Abstract
Immunologic adjuvants are essential for current vaccines to maximize their efficacy. Unfortunately, few have been found to be sufficiently effective and safe for regulatory authorities to permit their use in vaccines for humans and none have been approved for use with intradermal vaccines. The development of new adjuvants with the potential to be both efficacious and safe constitutes a significant need in modern vaccine practice. The use of non-damaging laser light represents a markedly different approach to enhancing immune responses to a vaccine antigen, particularly with intradermal vaccination. This approach, which was initially explored in Russia and further developed in the US, appears to significantly improve responses to both prophylactic and therapeutic vaccines administered to the laser-exposed tissue, particularly the skin. Although different types of lasers have been used for this purpose and the precise molecular mechanism(s) of action remain unknown, several approaches appear to modulate dendritic cell trafficking and/or activation at the irradiation site via the release of specific signaling molecules from epithelial cells. The most recent study, performed by the authors of this review, utilized a continuous wave near-infrared laser that may open the path for the development of a safe, effective, low-cost, simple-to-use laser vaccine adjuvant that could be used in lieu of conventional adjuvants, particularly with intradermal vaccines. In this review, we summarize the initial Russian studies that have given rise to this approach and comment upon recent advances in the use of non-tissue damaging lasers as novel physical adjuvants for vaccines.
Keywords: laser, vaccine adjuvant, near-infrared, influenza, cancer
Potential Role of Lasers as Vaccine Adjuvants
The challenge of adjuvant development for intradermal vaccines
Immunological adjuvants, (from the latin adjuvare, meaning to help), effect qualitative and quantitative changes in immune responses to a simultaneously administered vaccine antigen that result in sufficient immunological memory and protection against pathogens.1 Contemporary clinical vaccines generally use highly targeted recombinant molecules as antigens that are often poorly immunogenic in their own right and therefore require enhancement with immunologic adjuvants.2-6 Development of safe and potent immunologic adjuvants therefore represents an important element of current and future vaccine development.2,3 The immune potentiating effects of adjuvants are often accompanied by an increased risk of local reactogenicity or systemic toxicity. As a result, in spite of a steady proliferation of potential adjuvant candidates, the number of adjuvants that have actually been approved for use with human vaccines by regulatory agencies such as the FDA is surprisingly limited. Until the recent approval of AS04 (alum with monophosphoryl lipid A) for use in Cervarix® (GlaxoSmithKline) and AS03 (squalene-based oil-in-water emulsion) for Q-pan H5N1 influenza vaccine (GlaxoSmithKline), the FDA had only approved particulate aluminum-salt adjuvants for use with vaccines, mainly due to concerns over side effects. The European Medicines Agency (EMEA) has approved only 5.7-12
The tradeoff between efficacy and safety is evident in recent global experiences with influenza vaccines. Comparisons of adjuvanted to unadjuvanted vaccines in different populations consistently show that rates of seroconversion with adjuvanted vaccines is higher than with unadjuvanted vaccines, but that rates of injection site reactogenicity are also higher with the former.13-15 After the release of vaccines for H1N1 in 2009, the AS03-adjuvanted influenza vaccine Pandemrix® (GlaxoSmithKline) was linked to hundreds of cases of narcolepsy in the EU.16-19 In the US, where only unadjuvanted H1N1 vaccines were approved, no cases were reported.20 Since a potential link has been made between H1N1 epitopes and autoimmune narcolepsy,21 the AS03 adjuvant may have contributed to this adverse effect. The development of new vaccine adjuvants with improved safety profiles is a highly desirable factor in vaccine development.
The challenge of adjuvant development is increased when it comes to intradermal vaccines. There has been a growing interest in targeting vaccines to the skin due to the potential for the skin-based immune system to yield protective immune responses with smaller amounts of vaccine antigen.22-24 Vaccination at this epithelial surface can effectively prime the body to respond to pathogens22,23,25-28 and induce a robust recall immune response.29-31 The intradermal route of vaccination appears to be more effective than the conventional intramuscular route for many vaccines including influenza vaccines22,23,26,28 and hepatitis B vaccines.22-24 An intradermal influenza vaccine Fluzone Intradermal® (Sanofi Pasteur) was approved by the FDA in 2011 and the company has licensed such a vaccine in more than 40 countries.32
In spite of its promise, several distinct challenges have kept intradermal delivery from becoming a standard of practice in vaccination. One of these is the paucity of appropriate adjuvants.33,34 Most adjuvants used in licensed intramuscular vaccines like aluminum salt and oil-in-water adjuvants are simply too reactogenic when delivered intradermally.35,36 While some clinical trials have suggested that alum-adjuvanted vaccines are tolerable when given intradermally,37,38 reports of injection-site reactions with intradermally-delivered adjuvanted vaccines are much higher compared with non-adjuvanted formulations.39 Finally, current adjuvants may not be compatible with intradermal delivery or formulation requirements, especially with newer intradermal vaccination technologies.33,34 Not surprisingly, there is no adjuvanted intradermal vaccine licensed to date.
The use of non-destructive lasers to adjuvant vaccines
A number of studies conducted over the last 2 decades suggest that non-tissue damaging lasers can be used to modify local skin immune responses in a way that enhances systemic immune response to a vaccine introduced into the treated skin. Treating the skin with non-harmful laser light represents an adjuvanting approach that is potentially compatible with intradermal vaccination. This review focuses on the historical development, current status, and future prospect of lasers that do not breach or destroy skin tissue for this purpose. It is well established that skin injury such as scarification or burn can enhance immune responses40 and lasers can be used to induce such injuries to the skin.39,41-43 A number of investigators have explored the immune-stimulating effects of thermally-destructive lasers in treating cancer44,45 and a recent review has been published covering this approach.46 In addition, fractional laser devices used to enhance delivery of drugs and vaccines have been examined for their ability to alter immunologic responses in the skin to vaccines and immunotherapies.47 These lasers breach the tissue barrier by ablating tissue in small cylindrical volumes, creating a field of skin micropores whose diameter, depth, and density can be highly controlled. The tissue damage that results from the process of microporation can activate the immune system through release of damage-associated molecular patterns (DAMPs) from coagulated tissues, leading to expression of pro-inflammatory cytokines and activation of antigen presenting cells.47 This effect is enhanced by the cytokine cascades induced by the loss of cutaneous barrier integrity.48,49 The use of fractional lasers for vaccination has also been recently reviewed.50
The use of non-destructive lasers to alter tissue immune responses in a manner that can enhance systemic vaccine responses, (laser vaccine adjuvants or LVAs), is a novel approach that has just begun to be explored. LVA treatment of the skin is characterized by a combination of relatively low power densities or irradiances (0.7 to 6.0 W/cm2) combined with fairly high total fluences (energy dose supplied per unit area)—typically in the hundreds of Joules/cm2—a combination that is unique compared with most other clinical applications of lasers to the skin35,51-53 (summarized in Fig. 1). LVA exposures generate moderate but non-damaging thermal responses in the tissue that are quite distinct from “athermal” low-level laser therapy (LLLT), where both laser irradiances and fluences are typically 1–2 logs lower.54,55 The fluences applied with LVAs are well beyond the range where most simulative biological responses to LLLT have been identified. Such lasers also operate at irradiances about one log greater than high fluence, low power laser treatments used to induce apoptotic effects in a variety of cancer cell lines both in vitro and in vivo.56-59 With recently reported progress on a new type of LVAs, the combination of LVAs with skin-based vaccination now has the potential to yield more effective vaccine responses in a safe and cost-effective manner.
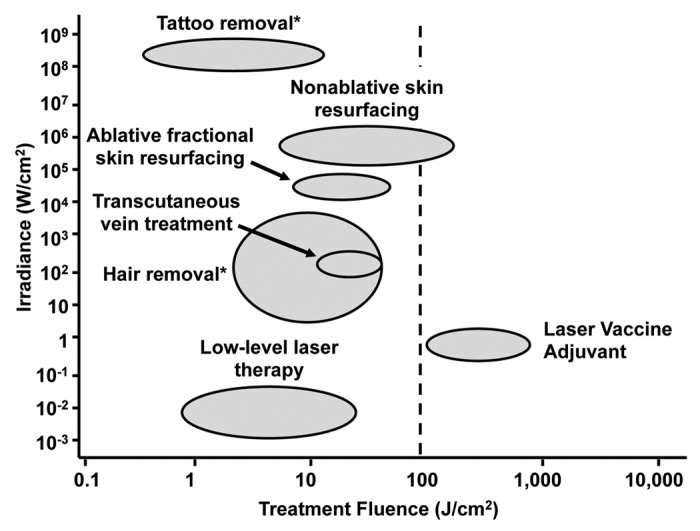
Figure 1. Comparison of the typical irradiances and fluences for laser dermatology procedures. A plot of irradiances vs. fluences for dermatologic applications of lasers is shown. Note that parameters of LVA are quite distinct from those of other applications. *Data represents single pulse treatment on a specific skin target.
History of Laser Vaccine Adjuvants
Initial Russian studies
The concept of using non-destructive lasers as vaccine adjuvants evolved from several decades of laser research in Russia, some of which was transferred to US laboratories over the decade. The origin of this work goes back to Russian investigations of photobiomodulation that began soon after the first descriptions of the effects of low-power laser on biological processes by Endre Mester in Hungary in 1967.60 One area of clinical application that attracted early medical interest was the promotion of wound healing in various tissues.61,62 Many of these early wound healing studies utilized low-power helium-neon lasers.63-66 In 1978, the copper vapor laser was clinically introduced in Russia. Unlike the continuous laser light emitted by helium-neon lasers, copper vapor (CV) lasers release very short duration pulses (10–25 ns) at very high repetition rates (5–20 kHz). CV laser light is emitted at 2 wavelengths—about 510 and 580 nm—representing the yellow-green part of the visible light spectrum. The distinctive laser emission characteristics of copper vapor lasers were of interest to Russian physicians to explore a variety of clinical applications, including wound healing.67 These explorations also gained impetus from medical research funded by the Soviet military during the Afghan conflict in the early 1980s.
While early Russian studies of CV lasers for wound healing featured either low-energy, athermal treatments at doses typical of LLLT, or high-power treatments to induce tissue coagulation,68,69 a small subset of studies in the 1980s and 1990s, many led by Dr Anatoly I Soldatov of the St. Petersburg Academy of Postgraduate Medical Education, utilized much higher irradiances and doses that induced significant photothermal and photoacoustic responses in the irradiated tissue but did not cause tissue damage typical of high power lasers. Some of these studies showed that this new type of treatment effectively promoted wound healing for specific chronic medical conditions such as gastric and duodenal ulcers, bronchitis, and bronchial hyperplasia.70-72
A group of St. Petersburg investigators, led by Dr Sergei Onikienko of the St. Petersburg Military Medical Academy, hypothesized that this higher-power but non-destructive type of laser treatment might also affect immune responses in healthy tissues. These scientists began to explore the potential of CV laser to improve vaccine responses and conducted a number of studies in mice and humans in the late 1990s and early 2000s that used CV lasers to enhance responses to both prophylactic and therapeutic vaccines. These studies, the majority of which were published in Russian, non-peer reviewed journals, showed that CV laser treatment of the skin improved responses to intradermal delivery of commercial influenza and hepatitis B vaccines in documented vaccine non-responders, and also potentiated the effects of experimental therapeutic vaccines for chronic hepatitis B and cancer.
Recent US studies
In 2004, a Massachusetts General Hospital team conducting scientific assessments as part of the BioIndustry Initiative of the US State Department—a program within the cooperative nonproliferation efforts between the US and Russia73—began to meet with these investigators, explore their approach and introduce them to US investigators for potential collaborations. In 2008, a US biotechnology company initiated an effort to replicate these earlier Russian studies using a more structured approach. The first of these studies, published by the Wellman Center for Photomedicine in 2010 provided support for the Russian preclinical results by showing that a 532 nm nanosecond pulsed Nd:YAG laser could enhance antibody titer responses to a model ovalbumin vaccine and a split-virion influenza vaccine.53 Chen et al. subsequently showed that this type of laser could enhance immune responses to a nicotine vaccine35,53 and a dendritic cell vaccine.74
In parallel studies conducted at the laboratories of the Vaccine and Immunotherapy Center (VIC) at Massachusetts General Hospital, Kashiwagi et al. showed that a Nd:YVO4 laser emitting either nanosecond pulsed light at 532 nm or continuous wave, near-infrared light at 1064 nm could enhance immune responses to a model vaccine (ovalbumin, OVA) and to a live attenuated influenza vaccine. Surprisingly, the 1064 nm laser provided superior efficacy to the 532 nm laser in a lethal challenge study.51 The responses to the 532 nm laser were anticipated based on the earlier work done in Russia and at Wellman, but the responses to the 1064 nm continuous wave laser were quite unanticipated and represent a promising avenue of exploration for this approach. Taken together, the research conducted by Russian and US scientists suggests that non-destructive lasers have the potential to enhance vaccine responses and are worthy of further exploration. In addition, the recent discovery by Kashiwagi et al. supports the view that potent vaccine responses can be induced by relatively simple, low power laser systems. This finding enhances the potential and clinical applicability for the commercial development of LVAs.
Immunologic Effects of Laser Vaccine Adjuvants
Systemic effects on vaccine responses
Three different research groups to date have shown systemic vaccine enhancement in response to LVA treatment. Table 1 summarizes the laser types and treatment parameters used by these different groups. The initial Russian studies on LVAs, summarized in Tables 2 and 3, provide a rationale to further pursue this approach but for the most part lack adequate descriptions of methods and are published largely in non-peer review publications. In these studies, Onikienko and his colleagues used a copper vapor (CV) laser (D.V. Efremov Institute of Electrophysical Apparatus) emitting light at both 510 and 578 nm (mix of 10% 510 nm and 90% 578 nm) with a pulse width of 10–12 ns and a pulse frequency of 10–20 kHz. The laser beam had a flat top profile. Irradiances were typically between 1–6 W/cm2 and skin exposures were typically 5 mm spots.
Table 1. Comparison of laser parameters used in different LVA studies.
| Study Source | Onikienko et al. | Chen et al. | Kashiwagi et al. | Kashiwagi et al. |
|---|---|---|---|---|
| Laser Type | Copper vapor | Q switched Nd:YAG | Q switched Nd:YVO4 | Nd:YVO4 |
| Wavelength | 578/511 nm | 532 nm | 532 nm | 1064 nm |
| Beam Profile | Flat top | Gaussian | Parabolic | Parabolic |
| Target Spot Diameter | 5 mm | 7 mm | 5 mm | 5 mm |
| Pulse Duration | 10 ns | 5–7 ns | 10 ns | Continuous |
| Pulse Frequency | 10 kHz | 10 Hz | 10 kHz | Continuous |
| Pulse Energy | 60 µJ | 30 mJ | 67 µJ | 1 J |
| Pulse Power | 1.5 kW | 5 MW | 2 kW | 1 W |
| Average Power | 0.15 - 0.3 W | 0.3 W | 0.2 W | 1 W |
| Irradiance | 0.8 to 3.0 W/cm2 | 0.78 W/cm2 | 1.0 W/cm2 | 5 W/cm2 |
| Exposure Duration | 1–3 min | 2 min | 4 min | 1 min |
| Fluence | 180–300 J/cm2 | 94 J/cm2 | 240 J/cm2 | 300 J/cm2 |
| References | 52,75,76 | 35,53,74 | 51 | 51 |
Nd:YAG, neodymium-doped yttrium aluminum garnet; Nd:YVO4, Neodymium-doped yttrium orthovanadate.
Table 2. Preclinical study of laser adjuvant.
| Laser | Animal strains | Vaccine | Pathway(s) activated | Immune responses | References |
|---|---|---|---|---|---|
| 510 + 578 nm Cu vapor | Mongrel white mice | Vaxigrip (Sanofi) | activation of APC by HSP70 release | Ab, CMI, protection | 52,77 |
| 532 nm Nd:YAG laser | BALB/c | OVA Fluvirin (Novartis) |
Enhancement of the motility of APCs | Ab, TH1, TH2, CTL | 53 |
| 532 nm Nd:YAG laser | BALB/c, C57Bl/6, B6.SJL | DC vaccine (MDDC) | Disruption of skin microstructure | CTL, protection | 74 |
| 532 nm Nd:YAG laser | BALB/c | Conjugated nicotine | ND | Ab | 35 |
| 532 nm Nd:YVO4 laser | C57Bl/6 | OVA whole inactivated influenza virus (PR/8) |
Chemokine release DC migration/maturation |
Ab, TH1 | 51 |
| 1064 nm Nd:YVO4 laser | C57Bl/6 | OVA whole inactivated influenza virus (PR/8) |
Chemokine release/DC migration/maturation | Ab, TH1, TH2, protection | 51 |
APC, antigen presenting cell; HSP, heat shock protein; Ab, antibody response; CMI; cell-mediated immunity; OVA, ovalbumin; TH1, type 1 helper T cell response; CTL, cytotoxic T lymphocyte; ND, not determined; DC, dendritic cell; MDDC, marrow-derived dendritic cell.
Table 3. Clinical study of laser adjuvant.
| Laser | Subjects | Vaccine | Positive response rate | Immune responses | References |
|---|---|---|---|---|---|
| 510 + 578 nm Cu vapor | 42 non-responders, 24 healthy controls | Vaxigrip (Sanofi Pasteur) 15 μg by needle injection or jet injector | 14/22 in laser 5/20 in control 20/24 in responders |
Ab, CMI, CTL | 77 |
| 510 + 578 nm Cu vapor | 17 non-responders | rHBsAg 20 μg (Combobiotech) by Mantoux technique | 7/9 in laser 0/8 in control (IL-2 treated) |
Ab | 77 |
| 510 + 578 nm Cu vapor | Not specified | HbsAg (not specified) | 5/9 in HBsAg id + laser 4/11 in control (lamivudine) |
CMI | 76 |
| 512 + 578 nm Cu vapor | stage IV cancer patients (18 colorectal, 7 gastric, 14 NSCLC, 5 RCC) | Auto-antigen + laser every week for 3 mo | delay in progression in 62% of patients for 1 y 1.6 times longer Survival time compared with no laser control |
CTL, APC function | 75 |
| 1064 nm Q-YAG 5 laser | 5 subjects with skin phototype V or VI | N/A | All 5 subject tolerated with no skin damage | N/A | 51 |
Ab, antibody response; CMI; cell-mediated immunity; CTL, cytotoxic T lymphocyte response; rHBsAg, recombinant hepatitis B surface antigen; NSCLC, non-small cell lung carcinoma; RCC, renal cell carcinoma; APC, antigen presenting cell.
Vaccine response studies were performed in both mice and humans. A vaccination study in white mongrel mice involved a single 1- or 2-min treatment of the ear skin at an irradiance of 1–3 W/cm2 followed by an intradermal injection of 50 μL of a split inactivated influenza vaccine (Vaxigrip, Sanofi Pasteur). CV laser treatment of the skin resulted in a 54% to 86% increase in anti-influenza antibody titer at 4 wk compared with vaccine alone.75,77 Induction of cell-mediated immune response was also shown using the leukocyte migration inhibition test (LMIT).78 In a follow up study, protective immunity was examined by exposure of 2 groups of 20 of the same breed of mice to a lethal dose of H3N2 influenza (А/Aichi/2/68) via an inhalation route 14 d after vaccination. 70% of the mice receiving vaccination and laser treatment survived compared with 35% of mice receiving vaccine only.75,77
Onikienko’s team extended the vaccination approach into clinical applications with prophylactic vaccines. In one study, 42 people with documented low antibody titer responses to influenza vaccination were intradermally vaccinated with a 15 μg dose of Vaxigrip via jet injector; 22 of these received skin site exposure to CV laser at an irradiance of 1.0 W/cm2 for 2 min (120 J/cm2 total dose) right before vaccination.77 Blood was drawn from each vaccine at 4 wk and a number of immune end points, including anti-influenza antibody titration, LMIT, lymphocyte cytotoxic activity, monocyte cytokine secretory activity, and the increase in activity of the lymphocyte enzymes, were examined and compared with 22 healthy controls who were also vaccinated. Based on assessment of these assays, it was determined that 14 of the 22 laser treated non-responder subjects showed a statistically significant increase in vaccine responses compared with only 5 of 20 non-responder subjects in the vaccine-only group. In the healthy control group, significant responses were measured in 20 out of 24 subjects.
A similar study was performed in 17 people documented as hepatitis B vaccine non-responders (failure to maintain HBsAb antibody titer of greater than 10 mIU/mL 6 mo after completing the course of vaccination).77 All subjects received a course of 3 intradermal injections (0, 1, and 3 mo) of 20 µg of a recombinant hepatitis B vaccine (Combiotech) using the Mantoux technique. Nine of the subjects received a 3 min treatment of a 5 mm spot on the shoulder skin with a CV laser at 1 W/cm2 average power (180 J/cm2 total dose) prior to each vaccination. Eight other subjects received hepatitis B vaccination with concomitant IL-2 injections (2 500 000 IE via subcutaneous injection) in a manner similar to Jungers et al.79 In the laser treated group, 7 out of 9 subjects reached the international standard for protection at 6 mo after the end of vaccination (10 IU/mL), while none of the IL-2 treated subjects did so.77
The St. Petersburg group also applied the laser approach to therapeutic vaccination. In one study, the laser was used to enhance responses to an investigational vaccine (recombinant HBsAg without alum, Combiotech) combined with laser treatment of the injection site. Subjects diagnosed with chronic hepatitis B for at least 2 y received either a series of 12 weekly intradermal vaccinations with or without CV laser skin pretreatment (1.0–1.5 W/cm2 on a 5 mm skin spot for 1–3 min). A control group received Lamivudine 100 mg daily for 12 wk. The effect of the treatment was evaluated by clinical indicators of disease including liver function tests, circulating HBV DNA by PCR and serum HBsAg, and immune responses using LMIT with HBsAg to measure cell-mediated immune response. At 12 wk, 5 of 9 of the laser-pretreated vaccines showed normalized ALT, HBV DNA copies below 300, and positive LMIT compared with 4 out of 11 of the Lamivudine-treated group.
The Wellman group’s initial studies at MGH similarly used a nanosecond pulsed laser operating in the green spectrum. This was a Q-switched neodymium-doped yttrium aluminum garnet (Q-Nd:YAG) laser emitting light at 532 nm with 6–7 ns pulse widths and repetition rate of 10 Hz. Their initial studies used 2 min exposures to 4 separate 6 mm spots of skin on BALB/c mice at an irradiance of 0.78 W/cm2 followed by intradermal injection of ovalbumin or inactivated influenza vaccines in each irradiated spot. The illumination of skin with the laser increased the motility of antigen presenting cells (APCs), leading to enhanced antigen uptake by APCs and helper T cell priming in the draining lymph nodes. This 2-min laser exposure increased humoral immune responses to a model vaccine (OVA) by 300 to 500% and a split-virion influenza vaccine (Fluvirin) by 400% in primary vaccination and 900% in booster vaccination compared with a non-adjuvanted group.53
In the most recently published LVA studies, the VIC group of Kashiwagi et al. at MGH used a neodymium-doped yttrium orthovanadate laser emitting light either at 532 nm in high frequency Q-switched mode (Q-Nd:YVO4) with an irradiance of 1.0 W/cm2, a pulse duration of 10 ns and a pulse repetition rate of 10 kHz, or in a continuous wave mode at the near-infrared spectrum at 1064 nm (CW-NIR) at an irradiance of 5 W/cm2. The study showed that the CW-NIR laser adjuvant induces the transient expression of a limited set of cytokines and chemokines in skin resulting in recruitment and activation of dendritic cells in skin draining lymph nodes (dLNs). Furthermore, a 1-min application of the CW-NIR laser augmented antibody response most efficiently to OVA and an influenza vaccine (whole inactivated PR8 virus) with a TH1-TH2 balanced T cell response, and conferred protection in a murine influenza lethal challenge model, whereas the 532 nm Q-Nd:YVO4 induced a TH1-skewed response with little impact on protection.51 Importantly, the protective immune responses induced by the CW-NIR were comparable to those induced by a licensed adjuvant and support the view that LVA might have utility in augmenting responses to intradermal vaccines.
Localized effects on irradiated tissues
In general, LVAs appear to work by modifying the immunologic environment within the tissue that receives the vaccine, resulting in enhancement of the vaccine response. The specific modifications in the local immune environment appear to differ depending on the type of laser used and are likely related to significant differences in the laser wavelength, pulse duration, pulse energy, and pulse frequency.
Effect on heat shock protein expression and release
A fundamental principle of vaccine adjuvant development, based on Matzinger’s danger theory of immune response80 is to trigger a danger signal to the immune system that can promote more vigorous and long-lasting responses to a vaccine antigen. These danger signals are often in the form of either DAMPs or pathogen-associated molecular patterns (PAMPs) that can trigger cytokine and chemokine cascades in the tissues, usually through Toll-like receptors (TLRs).81 Significant development work was put into developing adjuvants in the form of DAMPs or PAMPs that can trigger these TLR-mediated pathways to promote vaccine responses.7
Onikienko et al. identified an important role for heat shock protein 70 (HSP70) in mediating vaccine responses to CV laser treatment. HSPs are a family of ubiquitous intracellular molecules that function as molecular chaperones as part of numerous intracellular processes (e.g., protein folding and transport). Under conditions of stress, some of these play important roles in refolding or disposing of misfolded and denatured proteins,82 stabilizing cellular membranes and enhancing cell signaling,83 and inhibiting specific apoptotic pathways.84 The intracellular expression of many of these HSPs are significantly induced under stress conditions such as fever, radiation, infections, and neoplasia.85 Some HSPs, such as HSP70, play additional roles if and when released outside the cell. In these circumstances they can act as potent DAMP-like inducers of immunity and have been harnessed as adjuvants in experimental vaccines targeted to cancers and infections.86 HSPs expressed on the surface of stressed and damaged cells or released from necrotic cells can serve as a kind of danger signal87 and are recognized by APCs through specific receptors, such as TLRs, scavenger receptors (LOX-1), CD91, and CD14 resulting in increased antigen display by MHC class I and II molecules and priming T cells.86,88
Onikienko’s group showed that the CV laser treatment (irradiance of 1–3 W/cm2 with a pulse width of 10–12 ns and pulse repetition frequency of 10–20 kHz) to a 5 mm skin spot on a mouse ear for 3 min induced rapid, dose-related increases in extracellular HSP70 as determined by a whole-mount in vivo immunostaining of epidermal sheet of the mouse ear.52 Western blot analysis on epidermal tissue further showed an increased expression of HSP70 in the ear skin that persisted for 7–14 d.52 Since adjuvant effects in these mice similar to those caused by the CV laser could be induced by injection of exogenous by itself, Onnikienko et al. concluded that high-frequency pulsed laser treatment enhances immune responses via release and sustained expression of HSP70 by fibroblast and/or keratinocytes in the laser irradiated skin.
Cells in the skin harbor a high baseline level of intracellular HSP70 that could potentially be released under conditions of stress89 with the highest levels found in keratinocytes.90 While overexpression of heat shock proteins is a normal cellular response to stress, a number of investigators have shown that under conditions of stress a portion of constituent HSP70 is mobilized to the cell membrane and can be released from the cell through a variety of mechanisms.91-93 Subsequent LVA studies performed in the US and published to date have not linked LVA skin treatment to the release or overexpression of HSP70.51,53 This difference in expression and release profiles for HSP70 may be related to the differences in the laser parameters used by the different laboratories.
Effect on immune cell migration
The use of chemical adjuvants in skin-based vaccination studies directly activate and induce migration of APCs from the skin to the proximal dLN.94,95 LVAs similarly induce APC migration to the skin and dLNs, increasing the concentration of APCs in volume of treated tissue and enhancing their ability to activate, pick up antigen, and migrate to dLNs. The means by which they accomplish these effects may be different depending on the laser type.
Onikienko et al. showed by a histological analysis with electron microscopy that CV laser treatment resulted in a significant increase of Langerhans cells at the irradiation site. Chen et al. showed by intravital microscopy that 532 nm Q-Nd:YAG laser treatment increased the motility of MHC class II-positive APCs between 0.5 and 16 h after irradiation, resulting in a larger number of antigen-positive CD11c+ dendritic cells (DCs) within the dLNs after vaccination compared with vaccination without laser treatment.53 Their analysis of tissue samples from these studies suggests that this increase in motility and migration was related to the rearrangement of extracellular matrices including enlarged perforations in the perilymphatic basement membrane, disarray of collagen fibers and disruption of cell–matrix interactions in the dermis that persisted for at least 16 h after laser treatment.74 This led to their conclusion that the facilitation of APC migration was through alteration of extracellular matrices.
Kashiwagi et al. showed that the CW-NIR laser treatment at 5.0 W/cm2 on a 5 mm spot of mouse back skin for 1 min also resulted in CD11c+ dendritic cell recruitment in the irradiated skin at 6 h after exposure.51 This laser treatment did not appear to result in a significant increase in the migration of activated DCs to the draining skin-dLNs.51
Effects on inflammatory and chemokine signaling
With chemical and biological adjuvants, the activation and mobilization of APCs in the skin is a result of both autocrine and paracrine signaling through cytokines and chemokines.96 DCs are the most versatile APCs; licensed and experimental adjuvants activate DC-mediated innate immune responses that result in robust adaptive immune responses.7-9,97,98 Intradermal administration of adjuvants typically induces inflammatory responses including cytokine release and leukocyte infiltration.99 These adjuvants are effective also because the inflammatory responses mediated by chemical adjuvant lasts for several weeks.99 Unfortunately, this persistence of inflammatory signaling may also play a role in diminishing the safety of these adjuvants.12,100
LVAs appear to function quite distinctly from chemical adjuvants in that they result in tissue signaling and the activation of APCs, but do not appear to trigger significant inflammatory responses. Chen et al. reported that the activation and migration of APCs in the skin following 532 nm Q-Nd:YAG laser treatment was not accompanied by a significant increase in inflammatory cytokines including TNF-α, IL-1β, IL-6, and CCL2.36,53 The activation picture for APCs following exposure to the Q-Nd:YAG laser-treated skin was mixed. While the overall number of DCs migrating to skin-draining LNs was significantly increased by 24 h and activation markers including CD80 and MHC class I were upregulated in skin-dLNs, critical activation markers such as CD40 and MHC class II were not upregulated.74
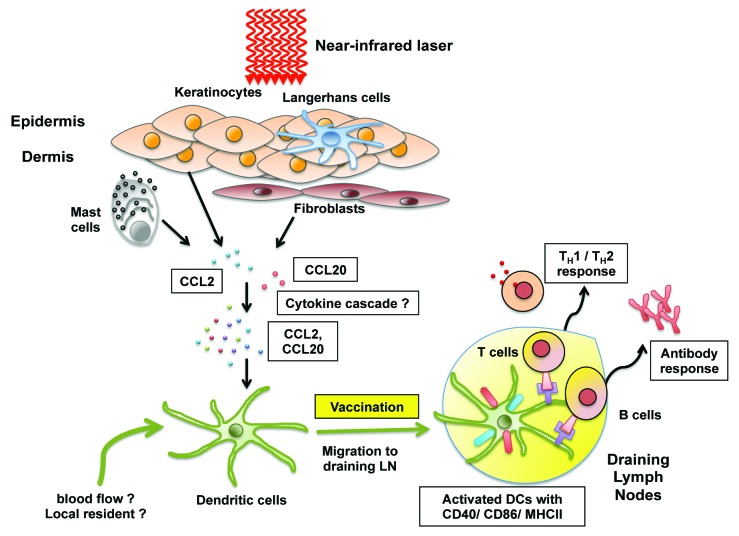
Kashiwagi et al. showed that the CW 1064 nm laser treatment did not result in the significant expression of proinflammatory cytokine genes such as Il1b, Il6, and Tnf and that, without introduction of a vaccine, cytokine responses and expression return to basal levels by 24 h after laser illumination.51 Nevertheless, CW-NIR laser treatment results in CD11c+ dendritic cell recruitment into the irradiated skin, increases the expression of MHC class II and co-stimulatory molecules including CD40 and CD86 on these cells, and increases the number of activated DCs in skin-dLNs 24 h after the laser irradiation.51 Since the magnitude of the CD4+ T cell responses is proportional to the number and quality of DCs that reach the dLNs,101 it is not surprising that the CW-NIR laser adjuvant enhances CD4+ T cell responses. These qualitative and quantitative DC responses correlate to the transient expression (measured 6 h after the CW-NIR laser treatment) of a set of chemokine genes including Ccl2, Ccl6, Ccl11, Ccl17, Ccl20, and Ccr7 that mediate DC migration96,102-105 (Fig. 2). The migration of mature DCs to dLNs through afferent lymphatic vessels is regulated by multiple cytokines and chemokines.101,106 The transient tissue response to the CW-NIR laser illumination results in expression of chemokines sufficient to initiate DC migration and maturation in situ but may not be sufficient to provide DCs with additional guidance cues including CCL19/21 expression in lymphatic endothelial cells. Further investigations of the effects of LVA on DC migration and/or activation are needed.
Figure 2. Putative mechanisms of action of NIR laser adjuvant. Non-tissue damaging continuous wave (CW) near-infrared (NIR) 1064 nm laser given in short exposures to small areas of the skin is able to augment broad immunity including antibody, TH1 and TH2 immune responses to vaccination. NIR laser adjuvant stimulates the expression of a defined set of cytokines and chemokines including CCL2 and CCL20 (via undefined molecular mechanisms) that ultimately induce functional and migrational changes in DCs in the skin. Figure courtesy of Eugene L.Q. Lee (Imperial College London).
Mechanisms of Action for Laser Vaccine Adjuvants
Photobiological basis for laser adjuvant effects
A fundamental principle of laser medicine is that emitted photons must be absorbed in order to have a biological effect. The absorbers of laser light, called chromophores, have specificity for and sensitivity to particular wavelengths of light due to how specific wavelength photons interact with electrons within the molecular structure of the chromophore. As a result, tissues preferentially absorb some wavelengths of light over others, showing greater absorption efficiency at different wavelengths.54,55 Three key chromophores in the skin are melanin, hemoglobin and water. In the ultraviolet (UV) and visible spectrum, absorption by melanin and hemoglobin dominate. The effective absorption of melanin drops off quickly beyond 700 nm and ends at around 1100 nm. Hemoglobin has a high coefficient of absorption in the visible light range with a peak at 578 nm and also falls quickly beyond 700 nm; it plays a relatively insignificant role as a chromophore beyond about 1000 nm. Water has a much lower absorption coefficient of light compared with melanin and hemoglobin until about 1000 nm, but as it makes up almost 70% of the composition of the skin, it presents a large absorption target. Beyond 1000 nm, water becomes the dominant dermal chromophore. The relatively weak absorption of the 3 main skin chromophores between 700 and 1000 nm provides an “optical window” in the skin that permits laser light to penetrate much deeper (Fig. 3). This means that the 510/578 nm CV laser, 532 nm Q-Nd:YAG and 532 nm Q-Nd:YVO4 lasers will have a much shallower effective penetration depth (depth at which the intensity of the laser energy falls to 1/e or about 37% of the incident intensity) about 1 mm, as compared to CW-NIR laser, which will be about 4 mm.107 These differences in absorption efficiency in the skin account for most of the difference in emitted irradiance between visible light and NIR systems (i.e., 1 W/cm2 in the Q-Nd:YVO4 system at 532 nm and 5 W/cm2 in the CW Nd:YVO4 system at 1064 nm).
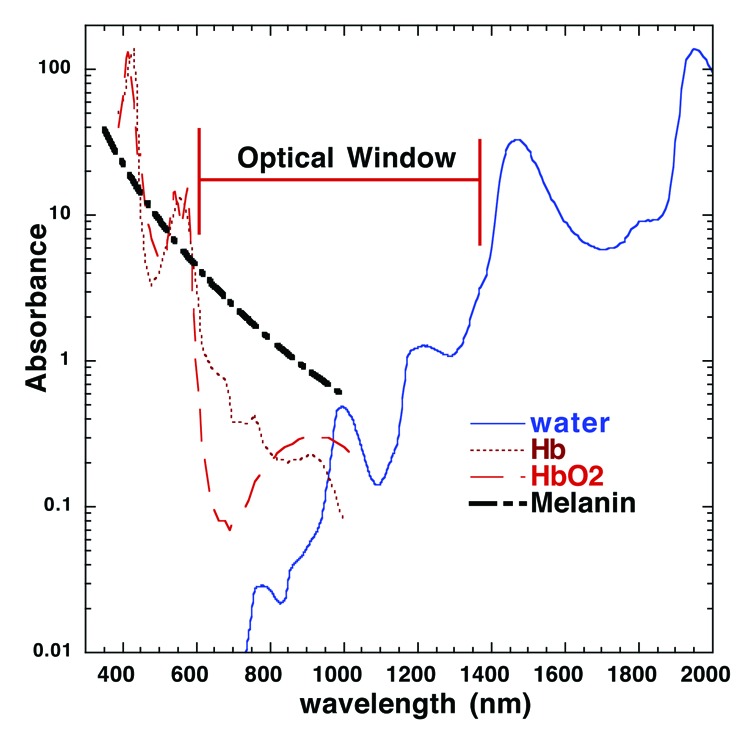
Figure 3. Absorption spectrum for major skin chromophores and the optical window. Light absorption in the skin is wavelength dependent. In the UV to near infrared portion of the spectrum, the predominant tissue chromophores are hemoglobin, melanin, and water. The absorption coefficients for both melanin and hemoglobin decline significantly after 600 nm and water does not increase significantly until after 1200 nm. This creates an “optical window” at red and near-IR wavelengths that maximizes penetration of light into the skin. Note that the 1064 nm NIR laser adjuvant is within the window whereas 510/578 or 532 nm pulsed laser adjuvants are not. Hb, hemoglobin; HbO2, oxygenated hemoglobin. Figure courtesy of Dr Michael Hamblin (Massachusetts General Hospital).
In most medical applications of lasers, much of the energy from photon absorption by chromophores is converted into heat (photothermal effect).108 In addition, some photon energy may induce chemical changes to the chromophore or surrounding molecules resulting in chemical reactions in the target tissue (photochemical effect). In living organisms these chemical alterations have biological effects, called photobiomodulation.54,55,109-111 Photobiomodulation forms the basis for low-level laser therapy (LLLT) approaches. In this setting, cytochrome c oxidase is a putative receptor of light stimuli with different redox states of the molecule absorbing different wavelengths of light. Photothermal effects likely play a larger role in LVA effects when lasers with nanosecond duration pulses at high power levels are used. It is also probable that, while the irradiance and dose of adjuvanting lasers exceed the effective dose range for LLLT by 1–2 log orders, photobiomodulation plays an important role in LVA effects as well especially for the CW-NIR laser.
Photothermal effects of adjuvanting lasers
All lasers that have been used as LVAs to date induce moderate thermal effects in the treated tissues, but irradiance and fluence are specifically calibrated to remain below the level that causes skin damage. Increases in skin temperature induced by these minute-range laser exposures do not reach the pain threshold (42–43 °C)51,53 much less than the temperatures known to induce skin damage within such exposure times.112-114 The non-destructive nature of the LVA exposures used in the recent US mouse studies were validated by both visible skin inspection and independent analyses of biopsy for histopathological evidence of tissue damage.51,53 In addition, a clinical safety study, performed with a 1064 nm nanosecond pulse laser using irradiances and fluences equivalent to those used in the murine laser vaccine enhancement experiments, was well tolerated in humans with no subject reporting uncomfortable skin sensations or pain and no significant skin damage or changes in skin appearance noted during or as a result of any laser exposure.51
While overall increases in temperature in the laser exposed skin is modest, lasers featuring high power, nanosecond duration laser pulses are nevertheless likely to cause significant thermal stresses in the skin. This is due to a phenomenon called thermal containment. When the time over which a laser pulse is absorbed into a volume of the skin around a chromophore (tp) is significantly shorter than the time the resulting heat can be dissipated from that volume to the surrounding tissue (tr), the result is a significant increase in the temperature within that volume relative to the surrounding tissue.108 This condition is known as thermal containment and is considered to be met when tp ≈ 0.25tr. As the duration of a laser pulse decreases, the volume in which the thermal energy is contained also decreases. Thermal confinement is the basis of selective photothermolysis, a phenomenon first described by Anderson and Parrish,115-117 and is the key mechanism of many medical laser applications.
In the nanosecond range, thermal containment is expected to occur at the organelle scale (e.g., 0.5 to 1.0 μm in diameter). In the visible light range where melanin is an important absorber, such targets are typically melanosomes in the basal epidermis.118,119 Given the same pulse energy, shorter pulse durations will result in larger temperature rises within more highly localized volumes around the chromophore. Eventually, sufficiently large pulse energies or sufficiently short pulse durations will result in temperature rises large enough to induce transient protein unfolding or permanent denaturation.120,121 Once these localized temperature spikes significantly exceed 100 °C, the result will be microcavitation or explosive vaporization of the water in and around the target chromophore.122-124 This phase transition can cause significant damage to the tissue. Laser pulse durations in the nanosecond range with pulse powers in the kW or MW range like the CV, Q-Nd:YAG, and Q-Nd:YVO4 lasers, contain sufficient energy to induce significant temperature perturbations at the subcellular level (microhyperthermia), resulting in cellular stress from heat shock.125,126 The propagation of high power (kW or MW) nanosecond-range pulses, repeated tens or thousands of times per second over a matter of minutes, likely triggers a number of stress responses in the skin that leads to enhanced immune processing of introduced vaccine antigens. As long as the combination of peak power, pulse duration and overall fluence are limited, these temperature spikes will not lead to significant irreversible damage to the tissue.
Aside from the tissue stress effects induced by highly localized generation of heat by nanosecond duration laser pulses, the modest overall increase of heat within the tissue does not appear to contribute significantly to the impact of the laser on the immune system. Chen et al. reported that skin heating did not result in a significant enhancement of immune responses.53 Kashiwagi et al. reported no correlation between measured maximal skin temperature and antibody titer in a model vaccine experiment.51
Photoacoustic effects of adjuvanting lasers
The photothermal effects generated by high energy, nanosecond pulsed lasers are accompanied by photoacoustic effects. The significant temperature discontinuities that high power, nanosecond duration laser pulses create between the absorbing target and the surrounding tissue results in different rates of thermal expansion and thus pressure differences.127,128 These pressure differences can generate an acoustic wave that propagates at a much slower rate than that of heat dissipation. When the laser pulse duration (tp) is shorter than the time required for these stress waves to propagate (tσ), a condition of acoustic containment is reached. At pulse durations below 100 ns, significant acoustic waves are generated in the tissue.129 The combination of microhyperthermia and shock wave generation from nanosecond pulse lasers can induce significant stress within cells and tissues, even when no significant damage is apparent.130-132 Not surprisingly, Chen et al. reported that laser treatment of mouse skin with 6–7 ns pulses with a peak power of about 5 MW at a frequency of 10 Hz over 2 min resulted in the disruption of the dense protein network in the skin tissue, resulting in disconnected tissue with collagen fibers, as determined by electron microscopy, even while the overall tissue appearance remained normal.74 Photoacoustic effects would be expected to be reduced in the pulse lasers with much lower peak powers, which may explain why such tissue changes were not reported by Kashiwagi et al.51 Finally, these effects are not expected with the CW-NIR laser. In this case, photochemical effects are more likely to be the mechanism.
Photochemical effects
One of the key photochemical effects of laser light is the generation of reactive oxygen and reactive nitrogen species (ROS and RNS).133 Generation of oxygen and nitrogen radicals from lasers have been demonstrated at a wide range of the spectrum including UV,134 blue,135 visible,136 and near-infrared.54,55,137-140 Generation of these radical species form the basis for a wide variety of biological effects. Since ROS and RNS (particularly nitric oxide, NO) have been shown to stimulate cytokine production in epithelial cells via activation of MAPKs (p38, ERK), JNKs, NF-κB, AP-1, soluble guanylate cyclase (sGC)/protein kinase G (PKG)141-145 and recently NLRP3 inflammasome pathways,146 it is possible that laser adjuvants can mediate activation (phosphorylation) of these pathways via ROS generation. Endogenous or exogenous ROS and NO have been shown to modulate the function of skin cells including keratinocytes147-150 and mast cells.151-153
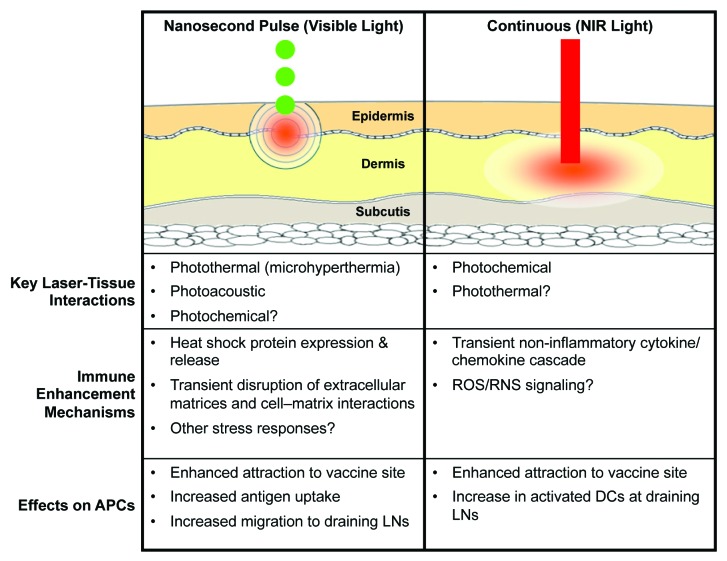
The results of published LVA studies to date clearly demonstrate that these lasers result in migrational and functional changes of DCs in the skin, which may be a common pathway for laser immune enhancement. To this end, different types of lasers appear to engage distinct molecular mechanisms. Photothermal and photoacoustic stress may play a more important role in immune signaling induced by nanosecond pulsed lasers, while photobiomodulation may play a larger role in the CW-NIR laser (Fig. 4). Elucidation of the exact photoreceptors and signaling pathways that mediate these effects is warranted.
Figure 4. The distinctions between pulsed and continuous wave laser vaccine adjuvants. There are 2 major types of laser vaccine adjuvant (LVA). Each class of LVA has a distinct mode of laser-tissue interaction, mechanisms of action, and the effect on antigen presenting cells (APCs). NIR, near-infrared; ROS, reactive oxygen species; RNS, reactive nitrogen species; DC, dendritic cell; LNs, lymph nodes.
Advantages and Limitations of Laser Vaccine Adjuvants
Advantages of LVA over conventional adjuvants
As an adjuvanting approach, lasers have several inherent advantages over chemical and biological adjuvants. (1) Laser light does not persist in the tissue or excessively perpetuate immune signaling, so it reduces the potential for toxic adjuvant effects. (2) It does not appear to depend on conventional inflammatory pathways, similarly reducing the potential for adverse events. (3) LVAs do not cause reactogenicity at the immunization site, a common factor with nearly all current vaccine adjuvants. (4) It cannot induce anti-adjuvant antibodies since it is not a chemical or biological substance. (5) There is little risk of allergic responses from lasers. Millions of people have been treated with visible light and NIR lasers for tattoo removal, hair removal, skin tightening and regeneration. While there are several published reports of allergic response after Nd:YAG laser treatment in tattoo removal, these were essentially delayed hypersensitivity reactions against the tattoo ink in the skin.154,155 (6) LVAs are separate from the vaccine antigen and do not require formulation with the vaccine. Some adjuvants are difficult to combine with the vaccine antigen because co-formulation may cause instability in the vaccine formulation. (7) The laser has no special storage requirements such as cold-chain requirements. Finally, as work by Chen et al. has shown, the laser can be used with several other conventional adjuvants to enhance their effect.35,36
Distinctions of the continuous wave, near-infrared laser for LVA over other laser parameters
When all LVA approaches to date are considered, the CW-NIR system has some compelling advantages over the nanosecond pulsed visible light lasers used in other studies. First, the CW-NIR system is much less sensitive to differences in skin pigmentation. Laser light in the green-yellow spectrum is significantly absorbed by melanin, resulting in highly variable absorption of the same laser dose across different skin phototypes.156,157 In addition, the melanin distribution in different types of skin is not uniform.114 This means that the laser dosing in dark skin may be different than in lighter skin, requiring a more careful recalibration of dose for different skin phototypes. Under these conditions, it may prove more challenging to tolerably treat a dark-skinned recipient due to the high efficiency of light absorption. While Russian investigators conducted initial studies of CV lasers in humans (Table 3), most of their subjects were fairly light skinned. Compared with the yellow-green spectrum, at 1064 nm in the NIR spectrum the coefficient of absorption of melanin is nearly 10-fold less.156,157 Differences in thermal responses to 1064 nm laser treatment between very light skinned and very dark skinned recipients appear to be modest and these differences appear to be shaped by absorption of laser light by blood.158,159 Initial pilot studies in humans using volunteers with skin phototypes V and VI have already established the probability that CW-NIR doses used in the mouse models will be tolerable in humans of all skin tones.33,51 The reduced differences in absorbance across skin phototypes makes the CW-NIR laser ideal for clinical use.
Second, the use of a device emitting continuous, low power laser light represents an engineering advantage for the development of LVA devices. Generation of nanosecond, high peak power laser pulses require technical specifications that may limit the compactness, simplicity and cost of devices to be deployed in the clinic or field. The generation of high frequency, high power pulses can be variable over the lifetime of the laser and the functional lifespan of a Q-switch diode laser is around 10 000 h. In contrast, CW 1064 nm lasers can be produced by very small and economic diode systems and have lifespans that exceed 100 000 h. Based on current technology capabilities, a handheld device for clinical use at under $1000 is feasible.
Limitations of LVA compared with conventional adjuvants
One key limitation in the use of LVA for potentiating vaccines is that their effects occur at a relatively shallow depth within exposed skin and require vaccination to occur intradermally in order to potentiate vaccine responses. This limitation may prove less troublesome as new technologies for intradermal and transcutaneous vaccines further develop.33,160
Another potential limitation of LVA pertains to the inherent limits of the immune response potential in treated tissue. LVA treatment alters DC responses to vaccine antigens based on transient light adjuvant exposure and without induction of significant inflammation. It remains to be seen whether this provides clinically meaningful increases in systemic immunity. Reported results of Russian experiments with the CV laser in human vaccine recipients are certainly provocative (Table 3) and Kashiwagi et al. have shown that the CW NIR laser outperforms alum in intradermal vaccination with model ovalbumin and influenza virus in the mouse model.51 Longer term, well-controlled studies are obviously needed. Further investigation will also be required to understand the optimal dose configurations for each type of system and to conduct comparative studies with other approved adjuvants.
Finally, the lack of appropriate laser devices for inducing vaccine responses is a key limitation in advancing the field. To date, laser devices used in studies of LVA have been either research prototypes or laser systems typically applied to cosmetic dermatology or manufacturing. While the treatment parameters needed to enhance vaccine responses can be produced by some of the existing clinical dermatologic laser systems if they are modified to generate significantly lower power levels and irradiances, such devices are relatively expensive, with the simplest devices typically costing more than $25 000, while more sophisticated devices can top $100 000. Lasers that are expensive and need extensive technical knowledge to operate and maintain reduce the opportunity for investigators working on vaccines, who typically do not have expertise in lasers, to test the potential of such an approach in many different vaccines. The development of a simple, low-cost, portable device would be essential for more rapid preclinical development. Ultimately, the use of LVA for mass vaccination will require simple, small, and economic systems that allow for repeated usage with little need for training or extensive maintenance.
Future Applications of Laser Vaccine Adjuvants
Research performed to date clearly underlines the promise of laser vaccine adjuvants. The technology is aligned with the long-term objectives of governmental and non-governmental organizations to reduce or eliminate chemical adjuvants whenever possible and promote the development of needleless vaccination approaches.34,161 LVA opens a new pathway toward those 2 important goals.
Use of LVA for prophylactic vaccines
In the near term, the most likely application for LVAs is to enhance intradermal vaccination. Clinical trials have been conducted on several different intradermal vaccines including influenza, HAV, HBV, polio, measles, and rabies.33,34 Some traditional vaccines such as the BCG (Bacille de Calmette et Guérin) and the smallpox vaccine are routinely given intradermally. BCG has an established track record as a safe vaccine and has been given to over 3 billion individuals, making it the most widely administered vaccine to date.162 Intradermal influenza vaccines are now available for clinical use.32 As a physically separate adjuvant, LVA represents a broadly-applicable technology for this approach to vaccine delivery. LVA could be easily combined with approved vaccines and experimental candidates, and could be developed into single devices for delivery of adjuvant and vaccine. Since, the efficacy of inactivated influenza vaccine in children and elderly has been suboptimal without adjuvant163 and BCG produces highly variable levels of protection against MTB especially in preventing adult pulmonary TB.164-166 These vaccines would be attractive and feasible candidates for addition of LVA. In addition, LVA could be also used to augment established vaccines when they fail to achieve clinical significance in certain populations. For example, HBV vaccination is suboptimal in immunocompromised populations on dialysis and immunosuppressive therapy. LVA appears to be effective in enhancing intradermal influenza and HBV vaccines in non-responders to previous vaccinations,77 which justifies exploration of the use of LVA this case. Further determination of the efficacy of LVA for currently approved vaccines is an important research goal.
Use of LVA for therapeutic vaccines
The versatility of dendritic cells (DC) and their key role in regulating adaptive immunity167,168 has led to extensive investigation of DC-based vaccines for cancer and chronic infectious diseases.168,169 Activation of cognate T cells in the dLN is also necessary.170 For such vaccines, the intradermal delivery route offers a way to generate superior T-cell induction.171 Some preliminary work has been done on the use of LVAs with DC vaccines. Onikienko et al. explored the use of DC vaccines loaded with laser-treated (photomodulated) antigens of hepatitis B virus (HBV) or tumor autoantigens for therapeutic HBV and cancer vaccinations.75 A recent study in the US showed that treatment of skin with 532 nm Q-Nd:YAG laser followed by a intradermal DC vaccine injection increased the efficacy of the cancer vaccine.74 In this study, the laser induced more efficient migration of intradermally injected DC vaccine into the skin-dLNs resulting in an increase of anti-tumor IFN-γ+CD8+ cytotoxic T cell responses and conferred a survival benefit in 4T1 breast tumor and B16F10 melanoma models in mice.74 Together, these data support the view that laser adjuvant may be able to augment and induce sustained anti-tumor immune response in the context of intradermal injection of autoantigen- or DC-based therapeutic cancer vaccines.
The use of a physical adjuvant for cancer vaccines has some distinct advantages over chemical or biological adjuvants. Unlike many cancer vaccine adjuvants, LVA provides a transient immune stimulation and does not produce a depot effect.51 Depot formation and the associated slow release of antigen is related to persistence of adjuvant in exposed tissues and induce a prolonged inflammatory cytokine response.172 This is a tried and true approach in prophylactic vaccines, but may not be optimal for cancer vaccines. A recent study shows that persisting vaccine depots could induce a prolonged inflammation at vaccination sites and reduce vaccine efficacy.173 In this study, persistent vaccine depots induced specific T cell sequestration, dysfunction and deletion at vaccination sites, while a non-persisting vaccine formulation shifted T cell localization toward tumors, inducing superior antitumor effects. The ability of LVAs to induce effective cancer vaccine responses without depot effect merits further exploration.
Conclusions
The clinical utility of vaccine adjuvants requires both safety and efficacy, with an overriding emphasis on safety for prophylactic vaccines.174 The scientific literature contains reports of several hundred adjuvants with efficacy profiles that could be better than most adjuvants that currently approved for use in licensed vaccines, but none of these are clinically relevant because of their unsatisfactory safety profiles.12,100 From this perspective, the potential of LVA for use in human vaccines is supported by evidence from the preclinical and clinical studies and a compelling safety profile. In the most recent published study, LVA doses equivalent to those shown to be immunogenic in mice were tolerable in humans and did not appear to cause any skin damage.51 As with any other adjuvant, the safety and efficacy of LVA will have to be demonstrated through further rigorous preclinical safety and toxicology studies, and ultimately through a series of clinical trials of the combination of a clinically approved vaccine with the laser adjuvant, but current evidence in mice and humans suggests efficacy without tissue damage or significant inflammatory changes. The use of LVAs would further enable the use of intradermal vaccines, which could also be delivered needle-free, as with patches or micro-needle, a tremendous advantage in resource-limited and epidemic situations. Additionally, the discovery of NIR laser adjuvant effects enables the development of low-cost, low-power, hand-held LVAs that could be used in such resource-limited situations.
Finally, the adjuvant effects of LVAs would permit additional antigen sparing, avoid chemical interactions with adjuvant, and could even potentially be applied to mucous membranes, where inflammatory adjuvants are unworkable. Finally, the ability of LVAs to activate dendritic cells and augment cell-mediated immunity, without reactogenicity or long pharmaceutical developmental time-lines, makes them ideal for inclusion in both prophylactic and therapeutic vaccines being developed for infectious agents ranging from influenza to dengue to HPV, as well as therapeutic vaccines targeting cancer, cell- or epitope-based.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr R Rox Anderson and Dr Apostolos Doukas (Masachusetts General Hospital), Dr Holger Schlüter (HIGHYAG Lasertechnologie GmbH), and Dr Gregory Altschuler (Dental Photonics) for their critical input into our understanding of lasers and their impact in medicine; we thank Dr Michael Hamblin (Masachusetts General Hospital) and Mr Eugene L.Q. Lee (Imperial College London) for their contributions to figures. This material is based upon work supported by Center for Integration of Medicine and Innovative Technology (CIMIT), Defense Advanced Research Projects Agency under Contract No. Space and Naval Warfare Systems Center Pacific Award N66001-10-1-2132, Bill and Melinda Gates Foundation (Grand Challenges Explorations OPP1046276), The Friends of VIC and National Institute of Allergy and Infectious Diseases grant (R01AI105131). Any opinions, findings and conclusions, or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the Defense Advanced Research Projects Agency. We also would like to acknowledge the additional support of the Edmund C. Lynch Jr. Cancer Fund.
Glossary
Abbreviations:
- AS03 (04)
adjuvant system 03 (04)
- APC
antigen presenting cell
- BCG
Bacille de Calmette et Guérin
- CMI
cell-mediated immunity
- CTL
cytotoxic T lymphocyte
- CV
copper vapor
- CW
continuous wave
- DAMP
damage-associated molecular pattern
- DC
dendritic cell
- dLN
draining lymph node
- EMEA
European Medicines Agency
- HA(B)V
hepatitis A (B) virus
- HBsAg
hepatitis B surface antigen
- HSP70
heat shock protein 70
- LLLT
low-level laser therapy
- LMIT
leukocyte migration inhibition test
- LVA
laser vaccine adjuvants
- NSCLC
non-small cell lung carcinoma
- Nd:YVO4
neodymium-doped yttrium orthovanadate
- Nd:YAG
neodymium-doped yttrium aluminum garnet
- NIR
near-infrared
- OVA
ovalbumin
- PAMP
pathogen-associated molecular pattern
- PW
pulsed wave
- RCC
renal cell carcinoma
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TLR
toll-like receptor
- UV
ultraviolet
References
- 1.Cox JC, Coulter AR. Adjuvants--a classification and review of their modes of action. Vaccine. 1997;15:248–56. doi: 10.1016/S0264-410X(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 2.Harandi AM, Davies G, Olesen OF. Vaccine adjuvants: scientific challenges and strategic initiatives. Expert Rev Vaccines. 2009;8:293–8. doi: 10.1586/14760584.8.3.293. [DOI] [PubMed] [Google Scholar]
- 3.Leroux-Roels G. Unmet needs in modern vaccinology: adjuvants to improve the immune response. Vaccine. 2010;28(Suppl 3):C25–36. doi: 10.1016/j.vaccine.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Perrie Y, Mohammed AR, Kirby DJ, McNeil SE, Bramwell VW. Vaccine adjuvant systems: enhancing the efficacy of sub-unit protein antigens. Int J Pharm. 2008;364:272–80. doi: 10.1016/j.ijpharm.2008.04.036. [DOI] [PubMed] [Google Scholar]
- 5.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357:1373–81. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 6.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11:865–72. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunner R, Jensen-Jarolim E, Pali-Schöll I. The ABC of clinical and experimental adjuvants--a brief overview. Immunol Lett. 2010;128:29–35. doi: 10.1016/j.imlet.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilar JC, Rodríguez EG. Vaccine adjuvants revisited. Vaccine. 2007;25:3752–62. doi: 10.1016/j.vaccine.2007.01.111. [DOI] [PubMed] [Google Scholar]
- 11.Tritto E, Mosca F, De Gregorio E. Mechanism of action of licensed vaccine adjuvants. Vaccine. 2009;27:3331–4. doi: 10.1016/j.vaccine.2009.01.084. [DOI] [PubMed] [Google Scholar]
- 12.Batista-Duharte A, Lindblad EB, Oviedo-Orta E. Progress in understanding adjuvant immunotoxicity mechanisms. Toxicol Lett. 2011;203:97–105. doi: 10.1016/j.toxlet.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 13.Waddington CS, Walker WT, Oeser C, Reiner A, John T, Wilkins S, Casey M, Eccleston PE, Allen RJ, Okike I, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ. 2010;340:c2649. doi: 10.1136/bmj.c2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villa M, Black S, Groth N, Rothman KJ, Apolone G, Weiss NS, Aquino I, Boldori L, Caramaschi F, Gattinoni A, et al. Safety of MF59-adjuvanted influenza vaccination in the elderly: results of a comparative study of MF59-adjuvanted vaccine versus nonadjuvanted influenza vaccine in northern Italy. Am J Epidemiol. 2013;178:1139–45. doi: 10.1093/aje/kwt078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poder A, Simurka P, Li P, Roy-Ghanta S, Vaughn D. An observer-blind, randomized, multi-center trial assessing long-term safety and immunogenicity of AS03-adjuvanted or unadjuvanted H1N1/2009 influenza vaccines in children 10-17 years of age. Vaccine. 2014;32:1121–9. doi: 10.1016/j.vaccine.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 16.Miller E, Andrews N, Stellitano L, Stowe J, Winstone AM, Shneerson J, Verity C. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ. 2013;346:f794. doi: 10.1136/bmj.f794. [DOI] [PubMed] [Google Scholar]
- 17.Partinen M, Saarenpää-Heikkilä O, Ilveskoski I, Hublin C, Linna M, Olsén P, Nokelainen P, Alén R, Wallden T, Espo M, et al. Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One. 2012;7:e33723. doi: 10.1371/journal.pone.0033723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, Himanen S-L, Hublin C, Julkunen I, Olsén P, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7:e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dauvilliers Y, Arnulf I, Lecendreux M, Monaca Charley C, Franco P, Drouot X, d’Ortho MP, Launois S, Lignot S, Bourgin P, et al. Narcoflu-VF study group Increased risk of narcolepsy in children and adults after pandemic H1N1 vaccination in France. Brain. 2013;136:2486–96. doi: 10.1093/brain/awt187. [DOI] [PubMed] [Google Scholar]
- 20.van der Most R, Van Mechelen M, Destexhe E, Wettendorff M, Hanon E. Narcolepsy and A(H1N1)pdm09 vaccination: Shaping the research on the observed signal. Hum Vaccin Immunother. 2013;10 doi: 10.4161/hv.27412. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.la Herran-Arita De AK, Kornum BR, Mahlios J, Jiang W, Lin L, Hou T, Macaubas C, Einen M, Plazzi G, Crowe C, et al. CD4+ T cell autoimmunity to hypocretin/orexin and cross-reactivity to a 2009 H1N1 influenza A epitope in narcolepsy. Sci Transl Med. 2013;5:216ra176. doi: 10.1126/scitranslmed.3007762. [DOI] [PubMed] [Google Scholar]
- 22.Sticchi L, Alberti M, Alicino C, Crovari P. The intradermal vaccination: past experiences and current perspectives. J Prev Med Hyg. 2010;51:7–14. [PubMed] [Google Scholar]
- 23.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-Based Vaccines. In: Current Topics in Microbiology and Immunology. Berlin, Heidelberg: Springer Berlin Heidelberg; 2009. pages 369–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert PH, Laurent PE. Intradermal vaccine delivery: will new delivery systems transform vaccine administration? Vaccine. 2008;26:3197–208. doi: 10.1016/j.vaccine.2008.03.095. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31:446–51. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahl M, Hermodsson S. Intradermal, subcutaneous or intramuscular administration of hepatitis B vaccine: side effects and antibody response. Scand J Infect Dis. 1987;19:617–21. doi: 10.3109/00365548709117195. [DOI] [PubMed] [Google Scholar]
- 27.Vankerckhoven V, Van Damme P. Clinical studies assessing immunogenicity and safety of intradermally administered influenza vaccines. Expert Opin Drug Deliv. 2010;7:1109–25. doi: 10.1517/17425247.2010.507668. [DOI] [PubMed] [Google Scholar]
- 28.Nicolas J-F, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008;7:1201–14. doi: 10.1586/14760584.7.8.1201. [DOI] [PubMed] [Google Scholar]
- 29.Sangaré L, Manhart L, Zehrung D, Wang CC. Intradermal hepatitis B vaccination: a systematic review and meta-analysis. Vaccine. 2009;27:1777–86. doi: 10.1016/j.vaccine.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 30.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, Jacob J, Prausnitz MR, Compans RW, Skountzou I. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J Infect Dis. 2011;204:582–91. doi: 10.1093/infdis/jir094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.del Pilar Martin M, Weldon WC, Zarnitsyn VG, Koutsonanos DG, Akbari H, Skountzou I, Jacob J, Prausnitz MR, Compans RW. Local response to microneedle-based influenza immunization in the skin. MBio. 2012;3:e00012–12. doi: 10.1128/mBio.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansaldi F, de Florentiis D, Durando P, Icardi G. Fluzone(®) Intradermal vaccine: a promising new chance to increase the acceptability of influenza vaccination in adults. Expert Rev Vaccines. 2012;11:17–25. doi: 10.1586/erv.11.154. [DOI] [PubMed] [Google Scholar]
- 33.Hickling JK, Jones KR, Friede M, Zehrung D, Chen D, Kristensen D. Intradermal delivery of vaccines: potential benefits and current challenges. Bull World Health Organ. 2011;89:221–6. doi: 10.2471/BLT.10.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hickling J, Jones R. Intradermal delivery of vaccines: A Review of the Literature and the Potential for Development for Use in Low- and Middle-Income Countries. Program for Appropriate Technology in Health (PATH) resource 2009. Available from: http://www.path.org/publications/files/TS_opt_idd_review.pdf
- 35.Chen X, Pravetoni M, Bhayana B, Pentel PR, Wu MX. High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine. 2012;31:159–64. doi: 10.1016/j.vaccine.2012.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Wu MX. Laser vaccine adjuvant for cutaneous immunization. Expert Rev Vaccines. 2011;10:1397–403. doi: 10.1586/erv.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson EAS, Lam HS, Choi KC, Ho WCS, Fung LWE, Cheng FWT, Sung RYT, Royals M, Chan PKS. A pilot randomized study to assess immunogenicity, reactogenicity, safety and tolerability of two human papillomavirus vaccines administered intramuscularly and intradermally to females aged 18-26 years. Vaccine. 2013;31:3452–60. doi: 10.1016/j.vaccine.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 38.Rahman F, Dahmen A, Herzog-Hauff S, Böcher WO, Galle PR, Löhr HF. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology. 2000;31:521–7. doi: 10.1002/hep.510310237. [DOI] [PubMed] [Google Scholar]
- 39.Sherwood KA, Murray S, Kurban AK, Tan OT. Effect of wavelength on cutaneous pigment using pulsed irradiation. J Invest Dermatol. 1989;92:717–20. doi: 10.1111/1523-1747.ep12721505. [DOI] [PubMed] [Google Scholar]
- 40.Kupper TS. Old and new: recent innovations in vaccine biology and skin T cells. J Invest Dermatol. 2012;132:829–34. doi: 10.1038/jid.2011.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldberg DJ, Silapunt S. Histologic evaluation of a Q-switched Nd:YAG laser in the nonablative treatment of wrinkles. Dermatol Surg. 2001;27:744–6. doi: 10.1046/j.1524-4725.2001.00353.x. [DOI] [PubMed] [Google Scholar]
- 42.Tanzi EL, Alster TS. Comparison of a 1450-nm diode laser and a 1320-nm Nd:YAG laser in the treatment of atrophic facial scars: a prospective clinical and histologic study. Dermatol Surg. 2004;30:152–7. doi: 10.1111/j.1524-4725.2004.30078.x. [DOI] [PubMed] [Google Scholar]
- 43.Prieto VG, Diwan AH, Shea CR, Zhang P, Sadick NS. Effects of intense pulsed light and the 1,064 nm Nd:YAG laser on sun-damaged human skin: histologic and immunohistochemical analysis. Dermatol Surg. 2005;31:522–5. doi: 10.1111/j.1524-4725.2005.31154. [DOI] [PubMed] [Google Scholar]
- 44.Isbert C, Ritz J-P, Roggan A, Schuppan D, Rühl M, Buhr HJ, Germer C-T. Enhancement of the immune response to residual intrahepatic tumor tissue by laser-induced thermotherapy (LITT) compared to hepatic resection. Lasers Surg Med. 2004;35:284–92. doi: 10.1002/lsm.20097. [DOI] [PubMed] [Google Scholar]
- 45.Ivarsson K, Myllymäki L, Jansner K, Stenram U, Tranberg K-G. Resistance to tumour challenge after tumour laser thermotherapy is associated with a cellular immune response. Br J Cancer. 2005;93:435–40. doi: 10.1038/sj.bjc.6602718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haen SP, Pereira PL, Salih HR, Rammensee H-G, Gouttefangeas C. More than just tumor destruction: immunomodulation by thermal ablation of cancer. Clin Dev Immunol. 2011;2011:160250. doi: 10.1155/2011/160250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Wang J, Shah D, Wu MX. An update on the use of laser technology in skin vaccination. Expert Rev Vaccines. 2013;12:1313–23. doi: 10.1586/14760584.2013.844070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Dermatol. 1994;30:535–46. doi: 10.1016/S0190-9622(94)70059-1. [DOI] [PubMed] [Google Scholar]
- 49.Tsai JC, Feingold KR, Crumrine D, Wood LC, Grunfeld C, Elias PM. Permeability barrier disruption alters the localization and expression of TNF alpha/protein in the epidermis. Arch Dermatol Res. 1994;286:242–8. doi: 10.1007/BF00387595. [DOI] [PubMed] [Google Scholar]
- 50.Scheiblhofer S, Thalhamer J, Weiss R. Laser microporation of the skin: prospects for painless application of protective and therapeutic vaccines. Expert Opin Drug Deliv. 2013;10:761–73. doi: 10.1517/17425247.2013.773970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kashiwagi S, Yuan J, Forbes B, Hibert ML, Lee EL, Whicher L, Goudie C, Yang Y, Chen T, Edelblute B, et al. Near-infrared laser adjuvant for influenza vaccine. PLoS One. 2013;8:e82899. doi: 10.1371/journal.pone.0082899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onikienko SB, Zemlyanoy AB, Margulis BA, Guzhova IV, Varlashova MB, Gornostaev VS, Tikhonova NV, Baranov GA, Lesnichiy VV. Diagnostics and correction of the metabolic and immune disorders. Interactions of bacterial endotoxins and lipophilic xenobiotics with receptors associated with innate immunity. Donosologiya (St Petersburg) 2007;1:32–54. [Google Scholar]
- 53.Chen X, Kim P, Farinelli B, Doukas A, Yun S-H, Gelfand JA, Anderson RR, Wu MX. A novel laser vaccine adjuvant increases the motility of antigen presenting cells. PLoS One. 2010;5:e13776. doi: 10.1371/journal.pone.0013776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang Y-Y, Chen ACH, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–83. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang Y-Y, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy - an update. Dose Response. 2011;9:602–18. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu S, Xing D, Gao X, Chen WR. High fluence low-power laser irradiation induces mitochondrial permeability transition mediated by reactive oxygen species. J Cell Physiol. 2009;218:603–11. doi: 10.1002/jcp.21636. [DOI] [PubMed] [Google Scholar]
- 57.Wu S, Xing D, Wang F, Chen T, Chen WR. Mechanistic study of apoptosis induced by high-fluence low-power laser irradiation using fluorescence imaging techniques. J Biomed Opt. 2007;12:064015. doi: 10.1117/1.2804923. [DOI] [PubMed] [Google Scholar]
- 58.Wu S, Zhou F, Wei Y, Chen WR, Chen Q, Xing D. Cancer phototherapy via selective photoinactivation of respiratory chain oxidase to trigger a fatal superoxide anion burst. Antioxid Redox Signal. 2014;20:733–46. doi: 10.1089/ars.2013.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang L, Wu S, Xing D. High fluence low-power laser irradiation induces apoptosis via inactivation of Akt/GSK3β signaling pathway. J Cell Physiol. 2011;226:588–601. doi: 10.1002/jcp.22367. [DOI] [PubMed] [Google Scholar]
- 60.Mester E, Szende B, Gärtner P. [The effect of laser beams on the growth of hair in mice] Radiobiol Radiother (Berl) 1968;9:621–6. [PubMed] [Google Scholar]
- 61.Conlan MJ, Rapley JW, Cobb CM. Biostimulation of wound healing by low-energy laser irradiation. A review. J Clin Periodontol. 1996;23:492–6. doi: 10.1111/j.1600-051X.1996.tb00580.x. [DOI] [PubMed] [Google Scholar]
- 62.Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M. Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg. 2005;31:334–40. doi: 10.1111/j.1524-4725.2005.31086. [DOI] [PubMed] [Google Scholar]
- 63.Averbakh MM, Sorkin MZ, Dobkin VG, Kosarev II, Ostapchenko EP. [Effect of helium-neon laser on the healing of aseptic experimental wounds] Eksp Khir Anesteziol. 1976;(3):56–9. [PubMed] [Google Scholar]
- 64.Gerasimenko N, Poddubnyĭ BK, Kuvshinov IuP, Ivanov AV, Efimov ON. [Use of nonhazardous laser radiation for treating the complications of patients undergoing radical surgery for esophageal and stomach cancer] Vopr Onkol. 1984;30:56–9. [PubMed] [Google Scholar]
- 65.Koliadenko VG, Shupen’ko NM. [Treatment of erosive-ulcerative processes in the skin with a low-intensity helium-neon laser] Vrach Delo. 1984;(10):98–100. [PubMed] [Google Scholar]
- 66.Aleksandrov MT, Baĭkova RA, Polnareva BD. [Use of helium-neon laser rays for treating diseases of the oral mucosa] Stomatologiia (Mosk) 1977;56:18–20. [PubMed] [Google Scholar]
- 67.Kazaryan MA. Copper vapor lasers in oncology. In: Pulsed Metal Vapor Lasers. Netherlands: Kluwer Academic Publishers; 1996. pages 409–14. [Google Scholar]
- 68.Matiushichev VB, Soldatov AI. [High-energy laser irradiation in the combined treatment of duodenal ulcer] Klin Med (Mosk) 1994;72:15–6. [PubMed] [Google Scholar]
- 69.Matiushichev VB, Soldatov AI, Linkevich EL. [High-energy laser therapy of stomach ulcers] Klin Med (Mosk) 1997;75:41–4. [PubMed] [Google Scholar]
- 70.Matiushichev VB, Soldatov AI, Titov VV. [Possibilities of intragastric laser therapy of septic ulcer] Klin Med (Mosk) 1987;65:6–9. [PubMed] [Google Scholar]
- 71.Soldatov AI, Matiushichev VB, Titov VV. Use of the copper-vapor laser in the combined treatment of peptic ulcer. Klin Khir. 1988;(8):64 [PubMed]
- 72.Cheremisina OV, Choinzonov EL, Yevtushenko VA, Soldatov AN, Pankova OV, Gerdt LV. Assessment of the efficacy of different treatments of the pre-cancerous changes of the bronchial epithelium. Siberian Oncology J. 2002;2:123–7. [Google Scholar]
- 73.U.S. State Department. U. S. - Russia: BioIndustry Initiative New Collaboration to Reduce the Threat of Bioterrorism. 2003. [Google Scholar]
- 74.Chen X, Zeng Q, Wu MX. Improved efficacy of dendritic cell-based immunotherapy by cutaneous laser illumination. Clin Cancer Res. 2012;18:2240–9. doi: 10.1158/1078-0432.CCR-11-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Onikienko BA, Zemlyanoy AV, Baranov GA, Smirnova MP, Tarasov VA, Filatov MV. Laser-based vaccine technology for cancer immunotherapy. St. Petersburg, Russia: 2005. pages 52–3. [Google Scholar]
- 76.Onikienko SB. New Approaches to the Immunotherapy of the persistent infections. 1. Hepatitis B. Sevastopol, Russia: 2005. [Google Scholar]
- 77.000 Npt Mbp Gormezis, Onikienko SB, Zemlyanoi, AV, Margulis BA, Guzhova, IV, Pimenova AA. Laser-based vaccine adjuvants. United States patent application US 12/754,081. 2008 Oct 6.
- 78.Bendixen G, Bendtzen K, Calusen JE, Kjaer M, Søborg M. Human leucocyte migration inhibition. Scand J Immunol. 1976;(Suppl 5):175–84. doi: 10.1111/j.1365-3083.1976.tb03867.x. [DOI] [PubMed] [Google Scholar]
- 79.Jungers P, Devillier P, Salomon H, Cerisier JE, Courouce AM. Randomised placebo-controlled trial of recombinant interleukin-2 in chronic uraemic patients who are non-responders to hepatitis B vaccine. Lancet. 1994;344:856–7. doi: 10.1016/S0140-6736(94)92829-0. [DOI] [PubMed] [Google Scholar]
- 80.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 81.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 82.Sharma SK, Christen P, Goloubinoff P. Disaggregating chaperones: an unfolding story. Curr Protein Pept Sci. 2009;10:432–46. doi: 10.2174/138920309789351930. [DOI] [PubMed] [Google Scholar]
- 83.Horváth I, Multhoff G, Sonnleitner A, Vígh L. Membrane-associated stress proteins: more than simply chaperones. Biochim Biophys Acta. 20081778:1653–64. doi: 10.1016/j.bbamem.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 84.Buzzard KA, Giaccia AJ, Killender M, Anderson RL. Heat shock protein 72 modulates pathways of stress-induced apoptosis. J Biol Chem. 1998;273:17147–53. doi: 10.1074/jbc.273.27.17147. [DOI] [PubMed] [Google Scholar]
- 85.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62:670–84. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Segal BH, Wang X-Y, Dennis CG, Youn R, Repasky EA, Manjili MH, Subjeck JR. Heat shock proteins as vaccine adjuvants in infections and cancer. Drug Discov Today. 2006;11:534–40. doi: 10.1016/j.drudis.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 87.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–89. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Srivastava PK, Amato RJ. Heat shock proteins: the ‘Swiss Army Knife’ vaccines against cancers and infectious agents. Vaccine. 2001;19:2590–7. doi: 10.1016/S0264-410X(00)00492-8. [DOI] [PubMed] [Google Scholar]
- 89.Manzerra P, Rush SJ, Brown IR. Tissue-specific differences in heat shock protein hsc70 and hsp70 in the control and hyperthermic rabbit. J Cell Physiol. 1997;170:130–7. doi: 10.1002/(SICI)1097-4652(199702)170:2<130::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 90.Trautinger F, Trautinger I, Kindas-Mügge I, Metze D, Luger TA. Human keratinocytes in vivo and in vitro constitutively express the 72-kD heat shock protein. J Invest Dermatol. 1993;101:334–8. doi: 10.1111/1523-1747.ep12365491. [DOI] [PubMed] [Google Scholar]
- 91.Broquet AH, Thomas G, Masliah J, Trugnan G, Bachelet M. Expression of the molecular chaperone Hsp70 in detergent-resistant microdomains correlates with its membrane delivery and release. J Biol Chem. 2003;278:21601–6. doi: 10.1074/jbc.M302326200. [DOI] [PubMed] [Google Scholar]
- 92.Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–57. doi: 10.4049/jimmunol.177.11.7849. [DOI] [PubMed] [Google Scholar]
- 93.Lancaster GI, Febbraio MA. Mechanisms of stress-induced cellular HSP72 release: implications for exercise-induced increases in extracellular HSP72. Exerc Immunol Rev. 2005;11:46–52. [PubMed] [Google Scholar]
- 94.Guebre-Xabier M, Hammond SA, Epperson DE, Yu J, Ellingsworth L, Glenn GM. Immunostimulant patch containing heat-labile enterotoxin from Escherichia coli enhances immune responses to injected influenza virus vaccine through activation of skin dendritic cells. J Virol. 2003;77:5218–25. doi: 10.1128/JVI.77.9.5218-5225.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Glenn GM, Rao M, Matyas GR, Alving CR. Skin immunization made possible by cholera toxin. Nature. 1998;391:851. doi: 10.1038/36014. [DOI] [PubMed] [Google Scholar]
- 96.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–42. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nestle FO, Di Meglio P, Qin J-Z, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–91. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Villadangos JA, Schnorrer P. Intrinsic and cooperative antigen-presenting functions of dendritic-cell subsets in vivo. Nat Rev Immunol. 2007;7:543–55. doi: 10.1038/nri2103. [DOI] [PubMed] [Google Scholar]
- 99.Vitoriano-Souza J, Moreira Nd, Teixeira-Carvalho A, Carneiro CM, Siqueira FAM, Vieira PM de A, Giunchetti RC, Moura SA de L, Fujiwara RT, Melo MN, et al. Cell recruitment and cytokines in skin mice sensitized with the vaccine adjuvants: saponin, incomplete Freund’s adjuvant, and monophosphoryl lipid A. PLoS One. 2012;7:e40745. doi: 10.1371/journal.pone.0040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Batista-Duharte A, Portuondo D, Carlos IZ, Pérez O. An approach to local immunotoxicity induced by adjuvanted vaccines. Int Immunopharmacol. 2013;17:526–36. doi: 10.1016/j.intimp.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 101.Martín-Fontecha A, Lanzavecchia A, Sallusto F. Dendritic cell migration to peripheral lymph nodes. Handb Exp Pharmacol. 2009;(188):31–49. doi: 10.1007/978-3-540-71029-5_2. [DOI] [PubMed] [Google Scholar]
- 102.Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Aït-Yahia S, Vicari A, Kaiserlian D, et al. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity. 2006;24:191–201. doi: 10.1016/j.immuni.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 103.Dieu-Nosjean MC, Massacrier C, Homey B, Vanbervliet B, Pin JJ, Vicari A, Lebecque S, Dezutter-Dambuyant C, Schmitt D, Zlotnik A, et al. Macrophage inflammatory protein 3alpha is expressed at inflamed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med. 2000;192:705–18. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakamura K, Williams IR, Kupper TS. Keratinocyte-derived monocyte chemoattractant protein 1 (MCP-1): analysis in a transgenic model demonstrates MCP-1 can recruit dendritic and Langerhans cells to skin. J Invest Dermatol. 1995;105:635–43. doi: 10.1111/1523-1747.ep12324061. [DOI] [PubMed] [Google Scholar]
- 105.Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/S1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 106.Platt AM, Randolph GJ. Dendritic cell migration through the lymphatic vasculature to lymph nodes. Adv Immunol. 2013;120:51–68. doi: 10.1016/B978-0-12-417028-5.00002-8. [DOI] [PubMed] [Google Scholar]
- 107.Peng Q, Juzeniene A, Chen J, Svaasand LO, Warloe T, Giercksky K-E, Moan J. Lasers in medicine. Rep Prog Phys. 2008;71:056701. doi: 10.1088/0034-4885/71/5/056701. [DOI] [Google Scholar]
- 108.Niemz MH. Laser-Tissue Interactions: Fundamentals and Applications (Biological and Medical Physics, Biomedical Engineering). 3rd ed. Springer; 2007. [Google Scholar]
- 109.Karu T. Mitochondrial mechanisms of photobiomodulation in context of new data about multiple roles of ATP. Photomed Laser Surg. 2010;28:159–60. doi: 10.1089/pho.2010.2789. [DOI] [PubMed] [Google Scholar]
- 110.Karu T. Is it time to consider photobiomodulation as a drug equivalent? Photomed Laser Surg. 2013;31:189–91. doi: 10.1089/pho.2013.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355–61. doi: 10.1089/pho.2005.23.355. [DOI] [PubMed] [Google Scholar]
- 112.Dewhirst MW, Viglianti BL, Lora-Michiels M, Hanson M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19:267–94. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]
- 113.Moritz AR, Henriques FC, Studies of Thermal Injury Studies of Thermal Injury: II. The Relative Importance of Time and Surface Temperature in the Causation of Cutaneous Burns. Am J Pathol. 1947;23:695–720. [PMC free article] [PubMed] [Google Scholar]
- 114.Stoll AM, Greene LC. Relationship between pain and tissue damage due to thermal radiation. J Appl Physiol. 1959;14:373–82. doi: 10.1152/jappl.1959.14.3.373. [DOI] [PubMed] [Google Scholar]
- 115.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524–7. doi: 10.1126/science.6836297. [DOI] [PubMed] [Google Scholar]
- 116.Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981;1:263–76. doi: 10.1002/lsm.1900010310. [DOI] [PubMed] [Google Scholar]
- 117.Anderson RR, Margolis RJ, Watenabe S, Flotte T, Hruza GJ, Dover JS. Selective photothermolysis of cutaneous pigmentation by Q-switched Nd: YAG laser pulses at 1064, 532, and 355 nm. J Invest Dermatol. 1989;93:28–32. doi: 10.1111/1523-1747.ep12277339. [DOI] [PubMed] [Google Scholar]
- 118.Murphy GF, Shepard RS, Paul BS, Menkes A, Anderson RR, Parrish JA. Organelle-specific injury to melanin-containing cells in human skin by pulsed laser irradiation. Lab Invest. 1983;49:680–5. [PubMed] [Google Scholar]
- 119.Polla LL, Margolis RJ, Dover JS, Whitaker D, Murphy GF, Jacques SL, Anderson RR. Melanosomes are a primary target of Q-switched ruby laser irradiation in guinea pig skin. J Invest Dermatol. 1987;89:281–6. doi: 10.1111/1523-1747.ep12471397. [DOI] [PubMed] [Google Scholar]
- 120.Phillips CM, Mizutani Y, Hochstrasser RM. Ultrafast thermally induced unfolding of RNase A. Proc Natl Acad Sci U S A. 1995;92:7292–6. doi: 10.1073/pnas.92.16.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Williams S, Causgrove TP, Gilmanshin R, Fang KS, Callender RH, Woodruff WH, Dyer RB. Fast events in protein folding: helix melting and formation in a small peptide. Biochemistry. 1996;35:691–7. doi: 10.1021/bi952217p. [DOI] [PubMed] [Google Scholar]
- 122.Thompson CR, Gerstman BS, Jacques SL, Rogers ME. Melanin granule model for laser-induced thermal damage in the retina. Bull Math Biol. 1996;58:513–53. doi: 10.1007/BF02460595. [DOI] [PubMed] [Google Scholar]
- 123.Brinkmann R, Hüttmann G, Rögener J, Roider J, Birngruber R, Lin CP. Origin of retinal pigment epithelium cell damage by pulsed laser irradiance in the nanosecond to microsecond time regimen. Lasers Surg Med. 2000;27:451–64. doi: 10.1002/1096-9101(2000)27:5<451::AID-LSM1006>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 124.Mills BM, Connor TM, Foltz MS, Stolarski J, Hayes KL, Denton ML, Eikum DM, Noojin GD, Rockwell BA. Microcavitation and spot size dependence for damage of artificially pigmented hTERT-RPE1 cells. Proc SPIE 2004; 5319: Laser Interaction with Tissue and Cells XV. 2004
- 125.Lukianova-Hleb EY, Oginsky AO, Olson JS, Lapotko DO. Short laser pulse-induced irreversible photothermal effects in red blood cells. Lasers Surg Med. 2011;43:249–60. doi: 10.1002/lsm.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chidlow G, Shibeeb O, Plunkett M, Casson RJ, Wood JP. Glial cell and inflammatory responses to retinal laser treatment: comparison of a conventional photocoagulator and a novel, 3-nanosecond pulse laser. Invest Ophthalmol Vis Sci. 2013;54:2319–32. doi: 10.1167/iovs.12-11204. [DOI] [PubMed] [Google Scholar]
- 127.Douki T, Lee S, Dorey K, Flotte TJ, Deutsch TF, Doukas AG. Stress-wave-induced injury to retinal pigment epithelium cells in vitro. Lasers Surg Med. 1996;19:249–59. doi: 10.1002/(SICI)1096-9101(1996)19:3<249::AID-LSM1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 128.Chen X, Shah D, Kositratna G, Manstein D, Anderson RR, Wu MX. Facilitation of transcutaneous drug delivery and vaccine immunization by a safe laser technology. J Control Release. 2012;159:43–51. doi: 10.1016/j.jconrel.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Venugopalan V, Nishioka NS, Mikić BB. Thermodynamic response of soft biological tissues to pulsed infrared-laser irradiation. Biophys J. 1996;70:2981–93. doi: 10.1016/S0006-3495(96)79868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kuwahara K, Gladstone HB, Gupta V, Kireev V, Neel V, Moy RL. Rupture of fat cells using laser-generated ultra short stress waves. Lasers Surg Med. 2003;32:279–85. doi: 10.1002/lsm.10154. [DOI] [PubMed] [Google Scholar]
- 131.Umebayashi Y, Miyamoto Y, Wakita M, Kobayashi A, Nishisaka T. Elevation of plasma membrane permeability on laser irradiation of extracellular latex particles. J Biochem. 2003;134:219–24. doi: 10.1093/jb/mvg132. [DOI] [PubMed] [Google Scholar]
- 132.Ara G, Anderson RR, Mandel KG, Ottesen M, Oseroff AR. Irradiation of pigmented melanoma cells with high intensity pulsed radiation generates acoustic waves and kills cells. Lasers Surg Med. 1990;10:52–9. doi: 10.1002/lsm.1900100112. [DOI] [PubMed] [Google Scholar]
- 133.Kim YG. Laser mediated production of reactive oxygen and nitrogen species; implications for therapy. Free Radic Res. 2002;36:1243–50. doi: 10.1080/1071576021000028389. [DOI] [PubMed] [Google Scholar]
- 134.Lawley W, Doherty A, Denniss S, Chauhan D, Pruijn G, van Venrooij WJ, Lunec J, Herbert K. Rapid lupus autoantigen relocalization and reactive oxygen species accumulation following ultraviolet irradiation of human keratinocytes. Rheumatology (Oxford) 2000;39:253–61. doi: 10.1093/rheumatology/39.3.253. [DOI] [PubMed] [Google Scholar]
- 135.Kushibiki T, Hirasawa T, Okawa S, Ishihara M. Blue laser irradiation generates intracellular reactive oxygen species in various types of cells. Photomed Laser Surg. 2013;31:95–104. doi: 10.1089/pho.2012.3361. [DOI] [PubMed] [Google Scholar]
- 136.Jou MJ, Jou SB, Chen HM, Lin CH, Peng TI. Critical role of mitochondrial reactive oxygen species formation in visible laser irradiation-induced apoptosis in rat brain astrocytes (RBA-1) J Biomed Sci. 2002;9:507–16. doi: 10.1007/BF02254977. [DOI] [PubMed] [Google Scholar]
- 137.Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS, et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One. 2011;6:e22453. doi: 10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang F, Chen T-S, Xing D, Wang J-J, Wu Y-X. Measuring dynamics of caspase-3 activity in living cells using FRET technique during apoptosis induced by high fluence low-power laser irradiation. Lasers Surg Med. 2005;36:2–7. doi: 10.1002/lsm.20130. [DOI] [PubMed] [Google Scholar]
- 139.König K. Multiphoton microscopy in life sciences. J Microsc. 2000;200:83–104. doi: 10.1046/j.1365-2818.2000.00738.x. [DOI] [PubMed] [Google Scholar]
- 140.Tirlapur UK, König K, Peuckert C, Krieg R, Halbhuber KJ. Femtosecond near-infrared laser pulses elicit generation of reactive oxygen species in mammalian cells leading to apoptosis-like death. Exp Cell Res. 2001;263:88–97. doi: 10.1006/excr.2000.5082. [DOI] [PubMed] [Google Scholar]
- 141.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005–28. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 142.Stamler JS, Lamas S, Fang FC. Nitrosylation. the prototypic redox-based signaling mechanism. Cell. 2001;106:675–83. doi: 10.1016/S0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 143.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–99. doi: 10.1016/S0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 144.Connelly L, Palacios-Callender M, Ameixa C, Moncada S, Hobbs AJ. Biphasic regulation of NF-kappa B activity underlies the pro- and anti-inflammatory actions of nitric oxide. J Immunol. 2001;166:3873–81. doi: 10.4049/jimmunol.166.6.3873. [DOI] [PubMed] [Google Scholar]
- 145.Pilz RB, Casteel DE. Regulation of gene expression by cyclic GMP. Circ Res. 2003;93:1034–46. doi: 10.1161/01.RES.0000103311.52853.48. [DOI] [PubMed] [Google Scholar]
- 146.Rubartelli A. Redox control of NLRP3 inflammasome activation in health and disease. J Leukoc Biol. 2012;92:951–8. doi: 10.1189/jlb.0512265. [DOI] [PubMed] [Google Scholar]
- 147.Grossman N, Schneid N, Reuveni H, Halevy S, Lubart R. 780 nm low power diode laser irradiation stimulates proliferation of keratinocyte cultures: involvement of reactive oxygen species. Lasers Surg Med. 1998;22:212–8. doi: 10.1002/(SICI)1096-9101(1998)22:4<212::AID-LSM5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 148.Grange PA, Chéreau C, Raingeaud J, Nicco C, Weill B, Dupin N, Batteux F. Production of superoxide anions by keratinocytes initiates P. acnes-induced inflammation of the skin. PLoS Pathog. 2009;5:e1000527. doi: 10.1371/journal.ppat.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cho KA, Suh JW, Lee KH, Kang JL, Woo SY. IL-17 and IL-22 enhance skin inflammation by stimulating the secretion of IL-1β by keratinocytes via the ROS-NLRP3-caspase-1 pathway. Int Immunol. 2012;24:147–58. doi: 10.1093/intimm/dxr110. [DOI] [PubMed] [Google Scholar]
- 150.Kim DH, Byamba D, Wu WH, Kim TG, Lee MG. Different characteristics of reactive oxygen species production by human keratinocyte cell line cells in response to allergens and irritants. Exp Dermatol. 2012;21:99–103. doi: 10.1111/j.1600-0625.2011.01399.x. [DOI] [PubMed] [Google Scholar]
- 151.Swindle EJ, Metcalfe DD. The role of reactive oxygen species and nitric oxide in mast cell-dependent inflammatory processes. Immunol Rev. 2007;217:186–205. doi: 10.1111/j.1600-065X.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 152.Sekar Y, Moon TC, Muñoz S, Befus AD. Role of nitric oxide in mast cells: controversies, current knowledge, and future applications. Immunol Res. 2005;33:223–39. doi: 10.1385/IR:33:3:223. [DOI] [PubMed] [Google Scholar]
- 153.Gilchrist M, Savoie M, Nohara O, Wills FL, Wallace JL, Befus AD. Nitric oxide synthase and nitric oxide production in in vivo-derived mast cells. J Leukoc Biol. 2002;71:618–24. [PubMed] [Google Scholar]
- 154.Ashinoff R, Levine VJ, Soter NA. Allergic reactions to tattoo pigment after laser treatment. Dermatol Surg. 1995;21:291–4. doi: 10.1111/j.1524-4725.1995.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 155.England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215–7. doi: 10.1016/S1081-1206(10)61942-4. [DOI] [PubMed] [Google Scholar]
- 156.Boulnois JL. Photophysical processes in recent medical laser developments: A review. Lasers Med Sci. 1986;1:47–66. doi: 10.1007/BF02030737. [DOI] [Google Scholar]
- 157.Sliney DH, Palmisano WA. The evaluation of laser hazards. Am Ind Hyg Assoc J. 1968;29:425–31. doi: 10.1080/00028896809343029. [DOI] [PubMed] [Google Scholar]
- 158.Tanzi EL, Alster TS. Long-pulsed 1064-nm Nd:YAG laser-assisted hair removal in all skin types. Dermatol Surg. 2004;30:13–7. doi: 10.1111/j.1524-4725.2004.30007.x. [DOI] [PubMed] [Google Scholar]
- 159.Leclère FM, Magalon G, Philandrianos C, Unglaub F, Servell P, Mordon S. Prospective ex-vivo study on thermal effects in human skin phototypes II, IV and VI: a comparison between the 808, 1064, 1210 and 1320-nm diode laser. J Cosmet Laser Ther. 2012;14:7–13. doi: 10.3109/14764172.2011.634419. [DOI] [PubMed] [Google Scholar]
- 160.Kim YC, Jarrahian C, Zehrung D, Mitragotri S, Prausnitz MR. Delivery systems for intradermal vaccination. Curr Top Microbiol Immunol. 2012;351:77–112. doi: 10.1007/82_2011_123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kendall M. Getting under the skin. Mater Today. 2006;9:72. doi: 10.1016/S1369-7021(06)71771-0. [DOI] [Google Scholar]
- 162.Beresford B, Sadoff JC. Update on research and development pipeline: tuberculosis vaccines. Clin Infect Dis. 2010;50(Suppl 3):S178–83. doi: 10.1086/651489. [DOI] [PubMed] [Google Scholar]
- 163.Furuya Y. Return of inactivated whole-virus vaccine for superior efficacy. Immunol Cell Biol. 2012;90:571–8. doi: 10.1038/icb.2011.70. [DOI] [PubMed] [Google Scholar]
- 164.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–80. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 165.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. doi: 10.1001/jama.1994.03510330076038. [DOI] [PubMed] [Google Scholar]
- 166.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–45. doi: 10.1016/S0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 167.Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13:1155–9. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- 168.Van Brussel I, Berneman ZN, Cools N. Optimizing Dendritic Cell-Based Immunotherapy: Tackling the Complexity of Different Arms of the Immune System. Mediators Inflamm . 2012;2012:690643. doi: 10.1155/2012/690643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lesterhuis WJ, de Vries IJM, Schreibelt G, Lambeck AJA, Aarntzen EHJG, Jacobs JFM, Scharenborg NM, van de Rakt MWMM, de Boer AJ, Croockewit S, et al. Route of administration modulates the induction of dendritic cell vaccine-induced antigen-specific T cells in advanced melanoma patients. Clin Cancer Res. 2011;17:5725–35. doi: 10.1158/1078-0432.CCR-11-1261. [DOI] [PubMed] [Google Scholar]
- 172.Gupta RK, Rost BE, Relyveld E, Siber GR. Adjuvant properties of aluminum and calcium compounds. Pharm Biotechnol. 1995;6:229–48. doi: 10.1007/978-1-4615-1823-5_8. [DOI] [PubMed] [Google Scholar]
- 173.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang X-F, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, et al. Persistent antigen at vaccination sites induces tumor-specific CD8⁺ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19:465–72. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Edelman R. An Overview of Adjuvant Use. Vaccine Adjuvants. 2000;42:1–27. doi: 10.1385/1-59259-083-7:1. [DOI] [Google Scholar]