Abstract
As India is transitioning from high to intermediate hepatitis A endemicity, the need for hepatitis A vaccination programs increases. This study investigated the immunogenicity and safety of a virosomal hepatitis A vaccine (HAVpur Junior) compared with an aluminum-adsorbed hepatitis A vaccine (Havrix 720 Junior) in Indian children. Healthy children aged 18–47 months, stratified by age, were randomized to either HAVpur Junior or Havrix 720 Junior. The first dose of vaccine was administered on Day 1 and the second (booster) dose 6 months later. Antibodies against hepatitis A virus (HAV) were measured using a microparticle enzyme immunoassay. The primary objective assessed non-inferiority of HAVpur Junior to Havrix 720 Junior in terms of seroprotection rates (≥ 10 mIU/mL anti-HAV antibodies) at 1 month after the first vaccination. Non-inferiority was demonstrated if the lower limit of the 90% confidence interval of the group difference was greater than –10%. Local and systemic adverse events were recorded. The seroprotection rate at 1 month was 95.9% in the HAVpur Junior group and 96.6% in the Havrix 720 Junior group. As the lower limit of the 90% confidence interval of the group difference was greater than –10% (–4.7), non-inferiority of HAVpur Junior to Havrix 720 Junior was established. The overall incidence of adverse events (solicited and unsolicited) after each vaccination was similar in both groups. In conclusion, the aluminum-free virosomal vaccine HAVpur Junior induced a similar immune response to Havrix 720 Junior in healthy Indian children aged 18 to 47 months. Both vaccines were well tolerated. The study shows that the low-dose virosomal HAV vaccine is consistently efficacious and well tolerated in children of all age groups and is suitable for inclusion into Indian childhood vaccination schedules.
Keywords: pediatric, HAV vaccine, virosomes, immunogenicity, safety, India
Introduction
Hepatitis A is an acute, self-limiting inflammation of the liver caused by the hepatitis A virus (HAV). The severity of the illness depends on the age of the patient. In children the infection is usually asymptomatic, but with increasing age symptoms such as malaise, vomiting, and diarrhea are common.1 In more than 75% of older children and adults the characteristic manifestations of acute viral hepatitis, such as elevated liver enzymes, jaundice, and dark urine occur.2 Fatal cases are rare and the risk increases with age: the case fatality ratio is 0.1% among children under 15 y of age, 0.3% among adolescent and adults 15 to 39 y of age, and 2.1% among adults aged 40 y and older.1 Due to high hygienic standards, infection with HAV is rare in industrialized countries. In less developed countries hepatitis A is still highly endemic, causing primarily asymptomatic infections early in life.1,3 However, as socioeconomic conditions improve many developing countries experience a transition from high to intermediate endemicity with decreased exposure during early childhood.2 For example, data from India show that the population is no longer homogenous for its HAV exposure profile.2,4 As a consequence, occasional HAV outbreaks occur and a higher proportion of symptomatic infection among older children and adults with an associated increased risk of fatal disease can be seen.4
Hepatitis A can be easily prevented by vaccination with either live attenuated or inactivated HAV vaccines.1 Field studies with inactivated hepatitis A vaccines have proven their safety, immunogenicity and high protective efficacy.5-7 Furthermore, inactivated HAV vaccines have been shown to confer long-lasting immunity against HAV infection.8-10
At present, two classes of inactivated HAV vaccines are available: several aluminum-adsorbed vaccines (e.g., Havrix 720, Vaqta) and an aluminum-free virosomal vaccine (marketed under the names HAVpur or Epaxal). Aluminum-based adjuvants have a number of disadvantages, including inflammation at the injection site leading to mild adverse reactions, such as local pain, swelling and redness at the injection site in 50 to 60% of vaccinated subjects.11-14 In the virosomal HAV vaccine, an alternative type of antigen delivery system, the immunopotentiating reconstituted influenza virosome (IRIV), is used to replace aluminum as adjuvant.15,16 The virosomal HAV vaccine has been shown to be well tolerated and highly immunogenic in adults in a number of clinical studies.13,15-19
In an effort to reduce costs and to increase availability of the vaccine, a lower dosed pediatric presentation of the virosomal HAV vaccine (≥ 12 IU/0.25 mL; marketed under the names HAVpur Junior or Epaxal Junior) was tested to evaluate whether this dose was immunogenic enough to elicit a protective immune response in children. In a first small study conducted in 55 Thai children aged 8 to 12 y the pediatric dose of the virosomal HAV vaccine was shown to be highly immunogenic and well tolerated.20 A second study in 308 Belgian subjects 1 to 16 y of age21 demonstrated that the pediatric dose of the virosomal HAV vaccine (Epaxal Junior) was as immunogenic as the standard dose and an aluminum-containing comparator (Havrix 720 Junior). Furthermore, Epaxal Junior had a better tolerability profile regarding local injection site reactions than Havrix 720 Junior. A third study conducted in Chile in 360 children aged 1 to 16 y produced similar results to the Belgian study:22 the pediatric dose of the virosomal HAV vaccine elicited seroprotection rates comparable to those seen after immunization with the standard dose or with Havrix 720 Junior. The vaccines were well tolerated with relatively low incidences of adverse events. In a further study with 322 children in Israel, a co-administration of the pediatric virosomal HAV vaccine (Epaxal Junior) with routine childhood vaccines showed immunogenicity and safety equal to Epaxal Junior administered alone and with Havrix 720 Junior co-administered with routine childhood vaccines.23
The present study was designed to confirm the results obtained in the earlier trials20-23 and to increase the amount of clinical information available regarding safety and immunogenicity of the pediatric dose of the virosomal HAV vaccine.
Results
Study population
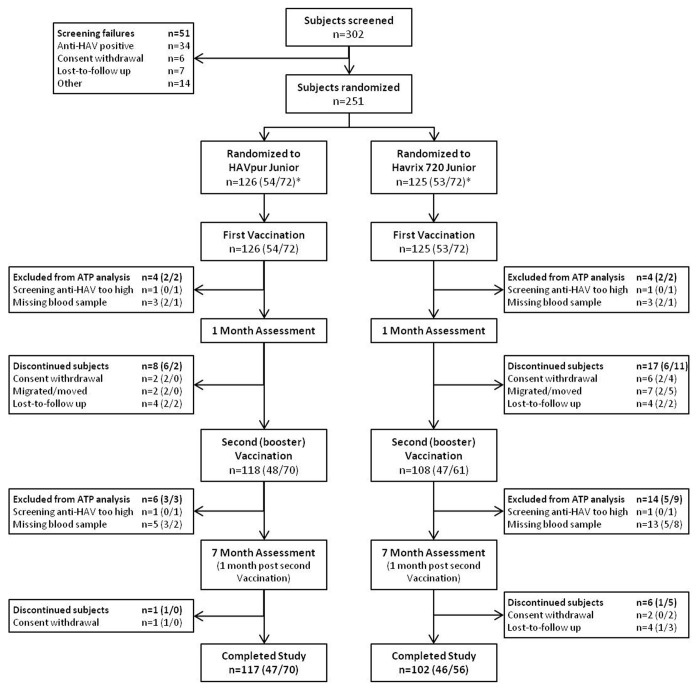
A total of 251 subjects were randomized and received the first vaccine dose: 126 subjects were vaccinated with HAVpur Junior and 125 subjects were vaccinated with Havrix 720 Junior (Fig. 1). The analyses following the first dose were performed in all 251 subjects in the safety population and 239 subjects in the ATP population (12 subjects were excluded from the ATP analyses due to protocol deviations). A total of 25 subjects discontinued after the first vaccination (HAVpur Junior, 8 subjects; Havrix 720 Junior, 17 subjects). The remaining 226 subjects (90.0%) received the second (booster) vaccination at Month 6, 118 subjects (93.7%) in the HAVpur Junior group and 108 subjects (86.4%) in the Havrix 720 Junior group. In total, 20 subjects were excluded from the ATP population of the second vaccination; thus, the analyses following the second dose included 226 subjects in the safety population and 206 subjects in the ATP population. Altogether 219 subjects (87.3%) completed the study, 117 (92.9%) in the HAVpur Junior group and 102 (81.6%) in the Havrix 720 Junior group.
Figure 1. Study profile. ATP, according-to-protocol; HAV, hepatitis A virus. * Number of subjects aged ≥ 18 to ≤ 23 vs. ≥ 24 to ≤ 47 mo.
About half (50.6%) of the randomized subjects were female (Table 1). The mean age of subjects was 27.8 mo (range, 18 to 48 mo); age and distribution by age were similar between vaccine groups. All subjects were Indian.
Table 1. Demographic characteristics at randomization (safety population).
| Demographic characteristic | HAVpur Junior N = 126 |
Havrix 720 Junior N = 125 |
Total N = 251 |
|---|---|---|---|
| Female, n (%) | 59 (46.8) | 68 (54.4) | 127 (50.6) |
| Male, n (%) | 67 (53.2) | 57 (45.6) | 124 (49.4) |
| Mean age, months (SD) | 27.6 (8.45) | 27.9 (8.23) | 27.8 (8.33) |
| Age range, n (%) | |||
| ≥ 18 to ≤ 23 mo | 54 (42.9%) | 53 (42.40%) | 107 (42.6%) |
| ≥ 24 to ≤ 47 mo | 72 (57.1%) | 72 (57.6%) | 144 (57.4%) |
N, number of subjects in specified group; n, number of subjects in specified category; SD, standard deviation.
Immunogenicity
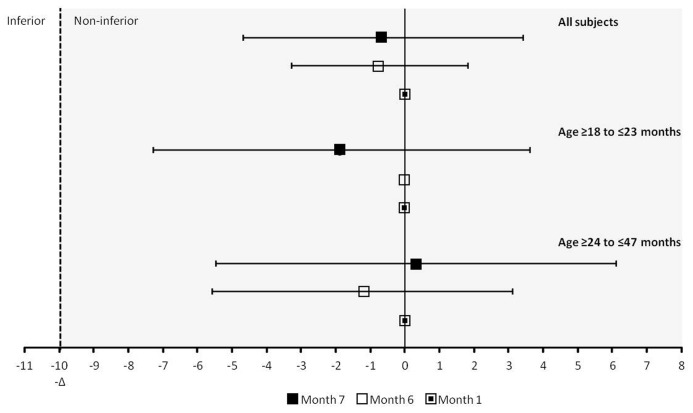
The seroprotection rate (anti-HAV concentration ≥ 10 mIU/mL) at Month 1 was 95.9% in the HAVpur Junior group and 96.6% in the Havrix 720 Junior group (Table 2). Since the lower limit of the 90% CI for the group difference between HAVpur Junior and Havrix 720 Junior was greater than –10% (–4.7), non-inferiority was demonstrated and the primary objective of this study was achieved (Fig. 2).
Table 2. Anti-HAV seroprotection rates (ATP population).
| HAVpur Junior N = 122 |
Havrix 720 Junior N = 117 |
|||
|---|---|---|---|---|
| n/n' (%) | 95% CI | n/n' (%) | 95% CI | |
| All subjects | ||||
| Month 1 (Day 29) | 117/122 (95.9) | 90.7, 98.7 | 113/117 (96.6) | 91.5, 99.1 |
| Month 6 | 113/115 (98.3) | 93.9, 99.8 | 104/105 (99.0) | 94.8, 100 |
| Month 7 | 112/112 (100) | 96.8, 100 | 94/94 (100) | 96.2, 100 |
| Age ≥ 18 to ≤ 23 mo | ||||
| Month 1 (Day 29) | 50/52 (96.2) | 86.8, 99.5 | 50/51 (98.0) | 89.6, 100 |
| Month 6 | 47/47 (100) | 92.5, 100 | 46/46 (100) | 92.3, 100 |
| Month 7 | 45/45 (100) | 92.1, 100 | 42/42 (100) | 91.6, 100 |
| Age ≥ 24 to ≤ 47 mo | ||||
| Month 1 (Day 29) | 67/70 (95.7) | 88.0, 99.1 | 63/66 (95.5) | 87.3, 99.1 |
| Month 6 | 66/68 (97.1) | 89.8, 99.6 | 58/59 (98.3) | 90.9, 100 |
| Month 7 | 67/67 (100) | 94.6, 100 | 52/52 (100) | 93.2, 100 |
Percentages of seroprotection (%) were based on n' ( = number of subjects with valid measurements in specified group and population). ATP, according-to-protocol; CI, confidence interval; N, number of subjects in specified group and population; n, number of subjects seroprotected.
Figure 2. Between-group comparison of seroprotection rates with HAVpur Junior and Havrix 720 Junior at different time points (ATP population). Error bars represent the two-sided 90% CI. Non-inferiority was concluded if the lower limit of the two-sided CI was greater than -10% (-Δ). ATP, according-to-protocol; CI, confidence interval.
The seroprotection rates before the second (booster) vaccination at Month 6 increased further to 98.3% in the HAVpur Junior group and 99.0% in the Havrix 720 Junior group and reached 100% at Month 7 in both groups (Table 2). Non-inferiority of HAVpur Junior to Havrix 720 Junior was established at both Months 6 and 7 (Fig. 2).
At Months 1 and 6, seroprotection rates were slightly higher in subjects aged ≥ 18 to ≤ 23 mo than in subjects aged ≥ 24 to ≤ 47 mo in both vaccine groups (Table 2). At Month 7, the seroprotection rates were 100% for all subjects.
Overall, GMCs induced by HAVpur Junior or Havrix 720 Junior were similar and no statistically significant differences were noted (Table 3). The values increased from around 50 mIU/mL at Month 1 to around 100 mIU/mL at Month 6 (before second vaccination) and to around 2000 mIU/mL at Month 7 (1 mo after second vaccination). There were no statistically significant differences in GMCs between HAVpur Junior and Havrix 720 Junior in the two age subgroups. However, there was a trend of numerically higher anti-HAV GMCs at Month 1 and Month 6 in the younger subjects compared with the older subjects.
Table 3. Anti-HAV GMCs (ATP population).
| HAVpur Junior N = 122 |
Havrix 720 Junior N = 117 |
Between group comparisons | ||||
|---|---|---|---|---|---|---|
| n' | GMC adjusted (95% CI) | n' | GMC adjusted (95% CI) | GMC ratio | p-value | |
| All subjects | ||||||
| Baseline (screening) | 122 | 1.3 (1.1, 1.4) | 117 | 1.2 (1.1, 1.3) | - | - |
| Month 1 (Day 29) | 122 | 51.5 (41.5, 64.0) | 117 | 53.3 (42.8, 66.5) | 0.966 | 0.824 |
| Month 6 | 115 | 96.0 (71.8, 128.4) | 105 | 113.0 (83.7, 152.5) | 0.850 | 0.437 |
| Month 7 | 112 | 1712.4 (1377.1, 2129.3) | 94 | 2226.4 (1758.4, 2819.1) | 0.769 | 0.098 |
| Age ≥ 18 to ≤ 23 mo | ||||||
| Baseline (screening) | 52 | 1.2 (1.0, 1.5) | 51 | 1.2 (1.0, 1.5) | - | - |
| Month 1 (Day 29) | 52 | 52.6 (36.2, 76.3) | 51 | 68.8 (47.2, 100.3) | 0.764 | 0.307 |
| Month 6 | 47 | 99.7 (56.9, 174.9) | 46 | 146.2 (83.4, 256.1) | 0.682 | 0.330 |
| Month 7 | 45 | 1355.6 (937.5, 1960.2) | 42 | 2105.3 (1435.7, 3087.2) | 0.644 | 0.083 |
| Age ≥ 24 to ≤ 47 mo | ||||||
| Baseline (screening) | 70 | 1.3 (1.1, 1.5) | 66 | 1.2 (1.093, 1.364) | - | - |
| Month 1 (Day 29) | 70 | 48.2 (37.4, 62.1) | 66 | 42.0 (32.3, 54.6) | 1.148 | 0.454 |
| Month 6 | 68 | 84.7 (63.0, 120.9) | 59 | 85.1 (67.3, 107.7) | 0.967 | 0.967 |
| Month 7 | 67 | 2056.1 (1570.2, 2692.3) | 52 | 2345.5 (1723.0, 3192.9) | 0.877 | 0.520 |
GMCs adjusted, GMC ratios, CIs, and p-values are based on an analysis of variance model with vaccine group, center and age stratum as factors. ATP, according-to-protocol; CI, confidence interval; GMC, geometric mean concentration; N, number of subjects in specified group and population; n', number of subjects with valid measurements in specified group and population.
Fourteen subjects from a single study site (Study Site No. 2) had unusual GMCs or GMC patterns, with two subjects (one from each study group) showing consistently high titers at each of the three study visits. All of these subjects received both the first and second (booster) vaccination. The analytical laboratory confirmed the results. These unusual values/patterns may indicate HAV infection (see Safety section below), or errors in sample collection and/or processing. Therefore, the immunogenicity analysis was repeated post hoc without the data from Study Site No. 2 to assess the potential influence of the unusual GMC values/patterns on the overall result. Exclusion of the study site from the analysis reduced the variability in the data but did not have an influence on the overall outcome of the study: seroprotection rates were similar in both vaccine groups and non-inferiority of HAVpur Junior to Havrix 720 Junior was established for Months 1, 6, and 7 (data not shown).
Safety
The overall incidence of adverse events (solicited and unsolicited) after each vaccination was similar in both groups (first vaccination: HAVpur Junior, 19.0%; Havrix 720 Junior, 19.2%; second vaccination: HAVpur Junior, 3.4%; Havrix 720 Junior 5.6%).
No deaths occurred and no subject discontinued the study due to an adverse event. Two serious adverse events, both of them unrelated to vaccination, occurred during the study. One subject in the Havrix 720 Junior group presented with enteric fever approximately 2 mo after the first dose. Another subject developed acute gastritis about 4½ months after the second dose of HAVpur Junior. Both events resolved without sequelae.
After the first vaccination with HAVpur Junior, pain at the injection site was reported for 2 subjects (1.6%), redness ≥ 5 mm for 2 subjects (1.6%), and swelling/induration ≥ 5 mm for 3 subjects (2.4%; Table 4). One subject (0.8%) in the Havrix 720 Junior group experienced injection site pain after the first dose. After the second (booster) vaccination, redness ≥ 5 mm was reported for 1 subject (0.9%) in the HAVpur Junior group, and pain at the injection site and redness ≥ 5 mm were each reported for 1 subject (1.0%) in the Havrix 720 Junior group. All solicited local adverse events were of mild intensity and most resolved within 1 or 2 d.
Table 4. Solicited local adverse events (safety population).
| First vaccination (Month 1) | Second vaccination (Month 6) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HAVpur Junior N = 125 |
Havrix 720 Junior N = 123 |
HAVpur Junior N = 117 |
Havrix 720 Junior N = 104 |
||||||
| n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | n (%) | 95% CI | ||
| Pain | 2 (1.6) | 0.2, 5.7 | 1 (0.8) | 0, 4.4 | 0 | 0, 3.1 | 1 (1.0) | 0, 5.2 | |
| Redness ≥ 5 mm | 2 (1.6) | 0.2, 5.7 | 0 | 0, 3.0 | 1 (0.9) | 0, 4.7 | 1 (1.0) | 0, 5.2 | |
| Swelling/induration ≥ 5 mm | 3 (2.4) | 0.5, 6.9 | 0 | 0, 3.0 | 0 | 0, 3.1 | 0 | 0.0, 3.5 | |
Percentages are based on N ( = the number of subjects who returned a diary). CI, confidence interval; n, number of subjects reporting the specified adverse event at least once.
Fever (a body temperature ≥ 38 °C) was the only solicited systemic adverse event and was observed in 1 subject (0.8%) in the Havrix 720 Junior group after the first vaccination.
The incidence of unsolicited adverse events after the first vaccination was comparable between groups (15.1% in the HAVpur Junior group and 18.4% in the Havrix 720 Junior group). The most common unsolicited adverse events after the first vaccination were fever (HAVpur Junior, 4.0%; Havrix 720 Junior, 4.0%) as well as upper respiratory tract infection (HAVpur Junior, 4.8%; Havrix 720 Junior, 2.4%). Other unsolicited adverse events reported for more than 3 subjects after the first vaccination were nasopharyngitis (HAVpur Junior, 1.6%; Havrix 720 Junior, 4.0%) and cough (HAVpur Junior, 1.6%; Havrix 720 Junior, 4.0%). Incidences of unsolicited adverse events after the second (booster) vaccination were low with 2.5% in the HAVpur Junior group and 3.7% in the Havrix 720 Junior group. Only single occurrences of adverse events were reported in both groups after the second dose. All but one unsolicited adverse event resolved without sequelae; one subject in the Havrix 720 Junior group had moderate diarrhea 4 mo after the first vaccination which resolved with sequelae. Most unsolicited adverse events were of mild intensity, followed by moderate intensity. No subjects had any of severe intensity. All unsolicited adverse events were considered unrelated to the study vaccines by the investigator.
The consistently high anti-HAV titers of one subject in the HAVpur Junior group and one subject in the Havrix 720 Junior group indicated a potential natural HAV infection, which may have occurred shortly before vaccination in this study. For this reason these two subjects and four other subjects with unusual high titers were invited for an additional health assessment after the end of the study. Two of these subjects had moved out of the study area and were therefore lost to follow up. According to the investigator, all assessed subjects were considered to be at high risk of HAV infection. Also according to the investigator, the health status of the remaining four subjects with unusual high titers was without specific clinical suspicion of diseases and there was no clinical evidence of hepatic abnormality. The results of the laboratory assessment were also considered to be within normal range by the investigator.
Discussion
Three previous randomized clinical studies compared the pediatric presentation (0.25 mL) of the virosomal HAV vaccine with the standard dose (0.5 mL) and an aluminum-adjuvanted HAV vaccine (Havrix 720 Junior). Two of the studies were conducted in children aged 1 to 16 y, with the majority being older than 4 y.21,22 The third study assessed the co-administration of hepatitis A and routine childhood vaccines in children aged 12 to 15 mo.23 In all three studies the immunogenicity of the pediatric dose was similar to the standard dose and Havrix 720 Junior. The present randomized, controlled study was designed to evaluate the safety and immunogenicity of the pediatric dose of the virosomal HAV vaccine (HAVpur Junior) specifically in healthy children aged ≥ 18 mo to ≤ 47 mo, which is the age range of children under-represented in the former studies. Havrix 720 Junior was used as a comparator; both vaccines were administered using a two-dose 0/6 mo immunization schedule.
The current study demonstrated that the immune response seen after immunization with HAVpur Junior was comparable to that elicited by immunization with Havrix 720 Junior. Non-inferiority of HAVpur Junior to Havrix 720 Junior was established for the primary endpoint of seroprotection rate at Month 1 and also for the secondary endpoints of seroprotection rates at Months 6 and 7. Overall, the immunogenicity results in both groups were similar to those observed in the previous randomized studies comparing the pediatric dose of the virosomal HAV vaccine and Havrix 720 Junior.21-23 The GMCs of anti-HAV antibodies increased from around 50 mIU/mL at Month 1 to around 100 mIU/mL at Month 6 (before second vaccination) and to around 2000 mIU/mL at Month 7 (1 mo after second vaccination). This increase in GMC from Month 1 to Month 6, observed in both groups in the present study, is unusual and may be explained by natural exposure to HAV in the HAV-endemic study area. An alternative explanation could be that in young children GMCs continue to increase for several months after the first vaccination; a modest increase in GMCs from Month 1 to 6 was also seen in subjects aged 12 to 15 mo in the Israel study.23 Overall, the GMCs observed in the present study were similar for both groups at Month 1 and numerically lower in the HAVpur Junior group than in the Havrix 720 Junior group at Month 7. A similar pattern was observed in the studies in Chile and Belgium21,22 while in the study in Israel consistently higher GMC values were detected with the pediatric dose of the virosomal HAV vaccine than with Havrix 720 Junior throughout the study.23 However, the small differences in HAV GMCs seen in the present study are not considered clinically relevant as the anti-HAV concentrations at Month 7 of most subjects were at least two orders of magnitude above the threshold (≥ 10 mIU/mL) commonly used to indicate seroprotection. Taken together, the immunogenicity data of the present and previous studies21-23 demonstrate that HAVpur Junior is equally efficacious in children of all age groups.
Unusual anti-HAV GMC values or GMC patterns occurred in a limited number of subjects (approximately 15%) in both vaccine groups of one study site. Therefore, it can be assumed that these unusual values did not have a substantial impact on the overall outcome of the study. Indeed, at all three time points assessed in this study, the GMCs were in the range observed in previous randomized studies comparing the pediatric virosomal HAV vaccine and Havrix 720 Junior.21-23 In addition, the GMCs were similar to the median antibody concentrations, indicating that the outliers did not have a substantial impact on the overall GMCs. However, the influence of the unusual values can be seen in increased mean values and particularly in high standard deviations, indicating an increased variability in the data. Nevertheless, a second immunogenicity analysis without the data from the affected study site confirmed the results obtained with the data from all sites: seroprotection rates were similar in both vaccine groups and non-inferiority of HAVpur Junior to Havrix 720 Junior was established for Months 1, 6, and 7 (data not shown).
The very high anti-HAV titers in one subject in the HAVpur Junior group and one subject in the Havrix 720 Junior group could suggest a potential natural HAV infection which may have occurred just shortly before the subjects were vaccinated in this study. An additional health assessment after the end of the study showed that the health status of both subjects was normal; there was no clinical evidence of hepatic abnormality, and the results of a safety laboratory assessment were also within normal range. The unusual titers in the other 12 subjects from study site 002 could have resulted from sample collection or processing errors, or, in some cases, a natural infection. When the most conservative approach is applied to all results (not excluding mix-up of samples) using a cut-off of 10 000 mIU/mL for any study sample, there may have been 12 (4.8%) cases of suspected infections among all study subjects who had negative anti-HAV results at baseline (8 cases from site 002 and 4 from site 003). It is a limitation of this study that the study protocol was not designed to investigate HAV infections in the study population, and thus it can only be speculated through post hoc analysis of the available data as to the potential cause of any unusual high titers. Nevertheless, the potential HAV cases highlight the need to vaccinate at an early age, i.e., in infancy, to stop transmission efficiently and eliminate hepatitis A from a given population.
The incidence of adverse events (solicited and unsolicited) was similar in both groups after each vaccination. The overall incidence of solicited adverse events was low, particularly due to a low incidence of pain. Solicited local reactions were more frequently reported in the HAVpur Junior group than in the Havrix 720 Junior group. It has to be taken into account though, that of the seven local solicited symptoms reported in the subject diaries in the HAVpur Junior group, the diameter of redness in two subjects and of swelling/induration in three subjects were just at the threshold (5 mm) at or above which the local event had to be recorded in the case report form. The relatively low numerical incidence of adverse events makes it difficult to detect relevant differences between the two groups. In the Belgian study incidence rates were higher and differences more obvious with an overall adverse event reporting rate of 52.8% for the low-dose virosomal vaccine (Epaxal Junior) and 69.4% for Havrix 720 Junior after the first dose.21 Particularly evident was a difference in the occurrence of injection site pain with 12.2% of subjects receiving Epaxal Junior and 30.6% of subjects receiving Havrix 720 Junior reporting this solicited symptom. Studies with the standard dose have also consistently shown a superior tolerability profile of the virosomal HAV vaccine compared with aluminum-adsorbed vaccines.19,24
With the improvement in socioeconomic conditions in India the population is no longer homogenous for HAV exposure.4 Thus, areas of intermediate endemicity may exist next to areas of high endemicity rendering a significant proportion of the Indian adolescent and adult population at risk of HAV infection with potentially severe clinical symptoms and morbidity.25 Close monitoring of HAV seroprevalence is therefore needed to identify susceptible populations,4 and large-scale vaccination campaigns are indicated to break the HAV transmission cycle and to prevent hepatitis A outbreaks in communities at risk.1 The occurrence of unusual high titers in our study indicates the need for further studies. A field efficacy trial for either one or two doses of hepatitis A vaccine in highly endemic regions or communities could provide information particularly on post-exposure protection of clinical hepatitis.
In conclusion, the pediatric presentation (0.25 mL) of the virosomal HAV vaccine HAVpur Junior was highly immunogenic and well tolerated in children aged 18 to 47 mo. The results of the present study provide valuable immunogenicity data on the Indian pediatric population in high endemic areas. This study together with previous studies in pediatric populations21-23 demonstrate that the low-dose virosomal HAV vaccine is consistently efficacious and well tolerated in children of all age groups and therefore suitable for inclusion into Indian childhood vaccination schedules.
Materials and Methods
Study design and participants
This Phase IV, open-label, age-stratified, randomized, controlled, multicenter, parallel-group study (NCT01349829) was conducted at three centers in India between March 2010 and April 2011. Figure 1 presents the study flow. At Visit 1 (Day -1 to -14) subjects were screened for eligibility. At Visit 2 (Day 1) a total of 251 healthy subjects aged 18 to 47 mo were enrolled in the study. Subjects were stratified into two age groups (18 to 23 mo and 24 to 47 mo). They were sequentially randomized in a 1:1 ratio according to the randomization scheme provided by Lambda Therapeutics Pvt. Ltd. to receive two doses of either HAVpur Junior or Havrix 720 Junior. Randomization numbers were provided in sealed envelopes. The lowest randomization number still available at the study center for the appropriate age stratum was allocated to the subject. Thereafter the corresponding sealed randomization envelope was opened to disclose the treatment group allocated to the selected randomization number. Unused envelopes were kept sealed and returned at the end of the study.
Subjects were excluded from study participation if they were seropositive for anti-HAV antibodies (≥ 10 mIU/mL), or if they had received a previous vaccination against hepatitis A. Furthermore, subjects who had received any investigational drug or vaccine 30 d preceding the first dose of study vaccine, or immune-modifying drugs 6 mo before the study, were not eligible to participate in the trial. Other reasons for exclusion were (planned) administration of a measles-containing vaccine within 4 wk prior to and after the first or second dose of study vaccine, a history of allergy to any component of the study vaccine, any immunodeficiency, major congenital defects, serious chronic illness, or acute disease at the time of enrolment.
The study was performed in compliance with the Declaration of Helsinki, the guidelines of the International Conference on Harmonization/Good Clinical Practice and the respective local legal requirements. The study was approved by each participating center’s Independent Ethics Committee. Parents/legal guardians confirmed their consent in writing before study entry and any study-specific procedure.
Vaccines
The composition of both vaccines has been described elsewhere.21,22 Vaccines were administered on Day 1 (first dose), and at Month 6 (booster dose) as a single intramuscular injection into the deltoid muscle.
Immunogenicity assessment
Blood samples were collected at the screening visit occurring within the 2 wk prior to the first dose (baseline samples), Month 1 (1 mo post first dose), Month 6 (6 mo post first dose), and Month 7 (1 mo post second dose). Antibody concentrations were quantified in serum using a microparticle enzyme immunoassay (AxSYM HAVAB 2.0 Quantitative, Abbott Diagnostics) at the department of Clinical Immunology Crucell Holland BV. An antibody concentration of ≥ 10 mIU/mL was regarded as seroprotective. The Abbott AxSYM HAVAB 2.0 Quantitative assay has a cut-off of 10 mIU/mL, with the assay being standardized to the international reference standard.
Safety assessment
Solicited and unsolicited adverse events were recorded as described for previous pediatric studies with the virosomal HAV vaccine.21-23 Body temperature was recorded by study personnel at the three study visits by placing a thermometer in the armpit with the arm pressed against the body for 3 min before taking the reading. The subject’s parents/legal guardians measured the body temperature, using the same method, in the evening and recorded the temperature in the Subject Diary. Should an additional temperature measurement have been performed at another time of the day, the highest temperature was recorded.
Statistical methods
The statistical analysis software used was SAS Version 9.1. The sample size determination was based on the primary objective to assess non-inferiority of HAVpur Junior to Havrix 720 Junior in terms of seroprotection rates at Month 1 (1 mo post first dose). Assuming a seroprotection rate of at least 95% for the HAVpur Junior group and a clinically significant non-inferiority limit of –10%, then 100 subjects per study group would have provided 90% power to show non-inferiority of HAVpur Junior to Havrix 720 Junior, using Chi-square test (normal approximation). Assuming a combined drop out and screening error rate of up to 25%, 250 subjects in total were planned to be randomized in a 1:1 ratio, 125 subjects to HAVpur Junior and 125 subjects to Havrix 720 Junior. In order to achieve an equal age distribution in the two vaccine groups, all eligible subjects were stratified into the age groups of 18 to 23 mo and 24 to 47 mo and then randomized according to the randomization scheme provided by Lambda Therapeutic Research Pvt. Ltd., Ahmedabad, India.
The study hypothesis is as follows
-Null hypothesis: The seroprotection rate at Day 29 for HAVpur Junior is inferior to the seroprotection rate at Day 29 for Havrix 720 Junior by more than -10%.
-Alternative hypothesis: The seroprotection rate at Day 29 for HAVpur Junior is not inferior to the seroprotection rate at Day 29 for Havrix 720 Junior by more than -10%.
The primary analysis (primary hypothesis testing) was performed on the according-to-protocol (ATP) population, which included all randomized subjects who received the first vaccination, had negative anti-HAV antibody concentration at screening, had no major protocol violations, and for whom a post first dose serum sample was available for the measurement of immunogenicity. Seroprotection was defined as anti-HAV antibody concentration ≥ 10 mIU/mL. Seroprotection rates and exact two-sided 95% confidence intervals (CI; Clopper-Pearson method) were provided for each vaccine at each time point. The seroprotection rate for HAVpur Junior was considered to be as good as the seroprotection rate for Havrix 720 Junior at Month 1 if the lower limit of the two-sided 90% CI for the difference in seroprotection rates (using normal approximation) was greater than –10%. If non-inferiority was demonstrated, the two-sided Fisher exact test was performed. Geometric mean concentrations (GMCs) and corresponding 95% CIs (normal approximation) were calculated as previously described.22
The safety analysis was performed on the safety population which included all subjects who received the respective vaccine dose (i.e., first or second vaccination). All solicited and unsolicited adverse events starting after the first vaccination were tabulated using descriptive statistics.
Disclosure of potential conflicts of interest
A Versteilen and M Sarnecki are employees of Crucell Switzerland AG. H Jain, V Kumavat and T Singh have no conflicts of interests to declare.
Acknowledgments
This study was sponsored by Crucell Switzerland AG. We would like to thank Dr Sonja Basta for writing the manuscript, and also all participants in the study.
Contributions
M Sarnecki was involved in study design and analysis, and critically reviewed the manuscript. A Versteilen was involved in analysis and critically reviewed the manuscript. H Jain, V Kumavat and T Singh were investigators, and involved in data collection, and critical review of the manuscript. All authors approved the final version of the manuscript.
Role of the funding source
Crucell Switzerland AG was involved in study design, analysis and interpretation of data, writing of the report and in the decision to submit the article for publication.
Glossary
Abbreviations:
- ATP
according-to-protocol
- CI
confidence interval
- GMC
geometric mean concentration
- HAV
hepatitis A virus
References
- 1.WHO position paper on hepatitis A vaccines – June 2012. Wkly Epidemiol Rec. 2012;87:261–76. [PubMed] [Google Scholar]
- 2.Franco E, Meleleo C, Serino L, Sorbara D, Zaratti L, Hepatitis A. Hepatitis A: Epidemiology and prevention in developing countries. World J Hepatol. 2012;4:68–73. doi: 10.4254/wjh.v4.i3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verma R, Khanna P. Hepatitis A vaccine should receive priority in National Immunization Schedule in India. Hum Vaccin Immunother. 2012;8:1132–4. doi: 10.4161/hv.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathur P, Arora NK. Epidemiological transition of hepatitis A in India: issues for vaccination in developing countries. Indian J Med Res. 2008;128:699–704. [PubMed] [Google Scholar]
- 5.American Academy of Pediatrics Committee on Infectious Diseases Hepatitis A vaccine recommendations. Pediatrics. 2007;120:189–99. doi: 10.1542/peds.2007-1088. [DOI] [PubMed] [Google Scholar]
- 6.Bovier PA. Epaxal: a virosomal vaccine to prevent hepatitis A infection. Expert Rev Vaccines. 2008;7:1141–50. doi: 10.1586/14760584.7.8.1141. [DOI] [PubMed] [Google Scholar]
- 7.Hendrickx G, Vorsters A, Van Damme P. Advances in hepatitis immunization (A, B, E): public health policy and novel vaccine delivery. Curr Opin Infect Dis. 2012;25:578–83. doi: 10.1097/QCO.0b013e328357e65c. [DOI] [PubMed] [Google Scholar]
- 8.Ott JJ, Irving G, Wiersma ST. Long-term protective effects of hepatitis A vaccines. A systematic review. Vaccine. 2012;31:3–11. doi: 10.1016/j.vaccine.2012.04.104. [DOI] [PubMed] [Google Scholar]
- 9.Bovier PA, Bock J, Loutan L, Farinelli T, Glueck R, Herzog C. Long-term immunogenicity of an inactivated virosome hepatitis A vaccine. J Med Virol. 2002;68:489–93. doi: 10.1002/jmv.10244. [DOI] [PubMed] [Google Scholar]
- 10.Van Herck K, Beutels P, Van Damme P, Beutels M, Van den Dries J, Briantais P, Vidor E. Mathematical models for assessment of long-term persistence of antibodies after vaccination with two inactivated hepatitis A vaccines. J Med Virol. 2000;60:1–7. doi: 10.1002/(SICI)1096-9071(200001)60:1<1::AID-JMV1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 11.Innis BL, Snitbhan R, Kunasol P, Laorakpongse T, Poopatanakool W, Kozik CA, Suntayakorn S, Suknuntapong T, Safary A, Tang DB, et al. Protection against hepatitis A by an inactivated vaccine. JAMA. 1994;271:1328–34. doi: 10.1001/jama.1994.03510410040030. [DOI] [PubMed] [Google Scholar]
- 12.Westblom TU, Gudipati S, DeRousse C, Midkiff BR, Belshe RB. Safety and immunogenicity of an inactivated hepatitis A vaccine: effect of dose and vaccination schedule. J Infect Dis. 1994;169:996–1001. doi: 10.1093/infdis/169.5.996. [DOI] [PubMed] [Google Scholar]
- 13.Holzer BR, Hatz C, Schmidt-Sissolak D, Glück R, Althaus B, Egger M. Immunogenicity and adverse effects of inactivated virosome versus alum-adsorbed hepatitis A vaccine: a randomized controlled trial. Vaccine. 1996;14:982–6. doi: 10.1016/0264-410X(96)00042-4. [DOI] [PubMed] [Google Scholar]
- 14.Fiore AE, Wasley A, Bell BP, Advisory Committee on Immunization Practices (ACIP) Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-7):1–23. [PubMed] [Google Scholar]
- 15.Loutan L, Bovier P, Althaus B, Glück R. Inactivated virosome hepatitis A vaccine. Lancet. 1994;343:322–4. doi: 10.1016/S0140-6736(94)91162-2. [DOI] [PubMed] [Google Scholar]
- 16.Just M, Berger R, Dreschsler H, Brantschen S, Glück R. A single vaccination with an inactivated hepatitis A liposome vaccine induces protective antibodies after only two weeks. Vaccine. 1992;10:737–9. doi: 10.1016/0264-410X(92)90506-F. [DOI] [PubMed] [Google Scholar]
- 17.Ambrosch F, Wiedermann G, Jonas S, Althaus B, Finkel B, Glück R, Herzog C. Immunogenicity and protectivity of a new liposomal hepatitis A vaccine. Vaccine. 1997;15:1209–13. doi: 10.1016/S0264-410X(97)00015-7. [DOI] [PubMed] [Google Scholar]
- 18.Bovier PA, Althaus B, Glueck R, Chippaux A, Loutan L. Tolerance and immunogenicity of the simultaneous administration of virosome hepatitis A and yellow fever vaccines. J Travel Med. 1999;6:228–33. doi: 10.1111/j.1708-8305.1999.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 19.Bovier PA, Farinelli T, Loutan L. Interchangeability and tolerability of a virosomal and an aluminum-adsorbed hepatitis A vaccine. Vaccine. 2005;23:2424–9. doi: 10.1016/j.vaccine.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 20.Pancharoen C, Mekmullica J, Thisyakorn U, Kasempimolporn S, Wilde H, Herzog C. Reduced-dose intradermal vaccination against hepatitis A with an aluminum-free vaccine is immunogenic and can lower costs. Clin Infect Dis. 2005;41:1537–40. doi: 10.1086/497266. [DOI] [PubMed] [Google Scholar]
- 21.Van Der Wielen M, Vertruyen A, Froesner G, Ibáñez R, Hunt M, Herzog C, Van Damme P. Immunogenicity and safety of a pediatric dose of a virosome-adjuvanted hepatitis A vaccine: a controlled trial in children aged 1-16 years. Pediatr Infect Dis J. 2007;26:705–10. doi: 10.1097/INF.0b013e31806215c8. [DOI] [PubMed] [Google Scholar]
- 22.Abarca K, Ibáñez I, de la Fuente P, Cerda L, Bergeret J, Frösner G, Ibarra H. Immunogenicity and tolerability of a paediatric presentation of a virosomal hepatitis A vaccine in Chilean children aged 1-16 years. Vaccine. 2011;29:8855–62. doi: 10.1016/j.vaccine.2011.09.095. [DOI] [PubMed] [Google Scholar]
- 23.Dagan R, Amir J, Livni G, Greenberg D, Abu-Abed J, Guy L, Ashkenazi S, Foresner G, Tewald F, Schätzl HM, et al. Concomitant administration of a virosome-adjuvanted hepatitis a vaccine with routine childhood vaccines at age twelve to fifteen months: a randomized controlled trial. Pediatr Infect Dis J. 2007;26:787–93. doi: 10.1097/INF.0b013e318060acbd. [DOI] [PubMed] [Google Scholar]
- 24.Clarke PD, Adams P, Ibáñez R, Herzog C. Rate, intensity, and duration of local reactions to a virosome-adjuvanted vs. an aluminium-adsorbed hepatitis A vaccine in UK travellers. Travel Med Infect Dis. 2006;4:313–8. doi: 10.1016/j.tmaid.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Mall ML, Rai RR, Philip M, Naik G, Parekh P, Bhawnani SC, Olowokure B, Shamanna M, Weil J. Seroepidemiology of hepatitis A infection in India: changing pattern. Indian J Gastroenterol. 2001;20:132–5. [PubMed] [Google Scholar]