Abstract
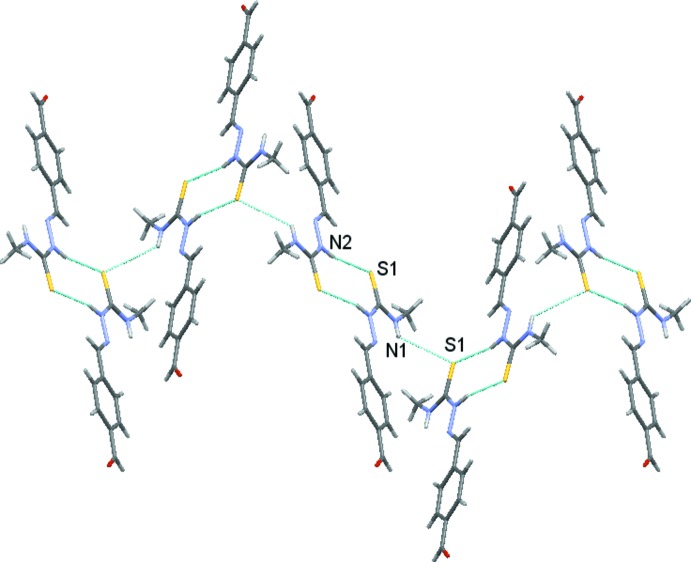
The structure of the title compound, C10H11N3OS, comprises an approximately planar molecule, with the r.m.s. deviation for the 15 non-H atoms being 0.089 Å. The conformation about the imine bond is E and an intramolecular N—H⋯N hydrogen bond is evident. Molecules are linked into a supramolecular chain along the b axis by N—H⋯S hydrogen bonds.
Keywords: crystal structure, thiosemicarbazone, thiourea, hydrogen bonding
Related literature
For the synthesis of the title compound, see: Jagst et al. (2005 ▶). For biological properties, see: Serda et al. (2012 ▶). For supramolecular studies of thiosemicarbazones, see: Alonso et al. (2002 ▶).
Experimental
Crystal data
C10H11N3OS
M r = 221.28
Orthorhombic,
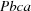
a = 13.1231 (3) Å
b = 8.8559 (2) Å
c = 19.3702 (4) Å
V = 2251.14 (9) Å3
Z = 8
Cu Kα radiation
μ = 2.38 mm−1
T = 296 K
0.14 × 0.13 × 0.05 mm
Data collection
Bruker CCD SMART 6000 diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2007 ▶) T min = 0.730, T max = 0.898
22698 measured reflections
1986 independent reflections
1798 reflections with I > 2σ(I)
R int = 0.046
Refinement
R[F 2 > 2σ(F 2)] = 0.034
wR(F 2) = 0.100
S = 1.08
1986 reflections
145 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.21 e Å−3
Δρmin = −0.16 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT (Bruker, 2007 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: Mercury (Bruno et al., 2002 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S1600536814016407/tk5328sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814016407/tk5328Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536814016407/tk5328Isup3.cml
. DOI: 10.1107/S1600536814016407/tk5328fig1.tif
The molecular structure of the title compound showing the atom-labelling scheme and displacement ellipsoids at the 50% probability level.
. DOI: 10.1107/S1600536814016407/tk5328fig2.tif
View of supramolecular chain formed by N—H⋯S interactions (dashed lines).
CCDC reference: 1014062
Additional supporting information: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H2N⋯S1i | 0.902 (19) | 2.53 (2) | 3.4154 (14) | 165.6 (16) |
| N1—H1⋯N3 | 0.84 (2) | 2.238 (18) | 2.6467 (18) | 109.9 (15) |
| N1—H1⋯S1ii | 0.84 (2) | 2.992 (19) | 3.5401 (15) | 124.6 (16) |
Symmetry codes: (i) 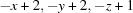 ; (ii)
; (ii) 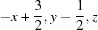 .
.
Acknowledgments
This research was supported by the European Rural Development Fund and the Spanish Ministry of Education and Science through Project CTQ2010–19386/BQU.
supplementary crystallographic information
S1. Chemical context
S2. Structural commentary
S3. Synthesis and crystallization
A solution of 4-methyl-3-thiosemicarbazide (392 mg, 3.72 mmol) in water (50 mL) was slowly added at 50°C to a solution of terephthaldicarboxaldehyde (500 mg, 3.73 mmol) in 100 mL water. Then the mixture was stirred at 50°C for 30 mins. Once cooled to room temperature, the yellow solid was filtered off and vacuum dried. Yellow single crystals suitable for X-ray diffraction were obtained by recrystallization from EtOH/H2O (1:1). Yield: 91%, M. pt: 213-216 °C. IR data (KBr, cm-1): 3368m, 3150m ν(N—H); 2838w, 2742w ν(C—H aldehyde); 1692 s ν(C═O); 1545 s, 1257m ν(C═N), 833m, 777w ν(C═S). 1H NMR data (DMSO-d6, ppm): 11.72 (s, 1H, N(2)—H); 10.03 (s, 1H, C(1)—H); 8.69 (s, 1H, N(2)—H); 8.11 (s, 1H, C(8)—H); 8.04 (d, 2H, J = 8.1 Hz, C(3,7)-H); 7.94 (d, 2H, J = 8.1 Hz, C(4,6)-H); 3.04 (s, 3H, C(10)—H).
S4. Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H = 0.95–0.99 Å) and were included in the refinement in the riding model approximation, with Uiso(H) = 1.2Ueq(C). The N-bound H-atoms were located in a difference Fourier map but were refined with distance restraints N—H = 0.84 (1) and 0.90 (1) Å, and with Uiso(H) = 1.2Ueq(N).
Figures
Fig. 1.
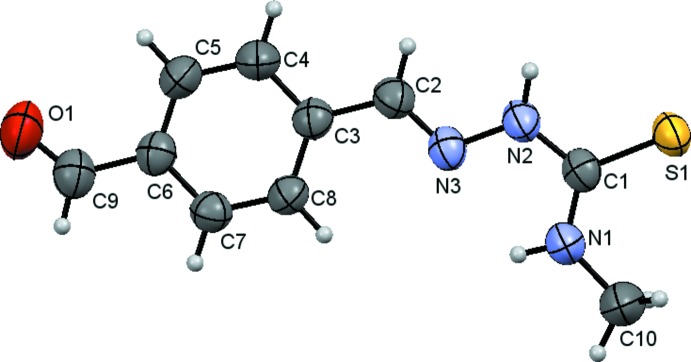
The molecular structure of the title compound showing the atom-labelling scheme and displacement ellipsoids at the 50% probability level.
Fig. 2.
View of supramolecular chain formed by N—H···S interactions (dashed lines).
Crystal data
| C10H11N3OS | F(000) = 928 |
| Mr = 221.28 | Dx = 1.306 Mg m−3 |
| Orthorhombic, Pbca | Cu Kα radiation, λ = 1.54178 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 9894 reflections |
| a = 13.1231 (3) Å | θ = 4.6–66.6° |
| b = 8.8559 (2) Å | µ = 2.38 mm−1 |
| c = 19.3702 (4) Å | T = 296 K |
| V = 2251.14 (9) Å3 | Plate, yellow |
| Z = 8 | 0.14 × 0.13 × 0.05 mm |
Data collection
| Bruker CCD SMART 6000 diffractometer | 1986 independent reflections |
| Radiation source: fine-focus sealed tube | 1798 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.046 |
| φ and ω scans | θmax = 66.6°, θmin = 4.6° |
| Absorption correction: multi-scan (SADABS; Bruker, 2007) | h = −15→15 |
| Tmin = 0.730, Tmax = 0.898 | k = −10→10 |
| 22698 measured reflections | l = −22→22 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.034 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.100 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.08 | w = 1/[σ2(Fo2) + (0.0559P)2 + 0.3598P] where P = (Fo2 + 2Fc2)/3 |
| 1986 reflections | (Δ/σ)max < 0.001 |
| 145 parameters | Δρmax = 0.21 e Å−3 |
| 0 restraints | Δρmin = −0.16 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.90393 (3) | 1.04683 (4) | 0.40705 (2) | 0.06222 (18) | |
| O1 | 0.88220 (12) | −0.01792 (18) | 0.72752 (8) | 0.0921 (5) | |
| N1 | 0.81376 (12) | 0.78044 (16) | 0.39148 (7) | 0.0655 (4) | |
| C1 | 0.87314 (11) | 0.86850 (16) | 0.42864 (8) | 0.0517 (3) | |
| N2 | 0.90945 (10) | 0.81086 (15) | 0.48868 (7) | 0.0579 (3) | |
| C2 | 0.92328 (12) | 0.62083 (19) | 0.56504 (9) | 0.0605 (4) | |
| H2 | 0.9617 | 0.6879 | 0.5913 | 0.073* | |
| N3 | 0.88781 (9) | 0.66473 (14) | 0.50704 (7) | 0.0542 (3) | |
| C3 | 0.90556 (11) | 0.46928 (19) | 0.59137 (8) | 0.0536 (4) | |
| C4 | 0.93836 (15) | 0.43343 (19) | 0.65777 (9) | 0.0678 (4) | |
| H4 | 0.9707 | 0.5066 | 0.6844 | 0.081* | |
| C5 | 0.92354 (14) | 0.2913 (2) | 0.68448 (9) | 0.0675 (4) | |
| H5 | 0.9454 | 0.2692 | 0.7290 | 0.081* | |
| C6 | 0.87602 (11) | 0.18070 (18) | 0.64526 (8) | 0.0554 (4) | |
| C7 | 0.84324 (11) | 0.21631 (18) | 0.57902 (8) | 0.0558 (4) | |
| H7 | 0.8112 | 0.1429 | 0.5524 | 0.067* | |
| C8 | 0.85745 (11) | 0.35836 (17) | 0.55224 (8) | 0.0543 (4) | |
| H8 | 0.8349 | 0.3805 | 0.5079 | 0.065* | |
| C9 | 0.86069 (14) | 0.0265 (2) | 0.67155 (10) | 0.0678 (4) | |
| H9 | 0.8313 | −0.0428 | 0.6415 | 0.081* | |
| C10 | 0.76953 (19) | 0.8229 (2) | 0.32581 (10) | 0.0924 (7) | |
| H10A | 0.7165 | 0.8957 | 0.3332 | 0.139* | |
| H10B | 0.7415 | 0.7351 | 0.3038 | 0.139* | |
| H10C | 0.8213 | 0.8660 | 0.2968 | 0.139* | |
| H2N | 0.9542 (15) | 0.865 (2) | 0.5138 (9) | 0.075 (5)* | |
| H1 | 0.8010 (15) | 0.695 (2) | 0.4085 (9) | 0.073 (6)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0655 (3) | 0.0429 (3) | 0.0782 (3) | −0.00218 (15) | −0.01004 (18) | 0.00628 (16) |
| O1 | 0.1022 (10) | 0.0890 (10) | 0.0851 (9) | 0.0013 (8) | −0.0037 (8) | 0.0320 (8) |
| N1 | 0.0797 (9) | 0.0507 (8) | 0.0662 (8) | −0.0120 (7) | −0.0135 (7) | 0.0075 (6) |
| C1 | 0.0458 (7) | 0.0469 (8) | 0.0622 (8) | 0.0028 (6) | 0.0023 (6) | 0.0008 (6) |
| N2 | 0.0550 (7) | 0.0479 (7) | 0.0707 (8) | −0.0065 (5) | −0.0097 (6) | 0.0089 (6) |
| C2 | 0.0566 (8) | 0.0555 (9) | 0.0693 (9) | −0.0066 (7) | −0.0097 (7) | 0.0047 (7) |
| N3 | 0.0477 (6) | 0.0487 (7) | 0.0663 (8) | −0.0014 (5) | −0.0001 (5) | 0.0069 (6) |
| C3 | 0.0468 (8) | 0.0555 (10) | 0.0585 (9) | 0.0000 (6) | −0.0032 (6) | 0.0047 (6) |
| C4 | 0.0759 (11) | 0.0641 (10) | 0.0635 (9) | −0.0119 (8) | −0.0176 (8) | 0.0023 (7) |
| C5 | 0.0763 (10) | 0.0707 (11) | 0.0555 (9) | −0.0044 (8) | −0.0131 (8) | 0.0106 (8) |
| C6 | 0.0504 (8) | 0.0573 (9) | 0.0585 (8) | 0.0032 (6) | 0.0018 (6) | 0.0059 (7) |
| C7 | 0.0539 (8) | 0.0544 (9) | 0.0590 (8) | −0.0013 (6) | −0.0037 (6) | −0.0017 (7) |
| C8 | 0.0536 (8) | 0.0564 (9) | 0.0531 (7) | 0.0013 (6) | −0.0071 (6) | 0.0040 (6) |
| C9 | 0.0656 (10) | 0.0653 (10) | 0.0724 (10) | 0.0035 (8) | 0.0001 (8) | 0.0122 (8) |
| C10 | 0.1231 (18) | 0.0784 (12) | 0.0756 (11) | −0.0277 (12) | −0.0324 (12) | 0.0131 (10) |
Geometric parameters (Å, º)
| S1—C1 | 1.6829 (15) | C3—C8 | 1.392 (2) |
| O1—C9 | 1.187 (2) | C3—C4 | 1.393 (2) |
| N1—C1 | 1.317 (2) | C4—C5 | 1.375 (2) |
| N1—C10 | 1.448 (2) | C5—C6 | 1.388 (2) |
| C1—N2 | 1.356 (2) | C6—C7 | 1.390 (2) |
| N2—N3 | 1.3718 (18) | C6—C9 | 1.471 (2) |
| C2—N3 | 1.277 (2) | C7—C8 | 1.373 (2) |
| C2—C3 | 1.454 (2) | ||
| C1—N1—C10 | 124.33 (15) | C4—C3—C2 | 118.99 (15) |
| N1—C1—N2 | 116.98 (14) | C5—C4—C3 | 120.82 (15) |
| N1—C1—S1 | 124.20 (12) | C4—C5—C6 | 120.26 (15) |
| N2—C1—S1 | 118.81 (12) | C5—C6—C7 | 118.98 (15) |
| C1—N2—N3 | 120.30 (13) | C5—C6—C9 | 121.80 (15) |
| N3—C2—C3 | 122.09 (15) | C7—C6—C9 | 119.21 (15) |
| C2—N3—N2 | 116.10 (13) | C8—C7—C6 | 120.97 (15) |
| C8—C3—C4 | 118.82 (15) | C7—C8—C3 | 120.15 (14) |
| C8—C3—C2 | 122.19 (14) | O1—C9—C6 | 126.24 (19) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H2N···S1i | 0.902 (19) | 2.53 (2) | 3.4154 (14) | 165.6 (16) |
| N1—H1···N3 | 0.84 (2) | 2.238 (18) | 2.6467 (18) | 109.9 (15) |
| N1—H1···S1ii | 0.84 (2) | 2.992 (19) | 3.5401 (15) | 124.6 (16) |
Symmetry codes: (i) −x+2, −y+2, −z+1; (ii) −x+3/2, y−1/2, z.
Footnotes
Supporting information for this paper is available from the IUCr electronic archives (Reference: TK5328).
References
- Alonso, R., Bermejo, E., Carballo, R., Castiñeiras, A. & Pérez, T. (2002). J. Mol. Struct. 606, 155–173.
- Bruker (2007). SADABS, APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruno, I. J., Cole, J. C., Edgington, P. R., Kessler, M., Macrae, C. F., McCabe, P., Pearson, J. & Taylor, R. (2002). Acta Cryst. B58, 389–397. [DOI] [PubMed]
- Jagst, A., Sánchez, A., Vázquez-López, E. M. & Abram, U. (2005). Inorg. Chem. 44, 5738–5744. [DOI] [PubMed]
- Serda, M., Mrozek-Wilczkiewicz, A., Jampilek, J., Pesko, M., Kralova, K., Vejsova, M., Musiol, R., Ratuszna, A. & Polanski, J. (2012). Molecules, 17, 13483–13502. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst. 43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, New_Global_Publ_Block. DOI: 10.1107/S1600536814016407/tk5328sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536814016407/tk5328Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536814016407/tk5328Isup3.cml
. DOI: 10.1107/S1600536814016407/tk5328fig1.tif
The molecular structure of the title compound showing the atom-labelling scheme and displacement ellipsoids at the 50% probability level.
. DOI: 10.1107/S1600536814016407/tk5328fig2.tif
View of supramolecular chain formed by N—H⋯S interactions (dashed lines).
CCDC reference: 1014062
Additional supporting information: crystallographic information; 3D view; checkCIF report