Abstract
This study aimed to calculate the cost-effectiveness of infant pneumococcal vaccination in the Netherlands, using the 13-valent PCV13 vs. the currently used 10-valent PCV10. We adapted a previously published model, using recent estimates of epidemiological and efficacy data. In 12 scenarios, we explored the impact of different assumptions on the incremental cost-effectiveness ratio (ICER) of PCV13 over PCV10.Taking only direct effects on invasive pneumococcal disease into account, PCV13 was not found to be cost-effective at a price difference of €11 per dose. If herd protection, replacement and non-invasive disease were also taken into account, the ICER of PCV13 compared with PCV10 was below €30 000/QALY gained in 11 of 12 scenarios. PCV13 was considered dominant in the primary scenario with a price difference below €2.63 per dose.
Keywords: Pneumococcal, cost-effectiveness, vaccines, infant vaccination, Netherlands, PCV
Introduction
In many ways, the world of vaccines and infectious diseases is a dynamic one. Two pneumococcal conjugate vaccines (PCV) have been licensed for use in the Dutch infant population since the 2006 introduction of a 7-valent vaccine (PCV7): PCV10 and PCV13, containing protection against respectively three and six extra serotypes. In 2010, members of our group calculated the cost-effectiveness (CE) of infant pneumococcal vaccination in the Netherlands.1,2 This study was influential in the following decision to offer PCV10 to Dutch infants within the Dutch National Immunization Program.3
Since then, epidemiological circumstances have changed, partly due to the vaccination effort as evidenced in literature from around the world.4-8 In addition, new efficacy and effectiveness data have been published, which were not available at that time.9-13 This study aimed to calculate the cost-effectiveness of PCV13 compared with PCV10 using the newly available data. Our study was performed to inform the Dutch Health Council, which has advised the Ministry of Health for a new tender, worth approximately 530 000 doses per year, that took place in the beginning of 2014.14 The cost-effectiveness of PCV13 over PCV10 will be driven completely by the effects of both vaccines on the 3 additional serotypes that are included in PCV13: 3, 6A and 19A. This study therefore focused completely on these three serotypes.
Results
In our primary scenario, using current epidemiological data, vaccination with PCV13 prevented 3.2 cases of IPD caused by serotypes 3, 6A, and 19A, in children younger than 5 y, compared with vaccination with PCV10 (Table 1). This corresponds to 34% less IPD cases per year caused by serotypes 3, 6A, and 19A, and 3.5% less total IPD cases. These 3.2 cases avoided would also lead to less sequelae: 0.15 deaths, 0.16 physical handicaps, and 0.26 cases of deafness. Vaccination with PCV13, compared with PCV10, would also lead to 70 fewer cases of pneumonia (-0.4%) and almost 860 fewer cases of AOM (-0.6%). Indirectly, due to herd protection, more than 140 cases of IPD could be avoided (40% of the IPD cases caused by serotype 3, 6A, and 19A, 8.3% of total IPD cases), and more than 40 deaths, of which almost 39 are in the population older than 65 y (not shown). Non-invasive diseases and sequelae other than death were not taken into account in the population older than 5 y.
Table 1. Differences in health outcomes between the two vaccines in scenario 1.
| Children < 5 y | Others (indirect effects) | |
|---|---|---|
| Disease cases avoided | ||
| Total invasive pneumococcal disease | 3.20 | 140.4 |
| Of which caused by: serotype 3 | 0.41 | 41.4 |
| serotype 6A | 0.15 | 11.1 |
| serotype 19A | 2.63 | 87.9 |
| Pneumonia, treated in the hospital | 20.0 | - |
| Pneumonia, treated by GP only | 52.3 | - |
| Acute Otitis Media (AOM) | 858.9 | - |
| Sequelae avoided | ||
| Death | 0.15 | 40.5 |
| Physical handicap | 0.16 | - |
| Deafness | 0.26 | - |
We used the ratio of the incremental costs in Euros, to the incremental benefits in terms of quality adjusted life years (QALY), of PCV13 over PCV10, called the incremental cost-effectiveness ratio (ICER). Our baseline price difference was €11 per dose. Looking at direct effects on invasive pneumococcal disease (IPD) only, the ICER was €670 000/QALY gained (Table 2). The ICER was higher than €390 000/QALY gained (not shown) for other scenarios. The price of PCV13 may be at most €1.06 per dose higher than PCV10, in order to yield an ICER below €50 000/QALY gained in the first scenario.
Table 2. Cost-effectiveness results in various scenarios at a price difference of €11 per dose.a.
| Incremental (PCV13 over PCV10) | |||
|---|---|---|---|
| QALYs | Total Costs | ICERb | |
| (x € 1,000) | |||
| Scenario 1 | |||
| Direct effects, IPD only | 10 | 7400 | € 670 000 (€ 394 000–1 500 000) |
| Direct and indirect effects, IPD only | 400 | 5900 | € 14 500 (€ 11 100–19 200) |
| Direct and indirect effects, IPD + non invasive disease | 440 | 5600 | € 12 700 (€ 9100–11 100) |
| Other scenarios (direct + indirect effects IPD + non invasive disease) | |||
| 2 Epidemiology based on Dutch post-PCV7 data | € 17 200 (€ 12 200–23 600) | ||
| 3 Efficacy PCV10 against IPD and pneumonia based on FINIP | € 17 400 (CI N/A)b | ||
| 4 Extra protection PCV10 against NTHi based on COMPAS | € 24 600 (CI N/A) b | ||
| 5 Extra protection PCV10 against NTHi based on POET | € 124 000 (CI N/A) b | ||
| 6 No cross-protection from PCV10 against serotype 19A | € 11 900 (€ 8200–16 200) | ||
| 7 Cross-protection from PCV10 against serotype 19A includes herd immunity | € 16 000 (€ 11 000–22 800) | ||
| 8 No reduced protection from PCV13 against serotype 3 | € 5 800 (€ 3500–8100) | ||
| 9 No herd protection PCV13 against serotype 3 | € 14 500 (€ 10 400–19 400) | ||
| 10 Excluding effects on pneumonia | € 13 200 (€ 9400–17 800) | ||
| 11 Herd protection and replacement based on UK data | € 4700 (€ 2800–6500) | ||
| 12 Reduced indirect effects based on UK-data (25%) | € 25 500 (€ 17 700–33 500) | ||
a QALY, Quality Adjusted Life Year. QALYs rounded to nearest tens, costs and ICERs rounded to nearest € 100; ICERs above € 100 000 rounded to the nearest € 1000;b 95% confidence interval (CI) given, when the difference in QALYs is significantly different from zero. Otherwise: CI N/A (not applicable)
In scenario 1, introducing indirect effects led to an ICER of €14 500/QALY gained. Also including non-invasive disease, changed the ICER slightly to €12 700/QALY gained. When indirect effects and non-invasive disease are taken into account, PCV13 was considered dominant in the first scenario with a price difference below €2.63 per dose (Fig. 1). At a price difference of €25 per dose, the ICER was €34 900/QALY gained. Each Euro price difference extra raised the ICER by approximately €1600/QALY gained.
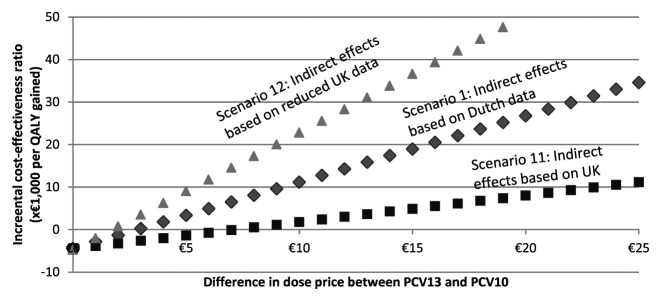
Figure 1. Cost-effectiveness by price difference, for three selected scenarios.
At a price difference of €11 per dose, the point estimates of the ICER when including indirect effects and non-invasive disease, were well below acceptable cost-effectiveness thresholds at €20 000/QALY in all but three scenarios.4,5,12 Scenarios 4 and 5 assumed extra protection against NTHi by PCV10, scenario 12 assumed reduced indirect effects. In scenarios 3, 4, and 5, using a price difference of €11 per dose, PCV10 dominates PCV13 in 10%, 6%, and 35% of the draws respectively: PCV10 has better health outcomes and is less costly than PCV13. At a threshold willingness-to-pay of €50 000/QALY gained, PCV13 can be considered cost-effective in approximately 80% of the draws in the probabilistic sensitivity analysis in scenarios 4 and 5, and in 38% of the draws in scenario 5 (not shown). The remaining uncertainty around estimates in other scenarios was relatively small, as can be seen in the presented confidence intervals.
Discussion and Conclusion
In this study, we analyzed a wide range of scenarios for the cost-effectiveness of PCV13 vaccination compared with PCV10 in the Netherlands. Since the difference between these two vaccines was only driven by three serotypes, namely 3, 6A, and 19A, we focused our study on these serotypes. Infant vaccination with pneumococcal vaccines has been part of the National Immunization Program for Dutch infants born after April 1, 2006. This program started with the 7-valent PCV7, followed by immunization with PCV10 from April 2011. The approximate burden of disease per 100 000 children younger than 5 y of age, after the introduction of PCV10, is 8.5 cases of IPD (April 2011-March 2013),15 2250 cases of cases of pneumonia and 16 000 cases of AOM (Primary Care Database). At the same time, the approximate disease burden per 100 000 Dutch inhabitants older than 5 y of age is 16.7 cases of IPD, 1350 cases of pneumonia and 1450 cases of AOM. As was already shown, introducing PCV13 would lead to lower disease burden in the infant population, but this effect would be relatively small, avoiding 3.2 cases of IPD over the course of five years in a single birth cohort. In relative terms, this would mean a drop of 3.5% in the yearly number of IPD. In the older population, via indirect effects, the impact was found to be slight larger: 140 cases or 8.3%. This relatively small drop is due to the small difference in the two vaccines. The efficacy estimates against VT serotypes for IPD are taken to be equal, due to a lack of better estimates. This means that the only difference between the two vaccines is due to the serotypes covered. The three serotypes covered by PCV13, but not by PCV10 -3, 6A and 19A- have only a small impact on the total disease burden. Together, these three serotypes caused 10% of the IPD in the period April 2011 to March 2013 within the infant population, and 20% within the population older than 5 y of age.15
This study has several limitations. First, in order to estimate the protection of PCV10 and PCV13 in non-invasive disease, we assumed the same serotype distribution as found in IPD. This was necessary, since no reliable information is available on serotype distribution of non-invasive disease cases in the Netherlands. Carrier information cannot be used since different serotypes have different effects on disease development. Therefore the serotype distribution for healthy children will be different from the serotype distribution in diseased children.
Efficacy effects for PCV13 are extrapolated from trials using PCV7, since no trials with PCV13 have been conducted. This extrapolation might be invalid, since interaction effects of the different vaccine components may influence VT efficacy.
Scenario 4 is based on the Clinical Otitis Media and Pneumonia Study (COMPAS),13 where the point estimate of protection against AOM was positive, but the statistical uncertainty around this estimate was very large and included no effectiveness against AOM at all. Scenario 5 is based on the Pneumococcal Otitis Efficacy Trial (POET) study. This study tested an 11-valent vaccine, not the currently available PCV10.16 In addition, the POET trial may not be comparable with other studies regarding acute otitis media (AOM).17,18 Furthermore, it has been shown that PCV10 does not impact carriage for NTHi.19 These two scenarios should therefore be considered with caution.
In the first two years after PCV10 was introduced, the growth in incidence of serotype 19A in the population older than 5 y, was stronger than that in the serotypes not covered by PCV13 (Table 3). Since the indirect effects in the model (Table 4) are based on post-PCV7 data,6,8 it is possible that these indirect effects reflect an underestimation of the effects on this specific serotype. This in turn may underestimate the incremental effects of PCV13 over PCV10, and overestimate the cost-effectiveness ratio.
Table 3. Number of cases per year of invasive pneumococcal disease (IPD).
| Post-PCV7 | Post-PCV10 | ||
|---|---|---|---|
| Apr 06 – Mar 2011 |
Apr 2011 – Mar 2013 |
Change | |
| IPD cases in children younger than five years of age.15 | |||
| Caused by serotypes covered in PCV7a | 28,8 | 10,1 | -19 (-65%) |
| Caused by serotypes covered in PCV10, not PCV7 b | 29,6 | 20,2 | -9 (-32%) |
| Caused by serotype 3 | 11,2 | 2,5 | -9 (-77%) |
| Caused by serotype 6A | 3,2 | 0,0 | -3 (-100%) |
| Caused by serotype 19A | 1,6 | 7,6 | +6 (+374%) |
| Caused by serotypes not covered in PCV13 | 32,8 | 58,1 | +25 (+77%) |
| IPD cases in patients between five and 64 y of age.15 | |||
| Caused by serotypes covered in PCV7 a | 288,0 | 106,1 | -182 (-63%) |
| Caused by serotypes covered in PCV10, not PCV7 b | 315,2 | 492,6 | +177 (+56%) |
| Caused by serotype 3 | 52,8 | 88,4 | +36 (+67%) |
| Caused by serotype 6A | 11,2 | 0,0 | -11 (-100%) |
| Caused by serotype 19A | 54,4 | 164,2 | +110 (+202%) |
| Caused by serotypes not covered in PCV13 | 319,2 | 591,2 | +272 (+85%) |
| IPD cases in patients older than five years of age.15 | |||
| Caused by serotypes covered in PCV7 a | 439,2 | 138,9 | -300 (-68%) |
| Caused by serotypes covered in PCV10, not PCV7 b | 231,2 | 356,2 | +125 (+54%) |
| Caused by serotype 3 | 89,6 | 151,6 | +62 (+69%) |
| Caused by serotype 6A | 28,8 | 25,3 | -4 (-12%) |
| Caused by serotype 19A | 94,4 | 255,2 | +161 (+170%) |
| Caused by serotypes not covered in PCV13 | 470,4 | 970,1 | +500 (+106%) |
a Includes serotypes 4, 6B, 9V, 14, 18C, 19F, 23F;b Includes serotypes 1, 5, 7F
Table 4. Indirect effects - herd protection and serotype replacement.a.
| Dutch data8 | UK data6 | |
|---|---|---|
| Percentage change in non-VT IPD, for age group: | ||
| < 2 y | 1.37 (0.77;2.46) | 1.68 (1.37;2.06) |
| 2–4 y | 1.22 (0.47;3.18) | 1.82 (1.30;2.55) |
| 5–14 y | 1.58 (0.77;3.26) | 0.82 (0.64;1.04) |
| 15–44 y | 1.22 (0.96;1.54) | 0.85 (0.71;1.02) |
| 45–64 y | 1.37 (1.11;1.70) | 0.96 (0.87;1.06) |
| 65+ year | 1.25 (1.09;1.43) | 1.48 (1.32;1.65) |
| Percentage change in VT IPD, for age group: | ||
| < 2 y | 0.01 (0.01;0.02)b | 0.02 (0.01;0.05) |
| 2–4 y | 0.19 (0.06;0.66) | 0.07 (0.04;0.13) |
| 5–14 y | 0.36 (0.12;1.14) | 0.25 (0.16;0.38) |
| 15–44 y | 0.81 (0.59;1.29) | 0.12 (0.07;0.21) |
| 45–64 y | 0.46 (0.33;0.63) | 0.15 (0.12;0.18) |
| 65+ year | 0.45 (0.37;0.55) | 0.19 (0.14;0.25) |
a Mean and confidence interval; IPD, invasive pneumococcal disease; VT, Vaccine-type; b No change in VT IPD in the original data; a continuity correction of 0.5 was applied, with an assumed confidence interval of Exp(ln(0.01)*1.1) to Exp(ln(0.01)*0.9).
Finally, this model does not take the exact dynamics of disease spread into account. As is true for many other infectious diseases, a dynamic model might be a future ideal, but the necessary information is scarce. It might be a good area for further investigation.
Taking only direct effects on IPD into account, PCV13 was not found to be cost-effective, at a price difference of €11 per dose. If herd protection, replacement and non-invasive disease are also taken into account, the ICER of PCV13 compared with PCV10 was below €30,000/QALY gained in 11 of 12 scenarios. PCV13 is considered dominant in the first scenario with a price difference below €2.63 per dose.
Material and Methods
Published model
We calculated the outcomes using an incremental analysis, meaning that only differences between the two vaccines are taken into account. We adapted and updated a previously published model.1 The model, build in Excel, was a decision tree, following a birth cohort for five years after vaccination (time horizon) and calculating the costs and effects linked to the number of diseased cases and their sequelae, from a societal perspective. We modeled IPD, non-invasive pneumonia and AOM and their sequelea.1 Uncertainty around model parameters is assessed by defining probability distributions for these parameters and taking 5,000 random draws in a probabilistic sensitivity analysis. Results were discounted using 4% for costs and 1.5% for health outcomes, according to the Dutch health-economic guidelines.20 All costs were updated to 2012 price levels, using the Dutch consumer price index.21 Cost-effectiveness results were calculated for 12 scenarios, in which the effects of different assumptions were calculated. We first explain the baseline data used in the following paragraphs, and next explain how different aspects are varied in scenarios.
Vaccine price
Drug price is a crucial element in any cost-effectiveness analysis. However, public information on price could not be used for our study, since the final price being offered will depend largely on strategic decisions from both pharmaceutical companies during the bidding process within the tender. We therefore modeled a price difference between the two vaccines, which we varied between €0 and €25, assuming that the higher number of serotypes covered would always render PCV13 more expensive. As a baseline price difference, we used €11 per dose, the approximate difference between the list price of PCV10 (€56.43) and PCV13 (€67,72).22
Epidemiological data
We used recent estimates of epidemiological data on IPD,15 non-invasive pneumonias treated in the hospital (ICD-9 480–486) provided by Kiwa Carity (Utrecht), and contacts with the general practitioner for non-invasive pneumonia (ICP code R81) and AOM (H71–72) provided by the Primary Care Database from the Julius Center (Utrecht) (Tables 3 and 5). Epidemiology as observed after the introduction of PCV10 was taken into account for all diseases. In scenario 2, we calculated the cost-effectiveness outcomes, using epidemiological data from the period between the introduction of PCV7 in April of 2007 and the introduction of PCV10 in March 2011.
Table 5. Epidemiological data on non-invasive disease in children younger than 5 y of age.
| Post-PCV7 | Post-PCV10 | |
|---|---|---|
| Apr 2006-Mar 2011 | Apr 2011-Dec 2012 | |
| Number of pneumonia cases per 100,000 inhabitants per year (Source: personal communication Kiwa Carity). | ||
| 0 y of age | 0,23 | 0,64 |
| 1 y of age | 0,47 | 1,74 |
| 2 y of age | 0,39 | 1,61 |
| 3 y of age | 0,27 | 1,51 |
| 4 y of age | 0,29 | 1,54 |
| Number of acute otitis media (AOM) cases per 100,000 inhabitants (Source: personal communication Primary Care Database). | ||
| 0 y of age | 1,57 | 4,58 |
| 1 y of age | 5,33 | 15,01 |
| 2 y of age | 3,96 | 10,88 |
| 3 y of age | 2,10 | 7,74 |
| 4 y of age | 2,02 | 7,14 |
| Number of GP contacts per acute otitis media (AOM) case (Source: personal communication Primary Care Database). | ||
| 0 y of age | 1,71 | 1,67 |
| 1 y of age | 1,95 | 2,03 |
| 2 y of age | 1,66 | 1,69 |
| 3 y of age | 1,51 | 1,50 |
| 4 y of age | 1,43 | 1,47 |
Efficacy data
Efficacy data was not changed from the original model,23-25 but we did look at the effects of newly available data in scenarios, coming from the Finnish Invasive Pneumococcal disease study (FINIP, scenario 3) and the Clinical Otitis Media and Pneumonia Study (COMPAS, scenario 4).11-13 Table 6 shows a summary of the efficacy data used in each of the scenarios. In the appendix (Table A1, A2 and A3), the background information of each of the studies used to calculate efficacy, is shown. In our primary scenario, we assumed a 30% efficacy of PCV10 against serotype 19A and PCV13 against serotype 3. The first assumption is based on indications of cross-protection.9,12 The second assumption is based on only modest observed reductions in IPD caused by serotype 310 and no difference in serotype 3 carriage between PCV7 and PCV13.26 Another study on the effects of pneumococcal vaccination on AOM, was the Pneumococcal Otitis Efficacy Trial (POET).16 Scenario 5 shows the cost-effectiveness when these efficacy numbers were used. Scenarios 6 and 7 explored the effect of the assumed cross-protection of PCV10 against serotype 19A. Scenarios 8 and 9 explored the effect of the assumed lesser efficacy of PCV13 against serotype 3. In a recent Cochrane review, no real difference was found between the different vaccines in the effect on pneumonia, despite the differences between vaccines in number of serotypes.27 In scenario 10, we therefore investigated the assumption that no difference can be found between vaccines, by disregarding the effect of pneumonia in the outcomes.
Table 6. Efficacy estimates used in the study.
| Scenarios | Reduction of disease cases1 | Source | |
|---|---|---|---|
| Invasive pneumococcal disease (IPD) | |||
| Reduction of vaccine-type IPD cases. | 1,2,5–12 | 93.9% (0.029) | 23 |
| (...), for PCV10. | 3 | 100.0% (0.026) (Maximized at 100%) |
11 |
| Non-invasive disease 2 | |||
| Reduction of all-cause AOM, using PCV7. | 1–8,11,12 | 6.0% (0.051) | 25 |
| (...), using PCV10. | 3 | 19.0% (0.069) | 13 |
| (...), using PCV10. | 4 | 33.6% (0.060) | 16 |
| Reduction of number of pneumonia cases, as seen by a general practitioner, using PCV7. | 1–10,12 | 6.0% (0.032) | 24 |
| Reduction of number of pneumonia cases, admitted in a hospital, using PCV7. | 1–10,12 | 11.1% (0.043) | 24 |
| (...), using PCV10. | 3 | 28.6% (0.043) | 11 |
(1)Mean (standard deviation) used in the probabilistic sensitivity analysis, using a normal distribution;2 Efficacy for non-invasive disease recalculated to vaccine-type, assuming a serotype distribution and assuming only disease cases caused by vaccine-type serotypes or non-typable H. influenzae (acute otitis media for PCV10) will be affected.
Table A1. Detail of studies used to calculate vaccine efficacy.1.
| Name of the study | Source | |
|---|---|---|
| Kaiser Permanente Vaccine Study | 23 , 24 | PCV7 vs. meningococcal vaccine (control) USA (Northern California) Aim: To evaluate protective efficacy of PCV7 against invasive pneumococcal disease caused by vaccine serotypes, clinical episodes of otitis media and episodes of pneumonia. |
| Finnish Otitis Media Vaccine Trial | 25 | PCV7 vs. hepatitis vaccine (control) Finland Aim: To evaluate protective efficacy of PCV7 against episodes of acute otitis media. |
| Pneumococcal Otitis Efficacy Trial (POET) | 16 | PCV11 vs. hepatitis vaccine (control) Czech Republic and Slovakia. Aim: to evaluate protective efficacy against acute otitis media caused by pneumococci and nontypable H. influenza. |
| Finnish Invasive Pneumococcal disease (FinIP) | 11 | PCV10 vs. hepatitis vaccine (control) Finland Aim: To evaluate protection against several disease endpoints associated with S. pneumoniae and non-typeable H. influenzae. |
| Clinical Otitis Media and Pneumonia Study (COMPAS) | 13 | PCV10 vs. hepatitis vaccine (control) Latin America Aim: to evaluate protective efficacy against acute otitis media. |
(1) PCV: Pneumococcal Conjugate Vaccine
Table A2. Parameters used in the health-economic model.
| Parameter used | Source | |
|---|---|---|
| Vaccine effectiveness | ||
| Effectiveness after dose 2 mo | 0.0% | Assumption |
| Effectiveness after dose 3 mo | 30.0% | Assumption |
| Effectiveness after dose 4 mo | 90.0% | Assumption |
| Effectiveness after booster dose (11 mo) | 100.0% | Assumption |
| Vaccination level. first three shots | 95.7% | 28 |
| Sequalae | ||
| Case fatality rate | ||
| 0–2 y | 6.0% | 29 |
| 3–4 y | 4.8% | 29 |
| Physical handicap (Mental retardation, spasticity, epilepsy) | 6.0% | 29 |
| Needing institutionalized care | 25.0% | 30 |
| Needing special education | 50.0% | 30 |
| Hearing problems | 9.5% | 29 |
| Percentage needing cochlear device | 37.5% | 29 |
| Quality of life weights | ||
| Per year | ||
| Mental retardation | 0.620 | 31 |
| Spasticity | 0.619 | 32 |
| Epilepsy | 0.830 | 31 |
| Slight hearing problems | 0.910 | 31 |
| Bilateral hearing problems (first year) | 0.550 | 33 |
| Bilateral hearing problems (cochlear device) | 0.820 | 33 |
| Death | 0.000 | Assumption |
| All other states | 0.890 | Assumption |
| Hospital admission for non-inv pneumonia | 0.930 | 34 |
| Non-inv pneumonia treated by GP | 0.930 | 34 |
| AOM | 0.980 | 34 |
| Per Episode | ||
| Hospital admission for meningitis | 0.070 | 34 |
Table A3. Cost parameters used in the health-economic model.1.
| Direct costs | Direct costs | Indirect costs |
|---|---|---|
| Invasive pneumococcal disease (IPD) | ||
| 0–1 y | €6773.42 | €781.24 |
| 2–4 y | €2732.31 | €335.80 |
| Non-invasive disease | ||
| Non-invasive pneumonia (admitted to hospital) | €2809.59 | €338,23 |
| Non-invasive pneumonia (seen by GP) | €28.42 | €123,89 |
| Acute otitis media (simple) | €18.75 | €65,66 |
| Acute otitis media (complex, tympanostomy) | €409.81 | €247,78 |
| Acute otitis media (complex, no tympanostomy) | €102.75 | €61,95 |
| Sequalea | ||
| Special education (annual costs) | ||
| Primary school (until age 12) | €10 527.77 | |
| High school (age 12–18) | €18 225.49 | |
| Intitutional care (annual costs) | €42 532.02 | |
| Cochlear implantation | €60 853.42 |
Efficacy data against non-invasive disease is estimated in a non-vaccinated population. Since this situation is not applicable in The Netherlands, we calculated a vaccine-type (VT) efficacy, which is applied to the post-PCV10 incidence of non-invasive disease. We assumed that 42% of AOM cases are caused by non-typeable Hemophilus influenzae (NTHi) and 22% by pneumococcii.4 For pneumococcal AOM and non-invasive pneumonia, a serotype distribution equal to that found in IPD was assumed.
Indirect effects
Indirect effects –herd protection and serotype replacement- were calculated, using the effects of the vaccines on the incidence during one year. Several sources of data on indirect effects are available (Table 4), including a Dutch estimate.8 However, these do not take the inherent time trends in IPD incidence into account. UK data are also available, which do take these time trends into account,6 but it also includes the effects of the UK catch-up program which was absent in the Netherlands. In addition, indirect effects take time to accumulate. It is therefore highly uncertain how much indirect effects should be taken into account. We therefore calculated the outcomes using several definitions for the indirect effects. In our primary scenario, we assumed the Dutch indirect effects, which is approximately equal to using 50% of the UK-levels of indirect effects. In two other scenarios we also included 100% (scenario 11) and 25% (scenario 12) of the UK-levels of indirect effects. Indirect effects were taken into account by multiplying the estimates from Table 4 by the observed incidence in the post-PCV7 period (Table 3), and calculating the difference between when the two treatment arms, caused by the difference in serotype coverage. Obviously, including indirect effects raises uncertainty inherent in the uncertainty in our assumptions.
Appendix
Acknowledgments
The authors would like to thank W. Hoogen Stoevenbeld from Kiwa Carity (Utrecht) and the people at the Julius Center (Utrecht) for providing the necessary data. We acknowledge Prof Dr E.A.M. Sanders for useful comments on an earlier draft of this paper. Employees of both vaccine manufacturers, GSK and Pfizer, were allowed to comment twice on the text. It was fully at the discretion of the authors to incorporate these comments.
Financial statement
This study was funded by an unrestricted grant from GSK.
Glossary
Abbreviations:
- AOM
acute Otitis Media
- CE
cost-effectiveness
- COMPAS
Clinical Otitis Media & Pneumonia Study
- FinIP
Finnish Invasive Pneumococcal disease
- ICER
Incremental Cost-effectiveness Ratio
- IPD
Invasive Pneumococcal Disease
- NTHi
non-typeable Haemophilus influenzae
- PCV
pneumococcal conjugate vaccine
- PCV10
10-valent PCV
- PCV13
13-valent PCV
- POET
Pneumococcal Otitis Efficacy Trial
- VT
Vaccine-type
References
- 1.Rozenbaum MH, Sanders EAM, van Hoek AJ, Jansen AGSC, van der Ende A, van den Dobbelsteen G, Rodenburg GD, Hak E, Postma MJ. Cost effectiveness of pneumococcal vaccination among Dutch infants: economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines. BMJ. 2010;340(c2509):c2509. doi: 10.1136/bmj.c2509. [DOI] [PubMed] [Google Scholar]
- 2.Rozenbaum MH, Hoek AJ, Hak E, Postma MJ. Huge impact of assumptions on indirect effects on the cost-effectiveness of routine infant vaccination with 7-valent conjugate vaccine (Prevnar) Vaccine. 2010;28:2367–9. doi: 10.1016/j.vaccine.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Health Council of the Netherlands. Vaccinatie tegen pneumokokken [in Dutch “Vaccination of infants against pneumococcal infections”]. 2010;2010/02(2005/13).
- 4.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29:304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodenburg GD, de Greeff SC, Jansen AG, de Melker HE, Schouls LM, Hak E, Spanjaard L, Sanders EA, van der Ende A. Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg Infect Dis. 2010;16:816–23. doi: 10.3201/eid1605.091223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–8. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 7.Cohen R, Levy C, Bingen E, Bechet S, Derkx V, Werner A, Koskas M, Varon E. [Nasopharyngeal carriage of children 6 to 60 months during the implementation of the 13-valent pneumococcal conjugate vaccine] Arch Pediatr. 2012;19:1132–9. doi: 10.1016/j.arcped.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 8.van Deursen AM, van Mens SP, Sanders EA, Vlaminckx BJ, de Melker HE, Schouls LM, de Greeff SC, van der Ende A, Invasive Pneumococcal Disease Sentinel Surveillance Laboratory Group Invasive pneumococcal disease and 7-valent pneumococcal conjugate vaccine, the Netherlands. Emerg Infect Dis. 2012;18:1729–37. doi: 10.3201/eid1811.120329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Wals P, Lefebvre B, Defay F, Deceuninck G, Boulianne N. Invasive pneumococcal diseases in birth cohorts vaccinated with PCV-7 and/or PHiD-CV in the province of Quebec, Canada. Vaccine. 2012;30:6416–20. doi: 10.1016/j.vaccine.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan SL, Barson WJ, Lin PL, Romero JR, Bradley JS, Tan TQ, Hoffman JA, Givner LB, Mason EO., Jr. Early trends for invasive pneumococcal infections in children after the introduction of the 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2013;32:203–7. doi: 10.1097/INF.0b013e318275614b. [DOI] [PubMed] [Google Scholar]
- 11.Effectiveness of the 10-valent pneumococcal Haemophilus Influenzae Protein D COnjugate Vaccines (PHiD-CV10) against hospital-diagnosed pneumonia in infants - FINIP trial . 31st Meeting of the European Society for Paediatric Infectious Diseases (ESPID 2013) ; May 2013; ; 2013.
- 12.Palmu AA, Jokinen J, Borys D, Nieminen H, Ruokokoski E, Siira L, Puumalainen T, Lommel P, Hezareh M, Moreira M, et al. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet. 2013;381:214–22. doi: 10.1016/S0140-6736(12)61854-6. [DOI] [PubMed] [Google Scholar]
- 13.Efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae Protein D conjugate vaccine (PHiD-CV) against acute otitis media in children in Panama. . 9th International Symposium onAntimicrobial Agents and Resistance (ISAAR 2013); March 2013; ; 2013.
- 14.TenderNed. Aankondiging van een opdracht:Pneumococcal Vaccine -Rijksinstituut voor Volksgezondheid en Milieu (RIVM) [Contract notice: Pneumococcal Vaccine -Institute for Public Health and the Environment (RIVM)]. 2014; Available at: https://www.tenderned.nl/tenderned-web/aankondiging/detail/samenvatting/akid/f52bf20f62d2535a8d402a6492e72777. Accessed 02/17, 2014.
- 15.Wie wordt er ziek en van welke pneumokok? [Who gets sick and from which pneumococcal serotype?]. Symposium Vaccinonderzoek in Nederland; Het Laatste Nieuws; 2013.
- 16.Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels JP, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367:740–8. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 17.De Wals P, Erickson L, Poirier B, Pépin J, Pichichero ME. How to compare the efficacy of conjugate vaccines to prevent acute otitis media? Vaccine. 2009;27:2877–83. doi: 10.1016/j.vaccine.2009.02.102. [DOI] [PubMed] [Google Scholar]
- 18.Gezondheidsraad. Vaccinatie van zuigelingen tegen pneumokokkeninfecties (3). [Vaccination of infants against pneumococcal infections (3)]. 2013;2013/28.
- 19.van den Bergh MR, Spijkerman J, Swinnen KM, François NA, Pascal TG, Borys D, Schuerman L, Ijzerman EP, Bruin JP, van der Ende A, et al. Effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine on nasopharyngeal bacterial colonization in young children: a randomized controlled trial. Clin Infect Dis. 2013;56:e30–9. doi: 10.1093/cid/cis922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.College voor Zorgverzekeringen. Richtlijnen voor farmaco-economisch onderzoek; evaluatie en actualisatie [Guidelines for pharmacoeconomic research: evaluation and actualization]. 2008.
- 21.Statistics Netherlands. CBS Statline. 2013; Available at: http://statline.cbs.nl/statweb/. Accessed 05/14, 2013.
- 22.Medicijnkosten CVZ. 2013; Available at: www.medicijnkosten.nl. Accessed 05/17, 2013.
- 23.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G, et al. Northern California Kaiser Permanente Vaccine Study Center Group Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000;19:187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, Noyes J, Lewis E, Ray P, Lee J, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J. 2002;21:810–5. doi: 10.1097/00006454-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, Takala A, Käyhty H, Karma P, Kohberger R, et al. Finnish Otitis Media Study Group Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–9. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 26.Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013;57:952–62. doi: 10.1093/cid/cit428. [DOI] [PubMed] [Google Scholar]
- 27.Lucero MG, Dulalia VE, Nillos LT, Williams G, Parreno RA, Nohynek H, et al. Pneumococcal conjugate vaccines for preventing vaccine-type invasive pneumococcal disease and X-ray defined pneumonia in children less than two years of age. Cochrane Database Syst Rev. 2009 Oct 7;(4):CD004977 [DOI] [PMC free article] [PubMed]
- 28.Van Lier EA, Oomen PJ, Oostenbrug MWM, Zwakhals SLN, Drijfhout IH, De Hoogh PAAM, et al. Vaccinatiegraad Rijksvaccinatieprogramma Nederland. Verslagjaar 2009 [In Dutch: “Vaccination level in the Dutch National Immunization Program. Ned Tijdschr Geneeskd. 2009 May 16;153(20):950-7 [PubMed]
- 29.Jansen AG, Rodenburg GD, de Greeff SC, Hak E, Veenhoven RH, Spanjaard L, Schouls LM, Sanders EA, van der Ende A. Invasive pneumococcal disease in the Netherlands: Syndromes, outcome and potential vaccine benefits. Vaccine. 2009;27:2394–401. doi: 10.1016/j.vaccine.2009.01.127. [DOI] [PubMed] [Google Scholar]
- 30.Hubben GA, Bos JM, Glynn DM, van der Ende A, van Alphen L, Postma MJ. Enhanced decision support for policy makers using a web interface to health-economic models--illustrated with a cost-effectiveness analysis of nation-wide infant vaccination with the 7-valent pneumococcal conjugate vaccine in the Netherlands. Vaccine. 2007;25:3669–78. doi: 10.1016/j.vaccine.2007.01.088. [DOI] [PubMed] [Google Scholar]
- 31.Oostenbrink R, A Moll HA, Essink-Bot ML. The EQ-5D and the Health Utilities Index for permanent sequelae after meningitis: a head-to-head comparison. J Clin Epidemiol. 2002;55:791–9. doi: 10.1016/S0895-4356(02)00448-1. [DOI] [PubMed] [Google Scholar]
- 32.WHO. Global burden of disease 2004 update: disability weights for diseases and conditions. 2004.
- 33.Krabbe PF, Hinderink JB, van den Broek P. The effect of cochlear implant use in postlingually deaf adults. Int J Technol Assess Health Care. 2000;16:864–73. doi: 10.1017/S0266462300102132. [DOI] [PubMed] [Google Scholar]
- 34.RIVM. Volksgezondheid Toekomst Verkenning, Nationaal Kompas Volksgezondheid (National Public Health Compass) version 3.12. Bilthoven: National Institute for Public Health and the Environment; 2007. [Google Scholar]


