Abstract
The novel meningococcal serogroup B vaccine (4CMenB, Bexsero®), recently approved in Europe and Australia, may soon be included in routine infant immunization schedules, subject to guidance from national or regional recommending bodies. In the development of 4CMenB and consistent with other newly introduced vaccines, clinical studies have shown concomitant administration with routine infant vaccines induces an incremental increase in some reactions, including fever. As this may hinder acceptability, we examined the impact of prophylactic paracetamol on the occurrence of fever and other solicited reactions, as well as the immune responses to study vaccines, in a prospectively designed study. 4CMenB was administered as a 4-dose series at 2, 3, 4, and 12 months of age concomitantly with routine infant vaccines: DTaP-HBV-IPV/Hib and PCV7, with or without prophylactic paracetamol; a third group received MenC vaccine. Immune responses to 4CMenB were not decreased by the use of paracetamol prophylaxis and there were no clinically relevant effects on immune responses to routine vaccines. Occurrence of fever was higher in infants co-administered with 4CMenB compared with those given MenC vaccine, but was significantly decreased by prophylactic paracetamol, as were other solicited reactions to vaccination, both local and systemic. Co-administration of 4CMenB had an acceptable tolerability profile, with no withdrawals due to vaccination-related adverse events. Inclusion of 4CMenB in routine infant immunization schedules will be a major advance in the control of meningococcal disease, and our study indicates that by using paracetamol prophylaxis, post-vaccination reactions are reduced without clinically relevant negative consequences on vaccine immunogenicity.
Keywords: infants, meningococcal, serogroup B, vaccination, antipyretics
Introduction
Inclusion of new vaccines into infant immunization schedules generally necessitates concomitant administration with established routine vaccines, with consequent potential for increased reactogenicity. These reactions are typically of limited duration and without significant medical consequences. However, parental concern with their child’s discomfort due to evident reactions such as post-vaccination fever or injection site pain may lead to unscheduled physician visits and other medical utilization, and result in work absenteeism for the parent and increased healthcare costs.1,2 For this reason the use of antipyretic agents after pediatric vaccination has become routine practice in many countries.3 In the 1980s paracetamol was shown to have a clinically beneficial effect of on the frequency and severity of common adverse reactions in young children following diphtheria-tetanus-whole-cell pertussis-polio vaccination (DTPw-IPV),4,5 and subsequently prophylactic paracetamol was shown to significantly reduce fever in infants given diphtheria-tetanus-acellular pertussis (DTaP) vaccine and other currently recommended vaccines.6,7
Early reports found antibody responses in young children to DTPw vaccine were unaffected by a single prophylactic dose of paracetamol 4 h after vaccination,8 or by paracetamol administered therapeutically within 48 h of DTwP vaccination.9 However, in recent investigations of the co-administration of a novel pneumococcal conjugate vaccines [PHiD-CV] with the routine infant vaccine (diphtheria-tetanus-acellular pertussis-inactivated poliovirus-hepatitis B with Hemophilus influenzae type b [DTaP-HBV-IPV/Hib]), some of the present authors reported a statistically significant negative impact on the immune responses to several antigens components when 3 doses of paracetamol were administered prophylactically with priming and booster doses.7 Follow up of these children to 4 y of age suggests that immune memory and effects on pneumococcal carriage were unaffected, so the clinical consequences of such interference appeared to be minimal.10
A current unmet need in infant vaccination is a broadly protective vaccine against serogroup B Neisseria meningitidis. Following the successful implementation of vaccination campaigns against serogroup C, serogroup B is now the major cause of meningococcal disease in many parts of the developed world, including Europe, Canada, and Australia.11 The multicomponent meningococcal serogroup B vaccine (4CMenB, Bexsero®, Novartis Vaccines and Diagnostics), recently approved in Europe and Australia for use from 2 mo of age, is highly immunogenic, and generally well tolerated, but is associated with an incremental increase in the incidence of fever when administered concomitantly with routine infant vaccines.12-15
In a phase 2 randomized, controlled trial investigating different formulations of the vaccine components in 4CMenB in healthy infants (registered on clinicaltrials.gov, NCT00937521), we included one study arm to examine the effect of paracetamol prophylaxis and another to compare the tolerability of meningococcal serogroup C conjugate vaccine with the final 4CMenB formulation, when these vaccines were administered as a 4-dose series concomitantly with routine vaccines (DTaP-HBV-IPV/Hib and heptavalent pneumococcal vaccine). The effect of paracetamol prophylaxis on the immunogenicity of all vaccine antigens was examined, since this may affect how these vaccines may be used in clinical practice as 4CMenB is now licensed for routine use.
Results
Demographics
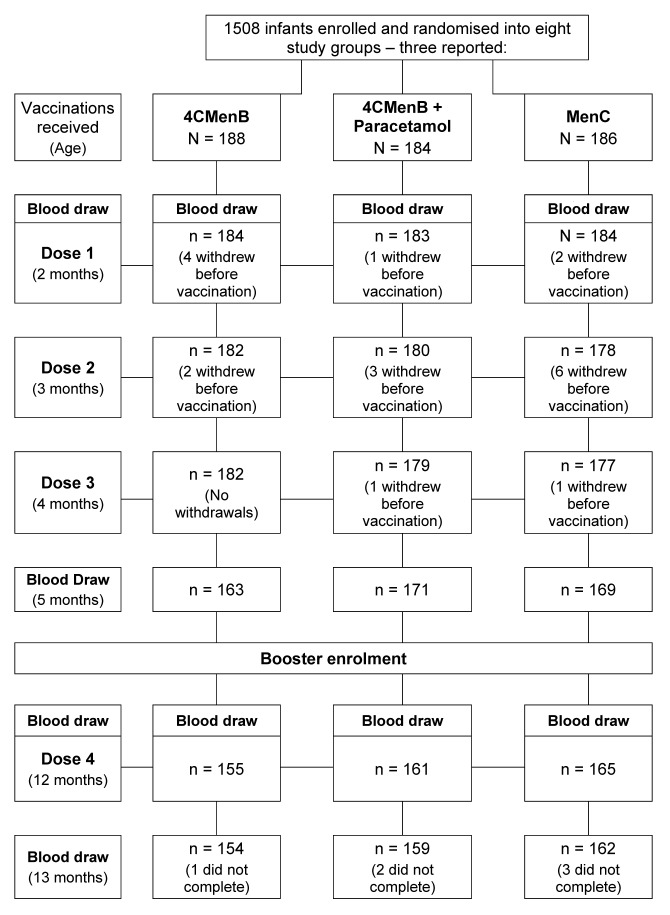
In the full study a total of 1507 infants were enrolled and randomly allocated to 8 study groups, mean age 74.6 d, of whom 558 were included in the 3 study groups described in this report—data on the other participants are presented in the accompanying paper.16 In the respective groups, in addition to their routine infant vaccines, 188 were vaccinated with 4CMenB, 184 with 4CMenB+prophylactic paracetamol, and 186 with MenC vaccine. Of these, 181, 179, and 177 received all 3 vaccination series and attended the fourth study visit 30 d after the primary 3-dose series, respectively, but 10, 10, and 13 subjects in these groups did not provide blood samples (Fig. 1). Main reasons for premature withdrawal were withdrawal of consent and loss to follow-up, but no withdrawals were due to an adverse event (AE).
Figure 1. Study flowchart.
At 12 mo of age, 506 subjects from the 3 groups were presented for the booster portion of the study, of whom 500 received one dose of 4CMenB—the fourth dose for those in the 4CMenB and 4CMenB+paracetamol groups, and the first of 2 4CMenB doses in the MenC group—and 470 completed the study.
Baseline demographic characteristics of enrolled subjects in all 3 groups were similar with respect to age (74.0–74.4 d), gender (53–61% male), weight (5.5–5.6 kg), height 58.8–59.4 cm), and ethnicity (89–96% Caucasian, 3–8% Hispanic).
Immunogenicity
Responses to 4CMenB
The immunogenicity of 4CMenB was assessed as hSBA titers against 3 indicator strains for antibodies against the fHbp, NadA, and NZOMV antigens, and as the proportion of subjects in each group who achieved a titer ≥ 5, which is associated with seroprotection against meningococcal infection.17
As illustrated in Figure 2 as percentages of subjects with hSBA titers ≥ 5, and shown as geometric mean titers in Table 1, there were low levels of hSBA against the 3 antigens prevaccination. These did not change up to the 12 mo assessment in the MenC group, but at 5 mo in the other 2 groups, after 3 doses of 4CMenB, there were strong immune responses; 100% and 99% of subjects had titers ≥ 5 against fHbp and NadA indicator strains, irrespective of the use of paracetamol, and 78% and 75% had titers ≥ 5 against the NZOMV strain without and with paracetamol. GMTs in both 4CMenB groups were similar.
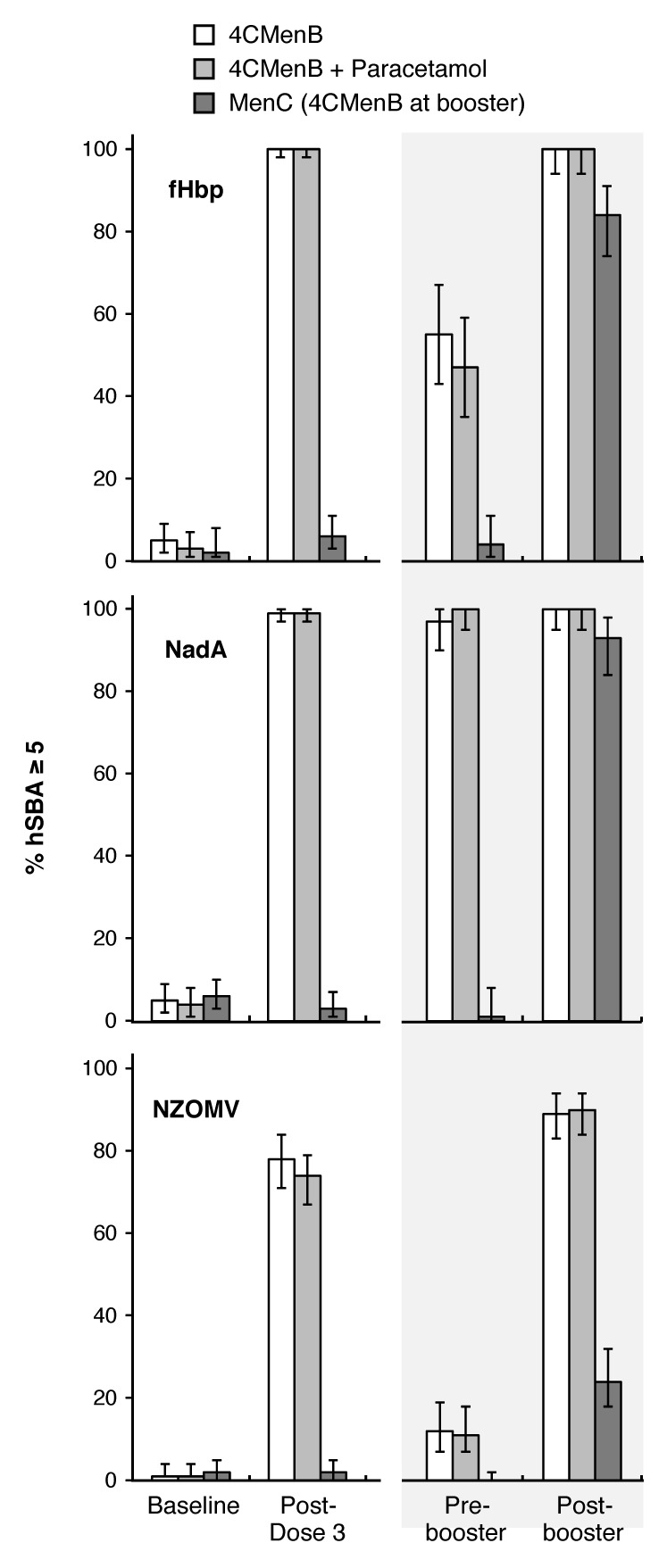
Figure 2. Proportions of subjects in each group with hSBA titers ≥ 5 before and 1 mo after any primary or booster vaccinations.
Table 1. hSBA Geometric mean titers in all study groups, and ratios of GMTs between 4CMenB groups.
| Study groups | Ratio of GMTs 4CMenB+Para and 4CMenB (95% CI) |
||||
|---|---|---|---|---|---|
| Antigen | Time-point | 4CMenB | 4CMenB + Para | Men C | |
| fHbp | Pre-vaccination |
1.25 (1.14–1.37) n = 166 |
1.18 (1.08–1.3) n = 166 |
1.16 (1.09–1.25) n = 168 |
0.95 (0.84–1.07) |
| Post-3rd dose |
101 (90–113) n = 170 |
102 (91–115) n = 167 |
1.24 (1.11–1.39) n = 132 |
1.02 (0.87–1.18) | |
| Pre-booster |
4.94 (3.76–6.5) n = 69 |
4.51 (3.43–5.95) n = 70 |
1.15 (1.03–1.29) n = 74 |
0.91 (0.63–1.32) | |
| Post-booster |
120 (95–150) n = 65 |
136 (107–172) n = 63 |
12 (10–16) n = 75 |
1.13 (0.83–1.55) | |
| NadA | Pre-vaccination |
1.18 (1.07–1.3) n = 162 |
1.07 (0.97–1.18) n = 157 |
1.21 (1.09–1.34) n = 161 |
0.91 (0.8–1.04) |
| Post-3rd dose |
396 (348–450) n = 165 |
455 (399–519) n = 160 |
1.15 (1.03–1.29) n = 159 |
1.15 (0.96–1.37) | |
| Pre-booster |
69 (53–88) n = 71 |
106 (82–136) n = 71 |
1.11 (0.95–1.29) n = 70 |
1.55 (1.1–2.16) | |
| Post-booster |
1950 (1573–2417) n = 73 |
2182 (1769–2691) n = 76 |
41 (29–57) n = 69 |
1.12 (0.85–1.48) | |
| NZOMV | Pre-vaccination |
1.02 (0.99–1.06) n = 170 |
1.02 (0.99–1.05) n = 169 |
1.06 (1–1.13) n = 171 |
0.99 (0.95–1.04) |
| Post-3rd dose |
10 (8.59–12) n = 171 |
8.48 (7.24–9.93) n = 168 |
1.05 (1.01–1.1) n = 168 |
0.85 (0.68–1.05) | |
| Pre-booster |
1.6 (1.43–1.8) n = 141 |
1.48 (1.32–1.66) n = 143 |
1.03 (1–1.06) n = 148 |
0.92 (0.79–1.08) | |
| Post-booster |
20 (16–24) n = 138 |
20 (17–25) n = 140 |
2.2 (1.89–2.57) n = 147 |
1.02 (0.78–1.33) | |
By 12 mo of age, titers had waned to varying extents (Table 1), with 97–100%, 50–53%, and 11–12% of subjects in the 2 groups still having titers ≥ 5 against NadA, fHbp, and NZOMV, respectively. Administration of a fourth dose of 4CMenB elicited booster responses against all 3 antigens, 100% achieving titers ≥ 5 against fHbp and NadA, and 88–89% against NZOMV, with similar GMTs in the 2 groups. At this time-point, 1 mo after their first dose of 4CMenB, 85%, 93%, and 23% of the MenC group had titers ≥ 5 against fHbp, NadA, and NZOMV, respectively, with much lower GMTs than the 4CMenB groups at 5 or 13 mo.
Responses to routine vaccines
Routine vaccines—DTaP-HBV-IPV/Hib antigens
Proportions of infants achieving the expected immunogenicity responses to 3 doses of DTaP-HBV-IPV/Hib and PCV7 vaccines were similar in all 3 groups (Supplementary Tables). The largest difference was a 6% lower rate for vaccine response to pertactin in the 4CMenB+paracetamol group compared with the 4CMenB control group. One month after the third vaccination, 97–100% of infants across the 3 groups had expected levels against diphtheria, tetanus, hepatitis B, Hib, and the poliovirus types 1, 2, and 3, and 91–100% demonstrated vaccine responses against the 3 acellular pertussis antigens. After the primary vaccination GMC/GMTs ratios for DTaP-HBV-IPV/Hib antigens of the 4CMenB+paracetamol and 4CMenB groups ranged from 0.82 for Poliotypes 1 and 2, to 1.15 for Hepatitis B antigen (Tables 2 and 3). After the booster doses administered at 12 mo rates were 99–100% against all antigens in all 3 groups.
Table 2. Geometric mean concentrations/titers for routine vaccine components (95% CI).
| Antigen (cut-off) |
Study groups | Ratio of GMTs 4CMenB+Para to 4CMenB (95% CI) | |||
|---|---|---|---|---|---|
| Timing | 4CMenB | 4CMenB + Para | Men C | ||
|
Diphtheria (ELISA IU/mL) |
Post-3rd dose |
1.93 (1.78–2.1) n = 140 |
1.84 (1.7–2.0) n = 135 |
2.43 (2.24–2.64) n = 132 |
0.95 (0.85–1.07) |
| Pre-booster |
0.38 (0.34–0.43) n = 119 |
0.37 (0.33–0.42) n = 115 |
0.46 (0.33–0.42) n = 131 |
0.97 (0.82–1.16) | |
| Post-booster |
4.48 (3.96–5.07) n = 118 |
4.31 (3.81–4.87) n = 120 |
5.23 (4.6–5.95) n = 119 |
0.96 (0.81–1.14) | |
|
Tetanus (ELISA IU/mL) |
Post-3rd dose |
1.83 (1.62–2.06) n = 140 |
1.58 (1.39–1.78) n = 135 |
2.0 (1.77–2.25) n = 132 |
0.86 (0.73–1.02) |
| Pre-booster |
0.39 (0.33–0.45) n = 119 |
0.35 (0.3–0.41) n = 115 |
0.41 (0.36–0.48) n = 131 |
0.91 (0.73–1.14) | |
| Post-booster |
4.31 (3.73–4.98) n = 118 |
3.76 (3.26–4.34) n = 120 |
4.8 (4.18–5.25) n = 119 |
0.87 (0.71–1.07) | |
|
PRP (µg/mL) |
Post-3rd dose |
1.6 (1.34–1.92) n = 140 |
1.55 (1.29–1.86) n = 135 |
1.72 (1.43–2.06) n = 132 |
0.96 (0.75–1.24) |
| Pre-booster |
0.81 (0.7–0.94) n = 119 |
0.76 (0.65–0.89) n = 115 |
0.76 (0.67–0.86) n = 131 |
0.94 (0.76–1.16) | |
| Post-booster |
11 (8.68–13) n = 118 |
9.8 (7.97–12) n = 120 |
14 (11–16) n = 119 |
0.92 (0.68–1.23) | |
|
Anti-HBs (mIU/mL) |
Post-3rd dose |
290 (211–398) n = 65 |
332 (243–455) n = 66 |
268 (192–375) n = 60 |
1.15 (0.73–1.79) |
| Pre-booster |
60 (46–78) n = 102 |
79 (60–102) n = 101 |
92 (67–126) n = 107 |
1.31 (0.9–1.9) | |
| Post-booster |
2492 (1875–3312) n = 98 |
2408 (1804–3214) n = 95 |
3408 (94–100) n = 94 |
0.97 (0.64–1.45) | |
|
Poliovirus 1/Dilution Post-3rd dose |
Poliovirus 1 |
120 (96–150) n = 123 |
98 (79–123) n = 120 |
127 (101–159) n = 119 |
0.82 (0.6–1.12) |
| Poliovirus 2 |
92 (72–118) n = 123 |
75 (58–97) n = 120 |
114 (90–144) n = 117 |
0.82 (0.57–1.16) | |
| Poliovirus 3 |
320 (263–391) n = 123 |
279 (228–341) n = 120 |
398 (322–492) n = 117 |
0.87 (0.66–1.15) | |
Table 3. Geometric mean ELISA pertussis antigen antibody concentrations (95% CI).
| Study groups | Difference between 4CMenB+Para and 4CMenB (95% CI) | |||
|---|---|---|---|---|
| 4CMenB | 4CMenB + Para | Men C | ||
| Pertussis Toxoid | ||||
| Post-3rd dose |
66 (60–72) n = 140 |
67 (61–74) n = 135 |
70 (63–78) n = 132 |
1.02 (0.89–1.16) |
| Pre-booster |
11 (9.7–13) n = 119 |
11 (9.9–13) n = 115 |
10 8.8–12) n = 131 |
1.02 (0.85–1.23) |
| Post-booster |
104 (92–119) n = 118 |
106 (94–121) n = 120 |
111 (97–127) n = 119 |
1.02 (0.85–1.23) |
| Filamentous hemagglutinin | ||||
| Post-3rd dose |
73 (66–81) n = 140 |
72 (65–80) n = 135 |
77 (69–86) n = 132 |
0.99 (0.85–1.14) |
| Pre-booster |
23 (20–26) n = 119 |
23 (21–27) n = 115 |
23 (21–27) n = 131 |
1.02 (0.85–1.22) |
| Post-booster |
197 (173–224) n = 118 |
209 (184–238) n = 120 |
224 (194–258) n = 119 |
1.06 (0.89–1.28) |
| Pertactin | ||||
| Post-3rd dose |
114 (97–133) n = 140 |
102 (87–120) n = 135 |
138 (122–156) n = 132 |
0.9 (0.72–1.13) |
| Pre-booster |
16 (13–19) n = 119 |
15 (12–17) n = 115 |
18 (16–21) n = 131 |
0.92 (0.72–1.17) |
| Post-booster |
193 (163–228) n = 118 |
174 (147–206) n = 120 |
275 (233–325) n = 119 |
0.9 (0.71–1.15) |
Routine vaccines—pneumococcal vaccines serotypes
Before vaccination proportions of infants with ELISA antibody levels ≥0.35 µg/mL against pneumococcal vaccine serotypes were similar in all 3 groups, with any differences being ≤5% (Supplementary Tables). One month after the third primary dose, antibody levels ≥0.35 µg/mL were achieved in 91–100% of infants across groups against 6 pneumococcal serotypes (4, 9V, 14, 18C, 19F, and 23F), and 76–81% against serotype 6B. One month after the booster dose 93–99% of toddlers achieved the threshold of 1.0 µg/mL against all 7 serotypes.
For pneumococcal antigens the range of GMC ratios was 0.79 (serotype 14) to 0.92 (serotype 9V), with the lower limit of the 95% confidence interval of the ratio ≥0.5 for all comparisons (Supplementary Tables). Following the booster dose of these vaccines, the booster responses to all antigens resulted in a range of ratios from 0.76 (pneumococcal serotype 19F) to 1.06 (pertussis FHA) with lower limit of the 95% CI of the ratio again ≥0.5 for all comparisons.
Reactogenicity
Almost every infant was reported to have some type of adverse reaction within 7 d of vaccination across the study: in the 4CMenB group 98%, 96%, and 92% of vaccinations 1, 2, and 3 were associated with reactions, respectively. Rates at similar study time points in 4CMenB+paracetamol and MenC groups were 99%, 100% and 96%, and 90%, 84% and 79%, respectively. At 12 mo, 96%, 96%, and 94% of the 3 groups displayed at least one reaction. The majority of reactions were mild to moderate in severity (Table 4 and Table 5), and were of limited duration.
Table 4. Percentages of infants experiencing any (severe in parentheses) solicited local reactions within 1 wk of vaccination at the injections sites of 4CMenB or MenC, DTaP-HBV-IPV/Hib, and pneumococcal conjugate vaccine (PCV7) for the 4 doses of each.
| Injection site | 4CMenB (or MenC) | DTaP-HBV-IPV/Hib | PCV7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th |
| 4CMenB, N | 182 | 182 | 181 | 155 | 182 | 182 | 181 | 155 | 182 | 182 | 181 | 155 |
| Tenderness (*) | 63 (15) | 66 (9) | 56 (9) | 75 (20) | 55 (13) | 59 (11) | 51 (7) | 65 (18) | 51 (11) | 55 (10) | 51 (7) | 67 (17) |
| Erythema (>50 mm) | 59 (2) | 57 (0) | 61 (1) | 58 (4) | 42 (0) | 51 (0) | 55 (0) | 50 (1) | 37 (0) | 49 (0) | 48 (0) | 48 (1) |
| Induration (>50 mm) | 55 (1) | 57 (0) | 54 (0) | 47 (2) | 41 (0) | 48 (0) | 48 (0) | 35 (1) | 29 (0) | 36 (0) | 33 (0) | 35 (1) |
| Swelling (>50 mm) | 32 (3) | 35 (1) | 31 (1) | 35 (5) | 21 (1) | 27 (0) | 27 (1) | 24 (2) | 17 (1) | 23 (0) | 22 (0) | 25 (1) |
| 4CMenB+Para, N | 179 | 179 | 179 | 159 | 179 | 179 | 179 | 159 | 179 | 179 | 179 | 159 |
| Tenderness (*) | 44 (4) | 47 (4) | 37 (4) | 58 (14) | 35 (6) | 34 (3) | 32 (4) | 49 (11) | 33 (7) | 36 (3) | 30 (3) | 51 (11) |
| Erythema (>50 mm) | 41 (0) | 53 (0) | 51 (1) | 51 (3) | 29 (0) | 43 (0) | 44 (0) | 46 (0) | 22 (0) | 38 (0) | 35 (0) | 43 (0) |
| Induration (>50 mm) | 46 (0) | 44 (0) | 45 (0) | 38 (1) | 31 (0) | 34 (0) | 36 (0) | 35 (0) | 23 (0) | 28 (0) | 28 (0) | 33 (0) |
| Swelling (>50 mm) | 23 (0) | 29 (0) | 26 (1) | 30 (3) | 15 (0) | 20 (0) | 23 (0) | 21 (0) | 12 (0) | 18 (0) | 17 (0) | 21 (0) |
| MenC, N | 177 | 177 | 177 | 162 a | 177 | 177 | 177 | 162 | 177 | 177 | 177 | 162 |
| Tenderness (*) | 27 (2) | 27 (3) | 24 (1) | 67 (20) | 37 (3) | 32 (4) | 28 (2) | 60 (15) | 36 (3) | 34 (3) | 25 (2) | 59 (16) |
| Erythema (>50 mm) | 25 (0) | 25 (0) | 36 (0) | 51 (3) | 33 (1) | 46 (0) | 46 (0) | 50 (1) | 33 (1) | 41 (0) | 40 (0) | 48 (1) |
| Induration (>50 mm) | 25 (14) | 21 (0) | 31 (0) | 38 (0) | 31 (0) | 46 (0) | 45 (0) | 44 (1) | 27 (0) | 34 (0) | 34 (0) | 35 (1) |
| Swelling (>50 mm) | 8 (0) | 12 (0) | 16 (0) | 24 (1) | 16 (1) | 23 (1) | 22 (0) | 26 (1) | 11 (1) | 18 (0) | 17 (0) | 20 (1) |
(*)Cried when injected limb was moved. aFirst toddler dose of 4CMenB.
Table 5. Percentages of infants experiencing any (severe in parentheses) solicited systemic reactions within 1 wk of vaccination in the 3 study groups for the 4-vaccination series. Maximum rectal temperature is shown according to the Brighton Collaboration guidelines.
| Study group | 4CMenB | 4CMenB+Paracetamol | MenC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th | 1st | 2nd | 3rd | 4th* |
| n = | 182 | 182 | 181 | 155 | 182 | 182 | 181 | 155 | 182 | 182 | 181 | 155 |
| Change in eating habits | 42 (2) | 34 (2) | 27 (1) | 48 (3) | 37 (3) | 30 (2) | 27 (2) | 42 (3) | 23 (2) | 19 (1) | 12 (1) | 36 (4) |
| Sleepiness | 66 (3) | 58 (1) | 41 (1) | 49 (1) | 65 (2) | 47 (1) | 42 (1) | 50 (3) | 52 (1) | 47 (1) | 42 (1) | 52 (4) |
| Vomiting | 13 (0) | 10 (0) | 4 (1) | 4 (0) | 12 (1) | 11 (0) | 11 (1) | 13 (2) | 10 (0) | 8 (1) | 6 (1) | 6 (0) |
| Diarrhea | 31 (0) | 24 (1) | 18 (0) | 19 (1) | 23 (0) | 22 (1) | 18 (0) | 19 (1) | 25 (0) | 22 (1) | 11 (0) | 16 (0) |
| Irritability | 70 (5) | 71 (7) | 64 (5) | 75 (6) | 54 (1) | 55 (5) | 47 (2) | 60 (3) | 45 (1) | 46 (2) | 43 (1) | 72 (6) |
| Unusual crying | 52 (5) | 49 (6) | 42 (3) | 48 (3) | 41 (2) | 40 (4) | 26 (3) | 31 (3) | 36 (1) | 25 (2) | 21 (0) | 45 (4) |
| Rash (Urticarial) | 3 (1) | 2 (2) | 1 (0) | 3 (2) | 3 (2) | 3 (0) | 3 (2) | 3 (3) | 2 (1) | 3 (1) | 3 (2) | 4 (2) |
| Max. rectal temperature | ||||||||||||
| <38.0 °C | 29 | 23 | 43 | 30 | 47 | 43 | 63 | 41 | 76 | 61 | 74 | 31 |
| 38.0°–38.9 °C | 49 | 62 | 50 | 50 | 48 | 51 | 34 | 50 | 23 | 34 | 23 | 46 |
| 39.0°–39.9 °C | 20 | 15 | 7 | 19 | 4 | 5 | 3 | 10 | 1 | 4 | 1 | 19 |
| ³40 °C | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 |
| Medically attended fever (within 3 d) | 4 (2) | 1 (1) | 0 | 1 (1) | 1 (1) | 0 | 1 (1) | 0 | 2 (1) | 1 (1) | 1 (1) | 3 (2) |
First toddler dose of 4CMenB
Solicited reactions—local
The rate of injection site reactions with 4CMenB was twice that of the MenC vaccine during the primary series, but the difference was less when compared with the routine vaccines, DTaP-HBV-IPV/Hib and PCV7 (Table 4). Prophylactic paracetamol diminished the frequency and severity of all local reactions for all vaccines. The most frequent reaction, tenderness, occurred in 63%, 66%, and 56% of infants at the 4CMenB injection site after doses 1, 2, and 3, respectively, and decreased to 44%, 47%, and 37%, respectively, when prophylactic paracetamol was administered. When concomitantly administered in the opposite limb to 4CMenB at doses 1, 2, and 3, the tenderness rates at the DTaP-HBV-IPV/Hib (range: 51–59%) and PCV7 (range: 51–55%) sites were also decreased by paracetamol prophylaxis, to 32–35% and 30–36%, respectively.
Rates of local reaction rates were generally consistent with subsequent doses in infants, booster doses at 12 mo of age eliciting slightly higher levels. The first dose of 4CMenB administered to the children who received 3 MenC vaccinations had a similar local reaction profile to the 4CMenB-primed children.
Solicited reactions—systemic
A solicited systemic reaction was reported for 92% and 84% (first vaccinations, P < 0.05), 92% and 82% (second vaccinations, P < 0.01), and 81% and 71% (third vaccinations, P < 0.05) of infants in the 4CMenB and 4CMenB+ paracetamol groups, respectively. The Men C group had lower rates at 77%, 71%, and 60%, respectively. The most frequently reported systemic reactions were sleepiness and irritability (Table 5). Severe systemic reactions were rare, and mainly consisted of severe irritability or unusual crying.
Figure 3 clearly illustrates that the main intention of paracetamol prophylaxis, a reduction in the incidence of fever ≥38.5 °C within 3 d of vaccination, was achieved. In the 4CMenB group, 128 of 182 (70.3%) of infants had a rectal temperature ≥38.5 °C at least once in the first 3 d after any primary dose, compared with 48 of the 177 (27.1%) in the Men C group. This increased fever profile with 4CMenB is similar to that observed when compared with routine vaccines alone observed in other studies. In the prophylactic paracetamol group, the incidence was reduced considerably, to 70 of 179 (39.1%). When assessed according to the Brighton Collaboration guidelines,18 there were decreases in fever between 38.0–38.9 °C, but the most striking impact of paracetamol was on incidence of fever ≥39.0 °C (Table 5). Fever ≥40.0 °C was rare in all 3 groups.
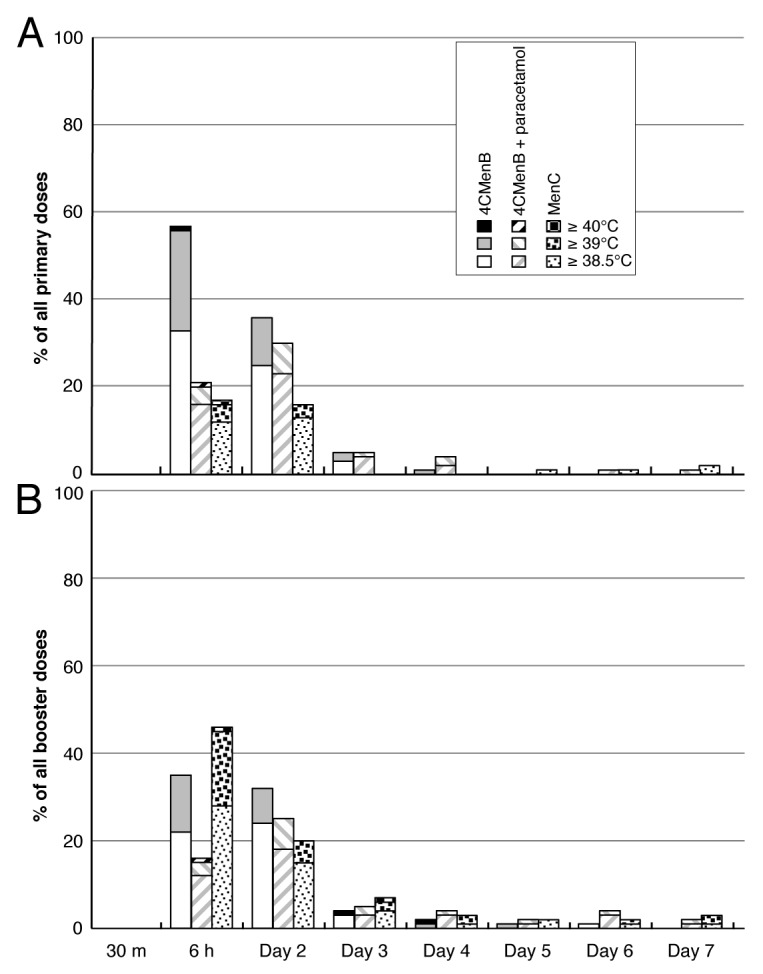
Figure 3. Proportions of subjects in each group with temperatures ≥ 38.5 °C for 7 d after any primary (A) or booster (B) vaccinations.
Medical attention for fever was uncommon, reported in 6 cases in the 4CMenB group, and 2 cases with prophylactic paracetamol. In the MenC group there were 4 cases of medical attention for fever during the 3-dose primary series, and 3 cases when the first dose of 4CMenB was given at 12 mo. In the 4CMenB group receiving routine paracetamol administration 56%, 55%, 36%, and 54% of subjects received some antipyretic after the first, second, third, and fourth vaccination visits, respectively, compared with 18%, 22%, and 11% in the MenC group after their first 3 vaccination visits, respectively. After their fourth visit, when the MenC group received a first dose of 4CMenB 57% received some anti-pyretic.
Spontaneously reported AEs
Overall rates of AEs were similar in the 3 groups; there were 157 AE’s reported in the 4CMenB group (in 85% of subjects) of which 76 (41%) were considered as possibly related to vaccination. Equivalent rates in the 4CMenB+paracetamol group were 80% (37% possibly related) and in the MenC group 73% (28% possibly related). The possibly related AEs were generally injection site reactions that extended beyond 7 d and by sponsor convention were considered therefore unsolicited reactions. Across all 3 groups there were 42 serious adverse events (SAE) reported, none of which were considered as possibly related to vaccination, and there were no seizures (febrile or afebrile), deaths or study withdrawals reported due to a vaccination-related AE.
Discussion
Our results from part of a phase 2, randomized, clinical trial show that prophylactic paracetamol in infants decreases fever and reactogenicity with no apparent clinically relevant impact on immune responses to the multicomponent meningococcal serogroup B vaccine (4CMenB), nor the concomitantly administered routine vaccinations (DTaP-HBV-IPV/Hib and PCV7). The administration of oral paracetamol at the time of vaccination, with 2 subsequent doses at 4–6 h intervals, significantly reduced the incidence of febrile reactions ≥38.5 °C over 7 d post-vaccination, and fewer infants experienced solicited local reactions. The proportion of infants experiencing any fever was lowered by 51–65% by paracetamol prophylaxis, and reports of rectal temperature >39.5 °C after any the 3-dose primary series were noticeably less common, i.e., 1.1% with paracetamol prophylaxis vs. 3–5% with no prophylaxis, representing an up to 80% decrease (after the first set of vaccinations). Similar decreases were observed after the booster dose at 12 mo. Medically attended visits for fever were rare, and the overall rate of unsolicited events did not differ between groups, but there was a trend toward fewer spontaneously reported AEs for the prophylactic paracetamol group within 3 d post-vaccination.
The beneficial effect of prophylactic paracetamol on the incidence of fever and common adverse reactions following pediatric immunization has already been demonstrated in clinical trials performed in the 1980s.4,5 Results from a recent trial in the US also found a significantly lower frequency of temperature ≥38 °C among infants aged between 24 wk and 10 mo given prophylactic paracetamol (13% in the prophylactic paracetamol group vs. 25% in the control group) following routinely recommended vaccinations.6 In addition, a trial of paracetamol prophylaxis in infants receiving 10-valent pneumococcal non-typeable Hemophilus influenzae protein D-conjugate vaccine (PHiD-CV) co-administered with a combination DTaP-HBV-IPV-Hib vaccine and oral human rotavirus vaccine reported that rectal temperature ≥38 °C within 4 d post-vaccination was significantly less frequent in the group with paracetamol prophylaxis (42%) than the no paracetamol group (66%).7 In this latter trial, however, an unexpected negative effect of paracetamol prophylaxis was observed on antibody responses to several vaccine antigens.7 In that study, conducted in many of the same sites as the current study, the point estimates of the GMC ratios of the pneumococcal antigens with and without paracetamol prophylaxis after primary vaccinations ranged from 0.45 for serotype 18C to 0.73 for serotype 7, and similar decreases were observed for other routine vaccine antigens; Hib (0.53), diphtheria (0.75), tetanus (0.61), and pertactin (0.76). Based on the lack of clear clinical benefit for use of prophylaxis in that setting and unknown clinical relevance of the decreased immune responses, the authors and accompanying editorial comment argued against the routine use of paracetamol during pediatric immunisations.7,19 Subsequently, the same authors found that this concomitant use of paracetamol had no effect on memory and nasopharyngeal carriage of pneumococcal species in those children at 4 y of age.10
In our study the proportions of infants with bactericidal antibodies against MenB strains and responses to routine vaccine antigens following a 4-dose series at 2, 3, 4, and 12 mo did not differ substantially between the groups with or without prophylactic paracetamol, when assessed after the primary or booster doses. Some trends observed in the GMC ratios in the pneumococcal responses suggest an impact, but the responses for both groups were similarly robust. For all serotypes the lower limit of the GMC ratio was higher than 0.5 (Supplementary Tables), the generally accepted threshold for non-inferiority analyses, although this does not definitively exclude an effect on protection. A clinical consequence of slightly lower anti-polio GMTs (PP:control ratios of 0.81–0.86) is unlikely in view of the known 19–35-fold increase in titers after booster IPV vaccination in the second year of life.20 Several exploratory analyses were performed to try to understand a reason for the different outcomes in the studies. Sub-analyses of the effect of prophylactic paracetamol just within the sites in the Czech Republic, where the PHiD-CV study was performed,7 did not show any meaningful differences vs. the larger study population (data not shown). There was no evidence of a “dose response” of the number of days receiving anti-pyretics when comparing those receiving no antipyretics vs. 1–3 d with antipyretics vs. 4–7 d with antipyretics. To our knowledge, only 2 other previously published trials, from the 1980s/90s, reported the effects of antipyretic drugs on vaccine responses in children and both found that the use of paracetamol did not interfere with antibody responses in young children receiving DTPw vaccine.8,9 Therefore, the recently reported negative effect of prophylactic paracetamol on postimmunisation immune responses is in contrast to the results from other studies and shows much higher magnitude of negative impact comparing with our study.
This study and the recent study by Prymula et al. on PHiD-CV7 were similar in design and in terms of strengths and weaknesses. Both were controlled, multicenter studies enrolling a comparable number of infants and at least partly randomized, but there were several differences in the study design: (1) different vaccination schedule (3, 4, and 5 mo of age vs. the 2, 3, and 4 mo of age in this study); (2) paracetamol administered via rectal route vs. oral route in this study, (3) use of a 10-valent pneumococcal conjugate vaccine (Synflorix®) vs. the 7-valent (Prevenar®) used in this study, and (4) concomitant administration of the novel 4CMenB meningococcal vaccine in the present study. Further limitations of the present study include the use of paracetamol being unblinded, and that therapeutic use of antipyretic drugs was discouraged but allowed at the discretion of the child’s parents or study physician in both groups. In the present study, a relatively high proportion in the control group used antipyretics therapeutically (36–55% across vaccine doses), the previous study reporting that therapeutic paracetamol was only administered after 9.2% of doses.7
Although pediatric paracetamol is generally considered to be safe when administered in the appropriate dosage, the unnecessary use of any pharmaceutical is to be discouraged. As noted by the American Academy of Physicians and other recommending bodies,23 parents of vaccinated children should be encouraged to ensure that their child is comforted in general, rather than focus on the management of any fever, which in itself is not an illness, but a physiological signal that the body is responding to an illness or other stimuli. Our data further confirm our previous observations15 that fever post-vaccination with 4CMenB is transient, and the majority of cases resolve within 1–2 d of the vaccination visit, but in addition, if parents do administer paracetamol, the expected immune responses will be generated to routine infant vaccines.
The development of an effective vaccine against MenB is a challenge due to the poor immunogenic potential of the capsular polysaccharide and the strain limitations of the outer membrane vesicles (OMV).21,22 Our results show that concomitant administration of the meningococcal serogroup B vaccine, 4CMenB, with routine immunizations induced large antibody responses in infants when administered as 3 doses at 2, 3, and 4 mo of age, eliciting hSBA titers ≥ 5 in 75–100% of subjects against the 3 reference strains, with or without prophylactic paracetamol. Robust immune responses were observed for all vaccine antigens of the concomitantly administered DTaP-HBV-IPV/Hib and pneumococcal vaccines. These responses were boosted by a fourth dose of 4CMenB at 12 mo, when the expected responses to booster doses of the routine vaccines were also observed.
Generally 4CMenB had acceptable reactogenicity; although we saw an increased frequency of local solicited reactions following vaccination with the 4CMenB compared with routine immunizations, consistent with the known reactogenicity of OMV-containing vaccines.24-26 However, all reactions were transient, mild to moderate in severity, and were less common after administration of prophylactic paracetamol. Results from this study are consistent with the results from other phase 2/3 trials of 4CMenB,11-15 which ultimately led to its approval in Europe and Australia.
In conclusion, these results from a phase 2 randomized clinical trial showed that prophylactic paracetamol in infants effectively decreased fever and reactogenicity without compromising immune responses following concomitant vaccination of a novel, multicomponent meningococcal serogroup B vaccine with routine immunizations. These results may be of topical interest as 4CMenB is now approved in Europe and Australia, where it may be widely used to meet the currently unmet need for vaccination against meningococcal serogroup B.21 Physicians may be reassured that in the context of 4CMenB administration, the use of paracetamol prophylaxis has no apparent clinically relevant effect on immunogenicity, while helping to allay parental fears of fever in children after concomitant vaccination with 4CMenB with routine infant vaccinations.
Methods
Study design and participants
This was a phase 2, randomized, controlled, multicenter trial, with booster phase, conducted in multiple centers in the Czech Republic (79), Italy (6), Hungary (8), Chile (1), and Argentina (1) between July 2009 and November 2010 to assess the effects of different formulations of a meningococcal serogroup B vaccine on safety and immunogenicity profiles. The study was designed according to Good Clinical Practice and the Declaration of Helsinki, with approval of the protocol by ethics committees of participating centers, and prior to enrolment, written, informed consent from the parents or guardian of each participant.
The full study included 8 study groups, including 2 groups described in this report who received the final 4CMenB formulation recently licensed for use in Europe (Novartis Vaccines, Rosia, Italy) 1 of which was designed to assess the impact of prophylactic administration of paracetamol, and a third group who received a licensed meningococcal serogroup C vaccine rather than 4CMenB vaccine. Other groups, as described in an accompanying report,16 received experimental formulations of the serogroup B vaccine. All 3 groups received 4CMenB or MenC administered concomitantly with routine infant vaccines (DTaP-HBV-IPV/Hib and heptavalent pneumococcal conjugate [PCV7]), and were assessed for reactogenicity, including body temperature, and the immunogenicity of antigen components of all vaccines.
Study participants were healthy infants aged 2 mo (55–89 d, inclusive) at the time of enrolment. Infants were excluded if they had been in receipt of any antipyretic medication in the previous 6 h or if they had a contraindication to paracetamol treatment. Other exclusion criteria were any history of disease caused by N. meningitidis or intimate exposure to an individual with laboratory confirmed N. meningitidis, or prior receipt of any meningococcal B or C, diphtheria, tetanus, pertussis, polio, Hib, or pneumococcal vaccines; significant acute or chronic infection within the previous 7 d or temperature ≥38 °C within the previous day; oral or parenteral antibiotic treatment in the 7 d prior to the scheduled blood draw; impairment/alteration of the immune system; receipt of blood, blood products and/or plasma derivatives or any parenteral immunoglobulin preparation; receipt of, or intent to immunize with any other licensed vaccine(s) (with the exception of rotavirus vaccines), from 28 d prior to enrolment to 28 d after the last study vaccination and; participation in another clinical trial.
For the booster phase, eligible subjects had completed the original primary series and had not displayed any of the exclusion criteria during the course of the primary study.
Vaccines
The Novartis meningococcal serogroup B vaccine (4CMenB; Bexsero®, Novartis Vaccines) was supplied in 0.5 mL doses in pre-filled syringes, containing 50µg each of 3 recombinant protein antigens—factor H binding protein (fHbp), Neisserial adhesin A (NadA), and Neisseria heparin binding antigen, and 25µg OMV from N. meningitidis strain NZ98/254, with 0.5 mg of Al3+ in the form of Al(OH)3 in 10 mM histidine/saline buffer.11-15
Each dose of DTaP-HBV-IPV/Hib vaccine (Infanrix hexa®; GlaxoSmithKline Biologicals) contained ≥30 IU diphtheria toxoid; ≥ 40 IU tetanus toxoid; 25 μg pertussis toxin (PT); 25 μg filamentous haemagglutinin (FHA); 8 μg pertactin (PRN); 10 μg recombinant hepatitis B surface antigen (HBsAg); 40D, 8D, and 32D antigen units of poliovirus types 1, 2, and 3, respectively; and 10 μg Hemophilus influenzae type b polyribosyl-ribitol-phosphate conjugated to tetanus toxoid (Hib). The vaccine was reconstituted by extemporaneous mixing the lyophilised Hib component with the liquid DTaP-HBV-IPV immediately prior to injection.
Each 0.5 ml dose of the PCV7 vaccine (Prevenar®; Wyeth) contained pneumococcal polysaccharide serotypes 4, 9V, 14, 18C, 19F, 23F (2 µg of each) and pneumococcal polysaccharide serotype 6B (4 µg). Each dose of the Men C vaccine, Menjugate® (Novartis Vaccines), was supplied as a lyophilised powder that was resuspended in phosphate-buffered saline just before use, to provide 10 µg MenC oligosaccharide conjugated to 12.5–25 µg CRM197 per 0.5 mL dose.
Randomization and procedures
In the full study local investigators enrolled infants who were randomly assigned using a web-based randomization system (1:1:1:1:1:1:1:1 ratio, block size = 8) supplied by the study sponsor into one of 8 study groups. Of the 2 groups who received the final 4CMenB formulation the first, the control group, received concomitant 4CMenB, DTaP-HBV-IPV/Hib, and PCV7 vaccines. The second group, the prophylactic paracetamol group, received the same vaccines with paracetamol administered prophylactically just before or at the time of vaccination. This was followed by 2 further administrations at 4–6 h intervals after vaccination by the parents/guardians. Paracetamol was supplied as a pediatric liquid formulation for oral administration at a dose of 10–15 mg/kg. The third group received 3 doses of meningococcal glycoconjugate serogroup C vaccine instead of 4CMenB. For the control and MenC groups, the use of prophylactic antipyretics in anticipation of fever or discomfort associated with the injections was strongly discouraged by the study personnel. If antipyretics were given therapeutically for treatment of fever, parents were instructed to take the temperature 4 h after the antipyretic dose to see if further treatment was necessary, but this was not required to recorded in in a systematic manner. As the control group did not receive a placebo drug the paracetamol treatment was not blinded and the investigators and parents were aware of the prophylactic antipyretic treatment assignment.
During the primary phase, 3 doses of each of the 3 respective vaccines were administered by intramuscular injection at 2, 3, and 4 mo of age; 4CMenB or MenC was administered intramuscularly into the right thigh, and DTaP-HBV-IPV/Hib and PCV7 vaccines were both administered intramuscularly into the left thigh at least 2.5 cm apart. At 12 mo of age, all groups received fourth doses of the routine vaccines, and the 4CMenB and 4CMenB+paracetamol groups received fourth doses of 4CMenB. The paracetamol group received the prophylactic paracetamol at each of the 4 4CMenB vaccinations. The MenC group received 2 doses of 4CMenB, the recommended “toddler catch-up” schedule, at 12 and 13 mo of age. At 13 mo of age a booster dose of MenC was offered to the MenC group, and all participants were offered a first dose of MenC at this time-point.
Blood samples were collected from all subjects immediately prior to the first primary dose, 30 d following the third dose, and before and 30 d after the booster dose for immunogenicity assessments. Sera were analyzed using validated methods at the Novartis laboratory (Clinical Laboratory Science, Novartis Vaccines, Marburg, Germany) or a designated contract laboratory. Immune responses to 4CMenB were assessed as serum bactericidal activity using human complement (hSBA) against 3 N. meningitidis test strains H44/76, 5/99 and NZ98/254, specific for 3 of the vaccine components (fHbp, NadA, and NZOMV, respectively), using a titer ≥ 5 as the lower limit of quantitation (LLQ) which represents with 95% confidence the traditional threshold hSBA titer (≥4) which is considered an indicator for protection.17
Immune responses to routine vaccines were assessed by standard ELISA or neutralization test methods. Seroprotection was defined as an antibody concentration ≥0.1 IU/mL for diphtheria and tetanus, 10 mIU/mL for hepatitis B, 1:8 dilution for poliovirus types 1, 2, and 3 and 0.35 µg/mL for pneumococcal serotypes 4, 6B, 9V, 14, 18C, 19F, 23F. For pertussis antigens, for which no serologic correlate of protection is established, a vaccine response was defined as an antibody concentration equal to or above the assay LLQ for initially seronegative subjects (pre-vaccination antibody concentration < LLQ), or at least maintenance of pre-vaccination antibody concentration for initially seropositive subjects (pre-vaccination antibody concentration ≥ LLQ), to account for loss of maternal antibodies. Percentages of subjects demonstrating seroprotection or vaccine responses, and geometric mean concentrations (GMC) or titers (GMT), as appropriate, were calculated.
In view of the blood small volumes available from infants, which may preclude performing all analyses on each individual serum sample, priority was given to analyses of 4CMenB antigen responses, and then concomitant vaccine antigens in this order: diphtheria-tetanus-acellular pertussis, Hib, pneumococcal, poliovirus, hepatitis B. Polio responses were only measured after primary vaccination.
Safety and reactogenicity
Subjects were observed for a minimum of 30 min after receipt of each vaccine dose to monitor for immediate adverse reactions, including a rectal temperature measurement. Parents then recorded on diary cards solicited reactions for 7 d post-vaccination. Solicited local reactions (i.e., injection site tenderness, erythema, induration, and swelling) and systemic reactions (i.e., change in eating habits, sleepiness, vomiting, diarrhea, irritability, unusual crying, and urticarial or other rash). Other indicators of reactogenicity were body temperature measured as rectal temperature with supplied study thermometers, (fever defined as rectal temperature ≥38.5 °C), and the use of antipyretic medication. Unsolicited adverse events (AEs) and serious adverse events (SAEs) were recorded throughout the study. Assessments of the causal relationship of adverse events to the vaccination were made by the investigators and study physicians (most conservative selected) and were classified as not related, possibly related, or probably related.
Statistical analyses
Immunogenicity analyses were performed on the per-protocol population, which consisted of subjects who received all the relevant doses of vaccine correctly, provided at least one evaluable serum sample at the relevant time points, and had no major protocol violations. Safety was analyzed for all subjects exposed to study vaccines. A minimum sample size of 140 subjects per group was estimated to provide sufficient power to detect differences between groups in antibody responses to the MenB strains. Population characteristics were summarized per vaccine group. The percentages of subjects with hSBA titers ≥ 5 against N. meningitidis serogroup B strains H44/76, NZ 98/254, and 5/99 and associated 2-sided 95% Clopper-Pearson Confidence Intervals (CIs) were computed for each group at baseline and 1 mo after the third vaccination. Percentages of subjects with antibody titers above pre-specified cut-offs for all antigens of the DTaP-HBV-IPV/Hib and PCV7 vaccines were calculated at baseline and 1 mo after the third vaccination. Differences between groups were calculated using the Miettinen-Nurminen method.27 Log10-transformed antibody titers were modeled using analysis of variance for each strain, and GMTs, GMCs, their ratios, and corresponding 2-sided 95% CI were calculated. Febrile rates per vaccine dose and group were summarized. Percentages of subjects with fever within 3 d (day 1–3) and 7 d (day 1–7) after each vaccination were tabulated by vaccine group and pair-wise comparisons were performed using the Pearson chi-square test, or the Fisher Exact test where appropriate. Safety and tolerability data were summarized by vaccine group providing the percentage of subjects reporting an event. For the analysis of group differences, a 2-sided P value less than 0.05 was required.
Supplementary Material
Disclosure of Potential Conflicts of Interest
R.P. has received research grants and honoraria from Novartis, GSK, Pfizer, Baxter and Sanofi Pasteur. S.E. has received research grants from Crucell, GSK, and Pfizer, and honoraria for consultancy work with Novartis and GSK. G.V.Z. received study fees from Novartis. F.X., D.T., I.K., and P.D. are full-time employees of Novartis Vaccines and Diagnostics.
Funding
The study was fully financed by Novartis Vaccine and Diagnostics. The study sponsor designed the study with the study investigators, drafted the protocol, supplied all materials, analyzed sera, collated data, and performed all statistical analyses
Acknowledgments
The authors are grateful to all the parents who volunteered their children to participate in this clinical trial, all the investigator physicians from the various study sites in Argentina, Chile, Czech Republic, Hungary, and Italy, and Drs Valentina Montinaro, Valentina Preti, Fabio Meneghin, and Francesca Penagini (all from the University of Milan, Italy) for their assistance in performing the study. The authors are also grateful to Dr Keith Veitch (keithveitch communications, Amsterdam, Netherlands) for preparing an initial draft manuscript and providing subsequent editorial assistance.
Author Contributions
R.P., S.E., G.V.Z., D.T., I.K., and P.D. participated in the conception and design of the trials. R.P., S.E., and G.V.Z. managed study sites and enrolled participants. D.T., I.K., and P.D. performed study management for the study sponsor, F.X. performed all statistical operations. All authors were involved in the interpretation of analyzed data and the decision to submit for publication, and commented and developed the manuscript from the initial draft.
Glossary
Abbreviations:
- 4CMenB
multicomponent meningococcal serogroup B vaccine
- hSBA
serum bactericidal activity with human complement
- DTwP
diphtheria-tetanus-whole cell pertussis vaccine
- DTaP
diphtheria-tetanus-acellular pertussis vaccine
- DTaP-HBV-IPV/Hib
diphtheria-tetanus-acellular pertussis-inactivated poliovirus-hepatitis B with Haemophilus influenzae type b combination vaccine
- PCV7
7-valent pneumococcal conjugate vaccine
- fHbp
factor H binding protein
- NadA
Neisserial adhesin A
- NZOMV
outer membrane vesicles from New Zealand serogroup B strain
References
- 1.Crocetti M, Moghbeli N, Serwint J. Fever phobia revisited: have parental misconceptions about fever changed in 20 years? Pediatrics. 2001;107:1241–6. doi: 10.1542/peds.107.6.1241. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan JE, Farrar HC, Section on Clinical Pharmacology and Therapeutics. Committee on Drugs Fever and antipyretic use in children. Pediatrics. 2011;127:580–7. doi: 10.1542/peds.2010-3852. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control (CDC) Pertussis immunization; family history of convulsions and use of antipyretics--supplementary ACIP statement. MMWR Morb Mortal Wkly Rep. 1987;36:281–2. [PubMed] [Google Scholar]
- 4.Ipp MM, Gold R, Greenberg S, Goldbach M, Kupfert BB, Lloyd DD, Maresky DC, Saunders N, Wise SA. Acetaminophen prophylaxis of adverse reactions following vaccination of infants with diphtheria-pertussis-tetanus toxoids-polio vaccine. Pediatr Infect Dis J. 1987;6:721–5. doi: 10.1097/00006454-198708000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lewis K, Cherry JD, Sachs MH, Woo DB, Hamilton RC, Tarle JM, Overturf GD. The effect of prophylactic acetaminophen administration on reactions to DTP vaccination. Am J Dis Child. 1988;142:62–5. doi: 10.1001/archpedi.1988.02150010072025. [DOI] [PubMed] [Google Scholar]
- 6.Jackson LA, Peterson D, Dunn J, Hambidge SJ, Dunstan M, Starkovich P, Yu O, Benoit J, Dominguez-Islas CP, Carste B, et al. A randomized placebo-controlled trial of acetaminophen for prevention of post-vaccination fever in infants. PLoS One. 2011;6:e20102. doi: 10.1371/journal.pone.0020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prymula R, Siegrist CA, Chlibek R, Zemlickova H, Vackova M, Smetana J, Lommel P, Kaliskova E, Borys D, Schuerman L. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: two open-label, randomised controlled trials. Lancet. 2009;374:1339–50. doi: 10.1016/S0140-6736(09)61208-3. [DOI] [PubMed] [Google Scholar]
- 8.Uhari M, Hietala J, Viljanen MK. Effect of prophylactic acetaminophen administration on reaction to DTP vaccination. Acta Paediatr Scand. 1988;77:747–51. doi: 10.1111/j.1651-2227.1988.tb10741.x. [DOI] [PubMed] [Google Scholar]
- 9.Long SS, Deforest A, Smith DG, Lazaro C, Wassilak GF. Longitudinal study of adverse reactions following diphtheria-tetanus-pertussis vaccine in infancy. Pediatrics. 1990;85:294–302. [PubMed] [Google Scholar]
- 10.Prymula R, Habib A, François N, Borys D, Schuerman L. Immunological memory and nasopharyngeal carriage in 4-year-old children previously primed and boosted with 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) with or without concomitant prophylactic paracetamol. Vaccine. 2013;31:2080–8. doi: 10.1016/j.vaccine.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 11.Halperin SA, Bettinger JA, Greenwood B, Harrison LH, Jelfs J, Ladhani SN, McIntyre P, Ramsay ME, Sáfadi MA. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(Suppl 2):B26–36. doi: 10.1016/j.vaccine.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Findlow J, Borrow R, Snape MD, Dawson T, Holland A, John TM, Evans A, Telford KL, Ypma E, Toneatto D, et al. Multicenter, open-label, randomized phase II controlled trial of an investigational recombinant Meningococcal serogroup B vaccine with and without outer membrane vesicles, administered in infancy. Clin Infect Dis. 2010;51:1127–37. doi: 10.1086/656741. [DOI] [PubMed] [Google Scholar]
- 13.Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, Findlow J, Yu LM, Borrow R, Ypma E, et al. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: a randomized comparative trial. Pediatr Infect Dis J. 2010;29:e71–9. doi: 10.1097/INF.0b013e3181f59f6d. [DOI] [PubMed] [Google Scholar]
- 14.Gossger N, Snape MD, Yu L-M, Finn A, Bona G, Esposito S, Principi N, Diez-Domingo J, Sokal E, Becker B, et al. Immunogenicity and tolerability of recombinant meningococcal serogroup B vaccine administered with or without routine infant vaccinations according to different immunization schedules: a randomized controlled trial. JAMA. 2012;307:573–82. doi: 10.1001/jama.2012.85. [DOI] [PubMed] [Google Scholar]
- 15.Vesikari T, Esposito S, Prymula R, Ypma E, Kohl I, Toneatto D, Dull P, Kimura A, EU Meningococcal B Infant Vaccine Study group Immunogenicity and safety of an investigational multicomponent, recombinant, meningococcal serogroup B vaccine (4CMenB) administered concomitantly with routine infant and child vaccinations: results of two randomised trials. Lancet. 2013;381:825–35. doi: 10.1016/S0140-6736(12)61961-8. [DOI] [PubMed] [Google Scholar]
- 16.Esposito S, Roman Prymula R, Zuccotti GV, Kittel C, Toneatto D, Kohl I, Dull P. A phase 2 randomised controlled trial of a multicomponent meningococcal serogroup B vaccine, 4CMenB, in infants (II): effects of variations of the OMV and protein content on immunogenicity and reactogenicity. Hum Vaccin Immunother. 2014 doi: 10.4161/hv.29218. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine. 2009;27(Suppl 2):B112–6. doi: 10.1016/j.vaccine.2009.04.065. [DOI] [PubMed] [Google Scholar]
- 18.Bonhoeffer J, Bentsi-Enchill A, Chen RT, Fisher MC, Gold MS, Hartman K, Heininger U, Hoet B, Jefferson T, Khuri-Bulos N, et al. Brighton Collaboration Methods Working Group Guidelines for collection, analysis and presentation of vaccine safety data in surveillance systems. Vaccine. 2009;27:2289–97. doi: 10.1016/j.vaccine.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 19.Chen RT, Clark TA, Halperin SA. The yin and yang of paracetamol and paediatric immunisations. Lancet. 2009;374:1305–6. doi: 10.1016/S0140-6736(09)61802-X. [DOI] [PubMed] [Google Scholar]
- 20.Heininger U, Sänger R, Jacquet J-M, Schuerman L, DTP-HBV-IPV-059 Study Group. DTP-HBV-IPV-096 Study Group Booster immunization with a hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus vaccine and Haemophilus influenzae type b conjugate combination vaccine in the second year of life: safety, immunogenicity and persistence of antibody responses. Vaccine. 2007;25:1055–63. doi: 10.1016/j.vaccine.2006.09.060. [DOI] [PubMed] [Google Scholar]
- 21.Tan LK, Carlone GM, Borrow R. Advances in the development of vaccines against Neisseria meningitidis. N Engl J Med. 2010;362:1511–20. doi: 10.1056/NEJMra0906357. [DOI] [PubMed] [Google Scholar]
- 22.Finne J, Bitter-Suermann D, Goridis C, Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol. 1987;138:4402–7. [PubMed] [Google Scholar]
- 23.Sullivan JE, Farrar HC, Section on Clinical Pharmacology and Therapeutics. Committee on Drugs Fever and antipyretic use in children. Pediatrics. 2011;127:580–7. doi: 10.1542/peds.2010-3852. [DOI] [PubMed] [Google Scholar]
- 24.Bjune G, Høiby EA, Grønnesby JK, Arnesen O, Fredriksen JH, Halstensen A, Holten E, Lindbak AK, Nøkleby H, Rosenqvist E, et al. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093–6. doi: 10.1016/0140-6736(91)91961-S. [DOI] [PubMed] [Google Scholar]
- 25.Holst J, Martin D, Arnold R, Huergo CC, Oster P, O’Hallahan J, Rosenqvist E. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine. 2009;27(Suppl 2):B3–12. doi: 10.1016/j.vaccine.2009.04.071. [DOI] [PubMed] [Google Scholar]
- 26.O’Hallahan J, McNicholas A, Galloway Y, O’Leary E, Roseveare C. Delivering a safe and effective strain-specific vaccine to control an epidemic of group B meningococcal disease. N Z Med J. 2009;122:48–59. [PubMed] [Google Scholar]
- 27.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–26. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.