Abstract
Pregnancy and early infancy are periods of relative immune suppression and increased vulnerability to infection. In these circumstances infections are associated with high morbidity and mortality. In particular, infants have high rates of invasive disease, higher than at any other stage of life with rates of 100 per 100 000 population. The concept of maternal vaccination is that maternal levels of pathogen-specific antibody are boosted and provide protection to the infant until the infant is able to mount an effective immune response to immunization. However, an important concern for women and healthcare providers is the safety of receiving vaccines during pregnancy. There are challenges associated with assessing safety in pregnant women. This review discusses the rationale for maternal vaccination, the concepts and mechanisms used. An assessment is made of the safety of vaccination during pregnancy, and the challenges associated with this are considered. In general terms, it is considered that the risk from disease far outweighs the small risk associated with vaccination during pregnancy and that they offer a new platform for preventing significant and serious infections in mothers and young infants.
Keywords: immunisation, maternal, infectious disease, antenatal, pregnancy, vaccination, infant
Introduction
The protective role of maternally derived immunity in infants was first observed in 1846 during a measles outbreak on the Faroe Islands. It was noted that if pregnant mothers survived the disease, their babies did not then become infected. In 1879, maternal immunization with vaccinia was found to protect infants against smallpox. In 1938 whole cell pertussis vaccine was used in pregnancy and later in 1961 tetanus toxoid. In Papua New Guinea during 1959–61 women received 3 injections of fluid formalinized tetanus toxoid during pregnancy, the third given in the last trimester, the incidence of neonatal tetanus was 10% in those where the mother received either no or only 1 injection, 3.42% in those that received 2 injections and 0.57% in those that received 3 injections.12
Rationale
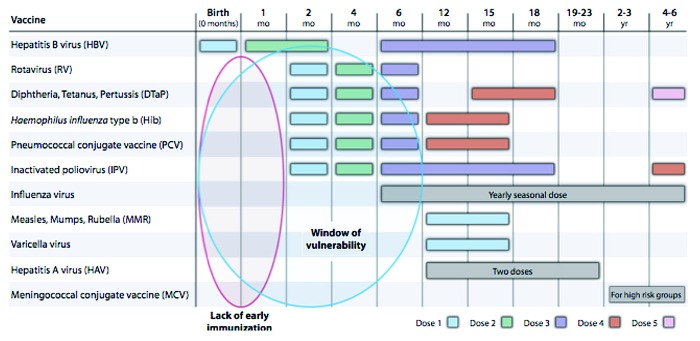
Pregnancy and early infancy are periods of relative immune suppression and increased vulnerability to infection. In these circumstances infections are associated with high morbidity and mortality. Infants have extremely high rates of invasive disease, higher than at any other stage of life with rates of 100 per 100 000 population suggesting that 0.1% of infants are affected by an invasive bacterial infection.1 Figure 1 shows the period between birth and 6 mo are the most vulnerable for infants in terms of infectious disease.11
Figure 1. The period of vulnerability for infant infectious disease (ref. 11).
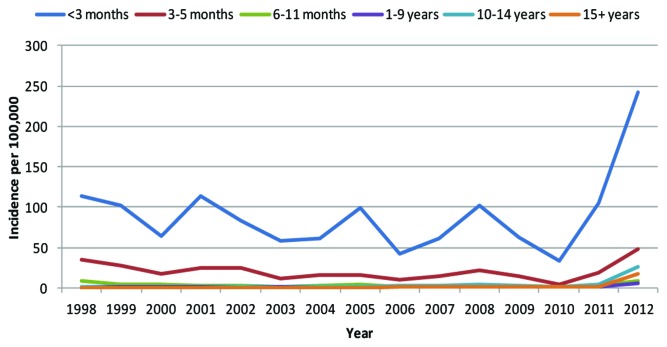
An example of this can be seen with pertussis in the UK (Fig. 2). The incidence of pertussis infection tends to peak every 3–4 y however there was a clear peak occurring in 2013, this peak is much higher than any previous peak year. Highest rates are observed in infants <3 mo of age with most in infants <6 wk of age.1 Similar rises have been seen in the USA and Canada, Australia, and New Zealand.
Figure 2. The recorded incidence of pertussis infection in the UK (ref. 1).
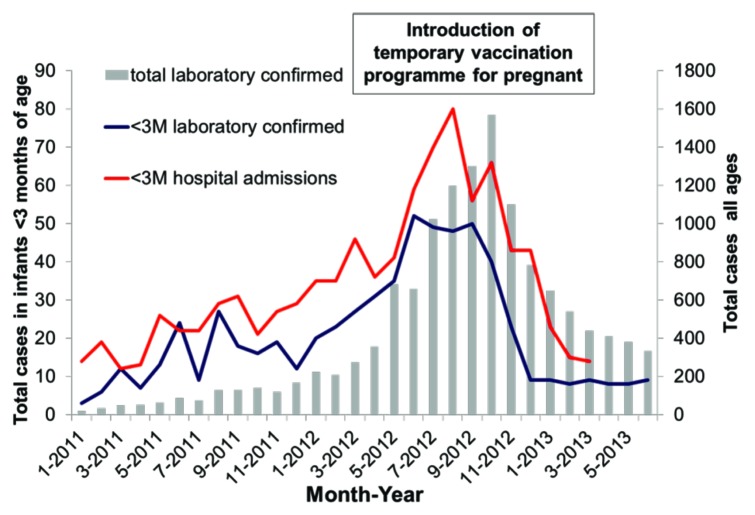
Whole cell pertussis vaccine studies in the 1930s demonstrated transfer of antibody to infants. It wasn’t until 2011 that pertussis vaccine was added to USA recommended list of immunizations during pregnancy for late second trimester/third trimester and it was recommended in UK in 2012. Figure 3 shows the impact of this introduction in pregnant women on the cases of pertussis infection in infants less than 3 mo of age. There was a 55% reduction in hospitalization.1
Figure 3. Laboratory confirmed pertussis cases and hospital admissions between January 2011 and June 2013, England only (ref. 1).
An example of an infection where vaccinating infants early would not benefit the infants most at risk is Group B Streptococcus (GBS). GBS usually manifest within the first 12 to 24 h of life (with 90% of GBS in first day of life and 90% have signs within the first 12 h of life), before any vaccine would be able to protect the infant.3 Intrapartum antibiotic prophylaxis (IAP) has no impact on late onset GBS and maternal infection.10 In this situation, maternal vaccination against GBS would be a very attractive option to provide protection even in utero. Studies have shown that maternal trivalent GBS vaccine is well-tolerated and immunogenic. In clinical safety trials it was found that all doses were well tolerated in pregnant women. In terms of immunogenicity all regimens tested induced specific antibody responses. However, large-scale pregnancy studies to demonstrate efficacy and safety of GBS trivalent vaccine need to be conducted.
Concept
The concept of maternal vaccination is that maternal levels of pathogen-specific antibody are boosted and provide protection to the infant until the infant is able to mount an effective immune response to immunization.
Infants born to mothers with low levels of pathogen-specific antibody have low levels of antibody which may not be sufficient to protect against disease. As these maternal antibody levels wane following birth, this leaves a window of vulnerability before the infant receives the first vaccinations and is able to mount their own antibody response. However, when an infant is born to a mother who was immunized during pregnancy, the mother and therefore the infant has high levels of antibody. These maternal antibody levels also wane at the same rate, but because the ‘starting’ point was higher the period of time where the infant has antibody levels below the protective threshold is reduced, therefore closing the window of vulnerability before vaccination.
Mechanisms
Antibody is transferred across the placenta using an active and selective receptor-mediated process. It begins at pregnancy week 17 and increases with gestation. By 33 wk, fetal levels match those of the mother and in many cases fetal levels exceed maternal levels at term due to the active transport process.
Antibody transfer is restricted to IgG and different subclasses are transferred with differing efficiency, IgG1 is most efficiently transported while IgG2 is the least well transported.8
Placental transport is affected by gestational age, maternal IgG levels and also infections.15 Maternal HIV and malaria are associated with reduced placental transfer. This is particularly relevant in preterm infants or infants born to mothers with HIV as lower levels of placental transfer means a shorter duration of protection.
Assessment of Safety
An important concern for women and healthcare providers is the safety of receiving vaccines during pregnancy. These may include local or systemic reactions with potential effects on pregnancy including induction of labor, preterm birth, fetal loss, and potential effects on the fetus including congenital abnormalities, prematurity and alteration of responses to infant vaccination.
There are challenges associated with assessing safety in pregnant women. In order to evaluate vaccine safety effectively an understanding of the background rates of adverse events, physiological and laboratory parameters in pregnancy needs to be established. Prior to the 2009 H1N1 influenza pandemic studies of vaccines in pregnancy were limited due to theoretical safety concerns and reluctance to enrol pregnant women in clinical trials. During the pandemic the risk of disease far outweighed the expected adverse events related to vaccination during pregnancy and studies in pregnant women were initiated.
A challenge to these studies was monitoring clinical and laboratory values, a recent study has developed a grading system for adverse events including vital signs grading and laboratory values.13
Influenza
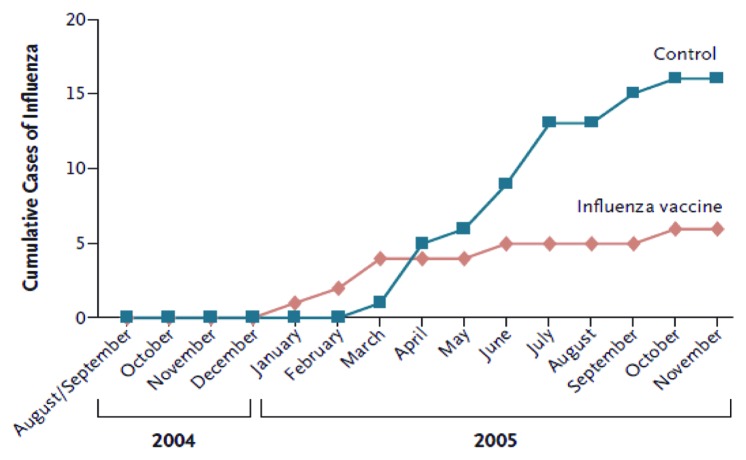
Influenza infection is responsible for excessive hospitalization in children especially infants under 6 mo of age.7 The safety of vaccination for mothers and infants was first established in the 1950s-60s.14 Influenza vaccines have been used in pregnancy since the 1950s in the USA and since 1997 has been recommended for pregnant women who fall into high risk categories. As shown in Figure 4, maternal influenza immunisation has been shown to be a strategy with substantial benefits for both mothers and infants.16
Figure 4. Cumulative cases of laboratory-proven influenza in infants whose mothers received influenza vaccine, as compared with control subjects (ref. 16).
Since the 2009 pandemic influenza vaccine has been routinely administered to pregnant women. A large prospective cohort study and a few randomized controlled clinical trials provide information on safety.6
The assessment of the safety of pandemic influenza vaccine is complicated by the fact that there are multiple manufactures in multiple areas of the world, with different formulations. Reactogenicity is mostly mild and self-limiting with no evidence of teratogenicity or adverse pregnancy outcomes. In USA alone, 2.4 million pregnant women have received influenza vaccine from Oct 2009–Feb 2010. There were 294 reports to Vaccine Adverse Event Reporting System (VAERS) but no pattern of adverse outcomes for mother or child.6 A nationwide register based cohort study in Denmark found no increase in fetal deaths following exposure to pandemrix (>7000 vaccinees). It was reported there was a reduced risk of stillbirths.9
Safety of Combined Tetanus, Diphtheria and Pertussis Vaccines (Tdap)
In a study of adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women there were 132 reports to VAERS between 2005 and 2010. There were no concerning patterns of maternal, infant or fetal outcomes.17
It has been reported that tetanus has been administered to millions of women worldwide without any serious adverse events of vaccination identified.13
In summary, there is no evidence of harm to the fetus from inactivated vaccines. Live vaccines are contraindicated for 28 d before and during pregnancy due to a theoretical risk of transmission of vaccine virus. In cases of inadvertent vaccination in pregnancy there has been no reported harm. It is considered that the risk from disease far outweighs the small risk associated with vaccination during pregnancy.
Challenges and Barriers
There are barriers to maternal vaccination on multiple levels. From the perspective of vaccine manufactures there has been reluctance toward developing, testing, and marketing vaccines for pregnant women and indeed pregnant women are routinely excluded from clinical trials. There is an understandable concern around liability for vaccine manufacturers. Vaccines that may be of great benefit are therefore not licensed for use in pregnancy.
From the healthcare providers there has been a failure to offer women vaccination. Notably, with influenza in the USA. Healthcare providers may have a perception of unwillingness of pregnant women to accept vaccination and therefore they may not even offer the vaccine. Vaccination during pregnancy has not been routine until relatively recently in the developed world setting and therefore there hasn’t been an established platform for delivery of vaccines. Midwives are not accustomed to delivering vaccines and consequently most are administered by GPs.
For pregnant women the concern regarding the safety and welfare of the unborn infant is very real. Women may perceive a potential risk and therefore not be willing to accept an intervention in pregnancy.
In a recent study online questionnaires were administered to 1013 women aged 18–44 y to assess the attitudes of women of child-bearing age in Great Britain toward antenatal immunization. They were asked “How likely would you be to have a GBS vaccine in pregnancy?”
As can be seen from Figure 5 providing information about GBS increased the proportion of women who thought that they would be likely to have a GBS vaccine in pregnancy increased the proportion of women from 72 – 82%.
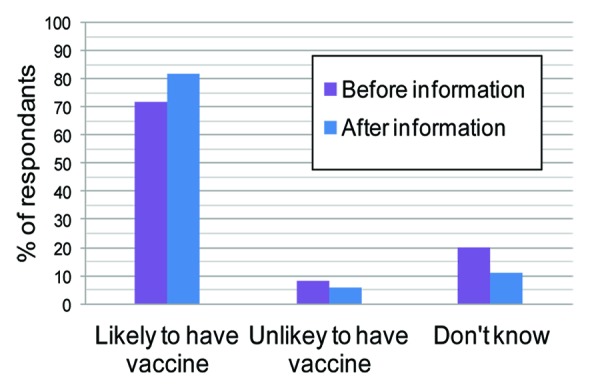
Figure 5. Response to the question “How likely would you be to have a GBS vaccine in pregnancy?” (ref. 5).
A third of women said that they would consider taking part in clinical trial if the vaccine had been tested in only 500 women, a scenario similar to the number enrolled in clinical trials of GBS to date. The role of health professionals in providing advice about immunizations to pregnant women is emphasized by the fact that women considered their GP, midwife and obstetrician the most important people in helping them make the decision about receiving a vaccine in pregnancy.5
More information about specific barriers and motivations to receiving vaccination during pregnancy are important to ensure optimal vaccine uptake in pregnancy.
Another concern has been the potential for maternal vaccines to interfere with the routine infant schedule. It was found that high maternally derived pre-existing pertussis toxin antibody was associated with a 28–56% reduction in pertussis toxin antibody in infants after receipt of 3 doses of DTwP (diphtheria, tetanus, and whole-cell pertussis) vaccine compared with infants with low pre-existing antibody. This effect was minimal with DTaP (diphtheria, tetanus, and acellular pertussis) vaccination. Moderate reductions in filamentous hemagglutinin (FHA) and fimbriae (FIM) antibodies were seen with both infant vaccines.2
It has been shown that higher antibody concentration at birth appears to inhibit the response to infant immunization for tetanus and pneumococcus; the effect was less marked for Hemophilus influenzae type b (Hib) and pertussis. However, the majority of infants tested achieved high antibody levels post-immunization. This supports maternal immunization, as high levels of maternally derived antibody at birth may not inhibit infants' immunization responses in a clinically relevant manner.4
Conclusion
Steady progress has been made in the acceptability of maternal vaccination programs and there is increasing data regarding their safety and effectiveness. However, better understanding is still required. Maternal vaccination provides a new platform for preventing significant and serious infections in mothers and young infants.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- DTaP
diphtheria, tetanus, and acellular pertussis vaccination
- DTwP
diphtheria, tetanus, and whole-cell pertussis combined vaccine
- FHA
filamentous hemagglutinin
- FIM
fimbriae antibodies
- GBS
Group B Streptococcus
- GP
general practitioner
- Hib
Haemophilus influenzae type b
- HIV
human immunodeficiency virus
- IAP
intrapartum antibiotic prophylaxis
- IgG
immunoglobulin G
- VAERS
Vaccine Adverse Event Reporting System
AUTHOR PLEASE PROVIDE SUBMITTED/ACCEPTED DATES
References
- 1.Courtesy of G.Amirthalingam, 2013 Amirthalingam G, Gupta S, Campbell H. Pertussis immunisation and control in England and Wales, 1957 to 2012: a historical review. Euro Surveill. 2013;18(38). pii: 20587. a. CMO Annual Report 2011. Accessed at https://www.gov.uk/government/publications/cmo-annual-report-2011-volume-one-on-the-state-of-the-public-s-health [DOI] [PubMed]
- 2.Englund JA, Anderson EL, Reed GF, Decker MD, Edwards KM, Pichichero ME, Steinhoff MC, Rennels MB, Deforest A, Meade BD. The effect of maternal antibody on the serologic response and the incidence of adverse reactions after primary immunization with acellular and whole-cell pertussis vaccines combined with diphtheria and tetanus toxoids. Pediatrics. 1995;96:580–4. [PubMed] [Google Scholar]
- 3.Heath PT, Balfour G, Weisner AM, Efstratiou A, Lamagni TL, Tighe H, O’Connell LA, Cafferkey M, Verlander NQ, Nicoll A, et al. PHLS Group B Streptococcus Working Group Group B streptococcal disease in UK and Irish infants younger than 90 days. Lancet. 2004;363:292–4. doi: 10.1016/S0140-6736(03)15389-5. [DOI] [PubMed] [Google Scholar]
- 4.Jones C, Pollock L, Barnett SM, Battersby A, Kampmann B. The relationship between concentration of specific antibody at birth and subsequent response to primary immunization. Vaccine. 2014;32:996–1002. doi: 10.1016/j.vaccine.2013.11.104. [DOI] [PubMed] [Google Scholar]
- 5.McQuaid F, Jones C, Stevens Z, Plumb J, Hughes R, Bedford H, Heath PT, Snape MD. Attitudes towards vaccination against group B streptococcus in pregnancy. Arch Dis Child. 2013 doi: 10.1136/archdischild-2013-305716. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 6.Munoz FM. Safety of influenza vaccines in pregnant women. Am J Obstet Gynecol. 2012;207(Suppl):S33–7. doi: 10.1016/j.ajog.2012.06.072. [DOI] [PubMed] [Google Scholar]
- 7.Neuzil KM, Mellen BG, Wright PF, Mitchel EF, Jr., Griffin MR. The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children. N Engl J Med. 2000;342:225–31. doi: 10.1056/NEJM200001273420401. [DOI] [PubMed] [Google Scholar]
- 8.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. doi: 10.1155/2012/985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasternak B, Svanström H, Mølgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, Hviid A. Vaccination against pandemic A/H1N1 2009 influenza in pregnancy and risk of fetal death: cohort study in Denmark. BMJ. 2012;344:e2794. doi: 10.1136/bmj.e2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, et al. Active Bacterial Core surveillance/Emerging Infections Program Network Epidemiology of invasive group B streptococcal disease in the United States, 1999-2005. JAMA. 2008;299:2056–65. doi: 10.1001/jama.299.17.2056. [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Schmitz G, Levy O. Development of newborn and infant vaccines. Sci Transl Med. 2011;3:90ps27. doi: 10.1126/scitranslmed.3001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schofield FD, Tucker VM, Westbrook GR. Neonatal tetanus in New Guinea. Effect of active immunization in pregnancy. Br Med J. 1961;2:785–9. doi: 10.1136/bmj.2.5255.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheffield JS, Munoz FM, Beigi RH, Rasmussen SA, Edwards KM, Read JS, Heine RP, Ault KA, Swamy GK, Jevaji I, et al. Research on vaccines during pregnancy: reference values for vital signs and laboratory assessments. Vaccine. 2013;31:4264–73. doi: 10.1016/j.vaccine.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 14.Tamma PD, Ault KA, del Rio C, Steinhoff MC, Halsey NA, Omer SB. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2009;201:547–52. doi: 10.1016/j.ajog.2009.09.034. [DOI] [PubMed] [Google Scholar]
- 15.van den Berg JP, Westerbeek EA, Berbers GA, van Gageldonk PG, van der Klis FR, van Elburg RM. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr Infect Dis J. 2010;29:801–5. doi: 10.1097/INF.0b013e3181dc4f77. [DOI] [PubMed] [Google Scholar]
- 16.Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 17.Zheteyeva YA, Moro PL, Tepper NK, Rasmussen SA, Barash FE, Revzina NV, Kissin D, Lewis PW, Yue X, Haber P, et al. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol. 2012;207:e1–7. doi: 10.1016/j.ajog.2012.05.006. [DOI] [PubMed] [Google Scholar]