Abstract
MENK, a penta-peptide is considered as being involved in the regulatory feedback loop between the immune and neuroendocrine systems, with marked modulation of various functions of human immune cells. The aim of the present work was to investigate change of lymphocyte subpopulations in peripheral blood of 50 cancer patients before and after treatment with MENK. Peripheral blood mononuclear cells (PBMCs) of peripheral blood from 50 cancer patients were isolated by density gradient centrifugation using Ficoll-Paque solution and cultured with MENK. We measured proliferation of total nucleated cells, subpopulations of individual CD4+T cells, CD8+T cells, CD4+CD25+ regulatory T cells (Treg), natural killer cells (NK) before and after treatment with 10-12M MENK in cell culture by flow cytometry (FCM). Our results indicated that MENK showed a strong inhibiting effect on Treg cells while it stimulated marked proliferation of other lymphocyte subpopulations. All data obtained were of significance statistically.
It was therefore concluded that MENK could work as a strong immune booster with great potential in restoring damaged human immune system and we could consider MENK as a drug to treat cancer patients, whose immune systems are damaged by chemotherapy or radiotherapy. Furthermore we could consider MENK as a chemotherapy additive, which would sustain immune system of cancer patients during the process of chemotherapy to get maximized efficacy with minimized side effect.
Keywords: methionine enkephalin, CD4+T cell, CD8+T cell, NK, CD4+CD25+ regulatory T cells, lymphocyte subpopulations, human peripheral blood
Introduction
There are increasing articles supporting that endogenous opioid peptides would be involved in the regulatory loop between the neuroendocrine and immune systems, and play a positive role in immune modulation. Several types of opioid receptors such as: mu, delta, and kappa receptors have been detected on the surface of various immune cells including T cells, NK cells, macrophages, and dendritic cells.1 MENK, composed of Tye-Gly-Gly-Phe-Met, is derived from pre-enkephalin and circulates in blood at low concentration in body.2 The data from both early documented studies3-5 and published results in our laboratory indicated that MENK at suitable range of concentrations could enhance activity of various types of immune cells, like: augmenting interactions between dendritic cells (DCs) and CD4+T cells, induction of phenotypic and functional maturation of DCs with increased antigen presentation, improvement of antitumor activity of DCs loaded with antigen, induction of macrophage polarization to the M1 phenotype in mouse model, eliciting CD8+T cell cytotoxicity, upregulating the secretion of cytokines such as IL-2, IL-12, IFN-γ, TNF-α, increasing the release of hydrogen peroxide and nitric oxide and stimulation of lymphocyte subpopulations in normal human donors.6-12 In addition, there have been studies indicating that MENK could inhibit tumor growth.13,14
However, so far there is little approach to the influence on lymphocyte subpopulations in large samples of cancer patients by MENK. Due to the importance of sustaining immunity in cancer patients and based on the data we have had on MENK we conducted the following approach to the clinical application of MENK for cancer therapy and immunotherapy.
Results
In general treatment of cancer patients with leukophoresis IL-2, anti-CD3 antibody, and TNF-α were used to stimulate proliferation of T cells and this method did induced proliferation of lymphocytes, however, when the huge number of expanded lymphocytes were infused back to patient body only limited efficacy was observed. We checked all subpopulations of lymphocytes and found that total Tregs were also increased. Probably that was the key to hinder the efficacy and this was why leukopharesis application in the past 30 y proceeded with no real progress.
Based on our previous discovery proving that MENK inhibited Tregs in human peripheral blood through opioid receptors and on our lot of published data on MENK we designed and developed this unique method with new mechanism to cancer immunotherapy. Our trial was a pioneer study.
After treatment with MENK for 7 d each lymphocyte subpopulation was checked with FCM. The concrete changes were as evidenced in following Table 1.
Table 1. Percentage of lymphocytes subpopulations.
| Before treatment | After treatment | P | |
|---|---|---|---|
| CD3+ T cell | 49.90 ± 4.06 | 76.04 ± 4.20 | P < 0.01 |
| CD8+ T cell | 33.33 ± 3.62 | 46.63 ± 4.78 | P < 0.01 |
| CD4+ T cell | 19.65 ± 3.48 | 33.81 ± 5.78 | P < 0.01 |
| NK cell | 6.98 ± 1.59 | 25.39 ± 4.42 | P < 0.01 |
| Treg cell | 4.62 ± 0.64 | 2.85 ± 0.48 | P < 0.01 |
| Total nucleated cell | 1.10 ± 0.13 | 4.01 ± 0.75 | P < 0.01 |
Table 1 Percentage of lymphocytes subpopulations
Clearly total lymphocytes were restored. If we compared these results before and after treatment by MENK statistically we could draw conclusion that total T cells were markedly increased (Figs. 1 and 2).
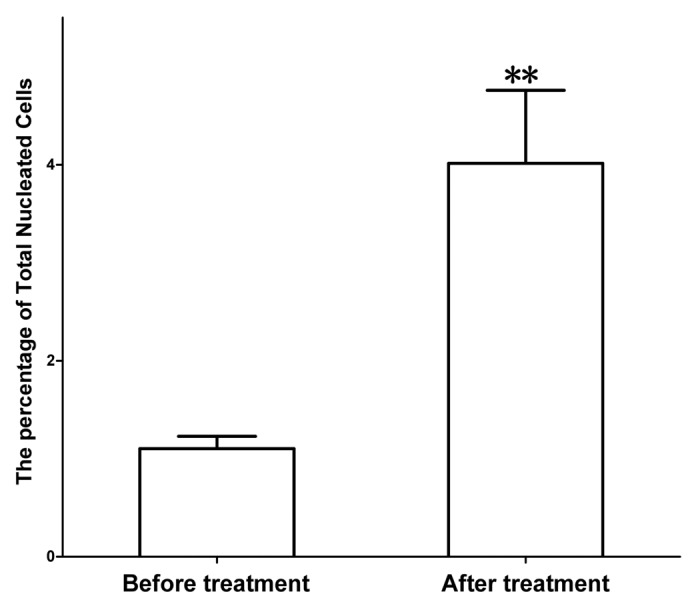
Figure 1. Proliferation of total nucleated cells (TNC) after treatment with MENK. Isolated TNC were cultured with MENK for 7 d in vitro and cell numbers were measured by FCM. The TNC grew into distinguished percentage as compared with that in control. We have proved there were more opioid receptors on T cell in our earlier publications and this was directly responsible for the proliferation of T cells because MENK binded to the receptors, triggering the response.
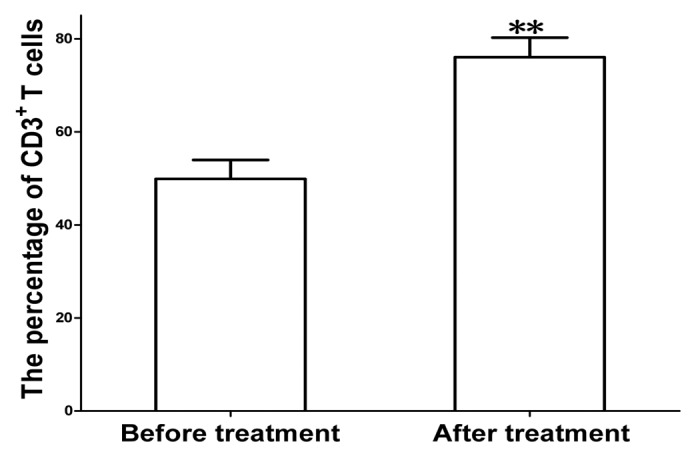
Figure 2. Proliferation of total CD3+ T cells after treatment with MENK. The cells were cultured with MENK for 7 d in vitro and cell numbers were measured by FCM. The cells grew into distinguished percentage. Especially the collected data indicated that CD4+T cells, CD8+T cells (Fig. 3), and NK cells (Fig. 4) were increased significantly, which were predominantly cell types for anti tumor activity were the cells of innate immune system. So these proved that MENK exerted a broad effect on the cells in both innate immune system and adaptive immune system and provided direct evidence of interaction between neuropeptide and immune system.
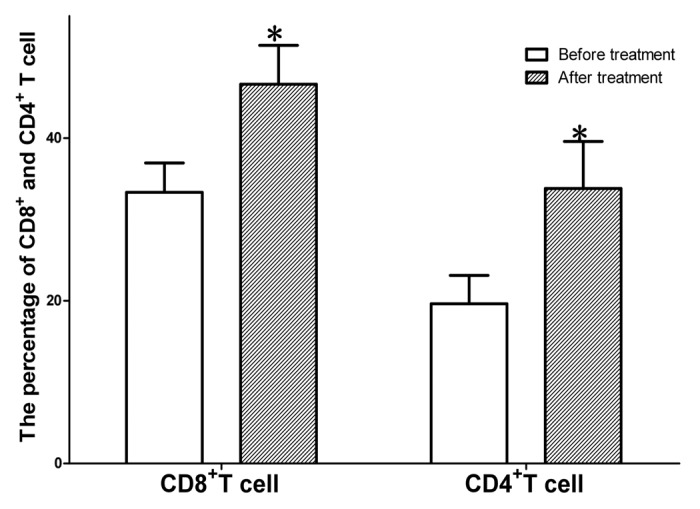
Figure 3. Proliferation of total CD4+ T cells /CD4+T cells after treatment with MENK. The peripheral cells were cultured with MENK for 7 d in vitro and cell numbers were measured by FCM. The cells grew into distinguished percentage.
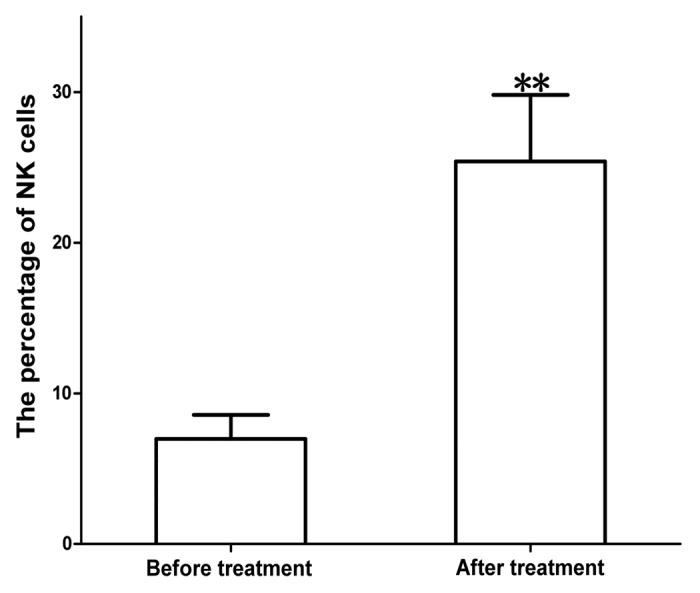
Figure 4. Proliferation of total NK cells after treatment with MENK.The cells were cultured with MENK for 7 d and cell number were measured by FCM. The cells grew into distinguished percentage. The most important finding in this study was that Tregs were downregulated markedly (Fig. 5). This was critical in cancer immunotherapy because in our previous study on cancer treatment we observed that in most cases, particularly in terminal cancer patients Tregs index were very high, which resulted in inhibition of activity of vast majority of immune cells. Inhibition of Tregs was our major target in this method and it was corresponding to the efficacy of treatment directly.
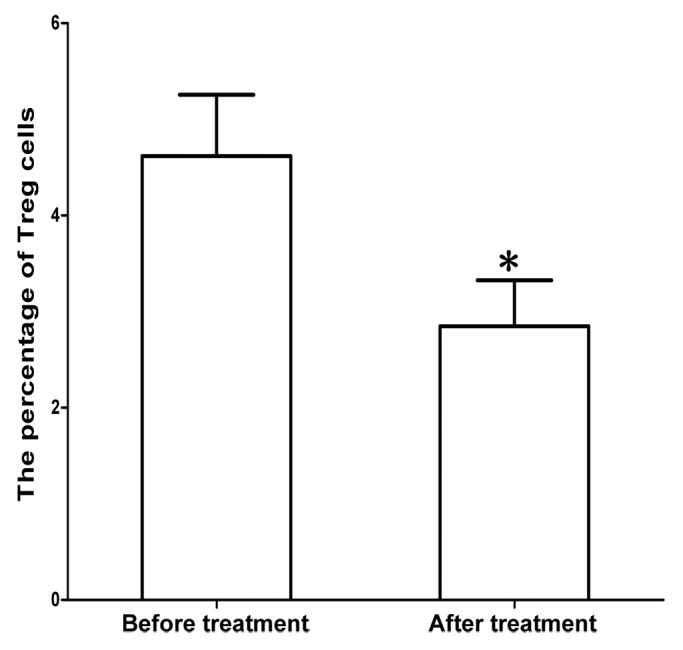
Figure 5. Inhibition of Treg cells after treatment with MENK.The Treg cells were cultured with MENK for 7 d and cell numbers were measured by FCM. Clearly cell growth was inhibited by MENK. Besides data above we compared cancer patient’s situation with number of Tregs and found the situation was closely related with number of Tregs in reverse relationship.
This was definitely due to the binding to the receptors on the surface of immune cells by MENK.
Discussion
MENK, as an important neuropeptide connecting endocrine and immune systems plays the modulating role coordinating and balancing 2 systems. Many results both from our laboratory and from other institutes on MENK’s function of upregulating immune system at suitable dose have been reported previously.15-21 Our documented record showed that considerable work of approaches with MENK on its impact on immune cells in vitro and in vivo has been done in our laboratory.7-11
There are predominantly mu, delta, and kappa—3 types of opioid receptors. The mu receptor is responsible for addiction and pain, while kappa and delta are responsible for immunity according to published data. The concentration of MENK we used was 10-12 M, the normal physiological concentration. So at this concentration MENK would bind to kappa or delta receptor on the immune cells rather than mu receptor, resulting in increased immunity and our previous exploration supported this conclusion.
We published an article on restore of immune system in 21 cancer patients with severely damaged immune systems by administration of MENK (in a Chinese journal). The present results showed MENK’s positive effect on the expansion of peripheral lymphocytes subpopulations in 50 cancer patients, which indicated the upregulating effect was marked, especially through the inhibition of Treg, which was of importance in cancer treatment. In addition the finding of MENK at used concentration could markedly stimulate the expansion of CD4+T cells, CD8+T cells, and NK cells would help understanding increased immunity.
The marked inhibition of the expansion of Tregs by MENK revealed a new mechanism by which MENK exerted positive regulation to the cells of immune system. Therefore this was unique highlight in this study.
The increased expansion of CD4+T cells, CD8+T cells, and NK cells under influence of MENK would work together to fight cancer cells by mounting innate immunity and adaptive immunity, so as to remove out or kill cancer cells. We do observed life prolongation in 50 cancer patients treated with MENK mediated leukopharesis. The vast majority of treated cancer patients felt much better with good appetite and weight gaining (under separate paper).
Tregs are a key component of the immune system, identified by scientists in recent years and they could suppress immune responses mounted by other cells. This is a necessary “self-check” built into the immune system to prevent body from getting excessive reactions causing autoimmune diseases. Regulatory T cells come in many forms with characteristic, being those that CD4+, CD25+, and Foxp3+ cells are responsible for maintaining immune tolerance, limiting body inflammatory diseases.22-26 However, Tregs also suppress beneficial response by restricting immunity like antitumor immunity, which is critical in cancer situation. Tregs identification is a key pace forward in the field of immunology and it directly provides a better understanding for T-cell-mediated immune response and immunosuppression. Although Tregs were identified for their potential to prevent organ-specific autoimmune disease at early time, continuously emerging evidence suggests that elevated Tregs play a key negative regulation in tumor related immunity and contribute to reason of tumor growth and progression, thereby exerting an important impact on the outcome of cancer patients.
The immune system is a diverse one controlled by endocrine system via signaling molecules that act as activating agents, and send feedback information to endocrine system. Thus, they are formulating a coordinated interaction between the immune and endocrine systems. In addition, there are internal interactions within the immune cells through cytokine network loop that interacts or even police each other as a dynamic part among immune system. This approach can therefore contribute to the understanding in depth, of MENK’s positive role in coordinating human immune system. Our results also provide a significant clue of action for MENK and highlighten the clinical potential of MENK in cancer immunotherapy, especially for the cancer patients with lower immunity post chemotherapy. According to our previous study in cancer therapy with MENK, application of MENK should include: (1) the application of MENK, before chemotherapy or with chemotherapy will sustain the immune system. This shows great synergistic, and minimize side effects during process of chemotherapy, (2) the application of MENK, post chemotherapy will restore immune system much fast, and minimize side effects during process of chemotherapy. Likewise, we may look at possibility of MENK as an adjuvant in vaccine designing against infectious diseases.
Of course this is only preliminary approach and there is much to be studied further to gain insight on MENK, such as how MENK inhibits Treg cells at molecular level, which will reveal nature of mechanism and will be of great importance.27,28
Also whether MENK can stop transformation of routine CD4+T cells to Tregs or just directly inhibits mature Tregs is another key issue to be clarified. MENK, plus tumor specific antigen may be better in expanding specific T cells, which can kill cancer cells more effectively and at same time Tregs will be inhibited. Furthermore we can separate cells in innate immune system from patient body and grow them with MENK in vitro, followed by infusing them to cancer patient to see more efficacy.
We are designing a modified method that is: purified NK, NKT, and gamma delta T cell from cancer patient were expanded in vitro under stimulation of MENK and re-infuse them back to cancer patient. The efficacy was then compared and evaluated with existing method. Initial test shows potential and this approach is in process deeply.
Conclusion
This approach helps us learn more knowledge about MENK’s action in rehabilitating human immune system and the conclusion of MENK as an immune enhancer, drawn from present study is fully supported by the data obtained. We believe that this is the first time that published data based on large samples show that MENK could stimulate proliferation of lymphocyte subpopulations by inhibiting Tregs in peripheral blood of cancer patients.
Materials and Methods
Key reagents
MENK was provided by Penta biotech. Inc. USA (≥97% purity). In our previous experiments we have tested a range of concentrations of MENK from 10−1 M to 10−15 M on the proliferation of human lymphocyte in vitro and the optimal concentration of 10−12 M to growth of immune cells was confirmed. So the current study was performed with use of 10−12 M of MENK. The mAbs used in this study included FITC anti-human CD4, CD8, Per CP anti- human CD3, FITC anti-human CD16, PE anti-human CD56, FITC anti-human CD1a and human Treg FlowTM Kit, which were all products of Biolegend or BD PharMingen. Ficoll-Paque solution was a product of Sigma-Wisconsin. Other chemicals or solvents frequently used in our laboratory were all certified products from sigma-Aldrich or BD PharMingen.
Patient information and lymphocytes isolation and culture
The patients recruited for this study were all with terminal cancers with broad metastasis, underwent chemotherapy and their immune system were damaged severely. They failed to respond to any therapy available and were desperate. After they signed informed consent we began to give treatment. The concrete cases distribution was as following:
Rectal cancer: 4, Colon cancer: 6, Stomach cancer: 3, Hepanocellular cancer: 2, Lung cancer: 7, Oval cancer: 3, Pancreatic cancer: 2, Breast cancer: 3, Urinary bladder cancer: 1
PBMCs were separated from heparinized peripheral blood of cancer patients by Ficoll density gradient centrifugation. The isolated PBMCs were then rinsed 3 times with phosphate buffer solution and the cell vitality was confirmed by trypan blue dye assay. The cell number were adjusted to 5 × 105/mL and the cells were grown in RPMI1640 supplemented with 10% fetal calf serum, 2 mL L-glutamine,1.2% sodium bicarbonate, and antibiotics (100 Units/mL penicillin, 100 μg/mL streptomycin, 100μg/mL kanamycin). The lymphocytes subpopulations grown with MENK and the proliferation of the lymphocyte were checked with FCM on 7 d of culture, while cells number were enriched to 109/mL. The proliferated cells were collected, washed 3 times with sterilized normal saline, dispersed in 500 mL sterilized normal saline and finally was infused back to patients. Unless otherwise indicated, all cells were grown under condition of a humidified atmosphere of 5% CO2 and 95% air at 37 °C.
Analysis by flow cytometry (FCM)
Lymphocyte subpopulations were assayed according to their immunophenotypes. We recorded the major lymphocyte subpopulations of total nucleated cells, total CD3+ cells, CD4+T cells, cytotoxic T cell (Tc,CD8+CD28+) and Treg (CD4+CD25+Foxp3+), and NK cells(CD16+CD56+). Fluoro-chrome, fluorescein (FITC), or phycoerythrin (PE) labeled monoclonal antibodies against surface markers were used and the surface markers of lymphocyte subpopulations were then analyzed with direct double immunofluorescence by using two-color flow cytometry (FACS-Calibur, Becton Dickinson) via acquired 10 000 events in the gating region. Each subpopulation was expressed as percentage of lymphocytes or as percentage of total T lymphocytes.
Statistical analysis
Statistical analysis was done using statistical program SPSS (Statistical Package for Social Sciences, Version 16.0) for Windows. All variables were presented as mean ± SE. The differences among the groups were evaluated by ANOVA for multiple groups and by the student test for 2 groups using the Prism (Graph Pad Software). Tukey test, used for post hoc analysis, when P < 0.05 by ANOVA indicated significance.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by project for construction of discipline platform in universities of Liaoning province, China and received funding from Liaoning science foundation (2009225008-7, 2012225016). We apologize to the researchers whose works could not be discussed here due to space limitations.
Glossary
Abbreviations:
- MENK
methionine enkephalin
- FCM
flow cytometry
- DC
dendritic cell
- NK
natural killer
- Treg
CD4+CD25+ regulatory T cells
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate buffered saline
References
- 1.Sasaki S, Tamaki Y, Nagata K, Kobayashi Y. Regulation of the estrous cycle by neutrophils via opioid peptides. J Immunol. 2011;187:774–80. doi: 10.4049/jimmunol.1002489. [DOI] [PubMed] [Google Scholar]
- 2.Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975;258:577–80. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- 3.Zagon IS, Donahue RN, Bonneau RH, McLaughlin PJ. B lymphocyte proliferation is suppressed by the opioid growth factor-opioid growth factor receptor axis: Implication for the treatment of autoimmune diseases. Immunobiology. 2011;216:173–83. doi: 10.1016/j.imbio.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Zagon IS, Donahue RN, McLaughlin PJ. Opioid growth factor-opioid growth factor receptor axis is a physiological determinant of cell proliferation in diverse human cancers. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1154–61. doi: 10.1152/ajpregu.00414.2009. [DOI] [PubMed] [Google Scholar]
- 5.Donahue RN, McLaughlin PJ, Zagon IS. Cell proliferation of human ovarian cancer is regulated by the opioid growth factor-opioid growth factor receptor axis. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1716–25. doi: 10.1152/ajpregu.00075.2009. [DOI] [PubMed] [Google Scholar]
- 6.Vujić V, Stanojević S, Dimitrijević M. Methionine-enkephalin stimulates hydrogen peroxide and nitric oxide production in rat peritoneal macrophages: interaction of mu, delta and kappa opioid receptors. Neuroimmunomodulation. 2004;11:392–403. doi: 10.1159/000080150. [DOI] [PubMed] [Google Scholar]
- 7.Shan F, Xia Y, Wang N, Meng J, Lu C, Meng Y, Plotnikoff NP. Functional modulation of the pathway between dendritic cells (DCs) and CD4+T cells by the neuropeptide: methionine enkephalin (MENK) Peptides. 2011;32:929–37. doi: 10.1016/j.peptides.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Chen W, Meng J, Lu C, Wang E, Shan F. Induction on differentiation and modulation of bone marrow progenitor of dendritic cell by methionine enkephalin (MENK) Cancer Immunol Immunother. 2012;61:1699–711. doi: 10.1007/s00262-012-1221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Meng J, Li X, Hua H, Yiming M, Wang Q, Wang E, Shan F. Methionine enkephalin (MENK) improved the functions of bone marrow-derived dendritic cells (BMDCs) loaded with antigen. Hum Vaccin Immunother. 2012;8:1236–42. doi: 10.4161/hv.21128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Liu J, Meng J, Lu C, Li X, Wang E, Shan F. Macrophage polarization induced by neuropeptide methionine enkephalin (MENK) promotes tumoricidal responses. Cancer Immunol Immunother. 2012;61:1755–68. doi: 10.1007/s00262-012-1240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng Y, Wang Q, Zhang Z, Wang E, Plotnikoff NP, Shan F. Synergistic effect of methionine encephalin (MENK) combined with pidotimod(PTD) on the maturation of murine dendritic cells (DCs) Hum Vaccin Immunother. 2013;9:773–83. doi: 10.4161/hv.23137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua H, Lu C, Li W, Meng J, Wang D, Plotnikoff NP, Wang E, Shan F. Comparison of stimulating effect on subpopulations of lymphocytes in human peripheral blood by methionine enkephalin with IL-2 and IFN-γ. Hum Vaccin Immunother. 2012;8:1082–9. doi: 10.4161/hv.20759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zagon IS, McLaughlin PJ. Targeting opioidergic pathways as a novel biological treatment for advanced pancreatic cancer. Expert Rev Gastroenterol Hepatol. 2012;6:133–5. doi: 10.1586/egh.11.106. [DOI] [PubMed] [Google Scholar]
- 14.Gredicak M, Supek F, Kralj M, Majer Z, Hollósi M, Smuc T, Mlinarić-Majerski K, Horvat S. Computational structure-activity study directs synthesis of novel antitumor enkephalin analogs. Amino Acids. 2010;38:1185–91. doi: 10.1007/s00726-009-0329-5. [DOI] [PubMed] [Google Scholar]
- 15.Carr DJ, Carpenter GW. Morphine-induced suppression of cytotoxic T lymphocyte activity in alloimmunized mice is not mediated through a naltrindole-sensitive delta opioid receptor. Neuroimmunomodulation. 1995;2:44–53. doi: 10.1159/000096847. [DOI] [PubMed] [Google Scholar]
- 16.Carr DJ, Carpenter GW, Garza HH, Jr., Baker ML, Gebhardt BM. Cellular mechanisms involved in morphine-mediated suppression of CTL activity. Adv Exp Med Biol. 1995;373:131–9. doi: 10.1007/978-1-4615-1951-5_18. [DOI] [PubMed] [Google Scholar]
- 17.Byun SW, Chang YJ, Chung IS, Moss SF, Kim SS. Helicobacter pylori decreases p27 expression through the delta opioid receptor-mediated inhibition of histone acetylation within the p27 promoter. Cancer Lett. 2012;326:96–104. doi: 10.1016/j.canlet.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiappelli F, Nguyen L, Bullington R, Fahey JL. Beta-endorphin blunts phosphatidylinositol formation during in vitro activation of isolated human lymphocytes: preliminary report. Brain Behav Immun. 1992;6:1–10. doi: 10.1016/0889-1591(92)90054-R. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin PJ, Zagon IS. The opioid growth factor-opioid growth factor receptor axis: homeostatic regulator of cell proliferation and its implications for health and disease. Biochem Pharmacol. 2012;84:746–55. doi: 10.1016/j.bcp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Garza HH, Jr., Prakash O, Carr DJ. Immunologic characterization of TAT72-transgenic mice: effects of morphine on cell-mediated immunity. Int J Immunopharmacol. 1994;16:1061–70. doi: 10.1016/0192-0561(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 21.Ghaffari K, Savadkuhi ST, Honar H, Riazi K, Shafaroodi H, Moezi L, Ebrahimkhani MR, Tahmasebi MS, Dehpour AR. Obstructive cholestasis alters intestinal transit in mice: role of opioid system. Life Sci. 2004;76:397–406. doi: 10.1016/j.lfs.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Noori S, Hassan ZM, Yaghmaei B, Dolatkhah M. Antitumor and immunomodulatory effects of salvigenin on tumor bearing mice. Cell Immunol. 2013;286:16–21. doi: 10.1016/j.cellimm.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Tauschmann M, Prietl B, Treiber G, Gorkiewicz G, Kump P, Högenauer C, Pieber TR. Distribution of CD4(pos) -, CD8(pos) - and regulatory T cells in the upper and lower gastrointestinal tract in healthy young subjects. PLoS One. 2013;8:e80362. doi: 10.1371/journal.pone.0080362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jawa V, Cousens LP, Awwad M, Wakshull E, Kropshofer H, De Groot AS. T-cell dependent immunogenicity of protein therapeutics: Preclinical assessment and mitigation. Clin Immunol. 2013;149(3PB):534–55. doi: 10.1016/j.clim.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Jang TJ. Progressive Increase of Regulatory T Cells and Decrease of CD8+ T Cells and CD8+ T Cells/Regulatory T Cells Ratio during Colorectal Cancer Development. Korean J Pathol. 2013;47:443–51. doi: 10.4132/KoreanJPathol.2013.47.5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li K, Wang C, Lu H, Gu X, Guan Z, Zhou P. Regulatory T cells in peripheral blood and cerebrospinal fluid of syphilis patients with and without neurological involvement. PLoS Negl Trop Dis. 2013;7:e2528. doi: 10.1371/journal.pntd.0002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee MK, 4th, Xu S, Fitzpatrick EH, Sharma A, Graves HL, Czerniecki BJ. Inhibition of CD4+CD25+ regulatory T cell function and conversion into Th1-like effectors by a Toll-like receptor-activated dendritic cell vaccine. PLoS One. 2013;8:e74698. doi: 10.1371/journal.pone.0074698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pucino V, De Rosa V, Procaccini C, Matarese G. Regulatory T cells, leptin and angiogenesis. Chem Immunol Allergy. 2014;99:155–69. doi: 10.1159/000353557. [DOI] [PubMed] [Google Scholar]


