Abstract
Background
Guidelines recommend metformin as the first-line oral treatment for type 2 diabetes. We conducted a systematic review to assess whether the use of second- and third-generation sulfonylurea agents is associated with benefits and harms in terms of patient-important outcomes compared with metformin.
Methods
We searched several electronic databases and other sources for randomized clinical trials published to August 2011. We included trials that compared sulfonylurea versus metformin monotherapy among patients 18 years or older with type 2 diabetes and that had an intervention period of at least 24 weeks. We assessed risk of bias and extracted data related to interventions and outcomes. The risk of random errors was assessed by trial sequential analysis.
Results
We included 14 trials (4560 participants). All trials were judged to be at high risk of bias. Data on patient-important outcomes were sparse. Compared with metformin, sulfonylurea did not significantly affect all-cause mortality (relative risk [RR] 0.98, 95% confidence interval [CI] 0.61 to 1.58) or cardiovascular mortality (RR 1.47, 95% CI 0.54 to 4.01). Sulfonylurea significantly decreased the risk of nonfatal macrovascular outcomes (RR 0.67, 95% CI 0.48 to 0.93). However, the definition of this outcome varied among trials, and trial sequential analysis showed that more trials are needed before reliable conclusions can be drawn. No differences between sulfonylurea and metformin were found for change in fasting blood glucose level or glycosylated hemoglobin concentration in the random-effects model. Sulfonylurea resulted in greater weight gain compared with metformin, a finding confirmed in the trial sequential analysis. Significantly more patients in the sulfonylurea arm than in the metformin arm had mild hypoglycemia (RR 2.95, 95% CI 2.13 to 4.07) and severe hypoglycemia (RR 5.64, 95% CI 1.22 to 26.00).
Interpretation
Some evidence suggests that, compared with metformin, second- and third-generation sulfonylureas may not affect all-cause or cardiovascular mortality but may decrease the risk of nonfatal macrovascular outcomes among patients with type 2 diabetes. They may also increase the risk of hypoglycemia. In general, the available data were too few and inconsistent to provide firm evidence concerning patient-important outcomes in relation to the benefits and harms of sulfonylurea versus metformin monotherapy.
The American Diabetes Association and the European Association for the Study of Diabetes consensus algorithm for the treatment of type 2 diabetes recommends beginning metformin treatment at diagnosis or soon after, along with lifestyle interventions.1 For patients who cannot use metformin, another oral antidiabetic agent might be prescribed, for example a sulfonylurea. The rationale for recommending metformin as the drug of choice for type 2 diabetes seems to be based on its perceived beneficial effect on conventional surrogate outcomes (e.g., weight, tolerability and cost),1 on the United Kingdom Prospective Diabetes Study (UKPDS) 34 outcomes in a selected subgroup of 342 obese patients2 and on findings from observational studies.3–6
Sulfonylureas are divided into classes. The first-generation agents (carbutamide, tolbutamide, acetohexamide, tolazomide and chlorpropamide) were introduced for diabetes treatment in the 1950s.1,7–9 The second-generation agents (e.g., glibenclamide, glipizide, glibornuride and gliclazide) and the third-generation agents (glimepiride, gliclazide modified-release and glipizide gastrointestinal therapeutic system) have almost completely replaced the first-generation drugs. The second- and third-generation sulfonylureas are preferred because of their perceived greater potency and perceived better safety profiles.1,7–9
The purpose of this systematic review was to determine whether the use of second- and third-generation sulfonylurea agents is associated with benefits and harms in terms of patient-important outcomes among patients with type 2 diabetes compared with the use of metformin.
Methods
This review follows the recommendations of The Cochrane Collaboration10 and is based on our published Cochrane protocol.11 We included randomized clinical trials comparing sulfonylurea monotherapy with other antidiabetic interventions, placebo or no intervention.11,12 Trials were analyzed according to the class of sulfonylurea used. In this article, we report our findings from the comparison of second- and third-generation sulfonylurea versus metformin monotherapy, because this comparison is currently of greatest clinical relevance. The main Cochrane review reports all comparisons.12 Sensitivity analyses for all dichotomous outcomes, including trials with 0 events, are reported in this article only and not the main Cochrane review.
Search strategy
We searched The Cochrane Library, MEDLINE, Embase, Science Citation Index Expanded, the Latin American and Caribbean Health Sciences Literature (LILACS) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) for randomized clinical trials published to August 2011 that compared sulfonylurea monotherapy with other antidiabetic interventions, placebo or no intervention in patients with type 2 diabetes. The terms and strategies used to search each database are provided in Appendix 1 (available at www.cmajopen.ca/content/2/3/E162/suppl/DC1). We also searched abstracts presented at congresses of the American Diabetes Association and the European Association for the Study of Diabetes. In addition, we searched reference lists of the included trials, systematic reviews, meta-analyses and health technology assessments. We contacted trial authors and a pharmaceutical company for additional trial data and to obtain information about additional unpublished trials; and we consulted the website of the US Food and Drug Administration.
Trial selection
To determine which documents to assess further, two authors (B.H. with J.B.S., L.H.L. or T.A.) independently screened the abstracts, titles or both. All potentially relevant documents were obtained as full text. Disagreements were resolved by discussion or, if necessary, by a third party (J.W. or C.G.).
A trial was considered eligible if it was a randomized clinical trial (crossover or parallel) evaluating adult patients (≥ 18 yr) with type 2 diabetes; it had a period of intervention of 24 weeks or more; and it compared sulfonylurea monotherapy versus metformin monotherapy.11,12 We included trials irrespective of outcomes reported or language or whether escape medicine was allowed if monotherapy failed.11,12
Data extraction and bias assessment
Two authors (B.H. with J.B.S., D.P.S., L.H.L. or T.A.) independently extracted information from each included trial using standard data extraction forms and assessed the risk of bias as advised in the Cochrane Handbook for Systematic Reviews of Interventions.10
We assessed the following risk-of-bias domains: sequence generation, concealment of allocation, blinding of participants and investigators, blinding of outcome assessors, completeness of outcome data, selective outcome reporting academic bias and sponsor bias. We classified risk of bias for each domain as low, uncertain or high11,12 (Appendix 2, www.cmajopen.ca/content/2/3/E162/suppl/DC1). Discrepancies between assessments were resolved by involvement of a third author (S.S.L., J.W., C.G. or A.V.). We divided the trials into those with a high risk of bias and those with a lower risk based on assessment of sequence generation, allocation concealment and blinding.10 When we judged those 3 domains to be adequate, we designated the trial as having a lower risk of bias.
We extracted baseline characteristics (e.g., age, duration of disease and glycosylated hemoglobin [HbA1c] concentration) and outcomes from the included trials. Our predefined outcomes were all-cause mortality, cardiovascular mortality, nonfatal macrovascular outcomes as a composite outcome, nonfatal myocardial infarction, nonfatal stroke, amputation of lower extremity, cardiac or peripheral revascularization, microvascular outcomes as a composite outcome, nephropathy, retinal photocoagulation, adverse events, serious adverse events, drop-outs due to adverse events, mild hypoglycemia, severe hypoglycemia, cancer, intervention failure, change in fasting blood glucose level from baseline, change in HbA1c concentration from baseline, change in body mass index (BMI) from baseline, change in weight from baseline, quality of life and costs of intervention.11,12 We sought relevant missing information from the original author(s) of the randomized trial. When we identified more than one publication of an original trial, we assessed these together to maximize data collection. In case of substantial disagreements between older and newer publications, we contacted the authors.11,12
Translators extracted data from all relevant non-English articles.
Statistical analysis
We analyzed data using Review Manager software (RevMan version 5.1.7; Nordic Cochrane Centre, The Cochrane Collaboration, 2011). Medians reported in the included trials were assumed to be close to the arithmetic mean. Reported standard errors and confidence intervals were converted to standard deviations. We used both a random-effects model and a fixed-effect model.13,14 In cases of differences in statistical significance of the effect estimate between the two models, we reported both results; otherwise, we reported results of the random-effects model.11,12
We tested for heterogeneity using the I2 statistic.10 An I2 value of 50% or more indicated substantial heterogeneity.10
Trial sequential analysis
Trial sequential analysis in a meta-analysis is similar to interim analysis of a single trial, where group sequential monitoring boundaries are used to decide whether a trial could be ended early if a p value is sufficiently small to show the anticipated effect.15–18 There is no reason why the standards for a meta-analysis should be less rigorous than those for a single trial. With trial sequential analysis, analogous boundaries for trial sequential monitoring can be applied to a meta-analysis.15–19 Cumulative meta-analyses of trials may increase the risk of random errors because of sparse data and repetitive testing when the required “information size” (analogous to the sample size of an optimally powered clinical trial) has not been obtained. Trial sequential analysis depends on quantification of the required amount of information (the meta-analysis sample size). In this context, the smaller the amount of required information, the more lenient the boundaries of trial sequential monitoring are and, accordingly, the more lenient the criteria for statistical significance will be.
We calculated the diversity-adjusted information size.18 We conducted the trial sequential analyses with the intention of maintaining an overall 5% risk of a type I error and 20% risk of a type II error for all the primary and for secondary outcomes showing statistical significance in both the random-effects and fixed-effect models. On the basis of predetermined criteria for the binary outcomes,11 we calculated the information size required to detect or reject a 10% reduction in relative risk resulting from the intervention. For the continuous outcomes, we estimated the amount of information required to detect or reject the observed differences between the interventions. We used trial sequential analysis software (TSA, version 0.9; Copenhagen Trial Unit, Copenhagen, Denmark, 2011).16
Results
Search results and study characteristics
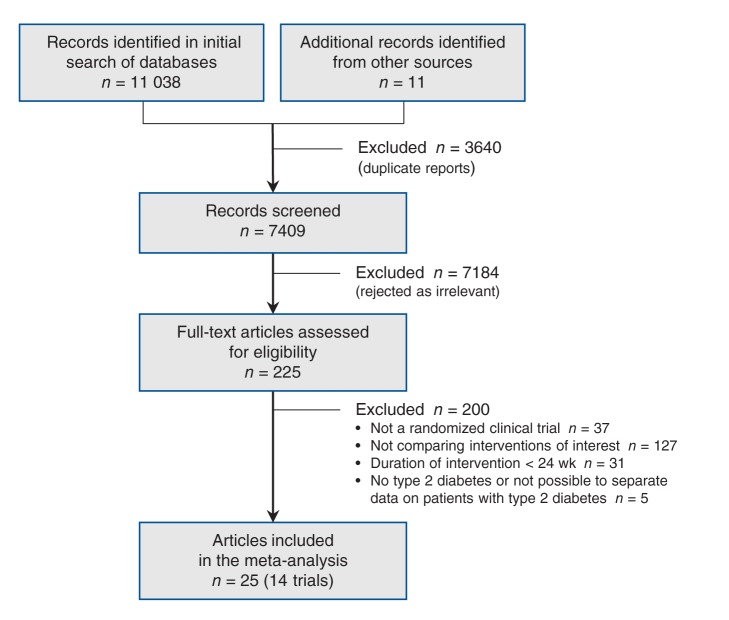
We identified 11 049 documents through the electronic and manual searches (Figure 1). After excluding duplicate reports, we screened the titles and abstracts of 7409 documents and rejected 7184 as irrelevant. After full-text review, a further 200 were excluded because they did not meet our criteria (the excluded documents are listed in Appendix 3, www.cmajopen.ca/content/2/3/E162/suppl/DC1). Twenty-five articles describing 14 randomized clinical trials met our inclusion criteria for the comparison of a second- or third-generation sulfonylurea versus metformin.2,20–43
Figure 1:
Selection of randomized clinical trials for the systematic review.
Most of the selected reports were published in English; 1 was published in Chinese.41 The trials included 4560 participants, of whom 2244 were randomly chosen to receive a second- or third-generation sulfonylurea versus 2313 randomly chosen to receive metformin. One trial did not describe the intervention group to which 3 of the participants were randomly assigned.30 Characteristics of the 14 included trials, the interventions and participants’ baseline characteristics are listed in Tables 1, 2 and 3, respectively. The number of participants in each trial ranged from 23 to 2902, and the duration of the intervention varied from 24 weeks to 10.7 years. Six trials used glibenclamide, 4 trials used gliclazide, and 1 trial used glipizide as the second-generation sulfonylurea. Three trials used a third-generation sulfonylurea, glimepiride.
Table 1: Characteristics of the 14 trials included in the meta-analysis.
| Trial | Location | Design | Treatment; no. of patients |
Duration of intervention | ||
|---|---|---|---|---|---|---|
| Sulfonylurea | Metformin | Total | ||||
| ADOPT, 200620–26 |
North America, Europe and Canada |
Parallel; blinding of investigators and participants |
1447 |
1455 |
2902 |
4 yr |
| Campbell et al., 199427 |
United Kingdom |
Parallel; open label |
24 |
24 |
48 |
1 yr |
| Collier et al., 198928 |
NR |
Parallel; open label |
12 |
12 |
24 |
6 mo |
| DeFronzo et al., 199529 |
United States |
Parallel; blinding of investigators and participants |
209 |
210 |
419 |
29 wk |
| Derosa et al., 200442 |
Italy |
Parallel; open label |
81 |
83 |
164 |
12 mo (+ 8-wk titration period) |
| Hermann et al., 1991a30 |
Sweden |
Crossover; open label |
10* |
12* |
25* |
6 mo |
| Hermann et al., 1991b31–34 |
Sweden |
Parallel; blinding of investigators and participants |
34 |
38 |
72 |
6 mo + 2–12 wk titration period |
| Kamel et al., 199735 |
Turkey |
Parallel; blinding of investigators and participants |
17† |
6 |
23 |
24 wk |
| Lawrence et al., 200436 |
United Kingdom |
Parallel; open label |
22 |
21 |
43 |
24 wk |
| Tang et al., 200441 |
China |
Parallel; open label |
33 |
29 |
62 |
6 mo |
| Tessier et al., 199937 |
Canada |
Parallel; open label |
19 |
20 |
39 |
24 wk |
| Tosi et al., 200338 |
Italy |
Crossover; blinding of investigators and participants |
22 |
22 |
44 |
6 mo |
| UKPDS 34, 19982,39,40 |
United Kingdom |
Parallel; open label |
277 |
342 |
619 |
10.7 yr |
| Yamanouchi et al., 200543 | Japan | Parallel; NR (we assume open label) | 37 | 39 | 76 | 12 mo |
Note: ADOPT = A Diabetes Outcome Progression Trial, NR = not reported, UKPDS = United Kingdom Prospective Diabetes Study. *Number of participants randomly selected for each intervention arm not reported; only those who completed the trial. †The 17 participants in the sulfonylurea arm were given either gliclazide (9 participants) or glibenclamide (8 participants).
Table 2: Interventions tested in the 14 trials included in the meta-analysis .
| Trial | Sulfonylurea intervention | Metformin intervention | Plan in case of treatment failure | Intervention arm not included in this analysis |
|---|---|---|---|---|
| ADOPT, 200620–26 |
Glibenclamide, PO; 2.5 mg/d initially, then up to 15 mg/d (7.5 mg twice daily) |
Metformin, PO; 500 mg/d initially, then up to 2 g/d (1 g twice daily) |
Escape medicine not allowed; participants excluded |
Rosiglitazone |
| Campbell et al., 199427 |
Glipizide, PO; 5 mg/d initially, increased to a maximum divided dose of 15 mg/d |
Metformin, PO; 500 mg/d initially, increased by 500 mg/d at each visit (every second week) to maximum of 3 g/d |
NR |
|
| Collier et al., 198928 |
Gliclazide, PO; 80–240 mg/d |
Metformin, PO; 1.5–3.0 g/d |
NR |
Healthy controls |
| DeFronzo et al., 199529 |
Glibenclamide, PO; 5 mg twice daily for first week, then 10 mg twice daily. Metformin placebo |
Metformin, PO; 500 mg/d initially. After 1 wk, increased to 1 g/d by adding 500-mg tablet at breakfast. After 2 wk, increased to 1.5 g/d by adding 500-mg tablet at lunch. After 3 wk, increased to 2 g/d by adding a second 500-mg tablet at evening meal. After 4 wk, increased to 2.5 g/d by adding a second 500-mg tablet at breakfast. Glibenclamide placebo |
Escape medicine not allowed; participants excluded |
Combination of metformin plus glibenclamide |
| Derosa et al., 200442 |
Glimepiride, PO; 1 mg/d initially, titrated to maximum of 4 mg/d (2 mg twice daily) |
Metformin, PO; 1 g/d initially, titrated to maximum of 3 g/d (1 g 3 times daily) |
Escape medicine allowed |
|
| Hermann et al., 1991a30 |
Glibenclamide, PO; 1.75–10.5 mg/d |
Metformin, PO; 0.5–3 g/d |
NR |
|
| Hermann et al., 1991b31–34 |
Glibenclamide, PO; 3.5 mg/d initially, increased to 14 mg/d. Tablets given shortly before breakfast; if daily dose exceeded 7 mg, then divided between breakfast and evening meal. Metformin placebo. |
Metformin, PO; 1 g/d initially, increased to 1–3 g/d in 2 doses shortly before breakfast and evening meal. Glibenclamide placebo. |
Escape medicine allowed |
Combination of metformin plus glibenclamide |
| Kamel et al., 199735 |
Gliclazide and glibenclamide |
Metformin |
NR |
Acarbose and placebo |
| Lawrence et al., 200436 |
Gliclazide, PO; 80 mg/d in single dose, titrated to 160 mg/d depending on fasting blood glucose level |
Metformin, PO; 500 mg twice daily, titrated to 1 g 3 times daily depending on fasting blood glucose level |
Escape medicine not allowed; participants excluded |
Pioglitazone |
| Tang et al., 200441 |
Glimepiride, PO; 1–2 mg/d |
Metformin, PO; 750–1500 mg/d |
NR |
|
| Tessier et al., 199937 |
Gliclazide, PO; titrated to glycemic target; doses were 80, 160, 240 and 320 mg/d divided into 2 doses with breakfast and evening meal |
Metformin, PO; titrated to glycemic target; doses were 750, 1500 and 2250 mg/d divided into 3 doses with each meal |
NR |
|
| Tosi et al., 200338 |
Glibenclamide, PO; starting dose was 1 tablet (5 mg) before lunch. Increased to 1 tablet twice daily (before breakfast and dinner), 2 tablets twice daily (before breakfast and dinner), and 2 tablets 3 times daily (before breakfast, lunch and dinner). For those given glibenclamide alone, the last 2 steps were 1 tablet of active drug + 1 tablet of placebo |
Metformin, PO; starting dose was 1 tablet (500 mg) before lunch. Increased to 1 tablet twice daily (before breakfast and dinner), 2 tablets twice daily (before breakfast and dinner), and 2 tablets 3 times daily (before breakfast, lunch and dinner). Therefore, scheduled dose steps were 0.5, 1, 2 and 3 g/d for metformin |
Escape medicine not allowed; participants excluded |
Combination of metformin plus glibenclamide |
| UKPDS 34, 19982,39,40 |
Glibenclamide, PO; 2.5–20 mg/d |
Metformin, PO; 850-mg tablet daily initially, increased to 850 mg twice daily, then 1700 mg in the morning and 850 mg with evening meal (maximum 2550 mg/d). If symptoms of diarrhea or nausea occurred, dose reduced to level that previously did not cause symptoms |
Escape medicine allowed |
Chlorpropamide and insulin |
| Yamanouchi et al., 200543 | Glimepiride, PO; 1–2 mg/d | Metformin, PO; 750 mg/d | Escape medicine allowed | Pioglitazone |
Note: ADOPT = A Diabetes Outcome Progression Trial, NR = not reported, po = oral dose, UKPDS = United Kingdom Prospective Diabetes Study.
Table 3: Baseline characteristics of trial participants*.
| Trial | Duration of diabetes, yr | Age, yr | HbA1c, % | Body mass index |
|---|---|---|---|---|
| ADOPT, 200620–26† |
Expressed in publication as: < 1 yr; 1–2 yr; and > 2 yr. Participants had to be diagnosed with type 2 diabetes within 3 yr from screening to trial |
SU: 56.4 ± 10.2 M: 57.9 ± 9.9 |
SU: 7.4 ± 0.9 M: 7.4 ± 0.9 |
SU: 32.3 ± 6.3 M: 32.1 ± 6.1 |
| Campbell et al., 199427 |
2.8 (3.9) / 2.3 (3.2) |
SU: 57 ± 9.0 M: 57 ± 10.0 |
SU: 11.8 ± 2.1 M: 11.5 ± 1.9 |
SU: 31.2 ± 6.6 M: 29.6 ± 5.6 |
| Collier et al., 198928 |
All newly diagnosed |
SU: 55.5 ± 5.1 M: 53.1 ± 5.1 |
SU: 11.7 ± 1.5 M: 12.1 ± 2.4 |
SU: 23.1 ± 1.3 M: 24.3 ± 1.4 |
| DeFronzo et al., 199529‡ |
8.7 (5.8) / 8.4 (5.8) |
SU: 56 ± 14.5 M: 55 ± 14.5 |
SU: 8.5 ± 1.4 M: 8.9 ± 1.4 |
SU: 29.1 ± 4.3 M: 29.0 ± 4.3 |
| Derosa et al., 200442 |
NR, but diabetes had to be diagnosed within 6 mo of enrolment |
SU: 54 ± 10.0 M: 56 ± 9.0 |
SU: 8.5 ± 1.2 M: 8.4 ± 1 |
SU: 27.6 ± 1.2 M: 28.1 ± 1.5 |
| Hermann et al., 1991a30§ |
All patients: 7.6 (range 4 mo to 24 yr) |
All patients: 58.9 ± 8.8 |
SU: 8.1 ± 1.0 M: 7.9 ± 1.6 |
All patients: 26.2 ± 3.8 |
| Hermann et al., 1991b31–34 |
All patients: 3.6 (range 0–38) |
All patients: 59.4 ± 8.8 |
SU: 6.7 ± 1.7 M: 6.9 ± 1.8 |
All patients: 28.3 ± 4.6 |
| Kamel et al., 199735 |
NR |
NR |
Gliclazide: 8.4 ± 1.1 Glibenclamide: 8.4 ± 1.1 M: 8.4 ± 0.5 |
NR |
| Lawrence et al., 200436** |
NR |
SU: 63.5 ± 11.4 M: 59.5 ± 9.3 |
SU: 7.9 ± 0.9 M: 8.0 ± 0.9 |
SU: 28.7 (28.3–34.4)** M: 29.2 (28.1–31.6)** |
| Tang et al., 200441 |
NR |
SU: 56.4 ± 8.8 M: 53.8 ± 9.7 |
SU: 6.8 ± 1.6 M: 7.2 ± 1.4 |
SU: 23.3 ± 1.7 M: 24.6 ± 2.2 |
| Tessier et al., 199937†† |
SU: 4.7 ± 6.1 M: 5.4 ± 6.5 |
SU: 59.3 ± 7.3 M: 59.1 ± 7.1 |
SU: 7.8 ± 1.8 M: 7.1 ± 1.7 |
SU: 28.6 ± 4.0 M: 29.3 ± 3.0 |
| Tosi et al., 200338 |
SU: 9.9 ± 6.6 M: 11.2 ± 9.6 |
SU: 57.9 ± 7.5 M: 58.2 ± 7.3 |
SU: 7.9 ± 1.0 M: 7.7 ± 0.9 |
SU: 26.3 ± 2.3 M: 26.4 ± 2.7 |
| UKPDS 34, 19982,39,40 |
All newly diagnosed |
SU: 53 ± 9.0 M: 53 ± 8.0 |
SU: 7.2 ± 1.5 M: 7.3 ± 1.5 |
SU: 31.5 ± 4.4 M: 31.6 ± 4.2 |
| Yamanouchi et al., 200543 | SU: 3.3 ± 2.6 M: 3.0 ± 2.5 |
SU: 55.6 ± 9.3 M: 54.7 ± 9.8 |
SU: 9.8 ± 0.7 M: 9.9 ± 0.7 |
SU: 25.6 ± 3.5 M: 26.2 ± 3.8 |
Note: ADOPT = A Diabetes Outcome Progression Trial, HbA1c = glycosylated hemoglobin, M = metformin, NR = not reported, SU = sulfonylurea, UKPDS = United Kingdom Prospective Diabetes Study. *Values are reported as means and standard deviations, unless stated otherwise. †Baseline characteristics reported only for the participants who received a dose of the study drug (glibenclamide 1441, rosiglitazone 1456, metformin 1454). ‡Standard deviations were calculated from standard errors. Fasting plasma glucose levels were converted from mg/dL to mmol/L. §Baseline characteristics reported only for the 22 participants who completed the trial. ¶Standard deviations for HbA1c concentration were calculated from standard errors. **Baseline variables reported only for the participants who completed the trial (20 in each intervention arm). Median (interquartile range) for body mass index. ††Baseline characteristics reported only for the participants who completed the trial (36 of 39).
The UKPDS 34 trial included overweight and obese participants with type 2 diabetes and compared intensive glycemic control using metformin with intensive glycemic control using other antidiabetic interventions (chlorpropamide, glibenclamide or insulin). The trial reported vascular outcomes and mortality for the metformin group versus the combined group of other interventions and not for the individual comparison groups.2 Our attempts to obtain separate data on sulfonylurea versus metformin were unproductive.
Two of the trials had a crossover design, and we used data only from the first period; the remaining 12 trials had a parallel design. Nine of the trials were open label, and in 5 trials, the investigators and participants were blinded to the treatment allocation. For 2 trials,35,43 the blinding of participants and investigators was not described. One of those35 involved an intervention arm with placebo; thus, we assumed blinding of the investigators and participants was part of the study design. The other43 was assumed to be open label.
All the trials were judged to be at high risk of bias in at least 1 domain (Table 4). Only 3 of the trials were considered to be at lower risk of bias based on assessment of sequence generation, allocation concealment and blinding.
Table 4: Risk-of-bias assessment of the trials included in the meta-analysis* .
| Trial | Sequence generation (selection bias) | Allocation concealment (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessors (detection bias) | Completeness of outcome data (attrition bias) | Selective reporting (reporting bias) | Academic bias | Sponsor bias | |
|---|---|---|---|---|---|---|---|---|---|
| ADOPT, 200620–26 |
Low |
Low |
Low |
Low |
Low |
Low |
Low |
High |
|
| Campbell et al., 199427 |
Unclear |
Unclear |
High |
High |
Low |
Unclear |
Low |
Unclear |
|
| Collier et al., 198928 |
Unclear |
Unclear |
High |
High |
Unclear |
Unclear |
Low |
High |
|
| DeFronzo et al., 199529 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
High |
|
| Derosa et al., 200442 |
Unclear |
Unclear |
High |
High |
Low |
Unclear |
Low |
Unclear |
|
| Hermann et al., 1991a30 |
Low |
Unclear |
High |
High |
Unclear |
Unclear |
Low |
High |
|
| Hermann et al., 1991b31–34 |
Low |
Low |
Low |
Low |
Low |
Low |
High |
High |
|
| Kamel et al., 199735 |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Unclear |
Low |
Unclear |
|
| Lawrence et al., 200436 |
Unclear |
Unclear |
High |
Low |
Low |
Unclear |
Low |
High |
|
| Tang et al., 200441 |
Unclear |
Unclear |
High |
High |
Unclear |
Unclear |
Low |
Low |
|
| Tessier et al., 199937 |
Unclear |
Unclear |
High |
High |
Low |
Unclear |
Low |
High |
|
| Tosi et al., 200338 |
Low |
Low |
Low |
Low |
Unclear |
Low |
Low |
High |
|
| UKPDS 34, 19982,39,40 |
Low |
Low |
High |
Low |
Unclear |
High |
Low |
High |
|
| Yamanouchi et al., 200543 | Low | Low | High | High | Low | Unclear | Low | Unclear | |
Note: ADOPT = A Diabetes Outcome Progression Trial, UKPDS = United Kingdom Prospective Diabetes Study. *The Cochrane risk-of-bias tool was used to assess the risk of bias for each study. Low risk = bias, if present, is unlikely to alter the results seriously, unclear risk = bias raises some doubt about the results, high risk = bias may alter the results seriously.10
Clinical outcomes
All-cause mortality
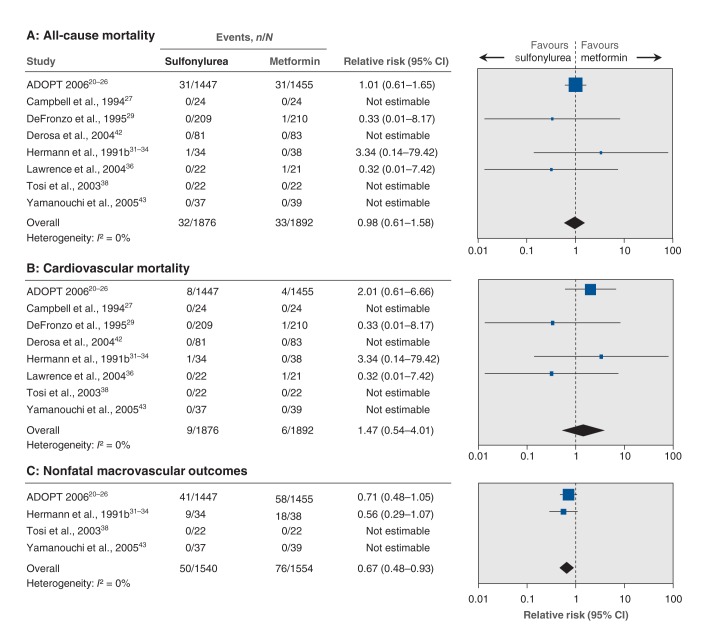
The effect estimate of all-cause mortality was dominated by the A Diabetes Outcome Progression Trial (ADOPT) trial, which contributed 62 of 65 fatal events. All-cause mortality was not significantly influenced by the interventions (relative risk [RR] 0.98, 95% confidence interval [CI] 0.61 to 1.58; I2 = 0%) (Figure 2A); the same was true when we included trials with 0 events (RR 0.98, 95% CI 0.62 to 1.56). Trial sequential analysis showed that only 2.3% of the diversity-adjusted required information size needed to detect or reject a 10% reduction in relative risk was accrued.
Figure 2:
Effect of sulfonylurea versus metformin monotherapy on all-cause mortality, cardiovascular mortality and nonfatal macrovascular outcomes. A relative risk of less than 1.0 indicates an effect in favour of sulfonylurea. CI = confidence interval.
Sensitivity analyses excluding the trial with the longest duration or excluding the trials that did not describe how the diagnosis of type 2 diabetes was established did not change the statistical significance of the effect estimate. Sensitivity analyses according to language of publication, funding source or publication status could not be conducted. Subgroup analyses were not conducted, because none of the primary outcomes showed statistically significant differences between the intervention groups.
Cardiovascular mortality
The total number of deaths due to cardiovascular disease was 15, of which 12 were reported in the ADOPT trial. Cardiovascular mortality in the sulfonylurea group was not significantly higher compared with the metformin group (RR 1.47, 95% CI 0.54 to 4.01; I2 = 0%) (Figure 2B). The same was true when we included trials with 0 events (RR 1.36, 95% CI 0.56 to 3.32). Trial sequential analysis showed that only 2.7% of the diversity-adjusted required information size needed to detect or reject a 10% reduction in relative risk was accrued.
Sensitivity analyses excluding the trial with the longest duration or excluding the trials that did not describe how the diagnosis of type 2 diabetes was established did not change the statistical significance of the effect estimate. Sensitivity analyses according to language of publication, funding source or publication status could not be done. Subgroup analyses were not conducted, because none of the primary outcomes showed statistically significant differences between the intervention groups.
Nonfatal macrovascular outcomes
Nonfatal macrovascular outcomes as a composite outcome were not fully in accordance with our predetermined definition of this outcome (for definitions of macrovascular events in the trials, see Appendix 4, www.cmajopen.ca/content/2/3/E162/suppl/DC1). The ADOPT trial and the trial conducted by Hermann and colleagues31–34 defined outcome in a way that may have included nonatherosclerotic cardiac outcomes. No cardiovascular events were observed in 1 trial,38 and another43 reported no adverse cardiac events. The ADOPT trial included fatal myocardial infarction in its composite cardiovascular outcome. In addition, nonfatal macrovascular outcomes in the ADOPT trial included congestive heart failure (9 participants in the glibenclamide group v. 19 in the metformin group), which may not have an atherosclerotic origin. Because of the definition of “cardiovascular disease” used in the ADOPT trial, we could not exclude congestive heart failure events.
We pooled nonfatal macrovascular events and found a significant reduction in favour of sulfonylurea (RR 0.67, 95% CI 0.48 to 0.93; I2 = 0%) (Figure 2C), and virtually no change in relative risk when we included trials with 0 events (RR 0.67, 95% CI 0.48 to 0.94). Trial sequential analysis showed that only 5% of the diversity-adjusted information required to detect or reject a 10% reduction in relative risk was accrued and that the trial sequential monitoring boundary for benefit was not crossed, which meant that firm evidence could not be established (Appendix 5A, www.cmajopen.ca/content/2/3/E162/suppl/DC1). That is, for the observed effect size (a reduction of 33% in relative risk of the outcome with sulfonylurea v. metformin [relative risk 0.67]) and despite the fact that the difference in treatments was significant (p = 0.02), the amount of available evidence was still smaller (only 5%) than required to detect or reject (at the 5% significance level) a 10% difference in relative risk as estimated from the observed event rate in the control group when adjusted for diversity (heterogeneity of trials).
Thirty-nine nonfatal myocardial infarctions were reported in 4 trials (n = 3061), of which 36 occurred in the ADOPT trial. The effect estimate of nonfatal myocardial infarctions was not significantly different between the intervention groups (RR 1.02, 95% CI 0.37 to 2.85; I2 = 15%). No meta-analysis of the remaining single components of the composite nonfatal macrovascular outcomes could be conducted because of lack of data.
Microvascular outcomes
Meta-analysis of microvascular outcomes could not be performed because of lack of data.
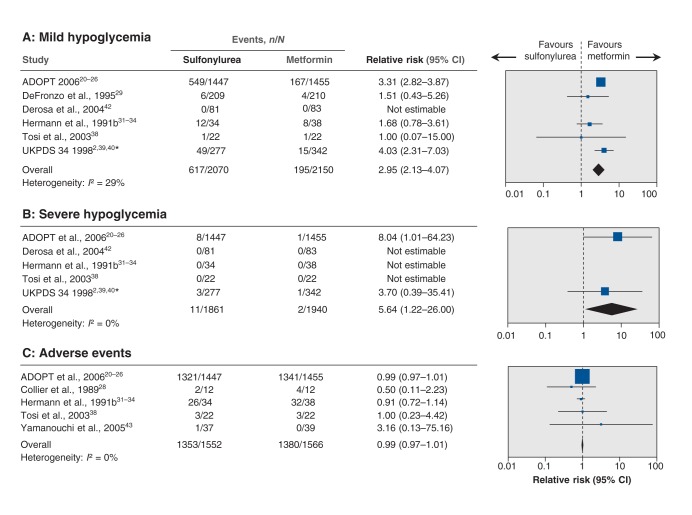
Hypoglycemia
Mild hypoglycemia was significantly increased with sulfonylurea (RR 2.95, 95% CI 2.13 to 4.07; I2 = 29%) (Figure 3A); when we included trials with 0 events, the effect estimate changed little (RR 3.01, 95% CI 2.29 to 3.97). Trial sequential analysis showed that only 2.7% of the diversity-adjusted information required to detect or reject a 10% increase in relative risk was accrued (Appendix 5B). Because of the way results were reported in the trials, meta-analysis of moderate hypoglycemia could not be performed. The risk of severe hypoglycemia was significantly increased with sulfonylurea (RR 5.64, 95% CI 1.22 to 26.00; I2 = 0%) (Figure 3B); the effect estimate decreased after we included trials with 0 events (RR 3.31, 95% CI 0.93 to 11.71). Trial sequential analysis showed that only 0.1% of the diversity-adjusted information required to detect or reject a 10% increase in relative risk was accrued. The UKPDS 34 researchers did not report the number of participants with hypoglycemia in each of the intervention arms at the end of the follow-up period; the data were obtained after 1 year of follow-up. Reporting of hypoglycemia in the trials is listed in Appendix 6 (www.cmajopen.ca/content/2/3/E162/suppl/DC1).
Figure 3:
Effect of sulfonylurea versus metformin monotherapy on mild hypoglycemia (A), severe hypoglycemia (B) and adverse events (C). A relative risk of less than 1.0 indicates an effect in favour of sulfonylurea. CI = confidence interval. *Data after 1 year of follow-up.
Adverse events
The effect estimate for adverse events was not significantly influenced by the interventions (RR 0.99, 95% CI 0.97 to 1.01; I2 = 0%) (Figure 3C); none of the trials reported 0 events in both intervention arms. The effect estimate for serious adverse events also did not show any significant difference between groups (RR 0.94, 95% CI 0.82 to 1.07; I2 = 0%) (Figure 4A); inclusion of trials with 0 events did not change the estimate (RR 0.94, 95% CI 0.82 to 1.07). A total of 641 participants reported a serious adverse event, of whom 639 were from the ADOPT trial. Drop-outs due to adverse events did not differ significantly between intervention groups (RR 1.18, 95% CI 0.98 to 1.41; I2 = 0%) (Figure 4B); inclusion of trials with 0 events did not change the estimate (RR 1.18, 95% CI 0.98 to 1.41). Definitions of adverse events and serious adverse events for the selected trials are listed in Appendix 6.
Figure 4:
Effect of sulfonylurea versus metformin monotherapy on serious adverse events (A), drop-outs due to adverse events (B) and intervention failure (C). A relative risk of less than 1.0 indicates an effect in favour of sulfonylurea. CI = confidence interval. *Data after 3 years of follow-up.
Cancer
Only the ADOPT trial provided data on cancer (55 of 1447 patients in the sulfonylurea arm; 50 of 1455 in the metformin arm). Meta-analysis could not be done because of lack of data.
Intervention failure
In the random-effects model, failure of the monotherapy intervention did not differ significantly between intervention arms (RR 1.00, 95% CI 0.66 to 1.53) (Figure 4C); the risk after inclusion of trials with 0 events was 1.01 (95% CI 0.68 to 1.52). However, the fixed-effects model showed that intervention failure was significantly more likely in the metformin arm (RR 1.34, 95% CI 1.16 to 1.55; I2 = 59%); the risk did not change after we included trials with 0 events (RR 1.34, 95% CI 1.16 to 1.54).
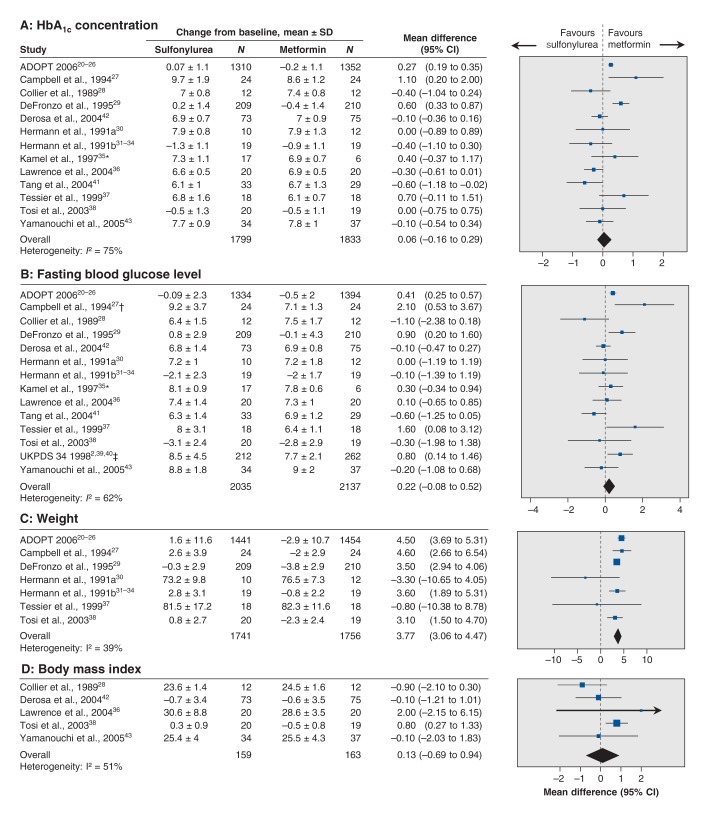
Glycemic control
Changes in HbA1c concentration from baseline did not differ significantly between the sulfonylurea and metformin groups in the random-effects model (mean difference 0.06%, 95% CI −0.16% to 0.29%) (Figure 5A). However, the fixed-effects model showed a significant difference in favour of metformin (mean difference 0.20%, 95% CI 0.13% to 0.28%; I2 = 75%). The changes in fasting blood glucose level from baseline also did not differ significantly in the random-effects model (mean difference 0.22 mmol/L, 95% CI −0.08 to 0.52 mmol/L; I2 = 62%) (Figure 5B). However, the fixed-effect model showed a significant difference favouring metformin (mean difference 0.30 mmol/L, 95% CI 0.18 to 0.43 mmol/L).
Figure 5:
Effect of sulfonylurea versus metformin monotherapy on changes from baseline in glycosylated hemoglobin (HbA1c) concentration (A), fasting blood glucose level (B), weight (C) and body mass index (D). A mean difference of less than 0 between the study groups indicates an effect in favour of sulfonylurea. CI = confidence interval. *Not stated in abstract whether values were standard deviations or standard errors. †Numbers taken from a figure. ‡Data after 3 years of follow-up.
Weight
Weight gain was significantly greater in the sulfonylurea group than in the metformin group (mean difference 3.77 kg, 95% CI 3.06 to 4.47 kg; I2 = 39%) (Figure 5C). Trial sequential analysis showed firm evidence for the difference in weight change between the 2 treatments, disregarding risk of bias (Appendix 5C). Change in BMI from baseline did not differ significantly between the 2 treatments (mean difference 0.13 kg/m2, 95% CI −0.69 to 0.94; I2 = 51%) (Figure 5D). However, only 2 of the trials included in this meta-analysis reported the actual change in mean BMI and standard deviation for each of the intervention groups.38,42 For the other three trials, the values from the end of the follow-up period were used.28,36,43 In all of these trials, the sample size was small, and the BMI was lower in the sulfonylurea groups than in the metformin groups at baseline and at the end of follow-up.28,36,43
Interpretation
Based on our published protocol,11 we identified and analyzed 14 randomized clinical trials comparing the effects of sulfonylurea with those of metformin in patients with type 2 diabetes. No significant differences were found between the interventions in terms of all-cause and cardiovascular mortality, but data were sparse. In contrast, a potential benefit of sulfonylurea over metformin was observed in relation to nonfatal macrovascular outcomes. This potential benefit should be interpreted with caution, because the definitions of composite cardiovascular outcome in the two trials contributing data to this meta-analysis made it impossible to identify clearly the number of events with an atherosclerotic origin.20–26,31–34 However, we cannot rule out the clinical relevance of the events reported in the trials, regardless of whether they are of atherosclerotic origin. Moreover, trial sequential analysis showed that the amount of evidence was insufficient to draw firm conclusions regarding mortality or any of the vascular outcomes. In agreement, the confidence intervals were broad, making the data inconclusive. All trials had a high risk of bias in one or more domains; only three trials were considered to have a lower risk of bias.20–26,31–34,38 Meta-analyses of patient-important outcomes were based on sparse data and, except for nonfatal macrovascular outcomes and severe hypoglycemia, and did not show any significant differences between the two drugs.
Metformin monotherapy was associated with a lower risk of hypoglycemia and less pronounced weight gain compared with sulfonylurea. However, weight changes could be confirmed only in the trial sequential analysis; thus, this constitutes the only firm evidence from randomized clinical trials, disregarding the risk of bias, to support the choice of metformin over a sulfonylurea as monotherapy. The change in BMI from baseline was not significantly different between the intervention groups, although we expected metformin to be of more benefit in this regard. The reason for lack of statistical significance is probably a result of reporting methods and the small number of trials contributing data.28,36,38,42,43
A Cochrane review compared metformin monotherapy with other antidiabetic interventions.44 However, it included only 6 randomized trials of 24 weeks or more comparing second- or third-generation sulfonylurea with metformin.2,27–29,31–34,37,39 Unlike our review, the Cochrane review was able to include mortality and vascular outcomes from the UKPDS trial because the authors compared metformin with any treatment and thus included data for the combined group of insulin and sulfonylurea reported by the UKPDS; however, like our review, they were unable to include data from the UKPDS trial comparing sulfonylurea versus metformin.44 The Cochrane review of metformin monotherapy did a pooled analysis of non-UKPDS trials for various comparators and found no significant difference in mortality or ischemic heart disease between treatments.44 The Cochrane review of metformin monotherapy also did a separate analysis of UKPDS only, which corroborated most of the previous conclusions from the UKPDS. However, a combined analysis of UKPDS and non-UKPDS trials was not made. Despite this, the Cochrane review concluded that metformin may be beneficial in terms of cardiovascular outcomes in overweight and obese patients with type 2 diabetes.44
The Cochrane review of metformin monotherapy found less hypoglycemia with metformin than with sulfonylurea and improved glycemic control in terms of fasting blood glucose level and HbA1c concentration.44We found a significantly lower risk of mild and severe hypoglycemia with metformin, but no significant difference in effect on glycemic control using the random-effects model. However, we did find a significantly lower blood glucose level and HbA1c concentration in favour of metformin in the fixed-effect model.
Several observational studies have shown an increased risk of death and cardiovascular disease with sulfonylurea than with metformin monotherapy.3–6 The data from these studies are based on a large number of patients, but they should be evaluated with caution.45 Our data, based on randomized clinical trials, did not find increased mortality with sulfonylurea compared with metformin monotherapy. In contrast, although reporting was heterogeneous, the composite nonfatal macrovascular outcome was significantly different between the 2 treatments in favour of sulfonylurea. For both outcomes (mortality and cardiovascular disease), we cannot exclude the risk of random errors, and more randomized clinical trials are needed. An observational study has shown that sulfonylureas may be associated with various risks of macrovascular disease, with gliclazide, putatively, exhibiting the most beneficial outcome profile.4 In our analysis, we were unable to differentiate between the effects of the various sulfonylureas because of the insufficient number of trials.
We were not able to include patient-important data from the longest follow-up period in the UKPDS trial.2 The importance of the UKDPS trial is based on the length of the intervention: about 10 years. According to the design description,39 the researchers planned to compare the subgroup of overweight and obese participants randomly assigned to receive sulfonylurea or metformin monotherapy. However, to our knowledge, these data have not been reported separately. Instead, the participants assigned to sulfonylurea or insulin were grouped together, which precludes direct comparison of sulfonylurea versus metformin.2,39 The largest trial reporting patient-important outcomes for sulfonylurea monotherapy compared with metformin was the ADOPT trial.20–26 It showed a significant benefit of metformin versus glibenclamide in terms of time to treatment failure (the primary outcome) and HbA1c concentration after about 4 years of follow-up. In contrast, fewer cardiovascular events occurred with sulfonylurea treatment than with metformin. However, like the UKPDS trial, no statistical analysis comparing sulfonylurea and metformin groups in terms of cardiovascular events appears to have been reported from ADOPT; this is available only from meta-analyses, such as ours. A later re-analysis of ADOPT data taking into account the differences in treatment time between interventions did not bring clarity to this issue.23
The literature search conducted for our review included trials reported up to August 2011. A cursory update of the MEDLINE search in December 2013 identified only one further randomized clinical trial of relevance to our systematic review.46 This trial, which included about 300 Chinese patients with type 2 diabetes and existing coronary artery disease, showed a significant benefit from metformin compared with glipizide for the primary composite cardiovascular outcome after about 3 years. Notably, the primary outcome was not reported after 3 years, but after a median follow-up period of about 5 years, i.e., about 2 years after the trial medication was stopped. Including the patient-important data from this trial in our meta-analysis did not change the significance of the effect estimates for the primary outcomes or for nonfatal myocardial infarction, although the composite outcome of nonfatal macrovascular complications was no longer statistically significant (RR 0.86, 95% CI 0.49 to 1.50 for sulfonylurea v. metformin). The discrepancy between the results of this relatively small trial and our current meta-analysis, which included substantially more patients, underscores the need for further randomized trials with a low risk of bias and, in particular, in broader populations to clarify the benefits and harms of sulfonylurea versus metformin in patients with type 2 diabetes.
Strengths and limitations
Our systematic review has several strengths. It is based on a published protocol,11 a comprehensive search strategy and rigid inclusion criteria for the randomized trials. Two of us independently selected trials and extracted data. We contacted corresponding authors of all trials to clarify details regarding methods and outcomes. We evaluated the strength of the available evidence by assessing the risks of bias47–49 and by using trial sequential analyses to control for the risks of random errors.15,17,50,51
The weaknesses of our analyses and conclusions mirror the weaknesses of the included trials. Most important, all of the included trials were judged to have a high risk of bias in one or more domains. Only three of the included trials were classified as having a lower risk of bias in terms of randomization, allocation and blinding. We did not have access to data at the patient level and therefore could not perform analyses taking length of treatment into account. Because we could not include mortality or vascular event data from the UKPDS trial,2 our review consists exclusively of trials that did not predefine mortality or vascular events as their primary outcome and instead reported them as adverse events. This might have led to bias arising from trial design features, such as lack of adjudication of events. In addition to a high risk of bias, our results also have a high risk of random errors, because the trial sequential analysis showed insufficient data for all outcomes except weight change.
The participants in the included trials represented a diverse sample of the population with type 2 diabetes. The results of our review should therefore be interpreted with caution. The inclusion criteria varied among the trials, but nearly all trials excluded participants with existing comorbidities, especially renal or hepatic disease. However, the diversity of patient characteristics is typical of real life, which may justify the clinical relevance of our results.
Differences between the protocol and the current review
Søren Lund is author Cochrane version of the review, but not the current review; David Sonne and Jeppe Schroll joined as authors after publication of the protocol.11 Christina Hemmingsen withdrew as an author after publication of the protocol. The title of the review is different from that of the protocol, because the Cochrane Metabolic and Endocrine Disorders Group asked us to focus on the sulfonylureas in the review. On the advice of the Cochrane Metabolic and Endocrine Disorder Group, we changed the inclusion criteria for the review to trials with a duration of 24 weeks or more and excluded combination therapies. We had not originally intended to search the US Food and Drug Administration website. We originally planned to assess baseline imbalance and early stopping as bias components, but did not do this, based on decisions made at the Cochrane Colloquium in 2010. We did not search for ongoing trials. The assessment of change in weight from baseline was not described in the protocol. When no differences in mean and standard deviations for the continuous outcomes were reported in trials, we used the values from the end of the follow-up period, if they were available.
Conclusion
Our review found that, compared with metformin, second- and third-generation sulfonylureas may not affect all-cause or cardiovascular mortality but may decrease the risk of nonfatal macrovascular outcomes among patients with type 2 diabetes. These agents may also increase the risk of hypoglycemia. However, the available data were too few and inconsistent to provide firm evidence concerning patient-important outcomes.
The most widely used guidelines recommend metformin as a first-line antidiabetic drug.1,52,53 This recommendation may be influenced by the results of the UKPDS trial for the subgroup of overweight and obese participants. However, this trial was of limited size and possibly biased in its reporting of the comparison of sulfonylurea and metformin, because it apparently did not adhere to the predefined statistical analysis plan described in the design article. Additional factors, such as sulfonylurea’s likely association with weight gain, as well as a number of potentially biased retrospective analyses, have all made sulfonylurea less used as monotherapy.2,39,54
Sulfonylurea is now largely prescribed as a part of a combination regimen.54 The use of sulfonylurea has to a large extent been replaced with the novel and, with respect to hard outcomes, as yet unproven but more expensive, dipeptidyl peptidase IV inhibitors.54 Future glucose-lowering interventions in type 2 diabetes need to be based on evidence from high-quality, long-term randomized clinical trials assessing patient-important outcomes.
Supplemental information
For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/2/3/E162/suppl/DC1
Supplementary Material
Acknowledgements
The authors thank Søren Lund for his substantial contribution to the interpretation and discussion of data and literature in this article. Søren Lund is an employee of Boehringer Ingelheim, Germany. Søren Lund’s contribution was his alone and does not necessarily reflect the official position of Boehringer Ingelheim. The authors thank Bernd Richter and the Cochrane Metabolic and Endocrine Disorder Group for their valuable assistance; Sarah Klingenberg, the trials search coordinator of the Cochrane Hepato-Biliary Group, for her assistance in developing the search strategy; Drs. Kåre Birkeland, Leif Hermann, Paolo Moghetti and Floris van de Laar for providing additional information on the trials in which they were involved; and Angel Rodriguez from Lilly for providing additional data. Additional data for the APPROACH and ADOPT trials were submitted by GlaxoSmithKline Pharmaceuticals, Metabolic and Cardiovascular Unit. Allan Vaag received financial support from the Danish National Type 2 Diabetes (DD2) study (http://dd2.nu/). This review is also published as a Cochrane systematic review in the Cochrane Database of Systematic Reviews 2013, issue 4. Cochrane reviews are regularly updated as new evidence emerges and in response to comments and criticism, and the Cochrane Database of Systematic Reviews should be consulted for the most recent version of the review.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577-96. [DOI] [PubMed] [Google Scholar]
- 2.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:854-65. [PubMed] [Google Scholar]
- 3.Roumie CL, Hung AM, Greevy RA, et al. Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med 2012;157:601-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schramm TK, Gislason GH, Vaag A, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J 2011;32:1900-8. [DOI] [PubMed] [Google Scholar]
- 5.Pantalone KM, Kattan MW, Yu C, et al. Increase in overall mortality risk in patients with type 2 diabetes receiving glipizide, glyburide or glimepiride monotherapy versus metformin: a retrospective analysis. Diabetes Obes Metab 2012;14:803-9. [DOI] [PubMed] [Google Scholar]
- 6.Tzoulaki I, Molokhia M, Curcin V, et al. Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 2009;339:b4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henquin JC. The fiftieth anniversary of hypoglycaemic sulphonamides. How did the mother compound work? Diabetologia 1992;35:907-12. [DOI] [PubMed] [Google Scholar]
- 8.Markkanen A, Oka M, Petola P. Carbutamide in diabetes: report of a long-term trial, with special reference to late failures. BMJ 1960;1:1089-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2009;52:17-30. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated Feb. 2008]. London (UK): Cochrane Collaboration; 2008. [Google Scholar]
- 11.Hemmingsen B, Lundstrøm LH, Hemmingsen C, et al. Non-incretin insulin secretagogues for patients with type 2 diabetes mellitus (protocol). Cochrane Database Syst Rev 2011;CD009008 [Google Scholar]
- 12.Hemmingsen B, Schroll JB, Lund SS, et al. Sulphonylurea monotherapy for patients with type 2 diabetes mellitus. Cochrane Database of Sys Rev 2013CD009008 [DOI] [PubMed]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
- 14.Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med 1987;6:341-50. [DOI] [PubMed] [Google Scholar]
- 15.Brok J, Thorlund K, Wetterslev J, et al. Apparently conclusive meta-analyses may be inconclusive — trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 2009;38:287-98. [DOI] [PubMed] [Google Scholar]
- 16.Thorlund K, Engstrøm J, Wetterslev J, et al. User manual for trial sequential analysis (TSA). Copenhagen (Denmark): Copenhagen Trial Unit, Centre for Clinical Intervention Research; 2011. Available: www.ctu.dk/tsa/downloads.aspx (accessed 2014 July 5).
- 17.Wetterslev J, Thorlund K, Brok J, et al. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008;61:64-75. [DOI] [PubMed] [Google Scholar]
- 18.Wetterslev J, Thorlund K, Brok J, et al. Estimating required information size by quantifying diversity in random-effects model meta-analyses. BMC Med Res Methodol 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogue J, Yusuf S. Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet 1998;351:47-52. [DOI] [PubMed] [Google Scholar]
- 20.GlaxoSmithKline. Diabetes study with rosiglitazone monotherapy versus metformin or glyburide/glibenclamide. ClinicalTrials.gov trial no. NCT00279045. Available: http://clinicaltrials.gov/show/NCT00279045 (accessed 2014 May 3).
- 21.Home PD, Kahn SE, Jones NP, et al. Experience of malignancies with oral glucose-lowering drugs in the randomised controlled ADOPT (A Diabetes Outcome Progression Trial) and RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes and Regulation of Glycaemia in Diabetes) clinical trials. Diabetologia 2010;53:1838-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy [published erratum in N Engl J Med 2007;356:1387-8]. N Engl J Med 2006;355:2427-43. [DOI] [PubMed] [Google Scholar]
- 23.Krall RL. Cardiovascular safety of rosiglitazone. Lancet 2007;369:1995-6. [DOI] [PubMed] [Google Scholar]
- 24.Lachin JM, Viberti G, Zinman B, et al. Renal function in type 2 diabetes with rosiglitazone, metformin, and glyburide monotherapy. Clin J Am Soc Nephrol 2011;6:1032-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viberti G, Kahn SE, Greene DA, et al. A diabetes outcome progression trial (ADOPT): an international multicenter study of the comparative efficacy of rosiglitazone, glyburide, and metformin in recently diagnosed type 2 diabetes. Diabetes Care 2002;25:1737-43. [DOI] [PubMed] [Google Scholar]
- 26.Viberti G, Lachin J, Holman R, et al. A Diabetes Outcome Progression Trial (ADOPT): baseline characteristics of Type 2 diabetic patients in North America and Europe. Diabet Med 2006;23:1289-94. [DOI] [PubMed] [Google Scholar]
- 27.Campbell IW, Menzis DG, Chalmers J, et al. One year comparative trial of metformin and glipizide in type 2 diabetes mellitus. Diabete Metab 1994;20:394-400. [PubMed] [Google Scholar]
- 28.Collier A, Watson HH, Patrick AW, et al. Effect of glycaemic control, metformin and gliclazide on platelet density and aggregability in recently diagnosed type 2 (non-insulin-dependent) diabetic patients. Diabete Metab 1989;15:420-5. [PubMed] [Google Scholar]
- 29.DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med 1995;333:541-9. [DOI] [PubMed] [Google Scholar]
- 30.Hermann LS, Karlsson JE, Sjöstrand A. Prospective comparative study in NIDDM patients of metformin and glibenclamide with special reference to lipid profiles. Eur J Clin Pharmacol 1991;41:263-5. [DOI] [PubMed] [Google Scholar]
- 31.Hermann LS, Bitzén PO, Kjellström T, et al. Comparative efficacy of metformin and glibenclamide in patients with non-insulin-dependent diabetes mellitus. Diabete Metab 1991;17:201-8. [PubMed] [Google Scholar]
- 32.Hermann LS, Kjellström T, Nilsson-Ehle P. Effects of metformin and glibenclamide alone and in combination on serum lipids and lipoproteins in patients with non-insulin-dependent diabetes mellitus. Diabete Metab 1991;17:174-9. [PubMed] [Google Scholar]
- 33.Hermann LS, Scherstén B, Melander A. Antihyperglycaemic efficacy, response prediction and dose-response relations of treatment with metformin and sulphonylurea, alone and in primary combination. Diabet Med 1994;11:953-60. [DOI] [PubMed] [Google Scholar]
- 34.Hermann LS, Scherstén B, Bitzén PO, et al. Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double-blind controlled study. Diabetes Care 1994;17:1100-9. [DOI] [PubMed] [Google Scholar]
- 35.Kamel AN, Cetinarslan B, Uysal AR, et al. Efficacy of monotherapy with acarbose, glibenclamide, gliclazide, metformin or placebo in NIDDM patients [abstract 1255]. Diabetologia 1997;40(suppl 1):A318 [Google Scholar]
- 36.Lawrence JM, Reid J, Taylor GJ, et al. Favorable effects of pioglitazone and metformin compared with gliclazide on lipoprotein subfractions in overweight patients with early type 2 diabetes. Diabetes Care 2004;27:41-6. [DOI] [PubMed] [Google Scholar]
- 37.Tessier D, Maheux P, Khalil A, et al. Effects of gliclazide versus metformin on the clinical profile and lipid peroxidation markers in type 2 diabetes. Metabolism 1999;48:897-903. [DOI] [PubMed] [Google Scholar]
- 38.Tosi F, Muggeo M, Brun E, et al. Combination treatment with metformin and glibenclamide versus single-drug therapies in type 2 diabetes mellitus: a randomized, double-blind, comparative study. Metabolism 2003;52:862-7. [DOI] [PubMed] [Google Scholar]
- 39.UK Prospective Diabetes Study (UKPDS) . VIII. Study design, progress and performance. Diabetologia 1991;34:877-90. [PubMed] [Google Scholar]
- 40.United Kingdom Prospective Diabetes Study (UKPDS) 13: Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ 1995;310:83-8. [PMC free article] [PubMed] [Google Scholar]
- 41.Tang JZ, Mao JP, Yang ZF, et al. Effects of glimepiride and metformin on free fatty acid in patients with Type 2 diabetes mellitus [article in Chinese]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2004;29:631-4. [PubMed] [Google Scholar]
- 42.Derosa G, Franzetti I, Gadaleta G, et al. Metabolic variations with oral antidiabetic drugs in patients with Type 2 diabetes: comparison between glimepiride and metformin. Diabetes Nutr Metab 2004;17:143-50. [PubMed] [Google Scholar]
- 43.Yamanouchi T, Sakai T, Igarashi K, et al. Comparison of metabolic effects of pioglitazone, metformin, and glimepiride over 1 year in Japanese patients with newly diagnosed Type 2 diabetes. Diabet Med 2005;22:980-5. [DOI] [PubMed] [Google Scholar]
- 44.Saenz A, Fernandez-Esteban I, Mataix A, et al. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev 2005; (3):CD002966. [DOI] [PubMed] [Google Scholar]
- 45.Jakobsen JC, Gluud C. The necessity of randomized clinical trials. Br J Med Med Res 2013;3:1453-68 [Google Scholar]
- 46.Hong J, Zhang Y, Lai S, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care 2013;36:1304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wood L, Egger M, Gluud LL, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savović J, Jones HE, Altman DG, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med 2012;157:429-38. [DOI] [PubMed] [Google Scholar]
- 49.Savović J, Jones H, Altman D, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta-epidemiological studies. Health Technol Assess 2012;16:1-82. [DOI] [PubMed] [Google Scholar]
- 50.Thorlund K, Imberger G, Walsh M, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis — a simulation study. PLoS ONE 2011;6:e25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol 2009;38:276-86. [DOI] [PubMed] [Google Scholar]
- 52.Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control [published erratum in Endocr Pract 2009;15:768-70]. Endocr Pract 2009;15:540-59. [DOI] [PubMed] [Google Scholar]
- 53.Garber AJ, Abrahamson MJ, Barzilay JI, et al. AACE comprehensive diabetes management algorithm 2013. Endocr Pract 2013;19:327-36. [DOI] [PubMed] [Google Scholar]
- 54.Alexander GC, Sehgal NL, Moloney RM, et al. National trends in treatment of type 2 diabetes mellitus, 1994-2007. Arch Intern Med 2008;168:2088-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.