cases in 2000 with a case-fatality rate of 1% resulting in 217 000 deaths.3 Pre-school and school-aged children are the most affected age groups.6-8 The global burden of disease attributable to S. Paratyphi A in 2000 was 5.4 million cases.3
Figure 1. Geographical distribution of A. enteric fever and B. invasive nontyphoidal Salmonella (iNTS) disease indicating countries with high (> 100 cases/100,000 population/year) and medium (10–100 cases/100,000 population/year) disease burden. Based on data from refs.2-4
In contrast, iNTS disease is a neglected disease and is mainly a problem in sub-Saharan Africa (Fig. 1B). Published global burden of disease estimates are not currently available, though case fatality rates, at 20–25%,9 are much higher than for enteric fever, with an overall annual mortality likely to be well in excess of 100,000 and not dissimilar from that of enteric fever. In sub-Saharan African countries, nontyphoidal Salmonellae (NTS) are either the leading or next most common pathogenic blood culture isolate after pneumococcus,4 for which vaccines are available and are being implemented in the region. The two main serovars responsible for iNTS disease are Typhimurium and Enteritidis and the two groups most affected by iNTS disease are children under two years and HIV-infected individuals. Fever surveillance across 12 sites in sub-Saharan Africa during the RTS,S/AS01 malaria vaccine phase 3 trials gave an incidence of iNTS disease in children under two years of around 500/100 000 children/year.10 It is not clear why invasive Salmonella disease is a problem in the developing world and not in high-income countries, particularly with respect to iNTS disease. This could be due to differences in transmission, host immunity or the bacteria themselves.11
Multi-locus sequencing typing (MLST) has been used to trace the evolutionary history of S. Typhi. This revealed the expansion of haplotype H58 in Asia and Africa associated with the acquisition of resistance to fluoroquinolones over the past 20 y.12 Whole genome studies of Salmonella isolates from Africa have identified new clades associated with iNTS disease, in particular, the S. Typhimurium ST313 pathovar.13,14 S. Typhimurium ST313 is characterized by genome degradation and pseudogene formation, similar to that found in S. Typhi, suggesting that it may be passing through an evolutionary bottleneck.13 The genomic differences present in the ST313 clade, compared with NTS strains in developed countries, could underlie alternative pathways of transmission which for ST313 appear to be primarily human-to-human, rather than zoonotic or through contaminated food products, as is common in industrialised nations.15,16 The emergence of the two known clades of ST313 has been traced back to independent origins in Malawi and DRC around 50 y ago and their transmission through sub-Saharan Africa has been linked to the occurrence of the HIV/AIDS pandemic in Africa.14
Why New Vaccines against Salmonella are Needed
There are two widely-available forms of vaccine licensed for use against Salmonella,17,18 yet neither have been implemented at country level. These are the live attenuated vaccine, Ty21a,17,18 and Vi capsular polysaccharide (Vi CPS).19 Part of the reason for their lack of use in at-risk populations is their poor immunogenicity in young children. Neither are licensed for use in under two year olds.17,18 There are other associated problems with these vaccines (see section “Past and Current Vaccines”) and both are targeted against S. Typhi, with no vaccine currently available against the other three key invasive serovars of Salmonella enterica, Paratyphi A, Typhimurium and Enteritidis. Although invasive forms of Salmonella disease are amenable to antibiotics, increasing frequencies of multi-drug resistance among invasive isolates threaten the effectiveness of such treatment.12,20 In Malawi, around 90% of iNTS isolates are multi-drug resistant.20
A key problem with the effective management of invasive Salmonella disease, particularly in Africa, is the lack of appropriate diagnostic facilities. Currently, these infections can only be detected by microbiological culture, and facilities for this are rare in developing countries, particularly in Africa. The Widal test, based on the detection of antibodies to the O- and H-antigens of S. Typhi by agglutination with patient serum, has been used in the past for diagnosis of typhoid fever. However, the sensitivity and specificity of this test is low, particularly in endemic areas where prior exposure to S. Typhi is common.21 Clinical diagnosis is difficult and for iNTS disease is simply not possible since there are no signs and symptoms that distinguish it from a number of other common infections. Fever is often the only presenting feature for iNTS disease resulting in confusion with malaria with which iNTS is well known to be associated.22 iNTS disease can also result in the same clinical presentation as severe pneumonia,23 the currently recommended antibiotic treatment for which is often ineffective against iNTS.
Even where blood culture facilities are available and a definitive diagnosis is possible, the clinical demise of individuals with iNTS disease is often rapid, with around half of children who die during their admission succumbing before the blood culture results are available. This often fatal condition is emphasized by the fact that the 20–25% case fatality rate comes from studies at sites where blood culture facilities are available. Another downside to these diagnostic problems is their contribution to the continuing poor awareness of invasive Salmonella infections, particularly iNTS disease, as a significant global disease burden. In short, new vaccines against Salmonella have the potential to make an enormous impact on global health.
Understanding the Modalities of Protective Immunity
Since Salmonellae are facultative intracellular pathogens, they are able to survive both extracellularly and within the intracellular niche in monocytes and macrophages. This presents a challenge to vaccine developers, since humoral immunity is key for dealing with extracellular bacteria, while cellular immunity, mediated by both CD4+ and CD8+ T cells,24,25 is required to eliminate bacteria within the monocyte/macrophage. Animal studies show that both modalities of immunity are required for the efficient control and elimination of Salmonella. Salmonellae have a conserved set of genes that allow them to survive within macrophages and mutations in these genes lead to a loss of virulence.26 Mice with defects in the oxidative burst mechanism27,28 and mice lacking T cells29 are unable to properly control Salmonella infections. Passive transfer studies indicate an important role for antibodies in protection against Salmonella.24,30 Antibodies have a key role in facilitating uptake and killing of Salmonella by phagocytes and preventing spread of disease which occurs principally via the blood,31 while T cells are required for the elimination of persistent infection within phagocytes.32,33
Studies in individuals with primary immunodeficiencies support the importance of cell-mediated immunity for protection against Salmonella disease. Patients with chronic granulomatous disease caused by defects in the oxidative burst mechanism,34,35 and those with deficiencies of T helper 1 cell activation, caused by deletions in the IL-12/23-IFNγ cytokine axis,36-38 are particularly susceptible to Salmonella disease. The best evidence for the role of antibodies in protection against Salmonella disease in man comes from efficacy studies of the Vi CPS vaccine, since this vaccine induces antibodies and not T cells. The importance of antibodies against iNTS disease in man is indicated by recent field studies from sub-Saharan Africa which have shown that the age-related prevalence of iNTS disease declines as specific antibody is acquired.39 Accompanying mechanistic laboratory studies have demonstrated that such antibodies have functional activity against the invasive disease-causing isolates, both through activation and deposition of complement on the bacterial membrane resulting in cell-free killing39 and through opsonisation for efficient uptake and killing by phagocytic cells.40 However, the time required for killing of extracellular Salmonella is sufficient for the bacteria to escape into the intracellular niche where they can no longer be targeted by antibody.41
Hence, the evidence from human studies also supports a role for both antibodies and cell-mediated immunity in protection against invasive Salmonella disease. The well-recognized clinical association between HIV infection and iNTS disease, particularly among individuals with CD4 counts below 200 cells/µl,42 also appears to provide clear support for the importance of cell mediated immunity in defense against Salmonella. However, the effects of HIV infection on host immunity are widespread, and there is recent evidence to indicate that HIV also increases susceptibility to NTS through dysregulation of humoral immunity43 and cytokine responses,44 and disruption of the integrity of the gastrointestinal mucosa.45 Vaccine strategies that induce protective mucosal immunity to prevent NTS gastroenteritis and invasion from the gastrointestinal tract could be particularly beneficial. No such association has been found between HIV infection and typhoid fever. Intriguingly, a study from Tanzania found that HIV-infected individuals were less susceptible to typhoid fever than those without HIV infection.46 These findings suggest differences in the immune mechanisms responsible for protection against iNTS disease and typhoid fever.
Targets of Protective Immunity
Antibodies mediating protection against Salmonella necessarily need to target moieties on the outer surface of the bacterium (Table 1). The Vi antigen, which forms a polysaccharide capsule around S. Typhi (hence Vi CPS) and also S. Paratyphi C and S. Dublin, O-antigen of lipopolysaccharide (somatic antigen) and flagellin (H-antigen), which forms the flagella of Salmonella, are all highly immunogenic. Antisera against Vi, O- and H-antigens have long been used for the typing of Salmonella serovars according to the Kauffmann-White scheme.48,49 All have been proposed as vaccine candidates with Vi CPS currently in use as a vaccine against typhoid fever.
Table 1. Potential vaccine coverage of main invasive Salmonella enterica serovars by candidate antigens .
| Clinical presentation | Enteric fever | iNTSa disease | ||
|---|---|---|---|---|
| Serovar of S. enterica | Typhi | Paratyphi A | Typhimurium | Enteritidis |
| Antigen | ||||
| A. Polysaccharide | ||||
| 1. Vi | + | - | - | - |
| 2. O:2 | - | + | - | - |
| 3. O:4,5 | - | - | + | - |
| 4. O:9 | + | - | - | + |
| B. Protein | ||||
| 1. Omp F/Omp C | + | + | + | + |
| 2. Omp D | - | + | + | + |
| 3. Other | +/−d | +/−d | +/−d | +/−d |
| C. Mixed | ||||
| 1. LAVb | +/−e | +/−e | +/−e | +/−e |
| 2. GMMAc | +/−e | +/−e | +/−e | +/−e |
a iNTS, invasive nontyphoidal Salmonella ; bLAV, Live Attenuated Vaccine; cGMMA, Generalized Modules for Membrane Antigens; d+/− for ‘Other’ protein antigens indicates dependency on identity of antigen; e+/− for ‘LAV’ and ‘GMMA’ indicates dependency on choice of production strain and presence/expression levels of key antigens in production strain and target serovar.47
Because the key iNTS serovars, Typhimurium and Enteritidis, are not host restricted to man, much work has been possible on NTS disease in the mouse model of salmonellosis. Experimental O-antigen-based conjugate vaccines can induce protection against otherwise lethal Salmonella challenge in mice.50,51 Passive transfer of monoclonal antibodies targeting O-antigen also protect against challenge,52,53 and most bactericidal antibody following immunisation of animals with heat-killed invasive African ST313 S. Typhimurium is directed against O-antigen.54 In man, antibodies against O-antigen are present in adults and children from an early age in both African55 and US56 populations and have bactericidal activity. However, high levels of antibodies against O-antigen in some HIV-infected African adults are associated with a lack of in vitro killing of Salmonella.43 While the in vivo significance of this finding is not clear, the implication is that antibody to O-antigen does not protect HIV-infected individuals from iNTS disease.
Host restriction of S. Typhi to man has resulted in less animal work in relation to the Vi antigen, though genetic manipulation of NTS strains to express Vi57 can help overcome this obstacle. Nevertheless, the bactericidal capacity of antibodies raised to Vi in rabbits and mice has been known for many years,58 and there is an inverse relationship between incidence of typhoid fever and serum bactericidal titer against S. Typhi.59 The strongest evidence for the importance of antibodies targeting Vi is the protective efficacy data for the Vi CPS vaccine17-19 and from Phase 3 studies with candidate Vi conjugate vaccine.60-63
While surface polysaccharides, by nature, are T-independent type 2 antigens (Ti2 antigens), and require conjugation to carrier proteins to induce T-dependent antibody responses,64,65 Salmonella proteins are able to recruit T cell help without further manipulation. Flagellin is the only Salmonella surface typing antigen that is a protein and therefore has been investigated for its ability to generate protective immune responses. Both immunisation with flagellin alone50,66,67 or as the carrier protein for an O-antigen glycoconjugate vaccine50,68 has been shown to induce protection in mice. Flagellin is also the key ligand for toll-like receptor 5 (TLR5) and through this interaction is involved in innate signaling to the immune system and can have immunomodulatory effects in mice.69-71 Potential problems with flagellin as a vaccine candidate are that in some serovars, notably S. Typhimurium, but not the other three main invasive serovars, there can be phase variable expression, and, in addition, it is not constitutively expressed by Salmonella during infection.
While Salmonella O-antigens and flagellin are antigenically diverse and vary between serovars, some surface proteins are highly-conserved and thus have potential for use in broadly protective vaccines. Monoclonal antibodies raised against Salmonella outer membrane proteins protect against challenge following passive transfer,53 indicating their potential utility in vaccines. Much attention has been given to the porins which constitute particularly abundant outer membrane proteins. Immunisation with OmpC and OmpF,72 and OmpD73 has been shown to protect mice in challenge studies. These are widely-conserved proteins, although OmpD is not expressed by S. Typhi. As they have multiple membrane-spanning domains, their production for use in vaccines is not straightforward. Protein arrays have enabled the screening of sera for antibodies targeting thousands of Salmonella proteins. This approach has recently been used to demonstrate a common immune signature shared by mice immunised with live attenuated Salmonella and African children convalescing following iNTS disease, and to identify other potential candidate antigens, including SseB.74 These protein arrays have enabled the discrimination of immune responses of Vietnamese patients with acute typhoid from healthy controls75 and Bangladeshi patients with typhoid from those who are febrile due to other causes.76 Although the importance of T cell-mediated immunity for protection against Salmonella is well-known, far less work has been performed in order to identify the relevant T cell antigens, compared with B cells antigens. Interestingly, a recent study has found that the important T cell antigens are likely to be surface-associated in Salmonella,77 indicating that surface proteins may act as both B cell and T cell antigens.
The Challenge Of Changing Epidemiology and Breadth Of Coverage
As well as understanding the modalities and targets of protective immunity for Salmonella vaccines, it is important to understand which serovars of Salmonella enterica need to be targeted by vaccines. A drawback of the currently-available vaccines is that they are all directed against S. Typhi. As mentioned earlier, S. Paratyphi A causes enteric fever with the same geographic distribution as S. Typhi, and the diseases are clinically indistinguishable.78 Hence, for South and South-East Asia, a vaccine that can protect against both serovars would be more valuable than a vaccine that is restricted to one. In sub-Saharan Africa, the same is true for S. Typhimurium and S. Enteritidis, indicating the importance of a vaccine that can protect against both iNTS serovars for this region.
With subunit vaccines based on antigens that are specific to single serovars or groups of serovars (e.g., Vi, the O-antigens and flagellin), broad coverage can be achieved by combining multiple subunits, as has been done for the multivalent pneumococcal and meningococcal conjugate vaccines. However, this has the disadvantage of increasing costs, especially when glycoconjugate technology is used. As the invasive forms of Salmonella disease are a major problem in some of the poorest countries, vaccine affordability is a key consideration. A further drawback to the use of serovar-specific antigens for vaccine development is the evolving epidemiology of invasive Salmonella disease. In the time it takes to develop a new vaccine (minimum ten years), the epidemiology may change considerably. There is evidence for this occurring already in Africa, where S. Typhi has become an increasing problem in some areas. This appears to be partly driven by the spread of S. Typhi H58 into Africa from Asia79 and has been a particular problem in urban slums with poor sanitation.80,81 Surprisingly, in Blantyre, Malawi, where typhoid fever has previously been uncommon compared with iNTS disease, in the last year S. Typhi has been isolated more frequently from the blood of patients admitted to hospital than nontyphoidal serovars.82
Other nontyphoidal serovars are a problem in specific parts of sub-Saharan Africa, such as Dublin and Stanleyville in Mali,83 and could take over from Typhimurium and Enteritidis as the major cause of iNTS disease. Nevertheless, O-antigen-based conjugate vaccines should offer cross-protection against other non-encapsulated serovars within the same serogroup. Hence, O:1,4,[5],12 (to give its full O-antigen designation) S. Typhimurium conjugates should protect against other O:4 group (formerly B group) serovars,49 including Stanleyville (O:1,4,[5],12,27) and Paratyphi B (O:1,4,[5],12), since all express the dominant O:4 antigen. Likewise, O:1,9,12 S. Enteritidis conjugates should protect against O:9 group (formerly D group) serovars, such as Dublin (also O:1,9,12) and potentially Typhi (O:9,12[Vi]), provided the O-specific antibodies gain access through the Vi capsule to their target antigen. In view of the potential for cross-protection within serogroups, it has been proposed that a multivalent vaccine composed of 5–6 conjugates could cover almost all invasive Salmonella disease.84 An alternative strategy for developing vaccines with broad coverage is to use protein antigens that are highly conserved among different serovars of Salmonella. The increasing number of available whole genome sequences from different invasive Salmonella field isolates,13,14,85 combined with the reverse vaccinology approach,86 can facilitate the identification of such antigens.
Past and Current Vaccines
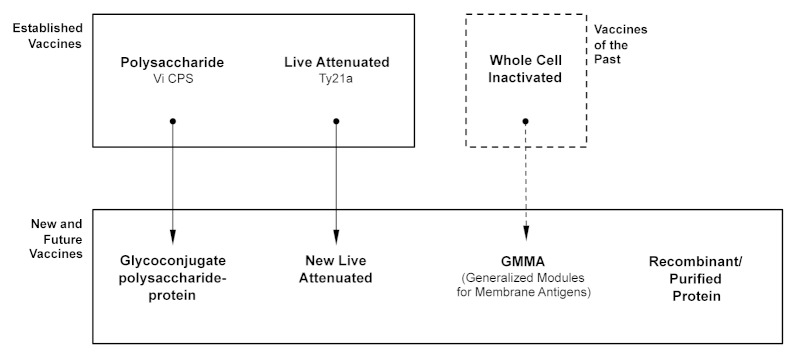
The first type of vaccine against Salmonella, an inactivated whole cell vaccine, was in use for over 100 y (Fig. 2, Table 2). Like Ty21a and Vi CPS, the inactivated whole cell vaccine targeted S. Typhi and was never implemented at a country-wide level. It was introduced in 1896,87 and used extensively by the British88 and US89 military resulting in a dramatic reduction in cases of typhoid fever and associated deaths. Of the three Salmonella vaccines, the inactivated whole cell vaccine has been the most effective with a three year cumulative efficacy of 73%.17 The major drawback, and reason why this vaccine is no longer used,17 is its high level of reactogenicity90,91 which, while previously tolerated by military personnel, is unacceptable for general use.
Figure 2. Established and new Salmonella vaccines, and how they relate to each other. (Adapted with permission from: MacLennan CA, Chapter 17 The challenge of developing global health vaccines against the invasive salmonelloses: enteric fever and invasive nontyphoidal Salmonella disease. Advanced Vaccine Research Methods for the Decade of Vaccines, Editors Bagnoli F, Rappuoli R, Publisher Caister Academic Press.)
Table 2. Advantages and disadvantages of past, present and future vaccines against Salmonella enterica.
| Vaccine | Advantages | Disadvantages |
|---|---|---|
| A Vaccines of the Past | ||
| Whole Cell Inactivated | 73% 3-y efficacy | Reactogenicity |
| B Established Vaccines | ||
| 1. Vi CPS | Single dose | Not licensed for infants |
| Low reactogenicity | Lack of memory response | |
| WHO prequalification | Lack of affinity maturation | |
| Only protects against S. Typhi | ||
| 2. Ty21a | Oral administration | Not licensed for infants |
| Some cross-protection against | Requires multiple doses | |
| S. Paratyphi B | ||
| C New and Future Vaccines | ||
| 1. Vi glycoconjugate | Higher efficacy than current vaccines | Only protects against S. Typhi |
| T-dependent antibody response | ||
| Memory induction | ||
| Affinity maturation | ||
| Low reactogenicity | ||
| 2. O-antigen glycoconjugate | As for Vi glycoconjugates | Only protects against serovars with same O-antigen specificity |
| 3. New Live Attenuated | Salmonella-specific B and T cell immunity | Attenuating for optimal balance of immunity and reactogenicity |
| Clearance of residual infection | Breadth of coverage may be limited by insufficient expression of key antigens | |
| Possibility of disease in immunocompromised subjects | ||
| 4. Recombinant Proteins | Salmonella-specific B and T cell immunity | Issues with antigen conformation may limit ability to induce effective B cell response |
| Potential for pan-specific immunity | ||
| Low reactogenicity | ||
| 5. Proteins purified from whole Salmonellae | Salmonella-specific B and T cell immunity | Difficulties with purification of integral membrane proteins |
| Potential for pan-specific immunity | ||
| Low reactogenicity | ||
| 6. GMMA | Salmonella-specific B and T cell immunity | Balance of reactogenicity and immunogenicity in man not currently known |
| Potential for pan-specific immunity | ||
| Enrichment of membrane antigens | ||
| Ease of manufacture Low cost-of-goods |
Adapted with permission from reference 47.
Ty21a and Vi CPS are fascinating for the immunologist and vaccinologist as they act through completely different, though still-to-be-properly-defined, mechanisms. Ty21a is a live attenuated vaccine that was developed through non-specific chemical mutagenesis.92 Though derived from the Vi-expressing S. Typhi Ty2 strain, surprisingly Ty21a does not express the Vi antigen and so none of its effects can be attributed to an immune response to this antigen. In contrast, the Vi CPS vaccine consists of purified Vi polysaccharide, although a recent study has suggested that other Salmonella components may be present in the vaccine.93 There are reduced rates of seroconversion following immunization with Ty21a in young children compared with adults.94 As an enteric-coated capsule, it is licensed for use in adults and children over five years. Multiple doses (routinely three) are needed and there are issues with thermal stability emphasizing the importance of a robust cold-chain. Despite these drawbacks, Ty21a has a cumulative three-dose efficacy of 51%17,18 and there is evidence that it can induce herd protection.95 The vaccine may also be amenable to increased thermal stability using a modified freeze-drying process.96
As a live attenuated vaccine, Ty21a has good potential to induce T cell immunity and cross-protection against non-Typhi serovars. Indeed, there is clinical evidence for some cross-protection against S. Paratyphi B97 and in vitro evidence for the induction of antibody-secreting cells with cross-reactivity against S. Paratyphi A and B.98,99 Such studies suggest that Ty21a may be acting primarily through the humoral immune response that it elicits, rather than through cell-mediated immunity. Recent evidence suggests that much of the B cell response is directed against O:9 which would indicate potential utility for the vaccine against the principal iNTS serovar, S. Enteritidis.100 S. Enteritidis, in common with S. Typhi, expresses O:9.
Being a Ti2 antigen, the Vi CPS is unlikely to be immunogenic in infants and is only licensed for children over two years of age. Its effectiveness in children between two and five years is uncertain, since two cluster randomized-controlled trials in this age group, in Kolkata101 and Karachi,102 gave differing results, with protective efficacy only demonstrated in the Kolkata study. The lack of T cell help in the immune response to pure polysaccharide vaccines classically results in a lack of immunoglobulin class-switching, affinity maturation through somatic hypermutation in germinal centers, in addition to a lack of immunogenicity in infants and young children.64,65 Further common findings are a lack of induction of immunological memory as well as limited duration of antibody response and hyporesponsiveness to subsequent vaccination.103 In this respect, it is perhaps surprising that Vi CPS has a similar three year efficacy against typhoid fever to that of Ty21a, at 55%,17,18 despite their different mechanisms of action. In contrast to Ty21a, the efficacy of Vi CPS is for a single vaccination dose, though Vi CPS has similar cold chain requirements. It is interesting to speculate what efficacy would result if both vaccines were given together, as their mechanisms of protection may well act in a complementary synergistic manner. As far as we are aware, no clinical trial has been conducted to investigate this. A further difference in the immune response elicited by Vi CPS and Ty21a is found in the profile of homing receptor expression in the circulating plasmablasts they induce. Those resulting from immunization with Vi CPS have a systemic homing profile with the large majority of cells expressing L-selectin, while plasmablasts after vaccination with Ty21a have very high expression of the mucosal homing receptor α4β7,93 similar to what occurs following natural infection.
New Vaccines
With the limitations of the two existing Salmonella vaccines, particularly their lack of effectiveness in young children, along with their lack of widespread uptake in endemic countries, the Salmonella community and global health policy makers are keenly awaiting the arrival of new vaccines against Salmonella (Table 3, 4 and 5). This has been a long wait given that the first Phase 3 study of a Vi glycoconjugate vaccine was reported over 12 y ago.118 This study found 91% efficacy with a vaccine consisting of Vi conjugated to recombinant Pseudomonas aeruginosa exoprotein A (Vi-rEPA) in Vietnamese children aged two to five years after 27 mo follow up, with 89% efficacy after 46 mo.119 The delay can most likely be attributed to the lack of a clear commercial incentive for developing vaccines against Salmonella.65 Invasive Salmonella infections are principally diseases of low-income countries. Nevertheless, in recent years there has been enhanced activity in the field of vaccine development against Salmonella, particularly in the development of conjugate vaccines against S. Typhi, with several different companies and institutions involved. These initiatives have partly been driven by the expanding network of vaccine manufacturers in the emerging economies, particularly India (BioMed, Shantha Biotechnics and Bharat Biotech International) and China (Lanzhou Institute of Biological Products). Several other manufacturers in the Developing Countries Vaccine Manufacturers Network,159 such as the Finlay Institute, Biological E, Biofarma, Chengdu, SK Chemical and EuBiologics are also developing vaccines against Salmonella. Impetus for the development of Salmonella vaccines has also come from global health vaccine institutes, particularly the International Vaccine Institute (IVI)160 in Seoul, South Korea, and Novartis Vaccines Institute for Global Health (NVGH)161 in Siena, Italy, as well as key academic institutions, notably the National Institutes of Health, USA, and Center for Vaccine Development (CVD) at the University of Maryland, Baltimore, USA.162
Table 3. Vaccines currently available and in development against S.Typhi.
| Name | Description | Developer | Stage of development | References |
|---|---|---|---|---|
| Ty21a | Live attenuated | Vivotif (Crucell) | licensed adults and children > 5 y | 17 , 18 , 104 - 111 |
| Vi CPS | Vi Polysaccharide | Typherix (GSK), Typhim Vi (Sanofi), Typbar Vi (Bharat Biotech), Typho Vi (BioMed); Vax-tyVi (Finlay Institute); > 6 other endemic countries manufacturers | licensed adults and children ≥ 2 y | 19 , 101 , 102 , 112 - 115 |
| Vi-TT | Vi Conjugate | Peda-Typh (BioMed) | Licensed in India | 62 , 116 |
| Typbar-TCV (Bharat Biotech) | Licensed in India | 62 , 117 | ||
| Vi-rEPA | Vi Conjugate | National Institutes for Health | Phase 3 | 61 , 118 , 119 |
| Lanzhou Institute (China) | Licensed in China | 62 | ||
| Vi-CRM197 | Vi Conjugate | NVGH (technology transfer to Biological E underway) | Phase 2 | 120 , 121 |
| Vi-DT | Vi Conjugate | International Vaccine Institute (IVI)/Shanta Biotech | Phase 1 | 62 , 122 - 124 |
| Vi conjugated to fusion protein PsaA-PdT | Vi Conjugate | Harvard Medical School | Preclinical | 125 |
| O:9-DT | O:9 Conjugate | International Vaccine Institute (IVI) | Preclinical | 126 |
| M01ZH09 | Live attenuated | Emergent Biosolutions | Phase 2 in adults and children; evaluation in S. Typhi human challenge | 127 - 132 |
| CVD 909 | Live attenuated | University of Maryland | Phase 2 | 133 - 137 |
| Ty800 | Live attenuated | Avant Immunotherapeutics | Phase 2 | 138 , 139 |
| OmpC and OmpF | Outer membrane protein | Instituto Mexicano del Seguro Social | Preclinical | 140 |
| OmpC and OmpF | Outer membrane protein | Instituto Mexicano del Seguro Social | Phase 1 in Mexico | 171 |
Table 4. Vaccines in development against S. Paratyphi A.
| Name | Description | Developer | Stage of development | References |
|---|---|---|---|---|
| O:2-TT | O:2 Conjugate | NIH | Phase 2 | 141 , 142 |
| Technology transfer from NIH to Lanzhou Institute (China) | Phase 2 | 143 | ||
| Technology transfer from NIH to Chengdu Institute (China) | Preclinical | 143 | ||
| Changchun Institute of Biological Products | Preclinical | 143 | ||
| O:2-DT* | O:2 Conjugate | IVI | Preclinical | 143 |
| O:2-CRM197† | O:2 Conjugate | NVGH (technology transfer to Biological E underway) | Preclinical | 144 , 145 |
| CVD 1902‡ | Live attenuated | University of Maryland | Phase 1 |
Notes: *development in combination with corresponding Vi-DT conjugate against S. Typhi; †development in combination with corresponding Vi-CRM197 conjugate against S. Typhi; ‡development in combination with CVD 909
Table 5. Vaccines in development against iNTS disease*.
| Name | Description | Developer | Stage of development | References |
|---|---|---|---|---|
| O:4,5/O:9-flagellin | O:4,5/O:9 Conjugate | University of Maryland | Preclinical | 50 , 68 |
| O:4,12-TT | O:4-TT Conjugate | NIH | Preclinical | 51 |
| Os-po | O:4-porin Conjugate | National Bacteriology Laboratory, Stockholm | Preclinical | 146 |
| O:4,5/O:9-CRM197 | O:4,5/O:9 Conjugate | NVGH | Preclinical | 145 |
| WT05 | Live attenuated | Microscience, Wokingham Berkshire | Phase 1 | 147 |
| CVD 1921 and CVD 1941 | Live attenuated | University of Maryland | Preclinical | 148 |
| S. Typhimuirum ruvB mutant | Live attenuated | Seoul National University | Preclinical | 149 |
| Salmonella hfq deletion mutant | Live attenuated | Indian Institute of Science Bangalore | Preclinical | 150 |
| SA186 | Live attenuated | Istituto Superiore di Sanità Roma | Preclinical | 151 |
| MT13 | Live attenuated | KIIT University Odisha | Preclinical | 152 |
| Various | Live attenuated, DNA adenine methylase mutants | University of California, Santa Barbara | Preclinical | 153 , 154 |
| Various | Live attenuated, regulated delayed attenuation | Arizona State University | Preclinical | 155 - 157 |
| Porins | S. Typhimurium porins | National Bacteriology Laboratory, Stockholm | Preclinical | 146 |
| OmpD | Outer membrane protein | University of Birmingham, UK | Preclinical | 73 |
| S. Typhimurium and S. Enteritidis GMMA | Generalized Modules for Membrane Antigens | NVGH | Preclinical | 65 , 158 |
an exhaustive list, particularly of all candidate vaccines in preclinical studies, is beyond the scope of this review
Such vaccines offer the prospect of inducing improved levels of protection over current vaccines and, importantly, providing protective immunity in children under two years of age where invasive Salmonella disease, particularly iNTS disease, is a particular problem. Therefore, these vaccines could be administered as part of national Expanded Programmes on Immunization (EPI), thus reducing delivery costs. Vaccines against typhoid and enteric fevers would likely be given at nine months,163 prior to the peak in age-related incidence in the second year of life,7 and vaccines against iNTS to young infants between two and four months of age, since peak incidence occurs around one year.39,164 While the first new vaccines against typhoid fever have already been licensed for in-country use in India and China, vaccines against iNTS disease lag a long way behind, despite the comparable burden of disease they cause. Since both enteric fever and iNTS disease are health problems primarily in low-income countries, this is likely to be attributable to a general lack of appreciation and awareness of the problem of iNTS in the global health community.11
Glycoconjugate Vaccines
Glycoconjugates are the most advanced of the new generation of vaccines against Salmonella and offer the advantages described above over pure polysaccharide vaccines such as Vi CPS. Salmonella glycoconjugate vaccines have the potential to recruit T cell help to the production of antibodies against Vi and O-antigen surface polysaccharides through covalent linkage to protein carrier molecules, thereby effectively converting these polysaccharides from T-independent to T-dependent antigens. This approach had been successfully applied to other encapsulated bacteria, particularly Hemophilus influenzae b, meningococcus and pneumococcus. Although S. Typhi is the only encapsulated serovar among the four Salmonella serovars responsible for the majority of invasive Salmonella disease, evidence from animal studies supports the development of conjugate vaccines against the three other principal invasive serovars through conjugation of their lipopolysaccharide O-antigens (O:1,2,12, O:1,4,[5],12 and O:1,9,12, for S. Paratyphi A, Typhimurium and Enteritidis, respectively)49 to suitable carrier proteins.84
The glycoconjugate strategy for new Salmonella vaccines is principally an antibody approach. The majority of Salmonella conjugate vaccines employ the familiar carrier proteins, tetanus toxoid (TT), diphtheria toxoid (DT) and the nontoxic recombinant form of diphtheria toxin (CRM197), as well as the less-commonly used protein, rEPA. The former three carrier proteins have been used extensively in existing glycoconjugate vaccines, are known to be safe and effective at inducing T-dependent responses to the carbohydrate moiety, but do not result in an immune response against any other Salmonella antigens. Vi-TT and Vi-rEPA vaccines are already licensed for in-country used in India and China. None of these carriers are Salmonella proteins. It has been argued that glycoconjugates employing Salmonella proteins could be more effective than those with exogenous carriers, as they would target the immune response to two different Salmonella antigens instead of one.84,146 Conjugation to Salmonella proteins also offers the possibility of inducing Salmonella-specific T cells. 35 y ago, Svenson and colleagues showed that O:4 conjugated to Typhimurium porins gave better protection in mice that vaccination with porin vaccines alone or an O:4-DT conjugate.146 More recently, investigators at the CVD have found enhanced immunogenicity and protective effect using iNTS vaccines with O:4,5 and O:9 conjugated to Salmonella flagellin compared with flagellin alone.50,68
Although only licensed in India, BioMed have been the first company to license a Salmonella conjugate vaccine: a Vi-TT vaccine, Peda Typh.116 Another Indian company, Bharat Biotech have registered their Vi-TT vaccine (Typbar-TT) in India, and the Lanzhou Institute of Biological Products, in partnership with the US National Institutes of Health, licensed their Vi-rEPA vaccine in China.61 The Vi-CRM197 vaccine developed by NVGH165-167 was tested in Phase 1 and 2 trials in adults in Europe,120 followed by Phase 2 trials evaluation in adults, children and infants in India, Pakistan and the Philippines.121 While being far more immunogenic than Vi CPS vaccine, anti-Vi response following revaccination was lower than after primary vaccination. Vi-CRM197 technology is currently being transferred to Biological E. Meanwhile, the International Vaccine Institute and Shanta Biotech have developed a Vi-DT vaccine.122-124 A related approach to that of the Vi glycoconjugate vaccines is protein capsular matrix vaccine (PCMV) technology by the Matrivax Research and Development Corporation that entraps the Vi polysaccharide in a matrix of CRM197.168
A key issue with these Vi-conjugate vaccines is that they offer no protection themselves against enteric fever caused by S. Paratyphi A or strains of Typhi that might not express Vi. Phase 1 and 2 clinical studies with an O:2-TT vaccine targeted against S. Paratyphi A141 were conducted 14 y ago in Vietnamese adults and children by the NIH group and found to be safe and immunogenic.142 The technology for this vaccine has subsequently been transferred to the Chengdu and Lanzhou Institutes of Biological Products, with the Lanzhou Institute currently conducting a Phase 2 trial with the vaccine. Other O:2 glycoconjugates using DT and CRM197 have been developed and tested in preclinical studies by IVI143,169 and NVGH144,145 respectively. Both have been developed alongside Vi conjugate vaccines in order to be used in bivalent combinations and protect against both main forms of enteric fever. The NVGH O:2-CRM197 technology is also being transferred to Biological E as part of a bivalent vaccine with Vi-CRM197. As mentioned previously, the development of conjugate vaccines against iNTS has lagged behind those for enteric fever, despite early preclinical proof of concept studies in mice at the National Bacteriology Laboratory in Sweden, using, among other candidate vaccines, O:4 conjugated to porin,146 and at the NIH with O:4-TT conjugates.51 In addition to the O:4,5- and O:9-flagellin vaccines of CVD, the technology for which is being transferred to Bharat Biotech, NVGH has developed and tested similar vaccines conjugated to CRM197 in preclinical studies.145 Given the emergence and spread of typhoid fever in sub-Saharan Africa, it will be important to know whether O:9 conjugates can protect against S. Typhi as well as S. Enteritidis.
Live-Attenuated Vaccines
Although new live-attenuated vaccines have received less attention recently compared with the glycoconjugate vaccines, this vaccine strategy has a number of potential advantages. Live-attenuated vaccines have excellent ability to elicit Salmonella-specific T cell responses required for clearance of residual infection, can be given orally and have good capacity to induce mucosal immunity through lymphocyte expression of mucosal homing receptors.100 The delivery of multiple Salmonella antigens to the immune system raises the possibility of inducing broad protective coverage across Salmonella serovars. Molecular biology has advanced greatly since the time when Ty21a was developed using random mutagenesis. The ability to introduce targeted mutations and genetic modifications combined with the full availability of the bacterial genomes from whole genome sequencing has considerably improved the capacity to rationally design new live-attenuated vaccines. The major challenge in the development of live-attenuated vaccines is in attaining an optimal level of attenuation without compromising immunogenicity. Attenuation is required both to prevent persistent infection and disease from the vaccine itself, a particularly important consideration in populations such as those in sub-Saharan Africa with high prevalence of HIV infection, and to minimise reactogenicity. Unfortunately, immunogenicity often decreases alongside reactogenicity when Salmonella are attenuated.170 A further consideration is to develop live vaccines that require fewer doses than Ty21a and are more heat-stable.
Three live attenuated vaccines against typhoid fever have been developed and tested in Phase 2 trials. CVD 909 is the latest in a series of live-attenuated vaccines developed by CVD. It has attenuating mutations in the aroC, aroD and htrA genes, but is distinguished from its predecessors by the replacement of the PtviA promoter, which controls Vi expression, with the strong constitutive Ptac promoter. This ensures constitutive Vi expression,133-136 which has been lacking from many live attenuated S. Typhi vaccines, either through complete lack of expression (as for Ty21a) or due to switching off of Vi expression during in vivo infection. The Ty800 vaccine developed by Avant Immunotherapeutics has a disrupted aroC gene and mutated ssaV gene.138,139 M01ZH09 from Emergent Biosolutions has mutations in the Pho/PhoQ regulator genes.127-130 All are based on the Ty2 parent strain and have good safety, tolerability and immunogenicity profiles inducing mucosal as well as systemic antibodies. As for the glycoconjugates, live attenuated oral vaccines against S. Paratyphi A and iNTS are further behind on the development pathway. CVD has tested its candidate live S. Paratyphi A vaccine CVD 1902 in a Phase 1 trial with a plan to use this in combination with CVD 909 to protect against both forms of enteric fever.
CVD have also developed and conducted preclinical studies with live iNTS vaccines. These consist of S. Typhimurium and S. Enteritidis with deleted guaBA and clpP genes and could protect against lethal challenge with the homologous serovar in mice.148 The only live-attenuated iNTS vaccine to be tested to date in man is WT05, a S. Typhimurium with the same aroC and ssaV attenuations as S. Typhi Ty800. This Phase 1 study found prolonged stool shedding in volunteers for up to 23 d,147 and the vaccine has not been tested further. Many other candidate live attenuated S. Typhimurium vaccines have been tested in mice,149-152 but none have so far been into clinical trials. These include Salmonella stains attenuated through mutation of DNA adenine methylase which acts as a global regulator of gene expression.153,154 Another promising strategy is the introduction of mutations in Salmonella that lead to regulated delayed attenuation in vivo through mechanisms including dependency on key nutrients that are absent in host tissues, and programmed lysis.155-157
Protein-Based Subunit Vaccines and GMMA
The main alternative subunit approach to Salmonella vaccines from the glycoconjugates is the development of vaccines from recombinant or purified proteins.73,140,171 These potentially have the advantage of cross-protection if carefully selected through bioinformatic analysis of whole genome sequences as part of a reverse vaccinology approach to vaccine antigen discovery.86 As proteins, such vaccines can induce both antibody and T cell responses. There are issues with preserving the conformation of proteins with multiple membrane-spanning domains that can result in the induction of antibodies with poor function on immunization. Such proteins may be better prepared by purification from whole Salmonella rather than recombinant technology.171
A better knowledge of the B cell and T cell epitopes of Salmonella would be of great help for advancing the protein-based vaccine approach. The proteins that have received most attention to date have been flagellin and porins OmpC, F and D. As described earlier, preclinical studies in the mouse model have demonstrated promise for all of these antigens with immunization resulting in protection against Salmonella challenge.50,68,72,73 OmpC and F induce long-lasting antibody responses in mice140 and have been found to be safe and immunogenic when tested in a Phase 1 study in man.171 However, such vaccines are not necessarily amenable to simple production methods. The importance of preserving the correct conformation of such antigens has been indicated by the failure of recombinant Salmonella porins to protect mice.172
An innovative strategy to maintain the conformational integrity of Salmonella antigens, while avoiding laborious purification steps, is GMMA technology. GMMA (Generalized Modules for Membrane Antigens)173,174 are small particles of 50 to 90 nm diameter consisting of blebs of outer membrane. There shedding from the surface of Gram-negative bacteria, such as Salmonella, is enhanced following the genetic deletion of proteins that span the periplasm and serve to maintain the integrity of the inner and outer membranes. Deletion of the tolR gene of Salmonella and Shigella65,158,173 results in the upregulation of this shedding process enabling the production of very high vaccine substance yields. Further deletions of genes, such as those encoding the late acyltransferases HtrB175 and MsbB,176 resulting in the removal of acyl groups from the lipid A moiety of LPS, are incorporated to reduce reactogenicity. Unlike the case with live-attenuated vaccines, there is no possibility of infection. Purification is straightforward and economical, consisting of two tangential flow filtration steps.158
Preclinical studies indicate that GMMA vaccines can deliver both surface polysaccharide and outer membrane proteins to the immune system and that they are more immunogenic than glycoconjugate vaccines. This is likely to be partly because antigens are delivered in their correct conformation and orientation, but also through the self-adjuvanting properties of GMMA which deliver innate signals through TLR ligands and other pathogen-associated molecular patterns (PAMPs). GMMA also have good potential to induce Salmonella-specific T cell immunity. The combination of high yield and a simple production process makes this a highly-affordable technology, particularly suited for the development of vaccines for low- and middle-income countries, where cost-of-goods is a key consideration.65 As the reactogenicity relative to the immunogenicity of GMMA vaccines in man is currently unknown, clinical trials (currently underway for a Shigella sonnei GMMA vaccine) are required to assess the safety and tolerability of this vaccine platform.
Conclusion
The licensure and large-scale implementation of S. Typhi glycoconjugate vaccines, foreseeable in the next few years, represents a major step forward for global health. The introduction of glycoconjugate vaccines has been a successful public health intervention against other encapsulated bacteria, particularly among infants and young children. It will be important to drive forward first vaccines against the other principal invasive Salmonella serovars: Paratyphi A, Typhimurium and Enteritidis. Improved surveillance of invasive Salmonella disease across the developing world, which is currently inadequate, will be key for assessing the impact of such new vaccines and detecting changes in the epidemiology of Salmonella disease which may be accelerated by their implementation. A key unresolved scientific question is whether the glycoconjugates, which rely on the induction of antibodies against surface polysaccharides as their principal mechanism of action, will be sufficient to effectively deal with the global problem of invasive Salmonella disease, particularly iNTS disease. While glycoconjugates are set to make a major impact, the possibility of serovar replacement and the challenge of iNTS disease among HIV-infected Africans, where lack of antibodies to Salmonella does not appear underlie susceptibility to NTS, supports the development of second and third generation vaccines. These include new live-attenuated, protein-based and GMMA-based vaccines, which could potentially induce broader protection together with Salmonella-specific T cell responses.
Potential conflict of interest
C.A.M., L.B.M. and F.M. are employees of the Novartis Vaccines Institute for Global Health. C.A.M. is the recipient of a clinical research fellowship from GlaxoSmithKline.
Glossary
Abbreviations:
- iNTS
invasive nontyphoidal Salmonella
- NTS
nontyphoidal Salmonella
- CPS
capsular polysaccharide
- Ti2
T-independent type 2
- TLR5
toll-like receptor 5
- rEPA
recombinant Pseudomonas aeruginosa exoprotein A
- IVI
International Vaccine Institute
- NVGH
Novartis Vaccines Institute for Global Health
- CVD
Center for Vaccine Development
- EPI
Expanded Programme on Immunization
- TT
tetanus toxoid
- DT
diphtheria toxoid
- CRM197
nontoxic recombinant form of diphtheria toxin
- PCMV
protein capsular matrix vaccine
- GMMA
Generalized Modules for Membrane Antigens
- PAMPs
pathogen-associated molecular patterns
- LAV
live attenuated vaccine
References
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–22. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Deen J, von Seidlein L, Andersen F, Elle N, White NJ, Lubell Y. Community-acquired bacterial bloodstream infections in developing countries in south and southeast Asia: a systematic review. Lancet Infect Dis. 2012;12:480–7. doi: 10.1016/S1473-3099(12)70028-2. [DOI] [PubMed] [Google Scholar]
- 3.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–53. [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:417–32. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochiai RL, Wang X, von Seidlein L, Yang J, Bhutta ZA, Bhattacharya SK, Agtini M, Deen JL, Wain J, Kim DR, et al. Salmonella paratyphi A rates, Asia. Emerg Infect Dis. 2005;11:1764–6. doi: 10.3201/eid1111.050168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinha A, Sazawal S, Kumar R, Sood S, Reddaiah VP, Singh B, Rao M, Naficy A, Clemens JD, Bhan MK. Typhoid fever in children aged less than 5 years. Lancet. 1999;354:734–7. doi: 10.1016/S0140-6736(98)09001-1. [DOI] [PubMed] [Google Scholar]
- 7.Saha SK, Baqui AH, Hanif M, Darmstadt GL, Ruhulamin M, Nagatake T, Santosham M, Black RE. Typhoid fever in Bangladesh: implications for vaccination policy. Pediatr Infect Dis J. 2001;20:521–4. doi: 10.1097/00006454-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Brooks WA, Hossain A, Goswami D, Nahar K, Alam K, Ahmed N, Naheed A, Nair GB, Luby S, Breiman RF. Bacteremic typhoid fever in children in an urban slum, Bangladesh. Emerg Infect Dis. 2005;11:326–9. doi: 10.3201/eid1102.040422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012;379:2489–99. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, Mordmüller B, et al. RTS,S Clinical Trials Partnership First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–75. doi: 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 11.MacLennan CA, Levine MM. Invasive nontyphoidal Salmonella disease in Africa: current status. Expert Rev Anti Infect Ther. 2013;11:443–6. doi: 10.1586/eri.13.27. [DOI] [PubMed] [Google Scholar]
- 12.Roumagnac P, Weill FX, Dolecek C, Baker S, Brisse S, Chinh NT, Le TA, Acosta CJ, Farrar J, Dougan G, et al. Evolutionary history of Salmonella typhi. Science. 2006;314:1301–4. doi: 10.1126/science.1134933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kingsley RA, Msefula CL, Thomson NR, Kariuki S, Holt KE, Gordon MA, Harris D, Clarke L, Whitehead S, Sangal V, et al. Epidemic multiple drug resistant Salmonella Typhimurium causing invasive disease in sub-Saharan Africa have a distinct genotype. Genome Res. 2009;19:2279–87. doi: 10.1101/gr.091017.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okoro CK, Kingsley RA, Connor TR, Harris SR, Parry CM, Al-Mashhadani MN, Kariuki S, Msefula CL, Gordon MA, de Pinna E, et al. Intracontinental spread of human invasive Salmonella Typhimurium pathovariants in sub-Saharan Africa. Nat Genet. 2012;44:1215–21. doi: 10.1038/ng.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kariuki S, Revathi G, Gakuya F, Yamo V, Muyodi J, Hart CA. Lack of clonal relationship between non-typhi Salmonella strain types from humans and those isolated from animals living in close contact. FEMS Immunol Med Microbiol. 2002;33:165–71. doi: 10.1111/j.1574-695X.2002.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 16.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Muyodi J, Githinji JW, Kagendo D, Munyalo A, Hart CA. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: zoonotic or anthroponotic transmission? J Med Microbiol. 2006;55:585–91. doi: 10.1099/jmm.0.46375-0. [DOI] [PubMed] [Google Scholar]
- 17.Engels EA, Falagas ME, Lau J, Bennish ML. Typhoid fever vaccines: a meta-analysis of studies on efficacy and toxicity. BMJ. 1998;316:110–6. doi: 10.1136/bmj.316.7125.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraser A, Paul M, Goldberg E, Acosta CJ, Leibovici L. Typhoid fever vaccines: systematic review and meta-analysis of randomised controlled trials. Vaccine. 2007;25:7848–57. doi: 10.1016/j.vaccine.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Khan MI, Ochiai RL, Clemens JD. Population impact of Vi capsular polysaccharide vaccine. Expert Rev Vaccines. 2010;9:485–96. doi: 10.1586/erv.10.43. [DOI] [PubMed] [Google Scholar]
- 20.Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, Molyneux E, Zijlstra EE, Heyderman RS, Hart CA, Molyneux ME. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica Serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008;46:963–9. doi: 10.1086/529146. [DOI] [PubMed] [Google Scholar]
- 21.Olopoenia LA, King AL. Widal agglutination test - 100 years later: still plagued by controversy. Postgrad Med J. 2000;76:80–4. doi: 10.1136/pmj.76.892.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mabey DC, Brown A, Greenwood BM. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis. 1987;155:1319–21. doi: 10.1093/infdis/155.6.1319. [DOI] [PubMed] [Google Scholar]
- 23.Graham SM, English M. Non-typhoidal salmonellae: a management challenge for children with community-acquired invasive disease in tropical African countries. Lancet. 2009;373:267–9. doi: 10.1016/S0140-6736(09)60073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mastroeni P, Villarreal-Ramos B, Hormaeche CE. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61:3981–4. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salerno-Goncalves R, Pasetti MF, Sztein MB. Characterization of CD8(+) effector T cell responses in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2002;169:2196–203. doi: 10.4049/jimmunol.169.4.2196. [DOI] [PubMed] [Google Scholar]
- 26.Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986;83:5189–93. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastroeni P, Vazquez-Torres A, Fang FC, Xu Y, Khan S, Hormaeche CE, Dougan G. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. II. Effects on microbial proliferation and host survival in vivo. J Exp Med. 2000;192:237–48. doi: 10.1084/jem.192.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez-Torres A, Jones-Carson J, Mastroeni P, Ischiropoulos H, Fang FC. Antimicrobial actions of the NADPH phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. I. Effects on microbial killing by activated peritoneal macrophages in vitro. J Exp Med. 2000;192:227–36. doi: 10.1084/jem.192.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha K, Mastroeni P, Harrison J, de Hormaeche RD, Hormaeche CE. Salmonella typhimurium aroA, htrA, and aroD htrA mutants cause progressive infections in athymic (nu/nu) BALB/c mice. Infect Immun. 1997;65:1566–9. doi: 10.1128/iai.65.4.1566-1569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar typhimurium. Infect Immun. 2000;68:3344–8. doi: 10.1128/IAI.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mastroeni P, Grant A, Restif O, Maskell D. A dynamic view of the spread and intracellular distribution of Salmonella enterica. Nat Rev Microbiol. 2009;7:73–80. doi: 10.1038/nrmicro2034. [DOI] [PubMed] [Google Scholar]
- 32.Blanden RV, Mackaness GB, Collins FM. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966;124:585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackaness GB, Blanden RV, Collins FM. Host-parasite relations in mouse typhoid. J Exp Med. 1966;124:573–83. doi: 10.1084/jem.124.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazarus GM, Neu HC. Agents responsible for infection in chronic granulomatous disease of childhood. J Pediatr. 1975;86:415–7. doi: 10.1016/S0022-3476(75)80975-9. [DOI] [PubMed] [Google Scholar]
- 35.Mouy R, Fischer A, Vilmer E, Seger R, Griscelli C. Incidence, severity, and prevention of infections in chronic granulomatous disease. J Pediatr. 1989;114:555–60. doi: 10.1016/S0022-3476(89)80693-6. [DOI] [PubMed] [Google Scholar]
- 36.MacLennan C, Fieschi C, Lammas DA, Picard C, Dorman SE, Sanal O, MacLennan JM, Holland SM, Ottenhoff TH, Casanova JL, et al. Interleukin (IL)-12 and IL-23 are key cytokines for immunity against Salmonella in humans. J Infect Dis. 2004;190:1755–7. doi: 10.1086/425021. [DOI] [PubMed] [Google Scholar]
- 37.Bustamante J, Boisson-Dupuis S, Jouanguy E, Picard C, Puel A, Abel L, Casanova JL. Novel primary immunodeficiencies revealed by the investigation of paediatric infectious diseases. Curr Opin Immunol. 2008;20:39–48. doi: 10.1016/j.coi.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Prando C, Samarina A, Bustamante J, Boisson-Dupuis S, Cobat A, Picard C, AlSum Z, Al-Jumaah S, Al-Hajjar S, Frayha H, et al. Inherited IL-12p40 deficiency: genetic, immunologic, and clinical features of 49 patients from 30 kindreds. Medicine (Baltimore) 2013;92:109–22. doi: 10.1097/MD.0b013e31828a01f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacLennan CA, Gondwe EN, Msefula CL, Kingsley RA, Thomson NR, White SA, Goodall M, Pickard DJ, Graham SM, Dougan G, et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J Clin Invest. 2008;118:1553–62. doi: 10.1172/JCI33998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gondwe EN, Molyneux ME, Goodall M, Graham SM, Mastroeni P, Drayson MT, MacLennan CA. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proc Natl Acad Sci U S A. 2010;107:3070–5. doi: 10.1073/pnas.0910497107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siggins MK, O’Shaughnessy CM, Pravin J, Cunningham AF, Henderson IR, Drayson MT, Maclennan CA. Differential timing of antibody-mediated phagocytosis and cell-free killing of invasive African Salmonella allows immune evasion. Eur J Immunol. 2014;44:1093–8. doi: 10.1002/eji.201343529. [DOI] [PubMed] [Google Scholar]
- 42.Gordon MA, Banda HT, Gondwe M, Gordon SB, Boeree MJ, Walsh AL, Corkill JE, Hart CA, Gilks CF, Molyneux ME. Non-typhoidal salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS. 2002;16:1633–41. doi: 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 43.MacLennan CA, Gilchrist JJ, Gordon MA, Cunningham AF, Cobbold M, Goodall M, Kingsley RA, van Oosterhout JJ, Msefula CL, Mandala WL, et al. Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science. 2010;328:508–12. doi: 10.1126/science.1180346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon MA, Gordon SB, Musaya L, Zijlstra EE, Molyneux ME, Read RC. Primary macrophages from HIV-infected adults show dysregulated cytokine responses to Salmonella, but normal internalization and killing. AIDS. 2007;21:2399–408. doi: 10.1097/QAD.0b013e3282f25107. [DOI] [PubMed] [Google Scholar]
- 45.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–8. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, Yang LY, Chow SC, Morpeth SC, Reyburn H, Njau BN, et al. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis. 2011;52:341–8. doi: 10.1093/cid/ciq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacLennan CA. Chapter 17 The challenge of developing global health vaccines against the invasive salmonelloses: enteric fever and invasive nontyphoidal Salmonella disease. Advanced Vaccine Research Methods for the Decade of Vaccines, Editors Bagnoli F, Rappuoli R, Publisher Caister Academic Press [Google Scholar]
- 48.Brenner FW, Villar RG, Angulo FJ, Tauxe R, Swaminathan B. Salmonella nomenclature. J Clin Microbiol. 2000;38:2465–7. doi: 10.1128/jcm.38.7.2465-2467.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grimont PA. D and Weill, F. X. WHO Collaborating Centre for Reference and Research on Salmonellae Antigenic formulae of Salmonella serovars 2007 9th edition. (cited 2007). Available from: https://www.pasteur.fr/ip/portal/action/WebdriveActionEvent/oid/01s-000036-089.
- 50.Simon R, Tennant SM, Wang JY, Schmidlein PJ, Lees A, Ernst RK, Pasetti MF, Galen JE, Levine MM. Salmonella enterica serovar enteritidis core O polysaccharide conjugated to H:g,m flagellin as a candidate vaccine for protection against invasive infection with S. enteritidis. Infect Immun. 2011;79:4240–9. doi: 10.1128/IAI.05484-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watson DC, Robbins JB, Szu SC. Protection of mice against Salmonella typhimurium with an O-specific polysaccharide-protein conjugate vaccine. Infect Immun. 1992;60:4679–86. doi: 10.1128/iai.60.11.4679-4686.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carlin NI, Svenson SB, Lindberg AA. Role of monoclonal O-antigen antibody epitope specificity and isotype in protection against experimental mouse typhoid. Microb Pathog. 1987;2:171–83. doi: 10.1016/0882-4010(87)90019-2. [DOI] [PubMed] [Google Scholar]
- 53.Singh SP, Williams YU, Benjamin WH, Klebba PE, Boyd D. Immunoprotection by monoclonal antibodies to the porins and lipopolysaccharide of Salmonella typhimurium. Microb Pathog. 1996;21:249–63. doi: 10.1006/mpat.1996.0059. [DOI] [PubMed] [Google Scholar]
- 54.Rondini S, Lanzilao L, Necchi F, O’Shaughnessy CM, Micoli F, Saul A, MacLennan CA. Invasive African Salmonella Typhimurium induces bactericidal antibodies against O-antigens. Microb Pathog. 2013;63:19–23. doi: 10.1016/j.micpath.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 55.MacLennan CA, Tennant SM. Comparing the roles of antibodies to nontyphoidal Salmonella enterica in high- and low-income countries and implications for vaccine development. Clin Vaccine Immunol. 2013;20:1487–90. doi: 10.1128/CVI.00465-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trebicka E, Jacob S, Pirzai W, Hurley BP, Cherayil BJ. Role of antilipopolysaccharide antibodies in serum bactericidal activity against Salmonella enterica serovar Typhimurium in healthy adults and children in the United States. Clin Vaccine Immunol. 2013;20:1491–8. doi: 10.1128/CVI.00289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jansen AM, Hall LJ, Clare S, Goulding D, Holt KE, Grant AJ, Mastroeni P, Dougan G, Kingsley RA. A Salmonella Typhimurium-Typhi genomic chimera: a model to study Vi polysaccharide capsule function in vivo. PLoS Pathog. 2011;7:e1002131. doi: 10.1371/journal.ppat.1002131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osawa E, Muschel LH. Muschel LH. The bacteridical actions of O and Vi antibodies against Salmonella Typhosa. J Immunol. 1964;92:281–5. [PubMed] [Google Scholar]
- 59.Pulickal AS, Gautam S, Clutterbuck EA, Thorson S, Basynat B, Adhikari N, Makepeace K, Rijpkema S, Borrow R, Farrar JJ, et al. Kinetics of the natural, humoral immune response to Salmonella enterica serovar Typhi in Kathmandu, Nepal. Clin Vaccine Immunol. 2009;16:1413–9. doi: 10.1128/CVI.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mai NL, Phan VB, Vo AH, Tran CT, Lin FY, Bryla DA, Chu C, Schiloach J, Robbins JB, Schneerson R, et al. Persistent efficacy of Vi conjugate vaccine against typhoid fever in young children. N Engl J Med. 2003;349:1390–1. doi: 10.1056/NEJM200310023491423. [DOI] [PubMed] [Google Scholar]
- 61.Thiem VD, Lin FY, Canh G, Son NH, Anh DD, Mao ND, Chu C, Hunt SW, Robbins JB, Schneerson R, et al. The Vi conjugate typhoid vaccine is safe, elicits protective levels of IgG anti-Vi, and is compatible with routine infant vaccines. Clin Vaccine Immunol. 2011;18:730–5. doi: 10.1128/CVI.00532-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szu SC. Development of Vi conjugate - a new generation of typhoid vaccine. Expert Rev Vaccines. 2013;12:1273–86. doi: 10.1586/14760584.2013.845529. [DOI] [PubMed] [Google Scholar]
- 63.Szu SC, Klugman KP, Hunt S. Re-examination of immune response and estimation of anti-Vi IgG protective threshold against typhoid fever-based on the efficacy trial of Vi conjugate in young children. Vaccine. 2014;32:2359–63. doi: 10.1016/j.vaccine.2014.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pollard AJ, Perrett KP, Beverley PC. Maintaining protection against invasive bacteria with protein-polysaccharide conjugate vaccines. Nat Rev Immunol. 2009;9:213–20. doi: 10.1038/nri2494. [DOI] [PubMed] [Google Scholar]
- 65.MacLennan CA. Vaccines for low-income countries. Semin Immunol. 2013;25:114–23. doi: 10.1016/j.smim.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Eom JS, Seok Kim J, Im Jang J, Kim BH, Young Yoo S, Hyeon Choi J, Bang IS, Lee IS, Keun Park Y. Enhancement of host immune responses by oral vaccination to Salmonella enterica serovar Typhimurium harboring both FliC and FljB flagella. PLoS One. 2013;8:e74850. doi: 10.1371/journal.pone.0074850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bobat S, Flores-Langarica A, Hitchcock J, Marshall JL, Kingsley RA, Goodall M, Gil-Cruz C, Serre K, Leyton DL, Letran SE, et al. Soluble flagellin, FliC, induces an Ag-specific Th2 response, yet promotes T-bet-regulated Th1 clearance of Salmonella typhimurium infection. Eur J Immunol. 2011;41:1606–18. doi: 10.1002/eji.201041089. [DOI] [PubMed] [Google Scholar]
- 68.Simon R, Wang JY, Boyd MA, Tulapurkar ME, Ramachandran G, Tennant SM, Pasetti M, Galen JE, Levine MM. Sustained protection in mice immunized with fractional doses of Salmonella Enteritidis core and O polysaccharide-flagellin glycoconjugates. PLoS One. 2013;8:e64680. doi: 10.1371/journal.pone.0064680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cunningham AF, Khan M, Ball J, Toellner KM, Serre K, Mohr E, MacLennan IC. Responses to the soluble flagellar protein FliC are Th2, while those to FliC on Salmonella are Th1. Eur J Immunol. 2004;34:2986–95. doi: 10.1002/eji.200425403. [DOI] [PubMed] [Google Scholar]
- 70.Flores-Langarica A, Marshall JL, Hitchcock J, Cook C, Jobanputra J, Bobat S, Ross EA, Coughlan RE, Henderson IR, Uematsu S, et al. Systemic flagellin immunization stimulates mucosal CD103+ dendritic cells and drives Foxp3+ regulatory T cell and IgA responses in the mesenteric lymph node. J Immunol. 2012;189:5745–54. doi: 10.4049/jimmunol.1202283. [DOI] [PubMed] [Google Scholar]
- 71.Gat O, Galen JE, Tennant S, Simon R, Blackwelder WC, Silverman DJ, Pasetti MF, Levine MM. Cell-associated flagella enhance the protection conferred by mucosally-administered attenuated Salmonella Paratyphi A vaccines. PLoS Negl Trop Dis. 2011;5:e1373. doi: 10.1371/journal.pntd.0001373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, Kenny SM, Khan M, Toellner KM, Lane PJ, et al. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. J Immunol. 2007;178:6200–7. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 73.Gil-Cruz C, Bobat S, Marshall JL, Kingsley RA, Ross EA, Henderson IR, Leyton DL, Coughlan RE, Khan M, Jensen KT, et al. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc Natl Acad Sci U S A. 2009;106:9803–8. doi: 10.1073/pnas.0812431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee SJ, Liang L, Juarez S, Nanton MR, Gondwe EN, Msefula CL, Kayala MA, Necchi F, Heath JN, Hart P, et al. Identification of a common immune signature in murine and human systemic Salmonellosis. Proc Natl Acad Sci U S A. 2012;109:4998–5003. doi: 10.1073/pnas.1111413109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang L, Juarez S, Nga TV, Dunstan S, Nakajima-Sasaki R, Davies DH, McSorley S, Baker S, Felgner PL. Immune profiling with a Salmonella Typhi antigen microarray identifies new diagnostic biomarkers of human typhoid. Sci Rep. 2013;3:1043. doi: 10.1038/srep01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Charles RC, Liang L, Khanam F, Sayeed MA, Hung C, Leung DT, Baker S, Ludwig A, Harris JB, Larocque RC, et al. Immunoproteomic analysis of antibody in lymphocyte supernatant in patients with typhoid fever in Bangladesh. Clin Vaccine Immunol. 2014;21:280–5. doi: 10.1128/CVI.00661-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barat S, Willer Y, Rizos K, Claudi B, Mazé A, Schemmer AK, Kirchhoff D, Schmidt A, Burton N, Bumann D. Immunity to intracellular Salmonella depends on surface-associated antigens. PLoS Pathog. 2012;8:e1002966. doi: 10.1371/journal.ppat.1002966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maskey AP, Day JN, Phung QT, Thwaites GE, Campbell JI, Zimmerman M, Farrar JJ, Basnyat B. Salmonella enterica serovar Paratyphi A and S. enterica serovar Typhi cause indistinguishable clinical syndromes in Kathmandu, Nepal. Clin Infect Dis. 2006;42:1247–53. doi: 10.1086/503033. [DOI] [PubMed] [Google Scholar]
- 79.Kariuki S, Revathi G, Kiiru J, Mengo DM, Mwituria J, Muyodi J, Munyalo A, Teo YY, Holt KE, Kingsley RA, et al. Typhoid in Kenya is associated with a dominant multidrug-resistant Salmonella enterica serovar Typhi haplotype that is also widespread in Southeast Asia. J Clin Microbiol. 2010;48:2171–6. doi: 10.1128/JCM.01983-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Breiman RF, Cosmas L, Njuguna H, Audi A, Olack B, Ochieng JB, Wamola N, Bigogo GM, Awiti G, Tabu CW, et al. Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS One. 2012;7:e29119. doi: 10.1371/journal.pone.0029119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tabu C, Breiman RF, Ochieng B, Aura B, Cosmas L, Audi A, Olack B, Bigogo G, Ongus JR, Fields P, et al. Differing burden and epidemiology of non-Typhi Salmonella bacteremia in rural and urban Kenya, 2006-2009. PLoS One. 2012;7:e31237. doi: 10.1371/journal.pone.0031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feasey NA, Heyderman RS, Gordon MA. Salmonella blood-stream infection in Malawi: A fall in nontyphoidal Salmonella and an outbreak of Typhoid. 8th International Conference of Typhoid Fever and Other Invasive Salmonelloses. Dhaka, Bangladesh: 2013. [Google Scholar]
- 83.Tennant SM, Diallo S, Levy H, Livio S, Sow SO, Tapia M, Fields PI, Mikoleit M, Tamboura B, Kotloff KL, et al. Identification by PCR of non-typhoidal Salmonella enterica serovars associated with invasive infections among febrile patients in Mali. PLoS Negl Trop Dis. 2010;4:e621. doi: 10.1371/journal.pntd.0000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simon R, Levine MM. Glycoconjugate vaccine strategies for protection against invasive Salmonella infections. Hum Vaccin Immunother. 2012;8:494–8. doi: 10.4161/hv.19158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holt KE, Parkhill J, Mazzoni CJ, Roumagnac P, Weill FX, Goodhead I, Rance R, Baker S, Maskell DJ, Wain J, et al. High-throughput sequencing provides insights into genome variation and evolution in Salmonella Typhi. Nat Genet. 2008;40:987–93. doi: 10.1038/ng.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sette A, Rappuoli R. Reverse vaccinology: developing vaccines in the era of genomics. Immunity. 2010;33:530–41. doi: 10.1016/j.immuni.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pfeiffer RW. Experimentelle Untersuchungen zur Frage der Schutzimpfung des Menschen gegen Typhus abdominalis. Dtsch Med Wochenschr. 1896;22:3. doi: 10.1055/s-0029-1204734. [DOI] [Google Scholar]
- 88.Wright AE, Leishman WB. Remarks on the Results which have been Obtained by the Antityphoid Inoculations and on the Methods which have been Employed in the Preparation of the Vaccine. Br Med J. 1900;1:122–9. doi: 10.1136/bmj.1.2038.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hawley PR, Simmons JS. The Effectiveness of Vaccines Used for the Prevention of Typhoid Fever in the United States Army and Navy. Am J Public Health Nations Health. 1934;24:689–709. doi: 10.2105/AJPH.24.7.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ivanoff B, Levine MM, Lambert PH. Vaccination against typhoid fever: present status. Bull World Health Organ. 1994;72:957–71. [PMC free article] [PubMed] [Google Scholar]
- 91.Wahdan MH, Sippel JE, Mikhail IA, Rahka AE, Anderson ES, Sparks HA, Cvjetanović B. Controlled field trial of a typhoid vaccine prepared with a nonmotile mutant of Salmonella typhi Ty2. Bull World Health Organ. 1975;52:69–73. [PMC free article] [PubMed] [Google Scholar]
- 92.Germanier R, Füer E. Isolation and characterization of Gal E mutant Ty 21a of Salmonella typhi: a candidate strain for a live, oral typhoid vaccine. J Infect Dis. 1975;131:553–8. doi: 10.1093/infdis/131.5.553. [DOI] [PubMed] [Google Scholar]
- 93.Kantele A, Pakkanen SH, Karttunen R, Kantele JM. Head-to-head comparison of humoral immune responses to Vi capsular polysaccharide and Salmonella Typhi Ty21a typhoid vaccines--a randomized trial. PLoS One. 2013;8:e60583. doi: 10.1371/journal.pone.0060583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cryz SJ, Jr., Vanprapar N, Thisyakorn U, Olanratmanee T, Losonsky G, Levine MM, Chearskul S. Safety and immunogenicity of Salmonella typhi Ty21a vaccine in young Thai children. Infect Immun. 1993;61:1149–51. doi: 10.1128/iai.61.3.1149-1151.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Levine MM, Ferreccio C, Black RE, Tacket CO, Germanier R. Progress in vaccines against typhoid fever. Rev Infect Dis. 1989;11(Suppl 3):S552–67. doi: 10.1093/clinids/11.Supplement_3.S552. [DOI] [PubMed] [Google Scholar]
- 96.Ohtake S, Martin R, Saxena A, Pham B, Chiueh G, Osorio M, Kopecko D, Xu D, Lechuga-Ballesteros D, Truong-Le V. Room temperature stabilization of oral, live attenuated Salmonella enterica serovar Typhi-vectored vaccines. Vaccine. 2011;29:2761–71. doi: 10.1016/j.vaccine.2011.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Levine MM, Ferreccio C, Black RE, Lagos R, San Martin O, Blackwelder WC. Ty21a live oral typhoid vaccine and prevention of paratyphoid fever caused by Salmonella enterica Serovar Paratyphi B. Clin Infect Dis. 2007;45(Suppl 1):S24–8. doi: 10.1086/518141. [DOI] [PubMed] [Google Scholar]
- 98.Pakkanen SH, Kantele JM, Kantele A. Cross-reactive gut-directed immune response against Salmonella enterica serovar Paratyphi A and B in typhoid fever and after oral Ty21a typhoid vaccination. Vaccine. 2012;30:6047–53. doi: 10.1016/j.vaccine.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 99.Wahid R, Simon R, Zafar SJ, Levine MM, Sztein MB. Live oral typhoid vaccine Ty21a induces cross-reactive humoral immune responses against Salmonella enterica serovar Paratyphi A and S. Paratyphi B in humans. Clin Vaccine Immunol. 2012;19:825–34. doi: 10.1128/CVI.00058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kantele A, Pakkanen SH, Siitonen A, Karttunen R, Kantele JM. Live oral typhoid vaccine Salmonella Typhi Ty21a - a surrogate vaccine against non-typhoid salmonella? Vaccine. 2012;30:7238–45. doi: 10.1016/j.vaccine.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 101.Sur D, Ochiai RL, Bhattacharya SK, Ganguly NK, Ali M, Manna B, Dutta S, Donner A, Kanungo S, Park JK, et al. A cluster-randomized effectiveness trial of Vi typhoid vaccine in India. N Engl J Med. 2009;361:335–44. doi: 10.1056/NEJMoa0807521. [DOI] [PubMed] [Google Scholar]
- 102.Khan MI, Soofi SB, Ochiai RL, Habib MA, Sahito SM, Nizami SQ, Acosta CJ, Clemens JD, Bhutta ZA, DOMI Typhoid Karachi Vi Effectiveness Study Group Effectiveness of Vi capsular polysaccharide typhoid vaccine among children: a cluster randomized trial in Karachi, Pakistan. Vaccine. 2012;30:5389–95. doi: 10.1016/j.vaccine.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 103.Poolman J, Borrow R. Hyporesponsiveness and its clinical implications after vaccination with polysaccharide or glycoconjugate vaccines. Expert Rev Vaccines. 2011;10:307–22. doi: 10.1586/erv.11.8. [DOI] [PubMed] [Google Scholar]
- 104.Wahdan MH, Sérié C, Cerisier Y, Sallam S, Germanier R. A controlled field trial of live Salmonella typhi strain Ty 21a oral vaccine against typhoid: three-year results. J Infect Dis. 1982;145:292–5. doi: 10.1093/infdis/145.3.292. [DOI] [PubMed] [Google Scholar]
- 105.Levine MM, Ferreccio C, Black RE, Germanier R. Large-scale field trial of Ty21a live oral typhoid vaccine in enteric-coated capsule formulation. Lancet. 1987;1:1049–52. doi: 10.1016/S0140-6736(87)90480-6. [DOI] [PubMed] [Google Scholar]
- 106.Levine MM, Ferreccio C, Cryz S, Ortiz E. Comparison of enteric-coated capsules and liquid formulation of Ty21a typhoid vaccine in randomised controlled field trial. Lancet. 1990;336:891–4. doi: 10.1016/0140-6736(90)92266-K. [DOI] [PubMed] [Google Scholar]
- 107.Black RE, Levine MM, Ferreccio C, Clements ML, Lanata C, Rooney J, Germanier R, Chilean Typhoid Committee Efficacy of one or two doses of Ty21a Salmonella typhi vaccine in enteric-coated capsules in a controlled field trial. Vaccine. 1990;8:81–4. doi: 10.1016/0264-410X(90)90183-M. [DOI] [PubMed] [Google Scholar]
- 108.Simanjuntak CH, Paleologo FP, Punjabi NH, Darmowigoto R, Soeprawoto, Totosudirjo H, Haryanto P, Suprijanto E, Witham ND, Hoffman SL. Oral immunisation against typhoid fever in Indonesia with Ty21a vaccine. Lancet. 1991;338:1055–9. doi: 10.1016/0140-6736(91)91910-M. [DOI] [PubMed] [Google Scholar]
- 109.Murphy JR, Grez L, Schlesinger L, Ferreccio C, Baqar S, Muñoz C, Wasserman SS, Losonsky G, Olson JG, Levine MM. Immunogenicity of Salmonella typhi Ty21a vaccine for young children. Infect Immun. 1991;59:4291–3. doi: 10.1128/iai.59.11.4291-4293.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Levine MM, Ferreccio C, Abrego P, Martin OS, Ortiz E, Cryz S. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine. 1999;17(Suppl 2):S22–7. doi: 10.1016/S0264-410X(99)00231-5. [DOI] [PubMed] [Google Scholar]
- 111.Salerno-Gonçalves R, Wahid R, Sztein MB. Immunization of volunteers with Salmonella enterica serovar Typhi strain Ty21a elicits the oligoclonal expansion of CD8+ T cells with predominant Vbeta repertoires. Infect Immun. 2005;73:3521–30. doi: 10.1128/IAI.73.6.3521-3530.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Robbins JD, Robbins JB. Reexamination of the protective role of the capsular polysaccharide (Vi antigen) of Salmonella typhi. J Infect Dis. 1984;150:436–49. doi: 10.1093/infdis/150.3.436. [DOI] [PubMed] [Google Scholar]
- 113.Acharya IL, Lowe CU, Thapa R, Gurubacharya VL, Shrestha MB, Cadoz M, Schulz D, Armand J, Bryla DA, Trollfors B, et al. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N Engl J Med. 1987;317:1101–4. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- 114.Klugman KP, Koornhof HJ, Robbins JB, Le Cam NN. Immunogenicity, efficacy and serological correlate of protection of Salmonella typhi Vi capsular polysaccharide vaccine three years after immunization. Vaccine. 1996;14:435–8. doi: 10.1016/0264-410X(95)00186-5. [DOI] [PubMed] [Google Scholar]
- 115.Yang HH, Wu CG, Xie GZ, Gu QW, Wang BR, Wang LY, Wang HF, Ding ZS, Yang Y, Tan WS, et al. Efficacy trial of Vi polysaccharide vaccine against typhoid fever in south-western China. Bull World Health Organ. 2001;79:625–31. [PMC free article] [PubMed] [Google Scholar]
- 116.BioMed. Peda Typh. (cited 2014 Feb 11). Available from: http://biomed.co.in/typh_vaccine.html.
- 117.Biotech B. (cited 2014 Feb 11). Available from: http://www.bharatbiotech.com/.
- 118.Lin FY, Ho VA, Khiem HB, Trach DD, Bay PV, Thanh TC, Kossaczka Z, Bryla DA, Shiloach J, Robbins JB, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001;344:1263–9. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
- 119.Mai NL, Phan VB, Vo AH, Tran CT, Lin FY, Bryla DA, Chu C, Schiloach J, Robbins JB, Schneerson R, et al. Persistent efficacy of Vi conjugate vaccine against typhoid fever in young children. N Engl J Med. 2003;349:1390–1. doi: 10.1056/NEJM200310023491423. [DOI] [PubMed] [Google Scholar]
- 120.van Damme P, Kafeja F, Anemona A, Basile V, Hilbert AK, De Coster I, Rondini S, Micoli F, Qasim Khan RM, Marchetti E, et al. Safety, immunogenicity and dose ranging of a new Vi-CRM₁₉₇ conjugate vaccine against typhoid fever: randomized clinical testing in healthy adults. PLoS One. 2011;6:e25398. doi: 10.1371/journal.pone.0025398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bhutta ZA, Capeding MR, Bavdekar A, Marchetti E, Ariff S, Soofi SB, Anemona A, Habib MA, Alberto E, Juvekar S, et al. Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: results from two randomised, observer-blind, age de-escalation, phase 2 trials. Lancet Infect Dis. 2014;14:119–29. doi: 10.1016/S1473-3099(13)70241-X. [DOI] [PubMed] [Google Scholar]
- 122.Cui C, Carbis R, An SJ, Jang H, Czerkinsky C, Szu SC, Clemens JD. Physical and chemical characterization and immunologic properties of Salmonella enterica serovar typhi capsular polysaccharide-diphtheria toxoid conjugates. Clin Vaccine Immunol. 2010;17:73–9. doi: 10.1128/CVI.00266-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.An SJ, Yoon YK, Kothari S, Kothari N, Kim JA, Lee E, Kim DR, Park TH, Smith GW, Carbis R. Physico-chemical properties of Salmonella typhi Vi polysaccharide-diphtheria toxoid conjugate vaccines affect immunogenicity. Vaccine. 2011;29:7618–23. doi: 10.1016/j.vaccine.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 124.An SJ, Yoon YK, Kothari S, Kim DR, Kim JA, Kothari N, Lee E, Park TH, Carbis R. Immune suppression induced by Vi capsular polysaccharide is overcome by Vi-DT conjugate vaccine. Vaccine. 2012;30:1023–8. doi: 10.1016/j.vaccine.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 125.Lu YJ, Zhang F, Sayeed S, Thompson CM, Szu S, Anderson PW, Malley R. A bivalent vaccine to protect against Streptococcus pneumoniae and Salmonella typhi. Vaccine. 2012;30:3405–12. doi: 10.1016/j.vaccine.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ali A, An SJ, Cui C, Haque A, Carbis R. Preparation and evaluation of immunogenic conjugates of Salmonella enterica serovar Typhi O-specific polysaccharides with diphtheria toxoid. Hum Vaccin Immunother. 2012;8:189–93. doi: 10.4161/hv.18350. [DOI] [PubMed] [Google Scholar]
- 127.Kirkpatrick BD, Tenney KM, Larsson CJ, O’Neill JP, Ventrone C, Bentley M, Upton A, Hindle Z, Fidler C, Kutzko D, et al. The novel oral typhoid vaccine M01ZH09 is well tolerated and highly immunogenic in 2 vaccine presentations. J Infect Dis. 2005;192:360–6. doi: 10.1086/431605. [DOI] [PubMed] [Google Scholar]
- 128.Kirkpatrick BD, Bentley MD, Thern AM, Larsson CJ, Ventrone C, Sreenivasan MV, Bourgeois L. Comparison of the antibodies in lymphocyte supernatant and antibody-secreting cell assays for measuring intestinal mucosal immune response to a novel oral typhoid vaccine (M01ZH09) Clin Diagn Lab Immunol. 2005;12:1127–9. doi: 10.1128/CDLI.12.9.1127-1129.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kirkpatrick BD, McKenzie R, O’Neill JP, Larsson CJ, Bourgeois AL, Shimko J, Bentley M, Makin J, Chatfield S, Hindle Z, et al. Evaluation of Salmonella enterica serovar Typhi (Ty2 aroC-ssaV-) M01ZH09, with a defined mutation in the Salmonella pathogenicity island 2, as a live, oral typhoid vaccine in human volunteers. Vaccine. 2006;24:116–23. doi: 10.1016/j.vaccine.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 130.Jain SK. M-01ZH09, an oral live attenuated Salmonella enterica serovar Typhi vaccine for the prevention of typhoid fever. Curr Opin Mol Ther. 2009;11:565–71. [PubMed] [Google Scholar]
- 131.Tran TH, Nguyen TD, Nguyen TT, Ninh TT, Tran NB, Nguyen VM, Tran TT, Cao TT, Pham VM, Nguyen TC, et al. A randomised trial evaluating the safety and immunogenicity of the novel single oral dose typhoid vaccine M01ZH09 in healthy Vietnamese children. PLoS One. 2010;5:e11778. doi: 10.1371/journal.pone.0011778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lyon CE, Sadigh KS, Carmolli MP, Harro C, Sheldon E, Lindow JC, Larsson CJ, Martinez T, Feller A, Ventrone CH, et al. In a randomized, double-blinded, placebo-controlled trial, the single oral dose typhoid vaccine, M01ZH09, is safe and immunogenic at doses up to 1.7 x 10(10) colony-forming units. Vaccine. 2010;28:3602–8. doi: 10.1016/j.vaccine.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 133.Wang JY, Noriega FR, Galen JE, Barry E, Levine MM. Constitutive expression of the Vi polysaccharide capsular antigen in attenuated Salmonella enterica serovar typhi oral vaccine strain CVD 909. Infect Immun. 2000;68:4647–52. doi: 10.1128/IAI.68.8.4647-4652.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wahid R, Salerno-Gonçalves R, Tacket CO, Levine MM, Sztein MB. Cell-mediated immune responses in humans after immunization with one or two doses of oral live attenuated typhoid vaccine CVD 909. Vaccine. 2007;25:1416–25. doi: 10.1016/j.vaccine.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wahid R, Salerno-Gonçalves R, Tacket CO, Levine MM, Sztein MB. Generation of specific effector and memory T cells with gut- and secondary lymphoid tissue- homing potential by oral attenuated CVD 909 typhoid vaccine in humans. Mucosal Immunol. 2008;1:389–98. doi: 10.1038/mi.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tacket CO, Levine MM. CVD 908, CVD 908-htrA, and CVD 909 live oral typhoid vaccines: a logical progression. Clin Infect Dis. 2007;45(Suppl 1):S20–3. doi: 10.1086/518135. [DOI] [PubMed] [Google Scholar]
- 137.Wahid R, Pasetti MF, Maciel M, Jr., Simon JK, Tacket CO, Levine MM, Sztein MB. Oral priming with Salmonella Typhi vaccine strain CVD 909 followed by parenteral boost with the S. Typhi Vi capsular polysaccharide vaccine induces CD27+IgD-S. Typhi-specific IgA and IgG B memory cells in humans. Clin Immunol. 2011;138:187–200. doi: 10.1016/j.clim.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hohmann EL, Oletta CA, Killeen KP, Miller SI. phoP/phoQ-deleted Salmonella typhi (Ty800) is a safe and immunogenic single-dose typhoid fever vaccine in volunteers. J Infect Dis. 1996;173:1408–14. doi: 10.1093/infdis/173.6.1408. [DOI] [PubMed] [Google Scholar]
- 139.Hohmann EL, Oletta CA, Miller SI. Evaluation of a phoP/phoQ-deleted, aroA-deleted live oral Salmonella typhi vaccine strain in human volunteers. Vaccine. 1996;14:19–24. doi: 10.1016/0264-410X(95)00173-X. [DOI] [PubMed] [Google Scholar]
- 140.Secundino I, López-Macías C, Cervantes-Barragán L, Gil-Cruz C, Ríos-Sarabia N, Pastelin-Palacios R, Villasis-Keever MA, Becker I, Puente JL, Calva E, et al. Salmonella porins induce a sustained, lifelong specific bactericidal antibody memory response. Immunology. 2006;117:59–70. doi: 10.1111/j.1365-2567.2005.02263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Konadu E, Shiloach J, Bryla DA, Robbins JB, Szu SC. Synthesis, characterization, and immunological properties in mice of conjugates composed of detoxified lipopolysaccharide of Salmonella paratyphi A bound to tetanus toxoid with emphasis on the role of O acetyls. Infect Immun. 1996;64:2709–15. doi: 10.1128/iai.64.7.2709-2715.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Konadu EY, Lin FY, Hó VA, Thuy NT, Van Bay P, Thanh TC, Khiem HB, Trach DD, Karpas AB, Li J, et al. Phase 1 and phase 2 studies of Salmonella enterica serovar paratyphi A O-specific polysaccharide-tetanus toxoid conjugates in adults, teenagers, and 2- to 4-year-old children in Vietnam. Infect Immun. 2000;68:1529–34. doi: 10.1128/IAI.68.3.1529-1534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sahastrabuddhe S, Carbis R, Wierzba TF, Ochiai RL. Increasing rates of Salmonella Paratyphi A and the current status of its vaccine development. Expert Rev Vaccines. 2013;12:1021–31. doi: 10.1586/14760584.2013.825450. [DOI] [PubMed] [Google Scholar]
- 144.Micoli F, Rondini S, Gavini M, Lanzilao L, Medaglini D, Saul A, Martin LBO. O:2-CRM(197) conjugates against Salmonella Paratyphi A. PLoS One. 2012;7:e47039. doi: 10.1371/journal.pone.0047039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Micoli F, Rondini S, Gavini M, Pisoni I, Lanzilao L, Colucci AM, Giannelli C, Pippi F, Sollai L, Pinto V, et al. A scalable method for O-antigen purification applied to various Salmonella serovars. Anal Biochem. 2013;434:136–45. doi: 10.1016/j.ab.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Svenson SB, Nurminen M, Lindberg AA. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979;25:863–72. doi: 10.1128/iai.25.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hindle Z, Chatfield SN, Phillimore J, Bentley M, Johnson J, Cosgrove CA, Ghaem-Maghami M, Sexton A, Khan M, Brennan FR, et al. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect Immun. 2002;70:3457–67. doi: 10.1128/IAI.70.7.3457-3467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tennant SM, Wang JY, Galen JE, Simon R, Pasetti MF, Gat O, Levine MM. Engineering and preclinical evaluation of attenuated nontyphoidal Salmonella strains serving as live oral vaccines and as reagent strains. Infect Immun. 2011;79:4175–85. doi: 10.1128/IAI.05278-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Choi J, Shin D, Ryu S. Salmonella enterica serovar Typhimurium ruvB mutant can confer protection against salmonellosis in mice. Vaccine. 2010;28:6436–44. doi: 10.1016/j.vaccine.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 150.Allam US, Krishna MG, Lahiri A, Joy O, Chakravortty D. Salmonella enterica serovar Typhimurium lacking hfq gene confers protective immunity against murine typhoid. PLoS One. 2011;6:e16667. doi: 10.1371/journal.pone.0016667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pesciaroli M, Aloisio F, Ammendola S, Pistoia C, Petrucci P, Tarantino M, Francia M, Battistoni A, Pasquali P. An attenuated Salmonella enterica serovar Typhimurium strain lacking the ZnuABC transporter induces protection in a mouse intestinal model of Salmonella infection. Vaccine. 2011;29:1783–90. doi: 10.1016/j.vaccine.2010.12.111. [DOI] [PubMed] [Google Scholar]
- 152.Vishwakarma V, Pati NB, Chandel HS, Sahoo SS, Saha B, Suar M. Evaluation of Salmonella enterica serovar Typhimurium TTSS-2 deficient fur mutant as safe live-attenuated vaccine candidate for immunocompromised mice. PLoS One. 2012;7:e52043. doi: 10.1371/journal.pone.0052043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Heithoff DM, Sinsheimer RL, Low DA, Mahan MJ. An essential role for DNA adenine methylation in bacterial virulence. Science. 1999;284:967–70. doi: 10.1126/science.284.5416.967. [DOI] [PubMed] [Google Scholar]
- 154.Heithoff DM, Enioutina EY, Daynes RA, Sinsheimer RL, Low DA, Mahan MJ. Salmonella DNA adenine methylase mutants confer cross-protective immunity. Infect Immun. 2001;69:6725–30. doi: 10.1128/IAI.69.11.6725-6730.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kong W, Wanda SY, Zhang X, Bollen W, Tinge SA, Roland KL, Curtiss R., 3rd Regulated programmed lysis of recombinant Salmonella in host tissues to release protective antigens and confer biological containment. Proc Natl Acad Sci U S A. 2008;105:9361–6. doi: 10.1073/pnas.0803801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li Y, Wang S, Scarpellini G, Gunn B, Xin W, Wanda SY, Roland KL, Curtiss R., 3rd Evaluation of new generation Salmonella enterica serovar Typhimurium vaccines with regulated delayed attenuation to induce immune responses against PspA. Proc Natl Acad Sci U S A. 2009;106:593–8. doi: 10.1073/pnas.0811697106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Curtiss R, 3rd, Wanda SY, Gunn BM, Zhang X, Tinge SA, Ananthnarayan V, Mo H, Wang S, Kong W. Salmonella enterica serovar typhimurium strains with regulated delayed attenuation in vivo. Infect Immun. 2009;77:1071–82. doi: 10.1128/IAI.00693-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Berlanda Scorza F, Doro F, Rodríguez-Ortega MJ, Stella M, Liberatori S, Taddei AR, Serino L, Gomes Moriel D, Nesta B, Fontana MR, et al. Proteomics characterization of outer membrane vesicles from the extraintestinal pathogenic Escherichia coli DeltatolR IHE3034 mutant. Mol Cell Proteomics. 2008;7:473–85. doi: 10.1074/mcp.M700295-MCP200. [DOI] [PubMed] [Google Scholar]
- 159.Pagliusi S, Leite LC, Datla M, Makhoana M, Gao Y, Suhardono M, Jadhav S, Harshavardhan GV, Homma A. Developing Countries Vaccine Manufacturers Network: doing good by making high-quality vaccines affordable for all. Vaccine. 2013;31(Suppl 2):B176–83. doi: 10.1016/j.vaccine.2012.11.060. [DOI] [PubMed] [Google Scholar]
- 160.International Vaccine Institute. (cited 2014 Feb 11). Available from: http://www.ivi.int.
- 161.Novartis Vaccines Institute for Global Health. (cited 2014 Feb 11). Available from: http://www.nibr.com/research/developing_world/NVGH/index.shtml.
- 162.University of Maryland School of Medicine. Center for Vaccine Development. (cited 2014 Feb 11). Available from: http://medschool.umaryland.edu/cvd/.
- 163.Podda A, Saul AJ, Arora R, Bhutta Z, Sinha A, Gaind R, Singhal T, Saha S, Brooks A, Martin LB, et al. Conjugate vaccines for enteric fever: proceedings of a meeting organized in New Delhi, India in 2009. J Infect Dev Ctries. 2010;4:404–11. [PubMed] [Google Scholar]
- 164.Feasey NA, Archer BN, Heyderman RS, Sooka A, Dennis B, Gordon MA, Keddy KH. Typhoid fever and invasive nontyphoid salmonellosis, Malawi and South Africa. Emerg Infect Dis. 2010;16:1448–51. doi: 10.3201/eid1609.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Micoli F, Rondini S, Pisoni I, Giannelli C, Di Cioccio V, Costantino P, Saul A, Martin LB. Production of a conjugate vaccine for Salmonella enterica serovar Typhi from Citrobacter Vi. Vaccine. 2012;30:853–61. doi: 10.1016/j.vaccine.2011.11.108. [DOI] [PubMed] [Google Scholar]
- 166.Micoli F, Rondini S, Pisoni I, Proietti D, Berti F, Costantino P, Rappuoli R, Szu S, Saul A, Martin LB. Vi-CRM 197 as a new conjugate vaccine against Salmonella Typhi. Vaccine. 2011;29:712–20. doi: 10.1016/j.vaccine.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rondini S, Micoli F, Lanzilao L, Hale C, Saul AJ, Martin LB. Evaluation of the immunogenicity and biological activity of the Citrobacter freundii Vi-CRM197 conjugate as a vaccine for Salmonella enterica serovar Typhi. Clin Vaccine Immunol. 2011;18:460–8. doi: 10.1128/CVI.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Killeen KP, Griffin TJ, Cartee RT, Thanawastien A. Development of protein capsular matrix platform technology. BioProcess International. 2013;11:S26–32. [Google Scholar]
- 169.Kothari S, Kim JA, Kothari N, Jones C, Choe WS, Carbis R. Purification of O-specific polysaccharide from lipopolysaccharide produced by Salmonella enterica serovar Paratyphi A. Vaccine. 2014;32:2457–62. doi: 10.1016/j.vaccine.2014.02.090. [DOI] [PubMed] [Google Scholar]
- 170.Galen JE, Simon R, Ernst RK. Salmonella expressing detoxified lipopolysaccharide is immunogenic and protective both as an attenuated vaccine and for delivery of foreign antigens. Expert Rev Vaccines. 2011;10:1679–82. doi: 10.1586/erv.11.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Salazar-González RM, Maldonado-Bernal C, Ramírez-Cruz NE, Rios-Sarabia N, Beltrán-Nava J, Castañón-González J, Castillo-Torres N, Palma-Aguirre JA, Carrera-Camargo M, López-Macías C, et al. Induction of cellular immune response and anti-Salmonella enterica serovar typhi bactericidal antibodies in healthy volunteers by immunization with a vaccine candidate against typhoid fever. Immunol Lett. 2004;93:115–22. doi: 10.1016/j.imlet.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 172.Toobak H, Rasooli I, Talei D, Jahangiri A, Owlia P, Darvish Alipour Astaneh S. Immune response variations to Salmonella enterica serovar Typhi recombinant porin proteins in mice. Biologicals. 2013;41:224–30. doi: 10.1016/j.biologicals.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 173.Berlanda Scorza F, Colucci AM, Maggiore L, Sanzone S, Rossi O, Ferlenghi I, Pesce I, Caboni M, Norais N, Di Cioccio V, et al. High yield production process for Shigella outer membrane particles. PLoS One. 2012;7:e35616. doi: 10.1371/journal.pone.0035616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Koeberling O, Ispasanie E, Hauser J, Rossi O, Pluschke G, Caugant DA, Saul A, Maclennan CA. A broadly-protective vaccine against meningococcal disease in sub-Saharan Africa based on Generalized Modules for Membrane Antigens (GMMA) Vaccine. 2014;32:2688–95. doi: 10.1016/j.vaccine.2014.03.068. [DOI] [PubMed] [Google Scholar]
- 175.Clementz T, Bednarski JJ, Raetz CR. Function of the htrB high temperature requirement gene of Escherchia coli in the acylation of lipid A: HtrB catalyzed incorporation of laurate. J Biol Chem. 1996;271:12095–102. doi: 10.1074/jbc.271.20.12095. [DOI] [PubMed] [Google Scholar]
- 176.Clementz T, Zhou Z, Raetz CR. Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A. Acylation by MsbB follows laurate incorporation by HtrB. J Biol Chem. 1997;272:10353–60. doi: 10.1074/jbc.272.16.10353. [DOI] [PubMed] [Google Scholar]