Abstract
The current study examined the safety and immunogenicity of 23-valent pneumococcal capsular polysaccharide vaccine (Pneumo23® [PPV23], Sanofi Pasteur) as a booster dose in 12- to 18-month-old children primed with heptavalent pneumococcal vaccine (PCV7; Prevnar®, Pfizer). This was a randomized, observer-blinded, 2-arm, controlled, multicenter phase III study performed in Thailand to assess and describe the immunogenicity and safety of PPV23 as a booster dose in children who had received the 3 primary doses of PCV7, the pneumococcal vaccine available during the study period. Children primed with 3 doses of PCV7 were randomized 1:1 to receive a booster immunization with PPV23 or PCV7. Pneumococcal antibody concentrations were measured by enzyme-linked immunosorbent assay and functional antibody levels by multiplex opsonophagocytosis assay on day 30. A total of 339 children were enrolled. Geometric mean serum antibody concentrations against serotypes common to PCV7 and PPV23 (4, 6B, 9V, 14, 18C, 19F, and 23F) increased in both groups but they were higher for serotypes 4, 9V, 18C, and 19F in the PPV23 group. Opsonization indices increased in both groups for all measured serotypes (1, 6B, 14, 19A, and 23F) and were higher for serotypes 6B, 14, and 23F in the PCV7 group and for serotypes 1 and 19A in PPV23 group. Solicited reactions and unsolicited adverse events were similar in the 2 groups and generally mild and transient. No treatment-related serious adverse events were reported. These results confirm that boosting with PPV23 is immunogenic and well tolerated in healthy toddlers primed with PCV7.
Keywords: 23-valent pneumococcal capsular polysaccharide vaccine, booster, clinical trial, heptavalent pneumococcal conjugate vaccine, immunization, infant, phase III, pneumococcal vaccines
Introduction
Pneumococcal disease is the leading cause of death in children under the age of 5 y.1 Every year, children less than 5 y of age suffer from an estimated 14.5 million episodes of serious pneumococcal disease and 826 000 pneumococcal-related deaths, which accounted for 11% of all deaths in this age group.2 This continues to be a significant problem in developing countries where approximately 90% children less than 5 y of age live.2,3
Treatment of S. pneumoniae with antibiotics has greatly reduced mortality due to pneumococcal disease, but antibiotic overuse has resulted in the emergence of resistant strains; therefore, vaccines are considered an important way of limiting the impact of pneumococcal disease.4 Prevnar® (Pfizer, formerly Wyeth Ltd.), a pneumococcal conjugate vaccine (PCV), is the most widely used.5 The heptavalent version of Prevnar (PCV7), available since 2000, contains CRM197 diphtheria toxin-conjugated polysaccharides from serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. Whereas the serotypes in PCV7 account for only 39% to 53% of disease-causing serotypes in Africa, Asia, Latin America, and the Caribbean, the 13-valent version (PCV13) contains additional polysaccharides from serotypes more common in these regions (1, 3, 5, 6A, 7F, and 19A).6 Although PCV7 has reduced pneumococcal disease, disease caused by non-PCV7 serotypes has increased gradually, which may be due to serotype replacement.7 This implies that vaccines with an even wider coverage than PCV13 may eventually be necessary.
Pneumo23® (PPV23; Sanofi Pasteur) is a pneumococcal vaccine containing unconjugated polysaccharide from 23 serotypes (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F). PPV23 is indicated for the prevention of bacteremia, meningitis, and pneumonia caused by S. pneumoniae in adults and children 2 y of age or older with underlying medical conditions. Although PPV23 is poorly immunogenic in children less than 2 y of age if used as the primary series, it can induce similar or stronger immunogenic responses as PCV8-12 when used as a booster in PCV-primed children. Therefore, PPV23 is recommended by the US Advisory Committee on Immunization Practices as a post-PCV booster in children at high risk of disease.13
This randomized phase III trial, performed in Thailand, investigated the immunogenicity and safety of PPV23 as a booster dose in children age of 12–18 mo primed with 3 doses of PCV7. The primary objective of the study was to assess and describe the immunogenicity and safety of PPV23 as a booster dose in children who had received the 3 primary doses of PCV7 (at 2, 4, and 6 mo of age), the pneumococcal vaccine available during the study period. Because limited blood samples could be taken from the children, we examined the 12 most dominant serotypes found in Thai children (1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) by ELISA and 5 representative serotypes (1, 6B, 14, 19A, and 23F) by multiplex opsonophagocytic activity (MOPA) assay.
Results
Subjects
Of 339 children enrolled, 170 were randomized to be vaccinated with PPV23 (PPV23 group) and 169 to be vaccinated with PCV7 (PCV7 group). Mean ages (14.8 ± 1.5 mo) and male-to-female ratios (0.92) were similar in the 2 groups. One child in the PPV23 group was withdrawn by the caregiver before being vaccinated. Two vaccinated children in the PCV7 group were lost to follow-up before the end of the study. Thus, 169 children in the PPV23 group and 167 in the PCV7 group completed the study.
Serum antibody concentrations as determined by ELISA
Serotypes common to both PCV7 and PPV23 (4, 6B, 9V, 14, 18C, 19F, and 23F)
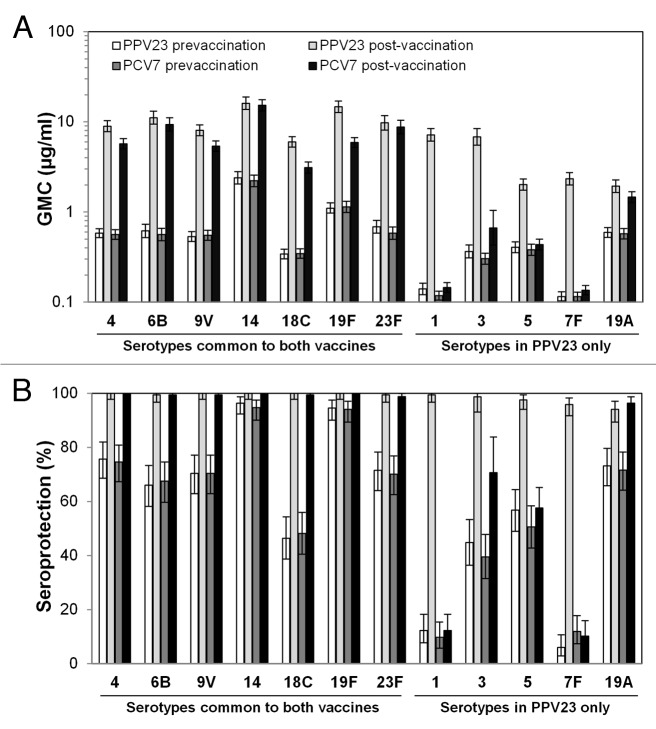
Following booster vaccination, geometric mean antibody concentrations (GMCs) for all 7 common serotypes increased in both groups. However, GMCs for serotypes 4, 9V, 18C, and 19F were significantly higher in the PPV23 group than in the PCV7 group (Fig. 1A). The post-boost seroprotection (≥0.35 μg/ml) rate was >99% of subjects in both groups (Fig. 1B).
Figure 1. Serum antibody concentrations. Before and 1 mo after booster vaccination, serum antibody concentrations were assessed by ELISA for the indicated serotypes. (A) GMCs. (B) Rates of seroprotection, defined as ≥0.35 μg/mL.
Serotypes exclusive to PPV23 (1, 3, 5, 7F, and 19A)
Following booster vaccination, GMCs to all 5 serotypes exclusive to PPV23 increased significantly in the PPV23 group, whereas only GMCs to serotype 3 and 19A increased significantly in the PCV7 group (Fig. 1A). In the PPV23 group, post-boost seroprotection rates for 5 serotypes ranged from 94.1% to 99.4% (Fig. 1B). In contrast, in the PCV7 group, post-boost seroprotection rates ranged from 10.2% to 96.4%, and only serotype 19A had a seroprotection rate over 75%.
Functional antibody concentrations as determined by MOPA
Serotypes common to PCV7 and PPV23 (6B, 14, 23F)
Following booster vaccination, opsonization indices (OIs) increased significantly for all 3 common serotypes in both treatment groups (Fig. 2A). However, OIs and post-vaccination-to-pre-vaccination geometric mean OI ratios for these serotypes were higher in the PCV7 group.
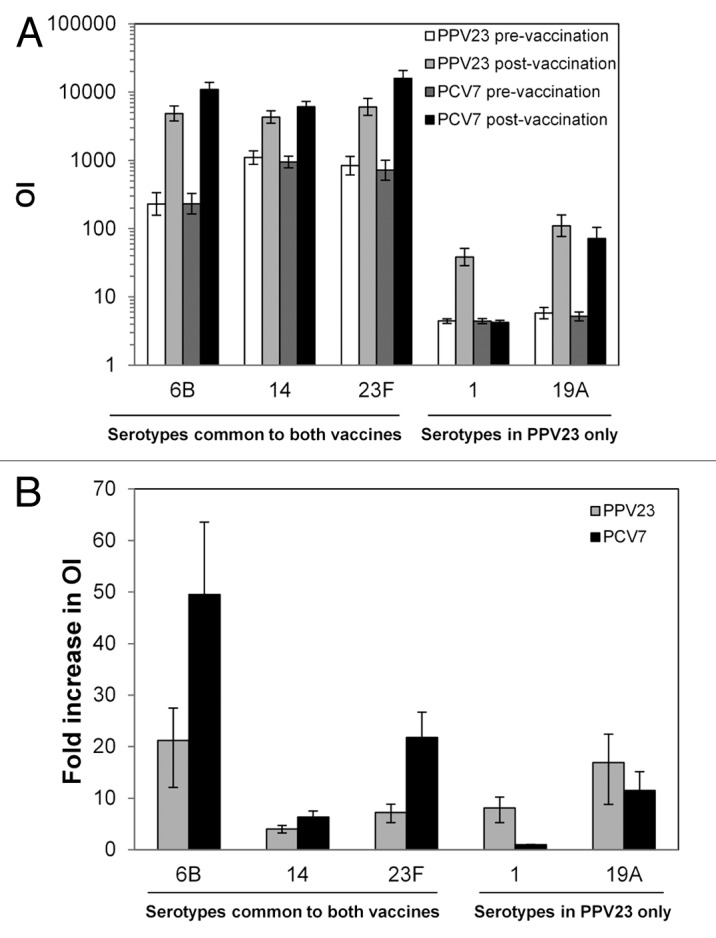
Figure 2. MOPA titers. Before and 1 mo after booster vaccination, functional antibody levels were assessed by MOPA for the indicated serotypes. (A) Geometric mean OIs. (B) Ratio of pre-vaccination geometric mean OI to post-vaccination geometric mean OI. In both panels, error bars indicate 95% confidence intervals. Values are shown for all subjects vaccinated according to the vaccine received.
Serotypes exclusive to PPV23 (1, 19A)
Following booster vaccination, OIs in the PPV23 group increased significantly for serotypes 1 and 19A (Fig. 2B). For PCV7 group, only the serotype 19A OIs increased.
Safety
Overall, 76.9% of subjects in the PPV23 group and 73.8% in the PCV7 group experienced solicited reactions (Table 1). Also, rates of individual solicited reactions were similar for the 2 vaccines (Fig. 3). Grade 3 reactions were experienced by 5 subjects boosted with PPV23 and 7 boosted with PCV7. The most common solicited local reaction was injection-site tenderness, and the most common solicited systemic reactions were irritability and abnormal crying. Most solicited reactions resolved within 3 d (Table S1). Two children experienced unsolicited adverse events (AEs) considered vaccination-related, both of which were injection-site bruising after PPV23 vaccination. Serious adverse events (SAEs) were reported by 6 subjects boosted with PPV23 (gastroenteritis [4 cases], viral croup [1 case], and near drowning [1 case]) and 1 boosted with PCV7 (gastroenteritis), but none of these was considered vaccination-related.
Table 1. Safety profiles between groups.
| Event | PPV23 n = 169 n (%) | PCV7 n = 169 n (%) |
|---|---|---|
| Solicited reactions | 130 (76.9) | 124 (73.8) |
| Grade 3 | 5 (3.0) | 7 (4.2) |
| Solicited local reaction | 114 (67.5) | 103 (61.3) |
| Grade 3 | 2 (1.2) | 3 (1.8) |
| Solicited systemic reaction | 88 (52.1) | 89 (53.3) |
| Grade 3 | 3 (1.8) | 5 (3.0) |
| Immediate unsolicited AE | 0 (0.0) | 1 (0.6) |
| Vaccination-related | 0 (0.0) | 0 (0.0) |
| Unsolicited AE | 72 (42.6) | 64 (37.9) |
| Vaccination-related | 2 (1.2) | 0 (0.0) |
| SAE | 6 (3.6) | 1 (0.6) |
| Vaccination-related | 0 (0.0) | 0 (0.0) |
Safety data are reported for all subjects vaccinated.
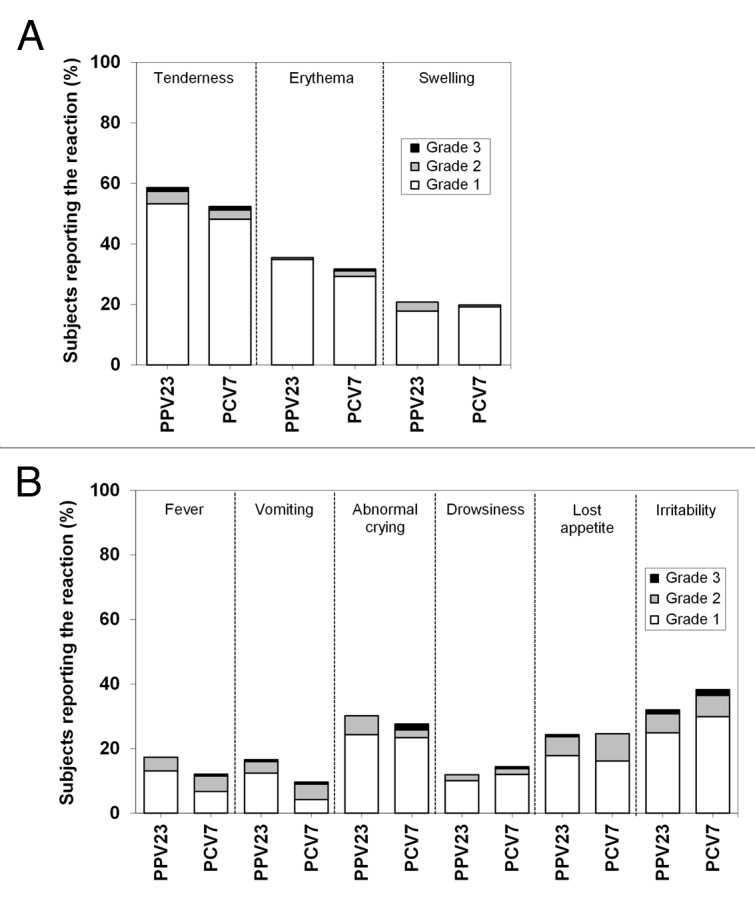
Figure 3. Solicited reactions. Solicited injection site (A) and systemic reactions (B) were assessed up to 7 d after vaccination. Solicited reactions were graded as follows: tenderness, 1 for a minor reaction when injection site is touched, 2 for child cries when injection site is touched, 3 for cries when injection site is moved or the movement of the injected limb is reduced; erythema and swelling, 1 for <2.5 cm in diameter, 2 for 2.5 to <5 cm in diameter, 3 for ≥5 cm in diameter; fever (axillary temperature), 1 for 38 °C to <38.5 °C, 2 for 38.5 °C to <39.5 °C, 3 for ≥39.5 °C; vomiting, 1 for 1 episode per 24 h, 2 for 2 to 5 episodes per 24 h, 3 for ≥6 episodes per 24 h; abnormal crying, 1 for <1 h, 2 for 1 to 3 h, 3 for >3 h; drowsiness, 1 for sleepier than usual or less interested in surroundings, 2 for not interested in surroundings or did not wake up for a feed/meal, 3 for sleeping most of the time or difficult to wake; loss of appetite, 1 for eating less than normal, 2 for missed 1 or 2 feeds/meals completely, 3 for refuses ≥3 feeds/meals or refuses most feeds/meals; irritability, 1 for easily consolable, 2 for requiring increased attention, 3 for inconsolable. Values are shown for all subjects vaccinated according to the vaccine received.
Discussion
PPV23 has been reported to boost antibody responses in children less than 2 y of age who had received PCV7 at 2, 4, and 6 mo of age.8,10,11,14-18 In particular, a previous phase II trial performed in Fiji showed that in children vaccinated with 1, 2, or 3 doses of PCV7, immune responses were improved by boosting with PPV23 compared with no booster.10,11 To extend these findings and to focus on immune responses in Asian children, we performed a phase III trial in which Thai children who had previously received the standard course of PCV7 at 2, 4, and 6 mo of age were randomized to boosting with PPV23 or PCV7 at 12 to 18 mo of age. We showed that boosting with PPV23 can increase serum pneumococcal antibody concentrations and functional antibody titers against certain PCV7 and non-PCV7 serotypes as much as or more than boosting by PCV7. This suggests that, although PPV23 alone is poorly immunogenic in children younger than 2 y of age,19 when used as a booster in PCV7-primed children, it can provide broader pneumococcal antibody responses than boosting with PCV7. The serum antibody response as measured by ELISA was stronger in the PPV23 group, which may have been due to the higher level of antigen (25 μg per strain). The study also confirmed that PPV23 and PCV7 have similar and good tolerability in toddlers.12
The findings are strengthened by the use of MOPA to measure functional antibody concentrations for 3 common serotypes (6B, 14, 23F) and 2 serotypes exclusive to PPV23 (1, 19A). Although MOPA is more cumbersome than the ELISA, the OI is generally considered to be a more reliable surrogate of efficacy than the serum antibody concentration.20-22 OIs for the 3 serotypes common to both vaccines were higher in children primed with PCV7. This might be due the need for multiple exposures to the same antigen to produce high-affinity antibodies: children in the PCV7 group were exposed 4 times to these strains, while those in the PPV23 group were exposed only once. Despite this, functional antibody responses were induced in the PPV23 group even to serotypes for which the children had not been primed. We also found that boosting with PCV7 induced both ELISA and MOPA responses to serotype 19A, which is not included in PCV7. This could have been due to cross-reactivity of antibodies to serotype 19F.11,20,23
Because limited blood samples could be taken from the children, measuring all pneumococcal serotypes in PPV23 and PCV7 was not possible. For the ELISA, we examined the 12 serotypes most dominant in Thai children between 2000 and 2005, the most recent data available at the time of the study.24 We selected only 5 serotypes because of the complexity of the assay and the need for larger volumes of serum. Serotypes 6B, 14, and 23 were included because they were 3 of the 4 serotypes most commonly found in invasive pneumococcal disease for children less than 5 y of age.24 We replaced 19F, also 1 of the 4 most common, with 19A because infections from it have been increasing in children less than 5 y of age,25,26 because it is present in both PPV23 and PCV13, and because antibodies against it cross-protect against 19F.11,20,23 Serotype 1 was selected because, like 19A, it was unique to PPV23. Data collected in 2006–2009 (i.e., after this study was performed) confirmed that serotypes 6B, 23F, 14, 19F, and 19A were the most common causes of invasive pneumococcal disease in Thai children less than 5 y of age at the time this study was performed (2007–2008).27 Although we do not know how effective boosting is for the serotypes that we did not assess, the results strongly suggest that boosting with PPV23 provides broader immune responses that boosting with PCV7.
A threshold of 0.35 μg/mL is recommended by the World Health Organization as a correlate of protection when using ELISA without 22F pre-absorption.28 Bridging studies have suggested that a lower threshold (0.1–0.2 μg/mL) can be used for the 22F pre-absorption ELISA in some cases,29 although the World Health Organization recommends retaining the 0.35 μg/mL threshold even when 22F pre-absorption is used.30 Accordingly, although we performed 22F pre-absorption ELISA in this study, we used the WHO threshold of 0.35 μg/mL, which we consider a more conservative estimate of protection. Regardless of the threshold, antibody concentrations are only correlates of protection and effectiveness studies are needed to determine to what extent PPV23 influences pneumococcal disease and carriage.
The US Advisory Committee on Immunization Practices guidelines state that PPV23 should be used as a booster vaccination in children 2 y and older at high risk for disease.13 This study, however, showed that PPV23 boosting in healthy children 12 to 18 mo old primed with a PCV can induce broader antibody responses and at a lower cost than boosting with PCV7, which is important in developing countries like Thailand. Whether this translates to clinical efficacy should be assessed in further clinical studies.
Patients and methods
Study design
This was a randomized, observer-blinded, 2-arm, controlled, multi-center phase III study performed in Thailand between November 2007 and March 2008 (ClinicalTrials.gov identifier: NCT00594347). The primary objective of the study was to assess and describe the immunogenicity and safety of PPV23 as a booster dose in children who had received the 3 primary doses of PCV7 (at 2, 4, and 6 mo of age), the pneumococcal vaccine available during the study period. The study was performed in compliance with Good Clinical Practice and the 2004 revision of the Declaration of Helsinki. Parents or legal guardians of all children gave written informed consent for their inclusion in the study.
Subjects
The children included in this study had participated in a previous phase III study where they were co-administered PCV7 and an experimental or a licensed hexavalent diphtheria-tetanus-pertussis-poliovirus-hepatitis B-hemophilus influenza type b vaccine at 2, 4, and 6 mo of age.31 Parents or guardians were invited to have their children participate in the current study if their child was 12 to 18 mo of age and had received all 3 PCV7 doses. Children were excluded if they had known or suspected congenital or acquired immunodeficiency or were receiving immunosuppressive therapy; systemic hypersensitivity to any of the vaccine components or history of a life-threatening reaction to the trial vaccine or to a vaccine containing any of the same substances; chronic illness at a stage that could interfere with trial conduct or completion; received blood or blood-derived products since birth that might interfere with the assessment of immune response; received any vaccine within the previous 4 wk; had a history of seizures; a history of seropositivity for human immunodeficiency virus, hepatitis B or C; a history of pneumococcal infection; previous booster vaccination against pneumococcal disease; thrombocytopenia, bleeding disorder, or received anticoagulants within the previous 3 wk; or febrile illness (temperature ≥38 °C), moderate or severe acute illness, or infection on the day of vaccination.
Treatments
Eligible children whose parents provided written informed consent for their participation in this study were randomized 1:1 to receive a booster immunization with 0.5 mL of PPV23 (batch B0112-1) or PCV7 (batch 25514). The randomization list was prepared by the permuted block method using SAS® version 9.1 (SAS Institute) and provided as a scratchable list. Treatments were assigned by the nurse administering the vaccine. PPV23 was provided in single-dose (0.5 mL) prefilled syringes, and PCV7 was provided in prefilled syringes or vials. All vaccinations were administered by intramuscular injection into the anterolateral area of the right deltoid.
Assessments
On day 30, antibody titers against serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F were measured by ELISA and functional antibody titers against serotypes 1, 6B, 14, 23F, and 19A were measured by MOPA. The vaccine serotypes and the serotypes measured in this study are summarized in Figure 4.
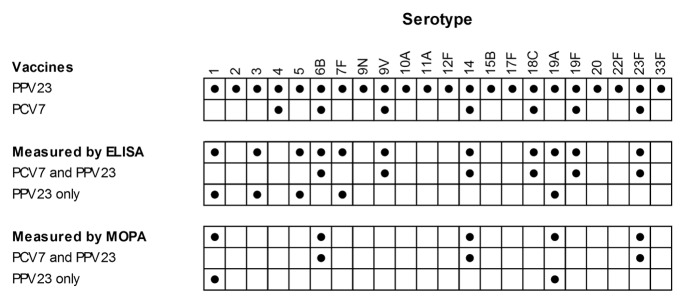
Figure 4. Pneumococcal serotypes present in PPV23 and PCV7 and measured by ELISA and MOPA.
The ELISA was performed at the World Health Organization Reference Laboratory for Pneumococcal Serology (UCL Institute of Child Health) and as described by Plikaytis et al.32 Briefly, sera and internal controls were incubated for with 22F pneumococcal capsular polysaccharide to reduce the interference of non-specific homologous antibodies.33 Samples and the reference standard (US Reference Pneumococcal Serum Lot 89SF; Centers for Biologics Evaluation and Research) were added as serial 2.5-fold dilutions to microtiter plates coated with type-specific pneumococcal capsular polysaccharide antigen (American Type Culture Collection), and after 2 h at room temperature or 18 h at 4 °C, bound antibody was detected with horseradish peroxidase-conjugated goat anti-human IgG conjugate, followed by conversion of 3,3′,5,5′-tetramethylbenzidine and spectrophotometric detection at 405 nm. Seroprotection was defined as an ELISA antibody concentration ≥0.35 μg/mL.34 Detection limits were 0.022 μg/mL for serotype 1, 0.011 μg/mL for serotype 3, 0.01 μg/mL for serotype 4, 0.024 μg/mL for serotype 5, 0.018 μg/mL for serotype 6B, 0.014 μg/mL for serotype 7F, 0.008 μg/mL for serotype 9V, 0.022 μg/mL for serotype 14, 0.006 μg/mL for serotype 18C, 0.027 μg/mL for serotype 19A, 0.013 μg/mL for serotype 19F, and 0.003 μg/mL for serotype 19F.
MOPA was performed as described by Burton and Nahm.35 Briefly, serially diluted test sera and controls were incubated with bacterial solutions (cassette 1, serotypes 4, 6B, 14 and 23F; cassette 2, serotypes 9V, 18C, 19F and 6A; and cassette 3, serotypes 1, 5, 7F, and 19A), followed by baby rabbit complement, and finally differentiated HL60 cells. Reactions were stopped by chilling on ice and were plated onto a solid medium and dried. Todd-Hewitt-yeast extract agar supplemented with different antibiotics (streptomycin, optochin, spectinomycin, or trimethoprim) was overlaid on the samples, and colonies were counted after overnight incubation at 37 °C. The OI was the reciprocal serum sample dilution yielding 50% killing compared with the growth in control wells containing no serum. The detection limit was 1:8 for all serotypes.
Safety
AEs were recorded as described by the International Conference for Harmonization E2A Guideline for Clinical Safety Data Management. Solicited injection site reactions (tenderness, erythema, and swelling) and systemic reactions (fever, vomiting, abnormal crying, drowsiness, appetite loss, and irritability) were recorded for 7 d, and unsolicited AEs and SAEs were recorded for the duration of the study.
Sample size
The sample size was based only on the number of children completing the previous study31 and eligible and willing to participate in this study.
Statistical analysis
Statistical analysis was performed using SAS® version 9.1 (SAS Institute). No statistical hypothesis was tested. Immunogenicity and safety calculations were made in all vaccinated subjects according to the vaccine they actually received. Missing data were not replaced.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was funded by Sanofi Pasteur. Medical writing was provided by Dr Phillip Leventhal (4Clinics, Paris, France) and funded by Sanofi Pasteur.
Glossary
Abbreviations:
- AE
adverse event
- ELISA
enzyme-linked immunoassay
- GMC
geometric mean antibody concentration
- multiplex opsonophagocytosis assay
MOPA
- OI
opsonization index
- PCV
pneumococcal conjugate vaccine
- PCV7
heptavalent pneumococcal conjugate vaccine
- PCV13
13-valent pneumococcal conjugate vaccine
- PPV23
23-valent unconjugated pneumococcal vaccine
References
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, et al. Child Health Epidemiology Reference Group of WHO and UNICEF Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.O’Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, Hib and Pneumococcal Global Burden of Disease Study Team Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 3.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/S1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 4.Lynch JP, 3rd, Zhanel GG. Streptococcus pneumoniae: epidemiology and risk factors, evolution of antimicrobial resistance, and impact of vaccines. Curr Opin Pulm Med. 2010;16:217–25. doi: 10.1097/MCP.0b013e3283385653. [DOI] [PubMed] [Google Scholar]
- 5.Improving Global Health by Preventing Pneumococcal Disease [Internet]. All-Party Parliamentary Group on Pneumococcal Disease Prevention in the Developing World; 2008.Available from http://www.path.org/vaccineresources/details.php?i=692
- 6.Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC) Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59(RR-11):1–18. [PubMed] [Google Scholar]
- 7.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–73. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blum MD, Dagan R, Mendelman PM, Pinsk V, Giordani M, Li S, Bohidar N, McNeely TB. A comparison of multiple regimens of pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine and pneumococcal polysaccharide vaccine in toddlers. Vaccine. 2000;18:2359–67. doi: 10.1016/S0264-410X(00)00021-9. [DOI] [PubMed] [Google Scholar]
- 9.Dagan R, Melamed R, Zamir O, Leroy O. Safety and immunogenicity of tetravalent pneumococcal vaccines containing 6B, 14, 19F and 23F polysaccharides conjugated to either tetanus toxoid or diphtheria toxoid in young infants and their boosterability by native polysaccharide antigens. Pediatr Infect Dis J. 1997;16:1053–9. doi: 10.1097/00006454-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Russell FM, Balloch A, Licciardi PV, Carapetis JR, Tikoduadua L, Waqatakirewa L, Cheung YB, Mulholland EK, Tang ML. Serotype-specific avidity is achieved following a single dose of the 7-valent pneumococcal conjugate vaccine, and is enhanced by 23-valent pneumococcal polysaccharide booster at 12 months. Vaccine. 2011;29:4499–506. doi: 10.1016/j.vaccine.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell FM, Carapetis JR, Burton RL, Lin J, Licciardi PV, Balloch A, Tikoduadua L, Waqatakirewa L, Cheung YB, Tang ML, et al. Opsonophagocytic activity following a reduced dose 7-valent pneumococcal conjugate vaccine infant primary series and 23-valent pneumococcal polysaccharide vaccine at 12 months of age. Vaccine. 2011;29:535–44. doi: 10.1016/j.vaccine.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell FM, Licciardi PV, Balloch A, Biaukula V, Tikoduadua L, Carapetis JR, Nelson J, Jenney AW, Waqatakirewa L, Colquhoun S, et al. Safety and immunogenicity of the 23-valent pneumococcal polysaccharide vaccine at 12 months of age, following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine in infancy. Vaccine. 2010;28:3086–94. doi: 10.1016/j.vaccine.2010.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Advisory Committee on Immunization Practices. Vaccines to prevent pneumococcal disease. Resolution No. 02/13-1, 2013. Available from: http://www.cdc.gov/vaccines/programs/vfc/downloads/resolutions/02-13-1-pneumo.pdf
- 14.Ahman H, Käyhty H, Lehtonen H, Leroy O, Froeschle J, Eskola J. Streptococcus pneumoniae capsular polysaccharide-diphtheria toxoid conjugate vaccine is immunogenic in early infancy and able to induce immunologic memory. Pediatr Infect Dis J. 1998;17:211–6. doi: 10.1097/00006454-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Choo S, Seymour L, Morris R, Quataert S, Lockhart S, Cartwright K, Finn A. Immunogenicity and reactogenicity of a pneumococcal conjugate vaccine administered combined with a haemophilus influenzae type B conjugate vaccine in United Kingdom infants. Pediatr Infect Dis J. 2000;19:854–62. doi: 10.1097/00006454-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Goldblatt D, Southern J, Ashton L, Richmond P, Burbidge P, Tasevska J, Crowley-Luke A, Andrews N, Morris R, Borrow R, et al. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 2006;25:312–9. doi: 10.1097/01.inf.0000207483.60267.e7. [DOI] [PubMed] [Google Scholar]
- 17.Huebner RE, Mbelle N, Forrest B, Madore DV, Klugman KP. Long-term antibody levels and booster responses in South African children immunized with nonavalent pneumococcal conjugate vaccine. Vaccine. 2004;22:2696–700. doi: 10.1016/j.vaccine.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Kilpi T, Ahman H, Jokinen J, Lankinen KS, Palmu A, Savolainen H, Grönholm M, Leinonen M, Hovi T, Eskola J, et al. Finnish Otitis Media Study Group Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin Infect Dis. 2003;37:1155–64. doi: 10.1086/378744. [DOI] [PubMed] [Google Scholar]
- 19.Bogaert D, Hermans PW, Adrian PV, Rümke HC, de Groot R. Pneumococcal vaccines: an update on current strategies. Vaccine. 2004;22:2209–20. doi: 10.1016/j.vaccine.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 20.Schuerman L, Wysocki J, Tejedor JC, Knuf M, Kim KH, Poolman J. Prediction of pneumococcal conjugate vaccine effectiveness against invasive pneumococcal disease using opsonophagocytic activity and antibody concentrations determined by enzyme-linked immunosorbent assay with 22F adsorption. Clin Vaccine Immunol. 2011;18:2161–7. doi: 10.1128/CVI.05313-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol. 2006;13:165–9. doi: 10.1128/CVI.13.2.165-169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee H, Nahm MH, Burton R, Kim KH. Immune response in infants to the heptavalent pneumococcal conjugate vaccine against vaccine-related serotypes 6A and 19A. Clin Vaccine Immunol. 2009;16:376–81. doi: 10.1128/CVI.00344-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hausdorff WP, Hoet B, Schuerman L. Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A? BMC Pediatr. 2010;10:4. doi: 10.1186/1471-2431-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phongsamart W, Srifeungfung S, Dejsirilert S, Chatsuwan T, Nunthapisud P, Treerauthaweeraphong V, Rungnobhakhun P, Chokephaibulkit K. Serotype distribution and antimicrobial susceptibility of S. pneumoniae causing invasive disease in Thai children younger than 5 years old, 2000-2005. Vaccine. 2007;25:1275–80. doi: 10.1016/j.vaccine.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Steenhoff AP, Shah SS, Ratner AJ, Patil SM, McGowan KL. Emergence of vaccine-related pneumococcal serotypes as a cause of bacteremia. Clin Infect Dis. 2006;42:907–14. doi: 10.1086/500941. [DOI] [PubMed] [Google Scholar]
- 26.Muñoz-Almagro C, Jordan I, Gene A, Latorre C, Garcia-Garcia JJ, Pallares R. Emergence of invasive pneumococcal disease caused by nonvaccine serotypes in the era of 7-valent conjugate vaccine. Clin Infect Dis. 2008;46:174–82. doi: 10.1086/524660. [DOI] [PubMed] [Google Scholar]
- 27.Srifeungfung S, Tribuddharat C, Comerungsee S, Chatsuwan T, Treerauthanaweeraphong V, Rungnobhakhun P, Nunthapisud P, Chokephaibulkit K. Serotype coverage of pneumococcal conjugate vaccine and drug susceptibility of Streptococcus pneumoniae isolated from invasive or non-invasive diseases in central Thailand, 2006-2009. Vaccine. 2010;28:3440–4. doi: 10.1016/j.vaccine.2010.02.071. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. WHO Expert Committee on Biological Standardization 54th report ed. Geneva: World Health Organization, 2005. [PubMed] [Google Scholar]
- 29.Poolman JT, Frasch CE, Käyhty H, Lestrate P, Madhi SA, Henckaerts I. Evaluation of pneumococcal polysaccharide immunoassays using a 22F adsorption step with serum samples from infants vaccinated with conjugate vaccines. Clin Vaccine Immunol. 2010;17:134–42. doi: 10.1128/CVI.00289-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siber GR, Chang I, Baker S, Fernsten P, O’Brien KL, Santosham M, Klugman KP, Madhi SA, Paradiso P, Kohberger R. Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine. 2007;25:3816–26. doi: 10.1016/j.vaccine.2007.01.119. [DOI] [PubMed] [Google Scholar]
- 31.Kosalaraksa P, Thisyakorn U, Benjaponpitak S, Chokephaibulkit K, Santos-Lima E. Immunogenicity and safety study of a new DTaP-IPV-Hep B-PRP-T combined vaccine compared to a licensed DTaP-IPV-Hep B//PRP-T comparator, both concomitantly administered with a 7-valent pneumococcal conjugate vaccine at 2, 4, and 6 months of age in Thai infants. Int J Infect Dis. 2011;15:e249–56. doi: 10.1016/j.ijid.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Plikaytis BD, Goldblatt D, Frasch CE, Blondeau C, Bybel MJ, Giebink GS, Jonsdottir I, Käyhty H, Konradsen HB, Madore DV, et al. An analytical model applied to a multicenter pneumococcal enzyme-linked immunosorbent assay study. J Clin Microbiol. 2000;38:2043–50. doi: 10.1128/jcm.38.6.2043-2050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Concepcion NF, Frasch CE. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2001;8:266–72. doi: 10.1128/CDLI.8.2.266-272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO Publication Pneumococcal vaccines WHO position paper - 2012 - recommendations. Vaccine. 2012;30:4717–8. doi: 10.1016/j.vaccine.2012.04.093. [DOI] [PubMed] [Google Scholar]
- 35.Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006;13:1004–9. doi: 10.1128/CVI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.