Abstract
Endothelial dysfunction is an early indicator of cardiovascular diseases. Increased stimulation of tumor necrosis factor-α (TNF-α) triggers the inflammatory mediator secretion of endothelial cells, leading to atherosclerotic risk. In this study, we investigated whether sulforaphane (SFN) affected the expression of intracellular adhesion molecule-1 (ICAM-1) in TNF-α-induced ECV 304 endothelial cells. Our data showed that SFN attenuated TNF-α-induced expression of ICAM-1 in ECV 304 cells. Pretreatment of ECV 304 cells with SFN inhibited dose-dependently the secretion of proinflammatory cytokines, such as interleukin (IL)-1β, IL-6, and IL-8. SFN inhibited TNF-α-induced nuclear factor-κB (NF-κB) DNA binding activity. Furthermore, SFN decreased TNF-α-mediated phosphorylation of IκB kinase (IKK) and IκBα, Rho A, ROCK, ERK1/2, and plasminogen activator inhibitor-1 (PAI-1) levels. Collectively, SFN inhibited the NF-κB DNA binding activity and downregulated the TNF-α-mediated induction of ICAM-1 in endothelial cells by inhibiting the Rho A/ROCK/NF-κB signaling pathway, suggesting the beneficial effects of SFN on suppression of inflammation within the atherosclerotic lesion.
Key Words: : atherosclerosis, proinflammation, Rho A, sulforaphane, tumor necrosis factor-α
Introduction
Atherosclerosis, a chronic inflammatory disease, results from interaction between modified lipoproteins, monocyte-derived macrophages, foam cells, T cells, and the normal cellular elements of the arterial wall.1 In general, endothelial dysfunction is an early indicator of cardiovascular diseases.2 Recent evidence suggests that stimulation of the pathway by inflammatory stimuli, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1, CD40, oxidized low-density lipoprotein (LDL), and reactive oxygen species (ROS), results in amplification and long-term maintenance of the vascular inflammatory burden, thus facilitating atherogenesis.3 Moreover, several studies have shown that visfatin, TNF-α, and leptin induce expression of inflammatory mediators, including monocyte chemotactic protein-1 (MCP-1), IL-6, IL-8, E-selectin, intracellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1), in human endothelial cells through the nuclear factor-κB (NF-κB) pathway.4–7
On the other hand, the recruitment of immune cells to the inflamed site is an important facet of an inflammatory response and represents a multistage process involving leukocyte rolling, adhesion to the endothelium, and migration through the endothelial cell junction into the surrounding tissues. Each stage of the inflammatory response is triggered by the expression of specific adhesion molecules on the surface of the vascular endothelium.8 Among various endothelial cell adhesion molecules, ICAM-1 is one of the most important active participants in the recruitment of leukocytes to endothelial and inflammatory lesions, which can be upregulated by various proinflammatory cytokines, including TNF-α.9 Blocking or inhibiting the expression of ICAM-1 can decrease the capability of leukocytes to adhere to the endothelium and subsequently inhibits leukocyte emigration.10 The upregulated expression of VCAM-1 and E-selectin11,12 on the endothelial cells alters the adhesive property of the vasculature and it is one of the key events that leads to indiscriminate infiltration of leukocytes across the blood vessels, thus causing inflammation.8
Small G proteins of the Rho family are now known to play a major role in many biological processes, including calcium sensitization of smooth muscle contraction, migration, gene transcription, proliferation, and transformation.13,14 They have also been implicated in the regulation of signal transduction cascades related to inflammation, particularly the JNK and NF-κB pathways.15 The cellular functions and transduction pathways may be relevant to atherogenesis. Although a role for Rho/Rho kinase activation has recently been reported in experimental models of vascular inflammation or injury,16–18 no information concerning the role of Rho kinase in atherosclerosis is available yet.
Growing evidence reveals that dietary supplements and exercise are emerging as effective adjunct therapies targeting endothelial dysfunction and vascular wall inflammation.19–21 In the biological kingdom, sulfur is an important element essential for human survival.22 Sulforaphane (1-isothiocyanato-4-methylsulfinylbutane [SFN]) is a naturally occurring aliphatic isothiocyanate currently under active research as a modulator of multiple cellular targets involved in cellular protection.23–25 SFN was discovered and its antioxidant effects and NF-κB inhibition in various diseases, such as cancer, skin disorders, and spinal cord injury, have been well investigated by Talalay et al.26–28 Furthermore, treating Raw 264.7 macrophages with SFN suppressed lipopolysaccharide (LPS)-induced nitric oxide (NO) generation, PGE2 production, TNF-α secretion, and inducible nitric oxide synthase and COX-2 expression.29 SFN inhibiting LPS-induced activation of AP-1 in Raw 264.7 cells and ultraviolet light-induced activation of AP-1 in human HaCaT keratinocytes were also proved.29–31 Previously, we reported that SFN could mediate migration of smooth muscle cells by reducing MMP-9 activity involving the suppression of the Ras and Rho A signaling pathways (accepted in Journal of Functional Foods). In this study, we further investigate the protective effects and mechanisms of SFN as a potential beneficial regulator of TNF-α-induced inflammation in endothelial cells.
Materials and Methods
Materials and cell line
SFN was obtained from LKT Laboratories, Inc. (St. Paul, MN, USA). TNF-α, dimethyl sulfoxide (DMSO), Tris-base, ethylenediaminetetraacetic acid (EDTA), sodium dodecyl sulfate (SDS), phenylmethylsulfonyl fluoride, bovine serum albumin, leupeptin, Nonidet P-40, deoxycholic acid, and sodium orthovanadate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Phosphate buffer solution (PBS), trypsin-EDTA, and powdered M199 were purchased from Gibco/BRL (Gaithersburg, MD, USA). Matrigel was obtained from BD Biosciences (Bedford, MA, USA). Antibody against ICAM-1 and phosphorylated proteins were purchased from Cell Signaling Tech. (Beverly, MA, USA). PI3K (p85), NF-κB (p65), Ras, Rho A, and ROCK antibodies were obtained from BD Transduction Laboratories (San Diego, CA, USA). The biotin-labeled and unlabeled double-stranded NF-κB consensus oligonucleotide and a mutant double-stranded NF-κB oligonucleotide for electrophoretic mobility shift assay (EMSA) were synthesized by MD Bio, Inc. (Taipei, Taiwan). The β-Galactosidase Enzyme Assay System with the Reporter Lysis Buffer and pSV-β-galactosidase control vector were purchased from Promega Corp., and Great EscAPe™ SEAP Chemiluminescence Detection Kits were obtained from Chontech Laboratories, Inc. (Mountain View, CA, USA). All other chemicals were of the highest quality available. Human endothelial cells ECV 304, obtained from the Clonetics and Bioresources Collection and Research Center, were grown in M199 (supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin). All cell cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 in air. For each experiment, ECV 304 cells at 80% confluence were incubated with SFN at the indicated concentrations for different durations and then stimulated with 5 ng/mL of TNF-α for an additional 6 h.
Analysis of cell viability (MTT assay)
To evaluate the cytotoxicity of SFN, a 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay was performed to determine cell viability. Briefly, ECV 304 cells were seeded in a 24-well plate overnight. Then, the cells were treated with SFN at various concentrations (0, 2.5, 5, and 10 μM) for various periods of time (1, 2, and 3 days). Afterward, the medium was changed and incubated with MTT solution (5 mg/mL/well) for 3 h. The medium was removed, and formazan was solubilized in isopropanol and measured spectrophotometrically at 563 nm.
Flow cytometric analysis
Incubated cells were trypsinized, centrifuged, and then labeled with either 1 μg of a purified mouse IgG1 isotype control (BD Biosciences PharMingen, San Diego, CA, USA), or mouse anti-human ICAM-1 in PBS/2% FBS for 60 min at 4°C. The cells were then washed twice in PBS and incubated with Alexa Fluor® 488 goat anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) diluted 1:500 in PBS/2% fetal calf serum, for 30 min at 4°C in the dark. They were washed, resuspended in 500 μL of PBS, and analyzed. ICAM-1 surface expression on ECV 304 cells (n=4) was assessed in a BD Biosciences FACScan with CellQuest™ Pro software.
RNA isolation and reverse transcriptase–polymerase chain reaction
Total RNA was isolated using Tri-Reagent™ (Molecular Research Center, Inc., Cincinnati, OH, USA), as described by the manufacturer, and RNA extracts were suspended in nuclease-free water. A total of 0.1 μg RNA was used as a template for cDNA synthesis. The reverse transcription was performed with MMMLV reverse transcriptase in a reaction buffer with a final volume of 20 μL comprising 1 mM of each deoxynucleotide triphosphate, 2.5 units RNase inhibitor, and 2.5 mM oligo (dT). For the synthesis of complementary DNA, reaction mixtures were incubated for 15 min at 45°C and stopped by denaturing the reverse transcriptase at 99°C for 5 min. The polymerase chain reaction (PCR) was performed in a thermal cycler (Gene Amp PCR System9700; Applied Biosystems, Foster City, CA, USA) with 1 mM specific primers, 0.2 mM dNTP, 1 mM MgCl2 and 0.05 units/μL DNA polymerase. The primer sequences for human ICAM-1 were 5′-ACTTGCTGCCTATTGGGTATG-3′ (forward) and 5′-TCTCTAATGCGTCATCCCGGA-3′ (reverse) and for mouse β-actin were 5′-CCACAGCTGAGAGGGAAATC-3′ (forward) and 5′-AAGGAAGGCTGGCTAGAGC-3′ (reverse). Amplification products (ICAM-1, 446 bp; β-actin, 192 bp) were resolved by 0.8% agarose gel electrophoresis stained with 0.5 μg/mL ethidium bromide and photographed with the BioDoc-It™ imaging system (Ultraviolet Products, Upland, CA, USA).
Quantification of IL-6, IL-1β, and IL-8 release by ELISA
After incubation with SFN at the indicated concentrations for 24 h and then with 5 ng/mL of TNF-α for an additional 6 h, the cultured medium was harvested and analyzed for the presence of IL-6, IL-1β, and IL-8 using specific quantitative enzyme-linked immunosorbent assay (ELISA) kits (R&D System Quantikine, Pittsburgh, PA, USA). Each sample was tested in triplicate.
Western blotting analysis
To analyze the migration-related proteins, western blotting was performed as follows. First, 50 μg purified protein was mixed with a 5×sample buffer and boiled for 10 min. Then, equal protein contents of total cell lysate from the control and SFN-treated samples were resolved on 10–12% SDS–polyacrylamide gel electrophoresis (PAGE) gels. Proteins were then transferred onto nitrocellulose membranes (Millipore, Bedford, MA, USA) by electroblotting using an electroblotting apparatus (Bio-Rad, Hercules, CA, USA). Nonspecific binding of the membranes was blocked with Tris-buffered saline (TBS) containing 0.01 g/mL nonfat dry milk and 0.1% (v/v) Tween-20 (TBST) for more than 2 h. Membranes were washed with TBST thrice for 10 min and incubated with an appropriate dilution of specific primary antibodies in TBST overnight at 4°C. Subsequently, the membranes were washed with TBST and incubated with an appropriate secondary antibody (horseradish peroxidase-conjugated goat antimouse or antirabbit IgG) for 1 h. After washing the membrane thrice for 10 min in TBST, band detection was revealed by enhanced chemiluminescence using ECL Western blotting detection reagents and exposed ECL hyperfilm in FUJFILM Las-3000 (Tokyo, Japan). Then, proteins were quantitatively determined by densitometry using FUJFILM-Multi Gauge V2.2 software.
Statistical analysis
Data are expressed as the mean±standard deviation of three independent experiments and analyzed by the Student's t-test (Sigmaplot 11.0). Significant differences were established at P≤.05.
Results
Cytotoxicity of SFN in ECV 304 cells for different time intervals
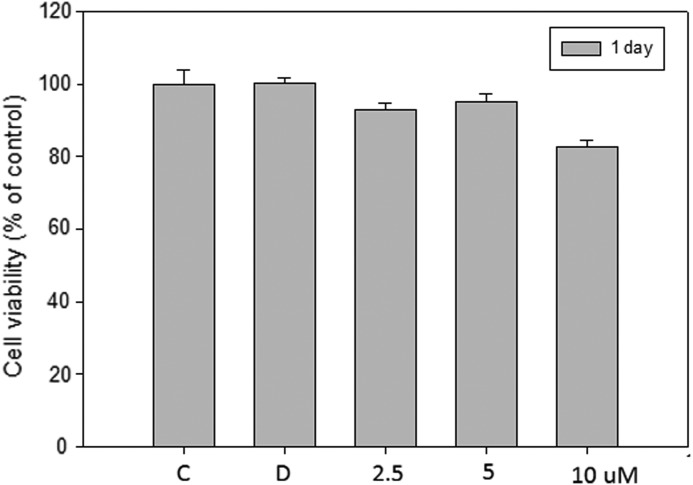
To assay the effect of SFN on cell viability, ECV 304 cells were treated with 2.5, 5, and 10 μM SFN for 24 h and viability determined by MTT assay. Cells treated with SFN at a concentration of 10 μM had 80% viability for 24 h (Fig. 1). After treatment with SFN (10 μM) for 2 and 3 days, over 50% of the viability of cells were decreased (data not shown), whereas 80% cell viability was observed at lower concentrations of SFN (2.5 and 5 μM). In the following experiments, SFN was used at doses below 10 μM for 1 day.
FIG. 1.
Effect of sulforaphane (SFN) on the viability of ECV 403 cells. Cells (1×104 cells/mL) were treated with various concentrations (0, 2.5, 5, and 10 μM) of SFN for 1 day. The viability of the cells was determined by MTT assay. The survival cell number was directly proportional to formazan, which was measured spectrophotometrically at 563 nm. Values are expressed as the mean±SD of three independent experiments. C, control group; D, dimethyl sulfoxide (DMSO) solvent control.
SFN inhibits ICAM-1 expression of ECV 304 cells
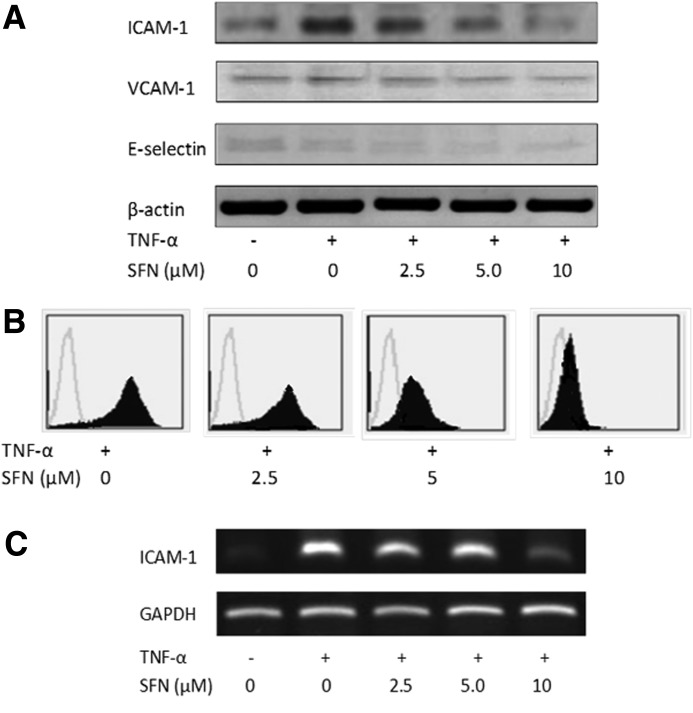
As cell adhesion molecules play an important role during inflammation, their expression in endothelial cells is a prerequisite for adhesion of neutrophils. Therefore, the effects of SFN on TNF-α-induced expressions of ICAM-1, VCAM-1, and E-selectin expression were analyzed by western blotting, as detailed in the Materials and Methods section. The unstimulated cells expressed low levels of ICAM-1, VCAM-1, and E-selectin. Our results demonstrated that VCAM-1 and E-selectin were expressed at low levels on ECV 304 cells upon stimulation with TNF-α (Fig. 2A). However, a 4.5-fold increase in the expression of ICAM-1 was observed upon stimulation with TNF-α compared with the untreated control group. Our data showed that SFN suppressed TNF-α-induced ICAM-1 expression in ECV 304 cells and similar results were observed by flow cytometry and reverse transcriptase–polymerase chain reaction (Fig. 2B, C). Collectively, these results suggested that SFN was effective for blocking the induced expression of ICAM-1.
FIG. 2.
Detection of cell surface expression of intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin. ECV 304 cells were pretreated with different concentrations of SFN for 18 h and induced by tumor necrosis factor-α (TNF-α) for 6 h. (A) The protein levels of ICAM-1, VCAM-1, and E-selectin were detected by western blotting. Flow cytometry (B) and reverse transcriptase–polymerase chain reaction (C) were used to assay the ICAM-1 expression suppressed by SFN. The figure is representative of three independent experiments with similar results.
SFN attenuated TNF-α-induced proinflammatory cytokines expression of ECV 304 cells
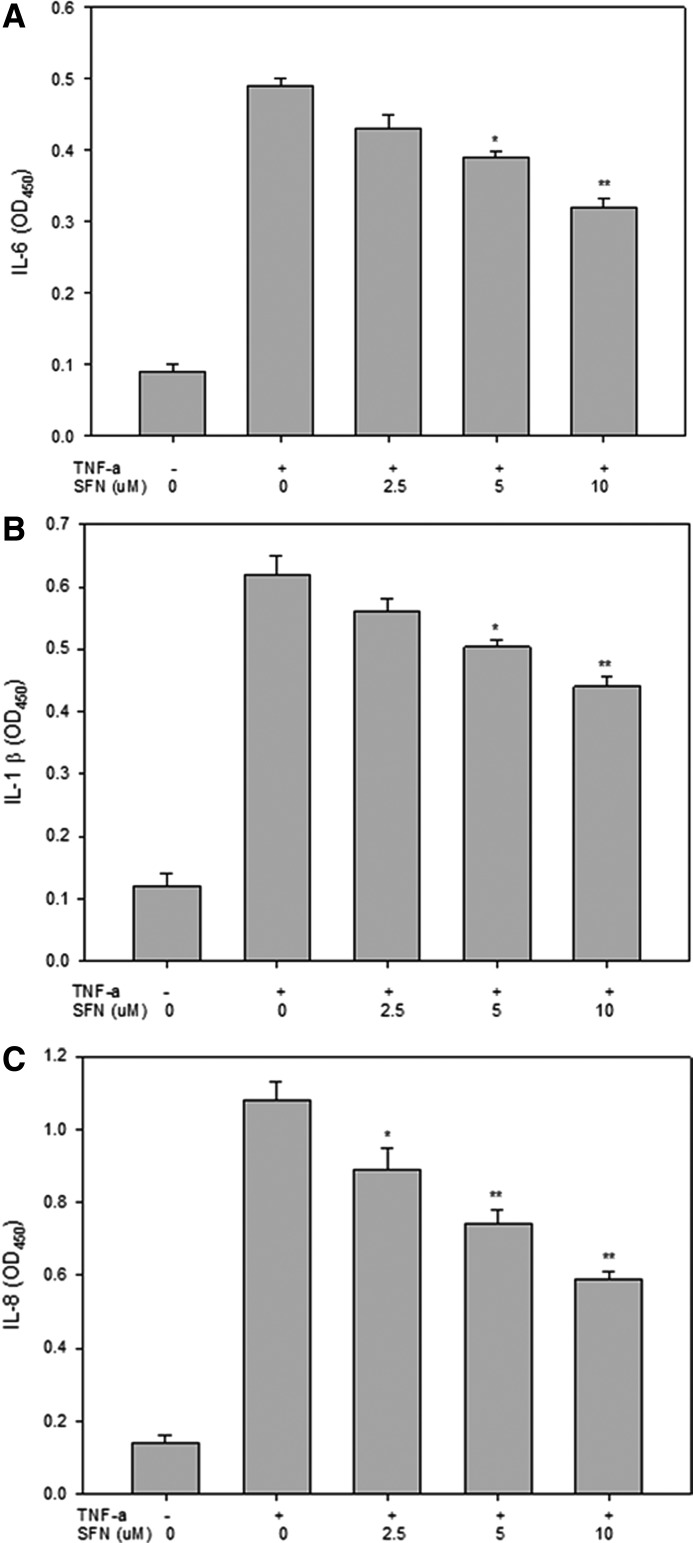
TNF-α is a potent inducer of inflammatory reactions and oxidative stress in endothelial cells. To investigate the effect of SFN on TNF-α-mediated secretion of proinflammatory mediators, we incubated ECV 304 cells with various concentrations of SFN for 18 h, followed by stimulation with 5 ng/mL of TNF-α for an additional 6 h. Then, IL-6, IL-1β, and IL-8 levels were measured in the cell culture medium using cell-ELISA. Unstimulated ECV 304 endothelial cells released low levels of IL-6, IL-1β, and IL-8 (Fig. 3). TNF-α treatment increased the release of these cytokines compared with non-TNF-α-treated control cells. As shown in Figure 3A and B respectively, SFN inhibited TNF-α-induced IL-6 and IL-1β secretion at 5 μM (P<.01) and 10 μM (P<.001). Furthermore, SFN at 2.5 and 5 μM significantly reduced IL-8 expression (P<.01 and P<.001, respectively; Fig. 3C).
FIG. 3.
SFN reduced TNF-α-induced IL-6, IL-1β and IL-8 expression in ECV 304 cells. After pretreatment with various concentrations of SFN and induced by TNF-α, the medium was collected. IL-6 (A), IL-1β (B), or IL-8 (C) protein secretions were measured by enzyme-linked immunosorbent assays. Statistical analysis was performed using the Student's unpaired t-test. Significant effect (*P<.001 and **P<.0001) on IL-6, IL-1β, and IL-8 protein secretion compared with the DMSO group.
Effect of SFN on TNF-α-induced NF-κB DNA binding activity
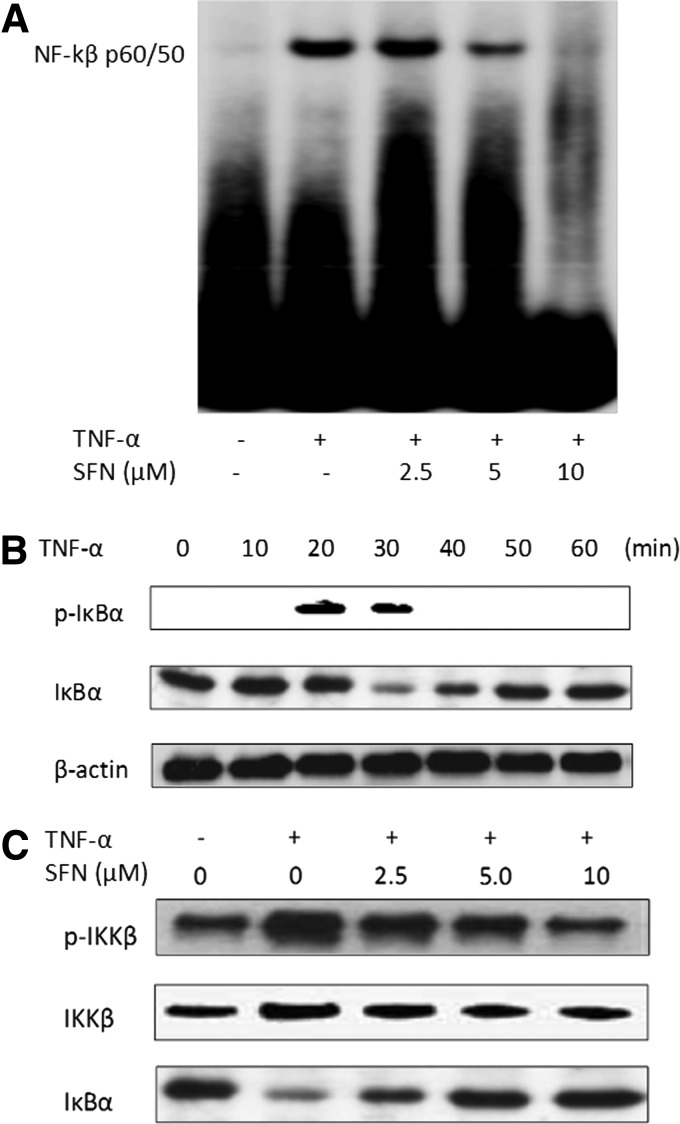
The presence of an NF-κB binding site is critical for ICAM-1 gene promoter activity; the effect of SFN on NF-κB was also assayed as follows. In Figure 4A, NF-κB was not detectable in the nucleus of unstimulated cells. Upon stimulation with TNF-α, there was an increase in NF-κB levels, thus causing substantial retardation in the mobility of the labeled oligonucleotide with high intensity of the shifted band. The enhanced DNA binding activity was dose dependently inhibited by pretreatment of SFN. Analysis of ECV 304 extracts using the IκBα antibody showed that rapid IκBα phosphorylation was caused by stimulating the cells with TNF-α for 20 min and its degradation occurred after 30 min of TNF-α exposure (Fig. 4B). In addition, we demonstrated that activation of IκB kinase (IKK)β, which is responsible for the phosphorylation and degradation of IκBα, was inhibited by SFN pretreatment (Fig. 4C). Taken together, these results clearly indicated that SFN inhibited the activation of the NF-κB induced by TNF-α.
FIG. 4.
SFN inhibited the activation of the nuclear factor-κB (NF-κB) pathway induced by TNF-α stimulation. (A) ECV 304 cells were pretreated for 18 h with indicated concentrations of SFN and stimulated with TNF-α subsequently. Nuclear extracts from each group were subjected to the electrophoretic mobility shift assay (EMSA) for analyzing the DNA binding activity of NF-κB. (B) The cells were preincubated with SFN and stimulated with TNF-α for the indicated periods. Cell lysates from each group were examined by immunoblotting analysis using p-IκBα and IκBα antibodies. (C) Cell lysates from each group were examined by immunoblotting analysis using p-IKKβ and IκB kinase (IKK)β antibodies. At least three independent experiments were performed, with similar results.
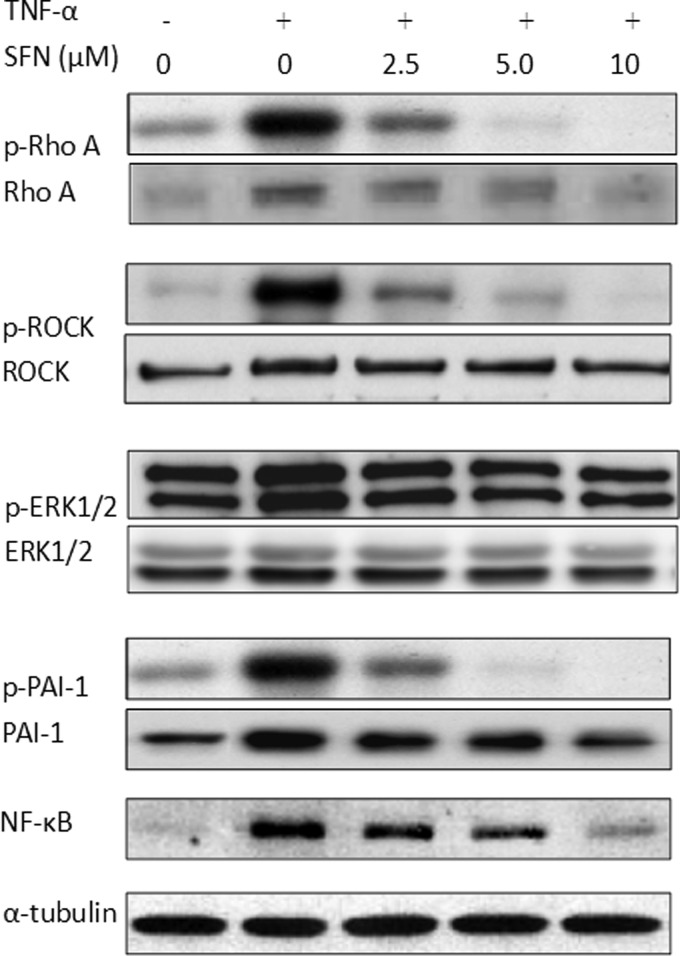
SFN inhibited TNF-α-induced Rho kinase and plasminogen activator inhibitor-1 expression
Some pathways regulated by TNF-α can promote inflammation of endothelial cells.32 We assayed the levels of Rho-related proteins, ERK1/2, and plasminogen activator inhibitor-1 (PAI-1) using western blotting. Exposure of endothelial cells to TNF-α increased phosphorylations of Rho A, ROCK, and PAI-1 levels, which were blocked by pretreatment with SFN for 24 h in a dose-dependent manner (Fig. 5). Sulforaphane also inhibited TNF-α-induced activation of p-ERK. We demonstrated that SFN attenuated both the NF-κB DNA binding activity and also the protein levels. These results indicated that SFN inhibited TNF-α-induced NF-κB-activated inflammation by regulating the Rho A-related pathways.
FIG. 5.
The expression of signal transduction proteins by the ECV 403 cells treated with SFN. Total cell lysate was extracted and the expression of Rho A, ROCK, ERK1/2, NF-κB, and plasminogen activator inhibitor-1 (PAI-1) proteins was assayed by western blotting; α-tubulin was the loading control. The figure is a representative of three independent experiments with similar results.
Discussion
TNF-α is known to amplify several signaling pathways leading to vascular inflammation, apoptosis, oxidative stress, and thrombosis, which are key mediators in the pathogenesis of vascular dysfunction. Accumulating evidence suggests that TNF-α induces vascular inflammation, monocyte adhesion to endothelial cells, vascular oxidative stress, apoptosis, and atherogenic response and participates in the regulation of thrombosis and coagulation through NF-κB, Sp1, JNK, p38, and STAT3 pathways.33 Dietary supplements and exercise training decrease TNF-α production and ameliorate TNF-α-mediated pathological changes in vasculature. Thus, the inhibitory effects of dietary supplements, including fish oil, dietary fibers, and various natural products, as well as physical exercise on TNF-α production and TNF-α signaling may contribute to their vasoprotective properties. However, the mechanisms underlying the vasoprotective benefits of dietary supplements and physical activity demand extensive investigation. In this study, we demonstrated that SFN inhibited the TNF-α-induced inflammation of endothelial cells through Rho A/ROCK and NF-κB pathways, and then decreased the secretion of proinflammatory mediators (IL-1, IL-6, and IL-8).
The involvement of Rho A/ROCK in cardiovascular pathophysiology is confirmed by the following evidences: (i) Rho A/ROCK activation of NAD(P)H oxidase,34 which contributes to the induction of oxidative stress; (ii) Rho A/ROCK control of the atherothrombogenetic PAI-135; (iii) demonstration of Rho A/ROCK signaling involvement in C-reactive protein (CRP)-induced atherothrombogenesis through the CRP-mediated Rho A/ROCK-induced increased NF-κB activity, resulting in an increase of the PAI-1 pathway36; and (iv) an abnormality in glucose transport and metabolism leading to angiotensin II-induced insulin resistance,37 and Rho A/ROCK-mediated inhibition of PI3K/Akt.38 Eto et al. reported that dominant negative Rho kinase suppressed neointimal formation after balloon injury in pigs.39 In addition, specific inactivation of Rho A/Cdc42/Rac1 reduced LPS-induced NF-κB phosphorylation and IL-6/TNF-α protein production.40 Hyperglycemia stimulates Rho-kinase activity through PKC- and oxidative stress-dependent pathways, leading to increased PAI-1 gene transcription.41 These results suggest that inhibition of Rho A/ROCK may be a novel therapeutic target for preventing thromboembolic complications of diabetes and cardiovascular disease. Accordingly, numerous studies have demonstrated that SFN inhibited TNF-α- or LPS-induced activation of NF-κB, ICAM-1, and VCAM-1 expression in endothelial cells.42,43 However, the expression of ICAM-1 was induced after cell activation by proinflammatory mediators such as IL-1β, IL-6, and IL-8 (Fig. 3). Furthermore, our data provided new information about the association between ICAM-1 and the important role of Rho A/ROCK under TNF-α induction. In this study, we showed that treatment of SFN attenuated TNF-α-induced Rho A- and NF-κB-related inflammation of endothelial cells, suggesting cardiovascular protection by SFN.
During the past few decades, dietary supplements, which delay or prevent the development of vascular disorders, have been found to act through regulating various adhesion molecules triggered by NF-κB. EGCG decreased TNF-α-induced fractalkine expression by suppressing NF-κB44 and inhibited TNF-α-induced MCP-1 production and the activation of AP-1 in vascular endothelial cells using heme oxygenase-1 (HO-1)-dependent mechanisms.45,46 Apigenin is a flavone that is the aglycone of apiin isolated from parsley and celery. Apigenin profoundly blocked monocyte adhesion to the monolayer of human umbilical vein endothelial cells (HUVECs) by suppressing TNF-α-stimulated upregulation of VCAM-1, ICAM-1, and E-selectin mRNA expression.47 Curcumin also decreased the intracellular ROS levels, phosphorylation of JNK, p38, and STAT3, and the expression of ICAM-1, MCP-1, IL-8, and lectin-like oxidized LDL receptor-1 (LOX-1) in TNF-α-stimulated HUVECs.48,49 In the present study, pretreatment with SFN inhibited significantly NF-κB-induced IL-1, IL-6, IL8, and ICAM expressions, indicating that SFN has similar anti-inflammatory and vasoprotective properties.
SFN has been known to have some very wide-ranging health effects, including anticarcinogenic, anti-inflammatory, and antimicrobial properties, in experimental models. Supporting its anti-inflammatory and cardiovascular protective properties, SFN can inhibit TNF-α-induced adhesion molecule expression through the inhibition of MAPK, NF-κB, and AP-1 signaling pathways and intracellular ROS production.50 Furthermore, SFN inhibits neointima formation using targeting adhesion molecules through the suppression of NF-κB/GATA6, and regulates migration and proliferation in VSMCs.51 SFN also induces multiple anti-oxidant enzymes through activation of Nrf2 transcription factor52 and inhibits EL expression through inhibition of NF-κB, which may have a beneficial effect on HDL cholesterol levels.53 This is the first report showing the inhibitory effect of SFN on TNF-α-induced expression of proinflammatory cytokines through the Rho A/ROCK pathway in endothelial cells. Thus, SFN may exert multiple effects in suppressing inflammation within the atherosclerotic lesion and may have potential for therapeutic use in preventing atherosclerotic lesions and other inflammatory diseases.
Acknowledgment
This work was supported by a grant from the National Science Council (NSC 99-2321-B-040-001), Taiwan.
Author Disclosure Statment
No potential conflicts of interest exist.
References
- 1.Glass CK, Witztum JL: Atherosclerosis. The road ahead. Cell 2001;104:503–516 [DOI] [PubMed] [Google Scholar]
- 2.Ding H, Triggle CR: Endothelial cell dysfunction and the vascular complications associated with type 2 diabetes: assessing the health of the endothelium. Vasc Health Risk Manag 2005;1:55–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sattar N: Inflammation and endothelial dysfunction: intimate companions in the pathogenesis of vascular disease? Clin Sci (Lond) 2004;106:443–445 [DOI] [PubMed] [Google Scholar]
- 4.Zoico E, Garbin U, Olioso D, Mazzali G, Fratta Pasini AM, Di Francesco V, et al. : The effects of adiponectin on interleukin-6 and MCP-1 secretion in lipopolysaccharide-treated 3T3-L1 adipocytes: role of the NF-kappaB pathway. Int J Mol Med 2009;24:847–851 [DOI] [PubMed] [Google Scholar]
- 5.Kim SR, Bae YH, Bae SK, Choi KS, Yoon KH, Koo TH, et al. : Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochim Biophys Acta 2008;1783:886–895 [DOI] [PubMed] [Google Scholar]
- 6.Lee WJ, Wu CS, Lin H, Lee IT, Wu CM, Tseng JJ, et al. : Visfatin-induced expression of inflammatory mediators in human endothelial cells through the NF-kappaB pathway. Int J Obes (Lond) 2009;33:465–472 [DOI] [PubMed] [Google Scholar]
- 7.Rahman I, Gilmour PS, Jimenez LA, MacNee W: Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol Cell Biochem 2002;234–235:239–248 [PubMed] [Google Scholar]
- 8.Krieglstein CF, Granger DN: Adhesion molecules and their role in vascular disease. Am J Hypertens 2001;14(6 Pt 2):44S–54S [DOI] [PubMed] [Google Scholar]
- 9.Ren DC, Du GH, Zhang JT: Inhibitory effect of the water-soluble extract of Salvia miltiorrhiza on neutrophil-endothelial adhesion. Jpn J Pharmacol 2002;90:276–280 [DOI] [PubMed] [Google Scholar]
- 10.Clark PR, Manes TD, Pober JS, Kluger MS: Increased ICAM-1 expression causes endothelial cell leakiness, cytoskeletal reorganization and junctional alterations. J Invest Dermatol 2007;127:762–774 [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Bussolino F, Introna M: Cytokine regulation of endothelial cell function: from molecular level to the bedside. Immunol Today 1997;18:231–240 [DOI] [PubMed] [Google Scholar]
- 12.Springer TA: Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 1994;76:301–314 [DOI] [PubMed] [Google Scholar]
- 13.Bar-Sagi D, Hall A: Ras and Rho GTPases: a family reunion. Cell 2000;103:227–238 [DOI] [PubMed] [Google Scholar]
- 14.Bishop AL, Hall A: Rho GTPases and their effector proteins. Biochem J 2000;348(Pt 2):241–255 [PMC free article] [PubMed] [Google Scholar]
- 15.Montaner S, Perona R, Saniger L, Lacal JC: Multiple signalling pathways lead to the activation of the nuclear factor kappaB by the Rho family of GTPases. J Biol Chem 1998;273:12779–12785 [DOI] [PubMed] [Google Scholar]
- 16.Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, et al. : Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 2002;39:245–250 [DOI] [PubMed] [Google Scholar]
- 17.Shibata R, Kai H, Seki Y, Kato S, Morimatsu M, Kaibuchi K, et al. : Role of Rho-associated kinase in neointima formation after vascular injury. Circulation 2001;103:284–289 [DOI] [PubMed] [Google Scholar]
- 18.Shimokawa H, Morishige K, Miyata K, Kandabashi T, Eto Y, Ikegaki I, et al. : Long-term inhibition of Rho-kinase induces a regression of arteriosclerotic coronary lesions in a porcine model in vivo. Cardiovasc Res 2001;51:169–177 [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, et al. : Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116:219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, et al. : Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation 2004;109:1981–1986 [DOI] [PubMed] [Google Scholar]
- 21.Watts K, Beye P, Siafarikas A, Davis EA, Jones TW, O'Driscoll G, et al. : Exercise training normalizes vascular dysfunction and improves central adiposity in obese adolescents. J Am Coll Cardiol 2004;43:1823–1827 [DOI] [PubMed] [Google Scholar]
- 22.Atmaca G: Antioxidant effects of sulfur-containing amino acids. Yonsei Med J 2004;45:776–788 [DOI] [PubMed] [Google Scholar]
- 23.Bertl E, Bartsch H, Gerhauser C: Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther 2006;5:575–585 [DOI] [PubMed] [Google Scholar]
- 24.Schwab M, Reynders V, Loitsch S, Steinhilber D, Schroder O, Stein J: The dietary histone deacetylase inhibitor sulforaphane induces human beta-defensin-2 in intestinal epithelial cells. Immunology 2008;125:241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thejass P, Kuttan G: Immunomodulatory activity of sulforaphane, a naturally occurring isothiocyanate from broccoli (Brassica oleracea). Phytomedicine 2007;14:538–545 [DOI] [PubMed] [Google Scholar]
- 26.Benedict AL, Mountney A, Hurtado A, Bryan KE, Schnaar RL, Dinkova-Kostova AT, et al. : Neuroprotective effects of sulforaphane after contusive spinal cord injury. J Neurotrauma 2012;29:2576–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD, Chen JG, et al. : Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Top Curr Chem 2013;329:163–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talalay P, Fahey JW, Healy ZR, Wehage SL, Benedict AL, Min C, et al. : Sulforaphane mobilizes cellular defenses that protect skin against damage by UV radiation. Proc Natl Acad Sci USA 2007;104:17500–17505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem 2001;276:32008–32015 [DOI] [PubMed] [Google Scholar]
- 30.Woo KJ, Kwon TK: Sulforaphane suppresses lipopolysaccharide-induced cyclooxygenase-2 (COX-2) expression through the modulation of multiple targets in COX-2 gene promoter. Int Immunopharmacol 2007;7:1776–1783 [DOI] [PubMed] [Google Scholar]
- 31.Zhu M, Zhang Y, Cooper S, Sikorski E, Rohwer J, Bowden GT: Phase II enzyme inducer, sulforaphane, inhibits UVB-induced AP-1 activation in human keratinocytes by a novel mechanism. Mol Carcinog 2004;41:179–186 [DOI] [PubMed] [Google Scholar]
- 32.Calo LA, Pessina AC: RhoA/Rho-kinase pathway: much more than just a modulation of vascular tone. Evidence from studies in humans. J Hypertens 2007;25:259–264 [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Zhang C: Vasoprotection by dietary supplements and exercise: role of TNFalpha signaling. Exp Diabetes Res 2012;2012:972679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, et al. : Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res 2003;93:767–775 [DOI] [PubMed] [Google Scholar]
- 35.Takeda K, Ichiki T, Tokunou T, Iino N, Fujii S, Kitabatake A, et al. : Critical role of Rho-kinase and MEK/ERK pathways for angiotensin II-induced plasminogen activator inhibitor type-1 gene expression. Arterioscler Thromb Vasc Biol 2001;21:868–873 [DOI] [PubMed] [Google Scholar]
- 36.Nakakuki T, Ito M, Iwasaki H, Kureishi Y, Okamoto R, Moriki N, et al. : Rho/Rho-kinase pathway contributes to C-reactive protein-induced plasminogen activator inhibitor-1 expression in endothelial cells. Arterioscler Thromb Vasc Biol 2005;25:2088–2093 [DOI] [PubMed] [Google Scholar]
- 37.Sowers JR: Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol 2004;286:H1597–H1602 [DOI] [PubMed] [Google Scholar]
- 38.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, et al. : Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol 2004;24:1842–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eto Y, Shimokawa H, Hiroki J, Morishige K, Kandabashi T, Matsumoto Y, et al. : Gene transfer of dominant negative Rho kinase suppresses neointimal formation after balloon injury in pigs. Am J Physiol Heart Circ Physiol 2000;278:H1744–H1750 [DOI] [PubMed] [Google Scholar]
- 40.Guo F, Xing Y, Zhou Z, Dou Y, Tang J, Gao C, et al. : Guanine-nucleotide exchange factor H1 mediates lipopolysaccharide-induced interleukin 6 and tumor necrosis factor alpha expression in endothelial cells via activation of nuclear factor kappaB. Shock 2012;37:531–538 [DOI] [PubMed] [Google Scholar]
- 41.Rikitake Y, Liao JK: Rho-kinase mediates hyperglycemia-induced plasminogen activator inhibitor-1 expression in vascular endothelial cells. Circulation 2005;111:3261–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen XL, Dodd G, Kunsch C: Sulforaphane inhibits TNF-a-induced activation of p38 MAP kinase and VCAM-1 and MCP-1 expression in endothelial cells. Inflamm Res 2009;58:513–521 [DOI] [PubMed] [Google Scholar]
- 43.Liu YC, Hsieh CW, Weng YC, Chuang SH, Hsieh CY, Wung BS: Sulforaphane inhibition of monocyte adhesion via the suppression of ICAM-1 and NF-kappaB is dependent upon glutathione depletion in endothelial cells. Vascul Pharmacol 2008;48:54–61 [DOI] [PubMed] [Google Scholar]
- 44.Lee AS, Jung YJ, Kim DH, Lee TH, Kang KP, Lee S, et al. : Epigallocatechin-3-O-gallate decreases tumor necrosis factor-alpha-induced fractalkine expression in endothelial cells by suppressing NF-kappaB. Cell Physiol Biochem 2009;24:503–510 [DOI] [PubMed] [Google Scholar]
- 45.Zheng Y, Toborek M, Hennig B: Epigallocatechin gallate-mediated protection against tumor necrosis factor-alpha-induced monocyte chemoattractant protein-1 expression is heme oxygenase-1 dependent. Metabolism 2010;59:1528–1535 [DOI] [PubMed] [Google Scholar]
- 46.Ahn HY, Xu Y, Davidge ST: Epigallocatechin-3-O-gallate inhibits TNFalpha-induced monocyte chemotactic protein-1 production from vascular endothelial cells. Life Sci 2008;82:964–968 [DOI] [PubMed] [Google Scholar]
- 47.Lee JH, Zhou HY, Cho SY, Kim YS, Lee YS, Jeong CS: Anti-inflammatory mechanisms of apigenin: inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch Pharm Res 2007;30:1318–1327 [DOI] [PubMed] [Google Scholar]
- 48.Kim YS, Ahn Y, Hong MH, Joo SY, Kim KH, Sohn IS, et al. : Curcumin attenuates inflammatory responses of TNF-alpha-stimulated human endothelial cells. J Cardiovasc Pharmacol 2007;50:41–49 [DOI] [PubMed] [Google Scholar]
- 49.Lee HS, Lee MJ, Kim H, Choi SK, Kim JE, Moon HI, et al. : Curcumin inhibits TNFalpha-induced lectin-like oxidised LDL receptor-1 (LOX-1) expression and suppresses the inflammatory response in human umbilical vein endothelial cells (HUVECs) by an antioxidant mechanism. J Enzyme Inhib Med Chem 2010;25:720–729 [DOI] [PubMed] [Google Scholar]
- 50.Kim JY, Park HJ, Um SH, Sohn EH, Kim BO, Moon EY, et al. : Sulforaphane suppresses vascular adhesion molecule-1 expression in TNF-alpha-stimulated mouse vascular smooth muscle cells: involvement of the MAPK, NF-kappaB and AP-1 signaling pathways. Vascul Pharmacol 2012;56:131–141 [DOI] [PubMed] [Google Scholar]
- 51.Kwon JS, Joung H, Kim YS, Shim YS, Ahn Y, Jeong MH, et al. : Sulforaphane inhibits restenosis by suppressing inflammation and the proliferation of vascular smooth muscle cells. Atherosclerosis 2012;225:41–49 [DOI] [PubMed] [Google Scholar]
- 52.Evans PC: The influence of sulforaphane on vascular health and its relevance to nutritional approaches to prevent cardiovascular disease. EPMA J 2011;2:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kivela AM, Makinen PI, Jyrkkanen HK, Mella-Aho E, Xia Y, Kansanen E, et al. : Sulforaphane inhibits endothelial lipase expression through NF-kappaB in endothelial cells. Atherosclerosis 2010;213:122–128 [DOI] [PubMed] [Google Scholar]