Abstract
The use of immunotherapy in the management of cancer is growing, and a range of new immunotherapeutic strategies is becoming available. It is important that people involved in the care of cancer understand how cancer immunotherapies differ from conventional chemotherapy and apply this knowledge to their clinical practice. Therefore, from August–September 2011 we undertook a survey of awareness, attitudes, and perceptions of cancer immunotherapy among 426 healthcare professionals (HCPs) in Europe with the aim of identifying and prioritizing educational needs. Nearly all (98%) HCPs were aware of cancer immunotherapy. While 68% of HCPs indicated a high level of interest in cancer immunotherapies, only 24% of the HCPs had direct experience with them. Overall perceptions of cancer immunotherapy among HCPs were largely positive (60%) and rarely negative (3%). The key advantages of cancer immunotherapy were perceived to be good safety and tolerability (75%), a targeted mechanism of action (61%) and good efficacy (48%). The leading barriers to use of immunotherapies were costs of treatment (58%), past clinical trial failures (45%), and access/formulary restrictions (44%). The results indicate that, among the respondents, awareness of cancer immunotherapy was high but that knowledge levels varied and direct experience with their use was limited. There appears to be a need for educational activities on cancer immunotherapy, as well as generation and communication of clinical data on long-term efficacy and safety.
Keywords: awareness, cancer immunotherapy, education, survey, vaccine
Introduction
Cancer immunotherapy encompasses a diverse variety of treatment approaches including “passive” administration of tumor-specific monoclonal antibodies and other immune system components as well as adoptive transfer of ex vivo modified T cells, “active” immunization to elicit or augment specific T cell-mediated immune responses against tumor cells, and non-specific enhancement of immune responsiveness with immune modulatory agents.1 Immunotherapy has already had a major impact on the management of a broad range of cancers, but this has been largely restricted to passive immunotherapy with monoclonal antibodies. Development of active antigen-specific cancer immunotherapies has provided many challenges and, to date, only one has been approved for clinical use.2
The field of cancer immunotherapy is complex and rapidly evolving. Immunotherapies differ from conventional chemotherapy in their mechanisms of action as well as the types of responses produced, and conventional response criteria may not provide a reliable assessment of the disease-modifying activity of immunotherapeutic agents.3 Many active immunotherapies incorporate multiple components (e.g., antigens, adjuvants, and delivery vehicles). Furthermore, it is increasingly recognized that robust therapeutic activity requires that immunization is combined with other immunomodulatory strategies directed at enhancing general immune responsiveness and overcoming the immunosuppressive mechanisms through which tumors promote a tolerogenic environment.
Healthcare professionals (HCPs) involved in the care of people with cancer need to understand how immunotherapies differ from chemotherapies so that they can interpret the growing body of clinical data and make optimal use of immunotherapies alongside established treatment modalities. We undertook a survey of HCPs involved in cancer care in Europe. The objectives of the survey were to assess current awareness, attitudes, and perceptions of cancer immunotherapy and to identify priorities for educational activities.
Results
Participants
A total of 3873 HCPs were contacted from August–September 2011 and invited to complete the online survey. Of all the HCPs contacted, approximately 80% did not respond, 6% did not meet the screening criteria (primarily due to not being recently active in the treatment of patients with cancer and/or non-small cell lung cancer), and 4% started but did not complete the survey. Results are presented here for the 426 HCPs who responded to the invitation to participate, met the screening criteria, and went on to complete the survey (completion rate = 11%). Approximately 85 HCPs completed the survey in each of the European countries (Table 1). Overall, 47% of completers were oncologists, 29% were surgeons, and 23% were nurses, with a similar ratio for each of the countries.
Table 1. Participant characteristics: country and specialty of healthcare professionals completing the online survey.
| France | Germany | Italy | Spain | United Kingdom | All countries | |
|---|---|---|---|---|---|---|
| All participants, N | 85 | 86 | 85 | 85 | 85 | 426 |
| Oncologist, n | 40 | 41 | 40 | 40 | 40 | 201 |
| Surgeon, n | 25 | 25 | 25 | 25 | 25 | 125 |
| Oncology nurse, n | 20 | 20 | 20 | 20 | 20 | 100 |
Telephone interviews were conducted with representatives from 2 patient advocacy groups in each of the 5 countries, including charities (France, Germany, and United Kingdom), hospital-based groups (Italy and Spain), and local government (France). Representatives interviewed included patient advocates, medical advisors/directors, oncologists, and a family doctor specializing in cancer care.
Awareness and experience of cancer immunotherapy
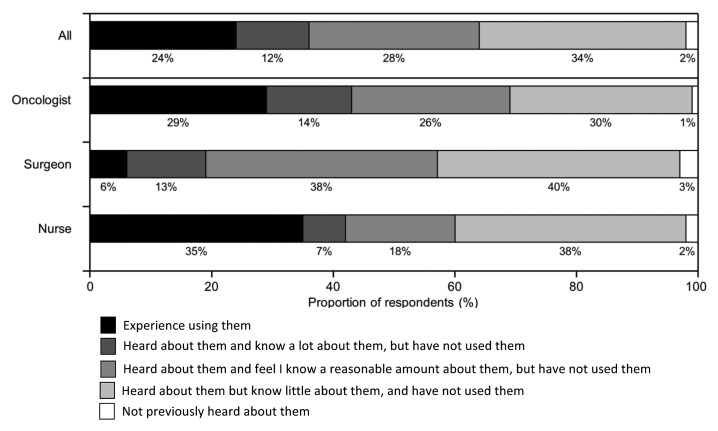
Nearly all (98%) of the HCPs surveyed indicated they were aware of cancer immunotherapeutics/therapeutic cancer vaccines (TCVs). While overall awareness was similar across the different specialties surveyed, oncologists and nurses appeared to have greater knowledge of cancer immunotherapeutics compared with surgeons. The proportion of respondents indicating they had experience with their use was approximately 30% for oncologists and nurses compared with just 6% for surgeons (Fig. 1). Overall, 68% of the HCPs indicated a high level of interest in cancer immunotherapeutics/TCVs, with higher rates of interest among nurses (78%) compared with surgeons (66%) and oncologists (63%). Only 5% of the respondents indicated they had little interest.
Figure 1. Awareness and experience of cancer immunotherapy. Participants were asked “Which of the following best describes your awareness of cancer immunotherapeutics/therapeutic cancer vaccines?”
Perceptions and attitudes toward cancer immunotherapy
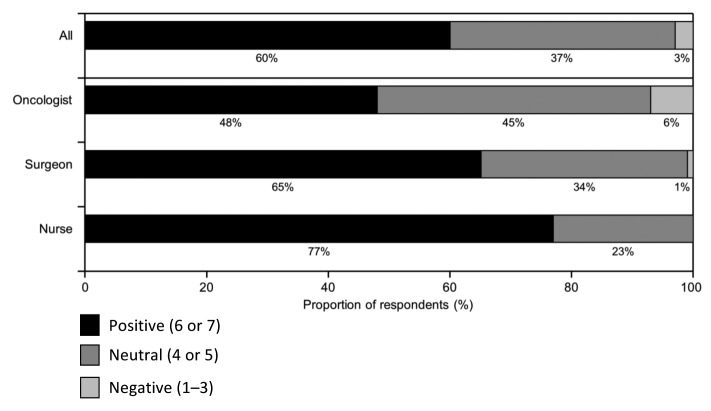
The majority (60%) of all respondents indicated they had an overall positive attitude toward cancer immunotherapeutics/TCVs, although there was considerable variation across the HCP specialties (Fig. 2). Nurses were the most positive, with 77% indicating an overall positive attitude, while oncologists were more or less equally divided between those with a positive attitude and those who were neutral (48% and 45%, respectively). A negative attitude was reported by only 3% of the HCPs, and predominantly among oncologists (6%).
Figure 2. Overall attitudes toward cancer immunotherapy. Participants were asked “Please indicate your overall attitude to cancer immunotherapeutics/therapeutic cancer vaccines where 1 means not at all favorable and 7 means extremely favorable.” Responses were classified as “Positive” (rating of 6 or 7), “Neutral” (4 or 5), or “Negative” (1–3).
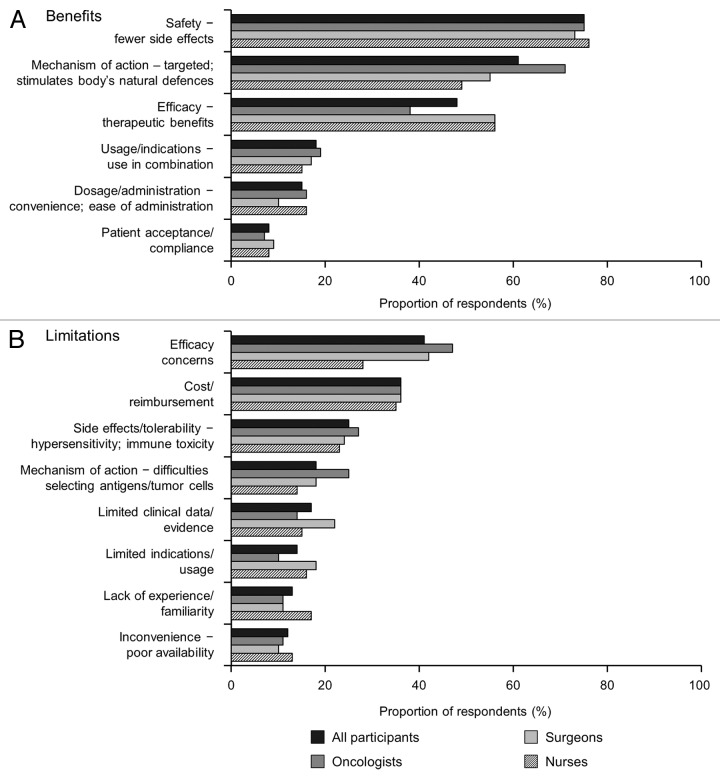
Perceptions of the key benefits and limitations of cancer immunotherapeutics/TCVs are presented in Figure 3. Key benefits were perceived to be: safety, with a better side-effect profile compared with established therapies (75%); a targeted mechanism of action that stimulates the body’s natural defense system (61%); and, efficacy/therapeutic benefits (48%). At the same time, concerns about lack of efficacy were the most frequently reported limitation (41%). The other limitations most frequently identified related to cost/reimbursement (36%) and safety concerns, particularly with regard to selection of antigens that may be expressed by normal as well as tumor cells (25%).
Figure 3. Perceptions of key (A) benefits and (B) limitations of cancer immunotherapy. Participants were asked “What if any do you feel are the key benefits/limitations associated with cancer immunotherapeutics/therapeutic cancer vaccines?”
Perceptions were broadly similar across the HCP specialties, other than that oncologists were more likely to see mechanism of action, but less likely to report efficacy, as a benefit. Also, fewer nurses than oncologists or surgeons reported efficacy concerns as a limitation.
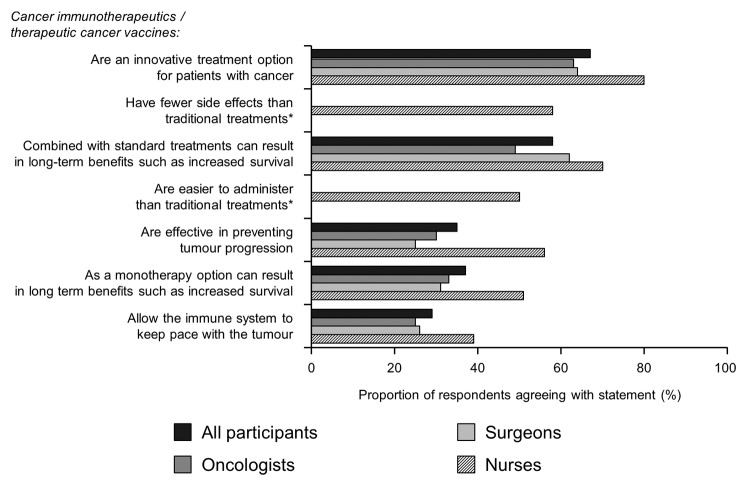
When asked to indicate the extent to which they agreed with a series of statements about cancer immunotherapeutics/TCVs, the greatest agreement across specialties (67%) was with their description as an innovative treatment option (Fig. 4). The extent of agreement with the statement that cancer immunotherapeutics/TCVs can result in long-term benefits depended on whether this was in the context of combination treatment with standard therapies (58%) or monotherapy (37%).
Figure 4. Perceptions and understanding of cancer immunotherapy. Participants were asked “To what extent do you agree or disagree with the following statements regarding cancer immunotherapeutics/therapeutic cancer vaccines? Please use a 7 point scale where 1 means “Disagree strongly” and 7 means “Agree strongly” Agreement was classified as rating of 5–7. *Statement given only to nurses.
Use of cancer immunotherapy and barriers to use
Across all countries, nearly 80% of the HCPs reported having spoken with their patients about cancer immunotherapeutics/TCVs (Fig. 5), with the highest rates in Germany (92%), followed by France and Italy (~75%) and then Spain and the UK (~70%). Where HCPs had discussed cancer immunotherapy with their patients, most (>90%) indicated the patients were receptive to the concept.
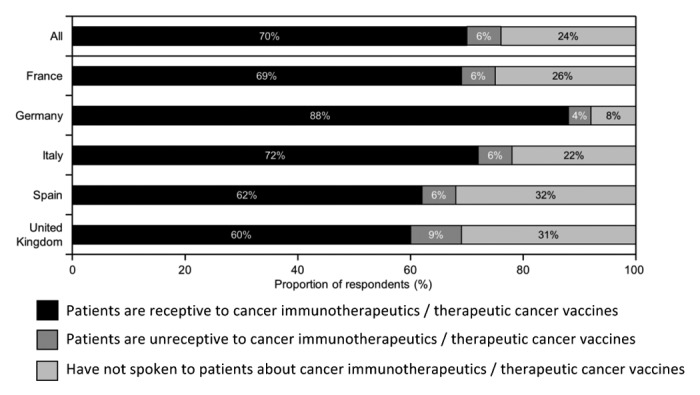
Figure 5. Patient receptivity to cancer immunotherapy. Participants were asked “How would you rate your patients’ receptivity to cancer immunotherapeutics/therapeutic cancer vaccines as a therapeutic option?”
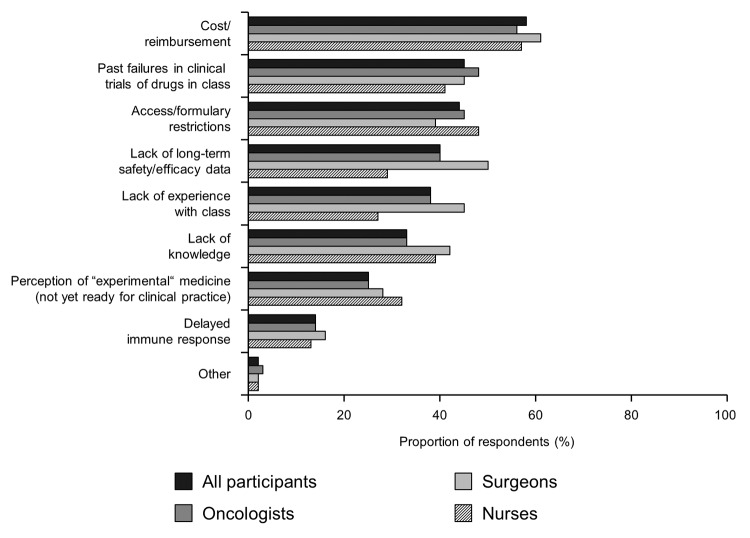
Across all of the HCP specialties, the key barrier to the use of cancer immunotherapy was perceived to be cost and reimbursement issues (58% overall, Figure 6). Additional key barriers were felt to include past failures in clinical trials of drugs in the class (45% overall), access/formulary restrictions (44%) and lack of long-term safety/efficacy data (40%).
Figure 6. Barriers to the use of cancer immunotherapy. Participants were asked “Which of the following barriers do you feel would prevent you from using cancer immunotherapeutics/therapeutic cancer vaccines based on your current experience and/or perceptions of this class?”
Education and information needs
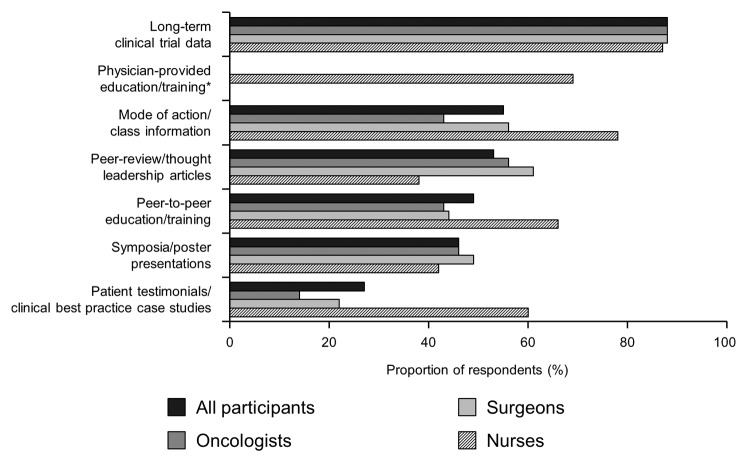
A large proportion (76%) of HCPs indicated they were interested in learning more about cancer immunotherapy. In terms of increasing knowledge of cancer immunotherapy, the most frequently reported need across specialties was for long-term clinical trial data (88% overall; Fig. 7). This figure was similar across HCP specialties. A total of 69% of nurses indicated that physician-provided education / training would be of value. Over half of HCP respondents (55%) indicated that more information on mode of action would be of value, with nurses reporting a greater requirement for this (78%) than oncologists (43%) or surgeons (56%).
Figure 7. Education and information needs. Participants were asked “What types of information would be useful to increase your knowledge/belief in cancer immunotherapeutics/therapeutic cancer vaccines?” *Statement given only to nurses.
The interviews with patient advocacy groups highlighted a need for materials that present and explain the results from clinical trials in a way that is understandable by patients. Some of those interviewed expressed concerns about the complexity of stimulating an immune response directed specifically against tumor cells and that, while there have been many attempts over several decades to achieve this, only a few of them have yielded positive results.
Discussion
The therapeutic potential of stimulating cell-mediated immune responses directed against tumor cells has long been recognized. The possibility of harnessing the specificity and powerful cytotoxic capacity of the body’s own immune defenses is attractive, but the development of treatments capable of delivering clinical efficacy has posed many challenges.4 Recent research has greatly advanced our understanding of the complex host-tumor interactions and the various strategies employed by tumors to evade immune surveillance. This knowledge has been applied to the development of new treatments that are now delivering promising results in clinical studies and the introduction of cancer immunotherapeutics/TCVs into clinical practice.2
The results of this survey among European HCPs involved in the care of patients with cancer indicate a high level of interest in cancer immunotherapeutics but also suggest a degree of skepticism as to their effectiveness. This is not perhaps surprising given the history of initial promise, followed by a failure to demonstrate clinical benefit that has typified the development of new treatments of this kind.4 Despite the challenges, the results of this survey indicate that most HCPs remain positive about the potential for cancer immunotherapeutics/TCVs.
Across all 3 specialties, nearly 90% of HCPs indicated a need for long-term clinical trial data. Extensive programmes of clinical trials across the diverse range of cancer immunotherapeutics are ongoing and results from a number of large phase III trials have been recently published.2,5-10 In order to apply this information in making evidence-based clinical decisions, it is important that HCPs are not only aware of the clinical trial data but also have the ability to interpret it. There are important differences in mechanisms of action of cancer immunotherapeutics compared with conventional chemotherapy that have implications for clinical use and the interpretation of clinical results. For example, responses to immunotherapy may not be adequately captured with the standard criteria used to assess responses to chemotherapy.3 The dynamics of therapeutic responses may also differ from those typical of chemotherapy—while the onset of activity of chemotherapy is essentially immediate it can take weeks or months for the therapeutic benefit of immunization against cancer to become apparent.11 From the survey, it is clear that mechanism of action is an important consideration for the HCPs, with the majority indicating they are interested in learning more about how they act.
While there have been many surveys of attitudes to the use of preventive cancer vaccines, particularly immunization against human papilloma virus infection to prevent cervical cancer,12-14 to our knowledge this is the first report of attitudes and awareness regarding the use of antigen-specific immunization to treat established cancer. Limitations of the study include the relatively low completion rate (11%). The sample consisted of those HCPs who responded to an invitation to participate which may have biased the results and limits the generalizability of the conclusions. The complex terminology and lack of standard definitions in the field of cancer immunotherapy is a further complication. While participants were given definitions of “cancer immunotherapeutics” and “therapeutic cancer vaccine” before they completed the survey, they may have had preconceptions of the meanings of these terms that differed from those given and may have influenced their responses and biased the results. Finally, cancer immunotherapy is a rapidly developing field and since the survey was conducted in 2011 a number of key clinical trials have reported results. In particular, recent trials of immunomodulatory monoclonal antibodies directed against the cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death-1 (PD-1) receptors have shown very positive results.15,16 Based on these and other recent developments, Science recognized cancer immunotherapy as the “Breakthrough of the Year” for 2013.17 Therefore, there might well have been a change in attitudes to cancer immunotherapy since the survey was conducted and, based on the above, a shift toward more positive perceptions seems most likely. A follow-up survey could be of interest in determining the extent to which advances in research and positive clinical trial results have reshaped opinions.
Conclusions
There was a high level of awareness of cancer immunotherapeutics/TCVs among the European HCPs involved in cancer care who completed the survey, although direct experience with their use was limited. Cancer immunotherapy was generally perceived as a positive addition to established treatment options, with safety profile perceived as a key potential benefit but concerns about efficacy based on failures in previous clinical trials. Further educational activities are needed, focusing on the dissemination and interpretation of long-term clinical data, as well as understanding of the mechanisms of action of different types of cancer immunotherapy.
Methods
Online survey of healthcare professionals
Healthcare professionals in 5 European countries (France, Germany, Italy, Spain, and the United Kingdom) were invited to participate in a survey of awareness and understanding of cancer immunotherapy. Respondents to the invitation were asked to complete a structured online questionnaire consisting of 8 sections. The first section included a number of screening questions designed to identify respondents who were oncologists, surgeons, or nurses directly and actively involved in the care of patients with cancer. Participation was stratified by country and medical profession, with the aim of achieving completed surveys from ~100 HCPs in each of the 5 countries and in an approximately 2:1:1 ratio for oncologists, surgeons, and nurses. Respondents satisfying the screening criteria were asked to complete the rest of the survey; otherwise, the survey was terminated at this point.
Eligible respondents were then asked about their awareness of new classes or types of oncology treatment, and specifically what they understood by the terms “immunotherapy” and “therapeutic cancer vaccines” within oncology. After completing these questions, they were given the following definitions:
“Cancer immunotherapeutic: Treatment to stimulate or restore the ability of the immune system to fight cancer by inducing, enhancing, or suppressing an immune response. Cancer immunotherapeutics result in targeted immune activity against a disease-specific antigen, either by increasing immune cell recognition of the target or by reducing disease-related immune suppression.”
“Therapeutic cancer vaccines are a new type of cancer immunotherapeutic designed to help the immune system recognize cancer cells by increasing exposure to tumor-associated antigens. Once activated, the immune system can target cancer, stop its growth, and prevent it from spreading or coming back. Unlike preventive cancer vaccines, such as those targeting viruses that cause cervical or liver cancer, therapeutic cancer vaccines are designed to be administered only after the disease is established.”
The respondents were then asked to answer a total of 30 questions related to cancer immunotherapeutics/therapeutic cancer vaccines. The questions were grouped into sections including current awareness and experience; attitudes and perceptions; drivers/barriers; communication; future—hopes and trends; and patient communications and dialogs.
Telephone interviews of patient advocacy groups
In addition to the online survey of healthcare professionals, 1–2 patient advocacy groups in each of the 5 countries were contacted by telephone and asked to complete a structured interview lasting approximately 30 min. Those agreeing to complete the interview were asked about the information sources they used to keep up-to-date with new cancer treatments and support. The participants were then read the same definitions of “cancer immunotherapeutics” and “therapeutic cancer vaccines” as given in the online survey. Interviewees were then asked a series of questions about their awareness and understanding of cancer immunotherapeutics/therapeutic cancer vaccines, both within the patient advocacy groups they represent and among patients and their families.
Disclosure of Potential Conflicts of Interest
H.M. has received honoraria and research funding from Merck KGaA. C.H. is an employee and has stock in BioNTech and GANYMED Pharmaceuticals, has provided consultancy to apceth, Bayer, Baxter, BioNTech, GANYMED, immatics, Merck KGaA, SuppreMol, and TRON, and received honoraria from the Kurume Cluster (Japan), CCR (UK), Swiss National Science Foundation and the Karolinska University, Stockholm, Sweden. I.M. has provided consultancy to and received honoraria and research funding from Bristol-Myers Squibb. G.P. has provided consultancy to CureVac and received honoraria from Recombio. N.T. and S.S. have provided consultancy and received honoraria from Merck KGaA. G.G. is employed by Ultimovacs AS and has stocks in Ultimovacs AS and Targovax AS and he has provided consultancy to and received honoraria from KaelGemvax and Lytix AS. W.G., J.W., and C.Z. have no conflicts of interest to disclose.
Acknowledgments
The POINT group receives funding from Merck KGaA, Darmstadt, Germany. Synovate Healthcare, London, United Kingdom partnered in the research survey and medical writing assistance was provided by Ian Faulkner, International Medical Press, London, UK, funded by Merck KGaA. Merck KGaA has performed a scientific review of the publication, but the views and opinions described in the publication do not necessarily reflect those of Merck KGaA.
Glossary
Abbreviations:
- HCP
healthcare professional
- TCV
therapeutic cancer vaccine
References
- 1.Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol. 2011;29:4828–36. doi: 10.1200/JCO.2011.38.0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. IMPACT Study Investigators Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 3.Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, Humphrey R, Blumenstein B, Old L, Wolchok J. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–97. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim NK, Murray JL, Zhou D, Mittendorf EA, Sample D, Tautchin M, Miles D. Survival advantage in patients with metastatic breast cancer receiving endocrine therapy plus sialyl Tn-KLH vaccine: post hoc analysis of a large randomized trial. J Cancer. 2013;4:577–84. doi: 10.7150/jca.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giaccone G, Bazhenova L, Nemunaitis J, Juhasz E, Ramlau R, van den Heuvel MM, Lal R, Dunlop DJ, Carrier E, Fakhrai H. A phase III study of belagenpumatucel-L therpaeutic tumor cell vaccine for non-small cell lung cancer (NSCLC). European Cancer Congress. Abstract #2-2013. [DOI] [PubMed] [Google Scholar]
- 7.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–27. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butts C, Socinski MA, Mitchell PL, Thatcher N, Havel L, Krzakowski M, Nawrocki S, Ciuleanu TE, Bosquée L, Trigo JM, et al. START trial team Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:59–68. doi: 10.1016/S1470-2045(13)70510-2. [DOI] [PubMed] [Google Scholar]
- 9.ASC University (Internet). Middleton GW, Valle JW, Wadsley J, Propper D, Coxon FY, Ross PJ, Madhusudan S, Roques T, Cunningham D, Corrie P et al. A phase III randomized trial of chemoimmunotherapy comprising gemcitabine and capecitabine with or without telmoerase vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer; Available from: http://meetinglibrary.asco.org/content/111300-132 [DOI] [PubMed]
- 10.Schuster SJ, Neelapu SS, Gause BL, Janik JE, Muggia FM, Gockerman JP, Winter JN, Flowers CR, Nikcevich DA, Sotomayor EM, et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J Clin Oncol. 2011;29:2787–94. doi: 10.1200/JCO.2010.33.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finke LH, Wentworth K, Blumenstein B, Rudolph NS, Levitsky H, Hoos A. Lessons from randomized phase III studies with active cancer immunotherapies--outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC) Vaccine. 2007;25(Suppl 2):B97–109. doi: 10.1016/j.vaccine.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 12.Marlow LA, Zimet GD, McCaffery KJ, Ostini R, Waller J. Knowledge of human papillomavirus (HPV) and HPV vaccination: an international comparison. Vaccine. 2013;31:763–9. doi: 10.1016/j.vaccine.2012.11.083. [DOI] [PubMed] [Google Scholar]
- 13.Perkins RB, Anderson BL, Gorin SS, Schulkin JA. Challenges in cervical cancer prevention: a survey of U.S. obstetrician-gynecologists. Am J Prev Med. 2013;45:175–81. doi: 10.1016/j.amepre.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Zhao FH, Tiggelaar SM, Hu SY, Zhao N, Hong Y, Niyazi M, Gao XH, Ju LR, Zhang LQ, Feng XX, et al. A multi-center survey of HPV knowledge and attitudes toward HPV vaccination among women, government officials, and medical personnel in China. Asian Pac J Cancer Prev. 2012;13:2369–78. doi: 10.7314/APJCP.2012.13.5.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–3. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]